Abstract
Background
Bicuspid aortic valve (BAV) is the most common congenital cardiac abnormality. A thoracic aortic aneurysm (TAA) is present in ≈50% of BAV patients, who also have an 8‐fold higher risk of aortic dissection than the general population. Because the health of the aorta is directly reflected in its stiffness and pulsatile hemodynamics, we hypothesized that measures of aortic stiffness and arterial load would be associated with TAA growth in BAV.
Methods and Results
Twenty‐nine unoperated participants with TAA due to BAV who had serial imaging were recruited. Aortic stiffness and steady and pulsatile arterial load were evaluated with validated methods that integrate arterial tonometry with echocardiography. TAA growth was assessed retrospectively based on available imaging, blinded to hemodynamic status. Multivariable linear regression assessed associations of aortic stiffness and hemodynamic variables with TAA growth, adjusting for potential confounders. Overall, 66% of participants were men. Mean±SD for age, baseline aneurysm size, growth rate, and follow‐up time were 57.2±8.3 years, 46.9±3.6 mm, 0.75±0.81 mm/y, and 2.9±3.3 years, respectively. We found that greater aortic stiffness (β±SE for carotid‐femoral pulse wave velocity: 0.30±0.13. P=0.03) and aortic characteristic impedance (β±SE: 0.46±0.18, P=0.02), as well as lower total arterial and proximal aortic compliance (β±SE: −0.44±0.21, P=0.05, and −0.63±0.16, P=0.001, respectively) were independently associated with faster aneurysm growth.
Conclusions
In patients with TAA due to BAV, measures of greater aortic stiffness and pulsatile arterial load indicate an association with accelerated aneurysm expansion. Assessing arterial hemodynamics may be useful for risk stratification and disease monitoring in TAA patients with BAV.
Keywords: aneurysm, aortic disease, arterial stiffness, bicuspid aortic valve, hemodynamics
Subject Categories: Aneurysm, Hemodynamics, Valvular Heart Disease
Clinical Perspective
What Is New?
Greater aortic stiffness and pulsatile arterial load are independently associated with faster thoracic aortic aneurysm growth in patients with bicuspid aortic valves.
These relevant hemodynamic markers may be useful in novel risk algorithms for thoracic aortic aneurysm risk prediction in patients with bicuspid aortic valves.
What Are the Clinical Implications?
Accelerated aneurysm growth is a risk factor for acute aortic syndromes. When added to existing cross‐sectional literature, our findings confirm that arterial hemodynamic measures that are associated with the health and function of the aorta may adequately represent thoracic aortic aneurysm disease activity in bicuspid aortic valves.
The relevant hemodynamic markers can be obtained noninvasively in the office setting, increasing the clinical applicability of our findings.
Introduction
Bicuspid aortic valve (BAV) is the most common congenital cardiac abnormality, affecting 1% to 2% of the population,1 with thoracic aortic aneurysm (TAA) formation being its second most frequent complication after aortic valve dysfunction (stenosis or insufficiency).2 Dilatation of any or all segments of the proximal aorta is present in up to 50% of patients with BAV.2 The 2 most likely mechanisms for aneurysm formation are hemodynamic flow disturbances, resulting in increased wall shear stress in the proximal aorta, and unidentified genetic or developmental abnormalities, resulting in congenital weakness of the aortic wall.3
Aortic dilation is a precursor to aortic rupture and dissection, both potentially fatal events.4 The risk of aortic dissection among BAV patients has been reported as 0.03% to 0.1% per year.5, 6 Despite the potentially morbid consequences of TAA in patients with BAV, no effective medical prophylactic measures are currently available to prevent progressive dilation or impact the remodeling of the aortic wall.7 This highlights a critical need to identify factors contributing to aneurysm growth among BAV patients in order to develop new risk stratification, disease monitoring, and therapeutic targets for this condition.
In the search for novel markers of TAA‐related risk in BAV, measures of aortic stiffness and hemodynamics have significant potential for clinical utility. Aortic stiffness increases as elastic fibers in the lamina media of the aorta are damaged and replaced by collagen fibers, causing a substantial increase in aortic impedance and resultant changes in the central pulse wave and the aortic pulse wave velocity.8, 9 As the aorta stiffens, its pressure‐buffering abilities are compromised, leading to an increase in the pulsatile arterial load.10, 11 Assessing aortic stiffness and hemodynamics provides a window into aortic health. Based on these concepts, we hypothesized that measures of aortic stiffness and pulsatile arterial load would be related to aneurysm expansion in individuals with BAV. In addition to addressing this hypothesis in the present study, we also aimed (1) to determine the value of central hemodynamic parameters over brachial blood pressure (the only hemodynamic measure routinely available to clinicians); (2) to prove a concept that only measures related to aortic health (ie, aortic stiffness and pulsatile arterial load but not systemic vascular resistance) are of value in assessing TAA disease activity in BAV; and (3) to select the best hemodynamic markers to be used in future, larger studies of this topic.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Participants and Their Characteristics
Study participants were recruited from the University of Ottawa Heart Institute's Aorta Clinic and included unoperated men and women with TAA. Participants were eligible if they had ≥2 previous aneurysm measurements at least 6 months apart. Exclusion criteria included a previous history of aortic dissection, rupture, intramural hematoma, or surgery; worse than mild aortic stenosis or worse than moderate aortic regurgitation (which could interfere with the noninvasive hemodynamic technique); and history of coarctation of the aorta. The studied participants had no history of connective tissue disorders or familial valve abnormalities. Participants gave informed consent, and the study was approved by University of Ottawa Heart Institute's research ethics board.
