Abstract
Background
Given that statins are increasingly being used for primary‐prevention, the public concerns regarding the risk of new‐onset diabetes mellitus associated with statin use may be an issue.
Methods and Results
Using healthcare data from the national health insurance examinees, our study comprised a cohort of adults aged ≥40 years with hypercholesterolemia who would be eligible for statin therapy for primary prevention from 2005 to 2012. The primary outcome was the occurrence of clinically relevant new‐onset diabetes mellitus requiring medical therapy. Among 2 162 119 adults with hypercholesterolemia who might be eligible for statin therapy, 638 625 (29.5%) ever used statins and 1 523 494 (70.5%) never used statins. In the propensity‐matched cohort of 518 491 pairs, during mean follow‐up of 3.9 years, being an ever‐user of statin was significantly associated with diabetes mellitus risk compared with being a never‐user of statin (13.4 versus 6.9 per 1000 person‐years; adjusted hazard ratio [HR], 1.88; 95% CI, 1.85–1.93). With increasing duration of statin use, the risk of diabetes mellitus was proportionally increased (HR 1.25 <1 year, HR 2.22 for 1–2 years, and HR 2.62 >2 years). An excess risk of diabetes mellitus was also associated with a higher intensity (HR 1.75 for low‐to‐moderate potency and HR 2.31 for high potency) and a cumulative dosing of statin (HR 1.06 for low‐tertile, HR 1.74 for middle‐tertile, and HR 2.52 for high‐tertile of defined‐daily‐disease).
Conclusions
In patients receiving statin therapy for primary prevention, there was a time‐ and dose‐dependent association of statin use with an increasing risk of new‐onset diabetes mellitus.
Keywords: diabetes mellitus, hypercholesterolemia, statin
Subject Categories: Risk Factors; Diabetes, Type 2; Cardiovascular Disease; Primary Prevention
Short abstract
See Editorial Robinson
Clinical Perspective
What Is New?
In the current practice pattern of statin therapy that is more broadly indicated for primary prevention, the adverse effect of statin therapy on increasing the risk of new‐onset diabetes mellitus is concerning.
However, given that indications of statin therapy are widely expanding, and more potent and long‐term use of statin is recommended in daily practice, a time‐ and dose‐dependent effect of statin on diabetes mellitus risks remains to be determined and is of particular relevance for primary‐prevention patients.
What Are the Clinical Implications?
In this analysis of data from the national health insurance examinees, we identified a strong association between statin therapy and the risk of new‐onset diabetes mellitus in primary prevention. There was a time‐ and dose‐dependent association of statin use with an increasing risk of incident diabetes mellitus.
It should be further discussed whether the absolute benefit of treatment substantially outweighs the diabetes mellitus risk in primary prevention in low‐risk patients, and further research is warranted to identify more vulnerable patients who are at a higher risk of developing incident diabetes mellitus.
Large‐scale evidence from randomized controlled trials (RCTs) shows that statin therapy effectively prevents the progression of atherosclerotic cardiovascular disease and reduces the risk of major cardiovascular events.1 On the basis of these proven benefits, the indications for statin therapy have expanded and more intensified strategies have been recommended for primary and secondary prevention.2 In addition, since statin therapy has been shown to reduce atherosclerotic cardiovascular disease risk during each year it continues to be taken, larger absolute benefits would accrue with more prolonged therapy, and these benefits persist long term.
However, the safety issue of statin therapy regarding an increased risk of incident diabetes mellitus is controversial.1, 3 Indeed, several trial data, meta‐analyses, and observational studies suggest that statin use is associated with an increased risk of new‐onset diabetes mellitus.4, 5, 6, 7, 8, 9 Although the benefits of statins outweigh the risks for many different patient populations and the implications of statin‐induced diabetes mellitus on the long‐term outcomes are uncertain,1 concerns regarding the statin‐induced diabetes mellitus risks have been continuously raised, particularly in primary prevention, for which statins are increasingly being used according to guideline changes.10, 11 Given that indications of statin therapy are widely expanding and more potent and long‐term use of statins is recommended in daily practice, a time‐ and dose‐dependent effect of statin on diabetes mellitus risks remains to be determined and is of particular relevance for primary‐prevention patients. Using large‐scale national healthcare data from the national health insurance examinees, we therefore examined the association between statin therapy and new‐onset diabetes mellitus according to duration, intensity, and cumulative dose of statins in the real‐world setting of primary prevention.
Methods
Data Sources
Anonymized data and study materials have been made publicly available.12 The analytic methods have been made available within the article to other researchers for purposes of reproducing the results or replicating the procedure.
We used healthcare data from national health insurance examinees endorsed by the National Health Insurance Services. As the single payer under the universal health coverage system, which covers ≈98% of the Korean population, the National Health Insurance Services provides a periodic general health examination for all insured individuals and their dependents.13 The databases of national health insurance examinees include comprehensive healthcare data, including demographics, socioeconomic status, self‐reported health‐related risk factors, smoking status, and biochemical data. The National Health Insurance Services databases also contain population‐level data on physician billing claims, on diagnoses, treatments, procedures, surgical history, and medical prescriptions, which are reimbursed by the government according to the National Health Insurance Act.12 The prescription claims data identified dispensed prescriptions, including medication, date filled, days supplied, pill number, and dosage. Based on these data sets, we collected information on demographics, clinical covariates, all diagnostic and procedure information at inpatient and outpatient encounters, statin prescription, and concomitant cardioactive medications of patients in the study cohort (Table S1). This study was approved by the institutional review board of the National Evidence‐based Healthcare Collaborating Agency (NECA‐A‐15‐002). The data are anonymous, and the requirement for informed consent was therefore waived.
