Abstract
Background
Hypertrophic cardiomyopathy is defined as unexplained left ventricular (LV) hypertrophy (wall thickness ≥15 mm) and is prevalent in 0.2% of adults (1:500) in population‐based studies using echocardiography. Cardiac magnetic resonance imaging (MRI) allows for more accurate wall thickness measurement across the entire ventricle than echocardiography. The prevalence of unexplained LV hypertrophy by cardiac MRI is unknown. MESA (Multi‐Ethnic Study of Atherosclerosis) recruited individuals without overt cardiovascular disease 45 to 84 years of age.
Methods and Results
We studied 4972 individuals who underwent measurement of regional LV wall thickness by cardiac MRI as part of the MESA baseline exam. American Heart Association criteria were used to define LV segments. We excluded participants with hypertension, LV dilation (≥95% predicted end‐diastolic volume) or dysfunction (ejection fraction ≤50%), moderate‐to‐severe left‐sided valve lesions by cardiac MRI, severe aortic valve calcification by cardiac computed tomography (aortic valve Agatston calcium score >1200 in women or >2000 in men), obesity (body mass index >35 kg/m2), diabetes mellitus, and current smoking. Sixty‐seven participants (aged 64±10 years, 9% female) had unexplained LV hypertrophy (wall thickness ≥15 mm in at least 2 adjacent LV segments), representing 1.4% (1 in 74) participants, 2.6% of men and 0.2% of women. Prevalence was similar across categories of race/ethnicity. Hypertrophy was focal in 17 (25.4%), intermediate in 44 (65.7%), and diffuse in 5 (7.5%) participants.
Conclusions
The prevalence of unexplained LV hypertrophy in a population‐based cohort using cardiac MRI was 1.4%. This may have implications for the diagnosis of patients with hypertrophic cardiomyopathy and will require further study.
Keywords: hypertrophic cardiomyopathy, magnetic resonance imaging, population‐based study
Subject Categories: Cardiomyopathy, Hypertrophy, Magnetic Resonance Imaging (MRI), Epidemiology
Clinical Perspective
What Is New?
This is the first study to evaluate the prevalence of unexplained left ventricular hypertrophy in a population‐based cohort utilizing cardiac magnetic resonance imaging.
A total of 1.4% participants had unexplained left ventricular hypertrophy, 2.6% of men and 0.2% of women, which represents a higher prevalence than in previous echocardiographic studies.
What Are the Clinical Implications?
It is unclear whether participants with unexplained hypertrophy have true hypertrophic (or infiltrative) cardiomyopathy or whether the diagnostic criteria for hypertrophic cardiomyopathy are overly sensitive when applied to cardiac magnetic resonance imaging.
This may have implications for the diagnosis of patients with hypertrophic cardiomyopathy and will require further study.
Introduction
Unexplained left ventricular (LV) hypertrophy is defined as wall thickness ≥15 mm in the absence of conditions that predispose to hypertrophy.1, 2 Based on echocardiograms of 4111 participants in the CARDIA (Coronary Artery Risk Development in Young Adults) study performed in 1990–1991, the prevalence of hypertrophic cardiomyopathy (HCM) is ≈0.2% (1 in 500) adults.3 This study included participants 23 to 35 years of age who were either African American or white. Other population‐based echocardiographic studies corroborated these findings.4, 5
In HCM, any segment of the left ventricle may be affected alone or in combination.6 In contrast to cardiac magnetic resonance imaging (MRI), not all myocardial segments may be easily visible by echocardiography. Given the limitations of echocardiography, MRI has emerged as a powerful complementary imaging modality in the contemporary management of HCM.7 Moreover, clinically relevant discrepancies in LV thickness measurements between echocardiography and cardiac MRI have been described.8 Given these differences between echocardiography and MRI, our goal was to investigate the prevalence of unexplained LV hypertrophy by MRI in a population‐based cohort.
Methods
Study Population
MESA (Multi‐Ethnic Study of Atherosclerosis), a multicenter, prospective cohort study designed to investigate subclinical cardiovascular disease, has been previously described.9 Briefly, 6814 community‐dwelling women and men who ranged in age from 45 to 84 years, were free of cardiovascular disease and self‐identified as either white, African American, Hispanic, or Chinese American, were enrolled from 6 communities in the United States (Baltimore County, MA; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan and Bronx, NY; St. Paul, MN) between 2000 and 2002. A total of 5098 underwent cardiac MRI and 5004 had technically adequate data. Informed consent was obtained at enrollment, and the study was approved by the institutional review boards at all participating centers. Data from the MESA study are available to other researchers via the National Heart, Lung, and Blood Institute's Biologic Specimen and Data Repository. Further information on the methods used in this manuscript may be obtained from the first author.
