Abstract
The radiosensitizing effect of 5-aminolevulinic acid (5-ALA) has been demonstrated in glioma and melanoma in a number of studies. Enhancing the radiosensitivity of colorectal cancer may improve survival rates and lessen adverse effects. The present study assessed the radiosensitizing effect of 5-ALA in colorectal cancer using the human colon cancer cell line HT29 in vitro and in vivo. In vitro, cells were pretreated with 5-ALA and exposed to ionizing radiation. Cells pretreated with or without 5-ALA were compared using a colony formation assay. In vivo, HT29 cells were implanted into mice subcutaneously and subsequently exposed to ionizing radiation. 5-ALA was administrated by intraperitoneal injection. Subcutaneous tumors treated with or without 5-ALA were compared. Single-dose and multi-dose irradiations were applied both in vitro and in vivo. Cells exposed to multi-dose irradiation and pretreated with 5-ALA in vitro had a significantly lower surviving fraction compared with cells without 5-ALA pretreatment. Following multi-dose irradiation in vivo, the volume of the subcutaneous tumors treated with 5-ALA was significantly lower compared with that of tumors without treatment. These results suggest that radiotherapy with 5-ALA may enhance the therapeutic effect in colon cancer.
Keywords: 5-aminolevulinic acid, protoporphyrin IX, colorectal cancer, radiotherapy, radiosensitizing effect, radiosensitivity
Introduction
In 2016, colorectal cancer was the second most common cancer type and the third most common cause of cancer-associated mortality worldwide (1). In Japan, due to changes in lifestyles, the incidence and mortality rates of colorectal cancer are increasing annually. In 2010, the incidence rate of colorectal cancer was second to gastric cancer, and in 2013, its mortality rate was second to lung cancer, in Japan (2). Despite advances in diagnostic techniques and the increasing prevalence of screening programs, surgical resection with lymph node dissection remains the most common curative therapy for colorectal cancer (3). The types of radiotherapy (RT) used for colorectal cancer are adjuvant and palliative RT (3). Adjuvant RT is used to prevent postoperative recurrence following surgery and reduce the tumor volume to enable preservation of the anal sphincter prior to surgery in locally advanced rectal cancer (4,5). Palliative RT is used to relieve the symptoms and prolong the lives of patients with unresectable or recurrent colorectal cancer, who have symptomatic lesions (6,7). However, curative RT has not yet been established, and it is controversial whether RT for colorectal cancer improves the survival rate (8–13). One reason for this may be the low radiosensitivity of colorectal cancer (14). RT has certain adverse effects in patients with colorectal cancer, including skin (pain, erythema, epilation, desquamation, edema, ulceration, hemorrhage and necrosis), gastrointestinal (nausea, diarrhea, abdominal or rectal discomfort, abdominal or rectal pain, mucous or blood discharge, ileus, obstruction, fistula and perforation), genitourinary (frequent urination, nocturia, dysuria, bladder spasm, hematuria and bladder obstruction), neurological (pain and a feeling of discomfort in the legs or gluteal region) and other adverse effects (15,16). In particular, late adverse effects, including anal dysfunction, bowel dysfunction and sexual dysfunction, depend on the radiation dose fraction size (8). The majority of adverse effects should lessen over time; however, certain adverse effects may continue after completing treatments, which often makes the continuation of treatments after radiotherapy difficult (5,8,11,17,18). The present study considers that a new RT for colorectal cancer is necessary, which has equivalent effectiveness to conventional RT but involves lower doses of radiation. In addition, enhancing the radiosensitivity of colorectal cancer may improve survival rates and lessen adverse effects. 5-aminolevulinic acid (5-ALA) is a natural amino acid that is biosynthesized in the mitochondria of animals. 5-ALA is an important precursor of heme and is synthesized from glycine and succinyl CoA by mitochondrial aminolevulinic acid synthase in animal cells (19). In tumor cells, exogenous administration of 5-ALA induces high accumulation of protoporphyrin IX (PpIX) resulting from high expression of peptide transporter 1 and low expression of ATP-binding cassette sub-family G member 2 (20,21). PpIX is used as a photosensitizer in photodynamic diagnosis (PDD) and photodynamic therapy (PDT) for various types of cancer (19,20,22–24). Hematoporphyrin derivatives (HpD) and photofrin are used in PDT, and their effectiveness has been demonstrated in several cancer types (25–27). Previous studies have reported that HpD and photofrin also act as significant radiosensitizers (28,29). Although the radiosensitizing effect of 5-ALA is controversial, it has been demonstrated in glioma and melanoma by numerous studies (30–33). The mechanisms remain unclear; however, a previous study has reported that the initial reactive oxygen species (ROS) generated from water radiolysis and delayed production of ROS in mitochondria following ionizing irradiation enhance radiosensitivity (33). The present study assessed the radiosensitizing effects of 5-ALA in colorectal cancer in vitro and in vivo using the human colorectal cell line HT29.
