Abstract
The 21-gene recurrence score (RS) predicts the prognosis of patients with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative early-stage breast cancer and the effectiveness of adding adjuvant chemotherapy on the basis of endocrine therapy to avoid excessive chemotherapy. The present study aimed to analyze clinicopathological characteristics and chemotherapeutic efficacy-related target genes with the 21-gene RS in hormone receptor-positive early-stage breast cancer in China. The prognostic value of chemotherapeutic efficacy-related target genes was also examined. In addition, this study investigated the postoperative adjuvant therapeutic decision-oriented role of 21-gene RS in hormone receptor-positive and lymph node-negative early-stage breast cancer. In the present retrospective study, 110 ER+/HER2− early-stage breast cancer patients were tested with the 21-gene RS. The analyses of clinicopathological characteristics and chemotherapeutic efficacy-related target genes with the 21-gene RS were performed using the χ2 test, the Wilcoxon rank-sum test and binary logistic regression. Kaplan-Meier survival plots were drawn in www.kmplot.com. Furthermore, the McNemar χ2 test was used to compare the changes of treatment decisions before and after the 21-gene test. The median RS of 110 patients was 16 (range, 2–47), and patients were categorized as low (59.1%), intermediate (34.5%) or high risk (6.4%). The results revealed that higher body mass index, invasive ductal carcinoma type, higher histological grade, luminal B molecular type and higher thymidylate synthetase (TYMS) and DNA topoisomerase IIα (TOP2A) gene expression levels were more likely to have a higher RS. Kaplan-Meier plots suggested that expression of TYMS, tubulin β3 class III (TUBB3) and TOP2A genes was significantly associated with relapse-free survival for ER+ breast cancer. Additionally, prior to 21-gene RS testing, 61 patients (55%) were recommended adjuvant chemotherapy and endocrine therapy; however, following 21-gene test, 32 patients (29%) were treated with only adjuvant endocrine therapy. TYMS, TUBB3 and TOP2A gene expression may have prognostic value for ER+ breast cancer. In addition, 21-gene RS testing may aid to avoid excessive postoperative adjuvant chemotherapy.
Keywords: breast cancer, 21-gene recurrence score, clinicopathological characteristics, gene expression, prognosis
Introduction
As the most prevalent cancer in women, breast cancer affects >1 million women worldwide and accounts for ~23% of all cases of cancer among women (1,2). In addition, the annual incidence of breast cancer in China was ~20,8000 cases in 2010, which accounted for 16.2% of all cancer cases in females (3). Breast cancer has become one of the main causes of death among young women in China (4). A multidisciplinary comprehensive treatment model has gradually been developed, including surgery, radiotherapy, chemotherapy and endocrine therapy (5). These treatments have improved the long-term survival of patients with breast cancer and reduced the rates of recurrence and metastasis (6). Nevertheless, chemotherapy has several side effects, and whether patients with low-risk early-stage breast cancer benefit from chemotherapy remains to be determined. Effective methods to accurately predict recurrence risk and simultaneously provide appropriate treatment to patients have not been established.
With the development of genomics (7), the treatment of breast cancer has stepped into a new era. The 21-gene recurrence score (RS) assay for patients with estrogen receptor (ER)-positive breast cancer has been demonstrated to be more accurate than clinicopathological indicators in predicting prognosis and metastasis (8–11). The 21-gene assay has already been recommended by the National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology for patients with early-stage breast cancer (12,13). Based on the results of National Surgical Adjuvant Breast and Bowel Project (NSABP) B-20 trials, a score <18 was categorized as low risk, a score between 18 and 30 was considered intermediate risk and a score ≥31 was categorized as high risk (8,14,15). This test helps screen out patients with low recurrence risk, thereby avoiding excessive chemotherapy. In a previous study, 70 surgeons at the Israeli Cancer clinics selected 300 Israeli patients with breast cancer to perform 21-gene RS tests (16). The results demonstrated that age and tumor size had no correlation with the RS value. In addition, the NSABP B-20 and Southwest Oncology Group-8814 trials revealed that the RS predicted benefit from adjuvant chemotherapy in tumor-node-metastasis (TNM) node stage (N)0–3 patients (14,17).
The 21-gene RS detection was obtained from large datasets abroad, but lacked the validation of large samples in China. In addition, the clinicopathological characteristics included in previous studies were limited. To the best of our knowledge, there have been no studies regarding the relationship between RS assay and chemotherapeutic efficacy-related target genes. Therefore, the present study aimed to analyze clinicopathological characteristics and chemotherapeutic efficacy-related target genes with 21-gene RS in patients with hormone receptor-positive early-stage breast cancer in China. The changes of treatment decisions before and after the 21-gene RS test were compared, and the prognostic value of chemotherapeutic efficacy-related target genes was examined to aid in the evaluation of the guiding significance of the 21-gene RS in the diagnosis and treatment of patients with breast cancer in China and to aid in the discovery of new prognostic markers.
Materials and methods
Data and patients
A retrospective, single-center study was conducted at The First Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China). The data from 110 patients with ER-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-negative T1-3N0M0 breast cancer who were tested for the 21-gene RS and underwent surgical treatment between June 2013 and December 2016 were reviewed. Exclusion criteria were patients with T4 tumors, those having received neoadjuvant therapy or those with distant metastasis occurring at first diagnosis. The study was approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University.
