Congenital heart disease (CHD), which is present in around 1.0% (1 in 110) of all live births in the United States, is the most common birth defect.1, 2, 3, 4, 5 Defined generally as malformations present at birth that involve the heart or major associated blood vessels, CHD includes a remarkably heterogeneous group of chronic conditions, with very different phenotypes, prevalence, risk factors, and outcomes. CHD is a significant contributor to birth‐defect–related morbidity, mortality, and healthcare costs6 in early life and increasingly among adolescents and adults.7 Because of their broad impact at the population level, a public health approach is needed to address the challenges of these common, critical, and costly conditions.8 We sought to create a framework to address CHD from a population‐based perspective, to serve as a model for a public health agenda for the United States, with a goal of improving the lives of those with or at risk for CHD. This framework is complementary to previous work outlining the Centers for Disease Control and Prevention's scientific priorities related to CHD,9 because implementation strategies are also needed in addition to addressing gaps in scientific knowledge.9, 10, 11
The CHD framework is a public health model for addressing disease at the population level, which emphasizes monitoring, interventions, and optimizing outcomes at the population level. Core components of a public health model are: (1) identifying or monitoring the occurrence and outcomes of a condition over time and among different subgroups in the population; (2) investigating factors that impact occurrence and outcomes, specifically causes of disease and modifiers of prognosis; (3) developing interventions and policies to reduce risks and improve outcomes; (4) implementing interventions and policies; and (5) evaluating the effectiveness of such interventions and policies.12 These components are interconnected in that improvements in each component lead to improvements in the entire framework and success in addressing public health challenges.
The main features of the public health framework for CHD, presented in language appropriate for a lay audience, have three key pillars: Identify and Investigate, Develop Interventions and Policies, and Implement and Evaluate (Figure 1). The scope encompasses everyone at risk for CHD or living with CHD to underscore the need for equitable and universal access to care delivery and services. In the following sections, components are discussed, along with key issues identified by the Congenital Heart Public Health Consortium (CHPHC) for improved outcomes. Key opportunities to advance a public health agenda for CHD are listed (Table 1).
Figure 1.
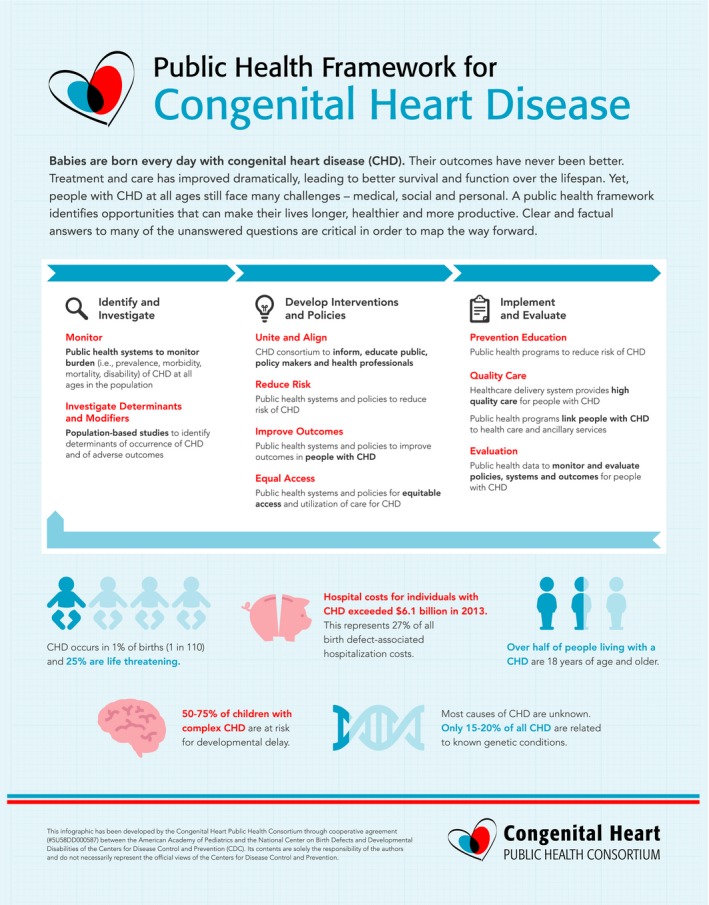
Congenital heart public health consortium public health framework for congenital heart disease.
Table 1.