Study Population
Using healthcare data from national health insurance examinees linked with administrative claims‐based data sets, we assembled a base cohort of adults with hypercholesterolemia (defined as total cholesterol levels of ≥240 mg/dL) between January 1, 2005 and December 31, 2012. During the study period, statin use for patients with hypercholesterolemia in primary prevention was fully reimbursed by government insurance, which was the most crucial factor determining statin prescription in routine clinical practice.11, 14 Thus, our study included adults aged ≥40 years who did not have a history of atherosclerotic cardiovascular disease and had a total cholesterol level of >240 mg/dL as primary‐prevention cohort who would be eligible for statin therapy. Among them, we then sequentially excluded, in descending order, patients who had received any lipid‐lowering therapy in the previous 3 years before cohort entry, in order to exclude patients who were not new users of statin; patients who received a diagnosis of diabetes mellitus in the year before base‐cohort entry; patients who had malignancy or cancer; patients who died at the year of cohort entry; patients who did not have health examination data; and patients who had follow‐up period <6 months after cohort entry (Figure 1).
Figure 1.
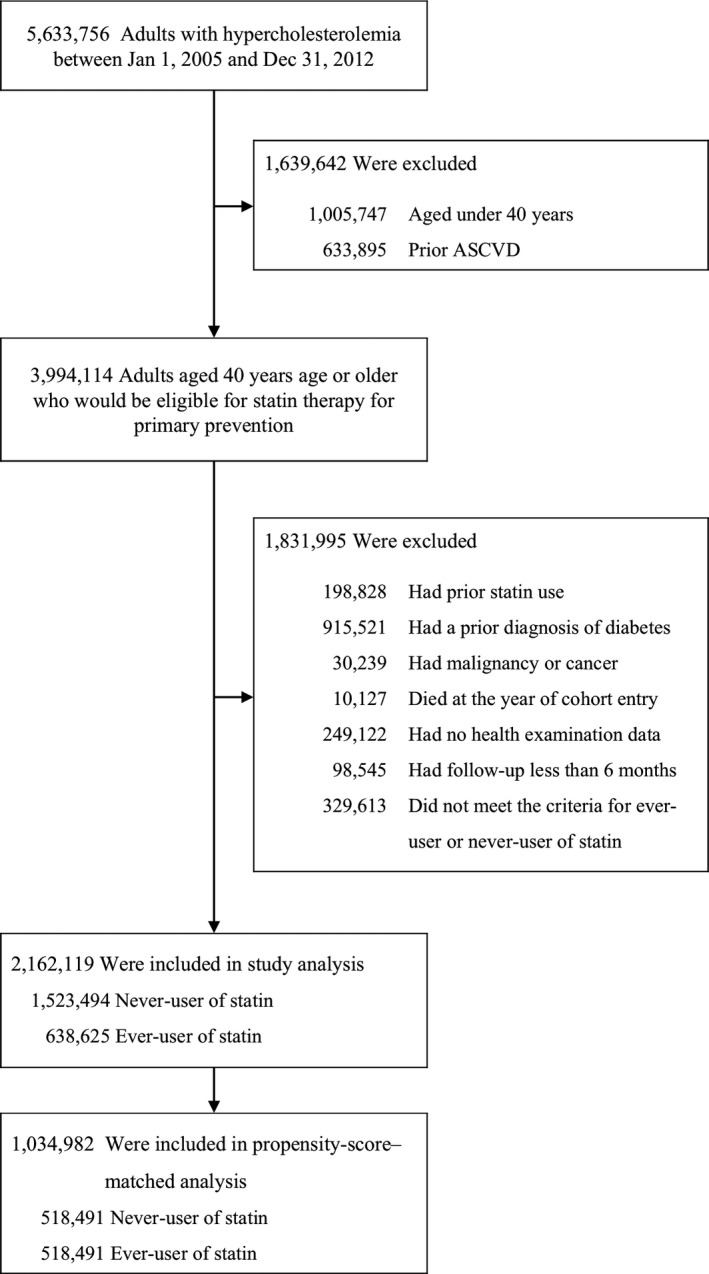
Creation of the study population. Hypercholesterolemia was defined as total cholesterol levels of ≥240 mg/dL. ASCVD indicates atherosclerotic cardiovascular disease.
Exposure and Outcome Assessment
The drug exposure of interest was ever or never use of statin therapy. Ever use of statin was defined as having filled at least 2 prescriptions for the drug within a 6‐month period. Once a patient met the exposure definition, he or she was considered exposed from that point forward: thus, we used the date of the second prescription as the index date for statin use. This time period between first and second prescriptions accounted for nonadherence and for the drug's biologic effect. Never use of statin was defined as patients who did not receive any statin therapy at any time period. We excluded patients who did not meet the criteria of ever‐ or never‐use exposure definition of statin (Figure 1). Among patients who received statin therapy, we subdivided cohorts according to the duration, intensity, and cumulative dose of statins. Duration of statin use was classified as <1, 1 to 2 years, and >2 years after the index date of statin use. For statin intensity, patients who received an atorvastatin dose of 40 to 80 mg/d or rosuvastatin dose of 20 to 40 mg/d was categorized as high‐intensity dose and other therapies were categorized as having low‐to‐moderate intensity. For computing the cumulative dose for statin, we applied the definition of defined daily disease by the World Health Organization.15 The defined daily disease is the assumed average maintenance dose per day for a drug used for its main indication in adults.
The primary outcome of the study was the newly developed, clinically relevant type 2 diabetes mellitus during follow‐up. Clinically relevant, new‐onset diabetes mellitus was defined on the basis of the new principal, inpatient or outpatient diagnostic code for type 2 diabetes mellitus (according to the International Classification of Diseases, Tenth Revision [ICD‐10], disease codes, E11 or E14) and the new prescription of oral antidiabetic drugs or insulin during follow‐up. Patients in each cohort were followed from the date of study‐cohort entry until study outcome occurred or data were censored, whichever occurred first.
Statistical Analysis
Propensity‐score matching was used as the primary tool to adjust for differences in the baseline characteristics between statin user group and statin nonuser group. The propensity score is a conditional probability of having a drug exposure given a set of baseline measured covariates.16 The propensity score was estimated with the use of a nonparsimonious multivariable logistic‐regression model,17 with statin user group as the dependent variable and all the baseline characteristics outlined in Table 1 as covariates. Matching was performed with the use of a nearest‐neighbor–matching algorithm with a “greedy” heuristic (a 1:1 matching protocol without replacement), with a caliper width equal to 0.2 of the SD of the logit of the propensity score. Standardized differences were estimated for all baseline covariates before and after matching to assess prematch imbalance and postmatch balance. Standardized differences of <10.0% for a given covariate indicate a relatively small imbalance.18
Table 1.
