Abstract
Background
Hypertension is a known risk factor for heart failure (HF), possibly via the mechanism of cardiac remodeling and left ventricular hypertrophy (LVH). We studied the extent to which blood pressure (BP) change and evolving LVH contribute to the effect that lisinopril, doxazosin, and amlodipine have on HF compared with chlorthalidone.
Methods and Results
We conducted causal mediation analysis of ALLHAT (Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial) data (1994‐2002; in‐trial follow‐up). ALLHAT participants with available serial ECGs and BP measurements were included (n=29 892; mean age 67±4 years; 32% black; 56% men): 11 008 were randomized to chlorthalidone, 5967 to doxazosin, 6593 to amlodipine, and 6324 to lisinopril. Evolving ECG LVH and BP lowering served as mediators. Incident symptomatic HF was the primary outcome. Linear regression (for mediator) and logistic regression (for outcome) models were adjusted for mediator‐outcome confounders (demographic and clinical characteristics known to be associated both with both LVH/hypertension and HF). A large majority of participants (96%) had ECG LVH status unchanged, but 4% developed evolving ECG LVH. On average, BP decreased by 11/7 mm Hg. In adjusted Cox regression analyses, progressing ECG LVH (hazard ratio [HR] 1.78 [95% CI 1.43‐2.22]), resolving ECG LVH (HR 1.33 [95% CI 1.03‐1.70]), and baseline ECG LVH (1.17 [95% CI 1.04‐1.31]) carried risk of incident HF. After full adjustment, evolving ECG LVH mediated 4% of the effect of doxazosin on HF. Systolic BP lowering mediated 12% of the effect of doxazosin, and diastolic BP lowering mediated 10% of the effect of doxazosin, 7% of the effect of amlodipine, and borderline 9% of the effect of lisinopril on HF.
Conclusions
Evolving ECG LVH and BP change account for 4% to 13% of the mechanism by which antihypertensive medications prevent HF.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00000542.
Keywords: antihypertensive agent, ECG, heart failure, hypertension, left ventricular hypertrophy
Subject Categories: Heart Failure, Hypertrophy, Hypertension, Treatment
Short abstract
See Editorial Ferdinand and Maraboto
Clinical Perspective
What Is New?
Patients with progressing and resolving left ventricular hypertrophy (LVH), as measured by serial ECGs, show an increase in heart failure risk compared with those with no evolving ECG LVH.
There is a U‐shaped association of blood pressure lowering and incident heart failure in which both greater increases and decreases in blood pressure over time are associated with an increased risk of heart failure.
What Are the Clinical Implications?
Hypertension and LVH are some of the pathways that can lead to heart failure, but simply trying to reverse these maladaptive pathways does not substantially improve mortality or symptoms from heart failure.
The protective effect of antihypertensive medications on heart failure is largely due to mechanisms outside of blood pressure lowering and evolving ECG LVH.
Although improving hypertension control and reducing/preventing LVH are clinically important, other aspects of cardiac health should be emphasized in order to lower risk of heart failure.
Introduction
Hypertension (HTN) is a major risk factor for heart failure (HF).1 HTN triggers cardiac remodeling and development of left ventricular hypertrophy (LVH), leading to subclinical organ damage, which evolves to clinically manifest HF and, ultimately, to death.2 The beneficial effect of antihypertensive treatment on HF risk is well known3 and reflected in the 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults.4 HTN treatment is associated with an ≈20% to 25% reduction in risk of incident HF.5
ALLHAT (the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial)6 was a multicenter, randomized, double‐blind, active‐controlled trial designed to compare cardiovascular outcomes in high‐risk antihypertensive patients assigned to the angiotensin‐converting enzyme inhibitor lisinopril, the calcium channel blocker amlodipine, and the α‐blocker doxazosin, in comparison to a thiazide‐type diuretic (chlorthalidone). Incident HF was a prespecified ALLHAT outcome. The rationale for the ALLHAT hypothesis was based on the previous demonstrations that angiotensin‐converting enzyme inhibitors and calcium channel blockers are more effective than diuretics in reducing left ventricular (LV) mass index measured by echocardiography.7 Contrary to expectations, the ALLHAT showed that chlorthalidone was superior to amlodipine, lisinopril, and doxazosin in preventing HF.8, 9 The subsequent ALLHAT HF validation study reinforced the original ALLHAT results.10, 11, 12
Although ALLHAT answered questions about the comparative effectiveness of antihypertensive treatments for HF prevention, the mechanisms behind this HF prevention remain incompletely understood. The extent to which the effect of a calcium channel blocker, an angiotensin‐converting enzyme inhibitor, and an α‐blocker (as compared with a diuretic) on incident HF is mediated by evolving LVH and blood pressure (BP) lowering per se remains unknown. This study aimed to quantify the extent to which the effects of lisinopril, amlodipine, and doxazosin (as compared with chlorthalidone) on incident HF are mediated by evolving LVH and BP lowering. We hypothesized that evolving ECG LVH and BP lowering are mechanisms behind previously observed differences in the rate of incident HF in hypertensive ALLHAT participants randomized to lisinopril, amlodipine, and doxazosin in comparison to those randomized to chlorthalidone.
Methods
For this study we used the ALLHAT data set, publicly available from the National Heart, Lung, and Blood Institute, via the Biologic Specimen and Data Repository Information Coordinating Center. The study was reviewed by an Oregon Health and Science University Institutional Review Board, which determined that it did not require further review due to the deidentified nature of the publicly available data set.
Study Population
The ALLHAT design and rationale have been described previously.6 Briefly, ALLHAT was conducted from 1994 to 2002 and enrolled adults age 55 and above with HTN and at least 1 risk factor (documented coronary heart disease [CHD], type 2 diabetes mellitus, LVH on ECG or echocardiogram, smoking, high‐density lipoprotein <35 mg/dL, or ST‐T ECG changes indicative of ischemia). Symptomatic HF patients or those with LV ejection fraction <35%, patients with recent myocardial infarction (MI), stroke, or poorly controlled HTN were excluded.
In this study we included ALLHAT participants with available assessment of evolving LVH status and dynamic BP changes during in‐trial follow‐up. We excluded participants with missing covariates. The final study population included 29 892 participants: 11 008 were randomized to chlorthalidone, 5967 to doxazosin, 6593 to amlodipine, and 6324 to lisinopril (Figure 1).
Figure 1.
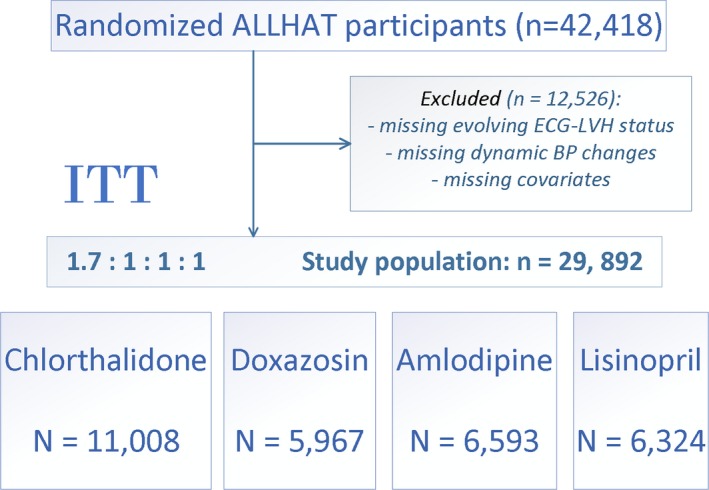
Flow diagram of exclusion criteria applied to achieve the final study population for this secondary analysis of ALLHAT (Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial) data. BP indicates blood pressure; LVH, left ventricular hypertrophy; ITT, intention to treat.
ECG Analysis: Evolving LVH During Follow‐Up
ECGs were recorded at the study sites at baseline and biannually during follow‐up. Minnesota coding13 of serial ECG changes was performed in trial, from 1994 to 2002, at the Minnesota ECG‐coding core center at the University of Minnesota in Minneapolis by reviewers who were blinded to treatment assignments. LVH was examined only in clinic (nonhospital) ECG recordings. The procedure for serial ECG coding has been previously described.14 First, Minnesota prevalence codes were assigned to all ECGs. Minnesota codes 3‐1 and 3‐3 are high left R amplitude patterns (relevant to LVH) as measured on the next‐to‐last complete normal beat. Code 3‐1 was coded if any of the following 3 criteria were present: (1) R amplitude >26 mm in either lead V5 or lead V6; (2) R amplitude >20 mm in any of leads I, II, III, or aVF; (3) R amplitude >12 mm in lead aVL. Code 3‐3 was coded if 1 or both of the following 2 criteria were present: R‐wave amplitude >15 mm but ≤20 mm in lead I; R‐wave amplitude in V5 or V6 plus S or QS amplitude in V1 >35 mm.