Clinicopathological data
Clinicopathological data were collected after obtaining informed consent from patients. Pathological examinations, including detection of ER, progesterone receptor (PR), HER2, nuclear protein Ki67, tumor protein p53 and cytokeratin 5/6 (CK5/6) protein expressions by immunohistochemistry (IHC), were conducted in the pathology department of The First Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China). The IHC analysis was conducted prior to the study. ER+ or PR+ results were defined as hormone receptor-positive. For HER2, the cytomembrane of cancer cells without staining was defined as HER2−. Any proportion of cancer cells exhibiting weak or incomplete cytomembrane staining or <10% of cancer cells exhibiting weak or complete cytomembrane staining were defined as HER2+; ≥10% of cancer cells exhibiting weak or moderately intact cytomembrane staining or <10% cancer cells exhibiting uniform, strong and complete cytomembrane staining were defined as HER2++; ≥10% of cancer cells exhibiting consistent, strong and intact cytomembrane staining were defined as HER2+++; HER2− or HER2+ was considered as HER2 low expression, which was considered negative in clinical therapy; HER2+++ was HER2 positive; and HER2++ required further detection with fluorescent in situ hybridization (FISH). If no gene expression was observed in the FISH analysis, HER2 was categorized as negative; otherwise, it was positive. For p53 and CK5/6, ≥10% staining of cancer cells was defined as positive. In addition to pathological data, the following data were recorded: Age, occupation, marital status, body mass index (BMI), menopausal status, tumor discovery time, tumor size, pathological type, histological grade, vascular tumor embolus state, TNM stage, clinical stage, molecular type, surgical scheme and tumor markers, including carcinoembryonic antigen and cancer antigen 15-3 tested by chemiluminescence microparticle I2000 immunoassay (Abbott, Abbott Park, IL, USA).
Patient occupations were classified as manual worker, skilled worker or unemployed. Manual workers included laborers and farmers. Skilled workers included clerks, national civil servants and professionals. The unemployed group also included retirees. The BMI (kg/m2) categories were based on the Working Group on Obesity in China with the support of the International Life Science Institute Focal point in China. Menopausal status referred to the definition of menopause as per the NCCN Guidelines (version 2, 2017). Tumor discovery time was calculated between the discovery of the breast lump and the initial breast cancer diagnosis. The diagnosis of TNM stage was based on the Cancer Staging Manual, Seventh Edition (American Joint Committee on Cancer, 2010; Springer Science Business Media) (18) and the Nottingham combined histologic grade was referenced. The molecular type was based on the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer (2013) (19).
Assay for 21-gene detection, chemotherapeutic efficacy-related target gene detection and RS definition
The 21-gene RS assay and the detection of chemotherapeutic efficacy-related target genes were performed on paraffin-embedded tumor samples at Surexam Medical Laboratory (Medical Institution Licensing permit no. 440116PDY707461; Guangzhou, China) using the DNA liquid chip technology to transfer the probe of a traditional gene chip from a solid carrier to liquid to increase the detection efficiency. The target genes were thymidylate synthetase (TYMS), ribonucleotide reductase catalytic subunit M1 (RRM1), tubulin β3 class III (TUBB3), DNA topoisomerase IIα (TOP2A) and phosphatase and tensin homolog (PTEN) (20–25). Gene expression did not translate or equate to protein expression of those markers by IHC due to differences in the detection methods. The expression levels of the following 21 gene mRNAs, which are related to recurrence risk, were detected and calculated to determine the RS: Ki67, aurora kinase A (STK15), Survivin, cyclin B1 (CCNB1), MYB proto-oncogene like 2 (MYBL2), growth factor receptor-bound protein 7 (GRB7), HER2, ER, PR, BCL2 apoptosis regulator (BCL2), signal peptide, CUB domain and EGF-like domain-containing 2 (SCUBE2), matrix metallopeptidase 11 (MMP11), cathepsin V (CTSL2), glutathione S-transferase µ1 (GSTM1), scavenger receptor class D, member 1 (CD68), BCL2-associated athanogene 1 (BAG1), β-actin (ACTB), GAPDH, ribosomal protein lateral stalk subunit P0 (RPLP0), β-glucuronidase (GUS) and transferrin receptor (TFRC). RS <18 was categorized as low risk, RS between 18 and 30 was considered to be intermediate risk, and RS ≥31 was categorized as high risk (8).
Kaplan-Meier Plotter database
As a tool for meta-analysis-based biomarker assessment, the Kaplan-Meier Plotter (http://www.kmplot.com) (26) was used to analyze the prognostic values of TYMS, RRM1, TUBB3, TOP2A and PTEN mRNA expression levels in ER+ breast cancer. Kaplan-Meier survival plots were drawn using data from the Kaplan-Meier database. A log P-value <0.01 was considered to indicate a statistically significant difference.
Therapeutic decision making and follow-up
Patients' data were discussed by the Multidisciplinary team (MDT) of The First Affiliated Hospital of Xi'an Jiaotong University. The MDT team independently made therapeutic decisions before and after the RS test. The clinical decision was made based on the Chinese Society of Clinical Oncology Breast Cancer diagnosis and treatment guidelines and NCCN Clinical Practice Guidelines in Oncology of Breast Cancer, St. Gallen Consensus. Finalized therapeutic decisions were made by combining the physician's clinical experience, the patients and their families' expectations, and patients' individual situation. Patients and their families were informed with the final decision and informed consent was obtained. All patients were followed up by a combination of outpatient and telephone visits to confirm recurrence, metastasis or death of breast cancer.