Key Opportunities to Advance Public Health for Individuals With Congenital Heart Disease
| Identify and Investigate | |
|---|---|
| Monitor | |
| 1 | Initiate comprehensive population‐based monitoring of the incidence, prevalence, morbidity and mortality of congenital heart defects across the life span |
| Investigate determinants and modifiers | |
| 2 | Leverage existing data to examine epidemiological and clinical factors associated with better and worse health outcomes health service delivery |
| Develop Interventions and Policies | |
|---|---|
| Unite and align | |
| 3 | Design universally accepted policies and interventions to improve access to appropriate care, including specialty care and services |
| Reduce risk | |
| 4 | Identify optimum timing of type of procedural medical intervention to inform treatment decisions in infancy, childhood, and adulthood |
| 5 | Research to identify strategies to reduce cardiac and noncardiac morbidity, including the brain, lungs, liver, and kidneys |
| 6 | Initiate practical, effective, and sustainable interventions for known modifiable risk factors for congenital heart disease that have public health importance (eg, maternal pregestational diabetes mellitus) |
| Improve outcomes | |
| 7 | Improve access to special education and/or other school‐based interventions for all children with congenital heart disease who have a neurodevelopmental impairment |
| 8 | Develop formal transition programs between pediatric and adult care and ongoing monitoring to assess the success or obstacles to transition efforts |
| 9 | Initiate programs to assure adequate support services for adults with neurocognitive decline |
| Equal access | |
| 10 | Encourage insurance availability for congenital heart disease care across the life span, including specialty care when necessary |
| 11 | Develop programs to assure that all people with congenital heart disease have primary care in a patient‐centered medical home that includes supports to family members and caregivers |
| Implement and Evaluate | |
|---|---|
| Prevention education | |
| 12 | Target educational programs to individuals and the medical community to disseminate information about proven and effective strategies to prevent congenital heart disease |
| Quality care | |
| 13 | Project workforce necessary to care for the growing population of adults with congenital heart disease and adjust the number of fellowship training programs and positions accordingly |
| 14 | Optimize healthcare systems with adequate specialty care for cardiac and noncardiac conditions to address the needs of people with congenital heart disease |
| Evaluation | |
| 15 | Develop and track key quality measures related to care for congenital heart disease and congenital heart disease–related population health |
Identify and Investigate
Identification includes monitoring both prevalence and outcomes—CHD prevalence at birth and across the life span as well as survival, morbidity, and disability. At any age, prevalence depends on both birth prevalence and survival. Disparities in survival based on race or ethnicity, or associated factors such as socioeconomic status or parental education, impact the prevalence of specific types of CHD at different ages.13 Reliable population‐based monitoring of CHD requires several elements: operational case definitions that are clear and consistent; a source population that is well defined in time and space; and a sustainable ascertainment system of CHD occurrence and outcomes that is accurate, complete, and timely. In the United States, there are well‐established programs for monitoring CHD at birth. The National Birth Defects Prevention Network has provided guidance for monitoring 16 specific types of CHD among births.14 In contrast, population‐based monitoring of CHD beyond infancy and childhood nationwide is much less developed and has significant gaps.15 These gaps pose a major challenge for population‐based public health programs aimed at addressing the needs of people with CHD across the life span. A fragmented system of care for those with CHD contributes to this challenge, particularly for adults. The situation in the United States is in contrast to a few successful models of population‐based monitoring of CHD throughout the life span.16, 17
Investigation includes epidemiological research to understand key elements such as: (1) factors that increase or decrease the risk of developing CHD and (2) factors, including genetic factors, that modify outcomes in those born with CHD, including survival, health, quality of life, societal integration, and other long‐term outcomes, such as neurodevelopmental and psychosocial outcomes and reproductive health. Currently, most causes of CHD are not known. A large fraction of CHD is thought to have a multifactorial etiology—that is, many cases are thought to be caused by a variable and mostly undetermined combination of environmental and genetic factors.18, 19 Determining the nature and contribution of these factors to the risk of developing CHD has proven remarkably difficult. Nevertheless, such research is crucial to design evidence‐based interventions aimed at primary prevention (reducing the number of newborn cases of CHD)9 and secondary prevention (reducing complications and improving outcomes for the many infants who continue to be born with CHD).20 The population of adolescents and adults with CHD continues to grow rapidly, underscoring a need to investigate modifiable population‐level factors that can be leveraged to improve outcomes across the life span. Recent evidence suggests ongoing risk for early mortality, even after repair of less‐severe CHD lesions.7 Multiple data sets are available and potentially useful to examine CHD outcomes and health services utilization.21
Key Issues Related to Monitoring
Infants with CHD are at increased risk for morbidity, mortality, and developmental disabilities not only in infancy, but also for decades later.13, 22, 23
Children and adults with CHD can develop problems in numerous other organ systems, most notably the neurological,23, 24, 25, 26 pulmonary,27 renal,28 gastrointestinal,29 and hematologic/oncological30 systems. Adults with complex CHD are at risk for lower functioning, achievement, executive function, memory, language, social interactions, and quality of life.31 Risk factors for brain injury are cumulative and synergistic.32, 33
There is no nation‐wide system for monitoring the number and health of people living with CHD.
There is no system to monitor outcomes of offspring of people with CHD, or to monitor the outcomes of pregnancies among women with CHD.
Limited information is available regarding racial, ethnic, and socioeconomic characteristics of people living with CHD.