Baseline Characteristics of Patients Before and After Propensity‐Score Matchinga
| Characteristic | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| Never User (N = 1 523 494) | Ever User (N = 638 625) | Standardized Differences (%) | Never User (N = 518 491) | Ever User (N = 518 491) | Standardized Differences, % | |
| Age | ||||||
| Mean, y | 51.9±9.1 | 55.5±8.6 | 1.7 | 54.9±9.1 | 55.0±8.6 | 1.8 |
| Distribution | ||||||
| 40–49 | 44.9 (684 364) | 24.7 (157 925) | 43.4 | 26.6 (138 212) | 27.8 (144 384) | 2.7 |
| 50–59 | 36.1 (549 988) | 45.2 (288 332) | 18.5 | 44.6 (231 153) | 44.5 (230 547) | 0.2 |
| 60–69 | 13.3 (202 934) | 23.0 (147 114) | 25.4 | 21.2 (109 754) | 20.7 (107 389) | 1.1 |
| ≥70 | 5.7 (86 208) | 7.1 (45 254) | 5.8 | 7.6 (39 372) | 7.0 (36 171) | 2.3 |
| Sex | ||||||
| Female | 50.0 (762 052) | 63.1 (402 839) | 26.6 | 62.1 (321 818) | 61.3 (317 757) | 1.6 |
| Male | 50.0 (761 442) | 36.9 (235 786) | 26.6 | 37.9 (196 673) | 38.7 (200 734) | 1.6 |
| Income tertile | ||||||
| Low‐tertile | 46.6 (709 963) | 48.0 (306 487) | 2.8 | 48.4 (251 001) | 48.4 (251 080) | 0.0 |
| Middle‐tertile | 29.2 (445 153) | 27.0 (172 458) | 4.9 | 27.3 (141 645) | 27.1 (140 289) | 0.6 |
| High‐tertile | 24.2 (368 378) | 25.0 (159 680) | 1.9 | 24.3 (125 845) | 24.5 (127 122) | 0.6 |
| Body mass indexb | ||||||
| Mean (kg/m2) | 24.3±2.9 | 24.5±2.9 | 8.7 | 24.4±3.0 | 24.4±2.9 | 0.0 |
| Distribution | ||||||
| <20 | 5.9 (89 326) | 4.4 (28 183) | 6.6 | 5.1 (26 447) | 4.9 (25 114) | 1.2 |
| 20–22.4 | 21.3 (324 421) | 19.4 (124 109) | 4.6 | 20.6 (107 029) | 20.3 (105 195) | 0.9 |
| 22.5–24.9 | 34.5 (525 346) | 35.0 (223 186) | 1.0 | 35.1 (181 859) | 35.0 (181 618) | 0.1 |
| ≥25 | 38.3 (584 401) | 41.2 (263 147) | 5.8 | 39.2 (203 156) | 39.8 (206 564) | 1.4 |
| Hypertension | 11.8 (180 423) | 39.6 (252 885) | 67.0 | 29.3 (151 887) | 30.4 (157 713) | 2.5 |
| Current smoking | 36.0 (548 281) | 26.7 (170 745) | 20.0 | 27.5 (142 817) | 28.1 (145 778) | 1.3 |
| Baseline fasting glucose level | ||||||
| Mean (mg/dL) | 98.2±20.6 | 96.4±15.3 | 10.1 | 97.2±18.0 | 96.4±15.5 | 4.8 |
| Distribution | ||||||
| <80 | 8.6 (131 063) | 8.8 (56 126) | 0.7 | 8.8 (45 752) | 8.8 (45 468) | 0.2 |
| 80–99 | 54.6 (832 116) | 56.8 (362 556) | 4.3 | 56.5 (292 869) | 56.7 (293 870) | 0.4 |
| 100–119 | 29.2 (445 167) | 28.9 (184 791) | 0.6 | 28.9 (149 852) | 28.8 (149 430) | 0.2 |
| ≥120 | 7.6 (115 148) | 5.5 (35 152) | 8.3 | 5.8 (30 018) | 5.7 (29 723) | 0.3 |
| Total cholesterol (mg/dL) | 256.7±19.4 | 259.8±32.5 | 11.6 | 262.5±24.6 | 257.6±30.9 | 17.6 |
| Physical activity (no. of exercise per wk) | ||||||
| 0 | 55.3 (842 689) | 55.0 (351 013) | 0.7 | 56.0 (290 340) | 55.3 (286 583) | 1.5 |
| 1–2 | 25.6 (389 288) | 22.9 (146 511) | 6.4 | 22.7 (117 928) | 23.2 (120 546) | 1.2 |
| 3–4 | 11.4 (174 391) | 12.7 (81 069) | 3.8 | 12.3 (63 529) | 12.5 (64 539) | 0.6 |
| ≥5 | 7.7 (117 126) | 9.4 (60 032) | 6.1 | 9.0 (46 694) | 9.0 (46 823) | 0.1 |
| Renal failure | 0.1 (865) | 0.3 (1688) | 5.2 | 0.1 (749) | 0.2 (886) | 0.8 |
| Charlson comorbidity index | ||||||
| Mean | 0.6±0.8 | 1.1±1.1 | 55.4 | 1.0±1.0 | 1.0±1.0 | 2.0 |
| Distribution | ||||||
| 0 | 60.3 (918 135) | 33.0 (211 078) | 56.7 | 36.3 (188 107) | 37.7 (195 541) | 3.0 |
| 1 | 28.2 (430 019) | 38.1 (243 153) | 21.0 | 38.6 (200 287) | 37.8 (196 122) | 1.6 |
| 2 | 8.5 (129 577) | 20.3 (129 550) | 34.0 | 18.2 (94 185) | 17.5 (90 576) | 1.8 |
| ≥3 | 3.0 (45 763) | 8.6 (54 844) | 24.1 | 6.9 (35 912) | 7.0 (36 252) | 0.2 |
| Concomitant cardioactive medications | ||||||
| Aspirin | 1.5 (22 857) | 7.0 (44 644) | 27.5 | 4.0 (20 494) | 4.6 (23 958) | 3.3 |
| β‐blockers | 5.3 (80 420) | 13.1 (83 858) | 27.4 | 10.7 (55 362) | 10.8 (55 936) | 0.4 |
| Calcium‐ channel blockers | 5.0 (75 425) | 16.4 (104 720) | 37.7 | 12.1 (62 778) | 12.7 (65 630) | 1.7 |
| ACE inhibitors or ARBs | 4.5 (67 855) | 19.4 (124 120) | 47.5 | 12.1 (62 533) | 13.5 (69 869) | 4.3 |
| Diuretics | 7.0 (107 100) | 20.2 (129 032) | 39.1 | 15.4 (79 884) | 15.9 (82 261) | 1.3 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker.