Statistical analysis
All categorical variables are expressed as the means of absolute numbers and percentages, whereas abnormally distributed continuous variables are expressed as medians and ranges. The associations between the 21-gene RS and clinicopathological characteristics that were categorical variables were analyzed by the χ2 test. Fisher's exact test was applied when the theoretical frequency was <5 or the total observation frequency was <20. For continuous variables, if they met the assumptions of normality and homogeneity, Student's unpaired t-test was performed. Otherwise, these variables were analyzed by the Wilcoxon rank-sum test. The relationships between the 21-gene RS and chemotherapeutic efficacy-related target genes were estimated using the Wilcoxon rank-sum test, and correlations were tested using Spearman's rank test. Univariate binary logistic regression of clinicopathological characteristics and chemotherapeutic efficacy-related target genes with 21-gene RSs and multivariate logistic analysis of independent variables associated with the 21-gene RS were analyzed. Furthermore, the McNemar χ2 test was used to compare the differences in treatment decisions before and after the 21-gene test. All statistical tests were two sided. P<0.05 was considered to indicate a statistically significant difference. All analyses were performed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient clinicopathological characteristics
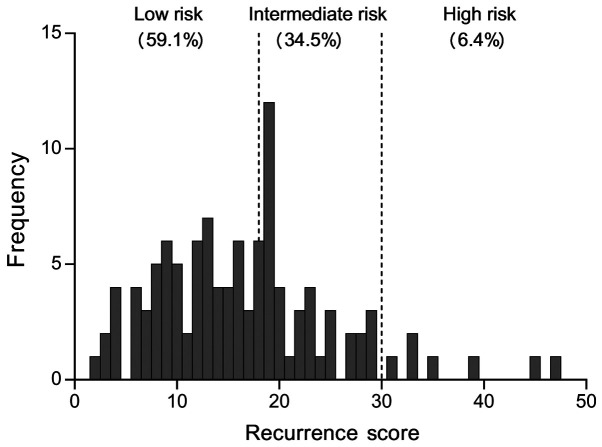
A total of 110 female patients with ER+ breast cancer who were tested with the 21-gene RS test and treated at The First Affiliated Hospital of Xi'an Jiaotong University were included in the present study (Fig. 1; Table I). Based on RS criteria, the majority of patients (59.1%; 65/110) were classified in the low-RS group, 34.5% (38/110) in the intermediate-RS group and 6.4% (7/110) in the high-RS group. Most patients (30.9%; 34/110) were skilled workers. Nearly 96% of patients were married, and the majority (60%; 66/110) had normal BMI. The proportions of postmenopausal and premenopausal patients were 51% (56/110) and 48% (53/110), respectively. A total of 93 (84.6%) patients had invasive ductal carcinoma; none of the patients had lymph node metastasis or a vascular tumor embolus. The clinical stages were I–II (100%). The baseline data are presented in Table I.
Figure 1.
Distribution of 21-gene recurrence score. The distribution of 21-gene recurrence scores of 110 female patients with estrogen receptor-positive breast cancer was used to categorize the patients into low, intermediate and high risk groups.
Table I.
Clinicopathological characteristics of patients with ER+ breast cancer.
| Clinicopathological characteristic | N | % | Valid%a |
|---|---|---|---|
| Age (years) | |||
| <45 | 33 | 30.00 | 30.00 |
| 45–59 | 50 | 45.45 | 45.45 |
| ≥60 | 27 | 24.55 | 24.55 |
| Occupationb | |||
| Manual worker | 28 | 25.45 | 31.11 |
| Skilled worker | 34 | 30.91 | 37.78 |
| Unemployed | 28 | 25.45 | 31.11 |
| Other | 20 | 18.18 | |
| Marital status | |||
| Married | 106 | 96.36 | 96.36 |
| Divorced | 2 | 1.82 | 1.82 |
| Not married | 2 | 1.82 | 1.82 |
| BMIc | |||
| Underweight: <18.5 | 6 | 5.45 | 5.45 |
| Normal weight: 18.5–23.9 | 66 | 60.00 | 60.00 |
| Overweight: ≥24.0 | 38 | 34.55 | 34.55 |
| Menopausal stated | |||
| Postmenopausal | 56 | 50.91 | 51.38 |
| Premenopausal | 53 | 48.18 | 48.62 |
| Other | 1 | 0.91 | |
| Tumor discovery time (days)e | |||
| t <1week | 11 | 10.00 | 10.00 |
| 1 week ≤ t <2 weeks | 16 | 14.55 | 14.55 |
| 2 weeks ≤ t <1 month | 18 | 16.36 | 16.36 |
| 1 month ≤ t <6 months | 30 | 27.28 | 27.28 |
| 6 months ≤ t <1 year | 14 | 12.73 | 12.73 |
| t ≥1 year | 21 | 19.09 | 19.09 |
| Tumor size (cm) | |||
| ≤2 | 73 | 66.36 | 66.36 |
| >2 | 37 | 33.64 | 33.64 |
| Pathological type | |||
| IDC | 93 | 84.55 | 84.55 |
| ILC | 9 | 8.18 | 8.18 |
| IPC | 4 | 3.64 | 3.64 |
| IMPC | 1 | 0.91 | 0.91 |
| MC | 3 | 2.73 | 2.73 |
| Vascular tumor embolus | |||
| Yes | 0 | 0 | 0 |
| No | 110 | 100 | 100 |
| Histological gradef | |||
| I | 13 | 11.82 | 12.50 |
| II | 63 | 57.27 | 60.58 |
| III | 28 | 25.45 | 26.92 |
| Unknown | 6 | 5.45 | |
| T stageg | |||
| T1 | 73 | 66.36 | 66.36 |
| T2 | 36 | 32.73 | 32.73 |
| T3 | 1 | 0.91 | 0.91 |
| N stageg | |||
| N0 | 110 | 100 | 100 |
| N1-3 | 0 | 0 | 0 |
| M stageg | |||
| M0 | 110 | 100 | 100 |
| M1 | 0 | 0 | 0 |
| Clinical stageg | |||
| I | 73 | 66.36 | 66.36 |
| II | 37 | 33.64 | 33.64 |
| III | 0 | 0 | 0 |
| Molecular typeh | |||
| Luminal A | 46 | 41.82 | 41.82 |
| Luminal B | 64 | 58.18 | 58.18 |
| Luminal B (−) | 57 | 51.82 | 51.82 |
| Luminal B (+) | 7 | 6.36 | 6.36 |
| Surgical scheme | |||
| BCS | 16 | 14.55 | 14.55 |
| BMS | 94 | 85.45 | 85.45 |
| ER | |||
| Positive | 110 | 100 | 100 |
| Negative | 0 | 0 | 0 |
| p53 | |||
| ≤10% | 74 | 67.27 | 71.84 |
| 11–50% | 20 | 18.18 | 19.42 |
| ≥51% | 9 | 8.18 | 8.74 |
| Unknown | 7 | 6.36 | |
| CK5/6 | |||
| Positive | 9 | 8.18 | 8.65 |
| Negative | 95 | 86.36 | 91.35 |
| Unknown | 6 | 5.45 | |
| Tumor markers CEA (ng/ml) | |||
| Negative, 0.00–3.40 | 100 | 90.91 | 92.59 |
| Positive, >3.40 | 8 | 7.27 | 7.41 |
| Unknown | 2 | 1.82 | |
| Tumor markers CA15-3 (ng/ml) | |||
| Negative 0.00–25.00 | 105 | 95.45 | 97.22 |
| Positive >25.00 | 3 | 2.73 | 2.78 |
| Unknown | 2 | 1.82 | |
Valid% means the proportion excludes ‘Others’ and ‘Unknown’.
