Abstract
Background
Hospitalization for acute myocardial infarction (MI) in the United States is both common and expensive, but those features alone provide little insight into cost‐saving opportunities.
Methods and Results
To understand the cost drivers during hospitalization for acute MI and in the following year, we prospectively studied 11 969 patients with acute MI undergoing percutaneous coronary intervention at 233 US hospitals (2010–2013) from the TRANSLATE‐ACS (Treatment With ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome) registry. Baseline costs were collected in a random subset (n=4619 patients, 54% ST‐segment–elevation MI [STEMI]), while follow‐up costs out to 1 year were collected for all patients. The mean index length of stay was 3.1 days (for both STEMI and non‐STEMI) and mean intensive care unit length of stay was 1.2 days (1.4 days for STEMI and 1.0 days for non‐STEMI). Index hospital costs averaged $18 931 ($19 327 for STEMI, $18 465 for non‐STEMI), with 45% catheterization laboratory–related and 20% attributable to postprocedure hospital stay. Patient factors, including severity of illness and extent of coronary disease, and hospital characteristics, including for profit status and geographic region, identified significant variations in cost. Intensive care was used for 53% of non‐STEMI and increased costs by $3282. Postdischarge 1‐year costs averaged $8037, and 48% of patients were rehospitalized (half within 2 months and 57% with a cardiovascular diagnosis).
Conclusions
While much of the cost of patients with acute MI treated with percutaneous coronary intervention is probably not modifiable by the care team, cost reductions are still possible through quality‐preserving practice efficiencies, such as need‐based use rather than routine use of intensive care unit for patients with stable non‐STEMI.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00097591.
Keywords: acute myocardial infarction, cost, percutaneous coronary intervention
Subject Categories: Myocardial Infarction
Short abstract
See Editorial Weintraub
Clinical Perspective
What Is New?
The drivers of the cost of care for patients with acute myocardial infarction treated with percutaneous coronary intervention beyond the acute care time period are examined in a contemporary, prospective, observational registry that assessed 1‐year outcomes and medical costs in a cohort of US patients.
What Are the Clinical Implications?
Some practice efficiencies, such as need‐based use rather than routine use of the intensive care unit for patients with stable non–ST‐segment–elevation myocardial infarction, together with detailed local microcosting analyses of the cost‐of‐care delivery offer potential ways to reduce acute myocardial infarction costs without adversely affecting guideline‐directed care.
Introduction
Hospitalization for acute myocardial infarction (MI) remains 1 of the top 5 most expensive principal diagnoses in the United States.1 One of the largest cost components of a hospitalization for MI is coronary revascularization, mostly with percutaneous coronary intervention (PCI).2 Hospitals and health systems, under pressure to reduce costs in response to actual or predicted shrinking payer reimbursements, often have unrealistic, and potentially harmful, expectations regarding the potential to achieve large cost savings in expensive diseases, such as acute MI.3 Knowing that a particular disease or condition is associated with high costs by itself is not sufficient.4 Identifying the portion of care expenses that can be reduced without reducing quality requires 2 complementary types of analyses: large, usually multi‐institution, cohort analyses to identify the major drivers of cost variation in each disease or condition of interest (eg, sociodemographic, severity of illness, comorbidity, variations in processes of care, institutional level factors, and regional factors), and hospital level analyses to identify disease‐specific inefficiencies (eg, cost or use of medical supplies and equipment, personnel costs attributed to individual patient care) in the local care processes that can be modified.5
Much of the literature pertaining to drivers of cost of care for cardiovascular disease is now more than 20 years old.6 The most recent US data for patients with acute MI treated with PCI are 2013 index and 30‐day readmission costs after PCI (58% of patients with acute MI at admission) and Medicare reimbursements from 2010 to 2014 for 90‐day PCI episodes in Michigan (38% with acute MI at admission).7, 8 Current US data regarding the frequency and cost of hospital care for patients with acute MI treated with PCI beyond the acute period are lacking, and data from other regions of the world are less directly informative for understanding US cost patterns because of both differences in patterns of care and often substantially different resource costs.9, 10
The TRANSLATE‐ACS (Treatment With ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome) study was a prospective, observational registry that assessed 1‐year outcomes and medical costs of patients with acute MI who underwent primary PCI.11 In this study, we use patient‐level resource use and cost data collected in the TRANSLATE‐ACS registry to describe the acute and longer‐term costs of care for a large cohort of US patients with acute MI treated with PCI and to identify factors that were associated with intensity and cost of care provided, both during the index MI hospitalization and during the subsequent year.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Data Source
Between April 2010 and October 2013, TRANSLATE‐ACS enrolled 12 365 patients who presented with ST‐segment–elevation MI (STEMI) or non–ST‐segment–elevation MI (NSTEMI) at 1 of 233 US hospitals and were treated with PCI and discharged on a P2Y12 inhibitor.11, 12 Clinical sites were recruited from those participating in the American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR). Designed to reflect the broad spectrum of “real‐world” practice, TRANSLATE‐ACS had minimal enrollment exclusions and involved no treatment protocols.11, 12 The most common P2Y12 inhibitor prescribed to patients at discharge was clopidogrel (72% of patients), followed by prasugrel (25%). Patients discharged on ticagrelor, which was approved midway through the study, or ticlopidine were excluded from the primary study cohort, leaving 11 969 patients. Prespecified comparative effectiveness analyses found no difference between prasugrel and clopidogrel groups in risk‐adjusted major adverse cardiac events or bleeding.12 All patients provided written informed consent and study protocol approval was obtained from either a site‐based or central institutional review board.
Data Collection
Index hospitalization
Study coordinators abstracted patients’ demographic and clinical characteristics, angiographic and procedural details, and in‐hospital outcomes from medical records into the TRANSLATE‐ACS case report form. Measures of healthcare resource use included length of stay, counts of diagnostic catheterizations, counts of transfusions, and counts and types of revascularization procedures. Primary inpatient service and discharge disposition were also recorded. Billing data (UB‐04 claim forms and itemized bills) for the index hospitalization were collected for a random sample of enrolled patients stratified by P2Y12 inhibitor treatment (n=4619) for the purposes of estimating cost (described below) and describing intensity of care in more depth (eg, time in intensive care). The use of random sampling for baseline cost data was performed for budgetary reasons and had a target of up to 5000 patients.
Follow‐up hospital encounters
Hospital‐based encounters occurring after PCI discharge were identified through centralized telephone interviews (performed by the Duke Clinical Research Institute Outcomes Call Center) with patients or their proxies at 6 weeks and 6, 12, and 15 months postdischarge. In addition, enrolling sites queried their medical record at the end of study follow‐up to ensure all encounters at their sites were captured. Billing data were requested from hospitals for all follow‐up hospitalizations, including observation stays and emergency department visits. In addition to supporting cost estimation, billing data provided length of stay by intensity of care, International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis and procedures codes, Medicare Severity––Diagnosis‐Related Groups, and discharge disposition. Vital status for patients without a documented in‐hospital death was determined from end‐of‐study interviews with patients or their proxies and publicly available data sources.
Hospital characteristics
Characteristics of enrolling hospitals were obtained from the Centers for Medicare & Medicaid Services Provider of Services file.13 Variables of interest included hospital size, geographic location, hospital control (eg, public, for profit, not‐for‐profit), and teaching status. Geographic regions were defined using US census divisions.
Cost Estimation
The cost of hospitalizations and emergency department visits were estimated using hospital billing data, with the charges for each hospital encounter converted to costs using department‐level cost‐to‐charge ratios available in each hospital's annual Medicare Cost Report.14 For encounters for which bills were unobtainable (0.5% baseline, 7.3% follow‐up), cost was imputed based on reported length of stay and median daily costs for the encounter type, estimated from collected bills. Hospital costs were adjusted to 2013 US dollars using the producer price index for hospital care.15 Physician costs were estimated by weighting counts of major physician services, as reported in the case report form and hospital billing data, with 2013 national Medicare physician reimbursement rates.16
Statistical Analyses
Characteristics of the baseline and follow‐up cohorts were described using summary statistics or proportions, as appropriate. Key elements of resource use and cost for the index hospitalization were summarized in a similar manner. Factors associated with variations in index costs were explored using generalized linear models, specified with a log‐link function that assumes multiplicative effects of covariates on cost. An inverse Gaussian error distribution, which assumes residual variance is proportional to the cube of the mean, was chosen based on deviance residual plots and results of the Park test.17 CIs were calculated using robust standard errors to account for clustering of patients within hospital. The initial model restricted explanatory variables to baseline patient characteristics. The model was then expanded sequentially to include hospital characteristics, procedure characteristics, and complications. Alternative specifications that considered established risk scores for patients with acute MI (GRACE [Global Registry of Acute Coronary Events] mortality,18 ACTION [Acute Coronary Treatment and Intervention Outcomes Network] mortality,19 and ACTION bleeding risk20 scores) were also examined. Finally, the association between intensive care unit (ICU) use and cost was explored in the NSTEMI subset, as treatment protocols for these patients are less standardized and variation in ICU use has been observed without apparent detriment to clinical outcomes.21 The influence of specific factors was expressed as the marginal effect of each factor on cost, calculated for each patient and averaged over the sample (average marginal effect [AME]).
Resource use during follow‐up was summarized for patients who were discharged alive and completed at least 1 follow‐up interview. Care was classified as cardiovascular or noncardiovascular on the basis of the diagnosis‐related group, diagnosis, and procedure codes appearing in billing data. Mean admission counts, length of stay, and costs were estimated for time intervals of interest (first month and quarterly intervals through 1 year) using inverse probability weighting to account for censored follow‐up.22 Percentile‐based CIs were estimated using a bootstrap approach (1000 samples with replacement). Factors associated with hospital encounters in the year after discharge were examined using a 2‐part model that estimated the probability of an encounter (part 1) using probit regression, and follow‐up costs for those with encounters (part 2) using a generalized linear model, with simultaneous estimation of variance.17 The model of follow‐up costs was specified with a gamma error distribution, which assumes residual variance is proportional to the square of the mean, and a log link. In these models, both baseline patient characteristics and events during the index admission were considered. The AMEs of individual factors were examined for the combined model of cost, as well for the 2 individual component models. In these follow‐up models, all patients with complete 1‐year follow‐up data were included. Analyses were performed using SAS version 9.4 (SAS Institute) and StataSE 15 (StataCorp).
Results
Baseline Characteristics
Of 11 969 patients in the TRANSLATE‐ACS primary study population, 14 died during the index hospitalization and 11 599 (97%) completed at least 1 follow‐up interview postdischarge. Complete follow‐up information was available through 1 year postdischarge or death within 1 year for 10 439 patients (mean=360 days, median=365 days). Length of follow‐up averaged 200 days for those with partial data. Those less likely to have complete follow‐up data were younger, lacked insurance coverage, and were more likely to smoke cigarettes than those with full follow‐up data, but the groups had similar cardiac disease profiles (Table 1).
