Abstract
Background
This longitudinal study aims to characterize longitudinal body mass index (BMI) trajectories during young adulthood (20–40 years) and examine the impact of level‐independent BMI trajectories on hypertension risk.
Methods and Results
The cohort consisted of 3271 participants (1712 males and 1559 females) who had BMI and blood pressure (BP) repeatedly measured 4 to 11 times during 2004 to 2015 and information on incident hypertension. Four distinct trajectory groups were identified using latent class growth mixture model: low‐stable (n=1497), medium‐increasing (n=1421), high‐increasing (n=291), sharp‐increasing (n=62). Model‐estimated levels and linear slopes of BMI at each age point between ages 20 and 40 were calculated in 1‐year intervals using the latent class growth mixture model parameters and their first derivatives, respectively. Compared with the low‐stable group, the hazard ratios and 95% CI were 2.42 (1.88, 3.11), 4.25 (3.08, 5.87), 11.17 (7.60, 16.41) for the 3 increasing groups, respectively. After adjusting for covariates, the standardized odds ratios and 95% CI of model‐estimated BMI level for incident hypertension increased in 20 to 35 years, ranging from 0.80 (0.72–0.90) to 1.59 (1.44–1.75); then decreased gradually to 1.54 (1.42–1.68). The standardized odds ratios of level‐adjusted linear slopes increased from 1.22 (1.09–1.37) to 1.79 (1.59–2.01) at 20 to 24 years; then decreased rapidly to 1.12 (0.95–1.32).
Conclusions
These results indicate that the level‐independent BMI trajectories during young adulthood have significant impact on hypertension risk. Age between 20 and 30 years is a crucial period for incident hypertension, which has implications for early prevention.
Keywords: Body mass index, Hypertension, Trajectory, Longitudinal study
Subject Categories: Obesity, Epidemiology, Hypertension
Short abstract
See Editorial Roush
Clinical Perspective
What Is New?
Four distinct trajectory groups of body mass index were identified, and the trajectory group membership was associated with the risk of incident hypertension independently of the body mass index levels.
The significant impact of level‐independent body mass index slopes during young adulthood on incident hypertension was found, and a critical period was determined.
What Are the Clinical Implications?
These findings emphasize the importance of controlling body mass index in young adulthood to prevent the incidence of hypertension later in life.
These observations suggest that the age of 20 to 30 years is a critical age window for obesity control to reduce hypertension risk.
Introduction
Hypertension is a major risk factor for cardiovascular diseases, and its prevalence has increased significantly in China in recent decades.1, 2 Hypertension has brought a heavy burden to the health system in Chinese population.3 Increased body mass index (BMI) or obesity is regarded as an important risk factor for hypertension. However, the BMI‐hypertension link over the life course has not been well characterized,4 especially during early stage of adult life. Many studies have shown that both high BMI level and excessive weight gain were associated with increased incidence of hypertension.5, 6, 7, 8 Most previous studies focused on BMI in a single or limited number of measurements, ignoring the dynamic BMI changes during the life course.
BMI trajectories reflect the potential BMI dynamic changing patterns during the life course. Despite numerous studies on the association between BMI and hypertension, there are significant gaps in understanding the relationship between BMI trajectories and hypertension during young adulthood. Several longitudinal studies have shown BMI trajectories in childhood, the first 2 decades in life, and late adulthood in relationship to elevated blood pressure (BP) and hypertension.9, 10, 11, 12 Buscot et al reported 6 distinct child‐to‐adult BMI trajectories and their associations with adult cardiometabolic risk.13 However, few studies have demonstrated BMI trajectories and their associations with incident hypertension during young adulthood. We hypothesized that participants in different trajectories have different levels of risk for hypertension in early adult life, and the rate at which BMI increases during this period may be predictive of incident hypertension, independent of BMI absolute values.
By using repeated measurements of BMI measured 4 to 11 times during 2004–2015 in a longitudinal cohort from Chinese population, the current study aimed to identify potential BMI dynamic changing trajectories during young adulthood, examine the association of BMI trajectories with incident hypertension and determine the potentially critical period for the development of hypertension related to rate of change in BMI.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Cohort
At the Health Management Center of Jining Medical University Hospital, 75 476 individuals, aged 20 to 80 years, underwent health examinations between 2004 and 2015. A total of 3271 participants (1712 males and 1559 females), aged 20 to 40 years, were identified based on the number of measurements of BMI and formed the longitudinal cohort in the current study. The mean baseline age was 28.7 years (20 to 37 years at baseline and 24 to 40 years at follow‐up). The mean follow‐up year was 5.5 (range=2.2 to 11.3 years). All the participants in this cohort were normotensive at the first examination and had BMI and blood pressure (BP) repeatedly measured 4 to 11 times during the follow‐up period. BMI data after the onset of hypertension (outcome) were not included in analyses.
The study protocol was approved by the ethics committee of School of Public Health, Shandong University, Jinan, China. Written informed consent was obtained from all participants.