Patient occupations were classified as manual worker, skilled worker, and unemployed. ‘Manual workers’ included laborers and farmers; ‘skilled workers’ included clerks, national civil servants and professionals; ‘unemployed’ included retired and unemployed people.
BMI (kg/m2) category was based on the Working Group on Obesity in China with the support of International Life Science Institute Focal point in China.
Menopausal status referred to the definition of menopause as per the NCCN Guidelines (version 2; 2017).
Tumor discovery time was calculated from the first time the patient touched the breast mass until the initial diagnosis of the breast cancer.
Nottingham combined histologic grade was referenced.
TNM stage is based on the American Joint Committee on Cancer Staging Manual (Seventh Edition; 2010; published by Springer Science+Business Media, LLC).
The molecular type referred to the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer (2013). BCS, breast conserving surgery; BMI, body mass index; BMS, breast mastectomy surgery; CA15-3, cancer antigen 15-3; CEA, carcinoembryonic antigen; CK5/6, cytokeratin 5/6; ER, estrogen receptor; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; IMPC, invasive micropapillary carcinoma; IPC, invasive papillary carcinoma; MC, mucinous carcinoma; TNM, tumor-node-metastasis.
Distribution of RS based on clinicopathological characteristics
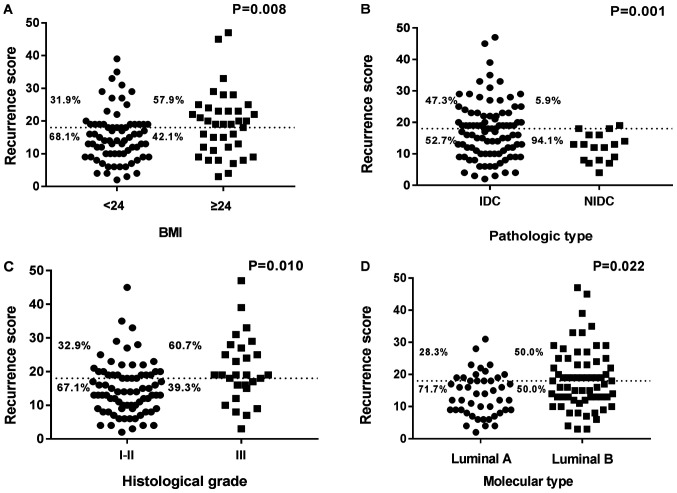
The associations between the 21-gene RS and clinicopathological characteristics are shown in Tables II and III. Median age at initial diagnosis of breast cancer in the low-RS group was 52 years old (range, 25–73), whereas median age was 50 years (range, 30–70) in the intermediate/high-RS group. The distribution of the 21-gene RS varied significantly according to BMI, pathological type, histological grade and molecular grade (P<0.05; Table II). Patients with higher BMI, invasive ductal carcinoma type, higher histological grade and luminal B molecular type were more likely to have higher RS compared with patients with lower BMI, non-invasive ductal carcinoma, lower histological grade and luminal A molecular type (Fig. 2). In histological grade I–II tumors, the proportions of low and intermediate/high RS were 67.1 and 32.9%, respectively, whereas in patients with histological grade III tumors, the proportions were 39.3 and 60.7% respectively (P=0.010; Fig. 2C). Similarly, compared with patients with lower BMI, non-invasive ductal carcinoma type and luminal A molecular type, patients with higher BMI, invasive ductal carcinoma type and luminal B molecular type were more likely to be categorized as the intermediate/high-RS group and less likely to be categorized as the low-RS group (P<0.05; Table II). In addition, univariate binary logistic regression analysis revealed that higher BMI [odds ratio (OR), 2.929; P=0.010], invasive ductal carcinoma type (OR, 14.367; P=0.011), higher histological grade (OR, 3.153; P=0.012) and luminal B molecular type (OR, 2.538; P=0.024) were independent risk factors for higher RS (Table III).
Table II.
