Abstract
Background
Evidence of the role of systolic blood pressure (SBP) in development of severe diabetic retinopathy is not strong, although the adverse effect of low diastolic blood pressure has been a partial explanation. We assessed the predictive ability of incident severe diabetic retinopathy between pulse pressure (PP) which considers both SBP and diastolic blood pressure, compared with SBP.
Methods and Results
Eligible patients (12 242, 83% men) aged 19 to 72 years from a nationwide claims database were analyzed for a median observational 4.8‐year period. Severe diabetic retinopathy was defined as vision‐threatening treatment‐required diabetic eye diseases. Multivariate Cox regression analysis revealed that hazard ratios (95% CI) of treatment‐required diabetic eye diseases for 1 increment of standard deviation and the top tertile compared with the bottom tertile were 1.39 (1.21–1.60) and 1.72 (1.17–2.51), respectively, for PP and 1.22 (1.05–1.41) and 1.43 (0.97–2.11), respectively, for SBP adjusted for age, sex, body mass index, hemoglobin A1c, fasting plasma glucose, lipids, and smoking status. In a model with SBP and PP simultaneously as covariates, the hazard ratios of only PP (hazard ratios [95% CI], 1.57 [1.26–1.96]) but not SBP (0.85 [0.68–1.07]) were statistically significant. Delong test revealed a significant difference in the area under the receiver operating characteristic curve between PP and SBP (area under the receiver operating characteristic curve [95% CI], 0.58 [0.54–0.63] versus 0.54 [0.50–0.59]; P=0.03). The strongest predictor remained as hemoglobin A1c (area under the receiver operating characteristic curve [95% CI], 0.80 [0.77–0.84]).
Conclusions
After excluding the significant impact of glycemic control, PP in comparison with SBP is a better predictor of severe diabetic retinopathy, suggesting a role of diastolic blood pressure and arterial stiffness in pathology.
Keywords: blood pressure, diabetic mellitus, hemoglobin A1c, pulse pressure, pulse pressure, systolic blood pressure, vision‐threatening treatment‐required diabetic eye diseases
Subject Categories: Epidemiology, Risk Factors
Short abstract
See Editorial Dart
Clinical Perspective
What Is New?
To our knowledge, this is the first longitudinal, historical cohort study to report that pulse pressure had a stronger predictive effect on the incidence of severe diabetic retinopathy than systolic blood pressure using a large number of nationwide participants over a long observational period.
What Are the Clinical Implications?
This study implies the importance of diastolic blood pressure in addition to systolic blood pressure and the difficulty of administering antihypertensive treatments for prevention of severe diabetic retinopathy leading to vision loss given the current lack of any therapy that exclusively targets pulse pressure.
These findings suggest the necessity of considering pulse pressure in addition to systolic blood pressure.
Introduction
Severe diabetic retinopathy (DR) has been a major cause of vision loss and an impaired quality of life in patients with diabetes mellitus. Generally, hypertension mainly focused on high systolic blood pressure (SBP) values is believed to be an excellent predictor of incident DR regardless of its severity. However, with regard to incident progressive DR, it has been reported that SBP is not necessarily associated with this outcome.1 Moreover, a recent meta‐analysis showed2 that strict blood pressure control is not significantly effective in preventing progressive DR, which has been partially explained by the adverse effect of low diastolic blood pressure (DBP). These results imply that evidence for a role of SBP in developing severe DR is not strong.
It is hypothesized that high pulse pressure (PP), which considers low DBP in addition to high SBP, is a better indicator of future development of severe DR compared with high SBP only. To test this hypothesis, we investigated the risk factors for severe retinopathy using our large longitudinal database on Japanese patients with diabetes mellitus.
Methods
Recruitment of Patients
In our analysis we used data from a national health insurance claim‐based database3 in Japan consisting of ≈3 000 000 people who are insured by a health insurance provider for company employees. Details of the claims data were described previously.3, 4 The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Although patients aged 19 to 72 years who had been followed for at least 3 years between April 1, 2008 and March 31, 2012 were initially included and followed up until August 31, 2015, patients for whom we did not have sufficient information initially for analysis, such as follow‐up periods, were subsequently excluded. Among 280 329 individuals who met these criteria, we included data on 17 158 individuals with diabetes mellitus in the present study. Of these, 4916 individuals who did not have full health examination data were further excluded. Finally, this study included 12 242 individuals (Figure S1).