Data are reported as means±SD or percentages (numbers). The standardized differences are reported as percentages; a difference of <10.0% indicates a relatively small imbalance.
The body mass index is the weight in kilograms divided by the square of the height in meters.
In the matched cohort, paired comparisons were performed with the use of McNemar's test for binary variables and a paired Student t test or paired‐sample test for continuous variables. To estimate the diabetes mellitus risks, the time to an event was calculated to obtain incidence rates per 1000 person‐years, which were age‐standardized after adjustment for sex. The comparative risks of outcomes were determined with the use of Cox regression model, with robust standard errors that accounted for the clustering of matched pairs. Survival curves were constructed with Kaplan–Meier estimates and compared according to methods appropriate for matched data.19
Analyses of diabetes mellitus risks were also performed in a separate propensity‐matched cohort of patients according to prespecified key subgroups: clinical subgroups were based on sex, age group (<60 and ≥60 years), clinical risk factors for developing diabetes mellitus (impaired fasting glucose, body mass index 25 kg/m² or higher, and lack of exercise), and types of statin (atorvastatin, rosuvastatin, simvastatin, pravastatin, or others).
This was an observational data analysis using national healthcare data sets. To carefully define the population of interest and minimize the data‐dredging processes, we prespecified study objectives, a hypothesis, and a statistical approach using a statistical analysis plan.20 All reported P values are 2‐sided and have not been adjusted for multiple testing. All the analyses were performed with the use of SAS software, version 9.3 (SAS Institute, Cary, NC).
Results
Study Population
We identified a total of 2 162 119 adults aged ≥40 years with hypercholesterolemia who would be eligible for statin therapy in primary prevention and who met our inclusion criteria (Figure 1). Among them, 638 625 (29.5%) ever used statin therapy and 1 523 494 (70.5%) never used statin. Before propensity‐score matching, there were differences between the 2 groups in several of the baseline variables (Table 1). With the use of propensity‐score matching, we identified 518 491 matched pairs of statin users and nonusers with similar baseline characteristics. After matching, the standardized differences were <10.0% for most of variables, indicating only small differences between the 2 groups (Table 1).
Risk of New‐Onset Diabetes Mellitus
The follow‐up time ranged from at least 6 months to 9 years (average follow‐up: 3.9 years). During follow‐up, statin use was founded to be significantly associated with an increased risk of new‐onset diabetes mellitus as compared with statin nonuse (13.4 versus 6.9 events per 1000 person‐years, respectively; adjusted HR, 1.88; 95% CI, 1.85–1.93) (Table 2 and Figure 2).
Table 2.
Incidence Rate and Hazard Ratios for the Association Between Statin Use and the Risk of New‐Onset Diabetes Mellitus in the Propensity‐Score‐Matched Cohorta
| Statin User | Duration of Statin Use, y | Intensity of Statin Therapy | Cumulative Dose of Statin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Never User | Ever User | <1 | 1 to 2 | >2 | Low or Moderate | High | First Tertile | Second Tertile | Third Tertile | |
| No. of new‐onset diabetes mellitus | 15 682 | 31 143 | 8777 | 6575 | 15 791 | 18 229 | 12 914 | 4047 | 8086 | 19 010 |
| Person‐y of follow‐up time | 2 006 261 | 2 074 998 | 940 003 | 402 667 | 732 328 | 1 347 976 | 727 021 | 522 036 | 618 248 | 934 713 |
| Incidence rate of new‐onset diabetes mellitusb | 6.9 | 13.4 | 8.2 | 14.6 | 19.8 | 12.0 | 16.0 | 6.7 | 11.5 | 18.6 |
| Hazard ratios (95% CI)c | 1 [referent] | 1.94 (1.90–1.98) | 1.21 (1.18–1.24) | 2.13 (2.07–2.19) | 2.67 (2.61–2.73) | 1.72 (1.68–1.75) | 2.29 (2.23–2.34) | 1.00 (0.97–1.04) | 1.70 (1.66–1.75) | 2.54 (2.48–2.59) |
| Adjusted Hazard ratios (95% CI)d | 1 [referent] | 1.88 (1.85–1.93) | 1.25 (1.21–1.28) | 2.22 (2.16–2.29) | 2.62 (2.56–2.67) | 1.75 (1.71–1.78) | 2.31 (2.26–2.37) | 1.06 (1.02–1.10) | 1.74 (1.70–1.79) | 2.52 (2.47–2.57) |
The propensity‐score–matched cohort included 518 491 patients in the statin ever‐user group and 518 491 patients in the statin never‐user group.
Incidence rate per 1000 person‐years.
Hazard ratios are for statin ever‐users as compared with statin never‐users in the propensity‐matched cohort.
Models were further adjusted for body mass index, baseline total cholesterol level, baseline fasting glucose level, and Charlson comorbidity index in the propensity‐matched cohort.
Figure 2.
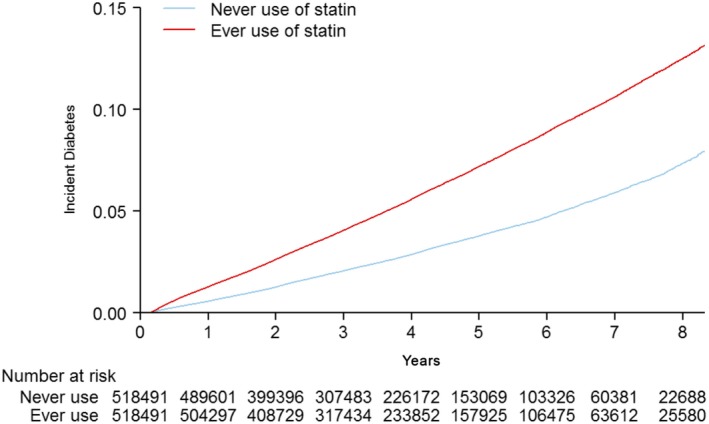
Cumulative risk of new‐onset diabetes mellitus in the matched cohort.
