Abstract
Background
Corticosteroid is an effective therapeutic option for inflammatory bowel disease flares, but its adverse effects may compromise treatment adherence and reduce patients’ quality of life. There is lack of data on the incidence of corticosteroid-induced mood changes in this patient population, which may be underappreciated by healthcare providers in clinical practice and interfere with optimal care. This study aimed to determine the rate of mood changes in this patient population.
Methods
In this prospective observational study, adult outpatients treated with prednisone for inflammatory bowel disease flares were considered for inclusion. Participants completed validated questionnaires (Beck Depression Inventory-II and Activation Subscale of Internal State Scale version two) before starting prednisone, after two weeks of prednisone, and at the end of prednisone taper to assess for mood changes. Harvey-Bradshaw Index and Simple Clinical Colitis Activity Index were used to monitor clinical disease activity.
Results
Fifty-three subjects were included in the analyses. The rate of mood change after two weeks of prednisone was 49.1%, primarily driven by increase in mood towards (hypo)mania. Younger age was an independent risk factor. Mood state returned to pretreatment level at the end of treatment. There was no correlation between clinical disease activity change and mood change.
Conclusions
Oral prednisone for inflammatory bowel disease flare is associated with high rate of mood change. As prednisone is a critical part of induction therapy, ways to minimize this adverse event must be studied. For now, healthcare providers should inform patients and monitor closely for this adverse event.
Keywords: Corticosteroid, Crohn’s disease, Inflammatory bowel disease, Mood, Prednisone, Ulcerative colitis
Since its introduction in 1948, corticosteroids (CS) have played a significant role in the treatment of allergic and immunological disorders such as asthma, giant cell arteritis, systemic lupus erythematosus, rheumatoid arthritis and inflammatory bowel disease (IBD). However, its use is primarily limited by the potential serious adverse effects, which can be systemic or neuropsychiatric (1–5). Current guidelines, therefore, recommend only short courses of CS in the treatment of acute IBD flares, with emphasis on CS-sparing maintenance therapy (6, 7).
While much attention has been paid to the systemic effects, relatively little is known about the neuropsychiatric effects that encompass a wide range of manifestations, most common of which include hypomania, depression and psychosis (8). Although previous studies have attempted to define the incidence of CS-induced mood changes (CIMC), most of the studies assessed either non-IBD conditions, which may have different baseline susceptibilities (9–18), or pediatric IBD patients (19, 20). Having a clear understanding of the neuropsychiatric adverse events will facilitate communication with patients and identification of such events more promptly, which is particularly important given that up to one-third of IBD patients may be nonadherent because of adverse medication effects (21). Education and early recognition may encourage dialogue between care providers and patients and prevent patients from simply discontinuing therapy. In the current study, we aimed to determine the rate of CIMC in patients with IBD undergoing outpatient oral CS therapy.
METHODS
Study Design and Patient Population
This is a prospective observational study at an outpatient clinic affiliated with an academic tertiary care centre (St. Paul’s Hospital, University of British Columbia, Vancouver, Canada). Adult patients (age ≥19 as per provincial definition) with inflammatory bowel disease, diagnosed on endoscopy and/or imaging, who were undergoing outpatient oral prednisone therapy for acute flares of IBD between October 2013 and April 2016 were considered for inclusion. Exclusion criteria included hospitalization for management of IBD within 2 weeks prior to study entry (due to possible use of intravenous CS), liver cirrhosis (due to potential effect on CS metabolism), use of medications that interfere with CS metabolism (e.g., clarithromycin, cyclosporine, ritonavir and ketoconazole), change in psychiatric medication within one month of study entry, and active recreational drug use or alcohol abuse.
Proposed CS course consisted of prednisone 40 mg daily for two weeks (6, 22–24), followed by a tapering course of 5 mg/day reduction every week. The treatment course could be adjusted at the physician’s discretion based on the subject’s clinical condition.
Participants completed two validated questionnaires for mood assessment at the time of CS initiation (first visit), at two-week follow-up after initiation of CS therapy (second visit), and at the end of the CS taper (third visit). Disease activity was also monitored at each visit using Harvey-Bradshaw Index (HBI) and Simple Clinical Colitis Activity Index (SCCAI) for Crohn’s disease and ulcerative colitis, respectively, by a physician, nurse or trained research assistant.
This study was approved by the institutional research ethics board and registered on clinicaltrials.gov (NCT01981889).