Relationship between 21-gene RS and clinicopathological characteristics.
| RS risk groups | |||
|---|---|---|---|
| Clinicopathological characteristics | Low risk (RS <18) | Intermediate/high risk (RS ≥18) | P-value |
| Median age, years (range) | 52 (25–73) | 50 (30–70) | 0.619 |
| Median tumor discovery time, days (range) | 56 (2–1680) | 28 (2–1680) | 0.317 |
| Occupation | 0.507 | ||
| Manual worker | 15 (26.8) | 13 (38.2) | |
| Skilled worker | 22 (39.3) | 12 (35.3) | |
| Unemployed | 19 (33.9) | 9 (26.5) | |
| BMI | 0.008 | ||
| <24 | 49 (75.4) | 23 (51.1) | |
| ≥24 | 16 (24.6) | 22 (48.9) | |
| Menopausal state | 0.902 | ||
| Postmenopausal | 34 (52.3) | 23 (51.1) | |
| Premenopausal | 31 (47.7) | 22 (48.9) | |
| Tumor size | 0.113 | ||
| ≤2 | 47 (72.3) | 26 (57.8) | |
| >2 | 18 (27.7) | 19 (42.2) | |
| Pathological type | 0.001 | ||
| IDC | 49 (75.4) | 44 (97.8) | |
| NIDC | 16 (24.6) | 1 (2.2) | |
| Histological grade | 0.010 | ||
| I–II | 51 (82.3) | 25 (59.5) | |
| III | 11 (17.7) | 17 (40.5) | |
| Clinical stage | 0.113 | ||
| Stage I | 47 (72.3) | 26 (57.8) | |
| Stage II–III | 18 (27.7) | 19 (42.2) | |
| Molecular type | 0.022 | ||
| Luminal A | 33 (50.8) | 13 (28.9) | |
| Luminal B | 32 (49.2) | 32 (71.1) | |
| Surgical scheme | 0.764 | ||
| BCS | 10 (15.4) | 6 (13.3) | |
| BMS | 55 (84.6) | 39 (86.7) | |
| Median P53% (range) | 8 (1–90) | 10 (1–90) | 0.099 |
| CK5/6 status | |||
| Positive | 4 (6.8) | 5 (11.1) | 0.670 |
| Negative | 55 (93.2) | 40 (88.9) | |
| Median CEA, ng/ml (range) | 1.50 (0.31–4.62) | 1.52 (0.20–5.73) | 0.771 |
| Median CA15-3, ng/ml (range) | 9.37 (4.67–25.48) | 11.12 (4.85–28.48) | 0.215 |
Bold indicates P<0.05. BCS, breast conserving surgery; BMI, body mass index; BMS, breast mastectomy surgery; CA15-3, cancer antigen 15-3; CEA, carcinoembryonic antigen; CK5/6, cytokeratin 5/6; IDC, invasive ductal carcinoma; NIDC, non-invasive ductal carcinoma; RS, recurrence score.
Table III.
Univariate binary logistics regression analysis of clinicopathological characteristics with 21-gene recurrence score.
| High/intermediate vs. low risk | |||
|---|---|---|---|
| Clinicopathological characteristics | OR | 95% CI | P-value |
| Age | 0.991 | 0.955–1.028 | 0.616 |
| Tumor discovery time | 1.000 | 0.998–1.001 | 0.664 |
| Occupation | 0.510 | ||
| Manual worker | 1.830 | 0.617–5.423 | 0.276 |
| Skilled worker | 1.152 | 0.399–3.324 | 0.794 |
| Unemployed | Ref | ||
| BMI | |||
| <24 | Ref | ||
| ≥24 | 2.929 | 1.300–6.601 | 0.010 |
| Menopausal state | |||
| Postmenopausal | Ref | ||
| Premenopausal | 1.049 | 0.490–2.245 | 0.902 |
| Tumor size (cm) | |||
| ≤2 | Ref | ||
| >2 | 1.908 | 0.855–4.260 | 0.115 |
| Pathological type | |||
| NIDC | Ref | ||
| IDC | 14.367 | 1.830–112.826 | 0.011 |
| Histological grade | |||
| I–II | Ref | ||
| III | 3.153 | 1.286–7.729 | 0.012 |
| Clinical stage | |||
| Stage I | Ref | ||
| Stage II–III | 1.908 | 0.855–4.260 | 0.115 |
| Molecular type | |||
| Luminal A | Ref | ||
| Luminal B | 2.538 | 1.132–5.692 | 0.024 |
| Surgical scheme | |||
| BCS | Ref | ||
| BMS | 1.182 | 0.397–3.523 | 0.764 |
| P53 (%) | 7.592 | 0.940–61.290 | 0.057 |
| CK5/6 status | |||
| Positive | Ref | ||
| Negative | 0.582 | 0.147–2.304 | 0.441 |
| CEA | 1.076 | 0.747–1.551 | 0.693 |
| CA15-3 | 1.042 | 0.971–1.118 | 0.254 |
Bold indicates P<0.05. BCS, breast conserving surgery; BMI, body mass index; BMS, breast mastectomy surgery; CA15-3, cancer antigen 15-3; CEA, carcinoembryonic antigen; CI, confidence interval; CK5/6, cytokeratin 5/6; IDC, invasive ductal carcinoma; NIDC, non-invasive ductal carcinoma; OR, odds ratio; Ref, reference.
Figure 2.
Association between RS risk groups and clinicopathological characteristics. (A) RS vs. BMI; (B) RS vs. pathological type; (C) RS vs. histological grade; (D) RS vs. molecular type. BMI, body mass index; IDC, invasive ductal carcinoma; NIDC, non-invasive ductal carcinoma; RS, 21-gene test recurrence score.
Distribution of RS according to chemotherapeutic efficacy-related target genes
Detection of PTEN, TYMS, RRM1, TUBB3 and other genes related to the efficacy and/or side effects of chemotherapeutic drugs predicted the therapeutic effects and/or side effects of chemotherapeutic drugs for each patient; the results were used to inform chemotherapy treatment. The associations between RS and chemotherapeutic efficacy-related target genes are demonstrated in Table IV. Differences in TYMS and TOP2A gene expression between the low- and intermediate/high-risk RS groups were statistically significant (P<0.05; Table IV). Patients with higher TYMS gene expression were more likely to have intermediate/high-risk RS compared with patients with lower TYMS gene expression (P=0.001; Table IV). In addition, the proportion of intermediate/high-risk RS was significantly higher among patients with higher TOP2A expression (P<0.001; Table IV). PTEN, RRM1 and TUBB3 had no impact on RS categories. Furthermore, univariate binary logistic regression analysis showed that TYMS (OR, 14.950; P=0.001) and TOP2A (OR, 14.846; P<0.001) gene expression levels were independent risk factors for higher RS (Table V).