Between 2014 and 2018, 29 consecutive participants were enrolled and their arterial hemodynamics were studied. At the time of enrollment, all participants completed a standard questionnaire about personal and family history and medication use, provided a blood sample, and had anthropometric data collected. Diabetes mellitus was defined by physician diagnosis and/or use of oral hypoglycemic medications or insulin. Hypertension was defined by physician diagnosis and/or use of antihypertensive medications. Smoking was defined as having smoked >100 cigarettes in the past. Weight was measured in kilograms using an electronic scale, and height was measured in meters by a stadiometer. Body mass index was calculated as weight (kg) divided by height (m2). Body surface area was calculated using the Gehan method.12 Brachial blood pressure was measured using an electronic sphygmomanometer with a microphone over the brachial artery to ensure fidelity of the measurements.13 It was measured 3 times, 2 minutes apart, and the average was used for analyses. Mean arterial pressure was calculated as a third of the systolic blood pressure plus two thirds of the diastolic blood pressure. Brachial pulse pressure was calculated as brachial systolic blood pressure minus diastolic blood pressure.
Assessment of Aortic Stiffness and Arterial Hemodynamics
Central arterial hemodynamics were noninvasively determined with validated methods that integrate arterial tonometry and transthoracic echocardiography, with simultaneous ECG recording, yielding measures of aortic stiffness (carotid–femoral pulse wave velocity [cfPWV]), steady arterial load (systemic vascular resistance) and pulsatile arterial load (aortic characteristic impedance [Zc], proximal aortic compliance [PAC], total arterial compliance [TAC], central systolic blood pressure, central pulse pressure, forward pressure wave amplitude [Pf], and reflected pressure wave amplitude). The methods for hemodynamic assessment have been described previously14, 15, 16 and will be briefly summarized. All studies were performed by trained cardiac sonographers, with participants lying supine in a darkened room with controlled temperature. Measurements from the suprasternal notch to the carotid and femoral tonometry sites were used to estimate aortic path length according to the subtraction method.17 After brachial blood pressure measurements (described earlier), applanation tonometry of the brachial, carotid, and femoral arteries was performed. Brachial systolic and diastolic blood pressures were used to calibrate the peak and trough of the signal‐averaged brachial pressure waveform13; diastolic and integrated mean brachial pressures were used to calibrate carotid pressure tracings. Then, transthoracic 2‐dimensional Doppler echocardiography was performed to measure the left ventricular outflow tract diameter (parasternal long‐axis view) and time velocity integral (apical 5‐chamber view, pulse wave Doppler). The left ventricular outflow tract velocity time integral was multiplied by outflow tract area to calculate aortic flow. This was followed, in rapid succession, by repeat tonometry of the carotid artery, which was used as surrogate for aortic pressure in pressure/flow analyses. All data were analyzed with validated software capable of pressure/flow analyses (NIHem; Cardiovascular Engineering Inc Norwood, MA, USA). The cfPWV was calculated as the estimated aortic path length in meters divided by the time delay between carotid and femoral pressure waveforms in seconds. From the calibrated central pressure waveform, central systolic and diastolic blood pressures were defined as the peak and trough of pressure, respectively, and central pulse pressure was calculated as their difference. TAC is the change in arterial blood volume due to a given change in pulsatile arterial blood pressure and is calculated using the area method during the latter two thirds of diastole.18 From pressure/flow analyses, Zc is calculated as the ratio of central pulsatile pressure to aortic flow. Systemic vascular resistance is calculated as impedance at zero frequency. PAC represents the compliance of the proximal portion of the aorta (the most compliant segment of the arterial tree) and is calculated from the Bramwell‐Hill equation PWV2=VΔP/ρΔV, where V is aortic volume, P is aortic pressure, and ρ is blood density. Once pressure and flow are known, Pf and reflected pressure wave amplitude can be separated and their amplitudes estimated. The global reflection coefficient, the reference standard for assessing wave reflections,19, 20 is calculated as reflected pressure wave amplitude divided by Pf.
Assessment of Aneurysm Size and Growth Rates
All previously performed imaging studies were done at our institution and were available for retrospective assessment. Imaging modalities assessed included echocardiography, computed tomography, and magnetic resonance imaging. Whenever possible, the same imaging modality was used to compare aneurysm growth. Maximum thoracic aorta size was measured systematically by an imaging cardiologist (T.C.), in diastole and blinded to hemodynamics. Our group has previously shown excellent reproducibility in TAA measurements across different imaging modalities.21 Aneurysm growth rate was calculated as the absolute growth of the aneurysm at its maximal location (ie, the root or ascending aorta) divided by the total follow‐up time (mm/y).