Questionnaire
Participants’ mood states were assessed at each visit using self-administered questionnaires that were validated in patients with various psychiatric disorders in the outpatient setting. Depressive symptoms were assessed using the Beck Depression Inventory II (BDI-II), a 21-item multiple choice questionnaire that asks participants to rate their symptoms such as sadness, loss of interest, guilt, agitation, suicidality, concentration and fatigue within the past two weeks (25). The total BDI-II possible score was 63. Hypomanic and manic symptoms were assessed using the Activation subscale of the Internal State Scale version 2 (AS-ISSv2), which consists of five questions that ask participants to rate the degree of manic symptoms such as impulsivity, rapid thought process, over-activity and restlessness over the past 24 hours (26). The total AS-ISSv2 score was 500, and a score of ≥155 was suggestive of mood elevation in the range of hypomania or mania and mixed state as seen in bipolar disorder. Of note is that hypomania and mania cannot be distinguished by this scoring system.
Mood changes were defined, a priori, as BDI-II increase by ≥10 and/or AS-ISSv2 increase by ≥50 between the first and second visit (i.e., after two weeks of prednisone). A change in BDI-II of 10 was chosen, as this increment also defines the various levels of depression severity. An AS-ISSv2 change of 50 was chosen because this is the minimum change required to appreciate symptom escalation in all five questions of this subscale and is also the amount previously observed in other studies with non-IBD conditions (13, 27).
Statistical Analysis
Our primary objective was to determine the rate of CIMC. A sample size calculation was performed based on an anticipated 40% rate of CS-induced mood change in IBD patients, based on previously published studies primarily focused on other immunological conditions (13, 28, 29) using a 95% confidence interval with precision of 0.15 and an anticipated drop out rate of 20%. The original target sample size was 50. However, given that a higher than expected drop out rate of ~33% after the initial 15 subjects, an updated sample size of 66 was calculated based on an estimated 40% drop out rate.
Baseline characteristics that are continuous variables were summarized as mean and standard deviation or median and quartiles, while categorical variables were reported as frequency and percentage. Comparisons between subjects were conducted using Chi-square test, Fisher exact test, t-test and Wilcoxon rank sum test, as appropriate, to identify potential risk factors. Univariate analysis was performed for participants who developed mood changes by the second visit. Multivariate logistic regression was performed for variables with P<0.20 in the univariate analysis. Correlations between mood score changes and IBD activity score changes were assessed using Spearman rank correlation coefficient. Pretreatment mood scores (baseline) and IBD activities were compared to those at follow-up visits using Wilcoxon signed-rank test.
Statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Statistical significance was defined as P value less than 0.05.
RESULTS
During the study period, 66 patients were considered for inclusion. Thirteen subjects were excluded (Figure 1). There was no significant difference in baseline characteristics (Table 1) between IBD patients who were excluded (n=13) compared with those who were included (n=53).
Figure 1.
Enrollment flow chart.
Table 1.
Baseline characteristics
| Variable | Included (n=53) | Excluded (n=13) | P |
|---|---|---|---|
| Age | 0.285 | ||
| Mean (SD) | 40.0 (15.0) | 45.1 (16.7) | |
| Median (IQR) | 36.0 (26.0, 56.0) | 48.0 (29.0, 57.0) | |
| Range | (21.0, 68.0) | (22.0, 70.0) | |
| Gender, n (%) | 0.420 | ||
| Female | 26 (49.1) | 8 (61.5) | |
| Male | 27 (50.9) | 5 (38.5) | |
| Diagnosis, n (%)* | 0.729 | ||