Clinical and Laboratory Measurements
The participating patients were classified as having diabetes mellitus based on their fasting plasma glucose (FPG), hemoglobin A1c (HbA1c) and claims database data. Criteria for diabetes mellitus were FPG ≥7.0 mmol/L or HbA1c ≥6.5% or both without antidiabetic drug prescription or the use of an antidiabetic drug prescription regardless of FPG or HbA1c.4 All facilities measured blood pressure (BP) in accordance with the guidelines of the Japanese Society of Hypertension.5 These guidelines recommended that BP is measured twice by the oscillometric method and the results averaged in medical checkups in Japan.5
Outcome Measures
Criteria for vision‐threatening treatment‐required diabetic eye diseases (TRDED) were a composite of: (1) the diagnosis of DR and/or diabetic maculopathy and/or diabetic macular edema (DME); and (2) the administration of medical procedures such as retinal photocoagulation treatment and/or pars plana vitrectomy and/or intraocular injection treatment with steroids and anti‐vascular endothelial growth factor agents. The incidence of these was determined according to claims using International Classification of Diseases, Tenth Revision (ICD‐10) codes for DR, diabetic maculopathy, or DME in E 103, 113, or 143, and medical procedures. The examination of the claims data enabled us to confirm that all participants had no past history of a previous TRDED before baseline.
Statistical Analysis
Categorical variables were expressed as numerals and percentages. Continuous variables were expressed as the mean±SD or median and interquartile range. For comparison between the cases and non‐cases groups, Chi squared tests were used for the categorical variables. For the continuous variables, either the unpaired Student t test or Mann‐Whitney U test was used, depending on the distribution of the 2 groups. Several Cox proportional‐hazards regression models identified variables related to the incidence of TRDED. Covariates included age, sex, body mass index, HbA1c, fasting plasma glucose, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, and smoking status.
To explore potential non‐linear relationships, we used multivariate‐adjusted generalized additive models with a spline function of 4 degrees of freedom. Unadjusted overall time to the incidence of TRDED was indicated by Kaplan–Meier analysis with log‐rank tests.
To compare the predictive ability between 2 variables, we assessed discriminative ability by calculating the area under the receiver operating characteristic curve (AUCROC) and compared the 2 AUCROCs using the DeLong method.6
Analyses were performed using SPSS (version 19.0, Chicago, IL) and SAS packages version 9.4 (SAS Institute Inc, Cary, NC). Statistical significance was considered for P<0.05. The study design was consistent with the tenets of the Declaration of Helsinki. This study was approved by the Ethics Committee of Niigata University and the requirement for informed consent was waived.
Results
Baseline Characteristics and Incidence of TRDED During Follow‐Up
Among 12 242 patients, a total of 165 TRDED occurred during the observational period of a median of 4.8 years. Cumulative incidence rate of TRDED was 2.83 per 1000 person‐years. Baseline characteristics of all patients as well as those who had or had not experienced TRDED during the observational period are summarized in Table 1. Individuals with TRDED were significantly older and had higher levels of SBP, PP, and HbA1c compared with those without.
Table 1.
Characteristics of Study Participants According to Presence or Absence of TRDED
| Characteristic | TRDED | |||
|---|---|---|---|---|
| Total | (−) | (+) | P Value | |
| (n=12 242) | (n=12 077) | (n=165) | ||
| Sex (male, %) | 10 158 (83) | 10 024 (83) | 134 (81) | 0.544 |
| Age (y), mean (SD) | 48±9 | 48±9 | 50±8 | 0.001 |
| Body mass index (kg/m2), mean (SD) | 25.8±4.6 | 25.8±4.6 | 26.1±4.5 | 0.386 |
| Systolic blood pressure (mm Hg), mean (SD) | 131±16 | 131±16 | 134±21 | 0.033 |
| Diastolic blood pressure (mm Hg), mean (SD) | 80±11 | 80±11 | 80±13 | 0.728 |
| Pulse pressure (mm Hg), mean (SD) | 50±11 | 50±11 | 54±14 | <0.001 |
| HbA1c (%), mean (SD) | 6.9±1.4 | 6.9±1.4 | 9.0±2.3 | <0.001 |
| HbA1c (mmol/mol), mean (SD) | 52±16 | 51±15 | 75±25 | <0.001 |
| Fasting plasma glucose (mmol/L), mean (SD) | 7.8±2.3 | 7.7±2.2 | 10.3±4.2 | <0.001 |
| LDL cholesterol (mmol/L), mean (SD) | 3.4±0.9 | 3.4±0.9 | 3.3±0.9 | 0.644 |
| HDL cholesterol (mmol/L), mean (SD) | 1.5±0.4 | 1.5±0.4 | 1.5±0.4 | 0.288 |
| Triglycerides (mmol/L), median (IQR) | 1.4 (1.0–2.2) | 1.4 (1.0–2.2) | 1.4 (1.0–2.2) | 0.511 |
| Current smoking (%) | 5009 (41) | 4949 (41) | 60 (36) | 0.231 |
Data are presented as numbers, means±SDs, median (IQR), or percentages. HbA1c indicates hemoglobin A1c; HDL, high‐density lipoprotein; IQR, interquartile; LDL, low‐density lipoprotein; TRDED, treatment‐required diabetic eye diseases.