Materials and methods
Chemicals
5-ALA was purchased from SBI Pharmaceuticals Co., Ltd., (Tokyo, Japan) and other chemicals [McCoy's 5A medium, phosphate-buffered saline (PBS), fetal bovine serum (FBS), trypsin/EDTA solution, antibiotics and anesthetic] were purchased from Wako Pure Chemical Industries, Ltd., (Osaka, Japan). 5-ALA was dissolved in fresh McCoy's 5A medium at a concentration of 1 mM for in vitro experiments. For the in vivo experiments, 50 mg 5-ALA was dissolved in 1 ml PBS and then the solution (0.1 ml) was administered by intraperitoneal injection. Details of each experiment are described in the following paragraphs.
Cell lines and animals
The human colorectal cancer cell line HT29 was purchased from the American Type Culture Collection (Manassas, VA, USA). These cells were grown in McCoy's 5A medium with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere with 5% CO2. A total of 24 6-week-old female BALB/c mice (16–21 g body weight) were used in the present study. The mice were housed in plastic cages with stainless steel grid tops in an air-conditioned environment at 23±1°C and a relative humidity of 50±5%, with a 12-h light/dark cycle. All mice were provided with food and water ad libitum. The animal protocols were approved by the Ethics Committee of Kyoto Prefectural University of Medicine (Kyoto, Japan).
Evaluation of the response of cells to irradiation in vitro
HT29 cells were seeded at 200 cells per well in six-well culture plates and incubated at 37°C for 24 h. For cells in the RT+5-ALA group in the single-dose experiments, the medium was replaced with fresh medium containing 1 mM 5-ALA. After 4 h, at 37°C, the medium was replaced with fresh culture medium. Control cells and cells of the RT only groups were subjected to the same procedure, but without 5-ALA treatment. In the RT and RT+5-ALA groups, cells were irradiated using an X-ray irradiator (M-150WE; SOFTEX Co., Ltd., Ebina, Japan) and exposed to 1 Gy ionizing radiation. In the multi-dose irradiation experiments, the RT+5-ALA group was subjected to the same procedure as the single-dose experiments and exposed to 0.3 Gy radiation on the first day. The following day, cells were treated with 5-ALA at 37°C for 4 h and then exposed to 0.3 Gy radiation again. These procedures were performed for 3 consecutive days (total radiation dose = 0.9 Gy). In the RT only groups, cells were subjected to the same procedure, but without 5-ALA treatment. At 10 days after the first irradiation, the cells were evaluated by a colony formation assay. Cells were fixed with 4% paraformaldehyde for 20 min and stained with Giemsa for 30 min at room temperature. The stained cells were observed under a Nikon 7S-100 light microscope (Nikon Corporation, Tokyo, Japan) at a magnification of ×40-100. Only colonies containing ≥50 cells were scored. The surviving fraction was calculated using the following formula: Surviving fraction = the number of colonies / the number of seeded cells prior to treatment.