Then, any specific Minnesota prevalence code change between baseline ECG and follow‐up was identified by a computer algorithm and used to select ECG pairs for direct serial comparison. Each Minnesota code change was evaluated for its importance by direct visual comparison of baseline and follow‐up waveforms and was either confirmed or not confirmed. Interobserver and intraobserver agreement was consistently high in the Minnesota coding center, as previously reported.14 The Minnesota code allowed objective classification of evolving LVH over time by setting limits to the percentage of change in voltage that occurs in serial ECGs.13 At the first step it was determined in which lead the most severe 3‐code occurred. Code 3‐1 was considered more severe than code 3‐3. If both ECGs had the same 3‐code, the follow‐up record determined which lead to use to compare with the reference ECG. If the 3‐code occurred in different leads, the following hierarchy was used to determine which lead to compare: V5/V6 (whichever R‐amplitude is higher) >I>II>III>aVL. In addition, several ECG LVH definitions were included (Sokolow‐Lyon, Cornell voltage, Cornell product, sum of 12 leads, 12‐leads product) using previously described thresholds.13 Evolving LVH was coded as either a significant progression/increase (including newly diagnosed ECG LVH) or a significant resolution/decrease (including complete resolution of ECG LVH). Absence of evolving ECG LVH was coded if the change in voltage was below the predefined threshold.13
BP Changes During Course of the Trial
BP was measured at every follow‐up visit (every 3 months for the first year and every 4 months thereafter). At each visit BP was recorded as an average of 2 measurements. To calculate achieved BP lowering during the trial, we subtracted baseline BP from the BP obtained at the latest in‐trial study visit available at year 1, 2, 3, 4, 5, or 6 from baseline, thus obtaining estimates of the “greatest” BP control. In addition, we conducted sensitivity analyses with 3 other definitions of BP lowering. By subtracting baseline BP from the BP obtained at the next in‐trial study visit available, we obtained estimates of the “fastest” BP control. We also divided the greatest and fastest BP control estimates by the baseline BP, obtaining relative greatest and fastest BP lowering.
Primary Outcome: Incident Heart Failure
Incident symptomatic congestive HF as defined by the ALLHAT investigators was a primary outcome in this study. Diagnosis of symptomatic congestive HF required the presence of both (1) paroxysmal nocturnal dyspnea or dyspnea at rest or New York Heart Association class III symptoms, or orthopnea and (2) rales or ankle edema (2+ or greater) or sinus tachycardia of 120 beats/min or more after 5 minutes at rest or cardiomegaly by chest x‐ray or chest x‐ray characteristic of congestive HF or S3 gallop or jugular venous distention. The incident HF outcome was validated by the ALLHAT HF validation study.10 In the current study hospitalized/fatal HF was included as a secondary outcome.
Covariates
Baseline BP was calculated as an average of 2 BP determinations taken at least 1 day apart, with each determination being an average of 2 measurements.
Baseline ECG LVH was based on any ECG within the past 2 years. Baseline ECG LVH definition included any 1 of the following: (1) R amplitude in V5 or V6 >26 mm, (2) R amplitude in V5 or V6 plus S amplitude in V1 >35 mm, (3) R amplitude in aVL >12 mm, (4) R amplitude in lead I >15 mm, (5) R amplitude in leads II or III or aVF >20 mm, (6) R amplitude in lead I plus S amplitude in lead III >25 mm, (7) R amplitude in aVL plus S amplitude in V3 >28 mm for men or >22 mm for women, or (8) computerized ECG machine–documented LVH.
Echocardiographic LVH was defined as combined wall (posterior wall plus interventricular septum) thickness ≥25 mm on any ECG in the past 2 years.
Baseline medical history was determined by the study investigators by a combination of chart review and questioning during a routine office visit. HTN history determined whether participants were treated for at least 2 months, were treated for <2 months, or were untreated. History of MI or stroke was at least 6 months old. History of revascularization included history of angioplasty, stenting, atherectomy, bypass surgery (coronary; peripheral vascular; carotid; vertebrobasilar), or aortic aneurysm repair. Presence of major ST‐segment depression or T‐wave elevation on any ECG in the past 2 years was identified. History of other atherosclerotic cardiovascular disease included documented peripheral artery disease or cerebrovascular disease. Baseline CHD history included known prior MI (including silent MI), angina, cardiac arrest, angiographically defined coronary stenosis more than 50%, reversible perfusion defects on cardiac scintigraphy, or prior coronary revascularization procedures. Type 2 diabetes mellitus was defined as fasting plasma glucose >140 mg/dL (7.77 mmol/L) or nonfasting plasma glucose >200 mg/dL (11.1 mmol/L) in the past 2 years and/or current treatment with insulin or oral hypoglycemic agents. History of high‐density lipoprotein cholesterol <35 mg/dL (0.91 mmol/L) on any 2 or more determinations within the past 5 years was included. History of smoking was also obtained.
Statistical Analyses
All continuous variables are presented as means±SD. ANOVA and χ2 test were used for unadjusted comparison of clinical characteristics in participants with evolving ECG LVH. To determine the association of clinical characteristics with achieved in‐trial BP changes, we used multivariable linear regression models, minimally adjusted for age, sex, and race/ethnicity. Intention‐to‐treat randomization assignment was used for definition of antihypertensive treatment groups.
Minimally adjusted (by age, sex, and race/ethnicity) Cox regression models were used to describe associations of baseline clinical characteristics, evolving ECG LVH, and BP lowering with 2 different definitions of incident HF, for comparison. Associations between BP lowering (continuous variable) and HF risk were also evaluated using adjusted (as above) Cox regression models incorporating cubic splines with 4 knots.
We conducted causal mediation analysis,15 allowing for treatment‐mediator interaction in the logistic regression, using counterfactual definitions of direct and indirect effects, as implemented by Valeri and Vanderweele.16 Two models were estimated: a linear model for the mediator conditional on treatment and covariates and a logistic model for the outcome conditional on treatment, the mediator, and covariates. Our study design was well suited for mediation analysis because randomization eliminated exposure‐outcome and exposure‐mediator confounding. Two mediators were studied (Figure 2): (1) evolving ECG LVH and (2) BP lowering over the course of the trial. We adjusted for mediator‐outcome confounders,11, 17 which were measured at baseline: demographic (age, sex, race and ethnicity) and clinical characteristics known to be associated both with LVH/HTN and HF; common risk factors (body mass index, smoking, diabetes mellitus), HTN history (levels of baseline systolic BP [SBP] and diastolic BP [DBP], baseline use of antihypertensive medications, ECG or echo LVH); CHD or cardiovascular disease history, coronary revascularization, major ST depression or T‐wave inversion, high‐density lipoprotein <35 mg/dL twice in the past 5 years, and participation in the lipid‐lowering ALLHAT trial. A natural direct effect represents the influence of antihypertensive treatment that is independent of evolving ECG LVH or BP lowering in the absence of evolving ECG LVH or BP changes (eg, via pleiotropic effects or drug‐specific pharmacodynamics). A controlled direct effect represents the effect of antihypertensive drug at a certain level of mediator (at progressing/resolving ECG LVH with a reference at absent evolving ECG LVH, and at tertiles of BP changes), allowing measurement of interaction between treatment and a mediator. A mediated effect represents the influence of antihypertensive drug that can be explained by its influence on evolving ECG LVH or dynamic BP changes achieved over the course of the trial. To assess the extent of mediation, we estimated the proportion mediated as a ratio of DE×(ME−1)/(DE×ME−1), where DE is direct effect and ME is mediated effect.
Figure 2.
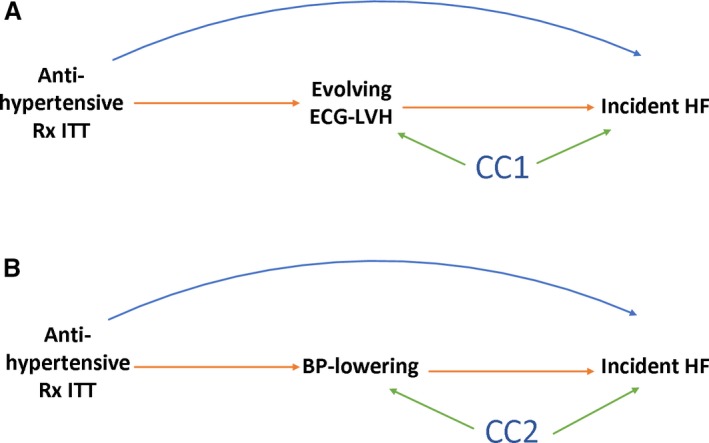
Directed acyclic graph to illustrate possible structural relationships between randomized antihypertensive treatment (Rx) in intention‐to‐treat (ITT) analysis evolving ECG left ventricular hypertrophy (LVH) (A) or blood pressure (BP) lowering (B), and incident heart failure (HF). CC denotes common causes (confounding factors), measured and unmeasured. The mediated effect is represented by the pathway from antihypertensive Rx to incident HF that goes through (A) evolving ECG LVH or (B) BP lowering. The direct effect is the pathway from antihypertensive Rx straight to incident HF.