Table 1.
Patient and Hospital Characteristics, by Follow‐up Status (n=11 955)a
| Complete Follow‐up (n=10 439) | Incomplete Follow‐up (n=1160) | No Follow‐up (n=356) | P Value | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age, y | ||||
| <55 | 30.9 | 47.3 | 44.1 | <0.001 |
| 55 to 64 | 33.6 | 33.2 | 30.9 | |
| 65 to 74 | 22.8 | 12.7 | 17.4 | |
| ≥75 | 12.6 | 6.8 | 7.6 | |
| Women | 27.8 | 29.5 | 25.6 | 0.30 |
| White | 89.0 | 83.4 | 89.0 | <0.001 |
| Hispanic | 3.1 | 4.9 | 4.5 | 0.003 |
| ≥High school education | 88.8 | 85.1 | 89.0 | 0.001 |
| Employed | 50.0 | 52.8 | 52.0 | 0.10 |
| Health insurance | ||||
| Private | 66.0 | 53.9 | 58.7 | <0.001 |
| Government | 20.4 | 23.0 | 17.4 | |
| None | 13.6 | 23.1 | 23.9 | |
| Comorbid illness | ||||
| Dyslipidemia | 66.6 | 61.7 | 58.1 | <0.001 |
| Hypertension | 67.6 | 67.1 | 59.6 | 0.006 |
| Peripheral arterial disease | 6.5 | 6.2 | 5.3 | 0.67 |
| Prior stroke/TIA | 5.4 | 5.9 | 3.9 | 0.33 |
| Diabetes mellitus | 26.1 | 30.0 | 26.4 | 0.017 |
| Chronic lung disease | 9.8 | 10.7 | 8.2 | 0.34 |
| Smoker (within the past year) | 36.2 | 54.1 | 49.2 | <0.001 |
| GI/GU bleeding in the past 6 mo | 1.1 | 1.0 | 1.7 | 0.52 |
| Creatinine | ||||
| <30 mg/dL | 3.9 | 3.8 | 2.0 | <0.001 |
| 30 to 44 mg/dL | 9.2 | 6.4 | 6.7 | |
| 45 to 59 mg/dL | 17.2 | 13.3 | 14.9 | |
| ≥60 mg/dL | 69.7 | 76.6 | 76.4 | |
| Severity of cardiac disease/presentation acuity | ||||
| Atrial fibrillation | 4.8 | 3.9 | 3.4 | 0.17 |
| Prior MI | 19.5 | 19.0 | 21.6 | 0.54 |
| Prior CABG | 9.5 | 8.0 | 9.0 | 0.28 |
| Prior PCI | 21.5 | 21.7 | 23.0 | 0.79 |
| HF | ||||
| Signs within 2 wks before admission | 6.6 | 7.2 | 6.2 | 0.90 |
| History without signs within 2 wks of admission | 3.4 | 3.2 | 3.4 | |
| EF <40% | 12.6 | 12.9 | 15.5 | 0.28 |
| Left main disease | 2.9 | 2.6 | 2.3 | 0.62 |
| No. of diseased vessels | ||||
| ≤1 | 50.5 | 54.5 | 56.5 | 0.01 |
| 2 | 31.9 | 28.6 | 26.1 | |
| ≥3 | 17.6 | 16.9 | 17.4 | |
| Lesion length (sum, mm) | ||||
| <15 | 20.1 | 19.3 | 19.9 | 0.94 |
| 15 to 22 | 29.1 | 29.1 | 27.5 | |
| 23 to 30 | 25.5 | 25.0 | 27.3 | |
| ≥31 | 25.4 | 26.6 | 25.3 | |
| Bifurcated culprit lesion | 11.2 | 10.7 | 11.8 | 0.81 |
| Culprit lesion in graft | 4.6 | 4.1 | 3.4 | 0.47 |
| STEMI on presentation | 51.6 | 52.9 | 50.3 | 0.61 |
| Cardiac arrest on presentation | 3.0 | 2.8 | 1.4 | 0.20 |
| Cardiogenic shock on presentation | 2.1 | 2.5 | 0.6 | 0.08 |
| Heart rate on presentation, beats per min | ||||
| <60 | 13.1 | 11.8 | 10.1 | 0.03 |
| 60 to 100 | 74.8 | 73.3 | 75.8 | |
| >100 | 12.1 | 14.9 | 14.0 | |
| Hemoglobin on presentation, g/dL | ||||
| <13 | 22.2 | 19.7 | 18.5 | 0.14 |
| 13 to <15 | 40.8 | 42.1 | 40.7 | |
| ≥15 | 37.0 | 38.2 | 40.7 | |
| GRACE index mortality risk score | ||||
| Low (<109) | 80.8 | 86.4 | 85.7 | <0.001 |
| Moderate (109–140) | 16.1 | 11.9 | 13.2 | |
| High (>140) | 3.1 | 1.7 | 1.1 | |
| ACTION bleed risk score | ||||
| Very low | 22.5 | 24.8 | 25.3 | 0.02 |
| Low | 56.6 | 54.0 | 59.0 | |
| Moderate | 18.0 | 17.3 | 15.4 | |
| High | 2.5 | 3.3 | 0.3 | |
| Very high | 0.4 | 0.5 | 0.0 | |
| Well‐being before admission | ||||
| EQ‐5D visual analog scaleb | 71 (19) | 68 (21) | 69 (21) | <0.001 |
| EQ‐5D scoresc | ||||
| Mobility (some problems/confined to bed) | 20.7/1.5 | 23.3/1.2 | 18.7/1.7 | 0.21 |
| Self‐care (some problems/unable) | 7.2/1.0 | 8.1/0.6 | 7.4/0.6 | 0.52 |
| Usual activities (some problems/unable) | 20.6/5.3 | 24.3/4.3 | 16.2/5.1 | 0.005 |
| Pain (moderate/extreme) | 30.6/3.3 | 34.8/4.2 | 24.7/3.7 | <0.001 |
| Anxiety/depression (moderate/extreme) | 2.8/24.4 | 27.2/5.0 | 24.9/2.8 | <0.001 |
| PHQ‐2 (Depression) | ||||
| Low (0–2) | 86.7 | 83.7 | 86.8 | 0.08 |
| Moderate (3–5) | 10.5 | 12.7 | 10.7 | |
| High (6) | 2.8 | 3.6 | 2.5 | |
| Process of care | ||||
| Prior use of P2Y12 inhibitor | 87.0 | 86.7 | 87.4 | 0.95 |
| Transferred from another hospital | 40.2 | 37.2 | 38.5 | 0.11 |
| Prasugrel (vs clopidogrel) | 25.6 | 30.2 | 28.1 | 0.003 |
| Primary service cardiology/cardiothoracic surgery | 88.6 | 87.9 | 85.7 | 0.20 |
| Hospital characteristicsd | ||||
| Urban location | 96.4 | 96.7 | 97.2 | 0.60 |
| Bed size, No. | ||||
| <300 | 17.9 | 15.5 | 14.9 | 0.01 |
| 300 to 499 | 27.8 | 25.1 | 27.5 | |
| ≥500 | 54.2 | 59.4 | 57.6 | |
| Teaching hospital | 73.2 | 72.8 | 75.6 | 0.57 |
| Control | ||||
| Not‐for‐profit | 83.3 | 80.7 | 80.0 | 0.08 |
| For profit | 3.7 | 3.7 | 3.9 | |
| Government | 13.0 | 15.6 | 16.1 | |
| Region | ||||
| Northeast | 15.6 | 15.6 | 19.4 | 0.11 |
| Midwest | 38.2 | 36.0 | 37.1 | |
| South | 32.4 | 34.7 | 27.5 | |
| West | 13.8 | 13.6 | 16.0 | |
| Procedure characteristics | ||||
| Stent use | ||||
| BMS | 25.4 | 30.7 | 28.7 | <0.001 |
| DES | 71.7 | 66.1 | 66.9 | |
| None | 2.9 | 3.2 | 4.5 | |
| Femoral access (vs radial) | 88.2 | 86.8 | 85.7 | 0.17 |
| No. of vessels dilated | ||||
| ≤1 | 78.1 | 77.2 | 80.1 | 0.36 |
| 2 | 17.8 | 19.5 | 16.3 | |
| ≥3 | 4.2 | 3.4 | 3.7 | |
| Successful procedure | 96.1 | 96.6 | 93.5 | 0.04 |
| Complications | ||||
| Bleeding | ||||
| Hemodynamically unstable | 0.5 | 0.6 | 0.6 | 0.30 |
| Transfusion for overt bleeding | 0.2 | 0.3 | 0.6 | |
| Other | 2.5 | 1.9 | 1.2 | |
| CABG | 0.3 | 0.4 | 0.3 | 0.66 |
| Other surgery | 1.2 | 1.2 | 1.7 | 0.74 |
| MI | 0.6 | 0.4 | 0.3 | 0.53 |
| Cardiogenic shock | 1.2 | 1.6 | 1.7 | 0.25 |
| HF | 1.5 | 2.1 | 1.4 | 0.30 |
| Stroke/CVA | 0.1 | 0.2 | 0.0 | 0.72 |
| Specialty of follow‐up provider at discharge | ||||
| Cardiology | 33.3 | 36.5 | 37.4 | 0.04 |
| Internal medicine | 5.0 | 5.1 | 3.4 | |
| Other | 1.7 | 2.4 | 2.5 | |
| None | 60.0 | 56.0 | 56.7 | |
ACTION indicates Acute Coronary Treatment and Intervention Outcomes Network; BMS, bare‐metal stent; CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; DES, drug‐eluting stent; EF, ejection fraction; GI/GU, gastrointestinal/genitourinary; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention; PHQ‐2, Patient Health Questionnaire‐2; STEMI, ST‐segment–elevation myocardial infarction; TIA, transient ischemic attack.
Expressed as a percentage unless otherwise indicated. Excludes 14 patients who died during index hospitalization.
Mean (SD).
Excludes missing values (0.4%).
Excludes missing values (0.5%).
The mean age of patients was 59 years, with 28% women, 12% nonwhite, and 15% of patients without health insurance. The most common chronic risk factors were hypertension (68%) and dyslipidemia (66%), followed by smoking (38%) and diabetes mellitus (27%). One fourth of patients previously underwent revascularization, and one fifth had a previous MI. Half of patients presented with STEMI. Most patients were treated at teaching hospitals (73%) and in urban locations (96%). Index hospitalization costs were collected for 4619 randomly selected patients stratified by P2Y12 inhibitor treatment. While the characteristics of the index cost sample generally reflected those of the overall follow‐up cohort, patients in the index sample had slightly lower levels of baseline clinical risk (Table 2). This lower risk profile stemmed from the stratified sampling, which balanced prasugrel and clopidogrel patients in the index group. In contrast, patients who took clopidogrel, who tended to have higher risk profiles, accounted for three quarters of the overall sample (74%).