Statistical Analyses
Continuous variables were reported as mean±SD, and nominal variables were reported as total number and percentage. The associations of brachial blood pressure and central hemodynamic variables with TAA growth were first assessed with Spearman correlation coefficients. This was followed by unadjusted and then multivariable linear regression models, using each hemodynamic measure separately as an independent variable. For the multivariable linear regression analyses, we created parsimonious models by evaluating the unadjusted associations of each covariate (age; sex; body surface area; mean arterial pressure; aneurysm location; baseline aneurysm size; time between studies; and history of hypertension, diabetes mellitus, and smoking) with aneurysm growth. Those associated with aneurysm growth at P≤0.20 were then added to the multivariable models. In addition, age, sex, and baseline aneurysm size were forced into all models because these variables are known to be associated with TAA outcomes and are considered by clinicians when evaluating TAA patients. All analyses were performed using JMP v13 (SAS Institute). P≤0.05 were considered statistically significant.
Results
Participant characteristics are summarized in Table 1. Overall, 66% of participants were men, consistent with the epidemiology of TAA.22 Mean±SD for age, baseline aneurysm size, growth rate, and follow‐up time was 57.2±8.3 years, 46.9±3.6 mm, 0.75±0.81 mm/y, and 2.9±3.3 years, respectively. Aneurysm growth was not different among those who had the same (mean±SD: 0.62±0.78 mm/y) or different (mean±SD: 0.45±0.65 mm/y) imaging modalities at baseline and follow‐up (P=0.36). Spearman correlations are depicted in Table 2, showing that lower PAC and TAC and higher Zc, Pf, and global reflection coefficient were moderately correlated with faster TAA growth. Notably, there were no significant correlations between brachial blood pressure parameters or steady arterial load (systemic vascular resistance) and TAA growth (P>0.05 for each). Figures 1 and 2 illustrate unadjusted associations of brachial and central hemodynamic variables with aneurysm growth, respectively.
Table 1.
Baseline Characteristics of the Study Participants (n=29)
| Variable | Mean±SD or n(%) |
|---|---|
| Age, y | 57.21±8.30 |
| Men | 19 (66) |
| Height, cm | 175±8.52 |
| Weight, kg | 90.62±19.23 |
| Body surface area, m2 | 2.06±0.23 |
| Body mass index, kg/m2 | 29.41±4.98 |
| Brachial SBP, mm Hg | 123±18 |
| Brachial DBP, mm Hg | 67±10 |
| MAP, mm Hg | 89±11 |
| Brachial PP, mm Hg | 56±15 |
| Hypertension | 9 (31) |
| Diabetes mellitus | 2 (6.9) |
| Smoking | 1 (3.4) |
| Maximal aneurysm location, n (root; ascending aorta) | 6; 23 |
| Baseline aneurysm size, mm | 46.91±3.62 |
| Imaging modality at baseline, n (TTE; CT; MRI) | 5; 19; 5 |
| Time between studies, y | 2.93±3.31 |
| Imaging modality at follow‐up, n (TTE; CT; MRI) | 7; 12; 10 |
| Concordant imaging modality | 15 (51) |
| Growth rate, mm/y | 0.75±0.81 |
| Arterial hemodynamic variables | |
| Central SBP, mm Hg | 121±14 |
| Central DBP, mm Hg | 71±11 |
| Central PP, mm Hg | 50±13 |
| cfPWV, m/s | 7.72±2.11 |
| SVR, dyne×s/cm5 | 1363±379 |
| TAC, mL/mm Hg | 2.82±1.22 |
| Zc, dyne×s/cm5 | 120±50 |
| PAC, 10−6 cm4/dyne | 14±9 |
| Forward pressure wave amplitude, mm Hg | 41±12 |
| Reflected pressure wave amplitude, mm Hg | 15±5 |
| Global reflection coefficient | 0.37±0.06 |
Data are shown as Mean±SD or n (%). cfPWV indicates carotid–femoral pulse wave velocity; CT, computed tomography; DBP, diastolic blood pressure; MAP, mean arterial pressure; MRI, magnetic resonance imaging; PAC, proximal aortic compliance; PP, pulse pressure; SBP, systolic blood pressure; SVR, systemic vascular resistance; TAC, total arterial compliance; TTE, transthoracic echocardiography; Zc, characteristic impedance of the aorta.
Table 2.
