Abstract
Background
Statins may reduce mortality after transcatheter aortic valve replacement (TAVR) through prevention of atherosclerotic events or pleiotropic effects. However, the competing mortality risks in TAVR patients may dilute any positive effect of statins. We sought to understand the association of statin use with post‐TAVR mortality.
Methods and Results
We included high– or intermediate–surgical risk patients who underwent TAVR as a part of the PARTNER (Placement of Aortic Transcatheter Valves) II and Sapien 3 trials and registries. Outcomes included 2‐year all‐cause, cardiovascular, and noncardiovascular mortality. We used propensity score matching to generate matched pairs between those discharged on a statin and those not on a statin after TAVR. Bias was explored with falsification end points (urinary infection, hip fracture). Among 3956 patients who underwent TAVR, we matched 626 patients on a statin with 626 patients not on a statin at discharge. Among matched patients, statin use was associated with lower risk of all‐cause (hazard ratio [HR] 0.65, 95% CI 0.49‐0.87, P=0.001), cardiovascular (HR 0.66, 95% CI 0.46‐0.96, P=0.030), and noncardiovascular mortality (HR 0.64, 95% CI 0.44‐0.99, P=0.045) compared with no statin use. The survival curves diverged within 3 months and continued to separate over a median follow‐up of 2.1 years. The falsification end points were similar among groups (urinary infection, P=0.66; hip fracture, P=0.64).
Conclusions
In an observational, propensity‐matched analysis of TAVR patients, statin use was associated with lower rates of cardiovascular and noncardiovascular mortality compared with no statin use. Given the early emergence of the apparent protective effect of statins, this result may be driven either by pleiotropic effects or by residual confounding despite propensity‐matching methodology.
Keywords: aortic stenosis, outcome, statin therapy, transcutaneous aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation
Clinical Perspective
What Is New?
In a propensity‐matched cohort, statin use at the time of hospital discharge for the index transcatheter aortic valve implantation was associated with lower risk of long‐term all‐cause mortality compared with non–statin use after transcatheter aortic valve replacement.
What Are the Clinical Implications?
If validated in prospective studies, this study suggests that statins may be used as an adjunct therapy after transcatheter aortic valve replacement to improve survival.
Introduction
Among patients with severe, symptomatic aortic stenosis, transcatheter aortic valve replacement (TAVR) improves survival and quality of life compared with medical therapy.1, 2 Despite the improvement in survival conferred by TAVR, mortality rates after TAVR remain high,3, 4 as patients undergoing TAVR are often elderly with multiple cardiovascular and noncardiovascular comorbidities. A number of strategies have been employed to reduce post‐TAVR mortality further, including improving patient selection, limiting periprocedural complications (eg, reducing sheath size to decrease vascular complications, computed tomography sizing, and sealing skirts to reduce paravalvular leaks), and improving postprocedure care (eg, less intensive care time and earlier mobilization). However, there is a continued need to investigate strategies that may provide further mortality reduction after TAVR.
Statins are well established as a key therapy to reduce the risk of cardiovascular mortality, especially among patients at high‐risk for cardiovascular events. However, the role of statins in older adults is uncertain. Due to competing mortality risks along with concerns for side effects and polypharmacy in the elderly population, current American clinical practice guidelines do not recommend statin therapy for patients >75 years of age for primary prevention with the decision to treat patients, and shared decision making is advised for secondary preventions.5 Despite the high rate of post‐TAVR mortality, it is unclear whether statins are associated with improved outcomes after TAVR. In addition to their cardiovascular benefits, statins may potentially reduce mortality via nonatherosclerotic or pleiotropic effects (eg, reducing acute kidney injury6 and other surgical complications).7 One study that examined the relationship of statins with post‐TAVR outcomes found that statin use was associated with lower mortality at 2 years compared with no statin use.8 A different study found no association of statins with mortality at 2 years after TAVR.9 However, because these were single‐center studies with relatively small sample sizes and the inherent risk of bias due to nonrandom treatment assignment in observational studies, leveraging statistical methods that increase the comparability between groups across key characteristics is important. We performed a propensity‐matched analysis among a large, prospective, adjudicated, multicenter cohort of patients who underwent TAVR to examine the association of statin use with the risk of mortality after TAVR.
Methods
Study Population
For data protection reasons, the data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results of this study. The current study included patients with severe symptomatic aortic stenosis who underwent TAVR as a part of the PARTNER II (Placement of Aortic Transcatheter Valve II) and Sapien 3 clinical trials or associated registries.10, 11 Briefly, the PARTNER II clinical trial enrolled patients who were felt to be at intermediate risk (cohort A: predicted risk of 30‐day mortality 4% to 8%, based on either the Society of Thoracic Surgeons [STS] mortality risk score or clinical assessment by a multidisciplinary heart team) or who were deemed inoperable (cohort B). The Sapien 3 trial enrolled patients who were felt to be intermediate (STS risk score 4% to 8% or heart team determination) and high (STS risk score >8% or heart team determination) surgical risk. The PARTNER II and Sapien 3 trials contained a number of nested registries that also enrolled high‐risk and inoperable patients (heart team estimation of ≥50% risk of death or serious, irreversible morbidity with surgery). Although the studies generally followed similar protocols with respect to inclusion and exclusion criteria, baseline data collection, and follow‐up, the PARTNER II studies used the Sapien‐XT valve, whereas the Sapien 3 studies used the Sapien 3 valve. The current analysis included only patients who underwent TAVR. The PARTNER study and registries were approved by the institutional review board at each participating site, and all patients provided written informed consent.