Examinations
Height and weight were measured while the subjects were wearing light clothing without shoes. BMI was calculated as weight (kg) divided by the square of height (m). Systolic BP (SBP) and diastolic BP (DBP) were recorded 3 times on the right upper arm using a mercury sphygmomanometer in a sitting position after a 5‐minute rest. The intra‐class correlations among 3 readings of BP measurements were 0.91 and 0.86 for SBP and DBP, respectively. The digit preference of blood pressure measurements was presented in Table S1. Information on smoking and alcohol use was obtained by means of a staff administered standard questionnaire. Smoking and drinking were defined as having a history of smoking at least 1 cigarette per day and consuming alcohol every day, respectively.
Outcome
Hypertension was defined as SBP/DBP ≥140/90 mm Hg or taking antihypertensive medication or diagnosis by medical records. The health examination database was linked to databases from the Office for Medical Insurance in Shandong Province, hospital admissions, and vital statistics from the Provincial Center for Disease Control and Prevention using a unique identification number for each participant. The histories of antihypertension medication and hypertension diagnosis were collected in the database, and the diagnostic date of hypertension was defined as the earliest recorded date.
Statistical Methods
The latent class growth mixed model (LCGMM)14 was used to identify different trajectory patterns of BMI. The latent class trajectories of BMI were specified as a function of age (centered to 31 years, the mean age of the cohort), with sex as a covariate. Multiple LCGMMs with different trajectory shapes including linear and non‐linear parameters were tested. Repeated trajectory analyses were performed to identify the latent classes by changing the number of groups from 2 to 5, with the same starting values calculated from the 1‐group model. The class memberships of subjects were determined by a latent discrete random variable, and its probability is described using a multinomial logistic model according to covariates. The shapes and optimal number of groups were determined by the following criteria15: (1) Bayesian information criterion decreased at least 20; (2) high mean posterior class membership probabilities (>0.65); (3) high mean posterior probabilities (>0.7). Estimation of latent class models were performed with lcmm (version 1.7.8) package in R (version 3.4.3). To avoid convergence towards local maxima, LCGMM models with 2 to 5 classes were performed for several times with different sets of random starting value based on 1‐class model. Finally, the best fitting model based on above criteria was cubic trajectories of 4 groups, and the final model was described as:
where υ=(υ0g, υ1g, υ2g, υ3g) is a vector of fixed‐effect parameters in the group “g”, u=(u0ig, u1ig, u2ig, u3ig) is a vector of random effect parameters of the individual “i” in the group “g”, εij is an unknown error term.
Cox proportional hazard models were used to investigate the association between the trajectory group membership and incident hypertension, adjusted for baseline age, sex, baseline BMI, baseline SBP, smoking, and alcohol drinking.
The LCGMM computes estimates of the cubic curve parameters, including fixed‐effect parameters (for a group) and random‐effect parameters (individual specific). The random‐effect coefficients represent the difference between the fixed‐effect parameters and the observed values for each individual. Then by combining the fixed and individual‐specific random‐effect parameters, 3271 different sets of the curve parameters were generated for each participant of the study cohort. The model‐estimated BMI levels and linear slopes were calculated at each age point in 1‐year intervals according to the model parameters and their first derivatives, respectively. Logistic regression analyses were used to examine the association of model‐estimated levels and linear slopes of BMI at each age point with incident hypertension. Before logistic regression analyses, the model‐estimated linear slope values of BMI at each age point were adjusted for their corresponding BMI levels and covariates mentioned above by regression residual analyses to avoid collinearity of levels and linear slopes in the same model. Standardized odds ratios (ORs) of levels and level‐adjusted slopes of BMI for incident hypertension were estimated.
Results
Table 1 summarizes the study variables by follow‐up incident hypertension. Sex, baseline BMI, baseline SBP, baseline DBP, smoking, and alcohol drinking were significantly different between normotensives and hypertensives. During the follow‐up period, 385 incident primary hypertensives were identified, with an incidence density 21.2 per 1000 person‐years. The exclusive percentages of incident hypertension identified by the 3 different methods (BP≥140/90, diagnosis by medical records, taking medications) were 10.4%, 1.0%, 0.4%, respectively. Table S2 summarizes the baseline and follow‐up characteristics by hypertension status at follow‐up. Hypertensives had higher follow‐up BMI, higher proportion of smokers and drinkers than normotensives. Table S3 presents the baseline characteristics of participants included and excluded.
Table 1.