Cardiac MRI
Cardiac MRI was performed with 1.5‐T magnets at all participating centers, with electrocardiographic gating, blood pressure monitoring, and a 4‐element phased‐array surface coil placed anteriorly and posteriorly, as previously described.10, 11 Briefly, a stack of short‐axis images covering the entire left ventricle was acquired using a flow‐compensated fast gradient echo sequence, with time to repetition/time to echo 8 to 10 ms/3 to 5 ms, flip angle 20°, 6‐mm slice thickness, 4‐mm gap, in‐plane resolution 1.4 to 1.6 mm (frequency direction)×2.2 to 2.5 mm (phase direction). Contouring of the endocardial and epicardial LV borders was performed using a semiautomated method (Q‐MASS 4.2, Medis, Leiden, The Netherlands) with manual correction for outliers. The left ventricle was divided on the basis of the midpoint of the LV septum, with superior levels defined as basal and more inferior locations as apical. Six segments per level were derived in the basal and midventricular levels and 4 segments at the apex.12, 13 Thickness of each segment was calculated by Q‐MASS software. LV volumes were adjusted for body size based on the following formula: %predicted LV volume=100×LV volume/(b×height^1.25×weight^0.43), where b=10.0 (women) or 10.5 (men) and volume is in milliliters, based on a previously derived allometric model.11, 14 LV dilation was defined as ≥95% predicted LV volume. The difference between the epicardial and endocardial areas in all levels was multiplied by the slice thickness and section gap, and then further multiplied by the myocardial specific gravity (1.04 g/mL) in order to determine the ventricular mass. Papillary muscle mass was included in the LV cavity and excluded from the LV mass. The technical error of measurement percent of the mean was 6% and 4% for LV mass and end‐diastolic volume, respectively, and the intraclass correlation coefficients were 0.98 and 0.98, respectively.10 Moderate‐to‐severe valve lesions were recorded prospectively by the interpreting physician. Information on infiltrative cardiomyopathies was not available.
Cardiac Computed Tomography
Participants underwent noncontrast cardiac computed tomography scans for evaluation of coronary and extracoronary calcification by the Agatston method, as previously described.15 Aortic valve calcification was defined as calcification focus with Agatston score >0 Agatston units in the aortic valve leaflets (not in the aortic annulus or a coronary artery).16 Severe aortic valve calcification was defined as >2000 Agatston units (men) and >1200 Agatston units (women) based on prior research correlating these parameters with echocardiographic severity.17 All scans were centrally read at the Harbor‐UCLA Research and Education Institute, Los Angeles, California.
Electrocardiography
Standard 12‐lead ECGs were recorded digitally at 10 mm/mV calibration and a speed of 25 mm/s using MAC 1200 ECG machines (Marquette Electronics, Milwaukee, WI) in all clinical centers. All ECGs were centrally read and visually checked for quality; ECG interpretation was performed automatically with the GE Marquette 12‐SL program 2001 version (GE Marquette, Milwaukee, WI). Finally, trained staff manually confirmed computer‐detected ECG abnormalities. The Cornell voltage criteria (R in lead aVL+S in lead V3 >2.8 mV (for men) and >2.0 mV (for women) were used to identify LV hypertrophy by ECG.
Covariates
Baseline demographic, clinical, and anthropometric information was obtained by trained research personnel, including self‐reported ethnicity, history of cardiovascular disease and risk factors, smoking status, physical activity, and medication inventory. Blood samples were obtained after a 12‐hour fast and analyzed by a central laboratory. Brachial blood pressure was measured 3 times at rest in a sitting position using a Dinamap model Pro 100 automatic oscillometric sphygmomanometer (Critikon, GE Healthcare, Waukesha, WI), and the second and third measurements were averaged. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg or treatment with antihypertensive medication in the presence of a self‐reported history of hypertension. Diabetes mellitus was defined as fasting glucose ≥126 mg/dL or use of antidiabetic medication. Impaired fasting glucose was defined as fasting glucose between 100 and 125 mg/dL. Current smoking was defined as having smoked a cigarette in the preceding 30 days.
Left Ventricular Hypertrophy by MRI
LV hypertrophy was primarily defined as LV wall thickness ≥15 mm in at least 2 adjacent segments of the standardized American Heart Association 16‐segment model.13 Adjacency was defined as neighboring segments either on the same level (basal, midventricular, or apical) or across levels. We used the following exclusion criteria to define the group of participants with unexplained LV hypertrophy: hypertension, LV dilation (≥95% predicted end‐diastolic volume) or dysfunction (ejection fraction ≤50%), moderate‐to‐severe left‐sided valve lesions by cardiac MRI or severe aortic valve calcification by cardiac computed tomography, diabetes mellitus, current smoking, and body mass index >35 kg/m2. Participants were classified as having secondary LV hypertrophy if they met at least one of the aforementioned criteria.
In addition to our main analysis, we applied less stringent sequential wall thickness criteria as sensitivity analyses: (1) LV wall thickness ≥15 mm in at least 1 segment, (2) borderline LV hypertrophy (defined as LV wall thickness ≥13 mm) in at least 2 adjacent segments, and (3) borderline LV hypertrophy in at least 1 segment (Figure 1).
Figure 1.