Table IV.
Relationship between 21-gene RS and chemotherapeutic efficacy-related target genes.
| RS risk groups N (%)a | |||
|---|---|---|---|
| Chemotherapeutic efficacy-related target genes | Low risk (RS <18) | Intermediate/high risk (RS ≥18) | P-value |
| TYMS | 28.4 (1–89) | 45.8 (4.5–96) | 0.001 |
| RRM1 | 44.8 (1–98.1) | 43.5 (1–99) | 0.975 |
| TUBB3 | 39.6 (1–99) | 52.3 (0.8–99) | 0.337 |
| TOP2A | 35.9 (1.1–94.6) | 63.1 (2.7–98.1) | <0.001 |
| PTEN | 64.7 (7.9–99) | 65.6 (1.4–99) | 0.681 |
Median percentage of gene expression (range). Bold indicates P<0.05. PTEN, phosphatase and tensin homolog; RRM1, ribonucleotide reductase catalytic subunit M1; RS, recurrence score; TOP2A, DNA topoisomerase IIα; TUBB3, tubulin β3 class III; TYMS, thymidylate synthetase.
Table V.
Univariate binary logistics regression analysis of chemotherapeutic efficacy- related target genes with 21-gene recurrence score.
| High/intermediate vs. low risk | |||
|---|---|---|---|
| Chemotherapeuticefficacy-related target genes | OR | 95% CI | P-value |
| TYMS | 14.950 | 2.863–78.082 | 0.001 |
| RRM1 | 0.972 | 0.257–3.670 | 0.966 |
| TUBB3 | 2.034 | 0.482–8.574 | 0.333 |
| TOP2A | 14.846 | 3.269–67.433 | <0.001 |
| PTEN | 0.620 | 0.154–2.495 | 0.501 |
Bold indicates P<0.05. CI, confidence interval; OR, odds ratio; PTEN, phosphatase and tensin homolog; RRM1, ribonucleotide reductase catalytic subunit M1; TOP2A, DNA topoisomerase IIα; TUBB3, tubulin β3 class III; TYMS, thymidylate synthetase.
Multivariate analysis of independent variables associated with the 21-gene RS
Multivariate logistic regression analysis demonstrated that BMI, histological grade and TOP2A were independent variables associated with RS (Table VI). Patients with BMI ≥24 were more likely to have high/intermediate-risk RS compared with patients with BMI <24 (OR, 3.590; 95% CI, 1.296–9.947; P=0.014). Histological grade III was associated with significantly higher odds of high/intermediate-risk RS (OR, 3.478; 95% CI, 1.139–10.627; P=0.029) compared with grade I–II. Additionally, the proportion of high/intermediate-risk RS was significantly higher among patients with high TOP2A gene expression compared with low TOP2A expression (OR, 16.056; 95% CI, 1.961–131.451; P=0.010).
Table VI.
Multivariate analysis of independent variables associated with 21-gene recurrence score
| High/intermediate vs. low risk | |||
|---|---|---|---|
| Clinicopathological characteristic | OR | 95% CI | P-value |
| BMI | |||
| <24 | Ref | ||
| ≥24 | 3.590 | 1.296–9.947 | 0.014 |
| Histological grade | |||
| I–II | Ref | ||
| III | 3.478 | 1.139–10.627 | 0.029 |
| Molecular type | |||
| Luminal A | Ref | ||
| Luminal B | 0.839 | 0.294–2.393 | 0.743 |
| TYMS | 3.865 | 0.401–37.294 | 0.242 |
| TOP2A | 16.056 | 1.961–131.451 | 0.010 |
Bold indicates P<0.05. BMI, body mass index; CI, confidence interval; OR, odds ratio; Ref, Reference; TOP2A, DNA topoisomerase IIα; TYMS, thymidylate synthetase.
Correlation between RS and chemotherapeutic efficacy-related target genes
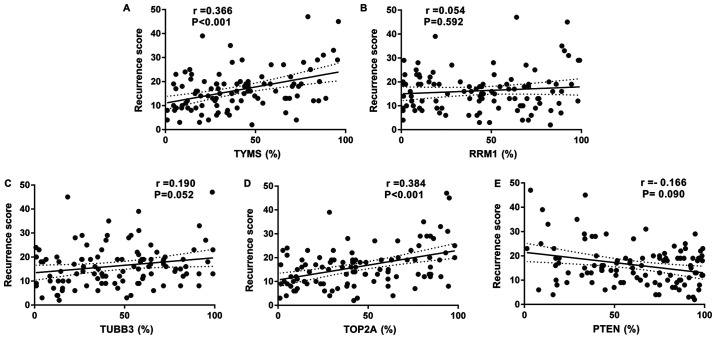
The correlation between TYMS gene expression and the RS value was determined to be statistically significant (r=0.366; P<0.001; Fig. 3A). Similar level of RRM1 gene expression was noted in both risk groups (Table IV) and no correlation was noted between RRM1 gene expression and the RS value (P=0.592; Fig. 3B). Although significantly higher TUBB3 gene expression was observed in the intermediate/high-risk RS group, the absolute difference between the two risk groups was not clinically significant and had no correlation (P=0.052; Fig. 3C). The correlation between TOP2A gene expression and the RS value was statistically significant (r=0.384; P<0.001; Fig. 3D). Higher PTEN gene expression was observed in the low-risk RS group, but there was no correlation (P=0.090; Fig. 3E).
Figure 3.
Correlation analysis between recurrence score (RS) and chemotherapeutic efficacy related target genes. (A) RS vs. TYMS; (B) RS vs. RRM1; (C) RS vs. TUBB3; (D) RS vs. TOP2A; (E) RS vs. PTEN. PTEN, phosphatase and tensin homolog; RRM1, ribonucleotide reductase catalytic subunit M1; RS, recurrence score; TOP2A, DNA topoisomerase IIα; TUBB3, tubulin β3 class III; TYMS, thymidylate synthetase.