Sensitivity Analyses
To test the robustness of our findings, we repeated analyses with different definitions of BP lowering, expressed as (1) fastest BP control, (2) relative greatest BP control, or (3) relative fastest BP control.
Statistical analyses were performed using STATA MP 15.1 (StataCorp LLC, College Station, TX). Given the many multivariate and interaction analyses performed, statistical significance at the 0.05 level should be interpreted cautiously.
Results
Study Population
The study population (Table 1) was identical to the previously reported ALLHAT population,8, 9 maintaining the treatment groups randomization ratio 1.7:1:1:1. After a median 3.1 years of follow‐up in the doxazosin group, and 5.0 years in the other 3 groups, there were 2049 incident HF outcomes, including 1598 hospitalized/fatal HF outcomes.
Table 1.
Clinical Characteristics of Study Participants With Evolving ECG LVH Increase or Decrease
| Characteristic | All (n=29 892) | Evolving ECG‐LVH Resolution (n=718; 2.4%) | Absent Evolving ECG LVH (n=28 493; 95.3%) | Evolving ECG LVH Progression (n=681; 2.3%) | P Value |
|---|---|---|---|---|---|
| Age (SD), y | 66.6 (7.4) | 66.7 (7.7) | 66.6 (7.4) | 67.5 (7.8) | 0.008 |
| Black race, n (%) | 9692 (32.4) | 372 (51.8) | 8982 (31.5) | 338 (49.6) | <0.0001 |
| Nonblack race, n (%) | 20 200 (67.6) | 346 (48.2) | 19 511 (68.5) | 343 (50.4) | <0.0001 |
| Men, n (%) | 16 819 (56.3) | 439 (61.1) | 16 028 (56.3) | 352 (51.7) | 0.002 |
| HTN treated >2 mo, n (%) | 26 122 (87.4) | 582 (81.1) | 24 923 (87.5) | 617 (90.6) | <0.0001 |
| BMI (SD), kg/m2 | 29.7 (5.8) | 27.8 (5.2) | 29.7 (5.8) | 28.3 (5.5) | <0.0001 |
| Baseline SBP (SD), mm Hg | 145.8 (15.6) | 151.2 (15.5) | 145.6 (15.6) | 146.8 (15.5) | <0.0001 |
| Baseline DBP (SD), mm Hg | 83.8 (10.0) | 85.6 (10.3) | 83.7 (10.0) | 83.4 (10.8) | <0.0001 |
| Hx of MI/stroke, n (%) | 6915 (23.1) | 141 (19.6) | 6636 (23.3) | 138 (20.3) | 0.014 |
| Hx of revascularization, n (%) | 4192 (14.0) | 80 (11.1) | 4052 (14.2) | 60 (8.8) | <0.0001 |
| Hx of ST‐T, n (%) | 3090 (10.3) | 81 (11.3) | 2931 (10.3) | 78 (11.5) | 0.431 |
| Hx of other CVD, n (%) | 7288 (24.4) | 132 (18.4) | 6987 (24.5) | 169 (24.8) | 0.001 |
| Hx of CHD, n (%) | 7854 (26.3) | 170 (23.7) | 7535 (26.5) | 149 (21.9) | 0.008 |
| Diabetes mellitus, n (%) | 10 249 (34.3) | 176 (24.5) | 9853 (34.6) | 220 (32.3) | <0.0001 |
| HDL <35 mg/dL, n (%) | 3781 (12.7) | 48 (6.7) | 3665 (12.9) | 68 (10.0) | <0.0001 |
| Smoking, n (%) | 6363 (21.3) | 197 (27.4) | 5990 (21.0) | 176 (25.8) | <0.0001 |
| Baseline ECG LVH, n (%) | 4857 (16.3) | 395 (55.0) | 4291 (15.1) | 171 (25.1) | <0.0001 |
| Baseline echo‐LVH, n (%) | 1450 (4.9) | 31 (4.3) | 1387 (4.9) | 32 (4.7) | 0.781 |
| LL trial, n (%) | 8206 (27.5) | 197 (27.4) | 7825 (27.5) | 184 (27.0) | 0.968 |
| Doxazosin ITT, n (%) | 5967 (20) | 122 (2.0) | 5698 (95.5) | 147 (2.5) | 0.001 |
| Chlorthalidone ITT, n (%) | 11 008 (37) | 262 (2.4) | 10 516 (95.5) | 230 (2.1) | 0.001 |
| Amlodipine ITT, n (%) | 6593 (22) | 193 (2.9) | 6270 (95.1) | 130 (2.00) | 0.001 |
| Lisinopril ITT, n (%) | 6324 (21) | 141 (2.4) | 6009 (95.0) | 174 (2.8) | 0.001 |
| SBP change (SD), mm Hg | −10.9 (20.0) | −16.2 (21.3) | −10.6 (19.9) | −6.2 (22.3) | <0.0001 |
| DBP change (SD), mm Hg | −7.3 (11.7) | −9.9 (12.2) | −7.3 (11.7) | −5.5 (12.5) | <0.0001 |
| Incident HF, n (%) | 2049 (6.9) | 65 (9.1) | 1901 (6.7) | 83 (12.2) | <0.0001 |
| Hospitalized/fatal HF, n (%) | 1598 (5.4) | 53 (7.4) | 1478 (5.2) | 67 (9.8) | <0.0001 |
BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; HF, heart failure; HTN, hypertension; Hx, history; ITT, intention‐to‐treat; LL, lipid‐lowering; LVH, left ventricular hypertrophy; MI, myocardial infarction; SBP, systolic blood pressure; ST‐T, major ST depression or T‐wave inversion on ECG.
Serial ECG Changes: Evolving ECG LVH
Overall, 58 366 serial ECG changes were evaluated. ECG LVH resolution was observed in about 2% of participants, and ECG LVH progression in another 2% (Table 1). The majority of participants had no evolving ECG LVH changes. ALLHAT participants with evolving ECG LVH were more likely black males, current smokers, with lower BMI but less likely to have CHD/MI history. As expected, baseline ECG LVH was more frequent in participants with resolving ECG LVH. Baseline LVH by ECG was similar in all 3 groups and was very infrequent (4% to 5%). Participants with resolving LVH by ECG were more likely diabetic, less likely to have been treated for HTN before the onset of the trial, and achieved the greatest degree of BP lowering in the trial. Incident HF was significantly more frequent in participants with evolving ECG LVH (Table 1). Doxazosin and lisinopril intention to treat was more likely to be associated with progressing ECG LVH and less likely associated with ECG LVH reduction. In contrast, chlorthalidone and amlodipine intention to treat was more likely to be associated with ECG LVH reduction and less likely associated with ECG LVH progression (Table 1).
Dynamic Changes in Blood Pressure in Trial
The first (Q1), second (Q2), and third (Q3) tertiles of the greatest BP lowering were −32/−19±10/6, −11/−7±5/3, and +11/6±12/7 mm Hg, respectively. Q1, Q2, and Q3 of the fastest BP lowering were −28/−16±10/6, −7/−4±5/3, and +14/8±12/6 mm Hg, accordingly. Hispanic ethnicity, previously untreated HTN, higher baseline levels of SBP/DBP (Figure 3) and baseline ECG LVH were associated with greater SBP and DBP lowering in trial (Table 2). In contrast, the presence of diabetes mellitus was associated with a SBP increase of nearly 2 mm Hg. Older age was associated with greater SBP lowering but slight DBP increase. A history of CHD/cardiovascular disease did not affect the degree of BP lowering in trial. Compared with chlorthalidone, doxazosin was associated with a significant SBP increase (by nearly 2 mm Hg), whereas amlodipine was associated with significant SBP and DBP decreases. Lisinopril was associated with greater DBP (but not SBP) lowering than chlorthalidone (Table 2). Participants in the doxazosin arm who developed HF had the greatest degree of BP lowering (both SBP/DBP) in trial (≈6/2 mm Hg lower than by diuretic), which contrasted with the overall weak BP‐lowering effect of doxazosin in the trial (Table 2).
Figure 3.