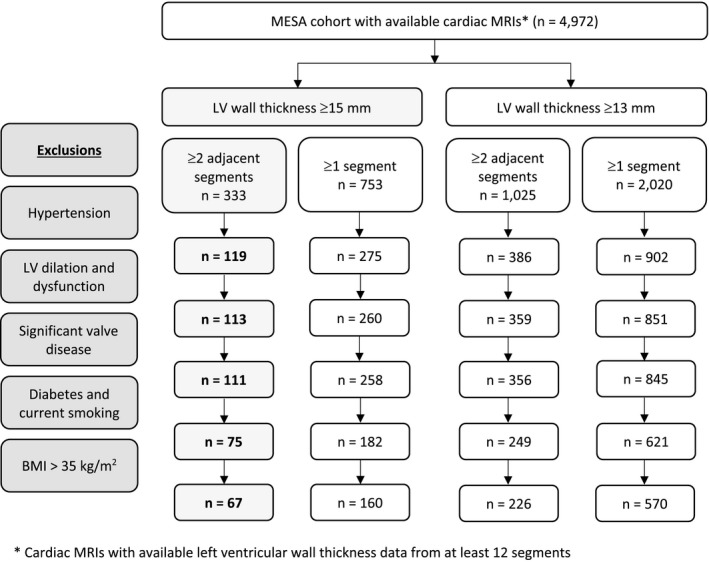
Flow diagram of screening strategy for participants with unexplained left ventricular hypertrophy. BMI indicates body mass index; LV, left ventricular, MESA, Multi‐Ethnic Study of Atherosclerosis; MRI, magnetic resonance imaging.
Participants with unexplained LV hypertrophy were categorized as having focal (2 segments), intermediate (3–7 segments), or diffuse (≥8 segments) hypertrophy. Furthermore, participants were categorized into the following morphologic phenotypes: asymmetric septal (ratio of any basal septal or midventricular septal segment to the average of lateral segments ≥1.5:1), apical (any apical segment ≥15 mm), midventricular (any midventricular segment ≥15 mm), basal (any basal segment ≥15 mm), and concentric (all segments ≥15 mm). Because the definition of LV hypertrophy requires 2 adjacent hypertrophied segments, which can occur across walls and levels, participants can be classified as having multiple morphological phenotypes.
Statistical Methods
Participants in the study sample were categorized by presence of unexplained, secondary or no LV hypertrophy. Continuous variables are presented as mean± SD and were compared with the unpaired Student t or ANOVA test, as appropriate. Categorical variables are presented as counts and proportions and were analyzed with the chi‐squared or Fisher's exact tests. For the comparison of phenotypes between categories of race/ethnicity, P values were calculated conditional on having unexplained LV hypertrophy using a Fisher's exact test. All analyses were performed using Stata 15.0 for Windows (StataCorp LP, College Station, TX). Figures were created with Prism 7.0e for Mac OS X (GraphPad Software, La Jolla, CA). A 2‐tailed P<0.05 was used to define statistical significance.
Results
Of 5004 MESA study participants with technically adequate data, 4972 had available wall thickness measurements from at least 12 segments and were included in the study sample. Mean (SD) age was 61.5 (10.1) years, and 52.3% were women (Table 1). A total of 333 (6.7%) participants had at least 2 adjacent hypertrophied LV segments (wall thickness ≥15 mm), and 266 participants were excluded for secondary causes (Figure 1). Sixty‐seven participants were classified as having unexplained LV hypertrophy, 61 men and 6 women. This represents a prevalence of 1.4% (1 in 74 participants), 2.6% of men, and 0.2% of women. Utilizing less stringent inclusion criteria of at least 1 LV segment with ≥15 mm wall thickness, 160 (3.2%) participants were classified as having unexplained LV hypertrophy (1 in 31); when applying criteria for borderline LV hypertrophy (≥13 mm), 226 (4.6%) participants were classified as having unexplained hypertrophy based on 2 adjacent LV segments, and 570 (11.5%) in at least 1 segment (Figure 1).
Table 1.
Characteristics by Categories of Unexplained, Secondary, and No Hypertrophy
| Characteristics | Unexplained Hypertrophy (n=67) | Secondary Hypertrophy (n=266) | No Hypertrophy (n=4639) | P Value |
|---|---|---|---|---|