Table 2.
Patient and Hospital Characteristics for Index and Follow‐up Cohorts
| Overall Follow‐up Cohort (n=11 599)a | Subgroup Randomly Selected for Index Hospital Bill Collection (n=4619) | |
|---|---|---|
| Sociodemographic characteristics | ||
| Age, y | ||
| <55 | 32.6 | 35.6 |
| 55 to 64 | 33.6 | 34.1 |
| 65 to 74 | 21.8 | 20.6 |
| ≥75 | 12.0 | 9.7 |
| Women | 28.0 | 26.0 |
| White | 88.4 | 87.7 |
| Hispanic | 3.3 | 4.0 |
| ≥High school education | 88.4 | 89.7 |
| Employed | 50.0 | 53.2 |
| Health insurance | ||
| Private | 64.8 | 66.1 |
| Government | 20.6 | 18.4 |
| None | 14.6 | 15.5 |
| Comorbid illness | ||
| Dyslipidemia | 66.1 | 64.4 |
| Hypertension | 67.5 | 65.3 |
| Peripheral arterial disease | 6.4 | 5.5 |
| Prior stroke/TIA | 5.4 | 4.7 |
| Diabetes mellitus | 26.5 | 26.1 |
| Chronic lung disease | 9.9 | 9.3 |
| Smoker (within the past year) | 38.0 | 39.3 |
| GI/GU bleeding in the past 6 mo | 1.1 | 1.0 |
| Creatinine | ||
| <30 mg/dL | 3.9 | 3.1 |
| 30 to 44 mg/dL | 9.0 | 8.1 |
| 45 to 59 mg/dL | 16.8 | 15.4 |
| ≥60 mg/dL | 70.4 | 73.4 |
| Severity of cardiac disease/presentation acuity | ||
| Atrial fibrillation | 4.7 | 4.3 |
| Prior MI | 19.5 | 17.8 |
| Prior CABG | 9.3 | 8.5 |
| Prior PCI | 21.6 | 19.8 |
| HF | ||
| Signs within 2 wks before admission | 6.6 | 5.0 |
| History without signs within 2 wks of admission | 3.4 | 2.8 |
| EF <40% | 12.6 | 12.5 |
| Left main disease | 2.9 | 2.6 |
| No. of diseased vessels | ||
| ≤1 | 50.9 | 52.2 |
| 2 | 31.6 | 30.5 |
| ≥3 | 17.5 | 17.3 |
| Lesion length (sum, mm) | ||
| <15 | 20.0 | 19.1 |
| 15 to 22 | 29.1 | 29.8 |
| 23 to 30 | 25.4 | 26.0 |
| ≥31 | 25.5 | 25.2 |
| Bifurcated culprit lesion | 11.2 | 11.3 |
| Culprit lesion in graft | 4.5 | 3.8 |
| STEMI on presentation | 51.8 | 54.1 |
| Cardiac arrest on presentation | 3.0 | 3.2 |
| Cardiogenic shock on presentation | 2.1 | 2.3 |
| Heart rate on presentation, beats per min | ||
| <60 | 12.9 | 12.7 |
| 60 to 100 | 74.7 | 73.7 |
| >100 | 12.4 | 13.6 |
| Hemoglobin on presentation, g/dL | ||
| <13 | 22.0 | 19.5 |
| 13 to <15 | 40.9 | 40.9 |
| ≥15 | 37.1 | 39.6 |
| GRACE index mortality risk score | ||
| Low (<109) | 81.4 | 84.4 |
| Moderate (109–140) | 15.6 | 13.3 |
| High (>140) | 3.0 | 2.3 |
| ACTION bleed risk score | ||
| Very low | 22.7 | 23.2 |
| Low | 56.3 | 57.5 |
| Moderate | 17.9 | 16.8 |
| High | 2.6 | 2.0 |
| Very high | 0.4 | 0.5 |
| Well‐being before admission | ||
| EQ‐5D visual analog scaleb | 71 (19) | 71 (19) |
| EQ‐5D scoresc | ||
| Mobility (some problems/confined to bed) | 20.9/1.4 | 18.4/1.5 |
| Self‐care (some problems/unable) | 7.3/0.9 | 6.6/0.9 |
| Usual activities (some problems/ unable) | 20.9/5.2 | 18.9/4.6 |
| Pain (moderate/ extreme) | 31.0/3.4 | 29.7/3.6 |
| Anxiety/depression (moderate/extreme) | 24.7/3.1 | 24.2/3.2 |
| PHQ‐2 (Depression) | ||
| Low (0–2) | 86.4 | 87.4 |
| Moderate (3–5) | 10.7 | 9.8 |
| High (6) | 2.9 | 2.8 |
| Process of care | ||
| Prior use of P2Y12 inhibitor | 87.0 | 88.3 |
| Transferred from another hospital | 39.9 | 32.9 |
| Prasugrel (vs clopidogrel) | 26.1 | 53.4 |
| Primary service cardiology/ cardiothoracic surgery | 88.5 | 86.8 |
| Specialty of follow‐up provider at discharge | ||
| Cardiology | 33.6 | 33.8 |
| Internal medicine | 5.0 | 3.3 |
| Other | 1.8 | 1.4 |
| None | 59.6 | 61.5 |
| Hospital characteristicsd | ||
| Urban location | 96.4 | 96.8 |
| Bed size, No. | ||
| <300 | 17.7 | 18.4 |
| 300 to 499 | 27.6 | 34.0 |
| ≥500 | 54.7 | 47.5 |
| Teaching hospital | 73.4 | 64.6 |
| Control | ||
| Not‐for‐profit | 83.0 | 85.0 |
| For profit | 3.7 | 5.1 |
| Government | 13.3 | 9.8 |
| Region | ||
| Northeast | 15.6 | 14.6 |
| Midwest | 37.9 | 35.0 |
| South | 32.7 | 34.0 |
| West | 13.8 | 16.5 |
| Procedure characteristics | ||
| Stent use | ||
| BMS | 25.9 | 24.7 |
| DES | 71.1 | 72.6 |
| None | 2.9 | 2.7 |
| Femoral access (vs radial) | 88.0 | 87.9 |
| No. of vessels dilated | ||
| ≤1 | 77.9 | 79.4 |
| 2 | 17.9 | 17.2 |
| ≥3 | 4.1 | 3.5 |
| Successful procedure | 96.1 | 96.6 |
| Complications | ||
| Bleeding | ||
| Hemodynamically unstable | 0.5 | 0.4 |
| Transfusion for overt bleeding | 0.2 | 0.2 |
| Other | 2.5 | 2.6 |
| CABG | 0.3 | 0.2 |
| Other surgery | 1.2 | 1.0 |
| MI | 0.6 | 0.7 |
| Cardiogenic shock | 1.2 | 1.2 |
| HF | 1.5 | 1.4 |
| Stroke/CVA | 0.1 | <0.1 |
| Discharge disposition | ||
| Expired | 0.0 | 0.1 |
| Home | 98.4 | 98.7 |
| Acute care facility | 0.3 | 0.3 |
| Nonacute care facility | 1.1 | 0.7 |
| Other | 0.2 | 0.2 |
Values are expressed as percentages unless otherwise indicated. ACTION indicates Acute Coronary Treatment and Intervention Outcomes Network; BMS, bare‐metal stent; CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; DES, drug‐eluting stent; EF, ejection fraction; GRACE, Global Registry of Acute Coronary Events; GI/GU, gastrointestinal/genitourinary; HF, heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention; PHQ‐2, Patient Health Questionnaire‐2; STEMI, ST‐segment–elevation myocardial infarction; TIA, transient ischemic attack.
Excludes 370 patients without follow‐up interviews (including 14 baseline deaths).
Mean (SD).
Excludes missing values (0.4%).
Excludes missing values (0.5%).
Index Hospitalization Resource Use and Costs
While most patients were admitted directly for primary PCI, one third were transferred from another hospital. Most procedures targeted a single vessel (79%) with femoral access (88%). Stent placement was almost universal (97%), with drug‐eluting stents being the predominant choice (73% of patients). Complications were unusual and included heart failure (HF; 1.4%), cardiogenic shock (1.2%), surgery other than coronary artery bypass grafting (1.0%), bleeding with hemodynamic instability or transfusion (0.6%), recurrent MI (0.7%), and coronary artery bypass grafting (0.2%). Most patients were treated on a cardiovascular service (87%) and discharged home (99%). Inpatient mortality was rare (0.1%).
Mean length of stay was 3.1 days, with 1.2 days on average spent in intensive care (Table 3). Although patients with STEMI and those with NSTEMI had similar total lengths of stay on average, patients with STEMI were more likely to be treated in the ICU (81% versus 53%, P<0.001), with mean ICU days of 1.4 versus 1.0 (P<0.001). Mean length of stay was shorter for transradial than transfemoral procedures (2.8 versus 3.1 days, P=0.002). Hospital costs averaged $18 931, including physician fees. Catheterization laboratory expenses (including implanted devices) accounted for 45% of hospital cost. Other major components of cost included room and board (22%), supplies (14%), and pharmacy (9%). In unadjusted univariate analyses, baseline costs were higher among elderly patients ($19 575 ≥65 years versus $18 652 <65 years, P=0.004) and among those presenting with STEMI versus NSTEMI ($19 327 versus $18 465, P=0.002). Transradial procedures were not significantly lower in cost compared with transfemoral cases ($18 546 versus $18 984, P=0.3).
Table 3.
Index Hospitalization Resource Use and Cost
| Total Index Sample (n=4619)a | STEMI (n=2498) | NSTEMI (n=2121) |
|---|---|---|
| Length of stay, d | ||
| Ward | ||
| 0.9 (1.4) | 0.7 (1.2) | 1.0 (1.6) |
| (0, 0, 1) | (0, 0, 1) | (0, 0, 2) |
| Stepdown | ||
| 1.0 (1.5) | 0.9 (1.4) | 1.1 (1.6) |
| (0, 0, 2) | (0, 0, 2) | (0, 1, 2) |
| Intensive care | ||
| 1.2 (1.3) | 1.4 (1.3) | 1.0 (1.3) |
| (0, 1, 2) | (1, 1, 2) | (0, 1, 1) |
| Total | ||
| 3.1 (2.0) | 3.1 (1.9) | 3.1 (2.2) |
| (2, 3, 3) | (2, 3, 3) | (2, 3, 4) |
| Index hospitalization cost ($2013) | ||
| Hospital | ||
| 17 637 (9102) | 17 997 (9502) | 17 212 (8591) |
| (12 390, 15 642, 20 222) | (12 706, 15 903, 20 401) | (12 072, 15 217, 19 951) |
| Physician services | ||
| 1294 (468) | 1330 (480) | 1253 (451) |
| (1074, 1186, 1366) | (1082, 1224, 1396) | (1014, 1172, 1336) |
| Total | ||
| 18 931 (9414) | 19 327 (9823) | 18 465 (8889) |
| (13 511, 16 825, 21 557) | (13 877, 17 172, 21 739) | (13 169, 16 453, 21 305) |
NSTEMI indicates non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Values are expressed as mean (SD) and (25th, 50th, 75th) percentiles unless otherwise indicated.