Hazard Ratios of BP Components for TRDED
Risk factors for incident TRDED investigated by the multivariate Cox model are shown in Table 2. HbA1c was the most strongly associated with the incidence of TRDED with an HR (95% CI) per 1‐SD for elevations of ≈ 2.2 (1.9–2.5) in any model. The hazard ratio (HR) (95% CI) of TRDED for an increment of 1‐SD was larger for PP (1.39 [1.21–1.60]) than for SBP (1.22 [1.05–1.41]) after adjusting for covariates (Table 2). In a model involving both SBP and PP simultaneously as covariates, the HR of only PP but not SBP was statistically significant (Table 2, model 3). When we reanalyzed our database adding a past history of cardiovascular diseases and the use of medications for dyslipidemia, hypertension, and diabetes mellitus at baseline as covariates, these relationships were unchanged (Table S1). In models involving either DBP or mean BP as the only covariate for BP, the HRs for TRDED for each 1‐SD for elevations were not significant; 0.93 (0.79–1.10) for DBP and 1.06 (0.91–1.24) for mean BP (Table S2).
Table 2.
HR With 95% CI of Baseline Values for Each Variable for TRDED Risk Analyzed by Cox Models
| Characteristic | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, y | 1.04 (1.02–1.06) | <0.001 | 1.03 (1.01–1.05) | 0.002 | 1.03 (1.01–1.05) | 0.001 |
| Sex, male | 1.22 (0.81–1.86) | 0.346 | 1.38 (0.90–2.11) | 0.140 | 1.41 (0.92–2.16) | 0.113 |
| Body mass index | ||||||
| Per 5 kg/m2 | 1.09 (0.91–1.31) | 0.368 | 1.10 (0.92–1.31) | 0.317 | 1.13 (0.94–1.36) | 0.190 |
| Per 1‐SD | 1.08 (0.91–1.28) | 1.09 (0.92–1.28) | 1.12 (0.95–1.33) | |||
| Systolic blood pressure | ||||||
| Per 10 mm Hg | 1.13 (1.03–1.23) | 0.008 | NA | 0.91 (0.79–1.04) | 0.175 | |
| Per 1‐SD | 1.22 (1.05–1.41) | NA | 0.85 (0.68–1.07) | |||
| Pulse pressure | ||||||
| Per 10 mm Hg | NA | 1.35 (1.19–1.53) | <0.001 | 1.50 (1.23–1.84) | <0.001 | |
| Per 1‐SD | NA | 1.39 (1.21–1.60) | 1.57 (1.26–1.96) | |||
| HbA1c | ||||||
| Per 1% (11 mmol/mol) | 1.70 (1.56–1.86) | <0.001 | 1.73 (1.59–1.89) | <0.001 | 1.73 (1.59–1.89) | <0.001 |
| Per 1 mmol/mol | 1.05 (1.04–1.06) | 1.05 (1.04–1.06) | 1.05 (1.04–1.06) | |||
| Per 1‐SD | 2.14 (1.89–2.41) | 2.19 (1.94–2.47) | 2.19 (1.94–2.47) | |||
| Fasting plasma glucose | ||||||
| Per 1 mmol/L | 1.02 (0.97–1.08) | 0.506 | 1.01 (0.96–1.07) | 0.714 | 1.01 (0.96–1.07) | 0.706 |
| Per 1‐SD | 1.04 (0.92–1.18) | 1.02 (0.91–1.16) | 1.02 (0.91–1.16) | |||
| HDL cholesterol | ||||||
| Per 1 mmol/L | 1.36 (0.88–2.11) | 0.171 | 1.35 (0.87–2.10) | 0.176 | 1.39 (0.90–2.17) | 0.141 |
| Per 1‐SD | 1.13 (0.95–1.34) | 1.13 (0.95–1.34) | 1.14 (0.96–1.36) | |||
| LDL cholesterol | ||||||
| Per 1 mmol/L | 0.77 (0.64–0.92) | 0.003 | 0.77 (0.64–0.92) | 0.004 | 0.77 (0.64–0.92) | 0.004 |
| Per 1 SD | 0.79 (0.67–0.92) | 0.79 (0.67–0.93) | 0.79 (0.67–0.93) | |||
| Log‐triglycerides | ||||||
| Per 1 | 0.84 (0.63–1.13) | 0.247 | 0.86 (0.65–1.14) | 0.299 | 0.88 (0.66–1.18) | 0.402 |
| Per 1‐SD | 0.90 (0.76–1.07) | 0.91 (0.77–1.09) | 0.93 (0.78–1.11) | |||
| Current smoker | 0.88 (0.63–1.23) | 0.450 | 0.84 (0.60–1.18) | 0.311 | 0.83 (0.59–1.16) | 0.265 |
A total of 165 patients developed TRDED. Model 1=adjusted for age, sex, body mass index, SBP, HbA1c, fasting plasma glucose, LDL cholesterol, HDL cholesterol, triglycerides, smoking status. Model 2=model 1 plus additional adjustment for pulse pressure and minus adjustment for SBP. Model 3=model 1 plus additional adjustment for pulse pressure. HbA1c indicates hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NA, not applicable; SBP, systolic blood pressure; TRDED, treatment‐required diabetic eye diseases.