Key Issues Related to Investigating Determinants and Modifiers
Primary Prevention
Nongenetic factors have been linked to increased risk for CHD, including maternal conditions such as uncontrolled pregestational diabetes, and pregnancy exposures, such as some infections and medications.18, 34, 35, 36 As a group, however, recognized environmental or maternal risk factors still account for a small fraction, likely ≤10%, of nonsyndromic CHD in the population.35, 36 Most women who give birth to children with CHD do not have or report exposures to known risk factors; even among those who report such exposures, the causal role of the exposure can be difficult to establish in any individual case.
Prenatal exposure to prescription opioids has been linked to risk of some types of CHD,37 in addition to the well‐documented causal relationship with neonatal abstinence syndrome. This type of risk factor with growing levels of prenatal exposure merits additional research to better understand the prevalence, timing, and correlates of prenatal exposure linked to greatest risk for infants.
The proportion of CHD cases attributed to genetic causes—chromosomal anomalies, genomic disorders (deletions or duplications), and single‐gene conditions—is still unclear. Chromosomal anomalies alone seem to account for ≈10% to 15% of cases of congenital heart defects.38, 39 The risk of some of these chromosomal anomalies (eg, trisomy 21, 18, and 13) are influenced by maternal age. As a group, single‐gene disorders (eg, the Noonan, Alagille, and CHARGE syndromes) probably account for a much smaller fraction of cases.38 However, recent findings using more‐advanced technology suggest that de novo mutations and novel copy number variants may account for an additional 10% to 15% of incident cases.40, 41, 42 What remains largely unclear is to what extent genetic loci contribute to disease risk and, in particular, to gene‐environment interactions that are modifiable by preventive interventions.
Secondary Prevention
Appropriate timing for repeat interventional procedures is not well established. Timing is important given that delayed repair may lead to sequelae such as irreversible myocardial dysfunction and arrhythmia. Conversely, procedures with limited durability may result in the need for additional procedures over the life span.
Emerging data suggest that older individuals with CHD are at increased risk for mortality, driven by coronary artery disease, heart failure, and ventricular dysfunction.43
Risk for coronary artery disease is related to age, hyperlipidemia, and hypertension.44 Certain types of CHD, such as coarctation of the aorta, may increase the risk for hypertension. Exercise restrictions may increase the risk for obesity. Little is known about how to prevent or treat acquired heart disease in people with CHD.
Develop Interventions and Policies
Public health interventions and policies that focus on CHD can improve the health and well‐being of people with CHD. Uniting and aligning efforts among stakeholders should accelerate effectively addressing CHD from a public health perspective. The CHPHC was formed in 2009 as an organization of stakeholders utilizing public health principles to affect change for those with CHD at the population level (http://www.chphc.org).10, 11 The CHPHC has over 200 members, including individuals and organizations, as well as liaisons from key federal agencies (Table 2). The activities of the CHPHC are coordinated by the American Academy of Pediatrics, with support from the Centers for Disease Control and Prevention. Since inception, the CHPHC has accomplished multiple initiatives, such as assembling information to disseminate key facts, identifying databases available for CHD surveillance and research, and public awareness campaigns on various topics related to CHD. The CHPHC can play a key role in aligning efforts to advance a public health agenda to improve lives for those affected by CHD.
Table 2.
Congenital Heart Public Health Consortium Steering Committee Member Organizations and Federal Liaisons
| Steering Committee Members | Federal Liaisons |
|---|---|
|
Adult Congenital Heart Association Alliance for Adult Research in Congenital Cardiology American Academy of Pediatrics Section on Cardiology and Cardiac Surgery American College of Cardiology Adult Congenital & Pediatric Cardiology Section American Heart Association Cardiovascular Disease in the Young Children's Heart Foundation Congenital Heart Surgeon's Society National Birth Defects Prevention Network Mended Little Hearts March of Dimes (2009–2017) Pediatric Congenital Heart Association Society for Thoracic Surgeons |
Centers for Disease Control and Prevention National Center on Birth Defects and Developmental Disabilities National Institutes of Health National Heart, Lung, and Blood Institute Agency for Healthcare Research and Quality Center for Quality Improvement and Patient Safety Health Resources and Services Administration |
To date, there are few specific policies or interventions designed to reduce the impact of CHD on the US population. An exception is the implementation of newborn screening using pulse oximetry to detect critical CHD at birth and decrease infant mortality resulting from undiagnosed CHD. Fortification of cereals with folic acid, although aimed at preventing other birth defects (ie, neural tube defects), may prevent some CHD.18 Because of the many gaps in current knowledge, more research—particularly translational research—is needed. However, several important opportunities for primary and secondary prevention of CHD are currently available based on what is already known about modifiable risk factors.
Key Issues Related to Uniting and Aligning
Stakeholders interested in improving CHD outcomes across the life span often work independently, without alignment.
Key Issues Related to Reducing Risk
Known modifiable risk factors for CHD include pregestational diabetes without adequate control, uncontrolled maternal phenylketonuria, and maternal pregnancy exposures, such as infections and the use of certain medications, continue to occur.