Baseline Characteristics by Incident Hypertension At Follow‐Up
| Variable | Total | Normotensives | Incident Hypertensives | P Value |
|---|---|---|---|---|
| n | 3271 | 2886 | 385 | |
| Age, y | 28.7 (4.7) | 28.8 (4.7) | 28.3 (4.3) | 0.075 |
| Males, n (%) | 1712 (52.3) | 1435 (49.7) | 277 (72.0) | <0.001 |
| BMI, kg/m2 | 22.4 (3.2) | 22.2 (3.1) | 23.3 (3.3) | <0.001 |
| SBP, mm Hg | 114.4 (11.1) | 113.9 (11.0) | 118.1 (11.2) | <0.001 |
| DBP, mm Hg | 72.0 (8.0) | 71.5 (7.9) | 75.6 (7.8) | <0.001 |
| Smoker, n (%) | 455 (13.9) | 367 (12.7) | 88 (22.9) | <0.001 |
| Drinker, n (%) | 641 (19.6) | 484 (16.8) | 157 (40.8) | <0.001 |
| Follow‐up, y | 5.5 (2.1) | 5.6 (2.2) | 4.9 (1.7) | <0.001 |
Study variables are presented as mean (SD) or n (%). BMI indicates body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table S4 shows the LCGMM results of fitting process. We fitted models from 1 class to 6 classes of linear, quadratic, and cubic curves. Results of 6 classes were not shown in this table because of low percentages of several group memberships (<1%). The model of quadratic curves with 5 groups was excluded because of the low percentage of group 3 (0.89%). According to the criteria mentioned above, a model of cubic parameters with 4 groups with lower Bayesian information criterion, relatively high posterior probabilities, and percentage of group memberships was chosen.
Figure 1 shows the 4 distinct trajectories of BMI, labeled as low‐stable (n=1497), medium‐increasing (n=1421), high‐increasing (n=291) and sharp‐increasing (n=62). In the low‐stable group, BMI persisted at a low level of 21 kg/m2 during young adulthood (20–40 years). In the medium‐increasing group, BMI increased gradually from 21 kg/m2 at age 20 years up to 25 kg/m2 at age 40 years. In the high‐increasing group, BMI increased gradually from 23 kg/m2 at age 20 years up to 29 kg/m2 at age 34 years and then kept stable up to age 40 years. In the sharp‐increasing group, BMI increased rapidly from 18 kg/m2 at age 20 years to 32 kg/m2 at age 40 years. The trajectory parameters (υ, u, β) were all significantly different from 0 (P<0.05). Table S5 shows curve parameters of fixed and random effects in the 4 trajectory groups. BMI trajectories in men and women were similar (Figure S1).
Figure 1.
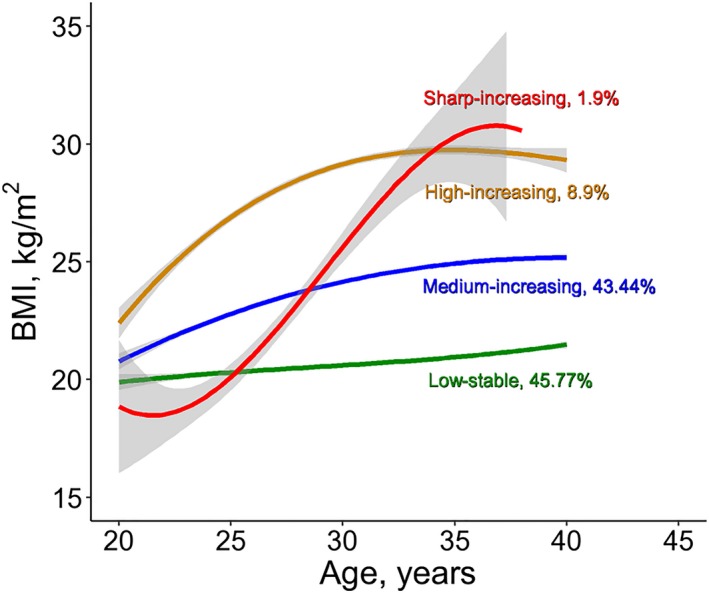
Trajectories of BMI during young adulthood. BMI indicates body mass index.
Table 2 summarizes the study variables at baseline by BMI trajectory group. Hypertension incidence densities were significantly different among the 4 BMI trajectory groups (P<0.001 for trend). Significant differences in sex, BMI, SBP, DBP, smoking, and alcohol drinking among the 4 trajectory groups were observed at baseline (P<0.001). Because of the lower proportion of males, the low‐stable and sharp‐increasing group had relatively fewer smokers and drinkers than the other 2 groups.
Table 2.