Study Exposure and Outcomes
Statin use was determined based on the discharge medication list from the index hospitalization. As a result, patients who died before discharge were excluded. The primary outcome was 2‐year all‐cause mortality. Secondary outcomes included cardiovascular mortality, noncardiovascular mortality, myocardial infarction, stroke, acute kidney injury, and bleeding over the 2 years after discharge. The clinical events committee adjudicated these outcomes according to the Valve Academic Research Consortium‐2 definitions.12, 13 We used rates of urinary tract infection and hip fractures as falsification end points to assess for bias. Falsification end points (negative controls) are outcomes that are not causally related to the main exposure under study. The absence of an association between the exposure and the falsification end point suggests a low bias.14 In our study urinary tract infections and hip fractures are not causally affected by statin use.15, 16
Statistical Analyses
Because patients were not randomized to statin therapy at discharge, and there might have been selection bias in who had been prescribed statins, we used propensity score methodology to create a cohort of patients with similar characteristics who were discharged on a statin versus not discharged on a statin after TAVR. Nonparsimonious logistic regression was used to calculate the probability of receiving a statin at discharge based on the covariates listed in Table 1, with spline terms included for all continuous variables. Covariates were selected based on their clinical importance and their potential to confound the relationship between statins and mortality. Matching was performed in a 1:1 ratio by greedy matching with a caliper width of 0.2 SD. Covariate balance before and after matching was assessed using standardized differences (>10% difference is considered clinically relevant). Furthermore, to assess for unmeasured confounding, we compared the rates of urinary tract infections and hip fractures at 2 years between matched groups.
Table 1.
Baseline Characteristics Before and After Propensity Score Matching
| Characteristic | Prematch | Postmatch | ||||
|---|---|---|---|---|---|---|
| Statin (n=2864) | No Statin (n=1092) | Stand Diff (%)a | Statin (n=626) | No Statin (n=626) | Stand Diff (%)a | |
| Age, y | 83 (78‐87) | 84 (79‐88) | 7.9 | 84 (78‐87) | 84 (80‐88) | 2.5 |
| Male | 59.8% | 48.4% | 22.3 | 49.0% | 48.4% | 0.7 |
| White | 93.7% | 94.0% | 1.7 | 95.2% | 93.6% | 5.7 |
| Hispanic | 1.7% | 2.6% | 5.2 | 2.1% | 2.4% | 2.3 |
| Body surface area, m2 | 1.9 (1.7‐2.1) | 1.9 (1.7‐2.0) | 18.4 | 1.9 (1.7‐2.0) | 1.8 (1.7‐2.0) | 4.3 |
| Diabetes mellitus | 36.9% | 30.6% | 14.8 | 30.0% | 31.3% | 3.2 |
| NYHA class IV | 23.4% | 25.7% | 3.4 | 27.0% | 25.6% | 4.3 |
| CCS class IV | 2.0% | 2.6% | 2.5 | 2.9% | 2.7% | 2.8 |
| Peripheral arterial disease | 33.3% | 28.0% | 11.6 | 28.0% | 28.8% | 2.2 |
| Prior stroke/TIA | 19.1% | 17.2% | 0.05 | 15.5% | 15.7% | 0.2 |
| Prior MI | 19.9% | 12.2% | 22.0 | 12.1% | 12.5% | 1.2 |
| Carotid stenosis | 35.0% | 19.8% | 16.4 | 19.0% | 16.1% | 7.3 |
| LAD stenosis >50% | 22.3% | 15.8% | 22.8 | 27.3% | 26.5% | 1.4 |
| LM stenosis >50% | 38.4% | 26.6% | 16.1 | 5.1% | 4.3% | 5.1 |
| Liver disease | 8.7% | 4.7% | 15.0 | 3.0% | 3.5% | 2.8 |
| Cirrhosis | 1.8% | 4.8% | 11.3 | 1.3% | 1.4% | 1.5 |
| COPD | 0.7% | 2.3% | 1.6 | 32.6% | 32.4% | 1.3 |
| O2‐dependent COPD | 32.9% | 32.7% | 0.1 | 6.7% | 6.4% | 1.1 |
| Prior endocarditis | 8.1% | 7.5% | 3.4 | 0.8% | 0.8% | 0.01 |
| Current smoker | 0.8% | 1.0% | 5.0 | 1.9% | 1.8% | 1.1 |
| Conduction defect | 2.6% | 2.1% | 5.1 | 44.6% | 41.4% | 0.7 |
| Atrial fibrillation/flutter | 43.3% | 40.3% | 4.3 | 40.1% | 39.0% | 2.4 |
| Permanent pacemaker | 38.2% | 40.4% | 5.7 | 16.9% | 18.1% | 5.2 |
| Antiarrhythmic | 14.7% | 16.8% | 3.2 | 9.6% | 8.9% | 3.1 |
| Heart rate, bpm | 71 (62‐80) | 71 (63‐82) | 11.4 | 71 (64‐81) | 71 (63‐81) | 3.0 |
| SBP, mm Hg | 130 (118‐145) | 130 (117‐145) | 0.5 | 131 (119‐144) | 132 (117‐146) | 0.3 |
| DBP, mm Hg | 67 (60‐74) | 68 (60‐75) | 6.5 | 68 (60‐75) | 68 (60‐75) | 0.8 |
| CABG | 35.0% | 20.1% | 35.9 | 18.2% | 18.7% | 1.5 |
| PCI | 35.0% | 19.8% | 33.9 | 20.8% | 21.1% | 1.1 |
| β‐Blocker | 62.7% | 54.7% | 18.4 | 53.4% | 52.4% | 2.2 |
| ACEI/ARB | 79.4% | 71.8% | 15.0 | 28.8% | 26.8% | 5.6 |
| Anticoagulant | 25.6% | 28.5% | 3.7 | 28.8% | 27.8% | 1.7 |