| Age, y | 64.3±10.1 | 65.2±9.3 | 61.3±10.1 | <0.001 |
| Female, n (%) | 6 (9.0%) | 50 (18.8%) | 2545 (54.9%) | <0.001 |
| Race/ethnicity, n (%) | <0.001 | |||
| White | 25 (37.3) | 82 (30.8) | 1834 (39.5) | |
| Chinese American | 6 (9.0) | 10 (3.8) | 633 (13.6) | |
| African American | 23 (34.3) | 125 (47.0) | 1132 (24.4) | |
| Hispanic | 13 (19.4) | 49 (18.4) | 1040 (22.4) | |
| Height, cm | 175.2±7.3 | 171.9±8.5 | 166.0±9.9 | <0.001 |
| Weight, kg | 86.7±13.3 | 89.3±15.0 | 76.2±15.9 | <0.001 |
| BMI, kg/m2 | 28.2±3.7 | 30.2±4.8 | 27.6±4.9 | <0.001 |
| Smoking status | <0.001 | |||
| Never | 28 (41.8%) | 101 (38.3%) | 2427 (52.5%) | |
| Former | 39 (58.2%) | 102 (38.6%) | 1633 (35.3%) | |
| Current | 0 (0.0%) | 61 (23.1%) | 567 (12.3%) | |
| Systolic blood pressure, mm Hg | 118.4±11.6 | 142.9±23.6 | 124.6±20.8 | <0.001 |
| Diastolic blood pressure, mm Hg | 72.5±8.0 | 79.0±11.6 | 71.4±10.1 | <0.001 |
| Hypertension, n (%) | 0 | 214 (80.5) | 1892 (40.8) | <0.001 |
| Antihypertensive medication, n (%) | 7 (10.4) | 177 (66.5) | 1570 (33.9) | <0.001 |
| Total cholesterol, mg/dL | 189.9±30.4 | 189.8±35.6 | 194.6±35.4 | 0.057 |
| LDL cholesterol, mg/dL | 119.2±29.4 | 116.3±32.1 | 117.2±31.3 | 0.79 |
| HDL cholesterol, mg/dL | 46.3±13.7 | 46.9±12.7 | 51.5±15.1 | <0.001 |
| Lipid‐lowering medication, n (%) | 4 (6.0) | 53 (20.0) | 736 (15.9) | 0.016 |
| Diabetes mellitus status, n (%) | <0.001 | |||
| Impaired fasting glucose | 17 (25.4) | 54 (20.3) | 570 (12.3) | |
| Diabetes mellitus | 0 (0.0) | 72 (27.1) | 503 (10.9) | |
| LV ejection fraction (%) | 66.1±6.6 | 65.5±9.8 | 69.2±7.2 | <0.001 |
| LV mass/BSA, g/m2 | 97.8±14.5 | 103.6±20.6 | 76.3±14.4 | <0.001 |
| LV end‐systolic volume/BSA, mL/m2 | 21.3±6.7 | 23.3±11.2 | 21.3±7.9 | <0.001 |
| LV end‐diastolic volume/BSA, mL/m2 | 62.2±13.7 | 65.6±17.3 | 68.4±13.2 | <0.001 |
| LV stroke volume/BSA, mL/m2 | 40.9±9.4 | 42.4±10.3 | 47.1±8.7 | <0.001 |
| LV hypertrophy by ECG (Cornell), n (%) | 2 (3.0) | 23 (8.6) | 158 (3.4) | <0.001 |
| Any major ECG abnormalities, n (%) | 10 (15.2) | 71 (26.7) | 406 (8.8) | <0.001 |
| Any minor ECG abnormalities, n (%) | 41 (62.1) | 181 (68.0) | 2025 (44.0) | <0.001 |
BMI indicates body mass index; BSA, body surface area; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LV, left ventricular.
Regional Distribution and Maximum Wall Thickness
Among those with unexplained hypertrophy, the most frequently hypertrophied segments in each level were the basal anterior septal (46%), midventricular anterior lateral (40%), and apical lateral (33%) segments (Figure 2). The thickest individual segments in each level were the basal inferior lateral (20 mm), the midventricular anterior lateral (21 mm), and the apical lateral segment (20 mm) (wall thickness in whole study group and by sex in Figure 3; by race/ethnicity in Figure S1).
Figure 2.
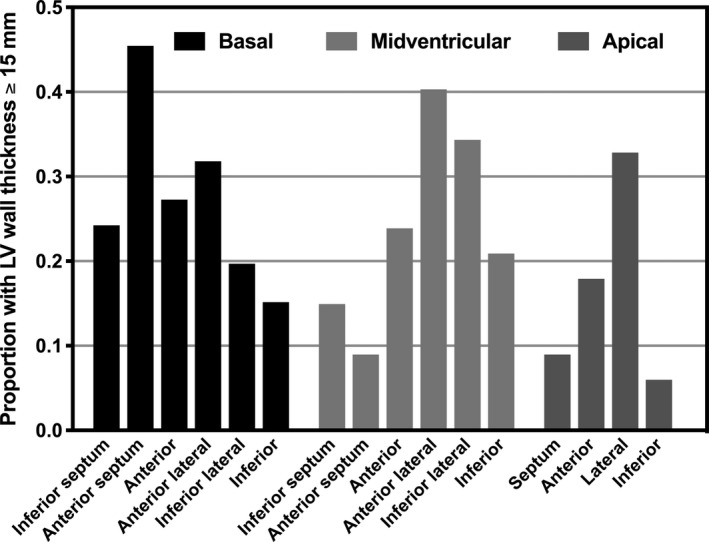
Proportion with LV thickness ≥15 mm by level and segment among a group of participants with unexplained LV hypertrophy. LV indicates left ventricular.
Figure 3.