Characteristics at Baseline and Incident Hypertension At Follow‐Up By BMI Trajectory Groups
| Variable | Low‐Stable (n=1497) | Medium‐Increasing (n=1421) | High‐Increasing (n=291) | Sharp‐Increasing (n=62) | P Trend |
|---|---|---|---|---|---|
| Baseline | |||||
| Age, y | 29.1 (4.8) | 28.8 (4.5) | 27.3 (4.5) | 25.0 (2.4) | <0.001 |
| Males, n (%) | 276 (18.4) | 1177 (82.8) | 253 (86.9) | 6 (9.7) | <0.001 |
| BMI, kg/m2 | 20.5 (2.1) | 23.4 (2.3) | 27.3 (3.3) | 20.3 (2.4) | <0.001 |
| SBP, mm Hg | 110.2 (10.2) | 117.7 (10.3) | 120.5 (10.6) | 110.6 (10.5) | <0.001 |
| DBP, mm Hg | 70.1 (8.0) | 73.5 (7.6) | 74.1 (7.9) | 71.5 (7.5) | <0.001 |
| Smoker, n (%) | 81 (5.4) | 306 (21.5) | 67 (23.0) | 1 (1.6) | <0.001 |
| Drinker, n (%) | 99 (6.6) | 447 (31.5) | 92 (31.6) | 3 (4.8) | <0.001 |
| Follow‐up, y | 5.5 (2.2) | 5.6 (2.1) | 5.2 (2.1) | 4.6 (1.6) | <0.001 |
| Follow‐up | |||||
| Incident hypertension, n (%) | 86 (5.7) | 206 (14.5) | 65 (22.3) | 28 (45.2) | <0.001 |
| Hypertension incidence density per 1000 person‐years (95% CI) | 10.5 (8.4, 12.9) | 25.8 (22.4, 29.6) | 42.6 (32.9, 54.3) | 97.3 (64.6, 140.6) | <0.001 |
Study variables are presented as mean (SD) or n (%). BMI indicates body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 3 presents hazard ratios and 95% CI for the association between the trajectory group membership and incident hypertension. Compared with the reference (low‐stable) group, the hazard ratios (95% CI) for the medium‐increasing, high‐increasing, and sharp‐increasing groups were 1.67 (1.19, 2.37), 2.78 (1.6, 4.82), 13.80 (9.12, 20.87), respectively, in model 2, with adjustment for age, sex, and baseline BMI level. After additional adjustment for baseline SBP level, smoking, and alcohol drinking, the trajectory group membership still had a positive association with incident hypertension.
Table 3.
Hazard Ratios and 95% CIs of BMI Trajectory Groups for Incident Hypertension
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Trajectory groups | ||||
| Low‐stable | Reference | Reference | Reference | Reference |
| Medium‐increasing | 2.42 (1.88, 3.11) | 1.67 (1.19, 2.37) | 1.63 (1.15, 2.31) | 1.67 (1.18, 2.36) |
| High‐increasing | 4.25 (3.08, 5.87) | 2.78 (1.60, 4.82) | 2.86 (1.64, 4.99) | 2.98 (1.71, 5.20) |
| Sharp‐increasing | 11.17 (7.60, 16.41) | 13.80 (9.12, 20.87) | 13.29 (8.75, 20.19) | 12.33 (8.06, 18.85) |
| Covariates | ||||
| Age, ya | ··· | 1.04 (1.01, 1.07) | 1.05 (1.02, 1.08) | 1.04 (1.01, 1.07) |
| Female | ··· | 0.61 (0.45, 0.82) | 0.72 (0.53, 0.97) | 0.97 (0.69, 1.37) |
| Baseline BMIa | ··· | 1.07 (0.91, 1.25) | 0.98 (0.83, 1.16) | 0.95 (0.81, 1.12) |
| Baseline SBPa | ··· | ··· | 1.40 (1.24, 1.58) | 1.39 (1.23, 1.56) |
| Smoker | ··· | ··· | ··· | 1.15 (0.89, 1.49) |
| Drinker | ··· | ··· | ··· | 1.77 (1.39, 2.25) |
| T2DM | ··· | ··· | ··· | 1.84 (0.88, 3.86) |
BMI indicates body mass index; SBP, systolic blood pressure; T2DM, Type 2 diabetes mellitus.
The standard deviations of continuous covariates (age, baseline BMI, baseline SBP) were 4.7 years, 3.2 kg/m2, 11.1 mm Hg, respectively.
Table S6 shows differences of model‐estimated levels and linear slopes between normotensives and hypertensives at each age point. For the model‐estimated levels, differences between normotensives and hypertensives were significant (P<0.01), except for age 22 to 24 years. For model‐estimated linear slopes, the differences between normotensives and hypertensives were all significant at each age point (P<0.001). The Pearson correlations between the model‐estimated and observed values of BMI levels were all significant (P<0.001) ranging from 0.83 to 0.89 at age points from 20 to 40 years. Correlations between the model‐estimated and observed slopes were not calculated because BMI was not measured annually, and the observed values of BMI slopes were not available.