| Antiplatelet | 81.3% | 71.8% | 21.0 | 72.8% | 70.30% | 6.4 |
| LVEF (%) | 58 (47‐65) | 59 (48‐65) | 3.4 | 60 (50‐65) | 60 (48‐66) | 2.4 |
| LVESd index, cm/m2 | 3.2 (2.7‐3.9) | 3.1 (2.6‐3.8) | 0.9 | 1.7 (1.4‐2.0) | 1.7 (1.4‐2.0) | 1.9 |
| LVEDd index, cm/m2 | 4.7 (4.3‐5.2) | 4.6 (4.2‐5.2) | 1.9 | 2.5 (2.3‐2.8) | 2.5 (2.3‐2.8) | 2.5 |
| Aortic insufficiency (mod‐severe) | 11.7% | 15.5% | 8.1 | 13.3% | 12.6% | 0.6 |
| Mitral insufficiency (mod‐severe) | 16.4% | 19.7% | 9.9 | 18.8% | 19.6% | 2.2 |
| Tricuspid insufficiency (mod‐severe) | 14.0% | 18.4% | 13.1 | 17.7% | 18.1% | 0.1 |
| BNP, ng/L | 350 (166‐843) | 416 (192‐986) | 2.7 | 354 (148‐824) | 413 (196‐944) | 6.3 |
| GFR, mL/min per 1.73 m2 | 61 (47‐76) | 62 (47‐79) | 5.5 | 70 (47‐77) | 63 (48‐79) | 1.7 |
| Albumin, g/dL | 3.9 (3.5‐4.2) | 3.8 (3.5‐4.1) | 14.8 | 3.9 (3.5‐4.1) | 3.8 (3.5‐4.1) | 3.7 |
| Hemoglobin, g/dL | 12 (11‐13) | 12 (11‐13) | 7.0 | 12 (11‐13) | 12 (11‐13) | 0.2 |
| Platelets, 109/L | 189 (156‐233) | 193 (154‐244) | 4.0 | 196 (160‐240) | 196 (157‐246) | 0.9 |
| History of cancer | 1.5% | 2.0% | 7.6 | 1.6% | 1.3% | 0.1 |
| Gait speed, m/s | 0.4 (0.1, 0.7) | 0.3 (0.1‐0.7) | 12.5 | 0.4 (0.1‐0.7) | 0.4 (0.1‐0.7) | 0.5 |
| KCCQ‐OS | 50 (33‐67) | 47 (29‐64) | 10.4 | 47 (31‐66) | 47 (30‐65) | 0.2 |
| MMSE | 28 (26‐29) | 28 (26‐29) | 2.8 | 28 (26‐30) | 28 (26‐29) | 1.5 |
| Prior nonaortic balloon valvuloplasty | 10.8% | 10.2% | 0.8 | 10.0% | 10.0% | 0.1 |
| Hostile chest | 5.8% | 6.5% | 5.0 | 5.6% | 5.3% | 3.2 |
| Porcelain aorta | 3.9% | 3.9% | 0.4 | 3.2% | 3.4% | 1.0 |
| Previous aortic valvuloplasty | 10.6% | 9.9% | 2.9 | 11.0% | 9.6% | 5.6 |
| Transfemoral access | 78.8% | 82.0% | 7.4 | 82.6% | 84.0% | 5.3 |
Values are median (IQR) or percentages. ACEI indicates angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; GFR, glomerular filtration rate; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; LAD, left anterior descending coronary artery; LM, left main coronary artery; LVEDd, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LVESd, left ventricular systolic diameter; MI, myocardial infarction; MMSE, mini mental status exam; mod, moderate; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; Stand Diff, standardized difference; TIA, transient ischemic attack.
Absolute standardized differences.
Continuous variables were reported using median (interquartile range) and compared by standardized difference. Categorical variables were reported using percentages and compared by standardized difference. Time‐to‐event variables were reported using Kaplan‐Meier estimations. Hazard ratios with 95% CIs and P‐values were reported using marginal Cox models, which accounted for intrapair correlation in a matched cohort. Competing risk models (all‐cause mortality as competing risk) were used to assess the association of statins with event rates (vascular complications, stroke, myocardial infarction, bleeding, endocarditis, and acute kidney injury). Statistical significance was defined at an α level of <0.05. All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Study Population
Among 3990 intermediate– and high–surgical risk patients who underwent TAVR as a part of the PARTNER II and Sapien 3 studies, 3956 patients survived to discharge and had use of statins documented, of whom, 2864 (72.4%) were discharged on a statin (Figure 1). Before matching, patients discharged on a statin were more likely to be men and to have diabetes mellitus and all forms of atherosclerosis (peripheral artery disease, carotid disease, coronary disease [including prior revascularization and myocardial infarction]) and were less likely to have liver disease as compared with patients not on a statin (Table 1). Patients on a statin (versus not) also had faster gait speeds, higher albumin levels, and better health status before TAVR. In unadjusted analyses among all eligible patients, patients discharged on a statin had lower rates of 2‐year death as compared with those not on a statin (Kaplan‐Meier–estimated 2‐year mortality rates: statin versus no statin (17.0% versus 23.4%, log‐rank P<0.0001; Figure 2).
Figure 1.
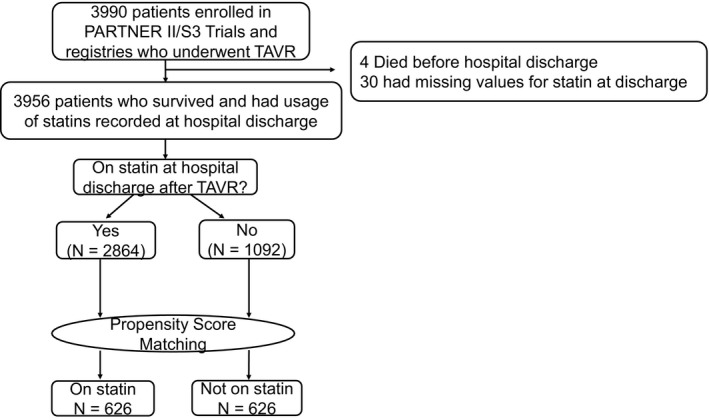
Derivation of analytic cohort. PARTNER indicates placement of aortic transcatheter valves; TAVR, transcatheter aortic valve replacement.
Figure 2.
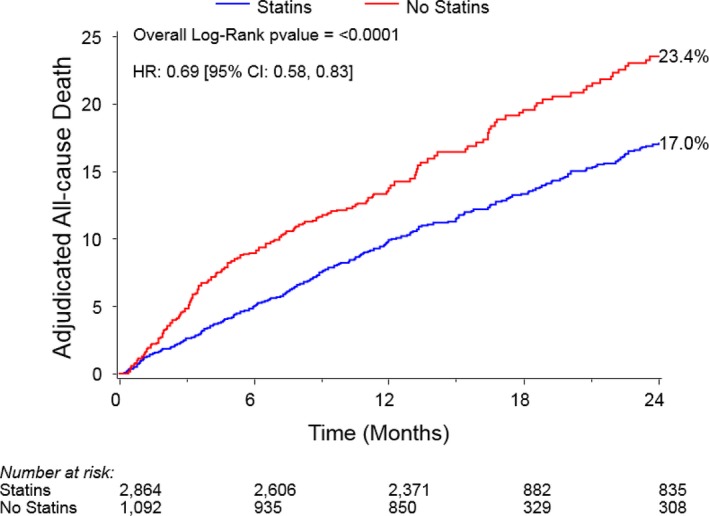
Two‐year all‐cause mortality for statin vs no‐statin groups in the unmatched cohort. HR indicates hazard ratio.
