Abstract
Background
Heart failure (HF) and atrial fibrillation (AF) are rising in prevalence and pose a substantial public health burden.
Methods and Results
We evaluated temporal trends specific to age, sex, race, and geographic region in rates of HF‐ and AF‐related morbidity, mortality, and years of potential life lost at age 75 years between 1991 and 2015 in the United States. For trends in hospitalization with a primary diagnosis of HF versus AF, we used data for patients aged ≥30 years from 1993 to 2014 from the Nationwide Inpatient Sample. For trends in death due to HF versus AF, we used data from 1991 to 2015 from the National Center for Health Statistics. Over the past 25 years, the age‐adjusted rates of hospitalization declined for HF (−1.72% per year) but increased for AF (+1.61% per year). HF mortality rates remained unchanged, whereas those for AF increased (+11.2% per year). Years of potential life lost increased for both HF (+0.4% per year) and AF (+9.8% per year). Trends in HF and AF morbidity rates varied moderately by age group, whereas mortality rates varied by age and race. HF and AF hospitalization and mortality rates rose for individuals aged <50 years. HF hospitalization rates declined in all 4 US census regions, whereas AF rates increased.
Conclusions
We observed divergent trends of decreasing hospitalization and mortality rates for HF versus increasing rates for AF. Variations in disease burden by race and geography warrant specific targeting of “at risk” groups in selected US regions. Additional studies are warranted to evaluate the rising burden of both conditions in younger adults.
Keywords: atrial fibrillation, epidemiology, heart failure, trends
Subject Categories: Epidemiology, Race and Ethnicity, Cardiovascular Disease
Clinical Perspective
What Is New?
We evaluated age‐, sex‐, race‐, and region‐specific temporal trends in morbidity and mortality due to heart failure (HF) and atrial fibrillation (AF) in the United States between 1991 and 2015.
Hospitalization rates for HF decreased and age‐adjusted death rates for HF remained constant, whereas both rates increased for AF.
These divergent trends for HF versus AF varied moderately by sex and race but varied markedly by region and age group, with both hospitalization and death rates due to HF and AF increasing in younger people aged <50 years.
What Are the Clinical Implications?
Variations in HF and AF disease burden by race and geography warrant specific targeting of “at risk” groups in selected US regions, along with additional studies to evaluate the rising burden of both conditions in younger adults.
Introduction
Morbidity and mortality due to cardiovascular disease have decreased substantially over the past 50 years in the United States and worldwide.1, 2, 3 Nevertheless, the prevalence of 2 cardiovascular conditions, namely, heart failure (HF) and atrial fibrillation (AF), continues to rise.1 These 2 conditions have been referred to as the “twin epidemics” of the present millennium.4 Recent epidemiological reports have evaluated trends in the incidence and prevalence of HF and AF in the community.5, 6, 7, 8, 9 These studies suggest a rising incidence of AF5, 6 that may be plateauing,10, 11 paralleled by a moderate decline in HF incidence.8, 9 The prevalence of both conditions has increased in recent decades, presumably because of the aging of the populations studied.6, 8 Other reports have used additional metrics to characterize the burden of HF and AF, such as hospitalizations rates,12, 13, 14, 15 geospatial heterogeneity in death rates,2 and rising economic costs.15, 16 Most prior studies of HF and AF trends assessed morbidity or mortality but not both. Few studies used age‐adjusted rates or assessed the impact of demographic factors (eg, age, sex, and race) and geography on temporal trends.
We investigated temporal trends in the burden of HF and AF in the United States over a 25‐year time period by evaluating age‐adjusted hospitalization and mortality rates and years of potential life lost (YPLL). We studied variations in these trends by age, sex, race, and geographic region.
Methods
The data that support the findings in this study can be obtained from the Nationwide Inpatient Sample (NIS)17 Healthcare Cost and Utilization Project (HCUP),18 US Agency for Healthcare Research and Quality (AHRQ), and the National Center for Health Statistics (NCHS).
Data Sources
HF and AF hospitalization
We used data from the NIS,17 HCUP,18 and AHRQ for estimating morbidity due to HF and AF. The NIS is the largest all‐payer inpatient database in the United States and contains all discharge data from hospitals located across 45 states from 1988 to 2015. States included from 1988 to 2015 are presented in Table S1. The NIS contains a ≈20% stratified sample of US community hospitals, and then uses sampling weights to calculate national estimates. One entry corresponds to 1 hospitalization and has 1 primary discharge diagnosis and a maximum of 24 secondary diagnoses during that index hospitalization. Each hospitalization also has information on patients’ demographic details and insurance status, presence of comorbidities, primary and secondary procedures performed, hospitalization outcomes, length of stay, and costs of care. We used data from 1993 to 2014 for assessing morbidity related to HF and AF because trend weights are provided from 1993 onward. We did not include 2015 because NIS data were coded using a mix of International Classification of Diseases, Ninth Revision (ICD‐9) and Tenth Revision (ICD‐10) codes that year. Weighted counts of hospitalizations with a primary diagnosis of HF and AF were obtained for patients aged ≥30 years in each year and for subgroups. Because state‐level information was available only from 1991 to 2011 and the data included or excluded specific states during each year, we used US division‐ and region‐level information. Annual subgroup‐level population counts by single year of age from 1993 to 2015 provided by the NCHS were used to calculate age‐adjusted rates per 100 000 population.19
HF and AF deaths
We used deidentified death records from the National Vital Statistics System provided by the NCHS for information on mortality due to HF and AF for the time period 1991 to 2015.20 These data contain records of deaths that occurred within the United States and include information on age, sex, and county of residence at the time of death for each decedent. Of note, these mortality data do not represent only hospitalization‐related mortality due to HF and AF (they capture all deaths attributed to these 2 conditions on death records). The registered underlying cause of death was coded according to the ICD‐9 for deaths before 1999 and the ICD‐10 for deaths that occurred in 1999 or later.21, 22 We did not consider multiple causes of death. Deaths were tabulated by single‐year ages (for adjustment purposes) and for strata defined by sex, race, county, and year.19
Hospitalization, mortality, and US population data were restricted to those aged ≥30 years. Institutional review board approval was not required because the study was a retrospective analysis of deidentified data.
Diagnosis Codes for HF and AF
The ICD‐9 codes were used to identify HF and AF hospitalizations (primary diagnosis), whereas ICD‐9 and ICD‐10 codes were used to identify cause of death due to HF and AF. The following ICD‐9 codes were used: 428.0, 428.1x, 428.2x, 428.3x, 428.4x, and 428.9x for HF and 427.31 for AF. The following ICD‐10 codes were used: I50, I50.0, I50.1, I50.2, I50.3, I50.4, and I50.9 for HF and I48, I48.0, I48.1, I48.2, I48.4, I48.91 for AF.
Subgroups
We assessed the population estimates overall and by sex, race/ethnicity (white, black, Hispanic, and other race), and age groups (30–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years). For hospitalization rates, estimates were derived by region (Northeast, Midwest, South and West) and division (New England, Middle Atlantic, East North Central, West North Central, South Atlantic, East South Central, West South Central, Mountain and Pacific). Because all states are not included in the national data, the estimates within each region and division may not be completely representative. In addition, for mortality estimates, we considered state‐specific mortality rates per 100 000 and YPLL.
Statistical Analysis
The weighted hospitalization counts were estimated using nationally representative hospitalization data from the NIS; mortality counts were derived using death records from the NCHS, and population counts from US census estimates. Age adjustment was performed by single age using population estimates for each age and year. All analyses were performed on the US national population and in subgroups by sex, race/ethnicity, and age group.
First, we analyzed hospitalization data from 1993 to 2014 to obtain weighted counts using survey‐weighted analysis within each year and each year of age. Population data for each year and for age were used as denominators to calculate the crude rates per 100 000 population for each year and age. Age adjustment was performed using weights calculated from the US 2000 Census population to reflect the burden of disease more appropriately and to offset in part the greater burden of disease in older people (see Data S1; Tables S2–S4).23, 24 The standard population of 2000 was used instead of 2010 because the estimates were found to be greater when using the 2010 versus 2000 population.24 We used random‐effects metaregression with year as a continuous covariate to estimate the annual change, its SE, and P for trend for hospitalization and mortality due to HF and AF. The annual percentage change for hospitalization was calculated from the changes in age‐adjusted rates from 1993 to 2014. Differences in temporal trends and annual changes in rates within subgroups were tested by incorporating appropriate statistical interaction terms into the metaregression.