Figure 2 presents odds ratio (OR) and 95% CI of model‐estimated levels and level‐adjusted linear slopes of BMI for incident hypertension, with adjustment for sex, smoking, drinking, and baseline SBP. The correlation coefficients of model‐estimated levels and slopes ranged from −0.38 to 0.42 (P<0.001 for all age points except for P=0.268 at age 33 and P=0.006 at age 34). In the logistic regression analyses for BMI linear slopes, the level‐adjusted standardized ORs were calculated with additional adjustment for their corresponding levels of BMI at each age point. The standardized ORs of model‐estimated BMI levels increased gradually from 0.80 (0.72–0.90) to 1.59 (1.44–1.75), then decreased gradually to 1.54 (1.42–1.68) during young adulthood. The association between model‐estimated BMI levels and incident hypertension became positive at age ≥27 years. The standardized ORs of level‐adjusted linear slopes increased from 1.22 (1.09–1.37) to1.79 (1.59–2.01) between ages 20 to 24 years; then decreased to 1.12 (0.95–1.32). The level‐adjusted linear slopes at age 20 to 37 years were significantly associated with incident hypertension.
Figure 2.
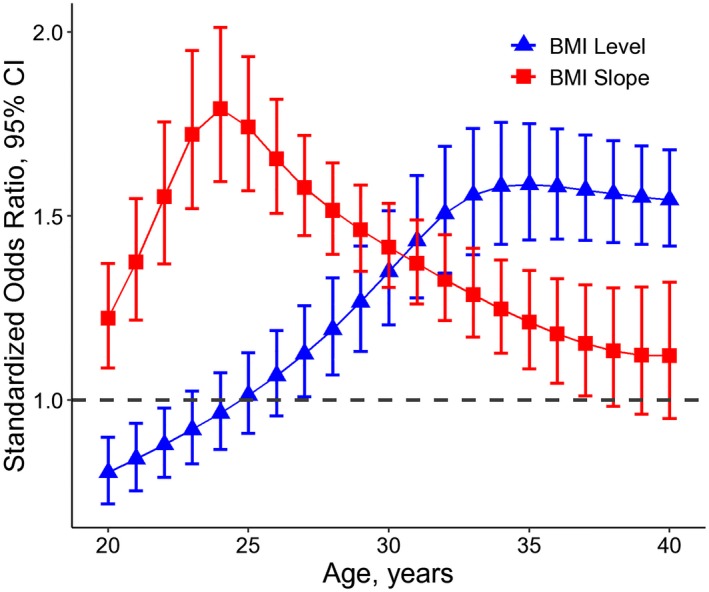
Standardized odds ratio and 95% CI of model‐estimated levels and level‐adjusted linear slopes of BMI during young adulthood by age for incident hypertension, adjusting for sex, smoking, alcohol drinking, and baseline SBP. BMI indicates body mass index; SBP, systolic blood pressure.
Figure S2 shows the association analyses of observed BMI levels with incident hypertension. The trends in ORs by ages were similar to those of model‐estimated BMI levels in Figure 2. Figure S3 shows the association analyses of hypertension with the BMI levels and slopes estimated in a quadratic curve model. The trends in ORs estimated in the quadratic curve model were similar to those in the cubic curve model as shown in Figure 2.
Discussion
In this longitudinal study, we identified 4 distinct trajectories of BMI during young adulthood in a Chinese cohort and found significant associations of BMI trajectory groups with incident hypertension. We further calculated the model‐estimated BMI levels and slopes at each age point in 1‐year intervals and examined their relationship with incident hypertension. Despite the available evidence on the predictive value of BMI levels for later life hypertension, no previous studies have concurrently considered the importance of linear slopes and levels of BMI at different age points during young adulthood for prediction of adult hypertension. The observations of this study provide new insights into origins of obesity‐related hypertension in early adult life and emphasize the importance of the level‐independent BMI trajectories during ages 20 to 30 years for assessing hypertension risk in later life.
Four distinct trajectories of BMI were identified for young Chinese adults in the current study. In the low‐stable group, BMI stayed stable at around 21 kg/m,2 while BMI of the other 3 groups increased with different baseline levels and incremental slopes. Particularly, a group of participants whose BMI increased from the lowest to the highest level was characterized. Previous studies have characterized BMI trajectories during certain periods of the life course in different populations, such as American, Canadian, Finnish, Netherlander, and Japanese.11, 13, 16, 17, 18, 19, 20 Among these studies, BMI trajectories were identified either by age or by follow‐up time and thus provided different changing patterns and different group numbers (range: 3–6). To our knowledge, there are no data available for comparison on BMI trajectories during young adulthood in relationship to hypertension risk in the Chinese population.