To determine the time‐ and dose‐dependent association of statin therapy with developing new‐onset diabetes mellitus, we further assessed the risk of incident diabetes mellitus according to the duration, intensity, and the cumulative dose of statin (Table 2 and Figure 3). There was evidence of a duration–response relationship between statin use and diabetes mellitus risks: with increasing duration of statin therapy, the risk of new‐onset diabetes mellitus substantially increased. Similar findings were observed with increasing potency of statin. On assessing the risks of incident diabetes mellitus according to the cumulative dose of statin therapy, we also found a proportional increase of the risk of developing new‐onset diabetes mellitus.
Figure 3.
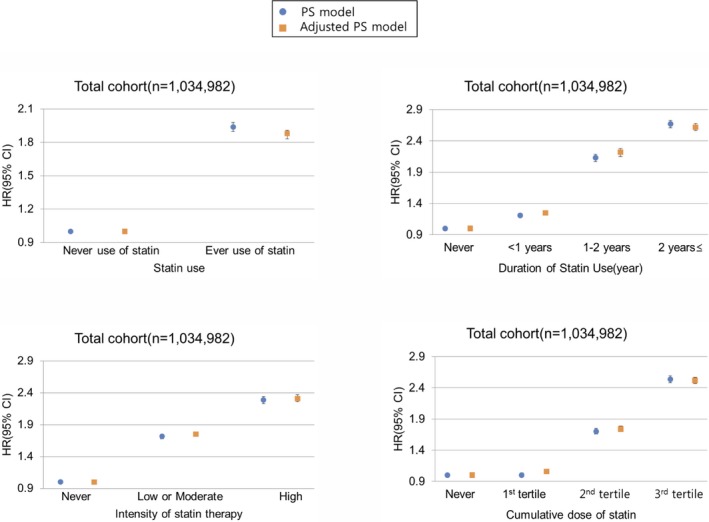
Association between statin therapy and the risk of new‐onset diabetes mellitus, according to duration, intensity, and cumulative dose of statins. Hazard ratios are for statin users as compared with statin nonusers in the propensity‐matched cohort. Adjusted PS Models were further adjusted for body mass index, baseline total cholesterol level, baseline fasting glucose level, and Charlson comorbidity index in the propensity‐matched cohort. HR indicates hazard ratio; PS, propensity‐score.
Subgroup Analyses
The associations of statin use with the risk of new‐onset diabetes mellitus stratified by the key clinical subgroups are shown in Table 3 and Figure 4. The effects of statin on diabetes mellitus risks were consistent for subgroups stratified by sex and age. When we performed analyses stratified on the basis of having clinical risk factors of diabetes mellitus (impaired fasting glucose, body mass index 25 kg/m² or higher, and lack of exercise), the magnitude of diabetes mellitus risks was not substantially modified by the presence or absence of diabetes mellitus risk factors. Similar results were obtained when statins were subcategorized according to specific types of drugs, suggesting no significant between‐drugs differences with respect to the risk of new‐onset diabetes mellitus.
Table 3.
Association Between Statin Use and Risk of New‐Onset Diabetes Mellitus in the Propensity‐Score–Matched Cohort, According to Major Clinical Subgroupsa
| Subgroups | No. of Matched Pairs | Incidence Rateb | Hazard Ratio (95% CI)c | P Value | ||
|---|---|---|---|---|---|---|
| Never User | Ever User | Never User | Ever User | |||
| Sex | ||||||
| Men | 197 192 | 197 192 | 8.72 | 16.12 | 1.82 (1.77–1.87) | <0.001 |
| Women | 317 909 | 317 909 | 5.73 | 11.67 | 2.01 (1.95–2.06) | <0.001 |
| Age | ||||||
| ≥60 y | 147 582 | 147 582 | 9.12 | 15.87 | 1.83 (1.77–1.89) | <0.001 |
| <60 y | 367 426 | 367 426 | 5.84 | 12.39 | 2.06 (2.01–2.11) | <0.001 |
| Diabetes mellitus risk factors | ||||||
| Impaired fasting glucosed | ||||||
| Yes | 180 224 | 180 224 | 15.23 | 28.25 | 1.88 (1.84–1.92) | <0.001 |
| No | 336 041 | 336 041 | 2.56 | 6.08 | 2.30 (2.21–2.38) | <0.001 |
| Body mass index | ||||||
| ≥25 kg/m2 | 203 814 | 203 814 | 9.93 | 19.34 | 1.93 (1.88–1.98) | <0.001 |
| <25 kg/m2 | 312 292 | 312 292 | 4.87 | 9.36 | 1.93 (1.88–1.99) | <0.001 |
| Lack of exercisee | ||||||
| Yes | 227 927 | 227 927 | 6.23 | 12.60 | 2.01 (1.95–2.07) | <0.001 |
| No | 288 509 | 288 509 | 7.27 | 14.03 | 1.90 (1.85–1.95) | <0.001 |
| Any risk factors | ||||||
| One or more | 422 188 | 422 188 | 7.99 | 15.60 | 1.95 (1.91–1.98) | <0.001 |
| None | 94 346 | 94 346 | 1.67 | 3.98 | 2.30 (2.11–2.52) | <0.001 |
| Types of statin | ||||||
| Atorvastatin | 233 554 | 233 554 | 6.54 | 14.06 | 2.17 (2.10–2.24) | <0.001 |
| Rosuvastatin | 25 586 | 25 586 | 6.17 | 14.11 | 2.55 (2.32–2.79) | <0.001 |
| Simvastatin | 121 127 | 121 127 | 7.34 | 15.03 | 2.04 (1.97–2.11) | <0.001 |
| Pravastatin | 10 877 | 10 877 | 7.29 | 14.96 | 2.12 (1.88–2.39) | <0.001 |
| Others | 48 979 | 48 979 | 6.90 | 14.74 | 2.10 (1.98–2.22) | <0.001 |
Patients were matched on the basis of the logit of the propensity score according to prespecified subgroups: sex (men or women), age (≥60 or <60 years), diabetes mellitus risk factors (impaired fasting glucose, higher body mass index, or lack of exercise) and type of statin (atorvastatin, rosuvastatin, simvastatin, pravastatin, or others).