After accounting for the differences in patient characteristics, there was adequate overlap in the distribution of propensity scores between the 2 cohorts (Figure 3), and we were able to identify 626 matched pairs of patients with similar propensity scores. The matched cohort had a median age of 84 years (interquartile range 79‐88), 51.3% were women, and 26.3% had New York Heart Association IV symptoms before TAVR (Table 1). The median STS mortality risk score was 6.1% (interquartile range 4.7% to 8.5%) in the matched cohort. Overall, there were some differences between patients who were matched and those who were not in the matched cohort. For example, the matched cohort had a higher proportion of women, New York Heart Association class IV heart failure, patients with permanent pacemaker, STS Predicted Risk of Mortality score 4 to 8, and use of transfemoral access compared with the unmatched patients (Table 2). Patients who were unmatched were more likely to have an STS Predicted Risk of Mortality score >8, diabetes mellitus, atherosclerotic disease, β‐blocker use, antiplatelet use, and oxygen‐dependent chronic obstructive pulmonary disease compared with the matched patients. Despite these clinical differences, there were no differences between cohorts in the rates of all‐cause death (matched versus unmatched patients 22.4% versus 20.5%, P=0.18), cardiovascular death (14.9% versus 13.1%, P=0.16), and noncardiovascular death (12.0% versus 11.1%, P=0.32). After matching, standardized differences were <10% for all covariates, indicating good covariate balance (Figure 4). There were no differences in the rates of the falsification end points between groups (statin versus no statin: urinary tract infection 14.0% versus 14.7%, P=0.66; hip fracture 8.8% versus 9.0%, P=0.64).
Figure 3.
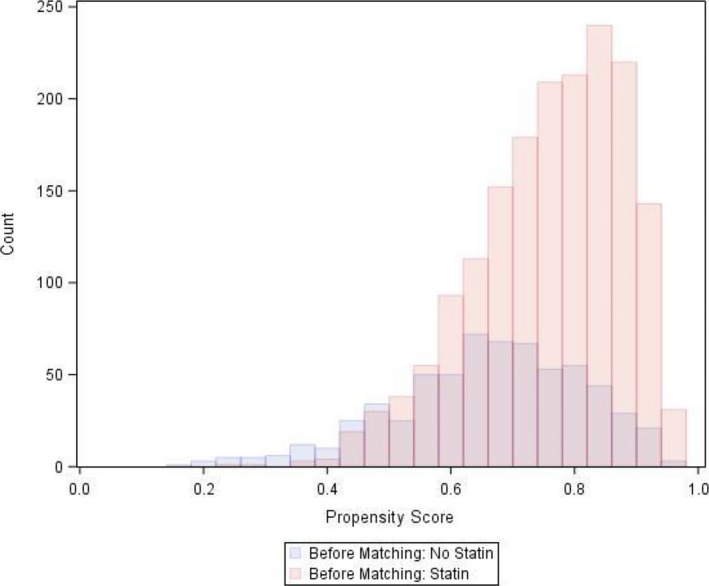
Distribution of propensity score before matching between statin groups.
Table 2.
Baseline Characteristics of Matched Versus Unmatched Patients
| Characteristics | Matched (n=1252) | Unmatched (n=2704) | P Value |
|---|---|---|---|
| Age, y | 84 (79‐88) | 83 (77‐87) | <0.01 |
| Male | 48.7% | 60.4% | <0.01 |
| White | 94.4% | 93.5% | 0.26 |
| Hispanic | 2.2% | 1.7% | 0.29 |
| Body surface area, m2 | 1.84 (1.69‐2.02) | 1.90 (1.71‐2.07) | <0.01 |
| Diabetes mellitus | 30.7% | 37.2% | <0.01 |
| NYHA class IV | 26.3% | 23.0% | 0.02 |
| CCS class IV | 2.8% | 1.9% | 0.07 |
| Peripheral arterial disease | 28.4% | 33.5% | <0.01 |
| Prior stroke or TIA | 15.6% | 20.0% | <0.01 |
| Prior MI | 12.3% | 20.3% | <0.01 |
| Carotid disease | 17.6% | 21.9% | <0.01 |
| LAD stenosis >50% | 26.9% | 39.0% | <0.01 |
| LM stenosis >50% | 4.7% | 8.9% | <0.01 |
| Liver disease | 3.3% | 2.3% | 0.07 |
| Cirrhosis | 1.4% | 1.0% | 0.38 |
| COPD | 32.5% | 33.0% | 0.74 |
| O2‐dependent COPD | 6.5% | 8.5% | 0.03 |
| Prior endocarditis | 0.8% | 0.9% | 0.87 |
| Current smoker | 1.8% | 2.7% | 0.09 |
| Conduction defect | 21.50% | 24.50% | 0.04 |
| Atrial fibrillation/flutter | 39.5% | 38.5% | 0.53 |
| Permanent pacemaker | 17.5% | 14.3% | 0.01 |
| Antiarrhythmic | 9.3% | 9.6% | 0.73 |
| Heart rate, bpm | 71 (64‐81) | 71 (62‐80) | 0.12 |
| SBP, mm Hg | 132 (118‐145) | 130.0 (118‐144) | 0.25 |
| DBP, mm Hg | 68 (60‐75) | 67 (60‐74) | 0.09 |
| CABG | 18.5% | 36.6% | <0.01 |
| PCI | 20.9% | 35.4% | <0.01 |
| β‐Blocker | 52.9% | 64.1% | <0.01 |
| ACEI/ARB | 42.4% | 45.9% | 0.03 |
| Anticoagulant | 28.3% | 25.5% | 0.07 |
| Antiplatelet | 71.6% | 81.9% | <0.01 |
| LVEF (%) | 60 (49‐65) | 57 (50‐65) | <0.01 |