Second, we used counts of HF and AF deaths for each year and age along with population data for each year and single ages to calculate the unadjusted mortality rates per 100 000 population. The calculation of rates, age‐adjustment, annual change and interaction tests were performed similar to the first part of the analysis. The annual percentage change for mortality was calculated from the changes in age‐adjusted rates between 1991 and 2015. In an effort to delineate HF with AF and AF with HF, we performed a sensitivity analysis (Data S2). The data collection approaches to multiple conditions were biased, and this prevented removing the possible “mixed” deaths.
Third, YPLL was calculated using counts of death and population as follows.25 YPLL is calculated by subtracting the age at death from the standard year of age and then summing the individual YPLL across each cause of death. YPLL calculation does not include people who died at the standard age or older. For example, if 3 people aged 52, 67, and 84 died from HF, the YPLL‐75 for that cause of death would be (75–52)+(75–67)=23+8=31. Choosing 75 as the standard age excludes people who died at age ≥75 years from the calculation of YPLL‐75. For each death, YPLLk.i=(number of deaths at a given age)×(weight for that age)=Dk.iwi, where k is the particular cause of death and i is age group or age of death. YPLL for each missed year is summed for each deceased patient. For example, if a person died at age 72, the first year of age would contribute a full year, the second year would contribute 1 discounted year (0.985221674), the third year would contribute a twice discounted year (0.985221674×0.985221674), so a total of 1+(0.985221674)+(0.985221674×0.985221674).
Weight of each age is calculated by weight for that age as W i=sum (weight for each year of life remaining)=å w j, where j is in range i…x. We then discounted the total years of life lost by 1.5%,25 choosing 75 years as the average life expectancy in the United States. The YPLL rate was then calculated per age by (YPLL per population under age 75 years)×100 000 and then age adjusted. We did not stratify YPLL by age group because YPLL is calculated from age.
Finally, we performed a post hoc analysis to understand the changes in age at death due to HF and AF within race/ethnicity groups.
All analyses were performed using STATA (StataCorp)26 and R (R Foundation for Statistical Computing).27
Results
Overall HF and AF Morbidity Trends
The cumulative count of HF hospitalizations from 1993 to 2014 was 22 828 980, with an estimated rate of 348.59 per 100 000 population (Table S5). The HF hospitalization rates dropped between 1993 and 2014, with an annual decline of −1.72%, (P<0.0001 for trend; Tables 1 and S5). The cumulative count of AF hospitalization from 1993 to 2014 was 7 378 978, with an estimated rate of 112.47 per 100 000 hospitalizations (Table S5). The AF hospitalization rates increased between 1993 and 2014, with an annual increase of +1.61% (P<0.0001 for trend; Tables 1 and S5).
Table 1.
Temporal Trends and Annual Change of Primary HF and AF Hospitalization Rates Among Patients ≥30 Years of Age, 1993–2014
| 1993 | 2014 | Change (SE) | % | P Trend | P Interaction | |
|---|---|---|---|---|---|---|
| HF | ||||||
| Overall | 407.4 | 271.3 | −7.02 (0.58) | −1.72 | <0.0001 | |
| By sex | 0.94 | |||||
| Women | 361.8 | 230.5 | −7.22 (0.67) | −1.99 | <0.0001 | |
| Men | 474.9 | 320.9 | −7.28 (0.52) | −1.53 | <0.0001 | |
| By race/ethnicity | 0.43 | |||||
| White | 371.4 | 234.2 | −7.21 (0.41) | −1.94 | <0.0001 | |
| Black | 694.0 | 530.2 | −8.73 (1.51) | −1.26 | <0.0001 | |
| Hispanic | 458.6 | 246.7 | −12.59 (2.37) | −2.75 | <0.0001 | |
| Other | 436.6 | 261.9 | −3.73 (2.06) | −0.86 | 0.084 | |
| By age group, y | <0.0001 | |||||
| 30–39 | 27.6 | 44 | 0.80 (0.14) | 2.91 | <0.0001 | |
| 40–49 | 95.7 | 122.2 | 1.05 (0.34) | 1.10 | 0.005 | |
| 50–59 | 335.8 | 281 | −3.86 (0.55) | −1.15 | <0.0001 | |
| 60–69 | 984.2 | 554.7 | −23.42 (1.78) | −2.38 | <0.0001 | |
| 70–79 | 2157.9 | 1238.2 | −46.87 (3.24) | −2.17 | <0.0001 | |
| ≥80 | 4510.9 | 3084.2 | −67.81 (4.70) | −1.50 | <0.0001 | |
| AF | ||||||
| Overall | 91.7 | 109 | 1.48 (0.22) | 1.61 | <0.0001 | |
| By sex | 0.87 | |||||
| Women | 83.6 | 100.7 | 1.40 (0.22) | 1.67 | <0.0001 | |
| Men | 99.9 | 115.3 | 1.45 (0.23) | 1.45 | <0.0001 | |
| By race/ethnicity | 0.75 | |||||
| White | 96.2 | 117.9 | 1.71 (0.23) | 1.78 | <0.0001 | |
| Black | 60.4 | 80.9 | 1.45 (0.17) | 2.40 | <0.0001 | |
| Hispanic | 70.9 | 72.4 | 0.26 (0.33) | 0.36 | 0.45 | |
| Other | 74.6 | 86.3 | 2.30 (0.63) | 3.08 | 0.002 | |
| By age group, y | <0.0001 | |||||
| 30–39 | 14.3 | 16.9 | 0.25 (0.05) | 1.73 | <0.0001 | |
| 40–49 | 33 | 44.1 | 0.98 (0.11) | 2.98 | <0.0001 | |
| 50–59 | 97.2 | 122.9 | 2.06 (0.20) | 2.12 | <0.0001 | |
| 60–69 | 261.8 | 271 | 2.13 (0.59) | 0.81 | 0.002 | |
| 70–79 | 515.5 | 564.1 | 5.13 (1.34) | 1.00 | 0.001 | |
| ≥80 | 724.4 | 992.2 | 18.09 (1.93) | 2.50 | <0.0001 | |
All rates except those by age groups are survey weighted and per 100 000 population. The rates by age groups are crude and per 100 000 population. Change denotes annual change in rate per 100 000 population and is calculated from a metaregression model with year as a continuous covariate. A negative value indicates decline and a positive value indicates increase in annual change per 100 000 and SE from 1993 to 2014. P for trend calculated using metaregression indicates the significance of the decline or the increase in hospitalization rates of primary heart failure hospitalizations from 1993 to 2014. P for interaction was calculated by adding an interactive term between the covariate and year in the model. AF indicates atrial fibrillation; HF, heart failure.
HF and AF Morbidity Trends in Subgroups
The rates of HF hospitalization declined among both sexes, in all race/ethnicity groups, and in older age groups (Table 1). There was no difference in the declining trends by sex and in different race/ethnicity groups, although Hispanic patients demonstrated the largest annual percentage decline. There was an increase in HF hospitalization rates among age groups of 30 to 39 and 40 to 49 years compared with the older age groups that consistently demonstrated a decline.
The rates of AF hospitalization increased in both sexes and in all race/ethnicity groups (except Hispanic patients) with no significant differences (Table 1). There was a significant difference in the increasing AF rates among the age groups, with the largest percentage increase among those aged 40 to 49 years. Figures 1A through 1D and 2A through 2D show these variations.
Figure 1.