In multivariable analyses, baseline patient characteristics associated with higher index hospitalization costs included severity of illness at presentation (recent HF symptoms, cardiac arrest, cardiogenic shock, low hemoglobin, STEMI presentation), extent of coronary artery disease (number of diseased vessels, total lesion length), and difficulty with self‐care (Figure 1). Of these, AMEs were highest for recent HF symptoms ($3070), long total lesion length ($3881), and difficulties with self‐care (unable to perform=$6159; moderate=$2957). Higher levels of bleeding risk, as measured by the ACTION bleeding risk score, were associated with increased costs, over and above the influence captured by individual risk factors (AMEs: very high risk=$7228; high risk=$3130). Female sex, older age, prior stroke, higher levels of depression, and treatment at a private for‐profit hospital were associated with somewhat lower index costs. Regional variation in cost was also apparent, with lowest costs observed in the south (AME=−$4150, relative to the west). When procedural factors and complications were added to the model, the effects of some related baseline characteristics were tempered, although results overall were not substantially altered. As expected, costs increased with the number of lesions treated and use of drug‐eluting stents. Access site did not significantly predict cost. Complications associated with increased cost (in addition to baseline risk) included need for coronary bypass graft surgery (AME=$42277), other surgeries ($13 317), stroke ($17 809), recurrent MI ($7293), HF ($4566), and bleeding ($2026) (Figure 2). In the NSTEMI subset, ICU care was associated with significantly increased cost, independent of baseline factors, procedural variables, and complications (AME=$3282, CI=$2593–$3971).
Figure 1.
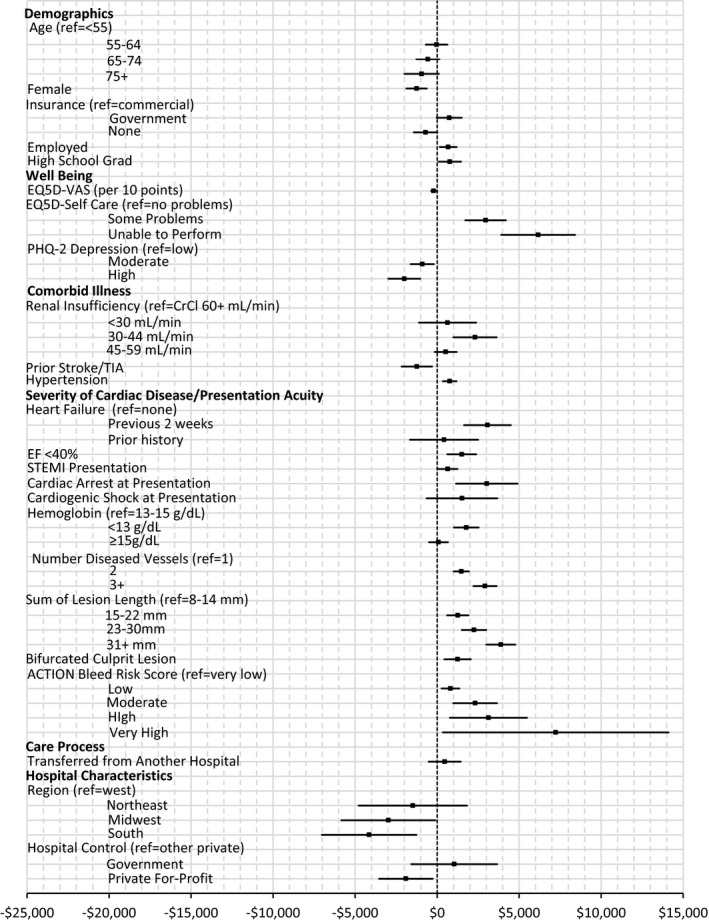
Average marginal effects of baseline factors on index hospitalization cost. Estimated marginal effects, averaged over the sample, of baseline factors on cost of index hospitalization (point estimate with 95% CI). n=4611 (8 patients excluded because of missing independent variables). ACTION indicates Acute Coronary Treatment and Intervention Outcomes Network; CrCl, creatinine clearance; EF, ejection fraction; PHQ, Patient Health Questionnaire; STEMI, ST‐segment–elevation myocardial infarction; TIA, transient ischemic attack; VAS, visual analog scale.
Figure 2.
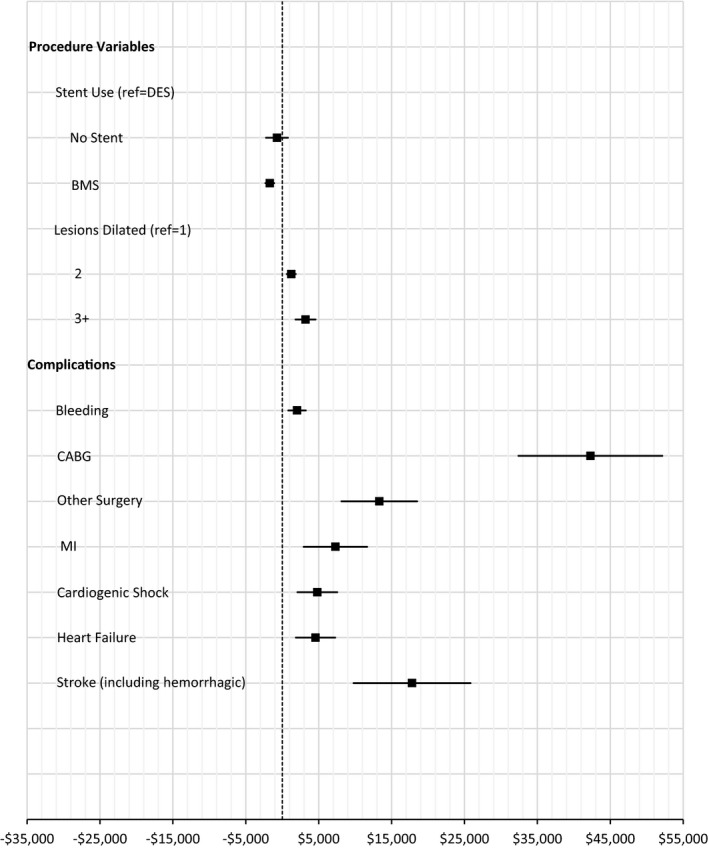
Average marginal effects of procedures and complications on index hospitalization cost. Marginal effects, averaged over the sample, of procedures and complications on cost of index hospitalization (point estimate with 95% CI). Procedures and complications were added to the model with baseline factors (Figure 1). n=4611 (8 patients excluded because of missing independent variables). BMS indicates bare‐metal stent; CABG, coronary artery bypass grafting; DES, drug‐eluting stent; MI, myocardial infarction.
Follow‐up Resource Use and Costs
In the year after discharge, 3% of patients died and 48% received additional in‐hospital care. One third of patients’ initial follow‐up encounters occurred within the first 30 days after discharge, and half occurred within 2 months (Figure 3A). One‐month costs, among those readmitted, averaged $8446. Follow‐up costs at 1 year, averaged over all patients, reached $8037, 57% of which were cardiovascular in nature (Figure 3B). Inpatient hospitalizations accounted for three quarters of average expenses ($6116), followed by outpatient hospital stays ($1334) and emergency department visits ($587).
Figure 3.
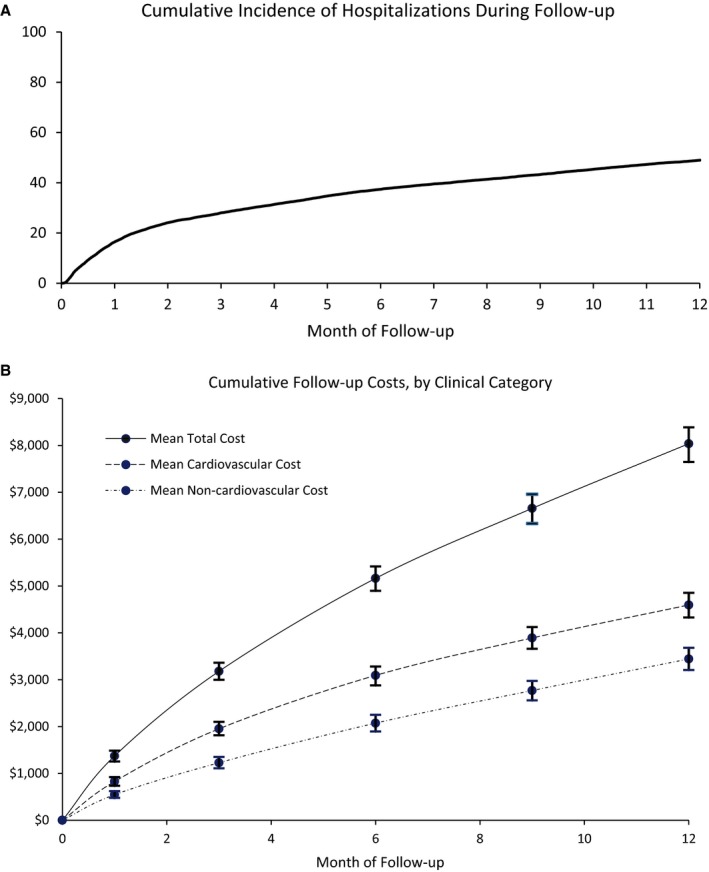
A, Cumulative incidence of hospitalizations during follow‐up. Cumulative incidence of first hospitalization (including emergency department encounters), accounting for the competing risk of death, over the 12‐month follow‐up period. B, Cumulative follow‐up costs, by clinical category. Cumulative mean costs at 1 month and at quarterly intervals over 1 year of follow‐up (total and stratified according to whether cardiovascular in nature). Vertical bars represent 95% CIs.