| Ulcerative colitis | 38 (71.7) | 8 (66.7) | |
| Crohn’s disease | 15 (28.3) | 4 (33.3) | |
| Presence of psychiatric condition, n (%)† | 1.000 | ||
| None | 48 (90.6) | 9 (90.0) | |
| Depression | 4 (7.5) | 1 (10.0) | |
| Anxiety | 1 (1.9) | 0 (0.0) | |
| Prior steroid exposure, n (%)† | 0.393 | ||
| No | 24 (45.3) | 6 (60.0) | |
| Yes | 29 (54.7) | 4 (40.0) | |
| IBD maintenance therapy, n (%)† | |||
| None | 7 (13.2) | 2 (10.0) | 0.573 |
| 5ASA | 33 (62.3) | 5 (50.0) | 0.467 |
| Immunomodulator | 7 (13.2) | 0 (0.0) | 0.223 |
| Biologic | 12 (22.6) | 2 (20.0) | 0.854 |
| Topical steroid | 4 (7.5) | 1 (10.0) | 1.000 |
| First visit SCCAI | 0.321 | ||
| Median (IQR) | 9.0 (8.0, 11.8) | 9.0 (6.3, 10.0) | |
| Mean (SD) | 9.2 (2.7) | 7.6 (3.1) | |
| Range | (3.0, 14.0) | (2.0, 10.0) | |
| First visit HBI‡ | 0.203 | ||
| Median (IQR) | 8.0 (5.5, 14.0) | 5.0 (4.5, 5.5) | |
| Mean (SD) | 9.9 (6.2) | 5.0 (1.4) | |
| Range | (2.0, 22.0) | (4.0, 6.0) | |
| First visit BDI-II§ | 0.881 | ||
| Median (IQR) | 9.0 (5.0, 13.0) | 11.0 (7.0, 12.0) | |
| Mean (SD) | 10.2 (7.3) | 12.1 (11.5) | |
| Range | (0.0, 25.0) | (2.0, 41.0) | |
| First visit AS-ISSv2¶ | 0.541 | ||
| Median (IQR) | 50.0 (0.0, 140.0) | 40.0 (0.0, 102.5) | |
| Mean (SD) | 79.6 (86.4) | 64.0 (78.3) | |
| Range | (0.0, 300.0) | (0.0, 200.0) |
5ASA, 5-aminosalicylic acid
*1 excluded patient with non-IBD diagnosis
†Data not available for three excluded patients
‡Data not available for two excluded patients
§Data not available for four excluded patients
¶Data not available for three excluded patients
Of the 53 subjects included in analyses, two subjects required hospitalization during the study period for escalation of therapy, including intravenous CS and surgery. Five subjects did not complete the third-visit questionnaire. Median follow-up time of those included in analyses was 64 (range 7–125, interquartile range [IQR] 57–72) days since start of prednisone therapy.
Thirty-three (62%) subjects completed the prednisone course per protocol. Seven subjects had prolonged taper due to persistent clinical symptoms, while 15 had rapid taper (one by physician during therapy due to insomnia; one by physician during therapy due to hypomania, which was also captured by AS-ISSv2; one by physician during therapy due to surgical cure obviating need for CS; one by physician during therapy due to poor response prompting therapy switch; four by physician during therapy due to exceptional response making longer therapy unnecessary; one by physician at initiation of therapy due to history of mood change; four by physician at initiation of therapy; two without specified reason).
Rate of Corticosteroid-Induced Mood Changes
Overall, the rate of mood changes with oral prednisone therapy was 49.1% (26 of 53) after a median of 15 (range 7–32, IQR 7–32) days on prednisone; the majority of the cases (25 of 26) were secondary to increases in manic symptoms as measured by AS-ISSv2, of which four also had concomitant depressive symptoms.
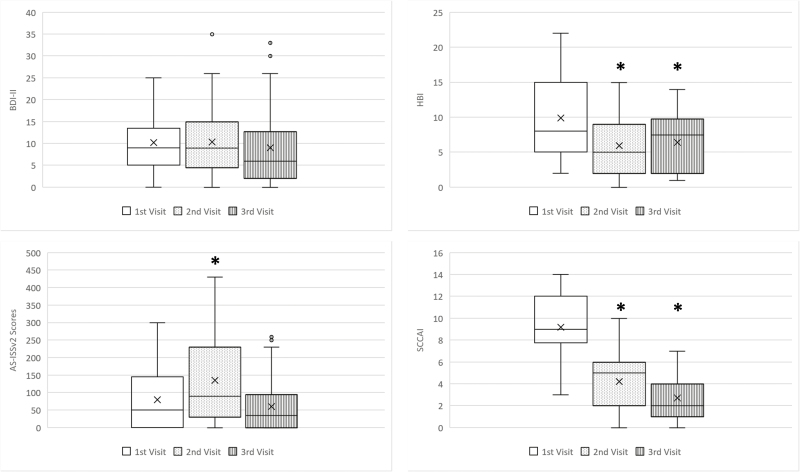
There was a significant increase in the AS-ISSv2 score with prednisone therapy at the second visit (mean 79.6 ± 86.4 versus 135.5 ± 120.2, P=0.004), which later returned to pretreatment level following the completion of tapering course at the third visit (mean 79.6 ± 86.4 versus 60.4 ± 72.5, P=0.250). However, no significant change in the BDI-II was seen between initial and subsequent visits. (Figure 2)
Figure 2.
Mood scores and disease activities before, during, and after steroid therapy.
* P < 0.05 compared to 1st visit
X mean
Clinical Disease Activities
Participants’ IBD symptoms improved with CS therapy both during and after completion of prednisone therapy. SCCAI improved from mean of 9.2 ± 2.7 to 2.7 ± 2.0 (P<0.001), and HBI improved from mean of 9.9 ± 6.2 to 6.4 ± 4.5 (P=0.024) at third visit after completion of CS treatment.