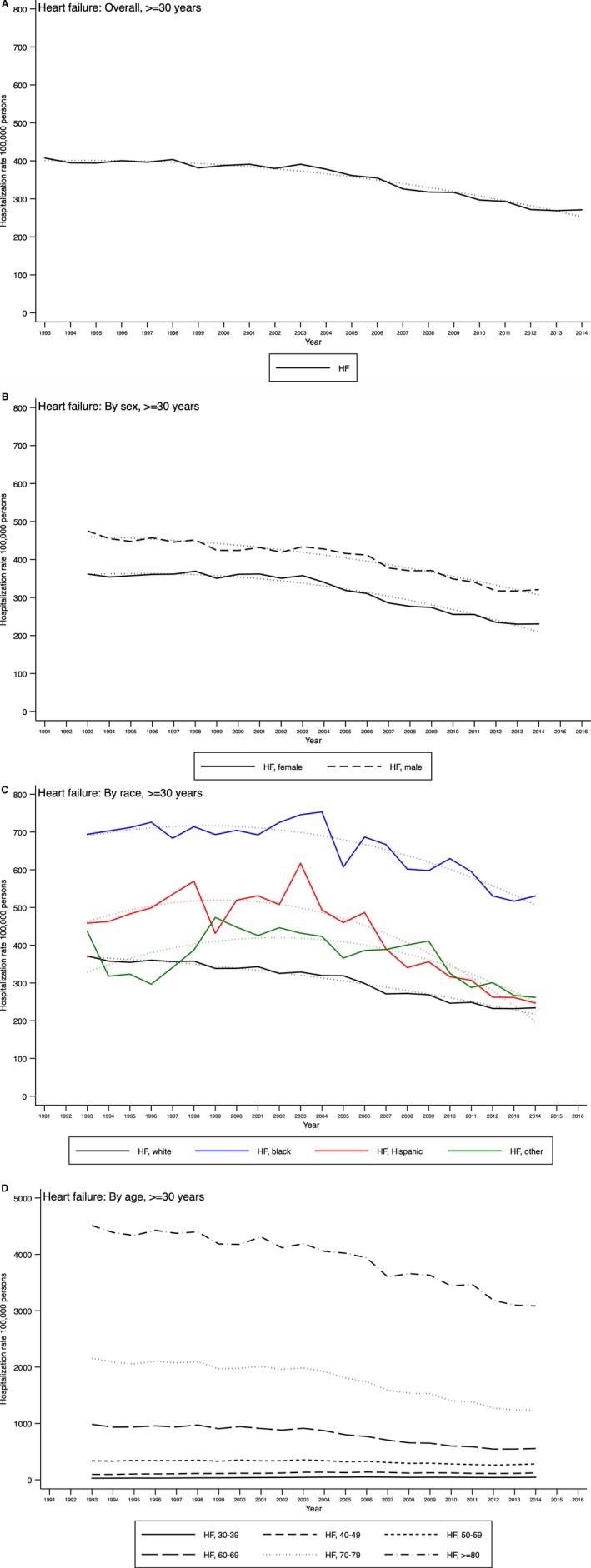
Temporal trends of primary heart failure (HF) hospitalization among hospitalizations of patients aged ≥30 years in the United States overall (A) and by sex (B), race (C), and age group (D), Nationwide Inpatient Sample 1993–2014.
Figure 2.
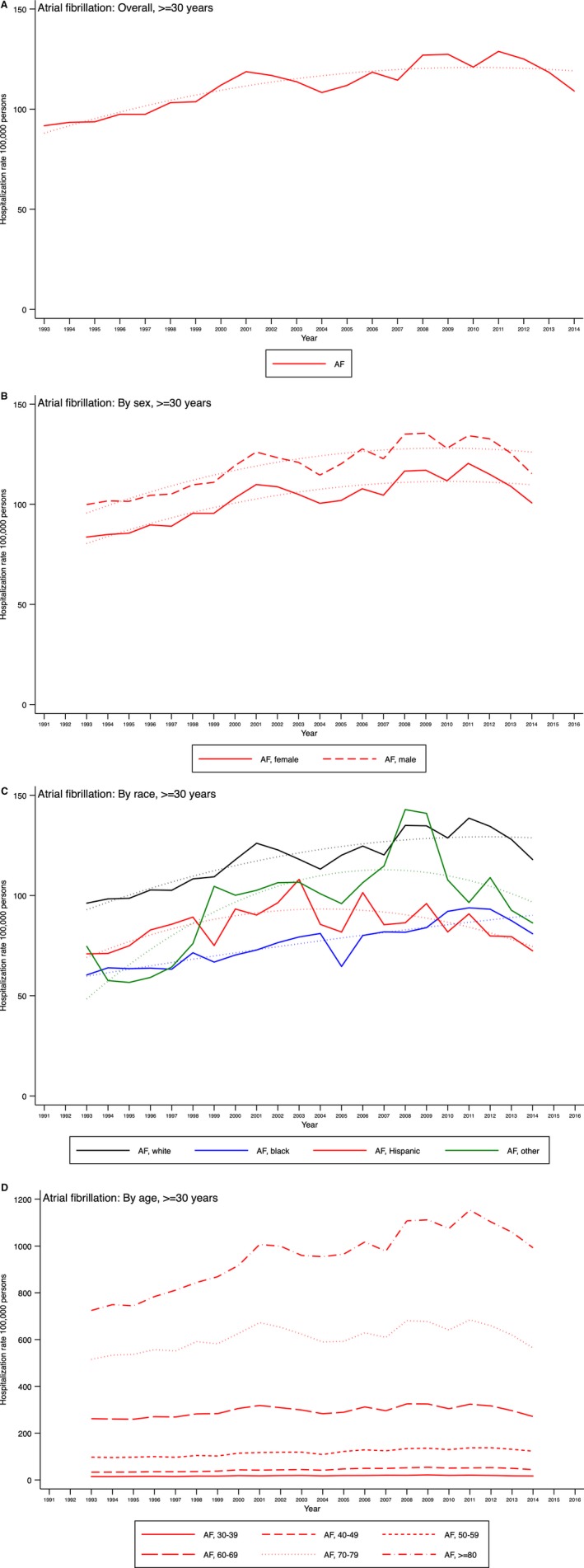
Temporal trends of primary atrial fibrillation (AF) hospitalization among hospitalizations of patients aged ≥30 years in the United States overall (A) and by sex (B), race (C), and age group (D), Nationwide Inpatient Sample 1993–2014.
Geographic Differences in HF and AF Morbidity Trends
The rates of HF hospitalization demonstrated a significant annual decline, whereas AF rates increased in all 4 US regions (Table S6). In the 7 US divisions, declining HF hospitalization rates were observed in Middle Atlantic, West North Central, South Atlantic, and Pacific states. In contrast, increasing AF rates were observed in the New England, East North Central, and Mountain divisions. The South Atlantic division demonstrated a significant decline in AF hospitalization rates.
Overall HF and AF Mortality Rate Trends
The cumulative count and the age‐adjusted rate of deaths due to HF between 1991 and 2015 were 1 373 756 and 18.6 per 100 000, respectively, remaining fairly constant over time (Table 2, Figure 3A). The cumulative count and the age‐adjusted rate of deaths due to AF between 1991 and 2015 were 289 173 and 3.9 per 100 000, respectively, with an annual increase of 11.2% (Table 2, Figure 4A).
Table 2.