Spearman Correlation Coefficients Depicting the Independent Associations of Each Hemodynamic Variable With TAA Growth in Patients With BAV
| Variable | Spearman's ρ | P Value |
|---|---|---|
| Brachial blood pressure measures | ||
| Brachial SBP | 0.21 | 0.28 |
| Brachial DBP | 0.05 | 0.79 |
| Brachial PP | 0.16 | 0.40 |
| Central blood pressure and arterial load measures | ||
| Central SBP | 0.38 | 0.04 |
| Central DBP | 0.25 | 0.20 |
| Central PP | 0.29 | 0.13 |
| SVR | 0.14 | 0.47 |
| Zc | 0.46 | 0.01 |
| PAC | −0.54 | 0.003 |
| TAC | −0.54 | 0.003 |
| Forward pressure wave amplitude | 0.42 | 0.02 |
| Reflected pressure wave amplitude | 0.11 | 0.56 |
| Global reflection coefficient | −0.37 | 0.05 |
| cfPWV | 0.35 | 0.06 |
BAV indicates bicuspid arterial valve; cfPWV, carotid–femoral pulse wave velocity; DBP, diastolic blood pressure; PAC, proximal aortic compliance; PP, pulse pressure; SBP, systolic blood pressure; SVR, systemic vascular resistance; TAA, thoracic aortic aneurysm; TAC, total arterial compliance; Zc, aortic characteristic impedance.
Figure 1.
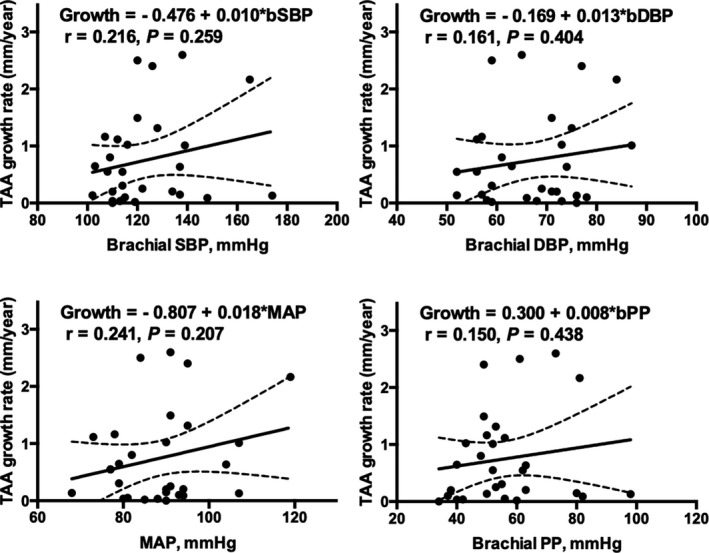
Unadjusted associations of brachial blood pressure with TAA growth. Brachial blood pressure measures were not significantly associated with aneurysm growth. bDBP indicates brachial diastolic blood pressure; bPP, brachial pulse pressure; bSBP, brachial systolic blood pressure; MAP, mean arterial pressure; TAA, thoracic aortic aneurysm.
Figure 2.
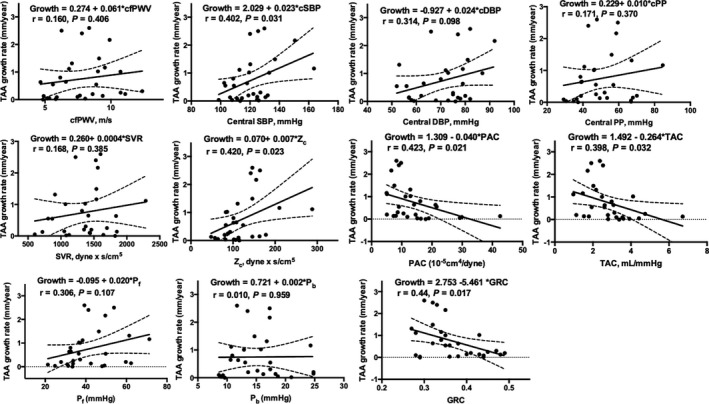
Unadjusted associations of central hemodynamic measures with TAA growth. Higher cSBP and aortic characteristic impedance and lower PAC, TAC, and GRC were significantly associated with aneurysm growth. cDBP indicates central diastolic blood pressure; cfPWV, carotid–femoral pulse wave velocity; cPP, central pulse pressure; cSBP, central systolic blood pressure; GRC, global reflection coefficient; PAC, proximal aortic compliance; Pb, reflected pressure wave amplitude; Pf, forward pressure wave amplitude; SVR, systemic vascular resistance; TAA, thoracic aortic aneurysm; TAC, total arterial compliance; Zc, characteristic impedance of the aorta.