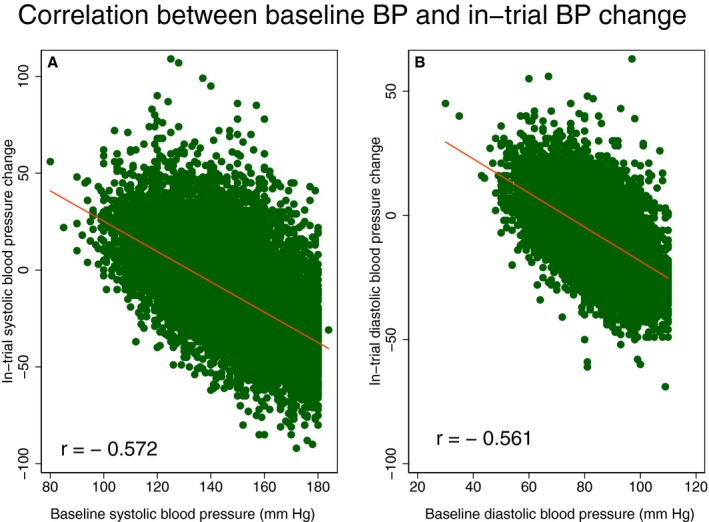
Scatterplots of (A) in‐trial systolic blood pressure (SBP) change (Y‐axis) against baseline SBP (X‐axis), and (B) diastolic blood pressure (DBP) change (Y‐axis) against baseline DBP (X‐axis). A line of the best linear fit is shown.
Table 2.
Associations of Clinical Characteristics With BP Change in Trial in Linear Regression Models
| Characteristic | Systolic BP Change (95% CI), mm Hg | P Value | Diastolic BP Change (95% CI), mm Hg | P Value |
|---|---|---|---|---|
| Age, per 1 y increase | −0.13 (−0.16 to −0.10) | <0.0001a | +0.02 (0.006‐0.04) | 0.009a |
| Race/ethnicity | ||||
| White non‐Hispanic | Reference | Reference | ||
| Black non‐Hispanic | +3.09 (2.57‐3.61) | <0.0001a | +1.59 (1.29‐1.90) | <0.0001a |
| White Hispanic | −3.64 (−4.42 to −2.86) | <0.0001a | −1.32 (−1.78 to −0.87) | <0.0001a |
| Black Hispanic | −3.79 (−5.37 to −1.19) | <0.0001a | −0.89 (−1.82 to 0.03) | 0.076 |
| Women | +0.44 (−0.02 to 0.90) | 0.063 | +0.67 (0.40‐0.94) | <0.0001a |
| HTN treated | ||||
| ≥2 months | Reference | Reference | ||
| <2 months | −6.89 (−8.16 to −5.61) | <0.0001a | −3.70 (−4.45 to −2.94) | <0.0001a |
| Not treated | −11.85 (−12.61 to −11.08) | <0.0001a | −5.74 (−6.19 to −5.29) | <0.0001a |
| BMI, per 1 kg/m2 increase | +0.04 (−0.0008 to 0.08) | 0.055 | −0.004 (−0.03 to 0.02) | 0.701 |
| Baseline SBP, per 1 mm Hg increase | −0.78 (−0.80 to −0.77) | <0.0001a | −0.29 (−0.29 to −0.28) | <0.0001a |
| Baseline DBP, per 1 mm Hg increase | −0.71 (−0.73 to −0.69) | <0.0001a | −0.72 (−0.73 to −0.71) | <0.0001a |
| Hx of MI/stroke | −0.22 (−0.77 to 0.32) | 0.423 | +0.22 (−0.10 to 0.54) | 0.178 |
| Hx of revascularization | +0.07 (−0.60 to 0.74) | 0.843 | +0.08 (−0.31 to 0.47) | 0.692 |
| Hx of ST‐T on ECG | −0.49 (−1.24 to 0.25) | 0.194 | −0.12 (−0.56 to 0.32) | 0.592 |
| Hx of other CVD | −0.32 (−0.85 to 0.22) | 0.245 | +0.30 (−0.01 to 0.61) | 0.061 |
| Hx of CHD | −0.62 (−1.15 to −0.09) | 0.022a | +0.03 (−0.28 to 0.34) | 0.827 |
| Diabetes mellitus | +1.64 (1.17‐2.11) | <0.0001a | +0.20 (−0.08 to 0.48) | 0.154 |
| HDL <35 mg/dL | +0.86 (0.17‐1.55) | 0.014a | +0.30 (−0.11 to 0.70) | 0.152 |
| Smoking | ||||
| Never | Reference | Reference | ||
| Past | −0.12 (−0.66 to 0.43) | 0.675 | −0.15 (−0.84 to 0.16) | 0.334 |
| Current | −1.13 (−1.77 to −0.49) | 0.001a | −0.46 (−0.84 to −0.09) | 0.016a |
| Baseline ECG LVH | −1.58 (−2.20 to −0.95) | <0.0001a | −0.95 (−1.31 to −0.58) | <0.0001a |
| Baseline echo LVH | −0.97 (−2.02 to 0.08) | 0.071 | +0.53 (−0.08 to 1.15) | 0.091 |
| Treatment arm | ||||
| Chlorthalidone ITT | Reference | Reference | ||
| Doxazosin ITT | +1.68 (1.05‐2.30) | <0.0001a | −0.29 (−0.66 to 0.08) | 0.124 |
| Amlodipine ITT | −1.25 (−1.86 to −0.65) | <0.0001a | −2.09 (−2.44 to −1.73) | <0.0001a |
| Lisinopril ITT | −0.16 (−0.77 to 0.46) | 0.616 | −1.37 (−1.73 to −1.01) | <0.0001a |
| Incident HF | −2.66 (−3.56 to −1.76) | <0.0001a | −1.23 (−1.76 to −0.70) | <0.0001a |
| Incident HF ## doxazosin | −5.33 (−7.86 to −2.80) | <0.0001a | −1.88 (−3.36 to −0.40) | 0.013a |
| Hospitalized/fatal HF | −2.56 (−3.57 to −1.55) | <0.0001a | −1.22 (−1.81 to −0.62) | <0.0001a |
| Hospitalized/fatal HF ## doxazosin | −5.97 (−8.85 to −3.09) | <0.0001a | −2.06 (−3.74 to 0.37) | 0.017a |
BMI indicates body mass index; BP, blood pressure; CHD, coronary heart disease; CVD, cardiovascular disease; DBP, diastolic BP; HDL, high density lipoprotein; HF, heart failure; HTN, hypertension; Hx, history; ITT, intention‐to‐treat; LVH, left ventricular hypertrophy; MI, myocardial infarction; SBP, systolic BP; ST‐T, major ST depression or T‐wave inversion on ECG. ## Significant interaction with treatment group. Participants in the doxazosin arm who developed HF or hospitalized/fatal HF
P<0.05.
Risk Factors for Heart Failure
As expected, age, ethnicity, history of HTN, CHD, and cardiovascular disease as well as ECG LVH were associated with increased risk of HF (Table 3). There were very small differences between risk factors of 2 incident HF outcomes: incident symptomatic HF and hospitalized/fatal HF.
Table 3.