| LVESd index, cm/m2 | 1.69 (1.44‐2.00) | 1.74 (1.47‐2.09) | <0.01 |
| LVEDd index, cm/m2 | 2.51 (2.26‐2.78) | 2.52 (2.28‐2.82) | 0.10 |
| Aortic insufficiency (mod‐severe) | 12.9% | 12.6% | 0.77 |
| Mitral insufficiency (mod‐severe) | 19.2% | 16.3% | 0.03 |
| Tricuspid insufficiency (mod‐severe) | 17.9% | 13.7% | <0.01 |
| GFR, mL/min per 1.73 m2 | 62 (47‐77) | 61 (46‐76) | 0.05 |
| BNP, ng/L | 384 (175‐880) | 358 (170‐866) | 0.42 |
| Albumin, g/dL | 3.8 (3.5‐4.1) | 3.9 (3.5‐4.2) | 0.01 |
| Hemoglobin, g/dL | 12.0 (10.8‐13.2) | 12.1 (11.0‐13.3) | 0.02 |
| Platelets, 109/L | 194 (159‐243) | 189 (154‐232) | 0.00 |
| History of cancer | 1.4% | 1.7% | 0.54 |
| Gait speed, m/s | 0.4 (0.1‐0.7) | 0.4 (0.1‐0.7) | 0.05 |
| KCCQ‐OS | 47 (30‐65) | 49 (33‐68) | 0.03 |
| MMSE | 28 (26‐29) | 28 (26‐29) | 0.04 |
| Prior non–aortic balloon valvuloplasty | 10.2% | 10.9% | 0.12 |
| Hostile chest | 5.4% | 6.2% | 0.33 |
| Porcelain aorta | 3.3% | 4.2% | 0.17 |
| Aortic valvuloplasty | 10.3% | 10.5% | 0.88 |
| STS‐PROMa | 6.1 (4.7‐8.5) | 6.4 (4.7‐9.2) | 0.02 |
| STS‐PROM Scorea | |||
| <4 | 7.4% | 8.6% | 0.21 |
| 4 to 8 | 64.4% | 57.7% | <0.01 |
| >8 | 28.2% | 33.7% | <0.01 |
| Transfemoral access (TF) | 83.3% | 78.0% | <0.01 |
Values are median (IQR) or percentages. Categorical variables compared using the Chi‐squared or Fisher exact test. Continuous variables are compared using ANOVA and the Wilcoxon Rank‐Sum test. ACEI indicates angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; GFR, glomerular filtration rate; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; LAD, left anterior descending coronary artery; LM, left main coronary artery; LVEDd, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESd, left ventricular systolic diameter; MI, myocardial infarction; MMSE, mini‐mental status exam; mod, moderate; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STS‐PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TF, transfemoral; TIA, transient ischemic attack.
Not included in the propensity model.
Figure 4.
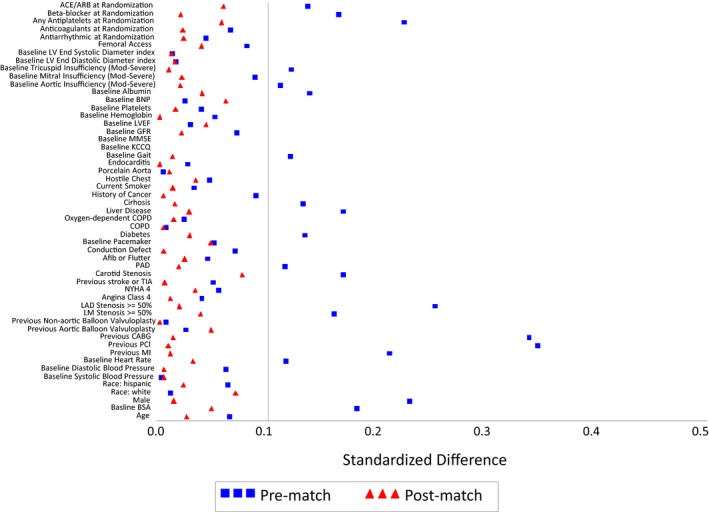
Standardized differences of covariates before and after propensity score matching. ACEI indicates angiotensin converting enzyme inhibitor; Afib, atrial fibrillation; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; BSA, body surface area; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; KCCQ, Kansas City Cardiomyopathy Questionnaire; LAD, left anterior descending artery; LM, left main; LV, left ventricle; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MMSE, mini mental status exam; NYHA, New York Heart Association; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Outcomes in Matched Cohort
The overall mortality at 2 years after TAVR in the matched cohort was 21.3%. Patients who were discharged on a statin had a lower rate of all‐cause mortality compared with patients who were not on a statin at discharge (Kaplan‐Meier–estimated 2‐year mortality rates of statin versus no statin: 18.1% versus 24.5%, log‐rank P=0.004; HR 0.65, 95% CI 0.49‐0.87, P=0.001, Figure 5). The survival curves diverged within ≈3 months and continued to separate over a median follow up of 2.1 years. Results were consistent for both cardiovascular (HR 0.66, 95% CI 0.46‐0.96, P=0.030, Figure 6A) and noncardiovascular mortality (HR 0.64, 95% CI 0.44‐0.99, P=0.045, Figure 6B).
Figure 5.
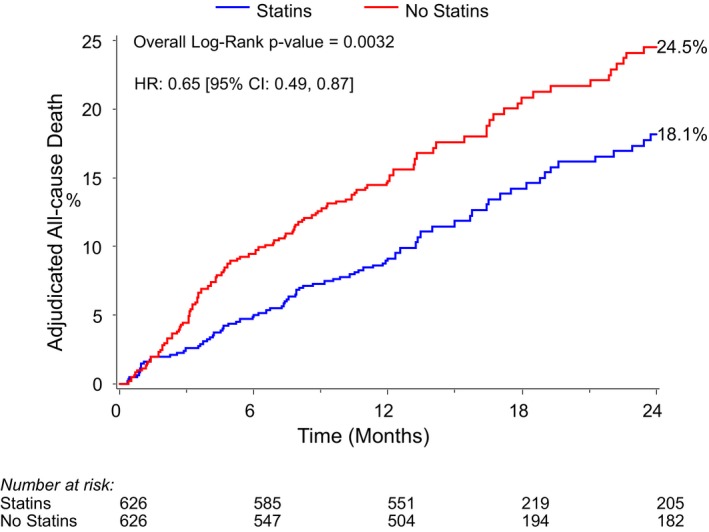
Kaplan‐Meier plot of 2‐year all‐cause mortality for statin vs no‐statin groups. HR indicates hazard ratio.