Notably, we identified a sharp‐increasing BMI growth trajectory, with BMI increasing rapidly from a low level to the obesity level. Although its proportion (1.9%) was small, our Cox regression analyses showed that the sharp‐increasing trajectory group had a markedly higher risk of hypertension than the low‐stable trajectory group, with a hazard ratio value as high as 12.33. The observations from this and other studies4, 21, 22 suggest that higher rate of change in body weight is important for the development of hypertension. For the medium‐increasing and high‐increasing groups, BMI levels instead of the rate of increase were considered the major difference compared with the low‐stable group, which indicated that high BMI levels in young adulthood were also a risk factor for hypertension. The results and concept are consistent with previous studies on the association between BMI levels and hypertension.4, 23, 24, 25, 26
In this study cohort, the average model‐estimated BMI levels increased with age from 21.9 to 26.1 years, while the average model‐estimated BMI slopes kept decreasing with age ranging from 0.42 to 0.14. Notably, model‐estimated BMI levels and linear slopes were highly correlated. Our association analyses showed that the level‐adjusted linear slopes had higher ORs than model‐estimated BMI levels before age 30 years. This phenomenon indicated that although BMI level is recognized as an important risk factor for hypertension, BMI slope which reflected the increasing velocity of body weight is a better predictor of hypertension in young adulthood. Further, a critical period was found at age 20 to 30 years by the analyses of level‐adjusted slopes. The results indicate that people whose BMI increases rapidly during age 20 to 30 years have a considerably higher risk of developing hypertension in later life, and BMI slope rather than BMI level during young adulthood is a better predictor for hypertension. To date, no data are available for comparison with our studies on the rate of increase in BMI in a critical window during young adulthood in relationship to the risk of hypertension.
According to the critical period model proposed by John Lynch and Ben‐Shlomo in the life‐course epidemiology theory,27, 28, 29 exposure at a specific period in the life span has a long‐lasting effect on the anatomical structure and physiological function that may eventually result in disease. People with higher increasing slope of BMI at age 20 to 30 years might have a series of changes in physiology and metabolism, including aorta root size increasing, early vascular aging and early endothelial dysfunction.30, 31, 32 Some of these changes may have long‐lasting and cumulative effects on the risk of developing hypertension in young adulthood. The increasement of BMI may lead to possible vascular alterations in young adulthood. For instance, significantly high prevalence of early vascular aging in age classes <40 years has been reported.31 Early endothelial dysfunction may also play an important role in incident hypertension in young adulthood.32 Early vascular aging and endothelial dysfunction may be caused by possible impact of body size on aorta root size. Aorta root size may play a causative role in the pathogenesis of systolic hypertension.30
For the sensitivity analysis, we repeated the association analyses of observed BMI levels with incident hypertension (Figure S2). The trends in ORs by ages were similar to those of model‐estimated BMI levels (Figure 2). The strong correlations of the model‐estimated with observed values of BMI levels and the similar trends in ORs of the estimated and observed BMI levels for hypertension showed the degree of accuracy of the model‐estimated BMI levels. In addition, we also did the association analyses of hypertension with the BMI levels and slopes estimated in a quadratic curve model (Figure S3). The trends in ORs estimated in the quadratic curve model were substantially similar to those in the cubic curve model as shown in Figure 2 although the magnitudes of ORs were slightly smaller as estimated in the quadratic curve than in the cubic curve.
The strengths of this study included the large study sample size, the availability of repeated measures of study variables over time and the use of LCGMM. The LCGMM can identify distinct trajectory subgroups to test the association of the trajectories with health outcomes in later life and allows for model‐estimated BMI slope parameters for each individual for the attempt to determine the critical age period. On the other hand, the current study had a few limitations. Firstly, the definition of incident hypertension was partly according to the medical records from the available database, and ambulatory blood pressure data were not available for the diagnosis of hypertension. Some incident hypertension patients have been missed. Secondly, data were from routine health examinations. The findings in this cohort may not be generalizable to the general population. Thirdly, the sharp‐increasing group had the shortest follow‐up years. The findings of this study need to be validated in further population studies.
Perspectives
The current study identified four distinct trajectories of BMI during young adulthood in the Chinese population and found significant associations of BMI trajectory groups with incident hypertension. The level‐independent rate of changes in BMI during young adulthood has an important impact on the development of hypertension in later life. Age between 20 and 30 years is a crucial period for the development of hypertension. The observations from this study provide new insights into the understanding of hypertension development and emphasize the importance of the level‐independent increasing velocity of BMI during ages 20 to 30 years for assessing hypertension risk in later life. Public health intervention should be underlined among young adults to prevent hypertension, especially for those who have high increasing velocity of body weight.
Sources of Funding
This study was supported by grants 81673271 and 81773547 from National Natural Science Foundation of China and the Young Scholars Program of Shandong University (2017WLJH36).
Disclosures
None.
Supporting information
Table S1. Digit Preference of Blood Pressure Measurements
Table S2. Descriptive Data of Baseline and Follow‐Up Characteristics by Incident Hypertension at Follow‐Up
Table S2. Descriptive Data of Baseline and Follow‐Up Characteristics by Incident Hypertension at Follow‐Up
Table S3. Baseline Characteristics of Participants Included and Excluded
Table S4. Latent Class Growth Mixture Model Results of Model Fitting Process
Table S5. Parameter Estimates for the Best Fitting 4‐Class Cubic Latent Class Growth Mixture Model
Table S6. Model‐Estimated Levels and Linear Slopes of BMI in Means (SD) by Incident Hypertension at Follow‐Up
Figure S1. Trajectories of body mass index by sex.