Temporal Trends and Annual Change of Death Rates Due to HF and AF, 1991–2015
| Deaths, 1991–2015 | 1991 | 2015 | Change (SE) | % | P Trend | P Interaction |
|---|---|---|---|---|---|---|
| HF, rate per 100 000a | ||||||
| Overall | 17.5 | 19.9 | −0.04 (0.03) | −0.2 | 0.25 | |
| By sex | 0.41 | |||||
| Women | 16.2 | 17.8 | −0.06 (0.03) | −0.3 | 0.105 | |
| Men | 19.5 | 22.6 | −0.02 (0.03) | −0.1 | 0.525 | |
| By race/ethnicity | <0.0001 | |||||
| White | 17.1 | 20.7 | 0.01 (0.03) | 0 | 0.853 | |
| Black | 20.6 | 22.8 | −0.02 (0.04) | −0.1 | 0.525 | |
| Hispanic | 8.2 | 11.8 | 0.08 (0.02) | 0.9 | 0.004 | |
| Other | 48.4 | 9.1 | −1.50 (0.24) | −3.1 | <0.0001 | |
| By age group, y | <0.0001 | |||||
| 30–39 | 0.3 | 0.5 | 0.01 (0.00) | 2.9 | <0.0001 | |
| 40–49 | 1.1 | 1.7 | 0.03 (0.00) | 2.3 | <0.0001 | |
| 50–59 | 4.0 | 5.8 | 0.04 (0.01) | 1 | <0.0001 | |
| 60–69 | 17.4 | 17.6 | −0.13 (0.03) | −0.7 | <0.0001 | |
| 70–79 | 61.8 | 62.4 | −0.40 (0.10) | −0.6 | 0.001 | |
| ≥80 | 347.2 | 441.1 | 0.88 (0.66) | 0.3 | 0.195 | |
| AF, rate per 100 000b | ||||||
| Overall | 1.7 | 6.3 | 0.18 (0.01) | 11.2 | <0.0001 | |
| By sex | 0.23 | |||||
| Women | 1.6 | 6.3 | 0.19 (0.01) | 11.3 | <0.0001 | |
| Men | 1.6 | 6.1 | 0.18 (0.01) | 11 | <0.0001 | |
| By race/ethnicity | <0.0001 | |||||
| White | 1.7 | 7 | 0.21 (0.01) | 12.6 | <0.0001 | |
| Black | 1.4 | 3.9 | 0.10 (0.00) | 7.4 | <0.0001 | |
| Hispanic | 0.9 | 3.1 | 0.09 (0.00) | 9.5 | <0.0001 | |
| Other | 4.2 | 3.3 | 0.00 (0.02) | 0 | 0.951 | |
| By age group, y | <0.0001 | |||||
| 30–39 | 0 | 0 | 0.00 (0.00) | 7.9 | <0.0001 | |
| 40–49 | 0 | 0.2 | 0.01 (0.00) | 14.7 | <0.0001 | |
| 50–59 | 0.3 | 1.0 | 0.03 (0.00) | 11.8 | <0.0001 | |
| 60–69 | 1.3 | 4.0 | 0.10 (0.01) | 7.9 | <0.0001 | |
| 70–79 | 6.0 | 19.0 | 0.51 (0.01) | 8.5 | <0.0001 | |
| ≥80 | 33.9 | 150.8 | 4.60 (0.15) | 13.6 | <0.0001 | |
All rates except those by age groups are per 100 000 population. The rates by age groups are crude and per 100 000 population. Change denotes annual change in rate per 100 000 population and is calculated from observed rates using a random‐effects metaregression model with year as a continuous covariate. A negative value indicates decline and a positive value indicates increase in annual change per 100 000 and SE from 1991 to 2015. P for trend indicates the significance of the decline or the increase in death rates of from 1991 to 2015. P for interaction was calculated by adding an interactive term between the covariate and year in the model. AF indicates atrial fibrillation; HF, heart failure.
HF deaths, 1991–2015: total: 1 373 756; rate per 100 000: 18.6.
AF deaths, 1991–2015: total: 289 173; rate per 100 000: 3.9.
Figure 3.
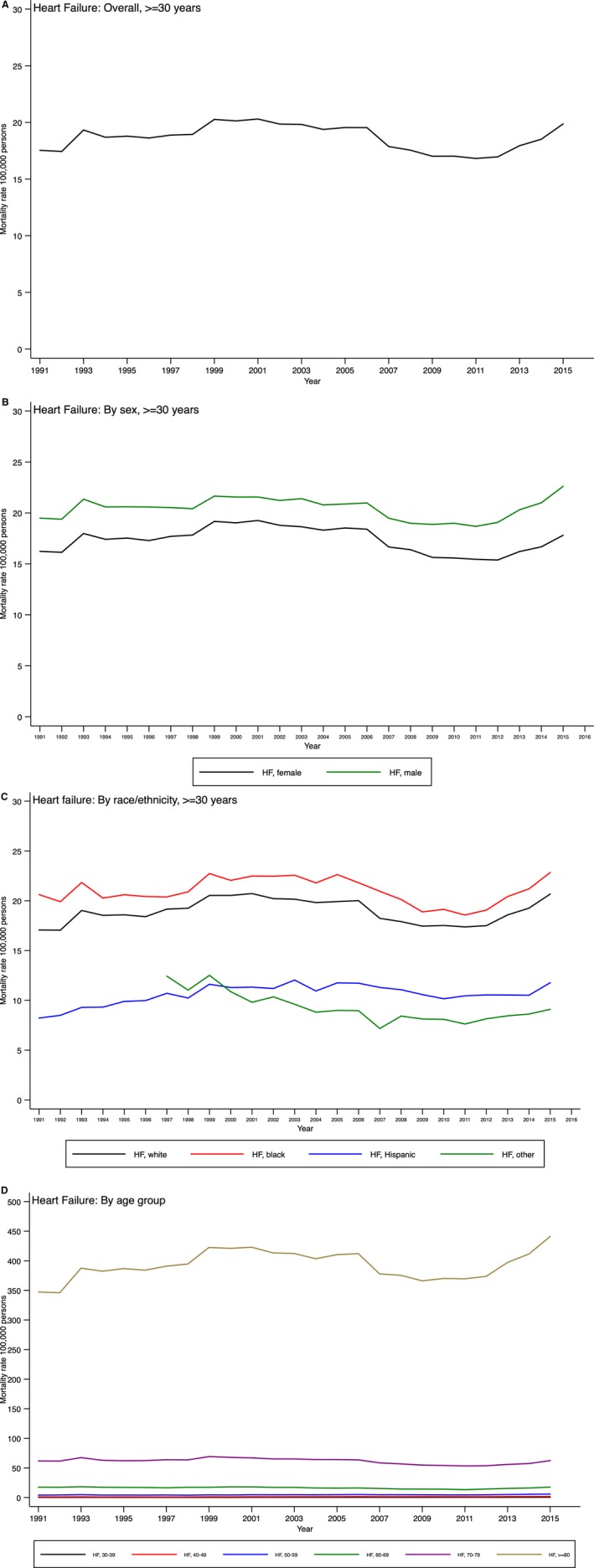
Temporal trends in heart failure (HF) mortality in the United States overall (A) and by sex (B), race (C), and age group (D).
Figure 4.
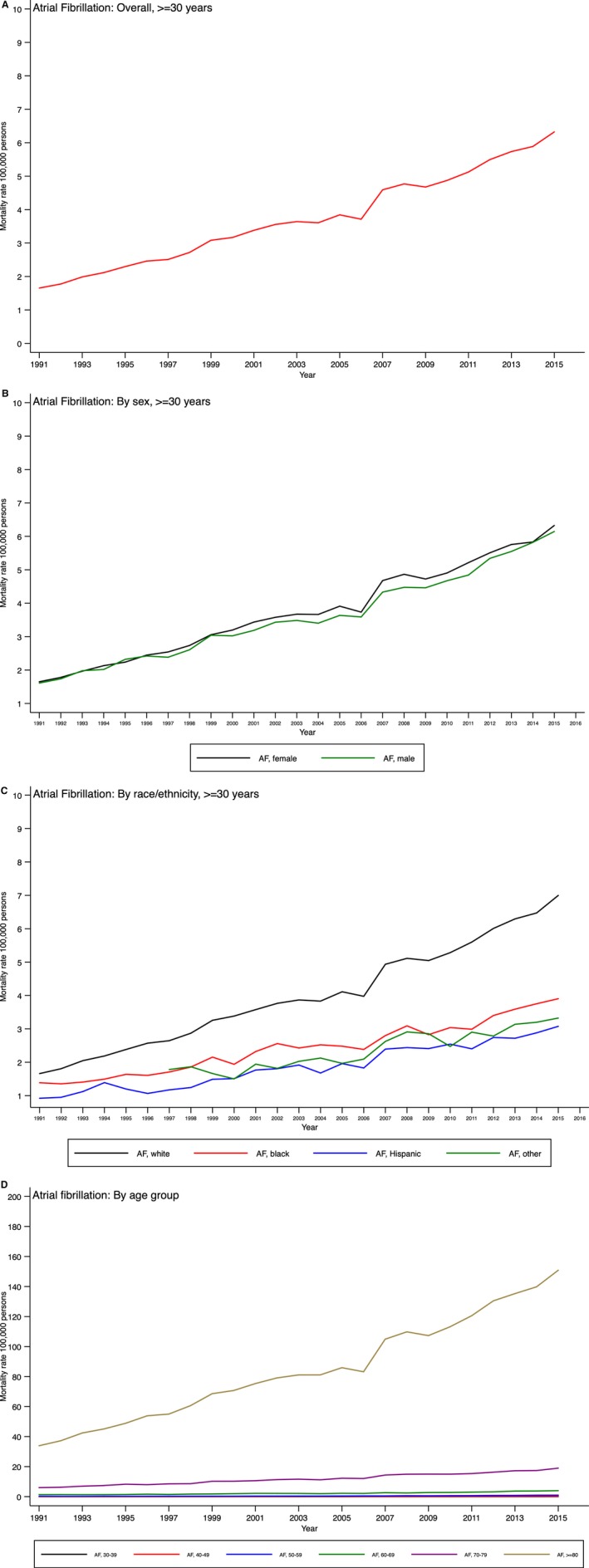
Temporal trends in atrial fibrillation (AF) mortality in the United States overall (A) and by sex (B), race (C), and age group (D).