Figure 6.
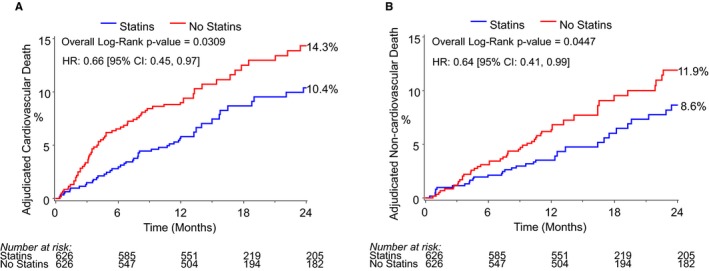
Kaplan‐Meier plot of 2‐year cardiovascular (A) and noncardiovascular (B) mortality for statin vs no‐statin groups. HR indicates hazard ratio.
At 2 years post‐TAVR, the rates of myocardial infarction (2.3% statin versus 1.5% no statin, log‐rank P=0.31), stroke (4.6% statin versus 5.4% no statin, log‐rank P=0.90), and bleeding (22.0% statin versus 21.6% no statin, log‐rank P=0.95) were similar between groups. There were trends toward lower rates of acute kidney injury (17.2% versus 24.2%, HR 0.70, 95% CI 0.48‐1.04, P=0.08) and endocarditis (0.5% versus 2.5%, HR 0.28, 95% CI 0.08‐1.01, P=0.13) in patients on statins compared with patients who were not on a statin at discharge (Table 3). Specifically in regard to the etiology of deaths, the difference in cardiovascular death appeared to be driven by lower rates of death from cardiac disease in the statin group (statin versus no statin: 3.0% versus 6.7%, HR 0.37, 95% CI 0.18‐0.73, P=0.004, Table 4). The difference in noncardiovascular death appeared to be driven by lower rates of infection (2.7% versus 6.6%, HR 0.38, 95% CI 0.19‐0.76, P=0.006) and malignancy (0.3% versus 2.3%, HR 0.21, 95% CI 0.04‐0.96, P=0.04) in the statin versus nonstatin groups, respectively.
Table 3.
Mortality‐Related Events at 2 Years After TAVR
| Statin, n (%) | No Statin, n (%) | HR (95% CI) | P Value | |
|---|---|---|---|---|
| Death | ||||
| All‐cause | 79 (18.1) | 113 (24.5) | 0.65 (0.49, 0.87) | 0.004 |
| Cardiovascular | 46 (10.4) | 65 (14.3) | 0.66 (0.46, 0.96) | 0.030 |
| Noncardiovascular | 33 (8.6) | 48 (11.9) | 0.64 (0.41, 0.99) | 0.045 |
| Cardiovascular deaths | ||||
| Procedure related | 2 (0.3) | 1 (0.2) | 1.95 (0.18, 21.42) | 0.58 |
| Cardiac disease | 11 (3.0) | 28 (6.7) | 0.37 (0.18, 0.73) | 0.004 |
| Non‐cardiovascular condition | 9 (2.1) | 9 (2.5) | 0.93 (0.37, 2.36) | 0.88 |
| Unknown | 24 (5.4) | 26 (5.4) | 0.86 (0.49, 1.52) | 0.61 |
| Noncardiovascular deaths | ||||
| Infectious | 11 (2.7) | 27 (6.6) | 0.38 (0.19, 0.76) | 0.006 |
| Pulmonary | 11 (3.3) | 5 (1.6) | 2.03 (0.70, 5.88) | 0.19 |
| Malignancy | 2 (0.3) | 9 (2.3) | 0.21 (0.04, 0.96) | 0.04 |
| Accidental/trauma | 2 (0.6) | 2 (0.3) | 0.93 (0.13, 6.47) | 0.94 |
| Renal causes | 4 (1.2) | 1 (0.5) | 3.64 (0.41, 32.73) | 0.25 |
HR indicates hazard ratio; TAVR, transaortic valve replacement.
Table 4.
Event Rates for Stroke, Myocardial Infarction, Bleeding, Endocarditis, and Acute Kidney Injury at 2 Years After TAVR (All‐Cause Death as Competing Risk)
| Outcome | Statin, n (%) | No Statin, n (%) | HR (95% CI) | P Value |
|---|---|---|---|---|
| Vascular complications | 13 (2.5) | 12 (3.6) | 1.10 (0.54, 2.27) | 0.79 |
| Stroke | 20 (4.6) | 20 (5.4) | 0.97 (0.54, 1.72) | 0.90 |
| Myocardial infarction | 7 (2.3) | 4 (1.5) | 1.89 (0.55, 6.48) | 0.31 |
| Bleeding | 49 (22.0) | 50 (21.6) | 1.01 (0.68, 1.50) | 0.95 |
| Endocarditis | 3 (0.5) | 10 (2.5) | 0.29 (0.08, 1.06) | 0.06 |
| Acute kidney injury | 0.74 (0.50, 1.09) | 0.13 | ||
| Any | 44 (17.2) | 61 (24.2) | 1.78 (0.43, 7.40) | 0.43 |
| Stage I | 5 (2.0) | 3 (1.0) | 0.70 (0.47, 1.06) | 0.09 |
| Stage II | 41 (15.1) | 60 (23.0) | 0.88 (0.30, 2.58) | 0.82 |
| Stage III | 5 (1.9) | 6 (2.5) | 1.10 (0.54, 2.27) | 0.79 |
HR indicates hazard ratio.
Discussion
The mortality rate of patients after TAVR remains high, necessitating further exploration of potential strategies or therapies that may improve mortality in this high‐risk group of patients. We found, in a large, nonrandomized cohort of patients undergoing TAVR, that those patients who were discharged on a statin after TAVR had lower rates of all‐cause, cardiovascular, and noncardiovascular death over a median follow up of 2.1 years compared with patients not on a statin. The survival curves diverged quite early, and there were no differences in the rates of atherosclerotic events after TAVR. Thus, these differences are likely not driven by the atherosclerotic effects of statins but likely either by pleiotropic effects, as evidenced by lower rates of infections and acute kidney injury, or by residual confounding, as evidenced by lower rates of deaths due to malignancy.