Key Issues Related to Improving Outcomes
Many children with CHD have difficulties in cognition, language development, visual construction and perception, visual motor integration, executive function, attention, impulsivity, and fine and gross motor skills.23 Poor executive functioning is closely associated with lower quality of life and school functioning in the CHD school‐aged population.45 Unrecognized or untreated neurodevelopmental impairments may lead to lower quality of life for children with CHD.25, 46, 47
Children with CHD, particularly those with more‐severe forms, can be screened for neurodevelopmental impairments.23
Adults with CHD also have altered cognition and neuropsychological and neurological impairments48 that can impact quality of life and workplace success.
Many adolescents and adults with CHD are not receiving specialty care.49, 50
Key Issues Related to Equal Access
Lack of healthcare providers with expertise in CHD, including cardiologists and cardiac surgeons, can preclude access, particularly in rural areas, or other geographical locations.
Insurance barriers can preclude access to necessary care for CHD, even when such care is available.
Unequal access to healthcare information related to CHD care may cause individuals or families to not seek appropriate care.
Implement and Evaluate
The measure of success of the public health approach aimed at improving both primary and secondary prevention is the extent to which it realizes a major reduction in the health impact of CHD in the entire population. While acknowledging that more research is needed, it is also important to develop and implement solutions based on what is already known. For example, maternal pregestational diabetes mellitus is an established risk factor for CHD,51, 52, 53 and primary prevention targeting diabetes mellitus before conception is possible today.54, 55, 56 It is also well established that diabetic women who are in optimal glycemic control immediately before and during pregnancy can reduce their risk of having a baby with CHD to nearly the level of those without pregestational diabetes mellitus.51, 54, 55 More concerted efforts could be undertaken to target screening and management of diabetes mellitus among childbearing‐aged women at high risk of diabetes mellitus, with implementation of both individual‐ and population‐level interventions. These efforts would include increasing awareness among childbearing‐aged women and healthcare providers about the risk of CHD associated with pregestational diabetes mellitus, as well as improving access to screening and care for diabetes mellitus to increase the proportion of childbearing‐aged women with pregestational diabetes mellitus in optimal glycemic control. Modeling has estimated that ≈2670 congenital heart defects could be prevented annually in the United States if interventions succeeded in ensuring all women with pregestational diabetes mellitus were in optimal glycemic control immediately before and during pregnancy.52
Similarly, secondary prevention should include comprehensive strategies with policy changes that improve access to specialty care across the life span, such as individual‐level education of cardiologists and patients regarding the importance of life‐long specialty care. Programs to improve secondary outcomes may target specific populations, such as implementation of neurodevelopmental screening for all children with CHD, as well as neurocognitive care and preventive care for adult patients as they age.32
Evaluation is instrumental in demonstrating program effectiveness and allowing effective pilot intervention programs to be expanded to reach a broader population. For example, newborn screening based on pulse oximetry for critical CHD in the United States began in 2011, and, as of 2015, 43 states had taken steps toward implementing universal screening.57 The goal of critical CHD screening is to reduce morbidity and mortality associated with delayed diagnoses, and evaluation will be essential in quantifying the impact of this public health intervention. Similarly, screening children and adults with CHD for neurodevelopmental/neurocognitive and psychosocial issues will provide secondary prevention opportunities to reduce the health impact of CHD on individuals over their entire life span through appropriate therapeutic interventions.23, 32, 33, 48, 58
Key Issues Related to Prevention Education
Few educational programs exist that are targeted to individuals and the medical community about reasonable strategies to prevent CHD, based on current knowledge.
Key Issues Related to Quality Care
Appropriately treating the medical and nonmedical needs of children with CHD is difficult. For providers, creating a patient‐centered medical home for children with CHD, particularly those with complex disease, is particularly important.59 For families, the toll on parents and siblings can be burdensome.60
People with CHD begin to leave specialty care around age 8 years, over half are lost to follow‐up by age 18 years,61, 62 and >40% of adult CHD patients note a prolonged gap in cardiology care, typically around age 19 to 20 years50, 63; those with gaps in care are more likely to have adverse outcomes.64, 65
Transition of care from pediatric cardiologists to adult cardiologists with expertise in CHD is inconsistent, and the optimum transition practice is not known.
Accurate projections of the workforce needed to care for the growing population of adults with CHD are lacking.
As neurodevelopmental sequelae in children with CHD evolve to cognitive decline or dementia during adulthood, a growing population of individuals living with CHD may require support services.
The American Board of Internal Medicine and American Board of Pediatrics have recently established fellowships in adult CHD, with different pathways and a board examination. The Adult Congenital Heart Association is developing program accreditation standards and center accreditation is being piloted.