HF and AF Mortality Rate Trends in Subgroups
Age‐adjusted HF mortality rates were not significantly different among men and women (Table 2, Figure 3B) but varied among race/ethnicity groups (P<0.0001 for interaction). During the 25‐year time period, HF death rates remained constant for both black and white patients but increased among Hispanic patients during this time period (although absolute rates still remained lower than those for white and black patients; Figure 3C). Of note, trends in HF death rates differed significantly by age groups: there were small statistically significant annual increases in those aged <60 years (with the largest increase being observed in those aged 30–39 years but significant decline in the age groups 60–69 and 70–79 years; Figure 3D).
Age‐adjusted AF mortality rates increased overall and consistently for both sexes and in the major race/ethnicity groups (Table 2, Figure 4A–4C). White patients experienced the largest increase in AF mortality rates, followed closely by Hispanic and black patients (P<0.0001 for interaction). Although AF death rates increased in all age groups, the percentage increase in those aged <60 years is noteworthy, with the highest increase in the age group 40 to 49 years (Table 2, Figure 4D).
Geographic Differences in HF and AF Mortality Trends
The age‐adjusted HF mortality rates declined from 1991 to 2015 in most US states (Table 3; Video S1). The states with the largest annual absolute and percentage decreases in rates were Arizona, Arkansas, Delaware, Nevada, South Dakota, Washington, and Vermont as well as the District of Columbia. Increases in HF death rates (>2%) were observed in California, Florida, Louisiana, New Hampshire, Rhode Island, Tennessee, and Texas. In 2015, the highest HF death rates (≥40/100 000) were observed in Alabama, Louisiana, and Mississippi.
Table 3.
Temporal Trends and Annual Change of Deaths Due to HF and AF in States and District of Columbia, 1991–2015
| State | Rates Per 100 000 Due to HF | Rates Per 100 000 Due to AF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age‐Adjusted Rate per 100 000 | Annual | P‐Trend | Age‐Adjusted Rate per 100 000 | Annual | P‐Trend | |||||||
| 1991 | 2015 | Change | SE | % | 1991 | 2015 | Change | SE | % | |||
| Alabama | 43.8 | 46.5* | −0.22 | 0.10 | −0.5 | 0.040 | 2.0 | 6.9 | 0.2 | 0.01 | 9.0 | <0.0001 |
| Alaska | 26.6 | 18.4 | −0.35 | 0.09 | −1.3 | 0.001 | 1.0 | 7.6 | 0.3 | 0.04 | 26.2* | <0.0001 |
| Arizona | 21.8 | 8.0 | −0.51 | 0.07 | −2.3 | <0.0001 | 1.5 | 5.6 | 0.1 | 0.01 | 7.4 | <0.0001 |
| Arkansas | 31.5 | 26.7 | −0.64 | 0.17 | −2.0 | 0.001 | 1.6 | 4.4 | 0.2 | 0.02 | 10.5 | <0.0001 |
| California | 10.0 | 15.0 | 0.20 | 0.03 | 2.0* | <0.0001 | 1.5 | 5.8 | 0.2 | 0.01 | 13.6 | <0.0001 |
| Colorado | 7.7 | 16.2 | −0.13 | 0.12 | −1.7 | 0.29 | 1.2 | 7.3 | 0.2 | 0.01 | 19.0 | <0.0001 |
| Connecticut | 14.8 | 18.6 | −0.01 | 0.04 | −0.1 | 0.83 | 2.0 | 9.4* | 0.3 | 0.01 | 13.4 | <0.0001 |
| Delaware | 18.7 | 11.4 | −0.42 | 0.05 | −2.2 | <0.0001 | 2.5 | 4.1 | 0.1 | 0.02 | 3.4 | <0.0001 |
| District of Columbia | 20.4 | 12.3 | −0.58 | 0.09 | −2.8 | <0.0001 | 2.5 | 5.3 | 0.0 | 0.02 | 0.5 | 0.59 |
| Florida | 5.2 | 11.5 | 0.22 | 0.02 | 4.3* | <0.0001 | 1.1 | 3.8 | 0.1 | 0.01 | 11.9 | <0.0001 |
| Georgia | 26.9 | 33.8 | 0.09 | 0.07 | 0.3 | 0.21 | 2.1 | 6.0 | 0.2 | 0.01 | 7.9 | <0.0001 |
| Hawaii | 12.0 | 14.4 | 0.05 | 0.04 | 0.4 | 0.23 | 2.0 | 4.8 | 0.1 | 0.01 | 7.1 | <0.0001 |
| Idaho | 16.7 | 20.3 | −0.08 | 0.06 | −0.5 | 0.17 | 2.7 | 9.6* | 0.3 | 0.02 | 10.4 | <0.0001 |
| Illinois | 20.8 | 25.6 | 0.01 | 0.04 | 0.1 | 0.70 | 1.8 | 5.9 | 0.2 | 0.01 | 9.4 | <0.0001 |
| Indiana | 27.5 | 23.5 | −0.25 | 0.07 | −0.9 | 0.003 | 1.5 | 6.6 | 0.2 | 0.01 | 14.8 | <0.0001 |
| Iowa | 8.9 | 9.6 | 0.10 | 0.04 | 1.2 | 0.018 | 1.2 | 5.6 | 0.2 | 0.01 | 16.6 | <0.0001 |
| Kansas | 24.5 | 24.8 | 0.00 | 0.06 | 0.0 | 0.97 | 1.1 | 5.3 | 0.2 | 0.01 | 15.8 | <0.0001 |
| Kentucky | 30.9 | 31.0* | −0.32 | 0.08 | −1.0 | 0.001 | 1.6 | 7.8 | 0.2 | 0.01 | 14.8 | <0.0001 |
| Louisiana | 23.1 | 41.0* | 0.47 | 0.08 | 2.0* | <0.0001 | 2.3 | 4.5 | 0.1 | 0.01 | 4.1 | <0.0001 |
| Maine | 16.6 | 20.5 | −0.06 | 0.04 | −0.4 | 0.15 | 2.4 | 7.0 | 0.2 | 0.02 | 7.7 | <0.0001 |
| Maryland | 18.5 | 12.3 | −0.36 | 0.03 | −1.9 | <0.0001 | 2.6 | 5.7 | 0.1 | 0.01 | 4.4 | <0.0001 |
| Massachusetts | 21.9 | 21.7 | −0.15 | 0.05 | −0.7 | 0.008 | 2.6 | 7.2 | 0.2 | 0.01 | 6.5 | <0.0001 |
| Michigan | 18.7 | 26.1 | 0.10 | 0.05 | 0.5 | 0.085 | 1.5 | 6.7 | 0.2 | 0.01 | 11.4 | <0.0001 |
| Minnesota | 17.4 | 16.7 | −0.17 | 0.05 | −1.0 | 0.002 | 2.0 | 7.9 | 0.2 | 0.01 | 11.5 | <0.0001 |
| Mississippi | 37.4 | 45.9* | 0.13 | 0.12 | 0.4 | 0.29 | 1.5 | 5.8 | 0.2 | 0.01 | 13.8 | <0.0001 |
| Missouri | 20.9 | 29.6 | 0.13 | 0.05 | 0.6 | 0.024 | 1.8 | 5.9 | 0.2 | 0.01 | 9.3 | <0.0001 |
| Montana | 23.6 | 24.6 | −0.17 | 0.09 | −0.7 | 0.061 | 1.9 | 9.4* | 0.2 | 0.03 | 12.6 | <0.0001 |
| Nebraska | 23.4 | 23.0 | −0.34 | 0.08 | −1.5 | <0.0001 | 1.4 | 9.9* | 0.3 | 0.02 | 20.7* | <0.0001 |
| Nevada | 26.1 | 16.1 | −0.66 | 0.14 | −2.5 | <0.0001 | 1.9 | 5.1 | 0.1 | 0.02 | 4.8 | <0.0001 |
| New Hampshire | 16.1 | 19.2 | 0.48 | 0.09 | 3.0* | <0.0001 | 2.1 | 8.4 | 0.2 | 0.02 | 10.9 | <0.0001 |
| New Jersey | 13.1 | 17.2 | 0.07 | 0.02 | 0.5 | 0.015 | 1.6 | 5.7 | 0.2 | 0.01 | 11.1 | <0.0001 |
| New Mexico | 17.3 | 14.2 | −0.17 | 0.06 | −1.0 | 0.012 | 1.5 | 4.3 | 0.1 | 0.02 | 8.5 | <0.0001 |
| New York | 12.1 | 12.9 | −0.04 | 0.03 | −0.3 | 0.25 | 1.6 | 4.8 | 0.1 | 0.01 | 8.4 | <0.0001 |