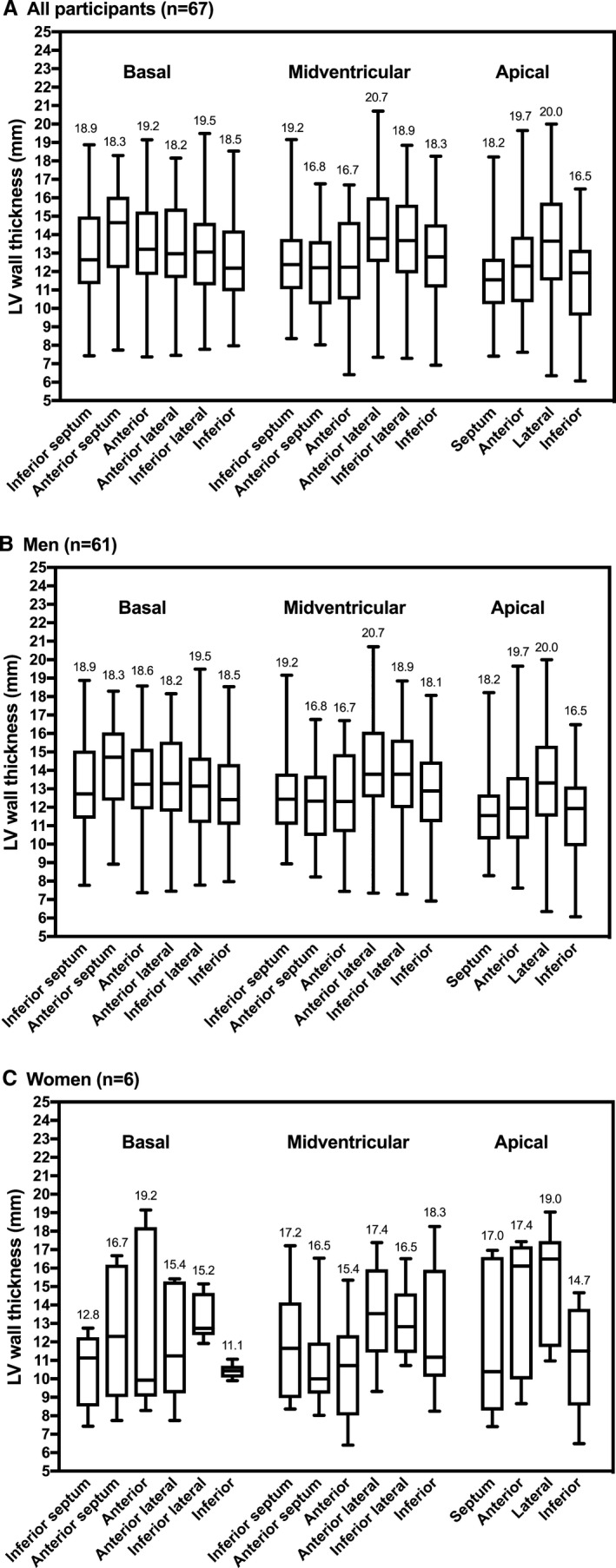
Distribution of left ventricular wall thickness by segment among all (A), male (B), and female (C) participants with unexplained LV hypertrophy. Maximum wall thickness in millimeters is noted for each segment. LV indicates left ventricular.
Extent of Hypertrophy
Hypertrophy was focal in 17 (25.4%), intermediate in 44 (65.7%), and diffuse in 5 (7.5%) participants (Table 2). Most Chinese American participants had focal hypertrophy (n=5 [83.3%]), while African American participants had the highest proportion of intermediate (n=16 [69.6%]) and diffuse (n=4 [17.4%]) hypertrophy. Mean (SD) total LV mass index was 88.2 (9.5) g/m2 in the focal group, 101.0 (14.9) g/m2 in the intermediate group, and 103.7 (11.7) g/m2 in the diffuse group. The number of hypertrophied LV segments and LV mass index were correlated (r=0.38, P=0.002).
Table 2.
Distribution of Participants With Unexplained Hypertrophy by Race/Ethnicity
| Total | White | Chinese | African American | Hispanic | P Value | |
|---|---|---|---|---|---|---|
| Unexplained hypertrophy, n (% total cohort) | 67 (1.4) | 25 (1.3) | 6 (0.9) | 23 (1.8) | 13 (1.2) | 0.38 |
| Extenta, n (% participants with unexplained hypertrophy) | 0.02 | |||||
| Focal (2 segments) | 17 (25.4) | 6 (24) | 5 (83.3) | 3 (13.0) | 3 (23.1) | |
| Intermediate (3–7 segments) | 44 (65.7) | 17 (68.0) | 1 (16.7) | 16 (69.6) | 10 (76.9) | |
| Diffuse (≥8 segments) | 5 (7.5) | 1 (4.0) | 0 | 4 (17.4) | 0 | |
| Locationb, n (% participants with unexplained hypertrophy) | ||||||
| Septum | 35 (52.2) | 13 (52.0) | 2 (33.0) | 13 (56.5) | 7 (53.9) | 0.83 |
| Lateral wall | 50 (74.6) | 19 (76.0) | 5 (83.3) | 16 (69.6) | 10 (76.9) | 0.93 |
| Anterior wall | 36 (53.7) | 14 (56.0) | 2 (33.0) | 12 (52.2) | 8 (61.5) | 0.76 |
| Inferior wall | 19 (28.4) | 10 (40.0) | 0 | 7 (30.4) | 2 (15.4) | 0.19 |
| Phenotypeb, n (% participants with unexplained hypertrophy) | ||||||
| Asymmetric septal | 21 (31.3) | 7 (28.0) | 2 (33.0) | 7 (30.4) | 5 (38.5) | 0.96 |
| Isolated basal septal | 4 (6.0) | 1 (4.0) | 1 (16.7) | 2 (8.7) | 0 | 0.36 |
| Apical | 26 (38.9) | 9 (36.0) | 2 (33.0) | 11 (47.8) | 4 (30.8) | 0.77 |
| Midventricular | 44 (65.7) | 17 (68.0) | 3 (50.0) | 15 (65.2) | 9 (69.2) | 0.89 |
| Basal | 47 (70.2) | 18 (72.0) | 3 (50.0) | 18 (78.3) | 8 (61.5) | 0.20 |
One participant was not classifiable because of missing data.
Participants can be counted multiple times because the definition of unexplained hypertrophy requires 2 adjacent hypertrophied segments in 1 or across levels.
Morphologic Phenotype
Asymmetric septal hypertrophy was present in n=21 (31.3%) participants, while the lateral wall (n=50 [74.6%]) and basal segments (n=47 [70.2%]) were the most commonly involved walls and segments, respectively. Isolated basal septal hypertrophy was present in 4 participants (all men). There was no statistically significant difference in race/ethnicity among distribution of hypertrophy across morphologic phenotypes (Table 2).