As with index costs, hospital costs during follow‐up increased with the baseline severity of cardiac illness, most notably multivessel disease (AME=$5277), chronic HF (AME=$4196), and low ejection fraction (AME=$4029) (Figure 4). During follow‐up, multiple baseline comorbid illnesses were significantly associated with increased costs. In addition to severe renal insufficiency (creatinine clearance <30 mL/min, AME=$3438) and hypertension (AME=$2003), which were also associated with higher index costs, presence of peripheral vascular disease (AME=$4037), diabetes mellitus ($2301), and chronic lung disease (AME=$1102) increased follow‐up costs. Diminished health‐related quality of life increased costs during follow‐up (AME=$1241–$3726 for moderate to severe self‐care difficulties, $1374 to $1701 for moderate to extreme pain, and $2102 for extreme anxiety). Process‐of‐care factors associated with follow‐up costs included angioplasty without stent placement (AME=+$4776), sole use of bare‐metal stents (+$3083), use of a P2Y12 inhibitor at baseline (AME=−$1812), and treatment on a cardiovascular service during the index admission (−$1618). While many of these factors affected both the probability of hospital encounters and the cost of care once hospitalized in a similar direction, consistent patterns of influence were not universal (Figures 5 and 6). For example, diabetes mellitus and STEMI presentation appeared to increase the cost of care for those hospitalized but had little effect on the likelihood of hospitalization. Conversely, chronic lung disease increased the probability of hospitalization but did not predict hospitalization costs (if hospitalized). Some factors, such as older but not advanced age and female sex, were related in opposite ways to the likelihood of hospitalization versus associated costs, resulting in a negligible net effect.
Figure 4.
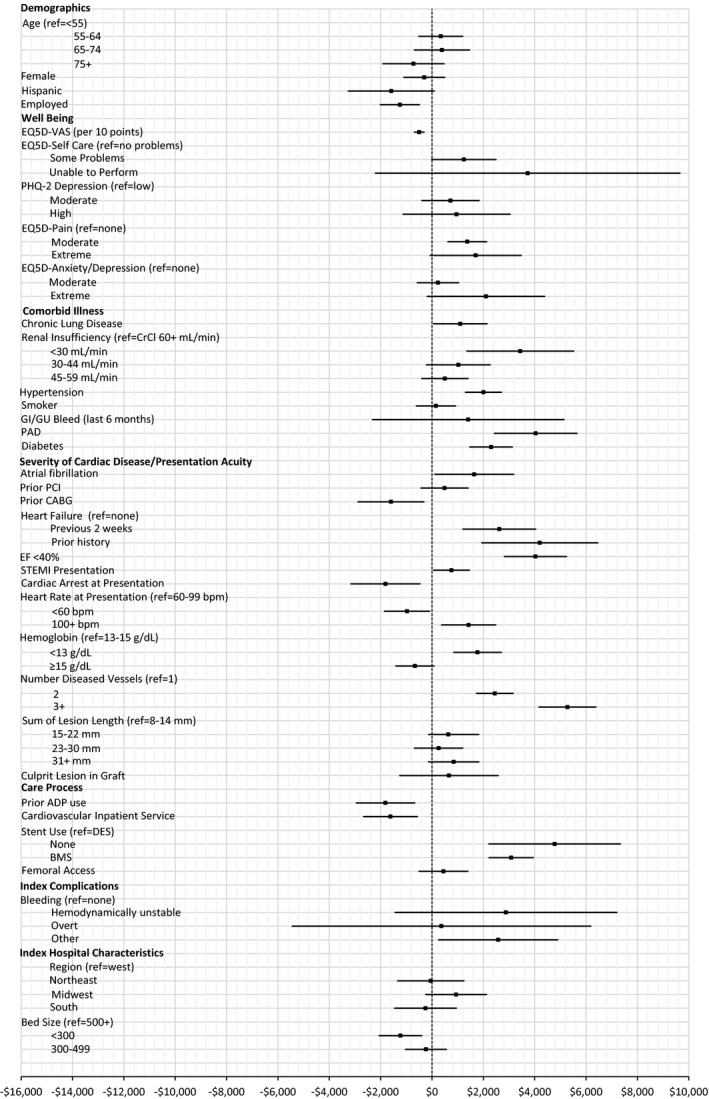
Average marginal effects of baseline and index factors on follow‐up cost. Estimated marginal effects, averaged over the sample, of baseline factors and events during index admission on cost of hospital care through 1 year (point estimate with 95% CI). n=10 328 (111 patients excluded due to missing independent variables). ADP indicates adenosine diphosphate; BMS, bare‐metal stent; bpm, beats per minute; CABG, coronary artery bypass grafting; CrCl, creatinine clearance; DES, drug‐eluting stent; EF, ejection fraction; GI/GU, gastrointestinal/genitourinary; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PHQ, Patient Health Questionnaire; STEMI, ST‐segment–elevation myocardial infarction; VAS, visual analog scale.
Figure 5.
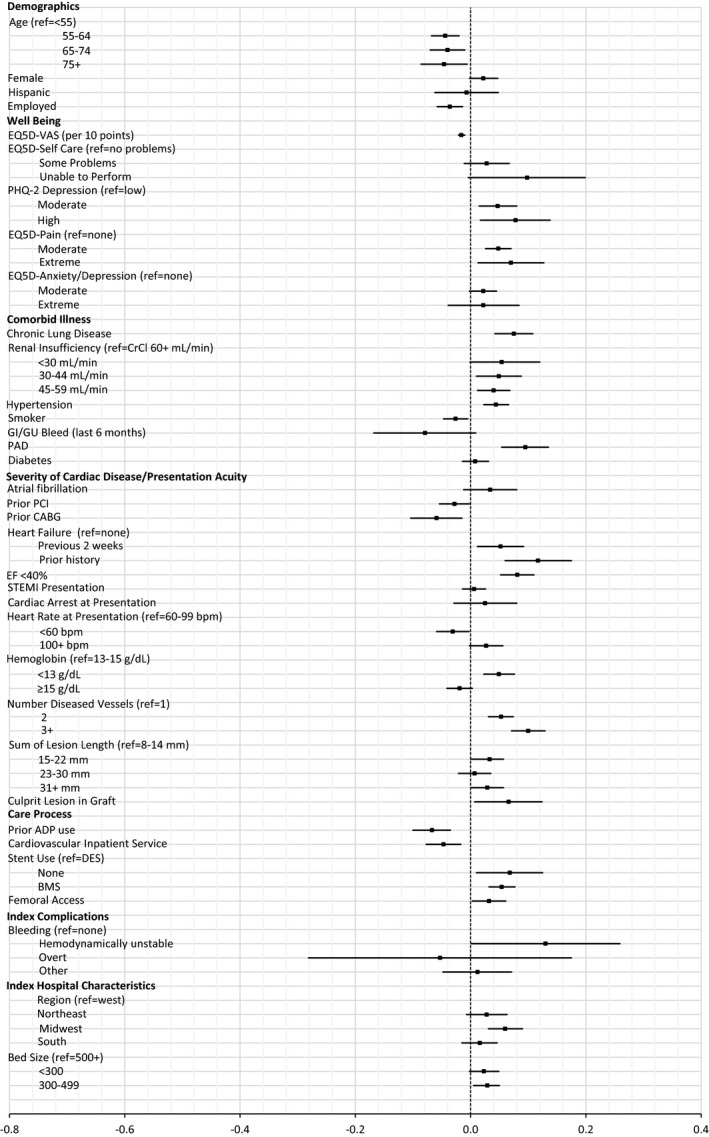
Estimated average marginal effects of baseline and index factors on probability of hospitalization. Estimated marginal effects, averaged over the sample, of baseline factors and events during index admission on probability of hospitalization through 1 year (point estimate with 95% CI). BMS indicates bare‐metal stent; CABG, coronary artery bypass grafting; CrCl, creatinine clearance; EF, ejection fraction; GI/GU, gastrointestinal/genitourinary; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; VAS, visual analog scale.
Figure 6.
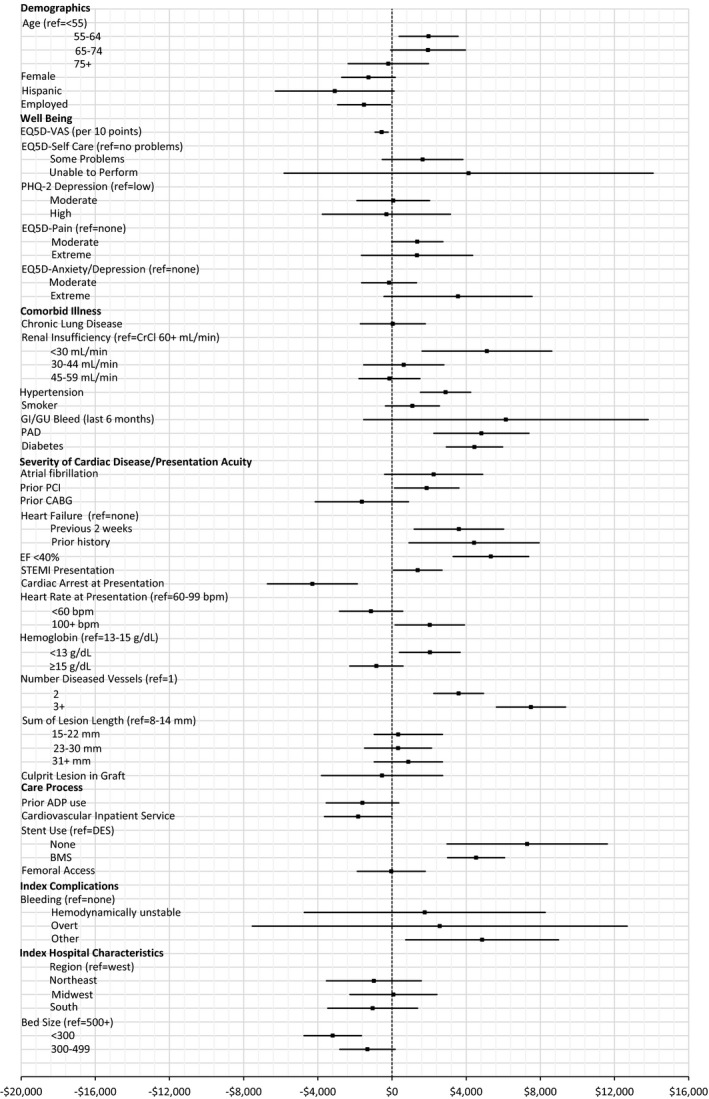
Estimated average marginal effects of baseline and index factors on hospitalization costs for those hospitalized. Estimated marginal effects, averaged over the sample, of baseline factors and events during index admission on hospitalization costs (for those hospitalized) through 1 year (point estimate with 95% CI). ADP indicates adenosine diphosphate; BMS, bare‐metal stent; bpm, beats per minute; CABG, coronary artery bypass grafting; CrCl, creatinine clearance; EF, ejection fraction; GI/GU, gastrointestinal/genitourinary; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; VAS, visual analog scale.