Figure S2. Standardized odds ratio and 95% CI of observed BMI levels for incident hypertension by age, adjusting for sex, smoking, alcohol drinking and baseline SBP.
Figure S3. Standardized odds ratio and 95% CI of model‐estimated levels and level‐adjusted linear slopes of BMI from a 4‐group quadratic model by age for incident hypertension, adjusting for sex, smoking, alcohol drinking, and baseline SBP.
Acknowledgments
This study is a joint effort of many investigators and staff members, and their contribution is gratefully acknowledged. We especially thank the Health Management Center of Jining Medical University Hospital and adults who participated in this study over years.
(J Am Heart Assoc. 2019;8:e011937 DOI: 10.1161/JAHA.119.011937.)
References
- 1. Li Y, Yang L, Wang L, Zhang M, Huang Z, Deng Q, Zhou M, Chen Z, Wang L. Burden of hypertension in China: a nationally representative survey of 174,621 adults. Int J Cardiol. 2017;227:516–523. [DOI] [PubMed] [Google Scholar]
- 2. Fang L, Song J, Ma Z, Zhang L, Jing C, Chen D. Prevalence and characteristics of hypertension in mainland Chinese adults over decades: a systematic review. J Hum Hypertens. 2014;28:649–656. [DOI] [PubMed] [Google Scholar]
- 3. Ye R, Liu K, Zhang Z, Gong S, Chen X. Health‐related quality of life of hypertension in China: a systematic review and meta‐analysis. J Cardiovasc Med. 2018;19:430–438. [DOI] [PubMed] [Google Scholar]
- 4. Shihab HM, Meoni LA, Chu AY, Wang N‐Y, Ford DE, Liang K‐Y, Gallo JJ, Klag MJ. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation. 2012;126:2983–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Juhaeri , Stevens J, Chambless LE, Tyroler HA, Rosamond W, Nieto FJ, Schreiner P, Jones DW, Arnett D. Associations between weight gain and incident hypertension in a bi‐ethnic cohort: the Atherosclerosis Risk in Communities Study. Int J Obes Relat Metab Disord. 2002;26:58–64. [DOI] [PubMed] [Google Scholar]
- 6. Curtis AB, Strogatz DS, James SA, Raghunathan TE. The contribution of baseline weight and weight gain to blood pressure change in African Americans: the Pitt County Study. Ann Epidemiol. 1998;8:497–503. [DOI] [PubMed] [Google Scholar]
- 7. Williams PT. Increases in weight and body size increase the odds for hypertension during 7 years of follow‐up. Obesity (Silver Spring). 2008;16:2541–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stringer MD, Dhawan A, Davenport M, Mieli‐Vergani G, Mowat AP, Howard ER. Choledochal cysts: lessons from a 20 year experience. Arch Dis Child. 1995;73:528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ziyab AH, Karmaus W, Kurukulaaratchy RJ, Zhang H, Arshad SH. Developmental trajectories of Body Mass Index from infancy to 18 years of age: prenatal determinants and health consequences. J Epidemiol Community Health. 2014;68:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Munthali RJ, Kagura J, Lombard Z, Norris SA. Childhood adiposity trajectories are associated with late adolescent blood pressure: birth to twenty cohort. BMC Public Health. 2016;16:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lear SA, Gotay CC, Richardson CG, Tu AW, Louise CM. Body mass index trajectories from ages 1 to 20 : results from two nationally representative Canadian longitudinal cohorts. Obesity (Silver Spring). 2015;23:1703–1711. [DOI] [PubMed] [Google Scholar]
- 12. Pryor LE, Tremblay RE, Boivin M, Touchette E, Dubois L, Genolini C, Liu XC, Falissard B, Cote SM. Developmental trajectories of body mass index in early childhood and their risk factors an 8‐year longitudinal study. Arch Pediatr Adolesc Med. 2011;165:906–912. [DOI] [PubMed] [Google Scholar]
- 13. Buscot M‐J, Thomson RJ, Juonala M, Sabin MA, Burgner DP, Lehtimäki T, Hutri‐Kähönen N, Viikari JSA, Raitakari OT, Magnussen CG. Distinct child‐to‐adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur Heart J. 2018;39:2263–2270. [DOI] [PubMed] [Google Scholar]
- 14. Proust‐Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Softw. 2015;78:1–56. [Google Scholar]