Evaluation of the response of subcutaneous tumors to irradiation in vivo
Mice were subcutaneously injected with 2.5×106 HT29 cells in 0.1 ml PBS. Once the subcutaneous tumors had grown to a diameter of 3 mm (defined as day 1), the mice were randomly divided into two groups for single-dose irradiation experiments and four groups for multi-dose irradiation experiments, and subjected to the following procedures. Single-dose irradiation experiments included an ionizing irradiation group (RT group; n=2) and ionizing irradiation with 5-ALA group (RT+5-ALA group; n=6). For single-dose irradiation, the mice were divided into the RT and the RT+5-ALA groups only, as comparison between the control and 5-ALA groups was performed in the multi-dose irradiation experiment. In the RT group, 0.1 ml PBS was administered by intraperitoneal injection. The RT+5-ALA group received an intraperitoneal injection of 5 mg 5-ALA at 4 h before irradiation. Both groups were irradiated at a dose of 1 Gy on day 1. Mice were covered with a lead shield, except for the tumor regions, to avoid excessive radiation exposure. Multi-dose irradiation experiments included a control group (n=3), 5-ALA administration group (5-ALA group; n=3), ionizing irradiation group (RT group: n=5), and ionizing irradiation with 5-ALA administration group (RT+5-ALA group; n=5). In the control and RT groups, 0.1 ml PBS was administered by intraperitoneal injection. The control group received no further treatment. The RT and RT+5-ALA groups were irradiated at a dose of 3 Gy/day on day s 1, 3, 5, 8 and 10. Mice were covered in the same manner as for the single-dose irradiation experiments. The 5-ALA and RT+5-ALA groups received an intraperitoneal injection of 5 mg 5-ALA. For these two groups, 5-ALA was administered at 4 h before irradiation on every day that the mice were irradiated. The tumor volume was calculated every 2–3 days according to the following formula: Tumor volume = the shortest diameter2 × the largest diameter × 0.5. The tumor specimens were removed and their final weight was measured on day 20.
Statistic analysis
Differences in tumor volume among the groups were analyzed using one-way analysis of variance (ANOVA) and a Dunnett's multiple comparisons test. Differences in tumor weight and cell viability among the groups were analyzed using a non parametric Mann-Whitney U test. P<0.05 was considered to indicate a statistically significant difference.
Results
Radiosensitizing effect of 5-ALA in the HT29 cell line in vitro
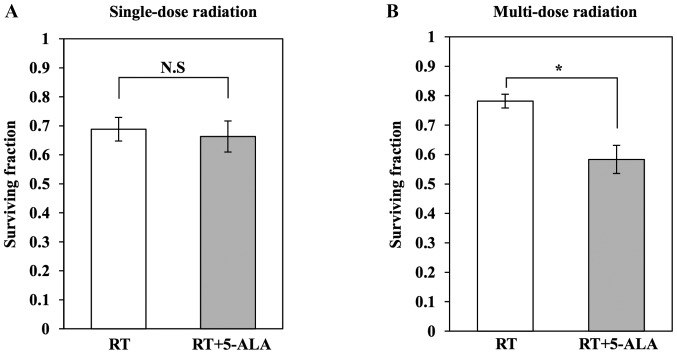
First, the radiosensitizing effect of 5-ALA in single-dose irradiation was evaluated. The surviving fraction of HT29 cells was compared using a colony formation assay. No significant difference was identified in the response of HT29 cells to single-dose irradiation between the RT group (n=3) and RT+5-ALA group (n=3) (P=0.210; Fig. 1A). Subsequently, the radiosensitizing effect in multi-dose irradiation was evaluated. In contrast to single-dose irradiation, a significant difference was identified in the response to multi-dose irradiation between the RT group (n=3) and RT+5-ALA group (n=3) (P=0.049; Fig. 1B). This result demonstrated that 5-ALA enhances the radiosensitivity of the HT29 cell line to multi-dose irradiation.
Figure 1.
Surviving fraction after single-dose and multi-dose radiations in vitro. (A) In the single-dose radiation experiment, the RT+5-ALA group tended to have a lower surviving fraction compared with that in the RT group; however, there was no significant difference. (B) In the multi-dose radiation experiment, the RT+5-ALA group had a significantly lower surviving fraction compared with that in the RT group. Data are presented as the mean ± standard deviation (n=3). *P<0.05. RT, radiotherapy; 5-ALA, 5-aminolevulinic acid.