At the univariable level, the following covariates were associated with TAA growth at P≤0.20: MAP (P=0.20), follow‐up time (P=0.03), diabetes mellitus (P=0.0007), and smoking (P=0.11). These variables, added to age, sex, and baseline aneurysm size, lead to the base model for our multivariable linear regression analyses, the results of which are summarized in Table 3. We found that measures of aortic stiffness (higher cfPWV) and pulsatile arterial load (higher Zc and Pf and lower PAC and TAC) remained independently associated with faster aneurysm growth (Table 3). In addition, when comparing standardized β coefficients, we observed that cfPWV, Zc, Pf, PAC, and TAC (standardized β: 0.59, 0.58, 0.57, −0.71, and −0.55, respectively) were superior to baseline aneurysm size (the parameter typically used for clinical decisions in TAA; standardized β: −0.11) when assessing aneurysm expansion. To consider the potential influence of same versus different imaging modalities at baseline and follow‐up and use of antihypertensive medications on the associations found, we repeated the multivariable models while also adjusting for the same or different nature of imaging modalities and antihypertensive medication use. We found that associations of higher cfPWV (β±SE for 1‐m/s increase: 0.22±0.11, P=0.05), Zc (β±SE for 1‐SD increase: 0.46±0.15, P=0.007) and Pf (β±SE for 5‐mm Hg increase: 0.21±0.08, P=0.02); and lower PAC (β±SE for 1‐SD increase: −0.61±0.14, P=0.0003) and TAC (β±SE for 1‐SD increase: −0.46±0.17, P=0.001) with TAA expansion rate remained statistically significant.
Table 3.
Summary of Multivariable Linear Regression Models Depicting the Independent Associations of Each Hemodynamic Variable With TAA Growth in Patients With BAV
| Variable | R 2 for the Model | β±SE | Standardized β | P Value |
|---|---|---|---|---|
| Base modela | 0.31 | ··· | ··· | |
| Brachial SBP, 5 mm Hg increase | 0.31 | 0.02±0.08 | 0.10 | 0.78 |
| Brachial DBP, 5 mm Hg increase | 0.34 | −0.16±0.17 | −0.37 | 0.36 |
| Brachial PP, 5 mm Hg increase | 0.32 | 0.03±0.06 | 0.12 | 0.60 |
| Central SBP, 5 mm Hg increase | 0.42 | 0.12±0.06 | 0.43 | 0.07 |
| Central DBP, 5 mm Hg increase | 0.35 | 0.11±0.10 | 0.30 | 0.29 |
| Central PP, 5 mm Hg increase | 0.36 | 0.10±0.07 | 0.232 | 0.22 |
| SVR, 1 SD increase | 0.34 | 0.18±0.20 | 0.22 | 0.37 |
| Zc, 1 SD increase | 0.53 | 0.47±0.15 | 0.58 | 0.006 |
| PAC, 1 SD increase | 0.63 | −0.57±0.14 | −0.71 | 0.0005 |
| TAC, 1 SD increase | 0.49 | −0.44±0.17 | −0.55 | 0.01 |
| Pf, 5 mm Hg increase | 0.46 | 0.19±0.08 | 0.57 | 0.03 |
| Pb, 5 mm Hg increase | 0.40 | 0.25±0.26 | 0.29 | 0.35 |
| GRC, 1 SD increase | 0.40 | −0.32±0.18 | −0.39 | 0.09 |
| cfPWV, 1 m/s increase | 0.43 | 0.22±0.10 | 0.59 | 0.04 |
BAV indicates bicuspid arterial valve; cfPWV, carotid–femoral pulse wave velocity; DBP, diastolic blood pressure; GRC, global reflection coefficient; PAC, proximal aortic compliance; Pb, reflected pressure wave amplitude; Pf, forward pressure wave amplitude; SBP, systolic blood pressure; SVR, systemic vascular resistance; TAA, thoracic aortic aneurysm; TAC, total arterial compliance; Zc, characteristic impedance of the aorta.
Base model included age, sex, mean arterial pressure, baseline aneurysm size, time between studies, and history of diabetes mellitus and smoking. Each hemodynamic variable reported in this table was added individually to the base model.
For comparison and validation, we identified 30 participants with heritable forms of TAA who had trileaflet aortic valves and who had characteristics similar to the BAV‐TAA patients (mean age: 55.3±11.2 years; 70% men; 65% with root aneurysm; baseline aneurysm size: 43.1±3.8 mm; follow‐up time: 3.3±2.9 years). In this group of non‐BAV participants with heritable TAA, only lower global reflection coefficient was independently associated with TAA expansion (β±SE for 1‐SD increase: −0.21±0.08, P=0.02). None of the other arterial stiffness and hemodynamic parameters that predicted aneurysm expansion in BAV participants were significant in this population (P>0.05 for each; analyses not shown), suggesting different pathophysiologies and greater roles for aortic stiffness and adverse pulsatile hemodynamics in disease activity in BAV‐TAA.
Discussion
In this study, we examined the associations of arterial hemodynamic markers with TAA growth in patients with BAV and found that greater aortic stiffness, Zc, and Pf, as well as lower TAC and PAC (measures that reflect aortic health and function) were significantly associated with TAA expansion. This study is the first, to the best of our knowledge, to comprehensively evaluate arterial hemodynamics and its association with TAA growth in patients with BAV.
Current guidelines for patients with BAV recommend yearly surveillance to monitor ascending aorta dilatation.23 The aortic diameter at baseline is an important predictor of aortic expansion24, 25, 26, 27; however, it does not fully account for the risk of adverse outcomes,28, 29, 30 highlighting the need to improve risk stratification in BAV‐TAA. Furthermore, novel medical therapies aimed at containing TAA disease progression are urgently needed. To achieve this, we must identify biological markers and physiologic measures that may play a role in risk stratification and disease monitoring but also highlight an opportunity to intervene for susceptible patients with adverse aortic wall biology.