Associations of Clinical Characteristics With Incident Heart Failure in Cox Regression Models
| Characteristic | Incident Symptomatic HF HR (95% CI) | P Value | Hospitalized/Fatal HF HR (95% CI) | P Value |
|---|---|---|---|---|
| Age, per 1 y increase | 1.06 (1.05‐1.06) | <0.0001a | 1.06 (1.05‐1.07) | <0.0001a |
| Race/ethnicity | ||||
| White non‐Hispanic | Reference | Reference | ||
| Black non‐Hispanic | 0.94 (0.86‐1.04) | 0.234 | 0.97 (0.87‐1.09) | 0.634 |
| White Hispanic | 0.41 (0.32‐0.51) | <0.0001a | 0.47 (0.37‐0.60) | <0.0001a |
| Black Hispanic | 0.50 (0.33‐0.77) | 0.002a | 0.54 (0.34‐0.88) | 0.013a |
| Women | 0.91 (0.84‐0.999) | 0.048a | 0.93 (0.84‐1.03) | 0.177 |
| HTN treated | ||||
| ≥2 months | Reference | Reference | ||
| <2 months | 1.04 (0.81‐1.34) | 0.732 | 1.21 (0.93‐1.58) | 0.148 |
| Not treated | 0.68 (0.57‐0.81) | <0.0001a | 0.71 (0.58‐0.87) | 0.001a |
| BMI, per 1 kg/m2 increase | 1.05 (1.04‐1.05) | <0.0001a | 1.04 (1.03‐1.05) | <0.0001a |
| Baseline SBP, per 1 mm Hg increase | 1.006 (1.004‐1.009) | <0.0001a | 1.009 (1.006‐1.01) | <0.0001a |
| Baseline DBP, per 1 mm Hg increase | 0.990 (0.986‐0.995) | <0.0001a | 0.990 (0.985‐0.995) | <0.0001a |
| Hx of MI/stroke | 1.75 (1.59‐1.91) | <0.0001a | 1.78 (1.61‐1.98) | <0.0001a |
| Hx of revascularization | 1.73 (1.55‐1.92) | <0.0001a | 1.65 (1.46‐1.87) | <0.0001a |
| Hx of ST‐T on ECG | 1.10 (0.96‐1.26) | 0.159 | 1.14 (0.98‐1.33) | 0.080 |
| Hx of other CVD | 1.26 (1.15‐1.39) | <0.0001a | 1.27 (1.14‐1.41) | <0.0001a |
| Hx of CHD | 1.66 (1.52‐1.82) | <0.0001a | 1.62 (1.46‐1.80) | <0.0001a |
| Diabetes mellitus | 1.71 (1.57‐1.87) | <0.0001a | 1.85 (1.67‐2.04) | <0.0001a |
| HDL <35 mg/dL | 0.98 (0.86‐1.11) | 0.731 | 0.97 (0.84‐1.13) | 0.718 |
| Smoking | ||||
| Never | Reference | Reference | ||
| Past | 1.19 (1.08‐1.32) | 0.001a | 1.21 (1.08‐1.36) | 0.001a |
| Current | 1.07 (0.94‐1.23) | 0.312 | 1.17 (1.01‐1.36) | 0.036a |
| Baseline ECG LVH | 1.17 (1.04‐1.31) | 0.008a | 1.16 (1.02‐1.32) | 0.023a |
| Baseline echo LVH | 1.00 (0.92‐1.21) | 0.972 | 1.06 (0.86‐1.32) | 0.576 |
| Evolving ECG LVH | ||||
| Absent | Reference | Reference | ||
| Resolving | 1.33 (1.03‐1.70) | 0.026a | 1.39 (1.05‐1.83) | 0.020a |
| Progressing | 1.78 (1.43‐2.22) | <0.0001a | 1.84 (1.44‐2.35) | <0.0001a |
| SBP lowering by 3 to 19 mm Hg (Q2): Reference | <0.0001a | |||
| By 20 mm Hg or more (Q1) | 1.34 (1.20‐1.49) | <0.0001a | 1.41 (1.25‐1.60) | <0.0001a |
| By 2 mm Hg or less (Q3) | 1.08 (0.97‐1.21) | 0.154 | 1.14 (1.01‐1.30) | 0.039a |
| DBP lowering by 2 to 11 mm Hg (Q2): Reference | ||||
| By 12 mm Hg or more (Q1) | 1.31 (1.18‐1.45) | <0.0001a | 1.32 (1.17‐1.49) | <0.0001a |
| By 1 mm Hg or less (Q3) | 1.09 (0.97‐1.21) | 0.155 | 1.12 (0.98‐1.27) | 0.088 |
BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; HF, heart failure; HR, hazard ratio; HTN, hypertension; Hx, history; LVH, left ventricular hypertrophy; MI, myocardial infarction; Q1/Q2/Q3, first/second/third tertile; SBP, systolic blood pressure; ST‐T, major ST depression or T‐wave inversion on ECG.
P<0.05.
Evolving ECG LVH was associated with incident HF (Figure 4), although progressing ECG LVH carried a larger risk, as compared with resolving ECG LVH. Evolving LVH was associated with incident HF in 3 out of 4 treatment groups (P interaction=0.056; Figure 5).
Figure 4.
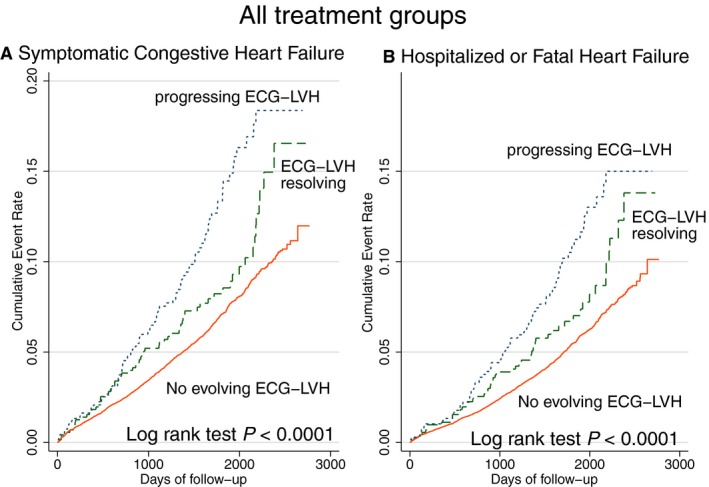
Unadjusted Kaplan‐Meier curves for probability of (A) incident symptomatic heart failure (HF) and (B) hospitalized or fatal HF in all treatment groups ALLHAT (Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial) participants with evolving ECG left ventricular hypertrophy (LVH) development (blue dotted line), resolution (green dashed line), or without evolving ECG changes (red solid line).
Figure 5.
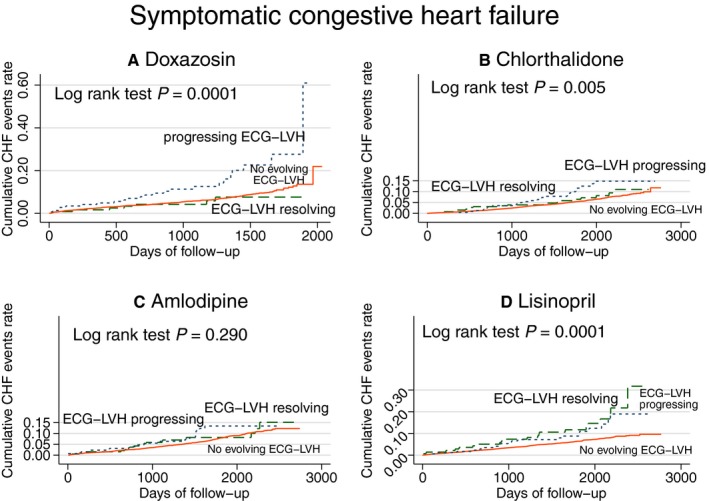
Unadjusted Kaplan‐Meier curves for probability of incident symptomatic heart failure (HF) in (A) doxazosin, (B) chlorthalidone, (C) amlodipine, and (D) lisinopril treatment groups. Evolving ECG left ventricular hypertrophy (LVH) groups as described in Figure 3 legend. CHF indicates congestive heart failure.
The association of in‐trial BP changes with HF was nonlinear (Figure 6). Both a large decrease and poor control of BP were associated with incident HF, but a large decrease in BP had a stronger effect than poor BP control on both primary and secondary outcomes (Table 3). A similar association of SBP lowering with incident HF was observed in 3 out of 4 treatment groups (Figure 7). In the amlodipine treatment group, SBP change was not associated with incident HF (P interaction=0.039; Figure 6). A noticeable U‐shaped association of DBP change with incident symptomatic HF was observed in the amlodipine and chlorthalidone treatment groups (Figure 7), whereas poor DBP control in the lisinopril and doxazosin treatment groups was not associated with incident HF.
Figure 6.
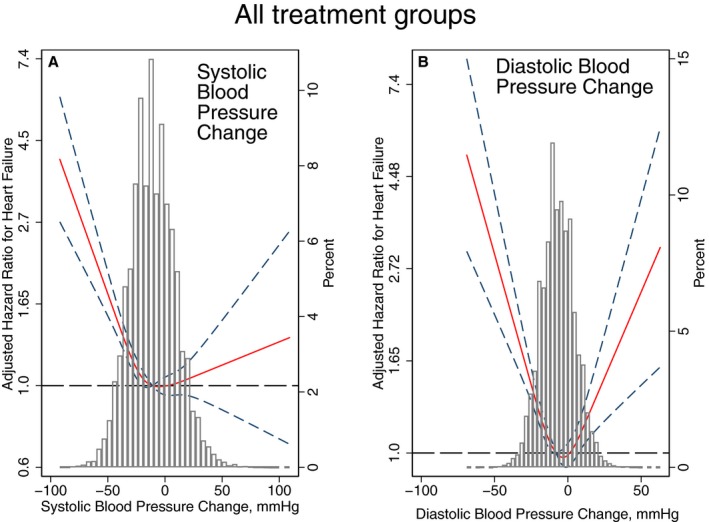
Adjusted (by age, sex, and race/ethnicity) risk of symptomatic congestive heart failure (HF) associated with achieved in‐trial greatest (A) systolic blood pressure (SBP) and (B) diastolic blood pressure (DBP) changes in all participants. Restricted cubic spline with 95% CI show change in hazard ratio (Y‐axis) in response to BP change (X‐axis). The 50th percentile of BP change is selected as the reference. BP indicates blood pressure.
Figure 7.