Radiosensitizing effect of 5-ALA in HT29 tumor-bearing mice in vivo
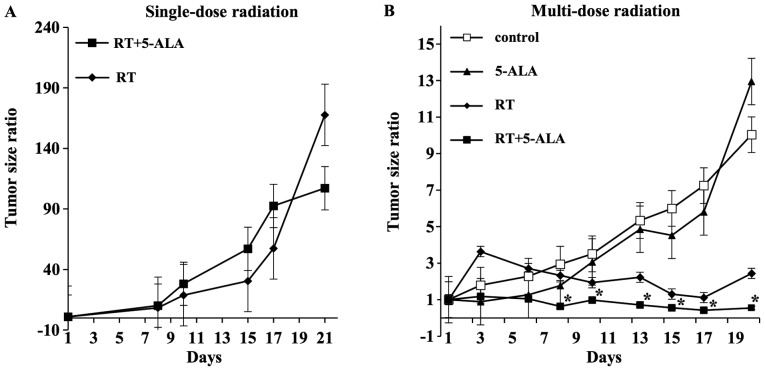
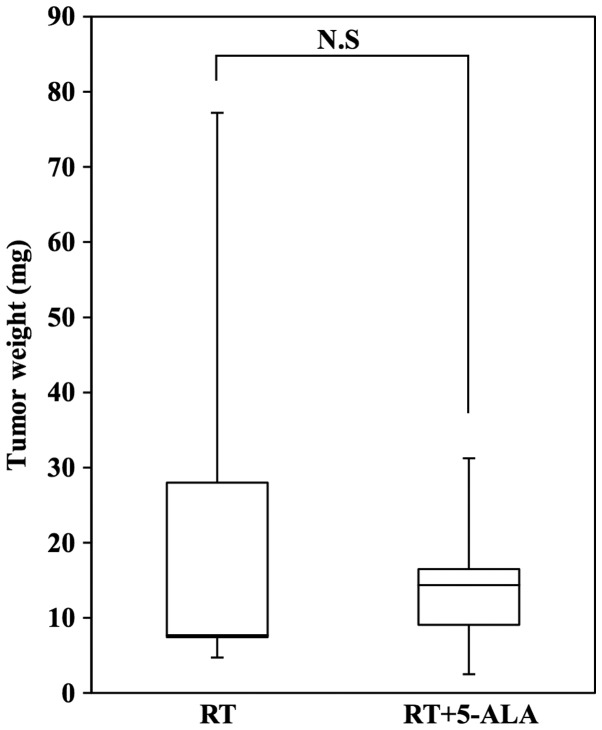
The HT29 subcutaneous tumor model was used to evaluate tumor suppression by RT in the presence of 5-ALA. Both single-dose and multi-dose irradiations were applied. In the single-dose irradiation experiment, the tumor size of the RT+5-ALA group was larger compared with that of the RT group at day 21 (Fig. 2A). In the multi-dose irradiation experiment, significant differences were revealed between the four groups at day 3 (P=0.0126), 8 (P=0.0482), 13 (P=0.0060), 15 (P=0.0102), 17 (P=0.0148) and 20 (P=0.0031) using ANOVA. With Dunnett's test, significant differences were identified between the control group and RT+5-ALA group at day 8 (P=0.0290), 10 (P=0.0365), 13 (P=0.0057), 15 (P=0.0088), 17 (P=0.0163) and 20 (P=0.0178). Finally, the subcutaneous tumors were removed and weighed. Although there was no significant difference in the tumor weights of the RT+5-ALA and the RT groups, the tumor weight in the RT+5-ALA group tended to be lower compared with that in the RT group (Fig. 3).
Figure 2.
Tumor size ratio after single-dose and multi-dose radiations in vivo. (A) In the single-dose radiation experiment, the tumor size of the RT+5-ALA group (n=6) was larger compared with that of the RT group (n=2) at day 21. (B) In the multi-dose irradiation experiment, significant differences were identified between the control group (n=3) and RT+5-ALA group (n=5) at day 8 (P=0.0290), 10 (P=0.0365), 13 (P=0.0057), 15 (P=0.0088), 17 (P=0.0163) and 20 (P=0.0178). Data are presented as the mean ± standard deviation. *P<0.05 vs. control. RT, radiotherapy; 5-ALA, 5-aminolevulinic acid.
Figure 3.
Tumor weights following treatment. No significant difference was identified in the tumor weights of the RT+5-ALA (n=5) and RT groups (n=5); however, the tumor weight in the RT+5-ALA group was markedly lower compared with that in the RT group (P=0.45). Data are presented as the mean ± standard deviation. RT, radiotherapy; 5-ALA, 5-aminolevulinic acid.