Summary and Recommendations
A public health framework is presented to guide a public health agenda for CHD in the United States. The framework includes: (1) identification and investigation, including public health monitoring systems and population‐based research; (2) development of interventions and policies, including aligning stakeholders, creating public systems and policies to reduce risk, improving outcomes, and ensuring equitable access and utilization of care; and (3) implementation and evaluation, including education and quality care programs; connecting individuals, health care, and ancillary services; and evaluation of systems. The CHPHC aligns key stakeholders as a public‐private partnership to reduce death and disability from CHD across the life span. Collective efforts within the framework by CHPHC members are addressing all components with improved coordination (Figure 2). Key opportunities to advance a public health agenda for CHD are listed in Table 1, including opportunities for research, monitoring, and implementation. The CHD population is growing, with significant risks, comorbidities, and enhanced need for healthcare resources. Knowledge gaps currently exist in many areas, and few policies and programs are specifically designed to reduce risk or improve outcomes for people with CHD. Future efforts aligned with the framework should accelerate knowledge and strategies to more rapidly reduce disease burden and improve outcomes at a population level for those affected by CHD.
Figure 2.
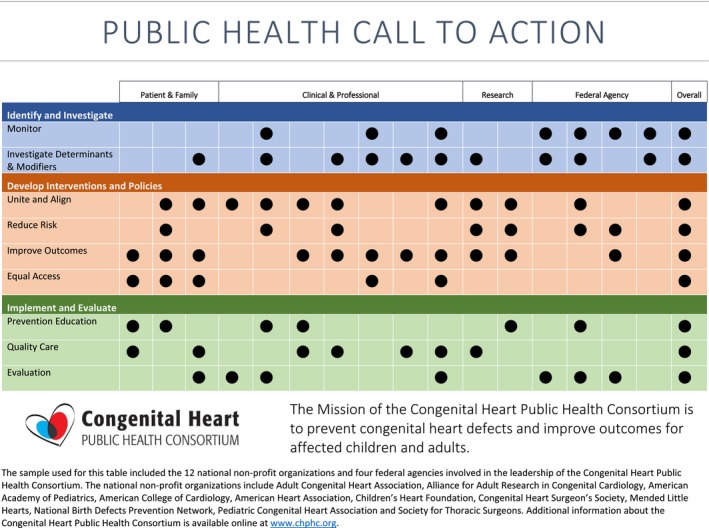
Grid displaying CHPHC member activities related to public health framework call to action. CHPHC indicates Congenital Heart Public Health Consortium.
Sources of Funding
The Congenital Heart Public Health Consortium is funded by a cooperative agreement between the Centers for Disease Control and Prevention (Atlanta, GA) and the American Academy of Pediatrics (Itasca, IL). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or member organizations of the Congenital Heart Public Health Consortium.
Disclosures
None.
Acknowledgments
The authors acknowledge Kristin Burns, MD, Jill Glidewell, MSN, MPH, and Kara Polen, MPH.
J Am Heart Assoc. 2019;8:e009450 DOI: 10.1161/JAHA.118.009450.
References
- 1. Centers for Disease Control and Prevention . Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978—2005. Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 2. Reller MD, Strickland MJ, Riehle‐Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 4. Moller JH, Taubert KA, Allen HD, Clark EB, Lauer RM. Cardiovascular health and disease in children: current status. A Special Writing Group from the Task Force on Children and Youth, American Heart Association. Circulation. 1994;89:923–930. [DOI] [PubMed] [Google Scholar]
- 5. Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66:1–55. [PubMed] [Google Scholar]
- 6. Arth AC, Tinker SC, Simeone RM, Ailes EC, Cragan JD, Grosse SD. Inpatient hospitalization costs associated with birth defects among persons of all ages—United States, 2013. Morb Mortal Wkly Rep. 2017;66:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spector LG, Menk JS, Knight JH, McCracken C, Thomas AS, Vinocur JM, Oster ME, St Louis JD, Moller JH, Kochilas L. Trends in long‐term mortality after congenital heart surgery. J Am Coll Cardiol. 2018;71:2434–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jenkins KJ, Honein MA. Public health approach to decrease mortality for congenital heart defects: dying too soon. J Am Coll Cardiol. 2018;71:2447–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oster ME, Riehle‐Colarusso T, Simeone RM, Gurvitz M, Kaltman JR, McConnell M, Rosenthal GL, Honein MA. Public health science agenda for congenital heart defects: report from a Centers for Disease Control and Prevention experts meeting. J Am Heart Assoc. 2013;2:e000256 DOI: 10.1161/JAHA.113.000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle‐Colarusso T, Nembhard WN, Xu P, Correa A, Jenkins K, Marelli AJ. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krasuski RA, Bashore TM. Congenital heart disease epidemiology in the United States: blindly feeling for the charging elephant. Circulation. 2016;134:110–113. [DOI] [PubMed] [Google Scholar]
- 12. Fielding J, Teutsch S, Breslow L. A framework for public health in the United States. Public Health Rev. 2010;32:174–189. [Google Scholar]
- 13. Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Birth Defects Prevention Network . Available at: http://www.nbdpn.org/. Webpage Header: Birth Defect Surveillance Guidelines. Updated 2019.