Prior Studies
Our study supports the findings from 1 prior single‐center study that examined the association of statins and post‐TAVR mortality among 294 patients.8 In this study mortality was similar at 30 days after TAVR between patients who were on a statin versus not on a statin at the time of TAVR. However, statin therapy was associated with lower rates of death at 1, 3, and 5 years of follow‐up compared with no statin, and high‐intensity statins demonstrated an even greater protective effect compared with low/moderate‐intensity statins. A different single‐center study of 342 patients who underwent TAVR for severe aortic stenosis found that although statin use at the time of TAVR was associated with lower rates of in‐hospital and 30‐day stroke/TIA compared with no statin use, there was no association of statin use with 30‐day, 6‐month, or 1‐ or 2‐year overall survival.9 The factors responsible for the seemingly conflicting results of these prior studies are unclear but may be related to the small sample size resulting in larger variance around outcome estimates. Additionally, the risk of treatment assignment bias is high in both studies. This is important, as we have shown that patients who are on statins at the time of TAVR have many clinical and frailty factors that differ from those not on statins. To address these limitations, we matched patients with similar likelihood for treatment with statins based on baseline covariates to limit bias related to treatment assignment. We also used falsification end points in our analysis to further assess for residual bias, and yet we still found similar results to the previous study. Due to the size of our study, we were able to delve further into nonfatal events and the etiology of fatal events to try to elucidate potential mechanisms for the findings.
Potential Explanations
Statins are well established in their ability to reduce the risk of all‐cause and cardiovascular mortality in patients who have established or are at high risk for atherosclerosis through reduction in ischemic events.17, 18 However, the benefits of statins in patients >75 years of age, particularly those with comorbidities, are controversial, given competing mortality risks and the duration of statin use needed to reduce atherosclerotic events. Given the burden of atherosclerosis among patients undergoing TAVR, the observed reduction in cardiovascular mortality with statins after TAVR may be due to a reduction in ischemic events. Because the survival curves diverged within ≈3 months, however, this difference is unlikely to be explained by a reduction in ischemic events, an effect that generally does not appear until >1 year in secondary prevention trials of statins versus placebo.19, 20 This is further supported by no evidence of lower rates of myocardial infarction or stroke in patients on a statin.
Thus, our findings may be explained by the anti‐inflammatory or other pleiotropic effects of statins. Statins have been shown to inhibit cytokine‐mediated induction of proadhesive and procoagulant substances.21 Additionally, statins reduced neointimal thickening after vascular injury without changing plasma LDL levels.22 Statins induce favorable vascular remodeling via induction of endothelial NO synthase and thrombomodulin.23 The role of statins in periprocedural complications remains an active area of study. For example, statins have been investigated as a tool to reduce contrast‐induced acute kidney injury in patients undergoing percutaneous coronary intervention with some24 but not all25 studies showing a benefit with statins. A recent prospective randomized trial, however, showed no reduction in acute kidney injury in patients who underwent cardiac surgery.26 Additionally, an assessment of statin use in noncardiac surgery in a large population of male veterans showed that statins were associated with reductions in 30‐day mortality and cardiac, infectious, respiratory, and renal complications at 30 days postsurgery.27 Similar to these findings, we noted lower risk of mortality due to infection and a trend toward a reduction in acute kidney injury in the statin group, but contrary to these studies, the benefit of statins did not emerge until after 30 days of follow‐up.
It is also plausible that statins have no impact on mortality after TAVR, and the observed differences are simply due to residual confounding despite propensity matching and examination of bias with falsification end points. For example, patients discharged on a statin were less likely to die from malignancy compared with those not on a statin. Despite advanced statistical methods, the observational nature of our analysis still harbors the risk of residual confounding. As a result, the association of statins with mortality after TAVR merits prospective assessment in a blinded randomized trial to generate a true estimate of the potential benefit and treatment effect of statins in this population.
Limitations
This study should be interpreted within the context of a few limitations. As described above, we cannot eliminate the possibility of residual confounding or infer causality despite the use of propensity matching and falsification end points. It is plausible that patients on statins may still be prognostically different from those not on a statin. The early emergence of a mortality benefit (within 3 months) is of unclear significance because the benefits of statin use are expected to accrue over time. As a result, this might be a signal for unmeasured confounding related to the different prognostic trajectories of patients on a statin versus those not on a statin in this study. Second, we did not have statin dosing or type in order to determine intensity of statin therapy and therefore examine a dose‐response association. Third, we assessed statin use only at discharge. Although statins are generally chronic medications in TAVR patients and likely remain fairly consistent over time, we were unable to assess any potential association between duration and change over time of statin use both before and after TAVR. We used statin prescription at discharge as a proxy for statin use after TAVR. This opens the possibility that some patients may have either discontinued or started statins during follow‐up. Finally, ezetimibe use was not collected in this study, which prevents us from examining the effect of a statin‐ezetimibe combination in this analysis.
Conclusion
In a large propensity‐matched analysis, patients discharged on a statin after TAVR experienced lower rates of mortality compared with those not discharged on a statin. Despite propensity matching and the use of falsification end points, this analysis cannot exclude residual confounding. Therefore, prospective studies are needed to further clarify the potential role of statins as an adjunctive therapy in patients with severe aortic stenosis treated with TAVR.
Sources of Funding
The PARTNER trial was sponsored by Edwards Lifesciences. This current study was self‐funded, and the funding organization for the trial did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Dr Peri‐Okonny is supported by a T32 training grant from the National Heart, Lung, and Blood Institute (T32 HL110837). Dr Arnold is supported by a Career Development Grant Award from the National Heart, Lung, and Blood Institute (K23 HL116799). The content of this study is solely the responsibility of the authors and does not necessarily the represent the official views of the Institutes of Health.
Disclosures
Dr Thourani has received grant support from Edwards Lifesciences and is a consultant for Edwards Lifesciences and Medtronic. Dr Kodali has been a consultant for Edwards Lifesciences, Merrill Lifesciences, and Claret Medical; has served on the advisory boards of Abbott Vascular, Biotrace Medical, Dura Biotech, Thubrikar Aortic Valve, Duratech, and VS Medtech and has equity in Thubrikar Aortic Valve, Dura Biotech, and Biotrace Medical. Dr Webb has been a member of the PARTNER Trial Executive Committee, for which he received no direct compensation and has been a consultant for Edwards Lifesciences. Dr Leon has been a member of the PARTNER Trial Executive Committee, for which he received no direct compensation. Dr Cohen has received research grant support from Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott Vascular and consulting income from Edwards Lifesciences and Medtronic. The remaining authors have no disclosures to report.