These gaps in clinical care motivated us to pursue alternative parameters for assessing TAA disease activity in BAV patients, focusing on arterial hemodynamics as correlates of aortic health. According to our hypothesis, we found that abnormalities in aortic stiffness and pulsatile arterial load, reflecting the health of the aortic wall and its pressure‐buffering function, were associated with faster aneurysm expansion. In contrast, measures of steady arterial load (reflecting resistive load imposed by peripheral arteries and arterioles), peripheral wave reflections and brachial blood pressure (the latter as the only measures typically available to clinicians in the office) had no independent relationship to TAA growth. This corroborates the notion that only the hemodynamic measures that track the health and function of the aorta are relevant for assessing TAA disease activity in BAV. Importantly, by comparing standardized β coefficients, we also demonstrated that measures of aortic stiffness and pulsatile arterial load were superior to baseline aneurysm size (the parameter currently use to guide TAA clinical decisions) in assessing aneurysm expansion, highlighting the need for better characterization of arterial hemodynamics to improve clinical evaluation of TAA disease activity.
Alterations in aortic stiffness and pulsatile arterial load in BAV may relate to adverse remodeling of the aneurysm wall. MMPs (matrix metalloproteinases) degrade matrix components and disrupt the media layer, affecting the structural flexibility of the aorta, and may play a role in the vascular disease in BAV patients.2, 4 As such, Fedak et al found increased MMP activity in the aortas of BAV patients, thus disrupting the aortic vessel wall integrity. Furthermore, the aortic media in patients with BAV has consistently been found to have focal abnormalities, such as elastin degradation, matrix disruption, loss of smooth muscle cells, and change in the content of collagen and glycoproteins.2, 4, 24 These degenerative processes can result in structural weakness of the aortic wall,4 reducing aortic compliance and increasing stiffness, thus predisposing patients to TAAs. These processes can be further exacerbated by altered hemodynamics caused by BAV, such as increased wall shear stress in certain regions of the aorta, resulting in accelerated degeneration of the aortic media31 and consequent stiffness of the aortic wall and accelerated growth of the aneurysm. Confirming these notions, a previous report demonstrated that aortic elasticity, assessed by M‐mode evaluation of the aortic root, is reduced in patients with BAV,32, 33 and the BAV predisposes patients to regional increases in wall shear stress.2, 34, 35, 36, 37, 38 Moreover, in a case–control study, Nistri et al found abnormal aortic elastic properties in patients with normally functioning BAVs compared with controls.39
Existing literature supports the concept of worse arterial health in BAV than controls. This observation helps put our findings into context, as these changes to the aortic wall structure and elasticity in BAV may impair the pressure‐buffering function of the aorta, leading to greater pulsatile arterial load. As such, it is possible that those patients with BAV‐TAA who have the greatest burden of abnormalities of the aortic wall may exhibit higher aortic stiffness and pulsatile load and, at the same time, be predisposed to accelerated aneurysm expansion. When added to existing cross‐sectional literature, our findings confirm that arterial hemodynamic measures associated with the health and function of the aorta may represent adequate markers of TAA disease activity in patients with BAV.
The other important findings from our study are (1) the lack of association between brachial blood pressure measures and aneurysm expansion and (2) the superiority of aortic stiffness and pulsatile arterial load measures over baseline TAA size in assessing aneurysm expansion. These observations are relevant because aneurysm size and brachial blood pressure are currently used for assessment of TAA‐related risk and clinical decisions about the TAA. Consequently, a significant opportunity exists to improve the clinical assessment of BAV‐TAA patients, and our data highlight that assessing aortic health, function, and hemodynamics allows us to better determine TAA disease activity in these individuals.
Limitations
The main limitation of our study is its retrospective nature, preventing us from making inferences regarding the causality or temporality of the associations found. Despite this, our observations add valuable, novel information to the existing literature and provide feasible nonsize arterial parameters that can be used in future prospective studies to enhance risk stratification of BAV‐associated TAA risk. In addition, the small sample size prevents us from correcting statistics for multiple testing. However, the concordance of our findings (ie, hemodynamic measures that were most closely related to aortic health and function were those associated with TAA growth in our study) enhance biological plausibility and motivate additional, larger studies of this topic. Furthermore, the small sample size precludes us from assessing the potential role of individual medication classes on aneurysm growth. This aspect remains amenable to further study in larger, population‐based cohorts. Not all participants had the same imaging modality at baseline and follow‐up. However, we have shown that our findings are independent of the concordant or discordant nature of the imaging modalities. In addition, this study reflects “real‐life” practice, increasing external validity. Last, TAA growth was used as the outcome of choice in our study, rather than acute aortic syndromes. Because acute aortic syndromes are not common among patients with known TAA undergoing careful surveillance,40 aneurysm growth is a more feasible surrogate end point for longitudinal studies and, as such, has been the outcome of choice for clinical trials in TAA.41, 42
Conclusions
In patients with TAA due to BAV, greater aortic stiffness and pulsatile arterial load are independently associated with faster aneurysm growth, whereas brachial blood pressure, wave reflections, and steady arterial load are not. Because accelerated aneurysm growth is a marker of disease activity and a risk factor for acute aortic syndromes, our findings highlight worse aortic wall and abnormal pressure‐buffering function, leading to greater pulsatile arterial load, as potentially clinically useful markers of TAA instability in BAV. Furthermore, the relevant hemodynamic markers can be obtained noninvasively in the office setting, increasing the clinical applicability of our findings. Our results motivate future, prospective studies of BAV patients with TAA to determine the potential role of assessing aortic stiffness and pulsatile arterial load in novel risk algorithms for TAA risk prediction. In addition, our findings support the need for studies targeting the aortic wall and its elastic properties as novel therapeutics to contain aneurysm expansion in BAV.