Discussion
Current RT for colorectal cancer can reduce the tumor size (4); however, there are certain adverse effects specific to the disease. These adverse effects often lower patient quality of life and make the continuation of treatment prior to RT difficult (5,8,11,17,18). A new RT approach for colorectal cancer is desirable, involving more effective tumor suppression with lower doses of radiation. PDT and PDD are based on excitation of a photosensitizer administered systemically or topically by light of a specific wavelength corresponding to the absorption peak of the photosensitizer (19,20). PDT and PDD are widely used for treatment of various cancer types (e.g., malignant glioma, bladder cancer, lung cancer, skin cancer, esophageal cancer and cholangiocarcinoma) due to their high selectivity for targeted tumors and non-invasiveness (19,20,22–27). Previous studies have demonstrated that HpD, photofrin, and 5-ALA are suitable photosensitizers for PDT and PDD of several types of cancer (e.g., malignant glioma, bladder cancer, lung cancer, skin cancer, esophageal cancer and cholangiocarcinoma) (19,20,22–27). However, there are certain side effects associated with these modalities. The main side effect is phototoxicity. The administration of a photosensitizer results in increased sensitivity of the skin and eyes of the patients to sunlight and bright indoor light for several weeks. Thus, the patient has to avoid exposure to bright light for a long period until the photosensitizer is metabolized in their body (34–36). HpD and photofrin require ~6 weeks of photosensitivity precautions (34–36). By contrast, PpIX synthesized from 5-ALA is cleared from the body within 24–48 h after systemic ALA administration (20). This is one of the main advantages of 5-ALA. HpD and photofrin have been demonstrated to act as radiosensitizers in previous studies (28,29). Photofrin has been reported to enhance radiosensitivity in human bladder cancer and human glioblastoma cell lines, but not in a colorectal cancer cell line (14). In general, ionizing radiation damages living cells via two mechanisms, namely by direct and indirect reactions. In the direct reaction, radiation is absorbed into the cells and affects DNA directly. In the indirect reaction, radiation induces water radiolysis and generates ROS, including superoxide, hydroxyl radicals and hydrogen peroxide, which damage DNA and cell organelles (37,38). Previous studies have reported that 5-ALA-induced PpIX serves an important role in the production of ROS (31,33). The radiosensitivity induced by HpD and photofrin is caused by higher intracellular concentrations of porphyrin compounds relative to 5-ALA-induced PpIX (39). Another study demonstrated that ionizing irradiation dose not decrease the quantity of 5-ALA-induced PpIX. Therefore, ionizing radiation does not influence the synthesis of 5-ALA-induced PpIX (33). Previous studies have demonstrated the radiosensitizing effects of 5-ALA in glioma and melanoma (30–33). Certain studies have reported that delayed production of ROS in mitochondria following ionizing irradiation, in addition to the initial ROS generated by water radiolysis, enhances radiosensitivity (33,40,41). The present study assessed the effects of 5-ALA in colorectal cancer using the human HT29 colorectal cancer cell line. To the best of our knowledge, this is the first study to demonstrate the possibility of the radiosensitivity of colorectal cancer as a result of 5-ALA both in vitro and in vivo. It was identified that 5-ALA radiosensitized HT29 cells following multi-dose irradiation but not single-dose irradiation. This result is similar to that reported in previous studies involving glioma cell lines (30,32,33). Pretreatment with 5-ALA prior to ionizing irradiation increased the delayed ROS production in the cytoplasm of glioma cells. Consequently, 5-ALA may act as a radiosensitizer (33). The current results suggest that radiotherapy with 5-ALA may enhance the therapeutic effect in colon cancer and the possibility of reducing the dose of radiation.