- 15. Centers for Disease Control and Prevention . Available at: http://cdc.gov/ncbddd/heartdefects/data.html. Webpage Header Data and Statistics on Congenital Heart Defects. Updated November 2, 2018.
- 16. Erikssen G, Liestol K, Seem E, Birkeland S, Saatvedt KJ, Hoel TN, Dohlen G, Skulstad H, Svennevig JL, Thaulow E, Lindberg HL. Achievements in congenital heart defect surgery: a prospective, 40‐year study of 7038 patients. Circulation. 2015;131:337–346; discussion, 346. [DOI] [PubMed] [Google Scholar]
- 17. Raissadati A, Nieminen H, Haukka J, Sairanen H, Jokinen E. Late causes of death after pediatric cardiac surgery: a 60‐year population‐based study. J Am Coll Cardiol. 2016;68:487–498. [DOI] [PubMed] [Google Scholar]
- 18. Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA, Webb CL. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995–3014. [DOI] [PubMed] [Google Scholar]
- 19. Pierpont ME, Basson CT, Benson DW Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. [DOI] [PubMed] [Google Scholar]
- 20. Marelli AJ, Ionescu‐Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–756. [DOI] [PubMed] [Google Scholar]
- 21. Riehle‐Colarusso TJ, Bergersen L, Broberg CS, Cassell CH, Gray DT, Grosse SD, Jacobs JP, Jacobs ML, Kirby RS, Kochilas L, Krishnaswamy A, Marelli A, Pasquali SK, Wood T, Oster ME. Databases for congenital heart defect public health studies across the lifespan. J Am Heart Assoc. 2016;5:e004148 DOI: 10.1161/JAHA.116.004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olsen M, Christensen TD, Pedersen L, Johnsen SP, Hjortdal VE. Late mortality among Danish patients with congenital heart defect. Am J Cardiol. 2010;106:1322–1326. [DOI] [PubMed] [Google Scholar]
- 23. Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH Jr, Li J, Smith SE, Bellinger DC, Mahle WT. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. [DOI] [PubMed] [Google Scholar]
- 24. Razzaghi H, Oster M, Reefhuis J. Long‐term outcomes in children with congenital heart disease: National Health Interview Survey. J Pediatr. 2015;166:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marino BS, Tomlinson RS, Wernovsky G, Drotar D, Newburger JW, Mahony L, Mussatto K, Tong E, Cohen M, Andersen C, Shera D, Khoury PR, Wray J, Gaynor JW, Helfaer MA, Kazak AE, Shea JA. Validation of the pediatric cardiac quality of life inventory. Pediatrics. 2010;126:498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lanz J, Brophy JM, Therrien J, Kaouache M, Guo L, Marelli AJ. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation. 2015;132:2385–2394. [DOI] [PubMed] [Google Scholar]
- 27. Alonso‐Gonzalez R, Borgia F, Diller GP, Inuzuka R, Kempny A, Martinez‐Naharro A, Tutarel O, Marino P, Wustmann K, Charalambides M, Silva M, Swan L, Dimopoulos K, Gatzoulis MA. Abnormal lung function in adults with congenital heart disease: prevalence, relation to cardiac anatomy, and association with survival. Circulation. 2013;127:882–890. [DOI] [PubMed] [Google Scholar]
- 28. Taylor ML, Carmona F, Thiagarajan RR, Westgate L, Ferguson MA, del Nido PJ, Rajagopal SK. Mild postoperative acute kidney injury and outcomes after surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2013;146:146–152. [DOI] [PubMed] [Google Scholar]
- 29. Rychik J, Veldtman G, Rand E, Russo P, Rome JJ, Krok K, Goldberg DJ, Cahill AM, Wells RG. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatr Cardiol. 2012;33:1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee YS, Chen YT, Jeng MJ, Tsao PC, Yen HJ, Lee PC, Li SY, Liu CJ, Chen TJ, Chou P, Soong WJ. The risk of cancer in patients with congenital heart disease: a nationwide population‐based cohort study in Taiwan. PLoS One. 2015;10:e0116844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tyagi M, Austin K, Stygall J, Deanfield J, Cullen S, Newman SP. What do we know about cognitive functioning in adult congenital heart disease? Cardiol Young. 2014;24:13–19. [DOI] [PubMed] [Google Scholar]
- 32. Marelli A, Miller SP, Marino BS, Jefferson AL, Newburger JW. Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation. 2016;133:1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Broomall E, McBride ME, Deal BJ, Ducharme‐Crevier L, Shaw A, Mazwi M, Backer CL, Monge MC, Costello J, Marino BS, DeFreitas A, Wainwright MS. Posterior circulation ischemia or occlusion in five adults with failing Fontan circulation. Ann Thorac Surg. 2016;101:2315–2320. [DOI] [PubMed] [Google Scholar]