Incidence rate per 1000 person‐year.
Hazard ratios are for statin user as compared with statin nonuser.
Impaired fasting glucose was defined as a fasting glucose ≥100 and <126 mg/dL.
Lack of exercise was defined as being engaged in <1 day of exercise per week.
Figure 4.

Hazard ratios for the risk of new‐onset diabetes mellitus in the propensity‐score–matched cohort, according to major clinical subgroups.
Discussion
Our study was designed to examine the effect of statin on the risk of new‐onset diabetes mellitus among a primary‐prevention patient cohort in the “real‐world” setting. The major findings from the present analysis, a nationwide population‐based study, are that (1) statin use was significantly associated with an increased risk of clinically relevant, new‐onset diabetes mellitus; (2) there was a time‐ and dose‐dependent association of statin use with an increasing risk of incident diabetes mellitus; (3) there was no substantial difference in the risk of new‐onset diabetes mellitus according to age, sex, and the presence or absence of diabetes mellitus risk factors; and (4) we did not find any differential risks of new‐onset diabetes mellitus associated with different types of statins.
Our study using healthcare data from national health insurance examinees combined with administrative claims‐based data sets has many unique aspects and advantages. Because the Korean National Health Insurance Services covers most of the population by obligation, data loss within the patient population was minimal and complete ascertainment for the development of new‐onset diabetes mellitus was feasible with a systematic linkage with hospital inpatient or outpatient diagnostic code and records of prescription‐drug dispensing data. In addition, because statin therapy for patients with hypercholesterolemia in primary prevention was fully reimbursed during the study period, many unrestricted incident users of statins were included. Thus, our study can provide clinically relevant information on diabetes mellitus risks of statin therapy in primary‐prevention patients encountered in routine care and can also assess a time‐ and dose‐dependent association of statin use with diabetes mellitus risks. Also, with 2.1 million patients under observation, we had the statistical power to robustly assess this important safety issue of statin.
Although the exact mechanism underlying diabetes mellitus risk of statin remains yet to be determined, several mechanisms have been suggested to explain this risk.3, 21, 22 New‐onset diabetes mellitus has been observed in clinical trials and in meta‐analyses involving statin therapy.4, 5, 6, 7, 23 The JUPITER trial was the first placebo‐controlled statin trial to formally report an increased risk of developing diabetes mellitus.4 The excess of diabetes mellitus risks seems to be limited to patients with major risk factors for diabetes mellitus.23 In subsequent meta‐analyses, standard dose of statin was associated with a proportional increase of ≈10% in reported diabetes mellitus, and more intensive doses with about a 10% further increase.5, 6, 7 Although the magnitude of the risk of new‐onset diabetes mellitus following statin use was very pronounced, several observational studies showed similar findings.8, 9, 24, 25, 26 Our population‐based study provided another evidence supporting the notion that statin use was significantly associated with an increased risk of incident diabetes mellitus in primary‐prevention patients. Although some studies showed conflicting findings with regard to overall dose and duration effects of statin on diabetes mellitus,27, 28 we found a time‐ and dose‐dependent association of statin use with increased diabetes mellitus risks. However, it is also recognized that people receiving statins have some common risk factors for diabetes mellitus that become worse over time and those with worse initial risk factors probably get more aggressive (higher dose) treatment with statins.
In the current study, we found that statins seem to have a class effect regardless of the individual type of statin. Prior studies suggested that different statins might impart different risks of diabetes mellitus.9, 29 Although some studies reported that a specific statin might be associated with neutral or beneficial effects on glucose metabolism,30 the current clinical guidelines, because of the limited evidence supporting varying risks of diabetes mellitus, do not recommend a specific statin type in populations at high risk of developing statin‐induced diabetes mellitus.2, 31
Previous studies revealed that patients having pre‐existing risk factors for developing diabetes mellitus (eg, metabolic syndrome, impaired fasting glucose, elevated body mass index, age, hypertension, increased fasting triglycerides, or elevated glycated hemoglobin A1C) had a higher diabetes mellitus risk.23, 32 By contrast, a pooled meta‐analysis showed conflicting results.7 In our study, the risks of new‐onset diabetes mellitus was not associated with the baseline characteristics of patients having a propensity for developing diabetes mellitus, including impaired fasting glucose, body mass index, or lack of exercise. Thus, further studies are required to optimally define more vulnerable people at high risk of developing diabetes mellitus and how to monitor such patients carefully.
Until recently, the clinical relevance of diabetes mellitus risks with statin use has been less clear. The cardiovascular benefit for people with atherosclerotic cardiovascular disease or for those at high cardiovascular risk strongly favors statin use. However, for people with a low cardiovascular risk who are usually indicated for primary prevention, the balance of vascular benefits and diabetes mellitus hazard of statin should be considered. Prior study reported that statin therapy was associated with a much greater number of vascular events or deaths reduction than the number of new cases of diabetes mellitus, which was already considered in the estimates of the overall benefits.23 Unfortunately, accurate assessment of the absolute benefit of statin on vascular events over the hazard of developing new‐onset diabetes mellitus is not feasible in our observational cohort. Although the magnitude of diabetes mellitus risks in our study were more pronounced than those observed in prior RCTs, overall findings should be interpreted with concerns that exaggerated claims about side‐effect rates with statin may be responsible for its underuse among individuals at increased risk of cardiovascular events.1, 33, 34
Different ethnicity also has been suggested to link between statin use and new‐onset diabetes mellitus: it is suggested that Asian ethnicity may be more prone to developing new‐onset diabetes mellitus with statin use.35 The genetic differences in statin pharmacokinetics and pharmacodynamics, as well as a genetic susceptibility to insulin resistance among Asian populations, likely play a role.36 In general, the Asian population achieves benefits similar to those of the Western population at lower statin doses, primarily because of genetically based differences in the statin metabolism at the level of hepatic enzymes and drug transporters.37 In addition, Asian populations, especially those of South Asian descent, are more prone to abdominal obesity and low muscle mass with increased insulin resistance compared with their Western counterparts.36 Thus, Asians are more genetically susceptible to insulin resistance and diabetes mellitus than whites. Although the mechanism that predisposes the higher rate of statin‐associated diabetes mellitus in Korean population remains unknown, a genetic susceptibility to insulin resistance in Asian populations and genetic differences in the metabolism of statins might play a role.