| North Carolina | 16.6 | 22.7 | 0.12 | 0.04 | 0.7 | 0.003 | 1.8 | 7.6 | 0.2 | 0.01 | 12.7 | <0.0001 |
| North Dakota | 20.2 | 16.6 | −0.35 | 0.13 | −1.8 | 0.010 | 0.9 | 6.7 | 0.2 | 0.02 | 23.1* | <0.0001 |
| Ohio | 20.8 | 21.8 | −0.23 | 0.08 | −1.1 | 0.009 | 2.1 | 7.6 | 0.2 | 0.01 | 10.4 | <0.0001 |
| Oklahoma | 28.2 | 19.6 | −0.27 | 0.12 | −1.0 | 0.033 | 1.2 | 7.2 | 0.3 | 0.02 | 22.2* | <0.0001 |
| Oregon | 14.1 | 19.6 | −0.09 | 0.06 | −0.6 | 0.18 | 2.1 | 11.5* | 0.4 | 0.02 | 17.2 | <0.0001 |
| Pennsylvania | 19.7 | 22.3 | −0.07 | 0.04 | −0.4 | 0.10 | 1.9 | 7.0 | 0.2 | 0.01 | 9.2 | <0.0001 |
| Rhode Island | 14.4 | 14.9 | 0.20 | 0.08 | 1.4 | 0.016 | 1.7 | 6.8 | 0.3 | 0.02 | 15.5 | <0.0001 |
| South Carolina | 20.6 | 21.7 | −0.06 | 0.05 | −0.3 | 0.22 | 2.1 | 6.7 | 0.2 | 0.01 | 8.1 | <0.0001 |
| South Dakota | 18.4 | 4.0 | −0.90 | 0.12 | −4.9 | <0.0001 | 1.1 | 3.6 | 0.1 | 0.02 | 7.9 | <0.0001 |
| Tennessee | 11.3 | 18.2 | 0.14 | 0.06 | 1.2 | 0.030 | 1.4 | 7.1 | 0.2 | 0.01 | 16.7 | <0.0001 |
| Texas | 12.2 | 21.7 | 0.16 | 0.08 | 1.3 | 0.054 | 1.0 | 6.7 | 0.2 | 0.01 | 20.1* | <0.0001 |
| Utah | 30.7 | 36.8* | 0.23 | 0.09 | 0.8 | 0.013 | 1.7 | 9.9* | 0.3 | 0.02 | 15.0 | <0.0001 |
| Vermont | 14.8 | 4.5 | −0.61 | 0.06 | −4.1 | <0.0001 | 3.5 | 7.1 | 0.2 | 0.02 | 5.0 | <0.0001 |
| Virginia | 26.4 | 21.5 | −0.20 | 0.03 | −0.8 | <0.0001 | 1.6 | 7.0 | 0.2 | 0.01 | 13.4 | <0.0001 |
| Washington | 16.1 | 9.3 | −0.50 | 0.07 | −3.1 | <0.0001 | 0.8 | 7.4 | 0.2 | 0.02 | 24.4* | <0.0001 |
| West Virginia | 24.8 | 25.7 | −0.10 | 0.05 | −0.4 | 0.040 | 1.8 | 8.0 | 0.2 | 0.01 | 13.5 | <0.0001 |
| Wisconsin | 21.2 | 19.6 | −0.13 | 0.04 | −0.6 | 0.005 | 2.1 | 7.0 | 0.2 | 0.01 | 8.3 | <0.0001 |
| Wyoming | 22.0 | 18.6 | 0.02 | 0.09 | 0.1 | 0.84 | 2.4 | 6.6 | 0.2 | 0.02 | 7.7 | <0.0001 |
| >30.0* | ≥+2* | >9* | >20* | |||||||||
AF indicates atrial fibrillation; HF, heart failure.
*Values indicate threshold levels that indicate very high rates and percentages.
The age‐adjusted AF mortality rates increased significantly in all 50 US states but not in the District of Columbia, which demonstrated no significant trend (Table 3; Video S2). The 5 states with the largest absolute annual increase were Connecticut, Idaho, Nebraska, Oklahoma, and Oregon. The states with the largest percentage increases were Alaska, Nebraska, North Dakota, Oklahoma, Texas, and Washington. In 2015, the highest AF death rates (approximately ≥9/100 000) were observed in Connecticut, Idaho, Montana, Nebraska, Oregon, and Utah.
Trends in YPLL Rates Due to HF and AF Overall and in Subgroups
Overall, YPLL rates due to HF increased by between 1991 and 2015 (Table S7, Figures S1–S4). YPLL rate changes were higher in men compared with women (P=0.038 for interaction) and varied by race/ethnicity (P<0.0001 for interaction) being higher for black and Hispanic patients compared with white patients. Post hoc analyses demonstrate that mean age of death due to HF during 1991 and 2015 was 75.0 and 73.9 years, respectively, among black patients and 79.7 and 80.7 years, respectively, among white patients.
YPLL rates due to AF increased steadily between 1991 and 2015. Men and white patients experienced the highest annual percentage changes in YPLL rate. Post hoc analyses demonstrate that mean age of death due to AF during 1991 and 2015 was 76.3 and 77.8 years, respectively, among black patients and 80.3 and 81.5 years, respectively, among white patients.
Geographic Differences in YPLL Rate Trends
YPLL rate due to HF from 1991 to 2015 demonstrated significant state‐specific heterogeneity in the rate of annual life loss (Table S8). The 3 states with the largest absolute annual increases in YPLL attributable to HF were Louisiana, Oklahoma, and Tennessee, and the states with the largest decreases were Arizona and South Dakota as well as the District of Columbia. The 3 states with the largest percentage increases in YPLL due to HF were Florida, Tennessee, and Hawaii, and the largest percentage decreases were in Vermont, South Dakota, and the District of Columbia. In 2015, the highest rates of YPLL due to HF (≥100/100 000) were in Mississippi, Louisiana, and Alabama.
YPLL rate due to AF increased significantly in most US states, except Alaska, Delaware, Montana, North Dakota, Rhode Island, South Dakota, Vermont and Wyoming and the District of Columbia. The 3 states with the largest absolute annual increase were Oklahoma, Kentucky, and Tennessee. The states with the largest percentage increase in YPLL due to AF were Kansas, Iowa, and Oklahoma. In 2015, the states with highest rates of YPLL due to AF (≥10/100 000) were Kentucky, Alabama, and West Virginia as well as the District of Columbia.
Discussion
Principal Findings
First, the 3 metrics used to evaluate burden offered complementary information. Second, HF accounts for twice as many hospitalizations, >3 times as many deaths, and >5 times as many YPLL compared with AF. Third, HF hospitalizations are decreasing, adjusted death rates seem to be stabilizing, and YPLL seems to be increasing. In contrast, all 3 metrics are increasing markedly for AF. Fourth, interesting differences in trends for the 3 metrics (for HF versus AF) emerged when evaluated by sex, age, race, and geography. Our investigation is incremental over several recent reports29, 30, 31 of trends in HF and AF hospitalization and mortality by virtue of evaluation of a 25‐year time period and geospatial trends (in addition to trends by age, sex, and race) and by comparing and contrasting HF versus AF trends.