- 34. Patel SS, Burns TL. Nongenetic risk factors and congenital heart defects. Pediatr Cardiol. 2013;34:1535–1555. [DOI] [PubMed] [Google Scholar]
- 35. Botto LD. Epidemiology and prevention of congenital heart defects In: Allen HD, Shaddy RE, Penny DJ, Feltes TF, Cetta F, eds. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult, 9th ed Philadelphia, PA: Lippincott; 2016:55–86. [Google Scholar]
- 36. Botto LD. Epidemiology and prevention of congenital heart defects In: Muenke M, Kruszka PS, Sable CA, Belmont JW, eds. Congenital Heart Disease: Molecular Genetics, Principles of Diagnosis and Treatment. Basel, Switzerland: Karger; 2015:28–45. [Google Scholar]
- 37. Lind JN, Interrante JD, Ailes EC, Gilboa SM, Khan S, Frey MT, Dawson AL, Honein MA, Dowling NF, Razzaghi H, Creanga AA, Broussard CS. Maternal use of opioids during pregnancy and congenital malformations: a systematic review. Pediatrics. 2017;139:e20164131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goldmuntz E, Crenshaw ME. Genetic aspects of congenital heart defects In: Allen HD, Shaddy RE, Penny DJ, Feltes TF, Cetta F, eds. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents, including the Fetus and Young Adult, 9th ed Philadelphia, PA: Lippincott; 2016:87–115. [Google Scholar]
- 39. Hartman RJ, Rasmussen SA, Botto LD, Riehle‐Colarusso T, Martin CL, Cragan JD, Shin M, Correa A. The contribution of chromosomal abnormalities to congenital heart defects: a population‐based study. Pediatr Cardiol. 2011;32:1147–1157. [DOI] [PubMed] [Google Scholar]
- 40. Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, Romano‐Adesman A, Bjornson RD, Breitbart RE, Brown KK, Carriero NJ, Cheung YH, Deanfield J, DePalma S, Fakhro KA, Glessner J, Hakonarson H, Italia MJ, Kaltman JR, Kaski J, Kim R, Kline JK, Lee T, Leipzig J, Lopez A, Mane SM, Mitchell LE, Newburger JW, Parfenov M, Pe'er I, Porter G, Roberts AE, Sachidanandam R, Sanders SJ, Seiden HS, State MW, Subramanian S, Tikhonova IR, Wang W, Warburton D, White PS, Williams IA, Zhao H, Seidman JG, Brueckner M, Chung WK, Gelb BD, Goldmuntz E, Seidman CE, Lifton RP. De novo mutations in histone‐modifying genes in congenital heart disease. Nature. 2013;498:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Al Turki S, Manickaraj AK, Mercer CL, Gerety SS, Hitz MP, Lindsay S, D'Alessandro LC, Swaminathan GJ, Bentham J, Arndt AK, Low J, Breckpot J, Gewillig M, Thienpont B, Abdul‐Khaliq H, Harnack C, Hoff K, Kramer HH, Schubert S, Siebert R, Toka O, Cosgrove C, Watkins H, Lucassen AM, O'Kelly IM, Salmon AP, Bu'lock FA, Granados‐Riveron J, Setchfield K, Thornborough C, Brook JD, Mulder B, Klaassen S, Bhattacharya S, Devriendt K, Fitzpatrick DF, Wilson DI, Mital S, Hurles ME. Rare variants in NR2F2 cause congenital heart defects in humans. Am J Hum Genet. 2014;94:574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Warburton D, Ronemus M, Kline J, Jobanputra V, Williams I, Anyane‐Yeboa K, Chung W, Yu L, Wong N, Awad D, Yu CY, Leotta A, Kendall J, Yamrom B, Lee YH, Wigler M, Levy D. The contribution of de novo and rare inherited copy number changes to congenital heart disease in an unselected sample of children with conotruncal defects or hypoplastic left heart disease. Hum Genet. 2014;133:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tutarel O, Kempny A, Alonso‐Gonzalez R, Jabbour R, Li W, Uebing A, Dimopoulos K, Swan L, Gatzoulis MA, Diller GP. Congenital heart disease beyond the age of 60: emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur Heart J. 2014;35:725–732. [DOI] [PubMed] [Google Scholar]
- 44. Giannakoulas G, Dimopoulos K, Engel R, Goktekin O, Kucukdurmaz Z, Vatankulu MA, Bedard E, Diller GP, Papaphylactou M, Francis DP, Di Mario C, Gatzoulis MA. Burden of coronary artery disease in adults with congenital heart disease and its relation to congenital and traditional heart risk factors. Am J Cardiol. 2009;103:1445–1450. [DOI] [PubMed] [Google Scholar]
- 45. Gerstle M, Beebe DW, Drotar D, Cassedy A, Marino BS. Executive functioning and school performance among pediatric survivors of complex congenital heart disease. J Pediatr. 2016;173:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mellion K, Uzark K, Cassedy A, Drotar D, Wernovsky G, Newburger JW, Mahony L, Mussatto K, Cohen M, Limbers C, Marino BS. Health‐related quality of life outcomes in children and adolescents with congenital heart disease. J Pediatr. 2014;164:781–788.e1. [DOI] [PubMed] [Google Scholar]