Our study had some limitations. First, our results are based on national administrative data records. There is a possibility of coding errors, missing data, lack of clinically relevant data because of unmeasured variables, or concomitant over‐the‐counter drug use that usually does not reflect in such data sources. In addition, the events of new‐onset diabetes mellitus were not centrally adjudicated, leaving a substantial risk of bias and misclassification of the end point. Second, this was a nonrandomized, observational study and hence suffers from potential selection and ascertainment bias despite robust propensity‐score matching. Third, the database did not have variables such as high‐density lipoprotein cholesterol or triglycerides that may result in more statin use and also increase the risk of diabetes mellitus; therefore, these variables could not be included in the propensity scores. Fourth, we defined statin‐eligible people on the basis of the reimbursement policy, which was the most crucial factor in determining statin prescription in our practice pattern. Thus, care should be used when considering the diabetes mellitus risks for other populations adopting different criteria of statin eligibility.2, 31, 38 Fifth, our findings could be influenced by a survivor bias: statin treatment makes people less likely to die and hence a statin user has an increased chance of developing diabetes mellitus. Sixth, we did not precisely account for treatment retention and adherence to statin therapy. Lastly, this study involves a single country. The direct applicability and generalizability of our results to our real‐world populations with different ethnic or backgrounds might be questionable.
Conclusion
In this nationwide observation cohort study of primary‐prevention patients for statin therapy, as compared with statin nonusers, being a statin user was significantly associated with an increased risk of new‐onset diabetes mellitus. There was a time‐ and dose‐dependent association of statin use with an increasing risk of diabetes mellitus. Our data support the findings from RCTs of statins and indicate caution in utilizing high‐dose statins for long‐term primary prevention. In addition, further studies are needed to evaluate the long‐term impact of new‐onset diabetes mellitus in patients receiving statin therapy for primary‐prevention.
Source of Funding
This study was supported by the National Evidence‐based Healthcare Collaborating Agency (NECA), Seoul, Korea (Project number NECA‐A‐15‐002).
Disclosures
None.
Supporting information
Table S1. Definitions of Clinical Risk Factors or Comorbid Conditions and Concomitant Cardioactive Medications on the Basis of Disease Codes and Prescriptions*
(J Am Heart Assoc. 2019;8:e011320 DOI: 10.1161/JAHA.118.011320.)
Contributor Information
Woo Je Lee, Email: lwjatlas@amc.seoul.kr.
Duk‐Woo Park, Email: dwpark@amc.seoul.kr.
References
- 1. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 2. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 3. Betteridge DJ, Carmena R. The diabetogenic action of statins—mechanisms and clinical implications. Nat Rev Endocrinol. 2016;12:99–110. [DOI] [PubMed] [Google Scholar]
- 4. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 5. Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta‐analysis. Diabetes Care. 2009;32:1924–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet. 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 7. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA. 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 8. Dormuth CR, Filion KB, Paterson JM, James MT, Teare GF, Raymond CB, Rahme E, Tamim H, Lipscombe L. Higher potency statins and the risk of new diabetes: multicentre, observational study of administrative databases. BMJ. 2014;348:g3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pencina MJ, Navar‐Boggan AM, D'Agostino RB Sr, Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population‐based sample. N Engl J Med. 2014;370:1422–1431. [DOI] [PubMed] [Google Scholar]
- 11. Ko MJ, Kim YJ, Park CM, Lee SM, Lee WJ, Pencina MJ, Navar‐Boggan AM, Park DW. Applicability and potential clinical effects of 2013 cholesterol guidelines on major cardiovascular events. Am Heart J. 2015;170:598–605e597. [DOI] [PubMed] [Google Scholar]
- 12. Song SO, Jung CH, Song YD, Park C‐Y, Kwon H‐S, Cha BS, Park J‐Y, Lee K‐U, Ko KS, Lee B‐W. Background and data configuration process of a nationwide population‐based study using the Korean National Health Insurance System. Diabetes Metab J. 2014;38:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim MK, Ko MJ, Han JT. Alcohol consumption and mortality from all‐cause and cancers among 1.34 million Koreans: the results from the Korea National Health Insurance Corporation's Health Examinee Cohort in 2000. Cancer Causes Control. 2010;21:2295–2302. [DOI] [PubMed] [Google Scholar]
- 14. Roh E, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, Song KH, Won JC, Kim DJ, Choi SH, Lim S, Cha BY. Prevalence and management of dyslipidemia in Korea: Korea National Health and Nutrition Examination Survey during 1998 to 2010. Diabetes Metab J. 2013;37:433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO collaborating centre for drug statistics methodology, guidelines for ATC classification and DDD assignment, 2018. Oslo; 2017. Available at: https://www.whocc.no/atc_ddd_index/. Accessed January 31, 2018. [Google Scholar]
- 16. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 17. Park DW, Seung KB, Kim YH, Lee JY, Kim WJ, Kang SJ, Lee SW, Lee CW, Park SW, Yun SC, Gwon HC, Jeong MH, Jang YS, Kim HS, Kim PJ, Seong IW, Park HS, Ahn T, Chae IH, Tahk SJ, Chung WS, Park SJ. Long‐term safety and efficacy of stenting versus coronary artery bypass grafting for unprotected left main coronary artery disease: 5‐year results from the main‐compare (revascularization for unprotected left main coronary artery stenosis: comparison of percutaneous coronary angioplasty versus surgical revascularization) registry. J Am Coll Cardiol. 2010;56:117–124. [DOI] [PubMed] [Google Scholar]