Risk Factors and Disease Activity Association
In the univariate analysis, younger age (32.8 versus 46.9 years) and female gender (65.4% versus 33.3%) were found to be associated with significant AS-ISSv2 or BDI-II changes, while IBD diagnosis, presence of psychiatric condition, type of maintenance therapy, initial disease activity score, duration of prednisone before mood reassessment, and positive response to treatment (defined as HBI/SCCAI reduction from ≥5 in first visit to <5 in second visit) were not. (Table 2) In the multivariate analysis, only younger age remained an independent risk factor (every five-year decrease, odds ration [OR] 1.41) (Table 3).
Table 2.
Univariate analysis of factors associated with mood score changes
| Variable | BDI-II increase by ≥10 and/or AS-ISSv2 increase by ≥50 | ||
|---|---|---|---|
| No (n=27) | Yes (n=26) | P | |
| Age | <0.001 | ||
| Mean (SD) | 46.9 (15.2) | 32.8 (11.1) | |
| Median (IQR) | 52.0 (33.0, 61.0) | 30.5 (24.0, 38.0) | |
| Range | (21.0, 68.0) | (21.0, 65.0) | |
| Female, n (%) | 9 (33.3) | 17 (65.4) | 0.020 |
| Diagnosis, n (%) | 0.317 | ||
| Ulcerative colitis | 21 (77.8) | 17 (65.4) | |
| Crohn’s | 6 (22.2) | 9 (34.6) | |
| Presence of psychiatric condition, n (%) | 0.610 | ||
| None | 24 (88.9) | 24 (92.3) | |
| Depression | 3 (11.1) | 1 (3.8) | |
| Anxiety | 0 (0.0) | 1 (3.8) | |
| Prior Steroid exposure, n (%) | 13 (48.1) | 16 (61.5) | 0.328 |
| IBD maintenance therapy, n (%) | |||
| None | 2 (7.4) | 5 (19.2) | 0.250 |
| 5ASA | 20 (74.1) | 13 (50.0) | 0.071 |
| Immunomodulator | 3 (11.1) | 4 (15.4) | 0.704 |
| Biologic | 6 (22.2) | 6 (23.1) | 0.941 |
| Topical steroid | 2 (7.4) | 2 (7.7) | 1.000 |
| First visit SCCAI | 0.280 | ||
| Median (IQR) | 9.0 (7.0, 11.0) | 9.0 (8.0, 12.0) | |
| Mean (SD) | 8.7 (3.0) | 9.8 (2.3) | |
| Range | (3.0, 14.0) | (6.0, 13.0) | |
| First visit HBI | 0.636 | ||
| Median (IQR) | 10.5 (5.0, 15.0) | 7.0 (6.0, 10.0) | |
| Mean (SD) | 10.8 (6.2) | 9.2 (6.4) | |
| Range | (4.0, 20.0) | (2.0, 22.0) | |
| Positive response to treatment, n (%) | 0.771 | ||
| No | 13 (54.2) | 14 (58.3) | |
| Yes | 11 (45.8) | 10 (41.7) | |
| No. of days between 1st and 2nd visit | 0.551 | ||
| Median (IQR) | 14.0 (14.0, 17.0) | 15.0 (14.0, 16.0) | |
| Mean (SD) | 15.3 (4.4) | 16.0 (3.5) | |
| Range | (7.0, 32.0) | (12.0, 27.0) | |
| Therapy protocol deviation, n (%) | 0.306 | ||
| Shorter | 10 (37.0) | 5 (19.2) | |
| Longer | 13 (48.1) | 18 (69.2) | |
| Per protocol | 4 (14.8) | 3 (11.5) | |
5ASA, 5-aminosalicylic acid
Table 3.
Multivariate analysis of factors associated with mood score changes.
| BDI-II increase by ≥10 and/or AS-ISSv2 increase by ≥50 | ||
|---|---|---|
| Variable | Odds ratio (95% CI) | P |
| Age (per 5-year decrease) | 1.41 (1.09, 1.82) | 0.010 |
| Male | 0.44 (0.12, 1.69) | 0.233 |
| Use of 5ASA | 0.32 (0.08, 1.21) | 0.093 |
5ASA, 5-aminosalicylic acid
Both initial assessment of SCCAI and HBI were found to have weak to moderate correlation with BDI-II (Spearman correlation of 0.37 [P=0.022] and 0.59 [P=0.020], respectively) but not with AS-ISSv2 at the initial visit prior to prednisone therapy. However, the correlation between IBD disease activity and BDI-II disappears during and after prednisone therapy (i.e., at second and third visits). There was also no significant correlation between the changes in the IBD disease activities and the changes in the mood symptoms between first and second visits.