Associated Factors
The prevalence of unexplained hypertrophy increased by decade of age: 14 (0.9%) in the 45‐ to 54‐year‐old group, 18 (1.3%) in the 55‐ to 64‐year‐old group, 23 (1.6%) in the 65‐ to 74‐year‐old group, and 12 (1.9%) in the 75‐ to 84‐year‐old group. Participants with unexplained LV hypertrophy were more likely to be male, white, and taller than those with secondary LV hypertrophy but had similar weight. They also had lower LV mass, LV end‐diastolic volumes and stroke volumes. Compared with participants without LV hypertrophy, those with unexplained or secondary LV hypertrophy were more likely to be male and have a higher body mass index.
ECG Abnormalities
Major ECG abnormalities were identified in 10 (15.2%) participants with unexplained hypertrophy and included complete left or right bundle branch block in 7, Q‐wave abnormalities with or without ST‐segment abnormalities in 3, and isolated ST‐segment or T‐wave abnormalities in 3 participants. Minor ECG abnormalities were identified in 41 (62.1%) participants with unexplained hypertrophy and included minor isolated Q, ST, or T changes; tall R waves; ST‐segment elevations; incomplete left or right bundle branch block; left axis deviation; first‐degree atrioventricular block; and frequent premature beats. Major and minor ECG abnormalities in participants with unexplained hypertrophy were as common as in participants with secondary hypertrophy but more common than in participants without hypertrophy (Table 1).
Discussion
In this study, we investigated the prevalence of unexplained LV hypertrophy by cardiac MRI in a population‐based cohort. While in echocardiographic studies the prevalence of unexplained hypertrophy among young adults was ≈0.2% (1 in 500 people),3 we found a prevalence of 1.4% (1 in 74 people), 2.6% of men and 0.2% of women, among middle‐aged and older adults who were free of clinical cardiovascular disease after exclusion of conditions associated with secondary hypertrophy. Whether these individuals have HCM or infiltrative cardiomyopathy or belong to the spectrum of normal wall thickness is unknown.
Phenotypic expression of HCM is variable, with incomplete and age‐dependent penetrance. Echocardiography is the most accessible imaging modality in the management of HCM. However, cardiac MRI enables more accurate and precise imaging of cardiac morphology and is commonly used as a “tiebreaker” in equivocal cases or in individuals with suboptimal echocardiographic image quality. Cardiac MRI covers the entire ventricle with high spatial resolution and allows wall thickness measurements of all LV segments with high fidelity attributable to the sharp contrast between blood and endocardial borders. Therefore, an updated estimate of the prevalence of unexplained LV hypertrophy has become necessary. Although we report an estimated prevalence of 1.4% based on 2 adjacent segments with increased wall thickness (≥15 mm), it may be as high as 3.3% based on just 1 hypertrophied segment, and higher still if a lower cutoff (≥13 mm) is used. Using the requirement for 2 adjacent hypertrophied segments may mitigate possible measurement errors introduced in the reporting process but may sacrifice sensitivity. Nevertheless, our prevalence is considerably higher than prior estimates.
There are 2 main possible explanations for these findings. First, the true prevalence of HCM may be higher than previously thought. Image quality and sensitivity for regional wall thickness measurements by cardiac MRI are better compared with echocardiography.8, 18 In clinical practice, basal septal and inferolateral wall thickness measurements are part of standardized echocardiographic protocols, while other segments may not be consistently measured. Cardiac MRI can identify hypertrophied segments that are undetected by echocardiography, especially in the anterior and lateral walls. Furthermore, discordances in wall thickness measurements between the 2 modalities are common: discrepancies of ≥10% were present in half of the study participants in one study; both over‐ and underestimation by echocardiography were common.8 In a study examining ECG abnormalities in 155 athletes, echocardiography identified 31 cases of HCM, while cardiac MRI added another 20 cases that were not found by echocardiography.19 Limitations of echocardiography that are not present in cardiac MRI include acoustic shadowing, poor endocardial definition, and predefined imaging views leading to gaps in myocardial visualization.20
In addition, age may explain the higher prevalence of unexplained hypertrophy. The MESA cohort was older (45–84 years at enrollment) compared with CARDIA (23–35 years). In individuals with sarcomeric mutations associated with HCM, the prevalence of hypertrophy increases with age,21 while in the general population wall thickness is not associated with age.22 Furthermore, the prevalence of known pathogenic sarcomeric mutations is 0.6% in population‐based studies23, 24 but may be higher considering that not all disease‐associated genes have yet been identified. Therefore, the true prevalence of HCM is potentially higher than previously thought.
The second possible explanation for our findings is the erroneous labeling of participants without pathology as having hypertrophy. Given the aforementioned advantages of MRI, diagnostic criteria for HCM may be overly sensitive and may inadvertently capture individuals with normal wall thickness. Guidelines for HCM define unexplained hypertrophy by the universal application of a 15‐mm cutoff in at least 1 segment despite substantial variability in normal wall thickness of different segments. Normal values for regional wall thickness in all segments have previously been published in a subset of MESA participants using the steady‐state free precession sequence.22 In this study, maximum wall thickness was highest in the basal, followed by the midventricular and apical segments, with differences of up to 4 mm between segments. Moreover, mean regional wall thickness was greater in men compared with women (1 mm difference), another factor that is unaccounted for in the current diagnostic criteria for HCM. Finally, although we excluded participants with body mass index >35 kg/m2 to eliminate outliers in weight, there is no established methodology to adjust wall thickness measurements for body size. The diagnostic criteria for HCM when applied to cardiac MRI may require refinement to reflect differences in normal wall thickness by segment, sex, and body size.