Sources of Funding
This research was supported by an Emerging Research Leaders Initiative grant from the Canadian Vascular Network and the Canadian Institutes of Health Research, and a Grant‐in‐Aid from the Heart and Stroke Foundation of Canada.
Disclosures
Coutinho is supported by a Clinician Scientist Stage I Award from the Heart and Stroke Foundation of Ontario and holds the Chair for Women's Heart Health at the University of Ottawa Heart Institute. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e010885 DOI: 10.1161/JAHA.118.010885.)
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–1929. [DOI] [PubMed] [Google Scholar]
- 3. Andreassi MG, Della Corte A. Genetics of bicuspid aortic valve aortopathy. Curr Opin Cardiol. 2016;31:585–592. [DOI] [PubMed] [Google Scholar]
- 4. Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106:900–904. [DOI] [PubMed] [Google Scholar]
- 5. Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM III, Enriquez‐Sarano M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. [DOI] [PubMed] [Google Scholar]
- 6. Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, Webb GD, Siu SC. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–1325. [DOI] [PubMed] [Google Scholar]
- 7. Shim CY, Cho IJ, Yang WI, Kang MK, Park S, Ha JW, Jang Y, Chung N. Central aortic stiffness and its association with ascending aorta dilation in subjects with a bicuspid aortic valve. J Am Soc Echocardiogr. 2011;24:847–852. [DOI] [PubMed] [Google Scholar]
- 8. O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658. [DOI] [PubMed] [Google Scholar]
- 9. Laurent S, Boutouryie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension. 2005;45:1050–1055. [DOI] [PubMed] [Google Scholar]
- 10. Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 1: pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension. 2010;56:555–562. [DOI] [PubMed] [Google Scholar]
- 11. Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure‐flow and pressure‐volume relations in humans. Hypertension. 2010;56:563–570. [DOI] [PubMed] [Google Scholar]
- 12. Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54:225–235. [PubMed] [Google Scholar]
- 13. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coutinho T, Pelikka PA, Bailey KR, Turner ST, Kullo IJ. Sex differences in the associations of hemodynamic load with left ventricular hypertrophy and concentric remodeling. Am J Hypertens. 2016;29:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum. Circulation. 2010;122:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laurent S, Cockroft J, Bortel LV, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 18. Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol. 1986;251:H588–H600. [DOI] [PubMed] [Google Scholar]
- 19. Tartiere JM, Logeart D, Safar ME, Cohen‐Solal A. Interaction between pulse wave velocity, augmentation index, pulse pressure and left ventricular function in chronic heart failure. J Hum Hypertens. 2006;20:213–219. [DOI] [PubMed] [Google Scholar]
- 20. Hughes AD, Park C, Davies J, Francis D, McG Thom SA, Mayet J, Parker KH. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS One. 2013;8:e59371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheung K, Boodhwani M, Chan K, Beauchesne L, Dick A, Coutinho T. Thoracic aortic aneurysm growth: role of sex and aneurysm etiology. J Am Heart Assoc. 2017;6:e003792 DOI: 10.1161/JAHA.116.003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population‐based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. [DOI] [PubMed] [Google Scholar]
- 23. Boodhwani M, Andelfinger G, Leipsic J, Lindsay T, McMurtry MS, Therrien J, Siu SC; Canadian Cardiovascular Society . Canadian Cardiovascular Society position statement on the management of thoracic aortic disease. Can J Cardiol. 2014;30:577–589. [DOI] [PubMed] [Google Scholar]
- 24. Dore A, Brochu MC, Baril JF, Guertin MC, Mercier LA. Progressive dilation of the diameter of the aortic root in adults with a bicuspid aortic valve. Cardiol Young. 2003;13:526–531. [PubMed] [Google Scholar]
- 25. Shimada I, Rooney SJ, Pagano D, Farneti PA, Davies P, Guest PJ, Bonser RS. Prediction of thoracic aortic aneurysm expansion: validation of formulae describing growth. Ann Thorac Surg. 1999;67:1968–1980. [DOI] [PubMed] [Google Scholar]