DISCUSSION
This is the first prospective study to formally assess CS-induced mood changes in adult patients with IBD using self-administered scoring systems that have been validated in general population with various psychiatric condition (25, 30). To the best of our knowledge, there is no evidence directly linking CIMC to treatment nonadherence or poor outcomes in IBD patients, but medication adverse events and psychological distress are associated with compromised adherence to therapy in IBD patients (21, 30). In addition, mood disturbances (e.g., depression, mania and hypomania) are associated with reduced quality of life in patients with bipolar disorder (31) and so is CIMC in pediatric oncology patients and their families (32). A better understanding of CIMC is therefore needed to allow healthcare providers and patients to better prepare for such an adverse event and minimize its impact.
The rate of 49.1% is consistent with the results from previous studies. For example, psychological and behavioural disturbances among IBD patients were observed at a rate of 65 events per 100 patient-years in a meta-analysis by Hoes et al., which included studies employing various types of CS and dichotomous adverse event outcomes (9). In a randomized controlled trial comparing different prednisolone formulations, Rhodes et al. reported 42% incidence of mood changes after two weeks of prednisolone (33). However, the incidence was defined based on patients’ perception of presence or absence of mood change instead of more formal assessment tools.
Previous reports demonstrated that hypomania and frank mania tend to occur more frequently than depressive symptoms in acute CS therapy for non-IBD conditions (12–14, 34). Similarly, symptoms of (hypo)mania (i.e., impulsivity, rapid thought process, overactivity, feeling “sped up” inside or restlessness) were the primary psychological or behavioural changes in this study population. Twelve participants (22.6%) developed new onset mood features in the range of hypomania or mania and mixed state of bipolar disorder (i.e., AS-ISSv2 ≥155) at the second visit (26), of whom eight had complete resolution at the third visit. It was not possible to pinpoint the onset of the changes given the study design, but previous studies demonstrated CIMC in as early as three days of therapy when at least a 40 mg equivalent of prednisone was used (13, 14). The clinical implication is less clear for the 13 patients who experienced increases in AS-ISSv2 by at least 50 points but did not meet cutoff for hypomania, mania or mixed state. Nonetheless, this detectable change likely remains clinically relevant and is an outcome of interest in this observational study.
It is worth noting in this study that mood changes simply reversed with tapering course of CS and did not require additional intervention such as psychotropic medications, which have been reported in the past (35). None of the subjects required hospitalization for the management of psychological symptoms.
Over one-third of the participants’ CS course deviated from the protocol, but the majority was guided by clinical response to therapy. Three subjects’ courses were shortened due to intolerance (hypomania, insomnia and history of mood change), while no clinical rationale was provided in six (including four whose shorter courses were determined at initiation). The person with a history of CIMC did develop symptoms consistent with hypomania or mania (i.e., AS-ISSv2 ≥155) during treatment but returned to pretreatment state at the end of the study. Given that the protocol deviations were primarily guided by clinical symptoms, they were understandably not associated with detectable psychological changes on the questionnaires.
The mood changes observed in this study appeared largely independent of the IBD activity, as was observed in obstructive airway diseases (13, 18). This is demonstrated by the return of the mood state to pretreatment level while clinical IBD activity remained stable or improved following completion of therapy. In addition, there was no correlation between the changes in clinical IBD activities and the changes in mood scores, which supports prednisone’s role in the mood score changes. It is also worth noting that neither clinical disease activity indices incorporated objective measures such as endoscopic assessment or biomarkers, but SCCAI was previously demonstrated to correlate well with endoscopic inflammation, as well as serum and fecal inflammatory markers (36). The Harvey-Bradshaw Index was chosen for its straightforward administration and good correlation with Crohn’s Disease Activity index, which has been utilized extensively in previous Crohn’s disease trials (37). Although HBI does not correlate well with endoscopic inflammation or fecal inflammatory marker (36, 38), use of such clinical disease activity index is warranted in absence of an objective modality that can accurately assess all phenotypes of Crohn’s disease.
Patients with psychiatric comorbidities only accounted for 9% (five of 53) of the study population, and all were taking stable doses of psychiatric medications. Only two of five developed detectable mood changes, which is comparable to the rest of the study population, but the numbers are too small to draw any meaningful conclusion. We did not include patients who had recent change in psychiatric medications to minimize the impact of their underlying mental illness.