Spline Curves for the Risk of TRDED With Regard to PP, SBP, and HbA1c
Figure 1 shows spline curves for the log risk of TRDED with regard to PP, SBP, and HbA1c. Compared with the linear model, the spline model improved the goodness of fit for HbA1c (P<0.01) but not for PP (P=0.48) and SBP (P=0.16). There was no clear threshold above which the risk of TRDED was greatly elevated in any of the 3 variables.
Figure 1.
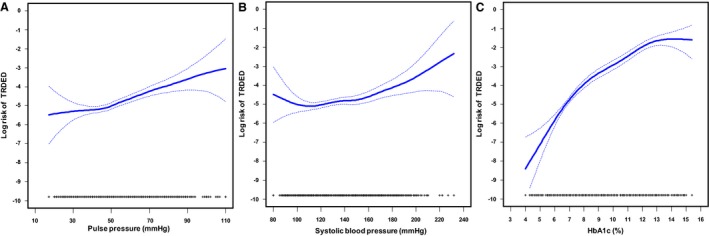
Spline curves of log risk of TRDED (solid line) with 95% CIs (broken line) in relationship to PP (A), SBP (B), and HbA1c (C), with rug plots describing distributions of PP, SBP, or HbA1c. (A and B) are adjusted for age, sex, bone mass index, HbA1c, fasting plasma glucose, LDL cholesterol, HDL cholesterol, triglycerides, and smoking status. Adjustment for (C) is (A) plus additional adjustment for SBP and minus adjustment for HbA1c. HbA1c indicated hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PP, pulse pressure; SBP, systolic blood pressure; TRDED, treatment‐required diabetic eye diseases.
HRs and Cumulative Incidence of TRDED for the Stratified Tertiles of PP, SBP, and HbA1c
We categorized the patients into tertiles according to HbA1c (≤6.4, 6.5–6.9, ≥7.0%), SBP (≤124, 125–136, ≥137 mm Hg), DBP (≤76, 77–84, ≥85 mm Hg), and PP (≤45, 46–54, ≥55 mm Hg). Multivariate Cox analysis shows the HRs for the stratified tertiles of PP, SBP, and HbA1c (Table 3). Figure 2 shows the corresponding cumulative incidences of TRDED for the stratified tertiles of these variables. Among the 3 variables, the HR for the top tertile compared with the bottom tertile was the largest for HbA1c (HR [95% CI], 5.04 [3.36–7.58]). In particular, when the HbA1c values were stratified into 6 categories with increments of 0.5% (Table S3, Figure S2), the HRs for TRDED among patients with HbA1c of 8.1% to 8.5% and ≥8.6% were 5.91 (2.87–12.17) and 14.10 (8.07–24.60), respectively, compared with those whose HbA1c was ≤6.5%. The HR of TRDED for the top tertile compared with the bottom tertile was larger for PP (HR [95% CI], 1.72 [1.17–2.51] than for SBP (HR [95% CI], 1.43 [0.97–2.11]).
Table 3.
HR With 95% CI for Baseline Values of PP, SBP, and HbA1c Tertiles for TRDED Risk Analyzed by Cox Models
| Variables | Cases/Total n | HR (95% CI) | P Value | P Trend |
|---|---|---|---|---|
| A. PP (mm Hg) | ||||
| Bottom tertile (≤45) | 48/4109 | 1.00 (reference) | 0.008 | |
| Middle tertile (46–54) | 48/4239 | 1.07 (0.71–1.60) | 0.754 | |
| Top tertile (≥55) | 69/3894 | 1.72 (1.17–2.51) | 0.006 | |
| B. SBP (mm Hg) | ||||
| Bottom tertile (≤124) | 53/4389 | 1.00 (reference) | 0.186 | |
| Middle tertile (125–136) | 48/3934 | 1.16 (0.77–1.72) | 0.481 | |
| Top tertile (≥137) | 64/3919 | 1.43 (0.97–2.11) | 0.071 | |
| C. HbA1c (% in National Glycohemoglobin Standardization Program) | ||||
| Bottom tertile (≤6.4) | 13/4389 | 1.00 (reference) | <0.001 | |
| Middle tertile (6.5–6.9) | 23/3876 | 2.29 (1.15–4.58) | 0.019 | |
| Top tertile (≥7.0) | 129/3977 | 7.97 (4.36–14.57) | <0.001 | |
B, C=adjusted for age, sex, body mass index, SBP, HbA1c, FPG, LDL cholesterol, HDL cholesterol, triglycerides, and smoking status. A=B plus additional adjustment for PP and minus adjustment for SBP. FPG indicates fasting plasma glucose; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; HR, hazard ratio; LDL, low‐density lipoprotein; PP, pulse pressure; SBP, systolic blood pressure; TRDED, treatment‐required diabetic eye diseases.