Acknowledgments
The authors thank all participants of the PARTNER and Sapien 3 trials and registries.
(J Am Heart Assoc. 2019;8:e011529 DOI: 10.1161/JAHA.118.011529.)
References
- 1. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 2. Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, Rihal CS, Brown DL, Smith CR, Leon MB, Cohen DJ. Health‐related quality of life after transcatheter or surgical aortic valve replacement in high‐risk patients with severe aortic stenosis: results from the PARTNER (Placement of Aortic Transcatheter Valve) Trial (Cohort A). J Am Coll Cardiol. 2012;60:548–558. [DOI] [PubMed] [Google Scholar]
- 3. Duncan A, Ludman P, Banya W, Cunningham D, Marlee D, Davies S, Mullen M, Kovac J, Spyt T, Moat N. Long‐term outcomes after transcatheter aortic valve replacement in high‐risk patients with severe aortic stenosis: the U.K. Transcatheter Aortic Valve Implantation Registry. JACC Cardiovasc Interv. 2015;8:645–653. [DOI] [PubMed] [Google Scholar]
- 4. Holmes DR Jr, Brennan JM, Rumsfeld JS, Dai D, O'Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, Tuzcu EM, Peterson ED, Brindis RG, Mack MJ. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–1028. [DOI] [PubMed] [Google Scholar]
- 5. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 6. Thompson K, Razi R, Lee MS, Shen A, Stone GW, Hiremath S, Mehran R, Brar SS. Statin use prior to angiography for the prevention of contrast‐induced acute kidney injury: a meta‐analysis of 19 randomised trials. EuroIntervention. 2016;12:366–374. [DOI] [PubMed] [Google Scholar]
- 7. Le Manach Y, Ibanez Esteves C, Bertrand M, Goarin JP, Fléron MH, Coriat P, Koskas F, Riou B, Landais P. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing vascular surgery. Anesthesiology. 2011;114:98–104. [DOI] [PubMed] [Google Scholar]
- 8. Huded CP, Benck LR, Stone NJ, Sweis RN, Ricciardi MJ, Malaisrie SC, Davidson CJ, Flaherty JD. Relation of intensity of statin therapy and outcomes after transcatheter aortic valve replacement. Am J Cardiol. 2017;119:1832–1838. [DOI] [PubMed] [Google Scholar]
- 9. Klinkhammer B. Statin therapy is not associated with improved overall survival after transcatheter aortic valve replacement (TAVR). J Cardiovasc Dis Res. 2018;9:54–58. [Google Scholar]
- 10. Webb JG, Doshi D, Mack MJ, Makkar R, Smith CR, Pichard AD, Kodali S, Kapadia S, Miller DC, Babaliaros V, Thourani V, Herrmann HC, Bodenhamer M, Whisenant BK, Ramee S, Maniar H, Kereiakes D, Xu K, Jaber WA, Menon V, Tuzcu EM, Wood D, Svensson LG, Leon MB. A randomized evaluation of the SAPIEN XT transcatheter heart valve system in patients with aortic stenosis who are not candidates for surgery. JACC Cardiovasc Interv. 2015;8:1797–1806. [DOI] [PubMed] [Google Scholar]
- 11. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 12. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G‐A, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. Eur Heart J. 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 13. Nuis RJ, van Mieghem NM, van der Boon RM, van Geuns RJ, Schultz CJ, Oei FB, Galema TW, Raap GB, Koudstaal PJ, Geleijnse ML, Kappetein AP, Serruys PW, de Jaegere PP. Effect of experience on results of transcatheter aortic valve implantation using a Medtronic CoreValve System. Am J Cardiol. 2011;107:1824–1829. [DOI] [PubMed] [Google Scholar]
- 14. Groenwold RH. Falsification end points for observational studies. JAMA. 2013;309:1769–1771. [DOI] [PubMed] [Google Scholar]
- 15. Mondul AM, Giovannucci E, Platz EA. A prospective study of statin drug use and lower urinary tract symptoms in older men. Am J Epidemiol. 2013;178:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ray WA, Daugherty JR, Griffin MR. Lipid‐lowering agents and the risk of hip fracture in a Medicaid population. Inj Prev. 2002;8:276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association between intensity of statin therapy and mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:47–54. [DOI] [PubMed] [Google Scholar]
- 19. Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, Pyorala K, Miettinen T, Wilhelmsen L, Olsson AG, Wedel H. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004;5:81–87. [DOI] [PubMed] [Google Scholar]
- 20. Heart Protection Study Collaborative Group . Randomized trial of the effects of cholesterol‐lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high‐risk conditions. J Vasc Surg. 2007;45:645–654.e641. [DOI] [PubMed] [Google Scholar]
- 21. Schonbeck U, Libby P. Inflammation, immunity, and HMG‐CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 2004;109:II18–II26. [DOI] [PubMed] [Google Scholar]
- 22. Chen Z, Fukutomi T, Zago AC, Ehlers R, Detmers PA, Wright SD, Rogers C, Simon DI. Simvastatin reduces neointimal thickening in low‐density lipoprotein receptor‐deficient mice after experimental angioplasty without changing plasma lipids. Circulation. 2002;106:20–23. [DOI] [PubMed] [Google Scholar]
- 23. Sen‐Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel‐like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. [DOI] [PubMed] [Google Scholar]
- 24. Liang M, Yang S, Fu N. Efficacy of short‐term moderate or high‐dose rosuvastatin in preventing contrast‐induced nephropathy: a meta‐analysis of 15 randomized controlled trials. Medicine (Baltimore). 2017;96:e7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toso A, Maioli M, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, Manzone C, Amato M, Bellandi F. Usefulness of atorvastatin (80 mg) in prevention of contrast‐induced nephropathy in patients with chronic renal disease. Am J Cardiol. 2010;105:288–292. [DOI] [PubMed] [Google Scholar]
- 26. Billings FT IV, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, Brown NJ. High‐dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA. 2016;315:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med. 2017;177:231–242. [DOI] [PubMed] [Google Scholar]