Discussion
Acute MI is 1 of the 5 most expensive causes of hospitalization (the estimated cost to the US health system was $12.1 billion in 2013), attributable to both the frequency with which it occurs and the cost associated with each acute hospitalization.23 Such statistics, however, provide little insight into the extent to which such costs can be recovered by decisions or actions of the care team without altering guideline‐directed care or other factors affecting quality. Understanding the opportunities for improved care efficiencies/cost reduction in a high‐cost condition such as acute MI requires 2 complementary forms of analysis: large cohort analyses to identify the major cost drivers, and local hospital microcosting analysis of the process of care for specific diseases or conditions.3 Large cohort studies are particularly useful for identifying the contribution of many different types of cost drivers that would be difficult for each hospital to identify properly from their own data alone. However, large cohort studies cannot obtain the granularity on hospital care delivery cost structures that allow judgment about whether some aspects of care can be made more efficient or less expensive at a specific institution.
This study focuses on the former type of analysis for patients with acute MI treated with PCI in 233 hospitals participating in the ACC NCDR. Much of the published cost data in this area are several decades old, pertain to health systems outside the United States, or use charges or reimbursements as a surrogate for costs.6 As part of the prospective TRANSLATE‐ACS research program, we planned an analysis of medical costs that would provide recent empirical cost data from a broad group of US hospitals participating in the ACC NCDR program. Many articles on the costs of different medical conditions do not distinguish the overall cost of care from the fraction of those costs that are potentially recoverable through changes in the process and/or efficiency of care. This distinction is important because most of the costs associated with high‐cost diseases are probably not recoverable through conventional means. For example, patients with complex comorbidities have a higher probability of developing a broad array of complications when hospitalized, regardless of the primary illness responsible for the admission or what interventions the medical team provide.24 By examining the major correlates of cost variations, we hoped to begin to develop useful insights into where potential opportunities might lie to reduce the costs of care associated with acute MI treated with PCI.
In the TRANSLATE‐ACS cohort, the cost of the index acute MI admission averaged $18 931 (median cost $16 825, 25th and 75th percentiles $13 511 and $21 557) (Table 3). A substantial portion of the index costs are a function of the standard way we currently treat acute MI, including the frequent use of invasive catheterization and PCI. In this study, the catheterization laboratory expenses accounted for almost half of the index hospital costs and the number of days in the hospital after the procedure accounted for an additional 20% of costs. Without changing the procedures performed or the standards for length of stay after a PCI in acute MI, this suggests that at most about one third of index costs might vary according to the specific patient being treated or the specifics of how they are treated. Given that all patients in the TRANSLATE‐ACS registry, by design, had a PCI, the cost effects of variations in the procedural details were generally modest (Figure 2). The primary exception was the need for coronary bypass surgery, which added about $40 000 to the cost of care (but was required in 0.2%). In this cohort, femoral artery catheterization was the dominant access method (88% of baseline cohort) and was associated with 0.3 days extra hospital stay (3.1 days versus 2.8 days). However, access site was not significantly related to index cost in unadjusted or adjusted analyses. The most expensive complication was stroke, which added over $17 000 to the hospitalization costs. Stroke is a prototypical example of a high‐cost, undesirable complication that is probably mostly not preventable with current methods of care, and thus the costs are not recoverable. Changes in the rates of stroke and associated costs will likely require new innovations in the PCI procedure or in the medical care given to protect the patient during the acute MI hospitalization.
Patient‐specific demographics and comorbid illnesses had a relatively modest effect on index costs, shifting mean index hospitalization costs <5%, if at all (Table 4). Extensive coronary disease (more diseased vessels, longer lesion length) had a greater impact, because of higher prevalence (≈20% of patients) and significant influence on cost (15–35% relative increase), but presents little opportunity for cost‐savings. Another factor that was both prevalent (20%) and costly ($2300–$7000 AME) without clear avenues for cost reduction was elevated risk of bleeding (Tables 2 and 4). Other factors with larger effect sizes were typically infrequent and not clearly a viable target for improving care efficiency/cost. For example, difficulties in self‐care (as measured by the EQ‐5D), reflecting frailty, were associated with ≈$3000 to $6000 higher index hospital costs (15–30% relative increase) and were present in 7% of the study cohort. Thus, the effect at the cohort level of this high‐cost condition is modest.
Table 4.
Average Marginal Effects for Index and Follow‐up Hospitalization Cost Models
| Average Marginal Effect, $a | |||
|---|---|---|---|
| Index Hospitalization Model With Baseline Risk Factorsb | Index Hospitalization Model With Baseline Risk Factors and Complicationsc | Follow‐up Hospitalization Modeld | |
| Sociodemographic characteristics | |||
| Age (reference: <55), y | |||
| 55 to 64 | −33 (−686 to 620) | −249 (−820 to 320) | 341 (−514 to 1196) |
| 65 to 74 | −564 (−1283 to 156) | −712 (−1380 to −44) | 388 (−688 to 1463) |
| ≥75 | −946 (−2000 to 108) | −1060 (−2015 to −104) | −723 (−1916 to 470) |
| Women | −1250 (−1872 to −630) | −1074 (−1688 to −460) | −301 (−1095 to 494) |
| Hispanic | n/a | n/a | −1587 (−3263 to 89) |
| Health insurance (reference: private) | |||
| Government | 738 (−25 to 1502) | 823 (33–1613) | n/a |
| None | −704 (−1417 to 9) | −501 (−1218 to 216) | |
| Employed | 672 (159–1186) | 356 (−105 to 817) | −1250 (−2011 to −489) |
| ≥High school education | 773 (84–1461) | 668 (26–1310) | n/a |
| Well‐being before admission | |||
| EQ‐5D visual analog scalec | −217 (−363 to −72) | −142 (−278 to −7) | −498 (−687 to −309) |
| EQ‐5D Self‐Care (reference: no problems) | |||
| Some problems | 2957 (1722–4191) | 1706 (690–2721) | 1241 (−9 to 2491) |
| Unable to perform | 6159 (3909–8410) | 5488 (3403–7572) | 3726 (−2204 to 9655) |
| PHQ‐2 depression (reference: low) | |||
| Moderate | −905 (−1615 to −195) | −710 (−1340 to −80) | 720 (−394 to 1834) |
| High | −2001 (−2973 to −1029) | −1724 (−2724 to −724) | 956 (−1131 to 3044) |
| EQ‐5D Pain (reference: none) | |||
| Moderate | n/a | n/a | 1374 (621–2129) |
| Extreme | 1701 (−74 to 3476) | ||
| EQ‐5D Anxiety/Depression (reference: none) | |||
| Moderate | n/a | n/a | 233 (−572 to 1038) |
| Extreme | 2102 (−180 to 4383) | ||
| Comorbid illness | |||
| Chronic lung disease | n/a | n/a | 1102 (62–2141) |
| Creatinine (reference: CrCl ≥60 mg/dL) | |||
| <30 mg/dL | 634 (−1105 to 2374) | 538 (−880 to 1957) | 3438 (1359–5517) |
| 30 to 44 mg/dL | 2307 (993–3622) | 2007 (915–3099) | 1026 (−217 to 2268) |
| 45 to 59 mg/dL | 522 (−143 to 1187) | 439 (−175 to 1053) | 502 (−400 to 1405) |
| Prior stroke/TIA | −1236 (−2162 to −310) | −758 (−1703 to 188) | n/a |
| Hypertension | 763 (344–1182) | 498 (80–916) | 2003 (1304–2701) |
| Smoker (within the past year) | n/a | n/a | 152 (−613 to 917) |
| GI/GU bleeding in the past 6 mo | n/a | n/a | 1409 (−2321 to 5140) |
| Peripheral arterial disease | n/a | n/a | 4037 (2427–5647) |
| Diabetes mellitus | n/a | n/a | 2301 (1473–3129) |
| Severity of cardiac disease/presentation acuity | |||
| Atrial fibrillation | n/a | n/a | 1643 (103–3184) |
| Prior PCI | n/a | n/a | 490 (−427 to 1406) |
| Prior CABG | n/a | n/a | −1597 (−2879 to −315) |
| HF (reference: none) | |||
| Signs within 2 wks before admission | 3070 (1646–4495) | 3191 (1798–4584) | 2622 (1205–4038) |
| History without signs within 2 wks of admission | 422 (−1656 to 2500) | 419 (−1534 to 2372) | 4196 (1940–6453) |
| EF <40% | 1504 (625–2383) | 1483 (638–2329) | 4029 (2817–5241) |
| STEMI on presentation | 644 (44–1244) | 875 (309–1440) | 758 (63–1454) |
| Cardiac arrest on presentation | 3031 (1156–4905) | 2423 (702–4143) | −1811 (−3160 to −462) |
| Cardiogenic shock on presentation | 1516 (−642 to 3674) | 1373 (−750 to 3496) | n/a |
| Heart rate at presentation (reference: 60–99 beats per min) | |||
| <60 | n/a | n/a | −971 (−1851 to −91) |
| >100 | 1425 (372–2478) | ||
| Hemoglobin on presentation (reference: 13–15 g/dL) | |||
| <13 | 1780 (1030–2530) | 1635 (966–2304) | 1772 (843–2701) |
| ≥15 | 83 (−491 to 658) | 130 (−430 to 691) | −666 (−1408 to 76) |
| No. of diseased vessels (reference: 1) | |||
| 2 | 1472 (1002–1941) | 1124 (677–1571) | 2446 (1729–3163) |
| ≥3 | 2920 (2215–3625) | 2292 (1641–2943) | 5277 (4167–6385) |
| Lesion length (sum) (reference: 8–14 mm) | |||
| 15 to 22 | 1260 (608–1913) | 1215 (590–1841) | 635 (−337 to 1607) |
| 23 to 30 | 2250 (1507–2993) | 1911 (1183–2639) | 259 (−683 to 1200) |
| ≥31 | 3881 (2993–4769) | 2992 (2055–3930) | 843 (−132 to 1818) |
| Bifurcated culprit lesion | 1251 (447–2054) | 1060 (275–1844) | n/a |
| Culprit lesion in graft | n/a | 659 (−1258 to 2575) | |
| ACTION bleed risk score (reference: very low) | |||
| Low | 813 (272–1353) | 602 (57–1148) | n/a |
| Moderate | 2316 (983–3650) | 2006 (698–3314) | |
| High | 3130 (778–5483) | 2444 (220–4668) | |
| Very high | 7228 (344–14112) | 5251 (−1411 to 11913) | |
| Process of care | |||
| Prior use of P2Y12 inhibitor | n/a | n/a | −1812 (−2949 to −675) |
| Transferred from another hospital | 463 (−514 to 1439) | 463 (−499 to 1424) | n/a |
| Primary service cardiology/cardiothoracic surgery | n/a | n/a | −1618 (−2665 to −572) |
| Procedure characteristics | |||
| Stent use (reference: DES) | |||
| None | n/a | −721 (−2231 to 790) | 4776 (2213–7338) |
| BMS | −1704 (−2315 to −1093) | 3083 (2222–3943) | |
| Femoral access (vs radial) | n/a | n/a | 445 (−506 to 1397) |
| No. of vessels dilated (reference: 1) | |||
| 2 | n/a | 1242 (612–1873) | n/a |
| ≥3 | 3194 (1822–4565) | ||
| Complications | |||
| Any bleeding | n/a | 2026 (823–3229) | n/a |
| Bleeding (reference: none) | |||
| Hemodynamically unstable | n/a | n/a | 2880 (−1435 to 7195) |
| Transfusion for overt bleeding | 368 (−5444 to 6180) | ||
| Other | 2577 (255–4898) | ||
| CABG | n/a | 42277 (32407–52147) | |
| Other surgery | n/a | 13317 (8125–18509) | n/a |
| MI | n/a | 7293 (2937–11648) | n/a |
| Cardiogenic shock | n/a | 4814 (2082–7546) | n/a |
| HF | n/a | 4566 (1870–7263) | n/a |
| Stroke/CVA | n/a | 17809 (9784–25833) | n/a |
| Hospital characteristics | |||
| Region (reference: west) | |||
| Northeast | −1485 (−4793 to 1822) | −1565 (−4744 to 1614) | −48 (−1339 to 1242) |
| Midwest | −2975 (−5838 to −111) | −2789 (−5615 to 36) | 939 (−245 to 2123) |
| South | −4150 (−7031 to −1270) | −4000 (−6807 to −1193) | −252 (−1456 to 951) |
| Control (reference: other private) | |||
| Government | 1043 (−1575 to 3662) | 1178 (−1255 to 3612) | n/a |
| Private for profit | −1904 (−3536 to −272) | −1725 (−3420 to −31) | |
| Bed size (reference: ≥500), No. | |||
| <300 | n/a | n/a | −1230 (−2057 to −403) |
| 300 to 499 | −236 (−1034 to 562) | ||
ACTION indicates Acute Coronary Treatment and Intervention Outcomes Network; BMS, bare‐metal stent; CABG, coronary artery bypass grafting; CrCl, creatinine clearance; CVA, cerebrovascular accident; DES, drug‐eluting stent; EF, ejection fraction; GI/GU, gastrointestinal/genitourinary; HF, heart failure; MI, myocardial infarction; n/a, not applicable (variable not in model); PCI, percutaneous coronary intervention; PHQ‐2, Patient Health Questionnaire‐2; STEMI, ST‐segment–elevation myocardial infarction; TIA, transient ischemic attack.