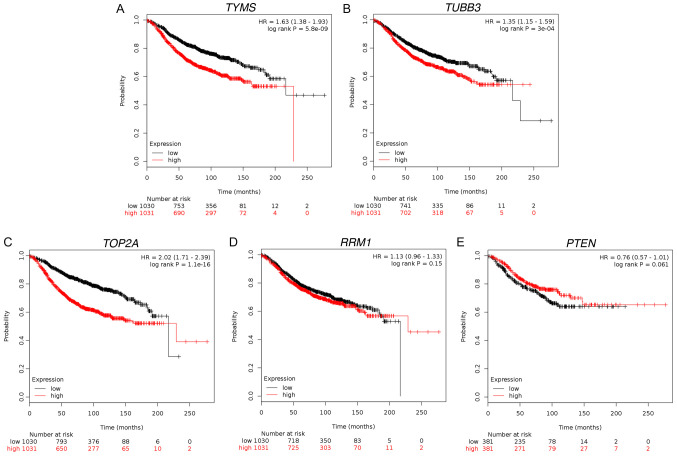
Prognostic values of the mRNA expression levels of chemotherapeutic efficacy-related target genes in ER+ breast cancer. Based on the correlations between RS and several of the tested chemotherapeutic efficacy-related target genes, the prognostic value of the mRNA expression levels of the five chemotherapeutic efficacy-related target genes in 2,061 patients with ER+ breast cancer was examined using a Kaplan-Meier plotter database. Among these genes, three were associated with relapse-free survival (RFS) for ER+ breast cancer (Fig. 4A-E). For TYMS (HR=1.63; 95% CI, 1.38–1.93; P<0.001; Fig. 4A), TUBB3 (HR=1.35; 95% CI, 1.15–1.59; P<0.001; Fig. 4B) and TOP2A (HR=2.02; 95% CI, 1.71–2.39; P<0.001; Fig. 4C), higher mRNA expression levels were associated with lower RFS in patients with ER+ breast cancer. However, RRM1 (HR=1.13; 95% CI, 0.96–1.33; P=0.15; Fig. 4D) and PTEN (HR=0.76; 95% CI, 0.57–1.01; P=0.061; Fig. 4E) were not associated with RFS. The threshold values for high and low expression levels were selected by the Kaplan-Meier plotter.
Figure 4.
Prognostic value of mRNA expression of TYMS, RRM1, TUBB3, TOP2A and PTEN in ER+ breast cancer. RFS curves of (A) TYMS (Affymetrix ID: 202589_at; n=2061), (B) RRM1 (Affymetrix ID: 201477_at; n=2061), (C) TUBB3 (Affymetrix ID: 213476_x_at; n=2061), (D) TOP2A (Affymetrix ID: 201292_at; n=2061) and (E) PTEN (Affymetrix ID:225363_at; n=762). ER, estrogen receptor; PTEN, phosphatase and tensin homolog; RFS, relapse-free survival; RRM1, ribonucleotide reductase catalytic subunit M1; RS, recurrence score; TOP2A, DNA topoisomerase IIα; TUBB3, tubulin β3 class III; TYMS, thymidylate synthetase.
Changes in treatment decisions before and after RS
The changes in doctor's treatment decision before and after RS are presented in Table VII. Among the patients who were classified as low risk group and intermediate risk group, 24 (39%) and 8 (20%) patients, respectively, were switched from adjuvant chemotherapy combined with endocrine therapy to endocrine therapy alone following the 21-gene RS test. In contrast, in the high risk group, one additional patient was prescribed adjuvant chemotherapy following the 21-gene RS test. Prior to the 21-gene RS test, a total of 61 patients (55%) were recommended adjuvant chemotherapy combined with endocrine therapy; following the 21-gene RS test, 32 patients (29%) were treated with adjuvant endocrine therapy alone. Therefore, the use of adjuvant chemotherapy was significantly reduced following the 21-gene RS test (P<0.001).
Table VII.
Treatment decisions before and after 21-gene RS test.
| Treatment decisions | Low RS n=62, n (%) | Intermediate RS n=4, n (%) | High RS n=7, n (%) | Total n=110, n (%) |
|---|---|---|---|---|
| No C recommended before RS | 34 (55) | 14 (34) | 1 (14) | 49 (45) |
| No C recommended after RS | 34 (55) | 14 (34) | 0 (0) | 48 (44) |
| C recommended after RS | 0 (0) | 0 (0) | 1 (14) | 1 (1) |
| C recommended before RS | 28 (45) | 27 (66) | 6 (86) | 61 (55) |
| C recommended after RS | 4 (6) | 19 (46) | 6 (86) | 29 (26) |
| No C recommended after RS | 24 (39) | 8 (20) | 0 (0) | 32 (29) |
| Overall change | 24 (39) | 8 (20) | 1 (14) | 33 (30) |
C, chemotherapy; RS, recurrence score.
Follow-up
By the end of August 2018, the median follow-up time was 33 months. Two patients had local recurrence (their RSs were 22 and 45, respectively). One patient with an RS of 29 had recurrence and liver metastasis. One patient with an RS of 29 had deceased. Four patients received postoperative adjuvant chemotherapy. Three patients (2.7%) were lost to follow-up due to change of contact details. All other patients survived without breast cancer recurrence or metastasis.