Figure 2.

Cumulative incidence of TRDED according to tertiles of PP, systolic blood pressure, and glycated hemoglobin determined by the Kaplan–Meier method. (A) Pulse pressure (dotted line, pulse pressure ≥55 mm Hg; black line, pulse pressure 46–54 mm Hg; dashed line, pulse pressure ≤45 mm Hg); (B) SBP (dotted line, systolic blood pressure ≥137 mm Hg; black line, systolic blood pressure 125–136 mm Hg; dashed line, systolic blood pressure ≤124 mm Hg) and (C) hemoglobin A1c (dotted line, HbA1c ≥7.0%; black line, hemoglobin A1c 6.5–6.9%; dashed line, hemoglobin A1c ≤6.4%) levels at baseline. TRDED indicates treatment‐required diabetic eye diseases.
HR for the Top Tertile Compared With the Combination of the Lower and Middle Tertiles With Regard to PP, SBP, and HbA1c
Similar to the above results, the HR for the top tertile versus middle+bottom tertiles was larger for PP (HR [95% CI], 1.66 [1.20–2.29] than for SBP (HR [95% CI], 1.33 [0.96–1.85]) (Table S4). This result was in line with the visual inspection of the Kaplan–Meyer analysis (Figure 2) indicating that gaps between curves for the top tertile and the middle+bottom tertiles were larger for PP than for SBP.
Combined Roles of SBP With DBP for TRDED
We additionally stratified the patients into tertiles according to DBP (≤76, 77–84, ≥85 mm Hg). Table S5 shows the result of Cox regression analysis using the stratified tertiles of DBP in addition to SBP, instead of PP. When the combination of the bottom tertile of SBP and the bottom tertile of DBP was the reference group, the HR of TRDED was larger for the bottom tertile of DBP than for the middle tertile of DBP in any SBP group. In particular, the HR was smaller in the low SBP and the middle DBP tertiles than in the reference group (HR [95% CI], 0.53 [0.25–1.14]).
Risk of TRDED in Each of the PP and SBP Tertiles Combined With HbA1c Tertiles
Figure S3 shows the risk of TRDED in each of the PP and SBP tertiles combined with the HbA1c tertiles. Compared with the referent (ie, combination of the bottom HbA1c tertile and bottom PP/SBP tertile), the HR (95% CI) for the extreme tertiles (ie, top HbA1c tertile and top PP/SBP tertile) was 12.51 (3.83–40.91) for PP and 5.68 (2.45–13.17) for SBP. The association between the PP tertiles and the risk of TRDED was not significant among individuals in the middle and bottom HbA1c tertiles. Among those in the top HbA1c tertile, a significantly positive association between tertiles and risk of TRDED was observed for PP (P trend=0.027) unlike that for SBP (P trend=0.517). However, interactions between HbA1c and PP were not statistically significant (P=0.298).
AUCROC for the Incidence of TRDED
Table 4 shows discriminative ability as indicated by the AUCROCs for HbA1c, PP, and SBP. The AUCROC (95% CI) was 0.80 (0.77–0.84) for HbA1c, 0.58 (0.54–0.63) for PP and 0.54 (0.50–0.59) for SBP. The Delong test indicated that the AUCROC for HbA1c was significantly larger than that for PP (P<0.001) and SBP (P<0.001); the AUCROC for PP was larger than that for SBP (P=0.03).
Table 4.
AUCROC for the Incidence of Treatment‐Required Diabetic Eye Diseases Using Conventional Risk Factors and Blood Pressure Values
| Variable | SBP | PP | HbA1c |
|---|---|---|---|
| AUCROC | 0.544 (0.498, 0.591) | 0.582 (0.535, 0.629) | 0.804 (0.770, 0.837) |
| PP vs SBP | PP vs HbA1c | HbA1c vs SBP | |
| P value | 0.027 | <0.001 | <0.001 |
Data are AUCROC and 95% CI. Results from AUCROC analysis with the DeLong test of area under the curve difference. AUCROC indicates area under the receiver operating characteristic curve; HbA1c, hemoglobin A1c; PP, pulse pressure; SBP, systolic blood pressure.