- 15. Yuan Z, Yang Y, Wang C, Liu J, Sun X, Liu Y, Li S, Xue F. Trajectories of long‐term normal fasting plasma glucose and risk of coronary heart disease: a prospective cohort study. J Am Heart Assoc. 2018;7:e007607 DOI: 10.1161/JAHA.117.007607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dhana K, van Rosmalen J, Vistisen D, Ikram MA, Hofman A, Franco OH, Kavousi M. Trajectories of body mass index before the diagnosis of cardiovascular disease: a latent class trajectory analysis. Eur J Epidemiol. 2016;31:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heianza Y, Arase Y, Kodama S, Tsuji H, Tanaka S, Saito K, Hara S, Sone H. Trajectory of body mass index before the development of type 2 diabetes in Japanese men: Toranomon Hospital Health Management Center Study 15. J Diabetes Investig. 2015;6:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tirosh A, Shai I, Afek A, Dubnov‐Raz G, Ayalon N, Gordon B, Derazne E, Tzur D, Shamis A, Vinker S, Rudich A. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364:1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiu C‐J, Wray LA, Lu F‐H, Beverly EA. BMI change patterns and disability development of middle‐aged adults with diabetes: a dual trajectory modeling approach. J Gen Intern Med. 2013;28:1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morris TT, Northstone K, Howe LD. Examining the association between early life social adversity and BMI changes in childhood: a life course trajectory analysis. Pediatr Obes. 2016;11:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xie YJ, Ho SC, Su X, Liu Z. Changes in body weight from young adulthood to middle age and its association with blood pressure and hypertension: a cross‐sectional study in Hong Kong Chinese women. J Am Heart Assoc. 2016;5:e002361 DOI: 10.1161/JAHA.115.002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang M, Zhao Y, Sun H, Luo X, Wang C, Li L, Zhang L, Wang B, Ren Y, Zhou J, Han C, Zhang H, Yang X, Pang C, Yin L, Feng T, Zhao J, Hu D. Effect of dynamic change in body mass index on the risk of hypertension: results from the Rural Chinese Cohort Study. Int J Cardiol. 2017;238:117–122. [DOI] [PubMed] [Google Scholar]
- 23. Dhuper S, Abdullah RA, Weichbrod L, Mahdi E, Cohen HW. Association of obesity and hypertension with left ventricular geometry and function in children and adolescents. Obesity (Silver Spring). 2011;19:128–133. [DOI] [PubMed] [Google Scholar]
- 24. Klumbiene J, Sileikiene L, Milasauskiene Z, Zaborskis A, Shatchkute A. The relationship of childhood to adult blood pressure: longitudinal study of juvenile hypertension in Lithuania. J Hypertens. 2000;18:531–538. [DOI] [PubMed] [Google Scholar]
- 25. Someya Y, Tamura Y, Kohmura Y, Aoki K, Kawai S, Daida H. Slightly increased BMI at young age is a risk factor for future hypertension in Japanese men. PLoS One. 2018;13:e0191170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Attard SM, Herring AH, Howard AG, Gordon‐Larsen P. Longitudinal trajectories of BMI and cardiovascular disease risk: the national longitudinal study of adolescent health. Obesity (Silver Spring). 2013;21:2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ben‐Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. [PubMed] [Google Scholar]
- 28. Kuh D, Ben‐Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. J Epidemiol Community Health. 2003;57:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. [DOI] [PubMed] [Google Scholar]
- 30. Farasat SM, Morrell CH, Scuteri A, Ting C‐T, Yin FCP, Spurgeon HA, Chen C‐H, Lakatta EG, Najjar SS. Pulse pressure is inversely related to aortic root diameter implications for the pathogenesis of systolic hypertension. Hypertension. 2008;51:196–202. [DOI] [PubMed] [Google Scholar]
- 31. Cunha PG, Cotter J, Oliveira P, Vila I, Boutouyrie P, Laurent S, Nilsson PM, Scuteri A, Sousa N. Pulse wave velocity distribution in a cohort study. J Hypertens. 2015;33:1438–1445. [DOI] [PubMed] [Google Scholar]
- 32. Scuteri A, Tesauro M, Rizza S, Iantorno M, Federici M, Lauro D, Campia U, Turriziani M, Fusco A, Cocciolillo G, Lauro R. Endothelial function and arterial stiffness in normotensive normoglycemic first‐degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2008;18:349–356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Digit Preference of Blood Pressure Measurements
Table S2. Descriptive Data of Baseline and Follow‐Up Characteristics by Incident Hypertension at Follow‐Up
Table S2. Descriptive Data of Baseline and Follow‐Up Characteristics by Incident Hypertension at Follow‐Up
Table S3. Baseline Characteristics of Participants Included and Excluded
Table S4. Latent Class Growth Mixture Model Results of Model Fitting Process
Table S5. Parameter Estimates for the Best Fitting 4‐Class Cubic Latent Class Growth Mixture Model
Table S6. Model‐Estimated Levels and Linear Slopes of BMI in Means (SD) by Incident Hypertension at Follow‐Up
Figure S1. Trajectories of body mass index by sex.
Figure S2. Standardized odds ratio and 95% CI of observed BMI levels for incident hypertension by age, adjusting for sex, smoking, alcohol drinking and baseline SBP.
Figure S3. Standardized odds ratio and 95% CI of model‐estimated levels and level‐adjusted linear slopes of BMI from a 4‐group quadratic model by age for incident hypertension, adjusting for sex, smoking, alcohol drinking, and baseline SBP.