- 18. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. [DOI] [PubMed] [Google Scholar]
- 19. Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. New York, NY: Springer–Verlag; 1997. [Google Scholar]
- 20. Thomas L, Peterson ED. The value of statistical analysis plans in observational research: defining high‐quality research from the start. JAMA. 2012;308:773–774. [DOI] [PubMed] [Google Scholar]
- 21. Brault M, Ray J, Gomez YH, Mantzoros CS, Daskalopoulou SS. Statin treatment and new‐onset diabetes: a review of proposed mechanisms. Metabolism. 2014;63:735–745. [DOI] [PubMed] [Google Scholar]
- 22. Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, Sofat R, Stender S, Johnson PC, Scott RA, Leusink M, Verweij N, Sharp SJ, Guo Y, Giambartolomei C, Chung C, Peasey A, Amuzu A, Li K, Palmen J, Howard P, Cooper JA, Drenos F, Li YR, Lowe G, Gallacher J, Stewart MC, Tzoulaki I, Buxbaum SG, van der A DL, Forouhi NG, Onland‐Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor‐Madry R, Stepaniak U, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Veglia F, Ford I, Jukema JW, Westendorp RG, de Borst GJ, de Jong PA, Algra A, Spiering W, Maitland‐van der Zee AH, Klungel OH, de Boer A, Doevendans PA, Eaton CB, Robinson JG, Duggan D, Kjekshus J, Downs JR, Gotto AM, Keech AC, Marchioli R, Tognoni G, Sever PS, Poulter NR, Waters DD, Pedersen TR, Amarenco P, Nakamura H, McMurray JJ, Lewsey JD, Chasman DI, Ridker PM, Maggioni AP, Tavazzi L, Ray KK, Seshasai SR, Manson JE, Price JF, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben‐Shlomo Y, Schreiner PJ, Fornage M, Siscovick DS, Cushman M, Kumari M, Wareham NJ, Verschuren WM, Redline S, Patel SR, Whittaker JC, Hamsten A, Delaney JA, Dale C, Gaunt TR, Wong A, Kuh D, Hardy R, Kathiresan S, Castillo BA, van der Harst P, Brunner EJ, Tybjaerg‐Hansen A, Marmot MG, Krauss RM, Tsai M, Coresh J, Hoogeveen RC, Psaty BM, Lange LA, Hakonarson H, Dudbridge F, Humphries SE, Talmud PJ, Kivimaki M, Timpson NJ, Langenberg C, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Reiner AP, Keating BJ, Hingorani AD, Sattar N. HMG‐coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the Jupiter Trial. Lancet. 2012;380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Culver AL, Ockene IS, Balasubramanian R, Olendzki BC, Sepavich DM, Wactawski‐Wende J, Manson JE, Qiao Y, Liu S, Merriam PA, Rahilly‐Tierny C, Thomas F, Berger JS, Ockene JK, Curb JD, Ma Y. Statin use and risk of diabetes mellitus in postmenopausal women in the women's health initiative. Arch Intern Med. 2012;172:144–152. [DOI] [PubMed] [Google Scholar]
- 25. Zaharan NL, Williams D, Bennett K. Statins and risk of treated incident diabetes in a primary care population. Br J Clin Pharmacol. 2013;75:1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen CW, Chen TC, Huang KY, Chou P, Chen PF, Lee CC. Differential impact of statin on new‐onset diabetes in different age groups: a population‐based case‐control study in women from an Asian country. PLoS One. 2013;8:e71817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ko DT, Wijeysundera HC, Jackevicius CA, Yousef A, Wang J, Tu JV. Diabetes mellitus and cardiovascular events in older patients with myocardial infarction prescribed intensive‐dose and moderate‐dose statins. Circ Cardiovasc Qual Outcomes. 2013;6:315–322. [DOI] [PubMed] [Google Scholar]
- 28. Izzo R, de Simone G, Trimarco V, Giudice R, De Marco M, Di Renzo G, De Luca N, Trimarco B. Primary prevention with statins and incident diabetes in hypertensive patients at high cardiovascular risk. Nutr Metab Cardiovasc Dis. 2013;23:1101–1106. [DOI] [PubMed] [Google Scholar]
- 29. Koh KK, Sakuma I, Quon MJ. Differential metabolic effects of distinct statins. Atherosclerosis. 2011;215:1–8. [DOI] [PubMed] [Google Scholar]
- 30. Vallejo‐Vaz AJ, Kondapally Seshasai SR, Kurogi K, Michishita I, Nozue T, Sugiyama S, Tsimikas S, Yoshida H, Ray KK. Effect of pitavastatin on glucose, HbA1c and incident diabetes: a meta‐analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis. 2015;241:409–418. [DOI] [PubMed] [Google Scholar]
- 31. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 32. Waters DD, Ho JE, DeMicco DA, Breazna A, Arsenault BJ, Wun CC, Kastelein JJ, Colhoun H, Barter P. Predictors of new‐onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol. 2011;57:1535–1545. [DOI] [PubMed] [Google Scholar]
- 33. Nielsen SF, Nordestgaard BG. Negative statin‐related news stories decrease statin persistence and increase myocardial infarction and cardiovascular mortality: a Nationwide Prospective Cohort Study. Eur Heart J. 2016;37:908–916. [DOI] [PubMed] [Google Scholar]
- 34. Matthews A, Herrett E, Gasparrini A, Van Staa T, Goldacre B, Smeeth L, Bhaskaran K. Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data. BMJ. 2016;353:i3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mach F, Ray KK, Wiklund O, Corsini A, Catapano AL, Bruckert E, De Backer G, Hegele RA, Hovingh GK, Jacobson TA, Krauss RM, Laufs U, Leiter LA, Marz W, Nordestgaard BG, Raal FJ, Roden M, Santos RD, Stein EA, Stroes ES, Thompson PD, Tokgozoglu L, Vladutiu GD, Gencer B, Stock JK, Ginsberg HN, Chapman MJ. Adverse effects of statin therapy: perception vs. the evidence—focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J. 2018;39:2526–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. [DOI] [PubMed] [Google Scholar]
- 37. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definitions of Clinical Risk Factors or Comorbid Conditions and Concomitant Cardioactive Medications on the Basis of Disease Codes and Prescriptions*