The present study had several limitations. First, the molecular mechanism underlying the radiosensitizing effect of 5-ALA was not investigated and the presence of increased intracellular ROS was not confirmed. In common with a previous study involving glioma, increased intracellular ROS may have led to the result that 5-ALA had a radiosensitizing effect in the HT29 cell line (33). However, other uncertain mechanisms may have contributed to the results. It has been reported that ROS generation is significantly higher in ALA-PDT-treated cells compared with control cells of the MKN-45 human gastric cancer cell line (42). In future studies the molecular mechanism underlying the radiosensitizing effect of 5-ALA should be investigated. Second, the RT alone group exhibited no radiosensitizing effect in the single-dose irradiation experiment in vivo. However, since single-dose irradiation has been performed clinically for certain types of neoplasm (e.g., breast cancer and metastatic brain tumor) (43,44). It is uncertain whether single-dose irradiation is not curative in colorectal cancer or whether 5-ALA exhibits no effect under the conditions used in the present study. Fractionated irradiation is widely applied to many cancer types based on the difference in the reactions of tumor and normal tissues (45,46) and fractionated irradiation is generally used for radiotherapy of colorectal cancer in Japan (3). Clinically, colorectal cancer demonstrates radiation resistance. A previous study has reported that human HT29 colorectal cancer cells have lower radiosensitivity compared with other cell lines (14). The result of the single dose irradiation experiment is possible in clinical practice. In the present study, the dose in the single dose irradiation experiment may be too low for tumor shrinkage. However, in the multi-dose irradiation experiment, the result was improved. Further experiments should be performed to verify this point. Third, images of the tumors were not obtained and were not presented in the current study. Photographs of the tumors should be presented to demonstrate the extent that the tumor sizes were altered. Fourth, although a significant difference was identified in the tumor size ratios of the control and the RT+5-ALA groups in the multi-dose irradiation experiment, no significant difference in tumor weight was revealed between the RT group and the RT+5-ALA group. A previous study has reported that a long interval between preoperative irradiation and surgery increases tumor downstaging. A long interval (6–8 weeks) between preoperative radiotherapy and surgery is associated with a significantly improved clinical tumor response and pathological downstaging (5). If observations were extended over a longer period, a significant difference may be identified between the two groups regarding tumor weight.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YM, YKa and EO designed the study. KY and YKa performed the experiments and acquired the data. KY, YM and YKa analyzed and interpreted the data. TA, TKo, HK, RM, AS, YKu, HI, TKu, MN, HF and KO assisted in preparing the experiments and interpreting the data. KY and YM were the major contributors in writing the manuscript. All authors were involved in the preparation and revision of the manuscript. All authors critically reviewed and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were conducted in accordance with the relevant guidelines for animal research without any unnecessary distress. The protocols were approved by the Ethics Committee of the Kyoto Prefectural University of Medicine (Kyoto, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, Anderson BO, et al. Global Burden of Disease Cancer Collaboration Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et al. The Editorial Board of the Cancer Statics in Japan (eds.), corp-author . Cancer Statics in Japan-2014. FPCR c/o National Cancer Center; Tokyo: 2015. Foundation for Promotion of Cancer Research; pp. 1–114. [Google Scholar]
- 3.Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et al. Japanese Society for Cancer of the Colon and Rectum Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207–239. doi: 10.1007/s10147-015-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skibber JM, Hoff PM, Minsky BD. Cancer of the rectum. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th edition. Lippincott, Williams and Wilkins; Philadelphia: 2001. pp. 1271–1318. [Google Scholar]
- 5.Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine P, Gerard JP. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: The Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396–2402. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 6.Wong CS, Cummings BJ, Brierley JD, Catton CN, McLean M, Catton P, Hao Y. Treatment of locally recurrent rectal carcinoma--results and prognostic factors. Int J Radiat Oncol Biol Phys. 1998;40:427–435. doi: 10.1016/S0360-3016(97)00737-2. [DOI] [PubMed] [Google Scholar]