- 47. O'Donovan CE, Painter L, Lowe B, Robinson H, Broadbent E. The impact of illness perceptions and disease severity on quality of life in congenital heart disease. Cardiol Young. 2016;26:100–109. [DOI] [PubMed] [Google Scholar]
- 48. Wilson WM, Smith‐Parrish M, Marino BS, Kovacs AH. Neurodevelopmental and psychosocial outcomes across the congenital heart disease lifespan. Prog Pediatr Cardiol. 2015;39:113–118. [Google Scholar]
- 49. Mylotte D, Pilote L, Ionescu‐Ittu R, Abrahamowicz M, Khairy P, Therrien J, Mackie AS, Marelli A. Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation. 2014;129:1804–1812. [DOI] [PubMed] [Google Scholar]
- 50. Gurvitz M, Valente AM, Broberg C, Cook S, Stout K, Kay J, Ting J, Kuehl K, Earing M, Webb G, Houser L, Opotowsky A, Harmon A, Graham D, Khairy P, Gianola A, Verstappen A, Landzberg M. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART‐ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol. 2013;61:2180–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Starikov R, Bohrer J, Goh W, Kuwahara M, Chien EK, Lopes V, Coustan D. Hemoglobin A1c in pregestational diabetic gravidas and the risk of congenital heart disease in the fetus. Pediatr Cardiol. 2013;34:1716–1722. [DOI] [PubMed] [Google Scholar]
- 52. Simeone RM, Devine OJ, Marcinkevage JA, Gilboa SM, Razzaghi H, Bardenheier BH, Sharma AJ, Honein MA. Diabetes and congenital heart defects: a systematic review, meta‐analysis, and modeling project. Am J Prev Med. 2015;48:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oyen N, Diaz LJ, Leirgul E, Boyd HA, Priest J, Mathiesen ER, Quertermous T, Wohlfahrt J, Melbye M. Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation. 2016;133:2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kitzmiller JL, Buchanan TA, Kjos S, Combs CA, Ratner RE. Pre‐conception care of diabetes, congenital malformations, and spontaneous abortions. Diabetes Care. 1996;19:514–541. [DOI] [PubMed] [Google Scholar]
- 55. Kitzmiller JL, Wallerstein R, Correa A, Kwan S. Preconception care for women with diabetes and prevention of major congenital malformations. Birth Defects Res A Clin Mol Teratol. 2010;88:791–803. [DOI] [PubMed] [Google Scholar]
- 56. Correa A. Pregestational diabetes mellitus and congenital heart defects. Circulation. 2016;133:2219–2221. [DOI] [PubMed] [Google Scholar]
- 57. Glidewell J, Olney RS, Hinton C, Pawelski J, Sontag M, Wood T, Kucik JE, Daskalov R, Hudson J. State legislation, regulations, and hospital guidelines for newborn screening for critical congenital heart defects—United States, 2011–2014. Morb Mortal Wkly Rep. 2015;64:625–630. [PMC free article] [PubMed] [Google Scholar]
- 58. Marino BS. New concepts in predicting, evaluating, and managing neurodevelopmental outcomes in children with congenital heart disease. Curr Opin Pediatr. 2013;25:574–584. [DOI] [PubMed] [Google Scholar]
- 59. Fernandes SM, Sanders LM. Patient‐centered medical home for patients with complex congenital heart disease. Curr Opin Pediatr. 2015;27:581–586. [DOI] [PubMed] [Google Scholar]
- 60. Caicedo C. Families with special needs children: family health, functioning, and care burden. J Am Psychiatr Nurses Assoc. 2014;20:398–407. [DOI] [PubMed] [Google Scholar]
- 61. Reid GJ, Irvine MJ, McCrindle BW, Sananes R, Ritvo PG, Siu SC, Webb GD. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113:e197–e205. [DOI] [PubMed] [Google Scholar]
- 62. Mackie AS, Ionescu‐Ittu R, Therrien J, Pilote L, Abrahamowicz M, Marelli AJ. Children and adults with congenital heart disease lost to follow‐up: who and when? Circulation. 2009;120:302–309. [DOI] [PubMed] [Google Scholar]
- 63. Heery E, Sheehan AM, While AE, Coyne I. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease: a systematic review. Congenit Heart Dis. 2015;10:413–427. [DOI] [PubMed] [Google Scholar]
- 64. de Bono J, Freeman LJ. Aortic coarctation repair—lost and found: the role of local long term specialised care. Int J Cardiol. 2005;104:176–183. [DOI] [PubMed] [Google Scholar]
- 65. Yeung E, Kay J, Roosevelt GE, Brandon M, Yetman AT. Lapse of care as a predictor for morbidity in adults with congenital heart disease. Int J Cardiol. 2008;125:62–65. [DOI] [PubMed] [Google Scholar]