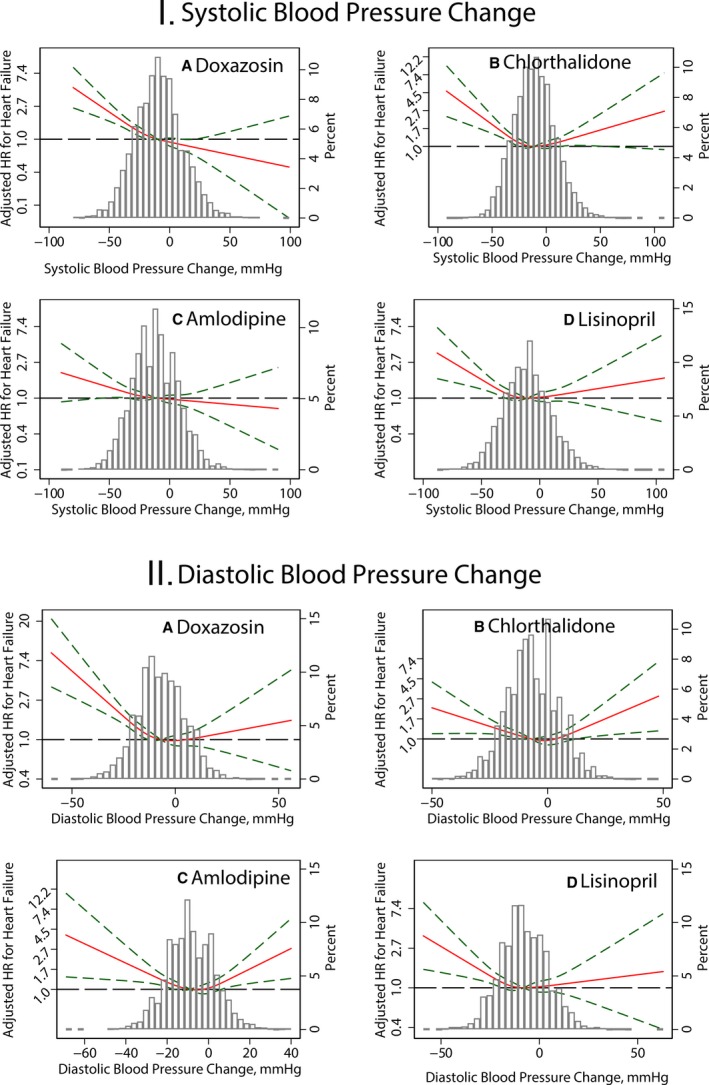
Adjusted risk of symptomatic congestive heart failure (HF) associated with achieved in‐trial greatest systolic blood pressure SBP (I) and diastolic blood pressure DBP (II) changes in (A) doxazosin, (B) chlorthalidone, (C) amlodipine, and (D) lisinopril treatment groups. See Figure 5 legend for details. HR indicates hazard ratio.
Mediation of HF Risk by Evolving LVH
In fully adjusted analyses, evolving LVH mediated 4% of the effect of doxazosin on HF (Table 4). Both direct and mediated pathways contributed to the increased HF risk in the doxazosin arm. The effects of amlodipine and lisinopril on HF were entirely independent of evolving LVH.
Table 4.
Fully Adjusted Effect of Antihypertensive Treatment on Incident Symptomatic Heart Failure (Total), Through Evolving ECG LVH or BP Changes (Mediated), and Independent of BP Lowering or Evolving ECG LVH (Direct)
| Treatment | Mediator | Controlled Direct Effect RR (95% CI) | Total Effect RR (95% CI) | Direct Effect RR (95% CI) | Mediated Effect RR (95% CI) | % Mediated |
|---|---|---|---|---|---|---|
| Doxazosin | Evolving ECG LVH (reference: none) | 1.16 (1.005‐1.33) | 1.18 (1.03‐1.36) | 1.18 (1.02‐1.36) | 1.006 (1.001‐1.015) | +3.9a |
| P interaction=0.082 | Resolving ECG LVH | 0.69 (0.38‐1.28) | ||||
| Progressing ECG LVH | 1.95 (0.93‐3.50) | |||||
| Amlodipine | Evolving ECG LVH (reference: none) | 1.41 (1.26‐1.61) | 1.40 (1.25‐1.61) | 1.40 (1.25‐1.61) | 0.999 (0.995‐1.002) | −0.2 (NS) |
| Resolving ECG LVH | 1.73 (0.85‐3.30) | |||||
| Progressing ECG LVH | 1.14 (0.60‐2.26) | |||||
| Lisinopril | Evolving ECG LVH (reference: none) | 1.17 (1.02‐1.32) | 1.17 (1.02‐1.32) | 1.17 (1.03‐1.32) | 0.999 (0.992‐1.003) | −0.9 (NS) |
| Resolving ECG LVH | 1.83 (0.80‐4.10) | |||||
| Progressing ECG LVH | 0.75 (0.31‐1.63) | |||||
| Doxazosin | SBP change Reference Q2 (−3 to −19 mm Hg) | 1.16 (1.02‐1.33) | 1.17 (1.01‐1.35) | 1.19 (1.03‐1.38) | 0.98 (0.97‐0.99) | −12.0a |
| P interaction<0.0001 | Q1 (−20 to −80 mm Hg) | 1.56 (1.22‐1.87) | ||||
| Q3 (−2 to +99 mm Hg) | 0.87 (0.70‐1.09) | |||||
| Amlodipine | SBP change Reference Q2 (−3 to −19 mm Hg) | 1.40 (1.25‐1.61) | 1.40 (1.25‐1.61) | 1.40 (1.25‐1.61) | 1.00 (0.997‐1.008) | +0.3 (NS) |
| Q1 (−20 to −90 mm Hg) | 1.47 (1.22‐1.80) | |||||
| Q3 (−2 to +90 mm Hg mm Hg) | 1.33 (1.07‐1.61) | |||||
| Lisinopril | SBP change Reference Q2 (−3 to −19 mm Hg) | 1.17 (1.02‐1.32) | 1.17 (1.03‐1.32) | 1.17 (1.03‐1.32) | 0.99997 (0.998‐1.002) | −0.1 (NS) |
| Q1 (−20 to −88 mm Hg) | 1.18 (0.97‐1.43) | |||||
| Q3 (−2 to +107 mm Hg) | 1.17 (0.95‐1.44) | |||||
| Doxazosin | DBP change Reference Q2 (−2 to −11 mm Hg) | 1.15 (0.98‐1.30) | 1.19 (1.02‐1.35) | 1.17 (0.998‐1.33) | 1.02 (1.007‐1.028) | +9.9a |
| Q1 (−12 to −60 mm Hg) | 1.38 (1.08‐1.67) | |||||
| Q3 (−1 to +56 mm Hg) | 0.96 (0.75‐1.22) | |||||
| Amlodipine | DBP change Reference Q2 (−2 to −11 mm Hg) | 1.38 (1.23‐1.59) | 1.41 (1.26‐1.62) | 1.38 (1.23‐1.59) | 1.02 (1.003‐1.037) | +6.9a |
| Q1 (−12 to −69 mm Hg) | 1.36 (1.15‐1.65) | |||||
| Q3 (−1 to +40 mmHg) | 1.40 (1.14‐1.70) | |||||
| Lisinopril | DBP change Reference Q2 (−2 to −11 mm Hg) | 1.16 (1.02‐1.31) | 1.17 (1.03‐1.32) | 1.16 (1.01‐1.31) | 1.01 (0.998‐1.03) | +8.8 (NS) |
| Q1 (−12 to −59 mm Hg) | 1.11 (0.91‐1.35) | |||||
| Q3 (−1 to +63 mm Hg) | 1.21 (0.99‐1.53) |
Proportion mediated=DE×(ME−1)/(DE×ME−1), where DE is direct effect and ME is mediated effect. Q1, Q2, Q3 are tertiles of blood pressure change. A controlled direct effect represents the effect of a drug at certain level of mediator (at absent evolving ECG LVH/progressing/resolving ECG LVH, and at tertiles of BP changes), allowing measurement of interaction between treatment and a mediator. BP indicates blood pressure; DBP, diastolic BP; LVH, left ventricular hypertrophy; RR, relative risk; SBP, systolic BP.
P<0.05.
Mediation of HF Risk by Dynamic BP Changes
After full adjustment for confounders, SBP lowering mediated 12% of the effect of doxazosin on HF (Table 4). Of note, the direct and mediated effects of doxazosin on HF were in opposite directions: the direct effect of doxazosin increased HF risk, whereas the SBP‐lowering–mediated effect reduced HF risk by 12%. There was significant (P<0.0001) interaction between doxazosin treatment and mediator: SBP‐lowering in Q1 and Q2 was associated with increased risk of HF, whereas Q3 SBP change (mean increase 11 mm Hg) was protective. The effects of amlodipine and lisinopril on HF were entirely independent of SBP changes.