Mean (CI).
Corresponds to Figure 1.
Corresponds to Figure 2.
Corresponds to Figure 4.
Over the first year of follow‐up, 3% of patients died and 48% required additional inpatient care, at an average cost of $8037. Half of readmissions occurred during the 2 months following the index hospitalization. Similar temporal readmission patterns have been reported for patients with acute MI treated at the Cleveland Clinic (3069 patients, 2008–2012) and in the Medicare fee for service population (674 799 patients, 2008–2010).25, 26 Early readmissions might, on the basis of temporal proximity, be considered as sources of high medical costs that could be at least partially avoided by actions that could be taken during or shortly after the index hospital admission. Patients found to be at increased risk for early readmission might be candidates for several different types of intervention. One such intervention is to have the patient come back soon after discharge for their first outpatient visit. However, observational data in a cohort of almost 26 000 patients with NSTEMI (2003–2006) did not find evidence that early physician follow‐up was associated with lower 30‐day readmission rates.27 Another strategy that has been considered promising for reduction of early readmissions involves efforts to enhance adherence to guideline‐directed secondary prevention therapies. A previous report from the TRANSLATE‐ACS registry found that low medication adherence (present in 4% of the cohort) was associated with a 35% greater relative risk for death or rehospitalization.28 Low medication adherence, in turn, was associated with depression and patient‐reported financial hardship as a result of the cost of medications. In the present study, we found that depression increased the likelihood of hospitalization (Figure 5) and that greater levels of anxiety/depression at baseline were associated with higher follow‐up costs of care (Figure 4). This does not mean, however, that treating depression and anxiety would improve medication compliance or otherwise reduce these costs. In a study of 550 patients with acute MI with depression measured at baseline using the Beck Depression Inventory, depressed patients were more likely to undergo revascularization and have post‐MI complications, as well as more frequent rehospitalizations.29 Depression may be a risk marker rather than a cause of these events.
Stated more generally, simple unidimensional solutions to multidimensional problems often disappoint. Comorbidities, including chronic lung disease, advanced renal insufficiency, diabetes mellitus, and peripheral vascular disease, also identified patients with elevated probability of follow‐up hospital care in the present study and in a registry of 206 869 patients with PCI (2013, 58% acute MI).7 HF and more extensive coronary artery disease were additional indicators of higher follow‐up costs specifically related to the severity of coronary artery disease. However, being able to predict a higher probability of readmission appears much easier than agreeing about its preventability, and many predictable readmissions may not be preventable with currently available tools.30, 31 The total risk facing any given patient of becoming a high‐cost outlier will reflect the interplay of all these factors, with their relative importance varying on an individual level.
While the societal perspective on medical costs is primarily concerned with the healthcare resource consumption as reflected by medical costs, hospitals are highly focused on the balance between the costs they incur to provide care and the corresponding payments they receive. Reimbursements to individual hospitals, even for a homogeneous condition such as acute MI treated with PCI can vary in complex ways that often have little evident relationship to standard clinical and economic factors. Experiments by payers with bundled payment models for conditions requiring acute hospitalization have generally not shown any significant reductions in resource use or payments, possibly because incentive signals in the programs have been insufficient to promote major shifts in efficacy of care.32, 33 The business implications of the continually shifting reimbursement patterns hospitals face can be properly determined only when one also understands the major clinical and system cost drivers for the condition in question (from large multicenter analyses such as the present one), the mix of patients seen by the institution with that condition, and the institutional cost structures involved in care for that type of patient (such as can be developed from activity‐based costing).3, 33
ICU use represents one area in which practice patterns could likely be modified without compromising quality of care. While ICU admission is the dominant practice and is generally accepted as standard of care for patients with STEMI, routine admission of patients with NSTEMI to the ICU is less universal. Consistent with a recent study of Medicare patients enrolled in the ACTION registry and with a Canadian study of NSTEMI admissions, we found that 53% of patients with NSTEMI spent time in the ICU and that practice varied considerably.9, 34 Given the sizeable (≈$3300) contribution of ICU use to admission costs in our study, and the lack of association found between ICU admission and short‐term clinical outcomes in previous work,9, 34 risk‐based rather than routine ICU admission of patients with NSTEMI may offer an opportunity for improved efficiency and value of care.
Caveats
Our analysis should be interpreted in the context of the following caveats. First, the subsample for whom we collected index costs was balanced by clopidogrel versus prasugrel use. Clopidogrel use in the overall cohort was 74%, reflecting the practice of the time (2010–2013). Data from a large insurance claims database showed that for PCI in patients with acute coronary syndrome, use of clopidogrel decreased from about 80% in 2010 to 52% in 2016, with a corresponding increase in the use of prasugrel or ticagrelor.35 Substantive impact of these changes in medication use patterns on the results of our multivariable analyses of index cost drivers is unlikely. Second, although we used a combination of patient interviews and enrolling site queries to identify rehospitalizations, admissions to nonenrolling hospitals were less likely to be captured for the small proportion of patients who did not complete all follow‐up interviews. We incorporated patients with incomplete follow‐up in estimates of mean follow‐up costs through inverse probability weighting, and excluded them from multivariable analyses of 1‐year follow‐up costs. Third, participation in the TRANSLATE‐ACS registry was voluntary, and the study sample differed somewhat from the national acute MI PCI population: slightly younger (69% versus 54% <65 years), fewer minority patients (12% versus 38%), and more often treated in teaching hospitals (65% versus 53%).36 While these differences may affect absolute estimates of cost and resource use and should be considered when extrapolating results, factors identified as influencing cost are likely more robust. Fourth, physician service costs, which were approximated by applying Medicare fees to services in the CRF (case report form) and bills, are subject to inaccuracies in service counts and differences between Medicare fee schedule payments and actual costs of providing care. However, given the relatively small proportion of inpatient costs accounted for by physician services, and the correlation between physician and hospital costs, overall results would be relatively insensitive to this approximation. Fifth, use of radial access has increased since our data were collected. However, access type was considered in models, and multivariable results should be largely unaffected. Sixth, as our study was not designed to provide robust information at the hospital level, we could not assess the influence of structural/managerial hospital factors (such as staffing patterns, supply management, and case volume) on costs of care. Seventh, as collection of cost data outside the hospital setting was beyond the scope of this study, our estimates exclude the costs of outpatient visits, medications, and other nonacute care. In a study of fee‐for‐service Medicare beneficiaries (2013–2014), outpatient costs at 180 days post‐MI were about 10% of the magnitude of inpatient care costs.37 Finally, as of 2011, PCI was performed in about 77% of patients with STEMI and 34% of patients with NSTEMI in the United States.2 Because our study was designed to include patients with acute MI treated with PCI, results do not pertain to patients with acute MI treated medically or with coronary bypass surgery, and consideration of potential savings from a reduction in inappropriate primary PCI procedures is beyond the scope of this study.
Conclusions
In a large multicenter cohort of patients with acute MI treated with PCI, index costs averaged about $19 000, with an additional average cost of about $8000 during the subsequent year. Many of the strongest predictors of cost were not clearly modifiable by the care team or the health system. Nonetheless, some practice efficiencies, such as need‐based use rather than routine use of ICU for patients with stable NSTEMI, together with detailed local microcosting analyses of care delivery, offer potential ways to reduce acute MI costs without adversely affecting guideline‐directed care.
Sources of Funding
This work was supported by grants from Daiichi Sankyo and Lilly USA.
Disclosures
Dr Cowper has received grant funding from AstraZeneca, Eli Lilly & Company, GE Healthcare, Bristol Myers Squibb, Pfizer, Tenax Therapeutics, Gilead, Merck, and Novartis (all significant). Dr Peterson has received research grants from Eli Lilly & Company and AstraZeneca and consulting support from AstraZeneca (all significant). Dr Wang has received research grants to the Duke Clinical Research Institute from Amgen (modest), AstraZeneca (significant), Bristol Myers Squibb (modest), Cryolife (significant), Novartis (significant), Pfizer (modest), Portola (significant), and Regeneron (significant), as well as consulting honoraria from Grifols (modest) and Gilead (modest). Dr Mark has received grant funding from Eli Lilly & Company, Merck & Company, Oxygen Therapeutics, and HeartFlow (all significant) and consulting fees from CeleCor, Cytokinetics, and Novo Nordisk (all modest). The remaining authors have no disclosures to report.