Female gender and younger age were associated with mood changes in the univariate analysis, but only age remained statistically significant in the multivariate analysis. Naber et al. reported that gender is not a risk factor for “neuropsychological and psychopathological changes” in patients receiving high-dose CS for ocular conditions (14). In contrast, higher incidence of CIMC in females was observed in earlier studies, but the interpretation was confounded by female predominance in certain autoimmune conditions such as rheumatoid arthritis and systemic lupus erythematosus (28, 39, 40).
Similar finding of emotional function impairment was observed in pediatric IBD patients treated with CS, compared with controls (IBD in remission) not taking CS (20). However, there is paucity of data on the relationship between age and risk of CIMC in the literature. Younger age has emerged as an independent risk factor for CIMC in our study, contrary to a previous report by Lewis and Smith examining psychiatric disturbances in a variety of conditions requiring CS for immunosuppression (28) and contrary to a prospective study by Brown et al. assessing mood changes during prednisone treatment of asthma (13). To our knowledge, there is no data directly comparing pediatric to adult population in terms of susceptibility to CIMC, but an earlier study found greater impact of CS on behaviour and mood in preschool children with acute lymphoblastic leukemia compared with school-aged children (41). It was postulated that the developing brain may be more susceptible to CS adverse effect (20), but this hypothesis is not applicable to adult patients. It is possible that the observed association was related to the greater proportion of younger patients with IBD in this study, compared with other autoimmune conditions included in previous reports. Alternatively, it may be related to a different phenotype of the disease in younger patients. Larger prospective data is required to draw more definitive conclusions regarding female gender and younger age as risk factors for CIMC among IBD patients.
This study has several limitations, including the single-centre design and the lack of a control group. However, as CS use is the standard of care in patients with IBD flares, ethical considerations preclude the institution of a placebo-controlled group. Comparison with an alternate induction agent such as anti-tumor necrosis factor alpha was also not feasible, as many patients were on concomitant CS or immunomodulator at the time of anti-tumor necrosis factor alpha initiation. To address this deficit, we incorporated a follow-up visit at the end of the CS treatment as internal control and demonstrated that the mood state returned to pretreatment level after completion of therapy. A potential confounder is patient adherence to prednisone therapy. As the first step to demonstrate the rate of CIMC in a real-life experience, patient adherence was not monitored; however, this information could be vital in future studies as an endpoint since nonadherence may be a result of CIMC.
The relatively small sample size limited the precision in assessing the rate of CIMC but did so without sacrificing accuracy. A larger sample size would have required a substantial, prohibitive amount of resources for recruitment. Therefore, we calculated the sample size based on a precision of 0.15. This study’s results are not generalizable to inpatients with IBD flares, which represent a significant portion of patients requiring systemic CS therapy at our centre. Inpatients were excluded to avoid potentially confounding effects of their severe disease activity and comorbidities, such as malnutrition and infection, on psychological state (42–44). Similarly, recent psychiatric medication changes and active alcohol or recreational drug use were excluded to minimize confounding. Further prospective study with larger sample size is needed to clarify the effect of CS on mood in these subgroups.
In conclusion, CS treatment was associated with frequent mood changes, affecting nearly half of the subjects in this study. The most common manifestation was mood elevation during therapy, but subsequently the affective state returned to pretreatment level upon completion of therapy without relapse of clinical disease activity. Healthcare providers should inform patients of such a risk and monitor for need of intervention. Future studies may help to clarify the role for concomitant therapy to ameliorate the mood-related side effects for patients requiring CS for management of their IBD.
Acknowledgements
The authors would like to thank Amy Wong and Oliver Takach for their assistance in subject recruitment and data collection. This work was supported by the 2014 Procter & Gamble-Canadian Association of Gastroenterology Resident Research Award. GO contributed to the conception and design of study, the acquisition, analysis and interpretation of data, and the drafting and final approval of the article. BB, NS, contributed to conception and design of study and the critical revision and final approval of the article. CG, EL, HHK, RE and JT contributed to acquisition of data and the critical revision and final approval of the article. TL contributed to the analysis and interpretation of data and the critical revision and final approval of the article. GR contributed to the conception and design of study, interpretation of data and critical revision and final approval of the article.