DBP lowering mediated 10% of the effect of doxazosin, 7% of the effect of amlodipine, and 9% of the effect of lisinopril on HF. In fully adjusted analyses (Table 4), mediation of the effect of lisinopril lost statistical significance. Both direct and mediated pathways had the same direction and contributed to the increased HF risk.
Sensitivity analyses with different definitions of BP lowering provided similar results (Table 5). The fastest SBP‐lowering mediated ≈13% of the effect of lisinopril on HF.
Table 5.
Effect of Antihypertensive Treatment on Incident Symptomatic Heart Failure (Total), Through Relative “Greatest” BP Changes, Absolute and Relative “Fastest” BP Changes (Mediated), and Independent of BP Lowering (Direct)
| Treatment | Mediator | Total Effect RR (95% CI) | Controlled Direct Effect RR (95% CI) | Direct Effect RR (95% CI) | Mediated Effect RR (95% CI) | % Mediated | |
|---|---|---|---|---|---|---|---|
| Relative greatest BP lowering | Doxazosin | SBP change Q2 | 1.17 (0.9996‐1.32) | 1.16 (1.00‐1.32) | 1.19 (1.02‐1.35) | 0.98 (0.97‐0.99) | −13.7a |
| Amlodipine | SBP change Q2 | 1.40 (1.25‐1.61) | 1.39 (1.25‐1.61) | 1.40 (1.25‐1.61) | 1.003 (0.998‐1.01) | +1.1 (NS) | |
| Lisinopril | SBP change Q2 | 1.17 (1.03‐1.32) | 1.17 (1.02‐1.32) | 1.17 (1.02‐1.32) | 1.00 (0.997‐1.003) | +0.02 (NS) | |
| Doxazosin | DBP change Q2 | 1.19 (1.02‐1.35) | 1.16 (0.99‐1.31) | 1.17 (0.998‐1.33) | 1.01 (1.006‐1.03) | +9.3a | |
| Amlodipine | DBP change Q2 | 1.41 (1.26‐1.62) | 1.38 (1.23‐1.59) | 1.38 (1.23‐1.59) | 1.02 (1.001‐1.04) | +7.0a | |
| Lisinopril | DBP change Q2 | 1.17 (1.03‐1.32) | 1.16 (1.02‐1.31) | 1.16 (1.01‐1.31) | 1.01 (0.998‐1.03) | +8.8 (NS) | |
| Fastest BP lowering | Doxazosin | SBP change Q2 | 1.17 (1.01‐1.34) | 1.16 (0.998‐1.32) | 1.16 (0.996‐1.32) | 1.008 (0.994‐1.25) | +5.6 (NS) |
| Amlodipine | SBP change Q2 | 1.40 (1.26‐1.62) | 1.39 (1.25‐1.60) | 1.39 (1.25‐1.60) | 1.006 (0.9995‐1.01) | +2.2 (NS) | |
| Lisinopril | SBP change Q2 | 1.17 (1.02‐1.32) | 1.15 (1.0005‐1.29) | 1.15 (0.999‐1.29) | 1.02 (1.009‐1.04) | +13.7a | |
| Doxazosin | DBP change Q2 | 1.18 (1.01‐1.34) | 1.18 (1.01‐1.34) | 1.18 (1.01‐1.33) | 1.0005 (0.999‐1.005) | +0.3 (NS) | |
| Amlodipine | DBP change Q2 | 1.41 (1.25‐1.62) | 1.39 (1.24‐1.60) | 1.40 (1.25‐1.61) | 1.004 (0.998‐1.01) | +1.2 (NS) | |
| Lisinopril | DBP change Q2 | 1.17 (1.02‐1.32) | 1.17 (1.02‐1.32) | 1.17 (1.02‐1.31) | 1.001 (0.9996‐1.006) | +0.9 (NS) | |
| Relative fastest BP lowering | Doxazosin | SBP change Q2 | 1.17 (1.01‐1.34) | 1.16 (0.998‐1.32) | 1.16 (0.995‐1.33) | 1.01 (0.994‐1.03) | +6.3 (NS) |
| Amlodipine | SBP change Q2 | 1.40 (1.26‐1.60) | 1.39 (1.25‐1.60) | 1.39 (1.25‐1.60) | 1.007 (0.99993‐1.02) | +2.4 (NS) | |
| Lisinopril | SBP change Q2 | 1.17 (1.02‐1.32) | 1.15 (1.003‐1.29) | 1.15 (0.999‐1.29) | 1.02 (1.009‐1.035) | +13.4a | |
| Doxazosin | DBP change Q2 | 1.18 (1.01‐1.33) | 1.18 (1.01‐1.34) | 1.18 (1.01‐1.33) | 1.0001 (0.999‐1.003) | +0.1 (NS) | |
| Amlodipine | DBP change Q2 | 1.40 (1.26‐1.62) | 1.40 (1.25‐1.61) | 1.40 (1.25‐1.61) | 1.003 (0.997‐1.01) | +1.05 (NS) | |
| Lisinopril | DBP change Q2 | 1.17 (1.02‐1.32) | 1.17 (1.02‐1.33) | 1.17 (1.02‐1.32) | 1.0006 (0.9994‐1.005) | +0.4 (NS) |
Proportion mediated=DE×(ME−1)/(DE×ME−1), where DE is direct effect and ME is mediated effect. Q2 is a medium tertile of blood pressure changes. A controlled direct effect represents the effect of a drug at the second tertile of BP changes. BP indicates blood pressure; DBP, diastolic blood pressure; NS, nonsignificant; RR, relative risk; SBP, systolic blood pressure.
P<0.05.
Discussion
The main finding of our study is that evolving ECG LVH and BP lowering explain up to 13% of the HF‐preventive effect of the diuretic chlorthalidone as compared with the preventive effect of antihypertensive treatment with the α‐blocker doxazosin, the angiotensin‐converting enzyme inhibitor lisinopril, and the calcium channel blocker amlodipine. This finding highlights the notion of HF as a complex multifactorial condition and underscores the importance of the use of diuretics for HF prevention, which targets mechanisms that are largely independent of BP lowering and evolving ECG LVH.
Heart Failure Prevention in Hypertension
HTN is the major risk factor of HF, associated with 2‐ to 3‐fold increased HF incidence in observational cohort studies.18 However, in randomized controlled trials HTN treatment is associated with only 20% to 25% reduction in HF risk.5 Our study provided consistent findings: BP lowering mediated only up to 13% of the effect of antihypertensive medications on incident HF. Such a disconnect between a risk factor and the effect of its modification is traditionally explained by poor BP control, irreversible damage of the heart over long‐time risk exposure, insufficient awareness of HTN, and inadequate assessment of HTN by a single BP measurement. Our study findings suggest that in order to achieve the most effective HF prevention, BP lowering should not be the only criterion of HTN treatment effectiveness. Moreover, because different antihypertensive treatments have different mediators, different criteria of effectiveness (beyond BP control) should be developed for each class of antihypertensive drugs.
Diuretics for HF Prevention
Our study showed that the mechanisms by which the thiazide diuretic chlorthalidone prevented HF were not restricted to BP lowering and prevention of LVH. The mechanisms responsible for the favorable effect of chlorthalidone on HF prevention in people with HTN are unknown. In addition to BP lowering, chlorthalidone has pleiotropic effects including improving endothethial function and reducing inflammation and oxidative stress.19 Better understanding of the mechanisms behind the effect of chlorthalidone on HF may lead to new drug formulations specifically targeting HF prevention in patients with HTN.
Left Ventricular Hypertrophy and Heart Failure
Long‐standing HTN and LVH can start a devastating cascade that leads to HF via myocyte growth, oxidative stress, and fibrosis.20 Although antihypertensive drugs have been shown to reduce and even reverse LVH, this study showed that reduction in ECG LVH increased the risk of HF, as compared with patients who remained free from LVH.
In the current study, evolving LVH mediated only 4% of the effect of doxazosin on HF. Consistent with our findings, previous analysis of Cornell voltage changes during the ALLHAT trial21 showed no difference in ECG LVH development/resolution between the amlodipine, lisinopril, or chlorthalidone treatment arms. There are known limitations of ECG LVH as a measure of the LV enlargement, as there are more than a dozen ECG LVH definitions with poor agreement among them.22 Differences between LVH measured by ECG and LV mass measured by imaging modalities22 reflect true differences between the cardiac anatomy and the electrophysiological substrate. ECG LVH characterizes an abnormal electrophysiological substrate, which is associated with sudden cardiac death and incident HF independent of LV mass and BP control.23, 24, 25 Additional ECG measures of electrophysiological substrate should be considered as potential mediators of antihypertensive treatment effect on HF. For example, the sum absolute QRST integral was shown to be associated with HF hospitalization or death in the MADIT II (Multicenter Automatic Defibrillator Implantation Trial II) study.26 Longitudinal changes in global electrical heterogeneity were associated with LV dysfunction.27 Comprehensive description of electrophysiological substrate beyond evolving LVH (eg, using the sum absolute QRST integral and global electrical heterogeneity) may improve understanding of the mechanisms responsible for HF development in the setting of HTN.