- 7.Lingareddy V, Ahmad NR, Mohiuddin M. Palliative reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1997;38:785–790. doi: 10.1016/S0360-3016(97)00058-8. [DOI] [PubMed] [Google Scholar]
- 8.Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N, Swedish Rectal Cancer Trial Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 9.Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008–1015. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- 10.Colorectal Cancer Collaborative Group Adjuvant radiotherapy for rectal cancer: A systematic overview of 22 randomised trials involving 8507 patients. Lancet. 2001;358:1291–1304. doi: 10.1016/S0140-6736(01)06409-1. [DOI] [PubMed] [Google Scholar]
- 11.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, et al. Dutch Colorectal Cancer Group Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 12.Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW, et al. Dutch Colorectal Cancer Group The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 13.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ, Dutch Colorectal Cancer Group Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 14.Kulka U, Schaffer M, Siefert A, Schaffer PM, Olsner A, Kasseb K, Hofstetter A, Dühmke E, Jori G. Photofrin as a radiosensitizer in an in vitro cell survival assay. Biochem Biophys Res Commun. 2003;311:98–103. doi: 10.1016/j.bbrc.2003.09.170. [DOI] [PubMed] [Google Scholar]
- 15.Marijnen CA, Kapiteijn E, van de Velde CJ, Martijn H, Steup WH, Wiggers T, Kranenbarg EK, Leer JW, Cooperative Investigators of the Dutch Colorectal Cancer Group Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: Report of a multicenter randomized trial. J Clin Oncol. 2002;20:817–825. doi: 10.1200/JCO.20.3.817. [DOI] [PubMed] [Google Scholar]
- 16.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 17.Marijnen CA, van de Velde CJ, Putter H, van den Brink M, Maas CP, Martijn H, Rutten HJ, Wiggers T, Kranenbarg EK, Leer JW, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: Report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847–1858. doi: 10.1200/JCO.2005.05.256. [DOI] [PubMed] [Google Scholar]
- 18.Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CA. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 19.Ishizuka M, Abe F, Sano Y, Takahashi K, Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S, Tanaka T. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int Immunopharmacol. 2011;11:358–365. doi: 10.1016/j.intimp.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky KE, Nesland JM. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997;79:2282–2308. doi: 10.1002/(SICI)1097-0142(19970615)79:12<2282::AID-CNCR2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 21.Hagiya Y, Fukuhara H, Matsumoto K, Endo Y, Nakajima M, Tanaka T, Okura I, Kurabayashi A, Furihata M, Inoue K, et al. Expression levels of PEPT1 and ABCG2 play key roles in 5-aminolevulinic acid (ALA)-induced tumor-specific protoporphyrin IX (PpIX) accumulation in bladder cancer. Photodiagn Photodyn Ther. 2013;10:288–295. doi: 10.1016/j.pdpdt.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utsuki S, Miyoshi N, Oka H, Miyajima Y, Shimizu S, Suzuki S, Fujii K. Fluorescence-guided resection of metastatic brain tumors using a 5-aminolevulinic acid-induced protoporphyrin IX: Pathological study. Brain Tumor Pathol. 2007;24:53–55. doi: 10.1007/s10014-007-0223-3. [DOI] [PubMed] [Google Scholar]
- 24.Fukuhara H, Inoue K, Satake H, Tamura K, Karashima T, Yamasaki I, Tatsuo I, Kurabayashi A, Furihata M, Shuin T. Photodynamic diagnosis of positive margin during radical prostatectomy: Preliminary experience with 5-aminolevulinic acid. Int J Urol. 2011;18:585–591. doi: 10.1111/j.1442-2042.2011.02789.x. [DOI] [PubMed] [Google Scholar]
- 25.Mimura S, Ito Y, Nagayo T, Ichii M, Kato H, Sakai H, Goto K, Noguchi Y, Tanimura H, Nagai Y, et al. Cooperative clinical trial of photodynamic therapy with photofrin II and excimer dye laser for early gastric cancer. Lasers Surg Med. 1996;19:168–172. doi: 10.1002/(SICI)1096-9101(1996)19:2<168::AID-LSM7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Corti L, Toniolo L, Boso C, Colaut F, Fiore D, Muzzio PC, Koukourakis MI, Mazzarotto R, Pignataro M, Loreggian L, et al. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med. 2007;39:394–402. doi: 10.1002/lsm.20513. [DOI] [PubMed] [Google Scholar]