Study Limitations
We acknowledge several limitations to our study. First, although we attempted to exclude all possible causes of secondary LV hypertrophy, the reported prevalence may be overestimated because of incomplete exclusion of secondary causes. Most importantly, we were unable to exclude participants with masked hypertension if they had normal blood pressure at the time of their in‐person examination, were not taking antihypertensive medication, and did not have a diagnosis of hypertension. Notably, 7 participants were taking antihypertensive agents presumably for other reasons because they did not report a diagnosis of hypertension. Similarly, because echocardiography was not performed in this cohort, cases of severe valve lesions may have been missed, although we attempted to capture those with severe aortic stenosis by surrogate measures of cardiac computed tomography calcium scoring. Of note, chronic kidney disease stage 4 or greater (estimated glomerular filtration rate <30 mL/min per kg2) was not present in the group with unexplained hypertrophy.
Second, of the 6814 total MESA participants, 5004 underwent cardiac MRI, of which 4972 (73%) had technically adequate data. Those who did not complete the cardiac MRI were most commonly ineligible because of metal implants, claustrophobia, or refusal, and were slightly older, more hypertensive, and had a higher body mass index.25 However, given the small differences, we believe that the prevalence of unexplained hypertrophy would not be meaningfully different among those who did not undergo cardiac MRI. Third, we were unable to provide information on symptoms or LV outflow tract gradients because this was not routinely reported in MESA. Fourth, gadolinium contrast and T1 mapping were not used in the baseline MESA exam, which may have provided valuable added information to better characterize our study group. In addition, rare cases of infiltrative diseases such as amyloid or Anderson‐Fabry cardiomyopathy may thus have been missed. Fifth, short‐axis wall thickness measurements of the basal and midventricular segments were only minimally larger compared with long‐axis measurements (6% and 10%, respectively), in contrast to measurements of apical segments, which were substantially larger (20%).22 Given that we used short axis measurements in our study, apical hypertrophy may have been overestimated.
Sixth, the fast gradient echo sequence used in this study has largely been replaced by the steady‐state free precession sequence in clinical practice, and there are known differences in LV mass measurements between sequences. However, wall thickness measurements in MESA on the latter were available in only a small subset of participants. In MESA, LV mass was found to be 4.8% higher on the fast gradient echo sequence compared with steady‐state free precession.26 Therefore, there may be a discrepancy in wall thickness as well. Because mass is proportional to volume, the discrepancy in 1‐dimensional wall thickness measurements is expected to be smaller yet.
Seventh, as the number of hypothesis tests presented in the tables is large, the possibility of false‐positive results exists. These associations will have to be confirmed in future studies. Finally, we were unable to include multivariable adjusted analyses given the arbitrary definition of unexplained hypertrophy that was driven by exclusion of participants. While the perfect cohort to answer the question of “true” hypertrophic cardiomyopathy prevalence would include echocardiography in parallel with cardiac MRI, MESA currently provides the closest possible opportunity available to attempt an estimation.
Conclusion
In a multiethnic population‐based cohort without clinical cardiovascular disease, the prevalence of unexplained LV hypertrophy by cardiac MRI is 1.4%, defined as wall thickness ≥15 mm in at least 2 adjacent LV segments. This estimate is higher than previously described by echocardiography, likely reflecting the better image quality and superior endocardial/epicardial border definition afforded by cardiac MRI and the older age of our sample. Additional studies are required to define the clinical implications of this higher MRI‐determined prevalence.
Sources of Funding
This research was supported by contracts HHSN268201500003I, N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute, and by grants UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420 from the National Center for Advancing Translational Sciences (NCATS). Massera was supported by The Glorney‐Raisbeck Fellowship Program, Corlette Glorney Foundation, and The New York Academy of Medicine. Kizer was supported by K24 Hl135413 from the National Heart, Lung, and Blood Institute.
Disclosures
Kizer reports stock ownership in Amgen, Gilead Sciences, Johnson & Johnson, and Pfizer. The remaining authors have no disclosures to report.
Supporting information
Figure S1. Distribution of LV wall thickness by segment by race/ethnicity, among white (A), Chinese (B), African American (C), and Hispanic (D) MESA participants with unexplained LV hypertrophy. Maximum wall thickness in mm is noted for each segment. LV indicates left ventricular; MESA, Multi‐Ethnic Study of Atherosclerosis.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
(J Am Heart Assoc. 2019;8:e012250 DOI: 10.1161/JAHA.119.012250.)