References
- 1. Manson SC, Brown RE, Cerulli A et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med 2009;103:975–94. [DOI] [PubMed] [Google Scholar]
- 2. Abroug F, Ouanes I, Abroug S et al. Systemic corticosteroids in acute exacerbation of COPD: A meta-analysis of controlled studies with emphasis on ICU patients. Ann Intensive Care. 2014;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kavanaugh A, Wells AF. Benefits and risks of low-dose glucocorticoid treatment in the patient with rheumatoid arthritis. Rheumatology (Oxford). 2014;53:1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sciascia S, Mompean E, Radin M et al. Rate of adverse effects of medium- to high-dose glucocorticoid therapy in systemic lupus erythematosus: A systematic review of randomized control trials. Clin Drug Investig. 2017;37:519–24. [DOI] [PubMed] [Google Scholar]
- 5. Proven A, Gabriel SE, Orces C et al. Glucocorticoid therapy in giant cell arteritis: Duration and adverse outcomes. Arthritis Rheum. 2003;49:703–8. [DOI] [PubMed] [Google Scholar]
- 6. Bressler B, Marshall JK, Bernstein CN et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: The Toronto consensus. Gastroenterology. 2015;148:1035–58.e1033. [DOI] [PubMed] [Google Scholar]
- 7. Terdiman JP, Gruss CB, Heidelbaugh JJ et al. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1459–63. [DOI] [PubMed] [Google Scholar]
- 8. Bhangle SD, Kramer N, Rosenstein ED. Corticosteroid-induced neuropsychiatric disorders: Review and contrast with neuropsychiatric lupus. Rheumatol Int. 2013;33:1923–32. [DOI] [PubMed] [Google Scholar]
- 9. Hoes JN, Jacobs JW, Verstappen SM et al. Adverse events of low- to medium-dose oral glucocorticoids in inflammatory diseases: A meta-analysis. Ann Rheum Dis. 2009;68:1833–8. [DOI] [PubMed] [Google Scholar]
- 10. Shimizu Y, Yasuda S, Kako Y et al. Post-steroid neuropsychiatric manifestations are significantly more frequent in SLE compared with other systemic autoimmune diseases and predict better prognosis compared with de novo neuropsychiatric SLE. Autoimmun Rev. 2016;15:786–94. [DOI] [PubMed] [Google Scholar]
- 11. Nishimura K, Omori M, Katsumata Y et al. Psychological distress in corticosteroid-naive patients with systemic lupus erythematosus: A prospective cross-sectional study. Lupus. 2016;25:463–71. [DOI] [PubMed] [Google Scholar]
- 12. Bolanos SH, Khan DA, Hanczyc M et al. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol. 2004;92:500–5. [DOI] [PubMed] [Google Scholar]
- 13. Brown ES, Suppes T, Khan DA et al. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22:55–61. [DOI] [PubMed] [Google Scholar]
- 14. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days’ corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21:25–31. [DOI] [PubMed] [Google Scholar]
- 15. Wolkowitz OM, Rubinow D, Doran AR et al. Prednisone effects on neurochemistry and behavior. Preliminary findings. Arch Gen Psychiatry. 1990;47:963–8. [DOI] [PubMed] [Google Scholar]
- 16. Gift AG, Wood RM, Cahill CA. Depression, somatization and steroid use in chronic obstructive pulmonary disease. Int J Nurs Stud. 1989;26:281–6. [DOI] [PubMed] [Google Scholar]
- 17. Joffe RT, Denicoff KD, Rubinow DR et al. Mood effects of alternate-day corticosteroid therapy in patients with systemic lupus erythematosus. Gen Hosp Psychiatry. 1988;10:56–60. [DOI] [PubMed] [Google Scholar]
- 18. Swinburn CR, Wakefield JM, Newman SP et al. Evidence of prednisolone induced mood change (‘steroid euphoria’) in patients with chronic obstructive airways disease. Br J Clin Pharmacol. 1988;26:709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castaneda AE, Tuulio-Henriksson A, Aronen ET et al. Cognitive functioning and depressive symptoms in adolescents with inflammatory bowel disease. World J Gastroenterol. 2013;19:1611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mrakotsky C, Forbes PW, Bernstein JH et al. Acute cognitive and behavioral effects of systemic corticosteroids in children treated for inflammatory bowel disease. J Int Neuropsychol Soc. 2013;19:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cerveny P, Bortlik M, Kubena A et al. Nonadherence in inflammatory bowel disease: Results of factor analysis. Inflamm Bowel Dis. 2007;13:1244–9. [DOI] [PubMed] [Google Scholar]