- 27.Cao LQ, Xue P, Lu HW, Zheng Q, Wen ZL, Shao ZJ. Hematoporphyrin derivative-mediated photodynamic therapy inhibits tumor growth in human cholangiocarcinoma in vitro and in vivo. Hepatol Res. 2009;39:1190–1197. doi: 10.1111/j.1872-034X.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 28.Luksiene Z, Kalvelyte A, Supino R. On the combination of photodynamic therapy with ionizing radiation. J Photochem Photobiol B. 1999;52:35–42. doi: 10.1016/S1011-1344(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 29.Schaffer M, Schaffer PM, Corti L, Gardiman M, Sotti G, Hofstetter A, Jori G, Dühmke E. Photofrin as a specific radiosensitizing agent for tumors: Studies in comparison to other porphyrins, in an experimental in vivo model. J Photochem Photobiol B. 2002;66:157–164. doi: 10.1016/S1011-1344(02)00237-3. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto J, Ogura S, Tanaka T, Kitagawa T, Nakano Y, Saito T, Takahashi M, Akiba D, Nishizawa S. Radiosensitizing effect of 5-aminolevulinic acid-induced protoporphyrin IX in glioma cells in vitro. Oncol Rep. 2012;27:1748–1752. doi: 10.3892/or.2012.1699. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi J, Misawa M, Murakami M, Mori T, Nomura K, Iwahashi H. 5-Aminolevulinic acid enhances cancer radiotherapy in a mouse tumor model. Springerplus. 2013;2:602. doi: 10.1186/2193-1801-2-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto J, Ogura S, Shimajiri S, Nakano Y, Akiba D, Kitagawa T, Ueta K, Tanaka T, Nishizawa S. 5-aminolevulinic acid-induced protoporphyrin IX with multi-dose ionizing irradiation enhances host antitumor response and strongly inhibits tumor growth in experimental glioma in vivo. Mol Med Rep. 2015;11:1813–1819. doi: 10.3892/mmr.2014.2991. [DOI] [PubMed] [Google Scholar]
- 33.Kitagawa T, Yamamoto J, Tanaka T, Nakano Y, Akiba D, Ueta K, Nishizawa S. 5-Aminolevulinic acid strongly enhances delayed intracellular production of reactive oxygen species (ROS) generated by ionizing irradiation: Quantitative analyses and visualization of intracellular ROS production in glioma cells in vitro. Oncol Rep. 2015;33:583–590. doi: 10.3892/or.2014.3618. [DOI] [PubMed] [Google Scholar]
- 34.Capella MA, Capella LS. A light in multidrug resistance: Photodynamic treatment of multidrug-resistant tumors. J Biomed Sci. 2003;10:361–366. doi: 10.1007/BF02256427. [DOI] [PubMed] [Google Scholar]
- 35.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 36.Vrouenraets MB, Visser GW, Snow GB, van Dongen GA. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003;23((1B)):505–522. [PubMed] [Google Scholar]
- 37.Hall EJ, Giaccia AJ. Physics and chemistry of radiation absorption. In: Hall EJ, Giaccia AJ, editors. Radiobiology for the Radiologist. 6th edition. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 5–15. [Google Scholar]
- 38.Hosokawa Y, Sakakura Y, Tanaka L, Okumura K, Yajima T, Kaneko M. Radiation-induced apoptosis is independent of caspase-8 but dependent on cytochrome c and the caspase-9 cascade in human leukemia HL60 cells. J Radiat Res (Tokyo) 2005;46:293–303. doi: 10.1269/jrr.46.293. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto J, Yamamoto S, Hirano T, Li S, Koide M, Kohno E, Okada M, Inenaga C, Tokuyama T, Yokota N, et al. Monitoring of singlet oxygen is useful for predicting the photodynamic effects in the treatment for experimental glioma. Clin Cancer Res. 2006;12:7132–7139. doi: 10.1158/1078-0432.CCR-06-0786. [DOI] [PubMed] [Google Scholar]
- 40.Saenko Y, Cieslar-Pobuda A, Skonieczna M, Rzeszowska-Wolny J. Changes of reactive oxygen and nitrogen species and mitochondrial functioning in human K562 and HL60 cells exposed to ionizing radiation. Radiat Res. 2013;180:360–366. doi: 10.1667/RR3247.1. [DOI] [PubMed] [Google Scholar]
- 41.Yamamori T, Yasui H, Yamazumi M, Wada Y, Nakamura Y, Nakamura H, Inanami O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial con-tent undercontrol of the cell cycle checkpoint. Free Radic Biol Med. 2012;15;53(2):260–70. doi: 10.1016/j.freeradbiomed.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 42.Hino H, Murayama Y, Nakanishi M, Inoue K, Nakajima M, Otsuji E. 5-Aminolevulinic acid-mediated photodynamic therapy using light-emitting diodes of different wavelengths in a mouse model of peritoneally disseminated gastric cancer. J Surg Res. 2013;185:119–126. doi: 10.1016/j.jss.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 43.Harris EER, Small W., Jr Intraoperative Radiotherapy for Breast Cancer. Front Oncol. 2017;7:317. doi: 10.3389/fonc.2017.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/S0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 45.Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: The 4 R's of radiobiology revisited. Stem Cells. 2010;28:639–648. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Withers HR. Advances in radiation biology. Lett JTAH (ed) Academic Press; New York: 1975. The four R's of radiotherapy. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.