References
- 1. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons . 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. [DOI] [PubMed] [Google Scholar]
- 2. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (YOUNG) Adults. Circulation. 1995;92:785–789. [DOI] [PubMed] [Google Scholar]
- 4. Maro EE, Janabi M, Kaushik R. Clinical and echocardiographic study of hypertrophic cardiomyopathy in Tanzania. Trop Doct. 2006;36:225–227. [DOI] [PubMed] [Google Scholar]
- 5. Codd MB, Sugrue DD, Gersh BJ, Melton LJ III. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population‐based study in Olmsted County, Minnesota, 1975–1984. Circulation. 1989;80:564–572. [DOI] [PubMed] [Google Scholar]
- 6. Klues HG, Schiffers A, Maron BJ. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two‐dimensional echocardiography in 600 patients. J Am Coll Cardiol. 1995;26:1699–1708. [DOI] [PubMed] [Google Scholar]
- 7. Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, De Santis M, Quarta G, Nistri S, Cecchi F, Salton CJ, Udelson JE, Manning WJ, Maron BJ. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:559–566. [DOI] [PubMed] [Google Scholar]
- 8. Hindieh W, Weissler‐Snir A, Hammer H, Adler A, Rakowski H, Chan RH. Discrepant measurements of maximal left ventricular wall thickness between cardiac magnetic resonance imaging and echocardiography in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2017;10:e006309. [DOI] [PubMed] [Google Scholar]
- 9. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 10. Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch‐Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in Multi‐Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–S365. [DOI] [PubMed] [Google Scholar]
- 11. Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan RT, Bluemke D, Gomes A, Burke G, Shea S, Liu K, Bahrami H, Sinha S, Wu C, Fernandes V, McClelland R, Lima JA. Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2011;57:1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 14. Brumback LC, Kronmal R, Heckbert SR, Ni H, Hundley WG, Lima JA, Bluemke DA. Body size adjustments for left ventricular mass by cardiovascular magnetic resonance and their impact on left ventricular hypertrophy classification. Int J Cardiovasc Imaging. 2010;26:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of Multi‐Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 16. Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O'Brien KD, Blumenthal RS, Carr JJ, Kronmal R. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the Multi‐Ethnic Study of Atherosclerosis. Acad Radiol. 2006;13:166–172. [DOI] [PubMed] [Google Scholar]
- 17. Clavel MA, Messika‐Zeitoun D, Pibarot P, Aggarwal SR, Malouf J, Araoz PA, Michelena HI, Cueff C, Larose E, Capoulade R, Vahanian A, Enriquez‐Sarano M. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329–2338. [DOI] [PubMed] [Google Scholar]
- 18. Valente AM, Lakdawala NK, Powell AJ, Evans SP, Cirino AL, Orav EJ, MacRae CA, Colan SD, Ho CY. Comparison of echocardiographic and cardiac magnetic resonance imaging in hypertrophic cardiomyopathy sarcomere mutation carriers without left ventricular hypertrophy. Circ Cardiovasc Genet. 2013;6:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schnell F, Riding N, O'Hanlon R, Axel Lentz P, Donal E, Kervio G, Matelot D, Leurent G, Doutreleau S, Chevalier L, Guerard S, Wilson MG, Carre F. Recognition and significance of pathological T‐wave inversions in athletes. Circulation. 2015;131:165–173. [DOI] [PubMed] [Google Scholar]
- 20. Hindieh W, Chan R, Rakowski H. Complementary role of echocardiography and cardiac magnetic resonance in hypertrophic cardiomyopathy. Curr Cardiol Rep. 2017;19:81. [DOI] [PubMed] [Google Scholar]
- 21. Charron P, Dubourg O, Desnos M, Isnard R, Hagege A, Millaire A, Carrier L, Bonne G, Tesson F, Richard P, Bouhour JB, Schwartz K, Komajda M. Diagnostic value of electrocardiography and echocardiography for familial hypertrophic cardiomyopathy in a genotyped adult population. Circulation. 1997;96:214–219. [DOI] [PubMed] [Google Scholar]
- 22. Kawel N, Turkbey EB, Carr JJ, Eng J, Gomes AS, Hundley WG, Johnson C, Masri SC, Prince MR, van der Geest RJ, Lima JA, Bluemke DA. Normal left ventricular myocardial thickness for middle‐aged and older subjects with steady‐state free precession cardiac magnetic resonance: the Multi‐Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2012;5:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bick AG, Flannick J, Ito K, Cheng S, Vasan RS, Parfenov MG, Herman DS, DePalma SR, Gupta N, Gabriel SB, Funke BH, Rehm HL, Benjamin EJ, Aragam J, Taylor HA Jr, Fox ER, Newton‐Cheh C, Kathiresan S, O'Donnell CJ, Wilson JG, Altshuler DM, Hirschhorn JN, Seidman JG, Seidman C. Burden of rare sarcomere gene variants in the Framingham and Jackson Heart Study cohorts. Am J Hum Genet. 2012;91:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Natarajan P, Gold NB, Bick AG, McLaughlin H, Kraft P, Rehm HL, Peloso GM, Wilson JG, Correa A, Seidman JG, Seidman CE, Kathiresan S, Green RC. Aggregate penetrance of genomic variants for actionable disorders in European and African Americans. Sci Transl Med. 2016;8:364ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch‐Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malayeri AA, Johnson WC, Macedo R, Bathon J, Lima JA, Bluemke DA. Cardiac cine MRI: quantification of the relationship between fast gradient echo and steady‐state free precession for determination of myocardial mass and volumes. J Magn Reson Imaging. 2008;28:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of LV wall thickness by segment by race/ethnicity, among white (A), Chinese (B), African American (C), and Hispanic (D) MESA participants with unexplained LV hypertrophy. Maximum wall thickness in mm is noted for each segment. LV indicates left ventricular; MESA, Multi‐Ethnic Study of Atherosclerosis.