Sex‐Related Differences in Trends for HF and AF Hospitalization, Death, and YPLL
Overall, we observed that men had a higher rate of hospitalization for both HF and AF and higher YPLL rates due to both conditions during the time period studied. Age‐adjusted death rates for HF were higher in men, but those for AF were slightly higher in women. Overall, women experienced a somewhat greater decline over time in hospitalization and death rates due to HF and experienced a slightly lesser increase in hospitalization rates due to AF; however, a formal test of an interaction was statistically nonsignificant. The rise in death rates due to AF over time was similar in the 2 sexes. Our findings differ from another report15 that noted higher hospitalization rates for AF in women (compared with men), but that report15 focused on a single decade of observation (2000–2010). In that report,15 the increase in hospitalization rates over the decade for AF in men was greater than the increase observed in women, a finding similar to ours. However, our observations are more consistent with sex‐related trends reported for HF hospitalizations in a recent report.31
Race‐Related Differences in Trends for HF and AF Hospitalization, Death, and YPLL
Overall, we observed that black patients had higher absolute rates of hospitalization, death, and YPLL due to HF, whereas white patients had higher rates of these metrics for AF. Across the decades of observation, hospitalization rates for HF fell but those for AF increased in all race/ethnicity groups except Hispanic patients. Death rates due to HF increased among Hispanic patients alone, whereas mortality due to AF increased in all race/ethnicity groups. YPLL rates for HF increased in black and Hispanic patients, with a minimal increase in white patients. Although YPLL rates for AF increased in all race/ethnicity groups, white and Hispanic patients (in that order) experienced the largest proportional increases. The discordant trends observed in Hispanic patients of decreasing HF and stable AF hospitalization rates in the face of increasing mortality rates due to both conditions are puzzling and raise some concern. Unfavorable trends in healthcare access among Hispanic patients may account for these trends—a premise that warrants further investigation. Prior studies conjointly evaluating race‐related differences in both hospitalization and mortality rates are lacking. A recent report did note a decrease in hospitalization rates for HF among Hispanic patients.31
Age‐Related Differences in Trends for HF and AF Hospitalization, Death, and YPLL
Not surprisingly, we observed that older patients had higher absolute rates of hospitalization and death due to both HF and AF. Our post hoc analysis indicates that black Americans who die of HF and AF are younger than white Americans, with the mean age of HF deaths among black patients signaling a lower age in 2015 than in 1991 for HF deaths, which may be considered a persistent racial gap. We observed a disturbing pattern of concordant increases in both hospitalization and death rates for HF and AF for individuals aged <60 years. Other recent reports have also underscored the increasing burden of HF and AF in younger individuals.32, 33, 34 The reasons for this disturbing trend are not clear and warrant further investigation.
Geographic Differences in Trends for HF and AF Hospitalization, Death, and YPLL
A very high disease burden due to HF hospitalization and mortality clusters (and associated YPLL) in selected parts of the southern United States (Alabama, Georgia, Louisiana, Mississippi). In comparison, both morbidity and mortality rates related to AF are similar across states. However, YPLL rates related to AF in 2015 are highest in the District of Columbia, Kentucky, Alabama, and West Virginia. Given the shared individual risk factors for HF and AF, these regional differences are intriguing and warrant further study. Similar regional differences for mortality due to HF and AF have been reported recently by others.2 Such regional differences in disease burden likely are multifactorial in origin and caused by socioeconomic differences and related behavioral patterns and risk factor distributions, variations in chronic disease management, and access to and quality of emergency services for managing these conditions.2
State mortality trends due to HF demonstrate high heterogeneity: the rates held steady in 17, declined in 23 and increased in 10 states. A more consistent pattern of an overall increase in mortality and YPLL due to AF across most states was noted. Additional investigation of regional “hotspots” for HF and AF is warranted.
Strengths and Limitations
We evaluated conjoint trends in HF and AF, conditions considered to constitute the twin epidemics,4 over a 25‐year period using 3 complementary metrics of disease burden and 2 large nationally representative databases. In addition, we elucidated variations in burden of HF and AF due to sex, race, age, and geography. Nevertheless, several limitations must be noted. Use of the NIS database precludes differentiation of initial admissions from readmissions, states were not sampled regularly over time, and the race/ethnicity variable in this database is noted to have differential missingness (in early versus late years). Moreover, the lack of methods to differentiate trends in initial admissions versus readmissions prevents performance of sensitivity analysis that may indicate whether there is an increased morbidity burden related to readmissions; therefore, the magnitude and nature of bias due to potential readmissions remains currently unassessed. Use of available databases also did not permit a distinction of trends in “all HF” from trends for its 2 subtypes characterized by reduced versus preserved left ventricular ejection fraction. The use of national vital statistics data for cause of death is error prone, and additional inaccuracies may be introduced by the transition from ICD‐9 to ICD‐10 disease codes for HF and AF during the 25‐year period evaluated. Furthermore, we evaluated trends in hospitalization for a primary diagnosis of HF and AF. Such analyses would underestimate the true burden of these conditions because hospitalizations with a secondary diagnosis of HF or AF were not considered. Our study was mainly descriptive, and future investigations must analyze potential factors underlying the divergent trends for HF versus AF and the variations in disease‐burden rates for these 2 conditions in subgroups. It is important to note that some of the observed trends in race/ethnicity subgroups (for HF and AF hospitalization and mortality) are visually nonlinear and thus should be interpreted with caution. Further research should be performed to identify whether there are inflection points that mark nonlinear trends and to identify the factors that drive these changes. In addition, changes in overall population mortality rates attributable to a disease entity may not adequately reflect the potential impact of health care on the disease. Temporal trends in survival after diagnosis may reflect the impact of health care. Considering the potential role of temporal trends in HF morbidity and mortality according to ejection fraction in HF, future studies should explore these temporal trends according to HF subtype (ie, reduced versus preserved ejection fraction). Such analyses may be more clinically meaningful. We do not consider temporal trends in multimorbidity, although it is likely that multiple diseases may occur in a given patient. In this context, the divergent trends in morbidity and mortality for HF versus AF should not overlook the strong mechanistic links between these 2 conditions (where presence of either condition often begets the other35). Last, but not least, we evaluated AF and HF as the “underlying cause” of death, as coded on death certificates. Although this approach is acceptable for mortality analyses,36 it is likely in AF that the arrhythmia triggers a series of more distal events (eg, thromboembolic stroke) that result in mortality.
Conclusions
Over a 25‐year period in the United States, hospitalization rates for HF have been decreasing and mortality rates stabilizing, whereas both have been escalating for AF. We noted modest variations in disease burden by age and race/ethnicity and more marked variations related to geographic region. Future studies should evaluate whether trends in putative risk factors for HF and AF may drive the morbidity and mortality trends that we observed. The increasing burden of both HF and AF in young adults is of concern because it may portend a reversal of our successes combating cardiovascular disease in middle‐aged and elderly individuals. The increase in HF and AF mortality rates in Hispanic patients in the face of potentially declining hospitalization rates for these conditions warrants further study. The regional patterns of disease burden emphasize the critical need for ongoing regional surveillance of HF and AF.
Disclosures
None.
Supporting information
Table S1. Summary of Nationwide Inpatient Sample States, 1988–2015
Data S1. Age standardization.
Table S2. Creating Weights Using 2000 Population
Table S3. Age Standardization of Overall Heart Failure Mortality Rate
Table S4. Age Standardization of Overall Heart Failure Mortality Rate Among Men
Data S2. Differentiating causes of death: primary (underlying cause of death) and secondary (multiple conditions).
Table S5. Temporal Trends of Primary Heart Failure and Atrial Fibrillation Hospitalization Among Hospitalization of Patients Aged ≥30 Years in the United States, Nationwide Inpatient Sample 1993–2014
Table S6. Temporal Trends and Annual Change of All Primary Heart Failure and Atrial Fibrillation Hospitalizations in Each Region and Division From 1993 to 2014
Table S7. Temporal Trends and Annual Change of Years of Potential Life Lost at Age 75 Years for Heart Failure and Atrial Fibrillation, 1991–2015
Table S8. Temporal Trends and Annual Change of Years of Potential Life Lost at Age 75 Years Due to Heart Failure and Atrial Fibrillation in States, 1991–2015
Figure S1. Temporal trends of years of potential life lost due to heart failure in the United States, 1991–2015: overall (A) and by sex (B) and race (C).
Figure S2. Temporal trends of years of potential life lost due to atrial fibrillation in the United States, 1991–2015: overall (A) and by sex (B) and race (C).
Figure S3. Mean age of heart failure deaths by race/ethnicity in the United States, 1991–2015.
Figure S4. Mean age of atrial fibrillation deaths by race/ethnicity in the United States, 1991–2015.
Video S1. Transitioning chloropleth map showing spatiotemporal changes in the mortality rates due to heart failure, 1990–2015 (best viewed with Windows Media Player).