Discussion
This is the first large‐scale, long‐term longitudinal study to compare PP and SBP in terms of their association with incident severe DR. All previous studies7, 8, 9, 10, 11, 12 except for 1 study13 that examined the associations between PP and DR used cross‐sectional research, which would fail to prove whether high PP preceded the incidence of DR. The only longitudinal study included only 86 patients and did not compare SBP with PP.13
We specified only cases with vision impairment requiring an ophthalmological intervention, which is of great significance because whether the DR is severe enough to require an ophthalmological intervention makes an enormous difference in urgency, the impact on quality of life and the medical cost between severity stages of DR indicating the need for an ophthalmological intervention. This still holds true even though there has been remarkable progress in the management of DR in the past few decades, including the use of anti‐vascular endothelial growth factor agents.14, 15
We indicated that compared with SBP, PP was not only more strongly associated with but also a stronger predictor of severe DR. Moreover, relevant to the stronger association of PP with TRDED than SBP, we confirmed adverse prognostic outcomes in patients with low DBP (≤76 mm Hg, in this study).
Although determining the underlying mechanisms for the enhancement of the progression of DR or DME through the superiority of elevated PP to that of elevated SBP is beyond an epidemiological study, there may be a plausible explanation for the current finding. For example, although only cross‐sectionally, arterial stiffness has been associated with DR in type 2 diabetes mellitus.16 It is possible that the arterial stiffness plays an important role in deteriorating DR given that an elevated PP, which is a surrogate marker of the arterial stiffness, is attributed to a decrease in DBP in addition to an increase in SBP.17 Possible contributors to progression of DR with which PP rather than SBP is predominantly associated are needed to be identified in future studies.
Of note, several analyses that we conducted indicated that it is most important to monitor HbA1c among possible traditional risk factors in predicting severe DR. At each level of HbA1c, PP is a much better predictor than SBP. In particular, the significant association between PP and the incidence of TRDED was observed in the top tertile of HbA1c (≥7.0%). This finding suggested that priority should be given to monitoring HbA1c to detect those at high risk for severe DR and that determining PP should be secondary to identifying patients with poor glycemic control.
In our study, serum low‐density lipoprotein levels were found to be lower in patients with TRDED and HRs for TRDED were significantly decreased in accordance with increases in low‐density lipoprotein cholesterol. Only the Tromsø Eye Study18 showed results similar to ours. When we reanalyzed our database adding the past history of cardiovascular disease and use of medications for dyslipidemia, hypertension, and diabetes mellitus at baseline as covariates (Table S1), the statistical significance of the negative relationship between low‐density lipoprotein cholesterol and incident TRDED disappeared. These factors might have affected and changed this negative relationship.
The strengths of this study are its large number of nationwide participants over a long observational period, an extremely low rate of lost‐to‐follow‐up participants, and accurate identification of TRDED based on actual medical practice. These strengths reflect the advantages of a claim‐based database. Moreover, thanks to the well‐organized public health insurance system that equally covers all citizens in Japan, ophthalmologists can choose treatment according to what is best for each patient's situation without considering economic status. This is another large advantage of the use of the claim‐based database or management‐based outcomes in this country.
Several limitations of the current study should be addressed. First, we had no information on types and duration of diabetes mellitus and fundus examinations. However, since it is well known that the prevalence of type 1 diabetes mellitus is extremely low in East Asia, most patients in our analysis were considered to have type 2 diabetes mellitus. Second, only baseline data were used for this analysis; therefore, therapeutic management during the observational period could have influenced the results.
Third, possible BP measurement errors could bias the results. Errors can be particularly large for PP rather than for SBP given that the difference between the 2 measurements (ie, SBP and DBP) enlarge the measurement error. However, since the strength of association tends to be weaker with the measurement error, the expected difference in the strength of association between PP and SBP would be rather enlarged compared with the observed difference. Therefore, we should not have to change the conclusion. Nevertheless, the possibility of measurement errors should always be considered when interpreting the results.
Fourth, instead of using outcomes predominantly based on criteria for results of fundus examinations (i.e, which would be more important and superior to definitions based on claims data), we identified TRDED based on comprehensive judgment of ophthalmologists in terms of the necessity for treatment in real‐world clinical settings, which might be vulnerable to observer bias. However, recent progress in examination and treatment technologies minimize this bias. Moreover, although fluorescein angiography has been widely used and remains a fundamental and useful diagnostic modality for classification of DR, severe adverse effects, including anaphylaxis, are of concern.