- 26. Della Corte A, Bancone C, Buonocore M, Dialetto G, Covino FE, Manduca S, Scognamiglio G, D'Oria V, De Feo M. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging. 2013;6:1301–1310. [DOI] [PubMed] [Google Scholar]
- 27. Detaint D, Michelena HI, Nkomo VT, Vahanian A, Jondeau G, Sarano ME. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: a comparative study with Marfan syndrome and degenerative aortopathy. Heart. 2014;100:126–134. [DOI] [PubMed] [Google Scholar]
- 28. Parish LM, Gorman JH III, Kahn S, Plappert T, St John‐Sutton MG, Bavaria JE, Gorman RC. Aortic size in acute type A dissection: implications for preventive ascending aortic replacement. Eur J Cardiothorac Surg. 2009;35:941–946. [DOI] [PubMed] [Google Scholar]
- 29. Pape LA, Tsai TT, Isselbacher EM, Oh JK, O'Gara PT, Evangelista A, Fattori R, Meinhardt G, Trimarchi S, Bossone E, Suzuki T, Cooper JV, Froehlich JB, Nienaber CA, Eagle KA; International Registry of Acute Aortic Dissection (IRAD) Investigators . Aortic diameter ≥ 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2007;116:1120–1127. [DOI] [PubMed] [Google Scholar]
- 30. Kim EK, Choi SH, Sung K, Kim WS, Choe YH, Oh JK, Kim DK. Aortic diameter predicts acute type A aortic dissection in patients with Marfan syndrome but not in patients without Marfan syndrome. J Thorac Cardiovasc Surg. 2014;147:1505–1510. [DOI] [PubMed] [Google Scholar]
- 31. Ng ACT, Wang WYS, Delgado V, Bax JJ. Bicuspid aortic valve disease: new insights. Struct Heart. 2017;1:9–17. [Google Scholar]
- 32. Nistri S, Grande‐Allen J, Noale M, Basso C, Siviero P, Maggi S, Crepaldi G, Thiene G. Aortic elasticity and size in bicuspid aortic valve syndrome. Eur Heart J. 2008;29:472–479. [DOI] [PubMed] [Google Scholar]
- 33. Grotenhuis HB, Ottenkamp J, Westenberg JJ, Bax JJ, Kroft LJ, de Roos A. Reduced aortic elasticity and dilatation are associated with aortic regurgitation and left ventricular hypertrophy in nonstenotic bicuspid aortic valve patients. J Am Coll Cardiol. 2007;49:1660–1665. [DOI] [PubMed] [Google Scholar]
- 34. Girdauskas E, Borger MA, Secknus MA, Girdauskas G, Kuntze T. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one‐sided argument. Eur J Cardiothorac Surg. 2011;39:809–814. [DOI] [PubMed] [Google Scholar]
- 35. Barker AJ, Markl M, Bürk J, Lorenz R, Bock J, Bauer S, Schulz‐Menger J, von Knobelsdorff‐Brenkenhoff F. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging. 2012;5:457–466. [DOI] [PubMed] [Google Scholar]
- 36. Hope MD, Hope TA, Meadows AK, Ordovas KG, Urbania TH, Alley MT, Higgins CB. Bicuspid aortic valve: four‐dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255:53–61. [DOI] [PubMed] [Google Scholar]
- 37. Cotrufo M, Della Corte A, De Santo LS, Quarto C, De Feo M, Romano G, Amarelli C, Scardone M, Di Meglio F, Guerra G, Scarano M, Vitale S, Castaldo C. Different patterns of extracellular matrix protein expression in the convexity and the concavity of the dilated aorta with bicuspid aortic valve: preliminary results. J Thorac Cardiovasc Surg. 2005;130:504–511. [DOI] [PubMed] [Google Scholar]
- 38. Della Corte A, Quarto C, Bancone C, Castaldo C, Di Meglio F, Nurzynska D, De Santo LS, De Feo M, Scardone M, Montagnani S, Cotrufo M. Spatiotemporal patterns of smooth muscle cell changes in ascending aortic dilatation with bicuspid and tricuspid aortic valve stenosis: focus on cell‐matrix signaling. J Thorac Cardiovasc Surg. 2008;135:8–18. [DOI] [PubMed] [Google Scholar]
- 39. Nistri S, Sorbo MD, Basso C, Thiene G. Bicuspid aortic valve: abnormal aortic elastic properties. J Heart Valve Dis. 2002;11:369–373; discussion 73‐4. [PubMed] [Google Scholar]
- 40. Filardo G, Powell JT, Martinez MA, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2012;3(3):CD001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC III. Angiotensin II blockade and aortic‐root dilation in Marfan's syndrome. N Engl J Med. 2008;358:2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shores J, Berger KA, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long‐term beta‐adrenergic blockade in Marfan's syndrome. N Engl J Med. 1994;330:1335–1341. [DOI] [PubMed] [Google Scholar]