- 22. Bar-Meir S, Chowers Y, Lavy A et al. Budesonide versus prednisone in the treatment of active Crohn’s disease. The Israeli Budesonide Study Group. Gastroenterology. 1998;115:835–40. [DOI] [PubMed] [Google Scholar]
- 23. Rutgeerts P, Lofberg R, Malchow H et al. A comparison of budesonide with prednisolone for active Crohn’s disease. N Engl J Med. 1994;331:842–5. [DOI] [PubMed] [Google Scholar]
- 24. Campieri M, Ferguson A, Doe W et al. Oral budesonide is as effective as oral prednisolone in active Crohn’s disease. The Global Budesonide Study Group. Gut. 1997;41:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beck AT, Steer RA, Ball R et al. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. [DOI] [PubMed] [Google Scholar]
- 26. Bauer MS, Vojta C, Kinosian B et al. The internal state scale: Replication of its discriminating abilities in a multisite, public sector sample. Bipolar Disord. 2000;2:340–6. [DOI] [PubMed] [Google Scholar]
- 27. Brown ES, Stuard G, Liggin JD et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Biol Psychiatry. 2005;57:543–8. [DOI] [PubMed] [Google Scholar]
- 28. Lewis DA, Smith RE. Steroid-induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5:319–32. [DOI] [PubMed] [Google Scholar]
- 29. Fardet L, Kassar A, Cabane J et al. Corticosteroid-induced adverse events in adults: Frequency, screening and prevention. Drug Saf. 2007;30:861–81. [DOI] [PubMed] [Google Scholar]
- 30. Jackson CA, Clatworthy J, Robinson A et al. Factors associated with non-adherence to oral medication for inflammatory bowel disease: A systematic review. Am J Gastroenterol. 2010;105:525–39. [DOI] [PubMed] [Google Scholar]
- 31. Vojta C, Kinosian B, Glick H et al. Self-reported quality of life across mood states in bipolar disorder. Compr Psychiatry. 2001;42:190–5. [DOI] [PubMed] [Google Scholar]
- 32. McGrath P, Pitcher L.‘Enough is enough’: Qualitative findings on the impact of dexamethasone during reinduction/consolidation for paediatric acute lymphoblastic leukaemia. Support Care Cancer. 2002;10:146–55. [DOI] [PubMed] [Google Scholar]
- 33. Rhodes JM, Robinson R, Beales I et al. Clinical trial: Oral prednisolone metasulfobenzoate (Predocol) vs. oral prednisolone for active ulcerative colitis. Aliment Pharmacol Ther. 2008;27:228–40. [DOI] [PubMed] [Google Scholar]
- 34. Sirois F. Steroid psychosis: A review. Gen Hosp Psychiatry. 2003;25:27–33. [DOI] [PubMed] [Google Scholar]
- 35. West S, Kenedi C. Strategies to prevent the neuropsychiatric side-effects of corticosteroids: A case report and review of the literature. Curr Opin Organ Transplant. 2014;19:201–8. [DOI] [PubMed] [Google Scholar]
- 36. Ricanek P, Brackmann S, Perminow G et al. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46:1081–91. [DOI] [PubMed] [Google Scholar]
- 37. Sandborn WJ, Feagan BG, Hanauer SB et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512–30. [DOI] [PubMed] [Google Scholar]
- 38. Jones J, Loftus EV Jr, Panaccione R et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218–24. [DOI] [PubMed] [Google Scholar]
- 39. Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Gen Psychiatry. 1981;38:471–7. [DOI] [PubMed] [Google Scholar]
- 40. Boye Nielsen J, Drivsholm A, Fischer F et al. Long-term treatment with corticosteroids in rheumatoid arthritis (over a period of 9 to 12 years). Acta Med Scand 1963;173:177–83. [DOI] [PubMed] [Google Scholar]
- 41. Mrakotsky CM, Silverman LB, Dahlberg SE et al. Neurobehavioral side effects of corticosteroids during active treatment for acute lymphoblastic leukemia in children are age-dependent: Report from Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. Pediatr Blood Cancer. 2011;57:492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Porcelli P, Leoci C, Guerra V. A prospective study of the relationship between disease activity and psychologic distress in patients with inflammatory bowel disease. Scand J Gastroenterol. 1996;31:792–6. [DOI] [PubMed] [Google Scholar]
- 43. Trindade IA, Ferreira C, Pinto-Gouveia J. Ulcerative colitis symptomatology and depression: The exacerbator role of maladaptive psychological processes. Dig Dis Sci. 2015;60:3756–63. [DOI] [PubMed] [Google Scholar]
- 44. Mardini HE, Kip KE, Wilson JW. Crohn’s disease: A two-year prospective study of the association between psychological distress and disease activity. Dig Dis Sci. 2004;49:492–7. [DOI] [PubMed] [Google Scholar]