Video S2. Transitioning choropleth map showing spatiotemporal changes in mortality rates due to atrial fibrillation, 1990–2015 (best viewed with Windows Media Player).
(J Am Heart Assoc. 2019;8:e010756 DOI: 10.1161/JAHA.118.010756.)
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roth GA, Dwyer‐Lindgren L, Bertozzi‐Villa A, Stubbs RW, Morozoff C, Naghavi M, Mokdad AH, Murray CJL. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980–2014. JAMA. 2017;317:1976–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ, Naghavi M. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 Study. Circulation. 2014;129:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. [DOI] [PubMed] [Google Scholar]
- 5. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 6. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Najafi F, Jamrozik K, Dobson AJ. Understanding the ‘epidemic of heart failure’: a systematic review of trends in determinants of heart failure. Eur J Heart Fail. 2009;11:472–479. [DOI] [PubMed] [Google Scholar]
- 8. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet. 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain AM, Gersh BJ, Alonso A, Chen LY, Berardi C, Manemann SM, Killian JM, Weston SA, Roger VL. Decade‐long trends in atrial fibrillation incidence and survival: a community study. Am J Med. 2015;128:260–267.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lane DA, Skjoth F, Lip GYH, Larsen TB, Kotecha D. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc. 2017;6:e005155 DOI: 10.1161/JAHA.116.005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munir MB, Sharbaugh MS, Thoma FW, Nisar MU, Kamran AS, Althouse AD, Saba S. Trends in hospitalization for congestive heart failure, 1996–2009. Clin Cardiol. 2017;40:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akintoye E, Briasoulis A, Egbe A, Dunlay SM, Kushwaha S, Levine D, Afonso L, Mozaffarian D, Weinberger J. National trends in admission and in‐hospital mortality of patients with heart failure in the United States (2001–2014). J Am Heart Assoc. 2017;6:e006955 DOI: 10.1161/JAHA.117.006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt M, Ulrichsen SP, Pedersen L, Botker HE, Sorensen HT. Thirty‐year trends in heart failure hospitalization and mortality rates and the prognostic impact of co‐morbidity: a Danish nationwide cohort study. Eur J Heart Fail. 2016;18:490–499. [DOI] [PubMed] [Google Scholar]
- 15. Sheikh A, Patel NJ, Nalluri N, Agnihotri K, Spagnola J, Patel A, Asti D, Kanotra R, Khan H, Savani C, Arora S, Patel N, Thakkar B, Patel N, Pau D, Badheka AO, Deshmukh A, Kowalski M, Viles‐Gonzalez J, Paydak H. Trends in hospitalization for atrial fibrillation: epidemiology, cost, and implications for the future. Prog Cardiovasc Dis. 2015;58:105–116. [DOI] [PubMed] [Google Scholar]
- 16. Echouffo‐Tcheugui JB, Bishu KG, Fonarow GC, Egede LE. Trends in health care expenditure among US adults with heart failure: the Medical Expenditure Panel Survey 2002–2011. Am Heart J. 2017;186:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. HCUP National Inpatient Sample (NIS) . Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed January 22, 2018. [Google Scholar]
- 18. HCUP Databases . Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2006. ‐2009. Available at: www.hcup-us.ahrq.gov/databases.jsp. Accessed December 11, 2017. [PubMed] [Google Scholar]
- 19. National Center for Health Statistics . Vintage 2016 postcensal estimates of the resident population of the United States (April 1, 2010, July 1, 2010‐July 1, 2016), by year, county, single‐year of age (0, 1, 2,.., 85 years and over), bridged race, Hispanic origin, and sex. Prepared under a collaborative arrangement with the U.S. Census Bureau. As of June 26, 2017, following release by the U.S. Census Bureau of the unbridged Vintage 2016 postcensal estimates by 5‐year age group on June 22, 2017. Available at: /nchs/nvss/bridged_race.htm. Accessed November 21, 2017.
- 20. National Center for Health Statistics . National Vital Statistics System: Multiple Cause of Death Data File, 1980–2015. Hyattsville, MD: National Center for Health Statistics; 2015. [Google Scholar]
- 21. World Health Organization . International Classification of Diseases, Ninth Revision. Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 22. World Health Organization . International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 23. Christie D, Gordon I, Heller R. Mortality and morbidity: comparisons of time and place. Epidemiology. 1994;4:26–36. [Google Scholar]
- 24. Li C, Ford ES, Zhao G, Wen XJ, Gotway CA. Age adjustment of diabetes prevalence: use of 2010 U.S. Census data. J Diabetes. 2014;6:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torgerson DJ, Raftery JB. Economics notes. Discounting. BMJ. 1999;319:914–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. StataCorp . Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 27. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. ISBN 3‐900051‐07‐0. Available at: www.R-project.org/. [Google Scholar]
- 28. Statistical Brief #236 . Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; February 2018. Available at: www.hcup-us.ahrq.gov/reports/statbriefs/sb236-Atrial-Fibrillation-Hospital-Stays-Trends.jsp. Accessed June 5, 2018. [Google Scholar]
- 29. Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles‐Gonzalez JF, Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371–2379. [DOI] [PubMed] [Google Scholar]
- 30. Sidney S, Quesenberry CP Jr, Jaffe MG, Sorel M, Go AS, Rana JS. Heterogeneity in national U.S. mortality trends within heart disease subgroups, 2000–2015. BMC Cardiovasc Disord. 2017;17:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH, Fonarow GC. National differences in trends for heart failure hospitalizations by sex and race/ethnicity. Circ Cardiovasc Qual Outcomes. 2017;10:e003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Christiansen MN, Kober L, Weeke P, Vasan RS, Jeppesen JL, Smith JG, Gislason GH, Torp‐Pedersen C, Andersson C. Age‐specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation. 2017;135:1214–1223. [DOI] [PubMed] [Google Scholar]
- 33. Deshmukh A, Pothineni NV, Patel N, Badheka A, Mulpuru SK, Paydak H, Noseworthy PA. Trends in hospitalizations of young patients with atrial fibrillation: a cause for concern? Int J Cardiol. 2016;203:164–165. [DOI] [PubMed] [Google Scholar]
- 34. Zhang W, Watanabe‐Galloway S. Ten‐year secular trends for congestive heart failure hospitalizations: an analysis of regional differences in the United States. Congest Heart Fail. 2008;14:266–271. [DOI] [PubMed] [Google Scholar]
- 35. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma J, Ward EM, Siegel RL, Jemal A. Temporal trends in mortality in the United States, 1969–2013. JAMA. 2015;314:1731–1739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of Nationwide Inpatient Sample States, 1988–2015
Data S1. Age standardization.
Table S2. Creating Weights Using 2000 Population
Table S3. Age Standardization of Overall Heart Failure Mortality Rate
Table S4. Age Standardization of Overall Heart Failure Mortality Rate Among Men
Data S2. Differentiating causes of death: primary (underlying cause of death) and secondary (multiple conditions).
Table S5. Temporal Trends of Primary Heart Failure and Atrial Fibrillation Hospitalization Among Hospitalization of Patients Aged ≥30 Years in the United States, Nationwide Inpatient Sample 1993–2014
Table S6. Temporal Trends and Annual Change of All Primary Heart Failure and Atrial Fibrillation Hospitalizations in Each Region and Division From 1993 to 2014
Table S7. Temporal Trends and Annual Change of Years of Potential Life Lost at Age 75 Years for Heart Failure and Atrial Fibrillation, 1991–2015
Table S8. Temporal Trends and Annual Change of Years of Potential Life Lost at Age 75 Years Due to Heart Failure and Atrial Fibrillation in States, 1991–2015
Figure S1. Temporal trends of years of potential life lost due to heart failure in the United States, 1991–2015: overall (A) and by sex (B) and race (C).
Figure S2. Temporal trends of years of potential life lost due to atrial fibrillation in the United States, 1991–2015: overall (A) and by sex (B) and race (C).
Figure S3. Mean age of heart failure deaths by race/ethnicity in the United States, 1991–2015.
Figure S4. Mean age of atrial fibrillation deaths by race/ethnicity in the United States, 1991–2015.
Video S1. Transitioning chloropleth map showing spatiotemporal changes in the mortality rates due to heart failure, 1990–2015 (best viewed with Windows Media Player).
Video S2. Transitioning choropleth map showing spatiotemporal changes in mortality rates due to atrial fibrillation, 1990–2015 (best viewed with Windows Media Player).