Lastly, among TRDED patients in general, there is large heterogeneity in severe eye diseases that are comprehensively designated as TRDED. However, unfortunately, we could not perform sensitivity analysis based on the underlying eye diseases. One reason is that some ophthalmological treatments (such as vitrectomy or retinal photocoagulation) are adapted not for a single treatment‐specific eye disease but for several severe eye diseases. Another reason is that some TRDED patients may not only have a single eye disease but ≥2 eye diseases (eg, proliferative DR and DME) simultaneously. It would therefore become complicated and difficult to clearly distinguish one from the other using information in the database.
We could not detect the thresholds of PP, SBP, and HbA1c above which the risk of TRDED was steeply elevated. For example, Hammes and colleagues showed that BP ≥150/90 mm Hg could impact on the development of advanced retinopathy by logistic regression analysis in patients with type 1 diabetes mellitus.20 However, few studies have investigated BP cut‐off values for DR risk. Further research is needed to determine the cut‐off value of PP for detection or early treatment of patients at high risk of sight‐threatening DR in terms of maintaining quality of life or controlling medical expenditures for ophthalmological interventions.
Conclusion
PP is not only more strongly associated with incident DR but is a stronger predictor of severe DR than SBP, suggesting the importance of DBP in addition to SBP and the difficulty of administering antihypertensive treatments for prevention of severe DR leading to vision loss given the current lack of any therapy that exclusively targets PP. However, priority should be given to monitoring HbA1c for identifying patients at high risk of severe DR.
Sources of Funding
This work is supported in part by the Japan Society for the Promotion of Science.
Disclosures
None.
Supporting information
Table S1. HR with 95% CI of baseline values for each variable for TRDED risk analyzed by Cox models
Table S2. HR with 95% CI of baseline values of each variable for TRDED risk analyzed by Cox models
Table S3. HR with 95% CI for baseline values of 6 categories of HbA1c for TRDED risk analyzed by Cox models
Table S4. HR with 95% CI of TRDED for the top tertile compared with the combination of the lower and middle tertiles with regard to PP, SBP, and HbA1c
Table S5. Combined roles of SBP with DBP for TRDED
Figure S1. Flow chart for the extraction of study participants. TRDED, treatment‐required diabetic eye diseases.
Figure S2. Cumulative incidence of TRDED according to 6 categories of HbA1c determined by the Kaplan–Meier method. Six categories of HbA1c (thick dotted line, HbA1c ≥8.6%; thick black line, 8.1‐8.5%; thick dashed line, 7.6‐8.0%; thin dotted line, 7.1‐7.5%; thin black line, 6.6‐7.0%; thin dashed line, HbA1c ≤6.5%). TRDED, treatment‐required diabetic eye diseases.
Figure S3. Combined roles of PP with HbA1c (A) and SBP with HbA1c (B) for TRDED. Each variable was stratified according to tertiles. The estimated log hazard ratios were plotted as connected points with confidence intervals. HbA1c (dotted line, HbA1c ≥7.0%; black line, HbA1c 6.5‐6.9%; dashed line, HbA1c ≤6.4%) levels at baseline. HbA1c, hemoglobin A1c; HR, hazard ratio; PP, pulse pressure; SBP, systolic blood pressure; TRDED, treatment‐required diabetic eye diseases.
Acknowledgments
The authors thank the Ethics Committee of Niigata University for approving this study. The authors also thank Mami Haga and Natsuko Tada, Niigata University Faculty of Medicine (1‐757 Asahimachi, Niigata, Niigata, Japan), for their excellent secretarial assistance.
(J Am Heart Assoc. 2019;8:e010627 DOI: 10.1161/JAHA.118.010627.)
Part of this study was presented in poster form at the American Diabetes Association Scientific Sessions, June 9 to 13, 2017, in San Diego, CA.