Blood Pressure Lowering and Heart Failure
Our findings are largely consistent with previous ALLHAT results and conclusions.28 Previous analysis of attributable risks due to BP lowering29 concluded that the effect of amlodipine on incident HF was BP independent, whereas BP lowering only partially explained the effect of lisinopril on HF. In our adjusted mediation analysis, the effect of both amlodipine and lisinopril on HF was entirely independent of SBP, whereas DBP lowering mediated 7% of the effect of amlodipine and 9% of the effect of lisinopril. Interestingly, we observed opposite directions of the direct effect of doxazosin (increased HF risk) and SBP‐lowering–mediated effect of doxazosin (reduced HF risk by 12%). DBP lowering mediated 10% of the effect of doxazosin and had the same direction as the direct effect of doxazosin. Because doxazosin remains a viable HTN treatment option for men with benign prostatic hyperplasia, complex effects of BP lowering on incident HF should be taken into account for patients on doxazosin. Overall, the very modest effect of BP lowering on incident HF highlights the importance of additional (beyond BP control) biomarkers for the assessment of effectiveness of antihypertensive drugs for HF prevention.
Strengths and Limitations
ALLHAT is the largest randomized controlled trial of antihypertensive treatment, allowing unbiased mediation analysis and strengthening 2 major assumptions of mediation analysis. Randomization eliminated exposure‐outcome and exposure‐mediator confounding. However, limitations of this study should be taken into account. Although we adjusted for known common causes of evolving ECG LVH, BP lowering, and incident HF, unmeasured confounding can affect the study estimates. ALLHAT enrolled high‐risk HTN patients, and thus the results of this study may not be generalizable to lower‐risk populations. In our study baseline BP displayed moderate correlation with in‐trial BP lowering (Figure 3), which at least partially explained the U‐shaped association of BP‐lowering with incident HF. We utilized modeling approaches that accounted for nonlinear associations, but it is possible that we underestimated the true effect of BP lowering on incident HF.
Sources of Funding
This work was partially supported by National Heart, Lung, and Blood Institute grant HL118277 to Tereshchenko. The ALLHAT study was supported by the National Heart, Lung, and Blood Institute (NO1‐HC‐35130). ALLHAT investigators received contributions of study medications supplied by Pfizer (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol‐Myers Squibb (pravastatin) and financial support provided by Pfizer.
Disclosures
None.
Acknowledgments
This article was prepared using ALLHAT Research Materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center.
(J Am Heart Assoc. 2019;8:e011961 DOI: 10.1161/JAHA.119.011961.)
References
- 1. Rapsomaniki E, Timmis A, George J, Pujades‐Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zanchetti A. Hypertension: cardiac hypertrophy as a target of antihypertensive therapy. Nat Rev Cardiol. 2010;7:66–67. [DOI] [PubMed] [Google Scholar]
- 3. Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Heckbert SR, Lemaitre RN, Wagner EH, Furberg CD. Health outcomes associated with antihypertensive therapies used as first‐line agents. A systematic review and meta‐analysis. JAMA. 1997;277:739–745. [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 5. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT Jr, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM. Rationale and design for the antihypertensive and lipid lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Research Group. Am J Hypertens. 1996;9:342–360. [DOI] [PubMed] [Google Scholar]
- 7. Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A meta‐analysis of randomized double‐blind studies. JAMA. 1996;275:1507–1513. [PubMed] [Google Scholar]
- 8. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Collaborative Research Group. JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 9. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 10. Einhorn PT, Davis BR, Massie BM, Cushman WC, Piller LB, Simpson LM, Levy D, Nwachuku CE, Black HR. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) heart failure validation study: diagnosis and prognosis. Am Heart J. 2007;153:42–53. [DOI] [PubMed] [Google Scholar]
- 11. Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S. Heart failure with preserved and reduced left ventricular ejection fraction in the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Circulation. 2008;118:2259–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis BR, Piller LB, Cutler JA, Furberg C, Dunn K, Franklin S, Goff D, Leenen F, Mohiuddin S, Papademetriou V, Proschan M, Ellsworth A, Golden J, Colon P, Crow R. Role of diuretics in the prevention of heart failure: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack trial. Circulation. 2006;113:2201–2210. [DOI] [PubMed] [Google Scholar]
- 13. Prineas RJ, Crow RS, Zhang Z‐M. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. London: Springer; 2010. [Google Scholar]
- 14. Crow RS, Prineas RJ, Hannan PJ, Grandits G, Blackburn H. Prognostic associations of Minnesota Code serial electrocardiographic change classification with coronary heart disease mortality in the Multiple Risk Factor Intervention Trial. Am J Cardiol. 1997;80:138–144. [DOI] [PubMed] [Google Scholar]
- 15. Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3:143–155. [DOI] [PubMed] [Google Scholar]
- 16. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure‐mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piller LB, Baraniuk S, Simpson LM, Cushman WC, Massie BM, Einhorn PT, Oparil S, Ford CE, Graumlich JF, Dart RA, Parish DC, Retta TM, Cuyjet AB, Jafri SZ, Furberg CD, Saklayen MG, Thadani U, Probstfield JL, Davis BR. Long‐term follow‐up of participants with heart failure in the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Circulation. 2011;124:1811–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. [DOI] [PubMed] [Google Scholar]
- 19. Roush GC, Buddharaju V, Ernst ME, Holford TR. Chlorthalidone: mechanisms of action and effect on cardiovascular events. Curr Hypertens Rep. 2013;15:514–521. [DOI] [PubMed] [Google Scholar]
- 20. Cacciapuoti F. Molecular mechanisms of left ventricular hypertrophy (LVH) in systemic hypertension (SH)—possible therapeutic perspectives. J Am Soc Hypertens. 2011;5:449–455. [DOI] [PubMed] [Google Scholar]
- 21. Ernst ME, Davis BR, Soliman EZ, Prineas RJ, Okin PM, Ghosh A, Cushman WC, Einhorn PT, Oparil S, Grimm RH Jr; Group ACR . Electrocardiographic measures of left ventricular hypertrophy in the antihypertensive and lipid‐lowering treatment to prevent heart attack trial. J Am Soc Hypertens. 2016;10:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jain A, Tandri H, Dalal D, Chahal H, Soliman EZ, Prineas RJ, Folsom AR, Lima JA, Bluemke DA. Diagnostic and prognostic utility of electrocardiography for left ventricular hypertrophy defined by magnetic resonance imaging in relationship to ethnicity: the Multi‐Ethnic Study of Atherosclerosis (MESA). Am Heart J. 2010;159:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leigh JA, O'Neal WT, Soliman EZ. Electrocardiographic left ventricular hypertrophy as a predictor of cardiovascular disease independent of left ventricular anatomy in subjects aged >/=65 years. Am J Cardiol. 2016;117:1831–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oseni AO, Qureshi WT, Almahmoud MF, Bertoni AG, Bluemke DA, Hundley WG, Lima JA, Herrington DM, Soliman EZ. Left ventricular hypertrophy by ECG versus cardiac MRI as a predictor for heart failure. Heart. 2017;103:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bang CN, Devereux RB, Okin PM. Regression of electrocardiographic left ventricular hypertrophy or strain is associated with lower incidence of cardiovascular morbidity and mortality in hypertensive patients independent of blood pressure reduction—a LIFE review. J Electrocardiol. 2014;47:630–635. [DOI] [PubMed] [Google Scholar]
- 26. Tereshchenko LG, McNitt S, Han L, Berger RD, Zareba W. ECG marker of adverse electrical remodeling post‐myocardial infarction predicts outcomes in MADIT II study. PLoS One. 2012;7:e51812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biering‐Sorensen T, Kabir M, Waks JW, Thomas J, Post WS, Soliman EZ, Buxton AE, Shah AM, Solomon SD, Tereshchenko LG. Global ECG measures and cardiac structure and function: the ARIC study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2018;11:e005961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davis BR, Furberg CD, Wright JT Jr, Cutler JA, Whelton P. ALLHAT: setting the record straight. Ann Intern Med. 2004;141:39–46. [DOI] [PubMed] [Google Scholar]
- 29. Proschan M, Ford CE, Cutler JA, Graumlich JF, Pavlik V, Cushman WC, Davis BR, Alderman MH, Gordon D, Furberg CD, Franklin SS, Blumenthal SS, Castaldo RS, Preston RA; LHAT Collaborative Research Group . How much effect of different antihypertensive medications on cardiovascular outcomes is attributable to their effects on blood pressure? Stat Med. 2013;32:884–897. [DOI] [PubMed] [Google Scholar]