Supporting information
Appendix S1. TRANSLATE‐ACS Investigators.
Acknowledgments
We are particularly indebted to the coordinators at the TRANSLATE‐ACS sites who enrolled the study patients and collected the study data and to the patients who agreed to participate in this study.
(J Am Heart Assoc. 2019;8:e011322 DOI: 10.1161/JAHA.118.011322.)
References
- 1. Torio C, Moore B. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013. HCUP Statistical Brief #204. May 2016. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.jsp. Accessed January 29, 2019. [PubMed] [Google Scholar]
- 2. Sugiyama T, Hasegawa K, Kobayashi Y, Takahashi O, Fukui T, Tsugawa Y. Differential time trends of outcomes and costs of care for acute myocardial infarction hospitalizations by ST elevation and type of intervention in the United States, 2001–2011. J Am Heart Assoc. 2015;4:e001445 DOI: 10.1161/JAHA.114.001445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaplan RS, Haas DA. How not to cut health care costs. Harv Bus Rev. 2014;92:142. [PubMed] [Google Scholar]
- 4. Dieleman JL, Squires E, Bui AL, Campbell M, Chapin A, Hamavid H, Horst C, Li Z, Matyasz T, Reynolds A, Sadat N, Schneider MT, Murray CJL. Factors associated with increases in US health care spending, 1996–2013. JAMA. 2017;318:1668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89:46–52, 54, 56‐61 passim. [PubMed] [Google Scholar]
- 6. Mark DB. Economics and cost effectiveness in cardiology In: Fuster V, Harrington RA, Narula J, Eapen ZJ, eds. Hurst's the Heart Manual of Cardiology. New York, NY: McGraw Hill; 2017. [Google Scholar]
- 7. Tripathi A, Abbott JD, Fonarow GC, Khan AR, Barry NGt, Ikram S, Coram R, Mathew V, Kirtane AJ, Nallamothu BK, Hirsch GA, Bhatt DL. Thirty‐day readmission rate and costs after percutaneous coronary intervention in the United States: a National Readmission Database Analysis. Circ Cardiovasc Interv. 2017;10:e005925. [DOI] [PubMed] [Google Scholar]
- 8. Alyesh DM, Seth M, Miller DC, Dupree JM, Syrjamaki J, Sukul D, Dixon S, Kerr EA, Gurm HS, Nallamothu BK. Exploring the healthcare value of percutaneous coronary intervention: appropriateness, outcomes, and costs in Michigan hospitals. Circ Cardiovasc Qual Outcomes. 2018;11:e004328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Diepen S, Tran DT, Ezekowitz JA, Zygun DA, Katz JN, Lopes RD, Newby LK, McAlister FA, Kaul P. The high cost of critical care unit over‐utilization for patients with NSTE ACS. Am Heart J. 2018;202:84–88. [DOI] [PubMed] [Google Scholar]
- 10. Janzon M, Henriksson M, Hasvold P, Hjelm H, Thuresson M, Jernberg T. Long‐term resource use patterns and healthcare costs after myocardial infarction in a clinical practice setting: results from a contemporary nationwide registry study. Eur Heart J Qual Care Clin Outcomes. 2016;2:291–298. [DOI] [PubMed] [Google Scholar]
- 11. Chin CT, Wang TY, Anstrom KJ, Zhu B, Maa JF, Messenger JC, Ryan KA, Davidson‐Ray L, Zettler M, Effron MB, Mark DB, Peterson ED. Treatment with adenosine diphosphate receptor inhibitors‐longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE‐ACS) study design: expanding the paradigm of longitudinal observational research. Am Heart J. 2011;162:844–851. [DOI] [PubMed] [Google Scholar]
- 12. Federspiel JJ, Anstrom KJ, Xian Y, McCoy LA, Effron MB, Faries DE, Zettler M, Mauri L, Yeh RW, Peterson ED, Wang TY; Treatment With Adenosine Diphosphate Receptor Inhibitors‐Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome (TRANSLATE‐ACS) Investigators. Comparing inverse probability of treatment weighting and instrumental variable methods for the evaluation of adenosine diphosphate receptor inhibitors after percutaneous coronary intervention. JAMA Cardiol. 2016;1:655–665. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Medicare and Medicaid Services . Provider of Services Current Files. 2017. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Provider-of-Services/. Accessed October 17, 2017.
- 14. Centers for Medicare and Medicaid Services . Cost Reports. 2017. Avaiable at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Cost-Reports. Accessed October 17, 2017.
- 15. Bureau of Labor Statistics . Databases, tables and calculators by subject. 2017. Accessed October 17, 2017.
- 16. Centers for Medicare and Medicaid Services . Physician fee schedule look‐up tool. 2017. Available at: https://www.cms.gov/apps/physician-fee-schedule. Accessed October 17, 2017.
- 17. Deb P, Norton EC, Manning WG. Health Econometrics Using Stata. College Station, TX: Stata Press; 2017. [Google Scholar]
- 18. The University of Edinburgh . Grace ACS Risk Score. 2017. Available at: http://gracescore.co.uk/risk-stratification. Accessed October 17, 2017.
- 19. Chin CT, Chen AY, Wang TY, Alexander KP, Mathews R, Rumsfeld JS, Cannon CP, Fonarow GC, Peterson ED, Roe MT. Risk adjustment for in‐hospital mortality of contemporary patients with acute myocardial infarction: the acute coronary treatment and intervention outcomes network (ACTION) registry‐get with the guidelines (GWTG) acute myocardial infarction mortality model and risk score. Am Heart J. 2011;161:e2. [DOI] [PubMed] [Google Scholar]
- 20. Mathews R, Peterson ED, Chen AY, Wang TY, Chin CT, Fonarow GC, Cannon CP, Rumsfeld JS, Roe MT, Alexander KP. In‐hospital major bleeding during ST‐elevation and non‐ST‐elevation myocardial infarction care: derivation and validation of a model from the ACTION Registry(R)‐GWTG. Am J Cardiol. 2011;107:1136–1143. [DOI] [PubMed] [Google Scholar]
- 21. Fanaroff AC, Chen AY, Thomas LE, Pieper KS, Garratt KN, Peterson ED, Newby LK, de Lemos JA, Kosiborod MN, Amsterdam EA, Wang TY. Risk score to predict need for intensive care in initially hemodynamically stable adults with non‐ST‐segment‐elevation myocardial infarction. J Am Heart Assoc. 2018;7:e008894 DOI: 10.1161/jaha.118.008894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika. 2000;87:329–343. [Google Scholar]
- 23. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 24. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto‐Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khot UN, Johnson MJ, Wiggins NB, Lowry AM, Rajeswaran J, Kapadia S, Menon V, Ellis SG, Goepfarth P, Blackstone EH. Long‐term time‐varying risk of readmission after acute myocardial infarction. J Am Heart Assoc. 2018;7:e009650 DOI: 10.1161/JAHA.118.009650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krumholz HM, Hsieh A, Dreyer RP, Welsh J, Desai NR, Dharmarajan K. Trajectories of risk for specific readmission diagnoses after hospitalization for heart failure, acute myocardial infarction, or pneumonia. PLoS One. 2016;11:e0160492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hess CN, Shah BR, Peng SA, Thomas L, Roe MT, Peterson ED. Association of early physician follow‐up and 30‐day readmission after non‐ST‐segment‐elevation myocardial infarction among older patients. Circulation. 2013;128:1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathews R, Peterson ED, Honeycutt E, Chin CT, Effron MB, Zettler M, Fonarow GC, Henry TD, Wang TY. Early Medication nonadherence after acute myocardial infarction: insights into actionable opportunities from the TReatment with ADP receptor iNhibitorS: Longitudinal Assessment of Treatment patterns and Events after Acute Coronary Syndrome (TRANSLATE‐ACS) study. Circ Cardiovasc Qual Outcomes. 2015;8:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lauzon C, Beck CA, Huynh T, Dion D, Racine N, Carignan S, Diodati JG, Charbonneau F, Dupuis R, Pilote L. Depression and prognosis following hospital admission because of acute myocardial infarction. CMAJ. 2003;168:547–552. [PMC free article] [PubMed] [Google Scholar]
- 30. van Galen LS, Cooksley T, Merten H, Brabrand M, Terwee CB, C HN, Subbe CP, Kidney R, Soong J, Vaughan L, Weichert I, Kramer MH, Nanayakkara PW. Physician consensus on preventability and predictability of readmissions based on standard case scenarios. Neth J Med. 2016;74:434–442. [PubMed] [Google Scholar]
- 31. Wasfy JH, Strom JB, Waldo SW, O'Brien C, Wimmer NJ, Zai AH, Luttrell J, Spertus JA, Kennedy KF, Normand SL, Mauri L, Yeh RW. Clinical preventability of 30‐day readmission after percutaneous coronary intervention. J Am Heart Assoc. 2014;3:e001290 DOI: 10.1161/JAHA.114.001290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joynt Maddox KE, Orav EJ, Zheng J, Epstein AM. Evaluation of Medicare's bundled payments initiative for medical conditions. N Engl J Med. 2018;379:260–269. [DOI] [PubMed] [Google Scholar]
- 33. Guduguntla V, Syrjamaki JD, Ellimoottil C, Miller DC, Prager RL, Norton EC, Theurer P, Likosky DS, Dupree JM. Drivers of payment variation in 90‐day coronary artery bypass grafting episodes. JAMA Surg. 2018;153:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fanaroff AC, Peterson ED, Chen AY, Thomas L, Doll JA, Fordyce CB, Newby LK, Amsterdam EA, Kosiborod MN, de Lemos JA, Wang TY. Intensive care unit utilization and mortality among medicare patients hospitalized with non‐ST‐segment elevation myocardial infarction. JAMA Cardiol. 2017;2:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dayoub EJ, Nathan AS, Khatana SAM, Seigerman M, Tuteja S, Kobayashi T, Kolansky DM, Groeneveld PW, Giri J. Use of prasugrel and ticagrelor in stable ischemic heart disease after percutaneous coronary intervention, 2009–2016. Circ Cardiovasc Interv. 2019;12:e007434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Afana M, Brinjikji W, Cloft H, Salka S. Hospitalization costs for acute myocardial infarction patients treated with percutaneous coronary intervention in the United States are substantially higher than Medicare payments. Clin Cardiol. 2015;38:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Likosky DS, Van Parys J, Zhou W, Borden WB, Weinstein MC, Skinner JS. Association between Medicare expenditure growth and mortality rates in patients with acute myocardial infarction: a comparison from 1999 through 2014. JAMA Cardiol. 2018;3:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. TRANSLATE‐ACS Investigators.