References
- 1. Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. Ukpds 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–163. [DOI] [PubMed] [Google Scholar]
- 2. Zhou JB, Song ZH, Bai L, Zhu XR, Li HB, Yang JK. Could intensive blood pressure control really reduce diabetic retinopathy outcomes? Evidence from meta‐analysis and trial sequential analysis from randomized controlled trials. Diabetes Ther. 2018;9:2015–2027. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fujihara K, Igarashi R, Yamamoto M, Ishizawa M, Matsubayasi Y, Matsunaga S, Kato K, Ito C, Koishi M, Yamanaka N, Kodama S, Sone H. Impact of glucose tolerance status on the development of coronary artery disease among working‐age men. Diabetes Metab. 2017;43:261–264. [DOI] [PubMed] [Google Scholar]
- 5. Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim‐Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253–390. [DOI] [PubMed] [Google Scholar]
- 6. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 7. Ishihara M, Yukimura Y, Aizawa T, Yamada T, Ohto K, Yoshizawa K. High blood pressure as risk factor in diabetic retinopathy development in NIDDM patients. Diabetes Care. 1987;10:20–25. [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto T, Iimuro S, Ohashi Y, Sone H, Yamashita H, Ito H; the Japanese Elderly Intervention Trial Research Group . Prevalence and risk factors for diabetic maculopathy, and its relationship to diabetic retinopathy in elderly Japanese patients with type 2 diabetes mellitus. Geriatr Gerontol Int. 2012;12:134–140. [DOI] [PubMed] [Google Scholar]
- 9. Jung C‐H, Jung S‐H, Kim K‐J, Kim B‐Y, Kim C‐H, Kang S‐K, Mok J‐O. Differential associations of central and brachial blood pressure with carotid atherosclerosis and microvascular complications in patients with type 2 diabetes. BMC Cardiovasc Disord. 2014;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knudsen ST, Poulsen PL, Hansen KW, Ebbehoj E, Bek T, Mogensen CE. Pulse pressure and diurnal blood pressure variation: association with micro‐ and macrovascular complications in type 2 diabetes. Am J Hypertens. 2002;15:244–250. [DOI] [PubMed] [Google Scholar]
- 11. Longo‐Mbenza B, Nkondi Mbadi A, Mbungu Fuele S. Higher pulse pressure, systolic arterial hypertension, duration of diabetes and family history of diabetes in central Africans. Int J Diabetes Metab. 2008;16:17–23. [Google Scholar]
- 12. Theilade S, Lajer M, Hansen TW, Joergensen C, Persson F, Andresdottir G, Reinhard H, Nielsen SE, Lacy P, Williams B, Rossing P. 24‐hour central aortic systolic pressure and 24‐hour central pulse pressure are related to diabetic complications in type 1 diabetes—a cross‐sectional study. Cardiovasc Diabetol. 2013;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel V, Sailesh S, Panja S, Kohner E. Retinal perfusion pressure and pulse pressure: clinical parameters predicting progression to sight‐threatening diabetic retinopathy. Br J Diabetes Vasc Dis. 2001;1:80–87. [Google Scholar]
- 14. Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(1695):1695.e1–1695.e15. [DOI] [PubMed] [Google Scholar]
- 15. Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006;26:699–700. [DOI] [PubMed] [Google Scholar]
- 16. Ogawa O, Hayashi C, Nakaniwa T, Tanaka Y, Kawamori R. Arterial stiffness is associated with diabetic retinopathy in type 2 diabetes. Diabetes Res Clin Pract. 2005;68:162–166. [DOI] [PubMed] [Google Scholar]
- 17. Steppan J, Barodka V, Berkowitz DE, Nyhan D. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 2011;2011:263585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bertelsen G, Peto T, Lindekleiv H, Schirmer H, Solbu MD, Toft I, Sjølie AK, Njølstad I. Tromsø eye study: prevalence and risk factors of diabetic retinopathy. Acta Ophthalmol. 2013;91:716–721. [DOI] [PubMed] [Google Scholar]
- 19. Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. [DOI] [PubMed] [Google Scholar]
- 20. Hammes HP, Kerner W, Hofer S, Kordonouri O, Raile K, Holl RW. Diabetic retinopathy in type 1 diabetes‐a contemporary analysis of 8784 patients. Diabetologia. 2011;54:1977–1984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. HR with 95% CI of baseline values for each variable for TRDED risk analyzed by Cox models
Table S2. HR with 95% CI of baseline values of each variable for TRDED risk analyzed by Cox models
Table S3. HR with 95% CI for baseline values of 6 categories of HbA1c for TRDED risk analyzed by Cox models
Table S4. HR with 95% CI of TRDED for the top tertile compared with the combination of the lower and middle tertiles with regard to PP, SBP, and HbA1c
Table S5. Combined roles of SBP with DBP for TRDED
Figure S1. Flow chart for the extraction of study participants. TRDED, treatment‐required diabetic eye diseases.
Figure S2. Cumulative incidence of TRDED according to 6 categories of HbA1c determined by the Kaplan–Meier method. Six categories of HbA1c (thick dotted line, HbA1c ≥8.6%; thick black line, 8.1‐8.5%; thick dashed line, 7.6‐8.0%; thin dotted line, 7.1‐7.5%; thin black line, 6.6‐7.0%; thin dashed line, HbA1c ≤6.5%). TRDED, treatment‐required diabetic eye diseases.
Figure S3. Combined roles of PP with HbA1c (A) and SBP with HbA1c (B) for TRDED. Each variable was stratified according to tertiles. The estimated log hazard ratios were plotted as connected points with confidence intervals. HbA1c (dotted line, HbA1c ≥7.0%; black line, HbA1c 6.5‐6.9%; dashed line, HbA1c ≤6.4%) levels at baseline. HbA1c, hemoglobin A1c; HR, hazard ratio; PP, pulse pressure; SBP, systolic blood pressure; TRDED, treatment‐required diabetic eye diseases.