Discussion
In the present study, DNA liquid chip technology was used to detect 21-gene RS and the expression profiles of chemotherapeutic efficacy-related target genes as gene expression did not translate or equate to protein expression of those markers identified by IHC. This technology detected >30 genes simultaneously without reverse transcription and polymerase chain reaction. Therefore, the present study reflected the real-life associations between RS and clinicopathological characteristics of breast cancer with more clarity compared with previous studies. As the DNA liquid chip technology had the advantages of parallel detection, high sensitivity, simple operation and a wide linear range, it was suitable for a number of types of samples. In addition, it did not require RNA extraction, reverse transcription and PCR, which reduced the multi-step error accumulation on the results. In addition to the correlation between RS and clinicopathological characteristics, chemotherapeutic efficacy-related target genes were also included in the study.
In the present study, 59.1% of the patients were classified as the low-risk RS group, which suggested that chemotherapy may not have been beneficial to at least 59.1% of patients examined between 2013 and 2016. Therefore, in clinical practice, <50% of the patients may have been overtreated with chemotherapy following surgery, resulting not only in adverse reactions, but also increasing medical costs. Furthermore, the present study also indicated that patients with higher BMI, invasive ductal carcinoma type, higher histological grade and luminal B molecular type were more likely to have higher RS. Similarly, the National Surgical Adjuvant Breast and Bowel Project B20 trial also found that patients with histological grade 3, T stage 2–3 and PR-negative tumors were more likely to be categorized as high-risk groups (14). Correlations between low RS and non-invasive ductal carcinoma type have also been reported (16). Owing to the cost of 21-gene detection, the assay was only applied in patients with ER+ early-stage breast cancer. The strong correlation between BMI, invasive ductal carcinoma type, histological grade, molecular type and recurrence score indicated that the clinicopathological characteristics are also important in the future recurrence risk prediction. Therefore, whether a model can be built to predict the long-term recurrence risk of patients with breast cancer who may not need or cannot afford the 21-gene RS test was under consideration. In a study by Orucevic et al, six clinicopathological variables of 27,719 21-gene-tested ER+/HER2−, lymph node-negative patients with 6–50 mm tumor size acquired from the National Cancer Database between 2010 and 2012 were assessed by logistic regression to predict high-risk or low-risk 21-gene test results (27); the results revealed that grade and progesterone receptor status were the highest predictors of both low-risk and high-risk RS, followed by age, tumor size, histologic tumor type and lymph-vascular invasion (27). The aforementioned study reported with confidence that clinicopathological variables may be used for prediction of low-risk or high-risk RS using nomogram models, which may help physicians and patients decide whether further 21-gene RS test is necessary and function as surrogates for patients for whom 21-gene RS test is not affordable or unavailable (27). Multivariate analysis in the present study demonstrated that BMI, histological grade and TOP2A were independent variables associated with RS. These results suggested that the 21-gene RS effectively integrated clinicopathological features and provided further information regarding breast cancer. The correlations reported in the present study indicated that traditional routine clinicopathological characteristics may aid in the prediction of RS.
There were certain discrepancies between RS and clinicopathological characteristics. A number of patients with risk factors for recurrence demonstrated low RS. For example, 24.6% (16/65) of patients with higher BMI (≥24) had RS <18. In addition, 17.7% (11/62) of patients with histological grade III tumors were categorized in the low-risk RS group. Therefore, the use of one or several clinicopathological characteristics to predict RS score may not be accurate; however, 21-gene test may help avoid these deviations and may be more comprehensive. These differences between RS and clinicopathological characteristics demonstrated that the 21-gene RS may provide more accurate and verifiable prognostic information compared with traditional clinicopathological characteristics.
A retrospective study by Stemmer et al demonstrated that in patients with ER+ breast cancer with up to three positive nodes, including micrometastases, tested using the Oncotype DX gene test, the chemotherapy use was lower compared with untested patients (24.5 vs. 70.1%), which suggested that Oncotype DX testing has a significant impact on reducing chemotherapy use (28). A retrospective analysis of a prospective designed registry, which included 1,801 patients with ER+/HER2−, lymph node-negative breast cancer (median follow-up, 6.2 years), demonstrated that estimates for distant recurrence and breast cancer mortality rate for the RS <18 patients were very low, supporting the use of endocrine therapy alone (29). Additional findings from another study supported the use endocrine therapy alone in patients with ER+/HER2− breast cancer with micrometastases, 1–3 positive nodes and recurrence score <18 (30). When patients with RS 11–25 were randomized to receive either chemotherapy or endocrine therapy, the results revealed that chemotherapy was not more effective compared with endocrine therapy (29). Lee et al conducted a study of Korean breast cancer patients, and the results demonstrated that 54.2% of the patients changed their treatment decisions following the 21-gene test, among which 51.4% of patients switched to hormone therapy without chemotherapy (31). Holt et al revealed that the use of 21-gene detection had a considerable impact on chemotherapy recommendation in early stage breast cancer (32). These data demonstrated that RS test may be essential to avoid unnecessary chemotherapy. Similarly, the present study revealed that for patients with hormone receptor-positive and lymph node-negative early-stage breast cancer, 21-gene RS test may aid to avoid excessive postoperative adjuvant chemotherapy and lessen the side effects of chemotherapy. The change rate of treatment decision before and after 21-gene RS detection in China is in the top 8 in the world (UK 10%, Italy 12%, USA 22%, Japan 26%, China 29%, Germany 30%, Spain 33% and South Korea 51%) (31–37).
Notably, the distribution of RS according to chemotherapeutic efficacy-related target genes was analyzed. The detection of PTEN and other chemotherapeutic efficacy-related target genes has been previously used to predict the curative effect of target drugs and to inform their use to help patients select appropriate chemotherapy drugs (21–25). However, the data revealed that the proportion of intermediate/high-risk RS was significantly higher among patients with higher TYMS and TOP2A expression. TYMS and TOP2A gene expression correlated with the RS value, which suggested that TYMS and TOP2A gene expression may also predict prognosis and recurrence risk in patients with breast cancer. As the sample size of the present study was small, these distinct gene expression profiles were further tested in Kaplan-Meier for survival analysis. The data (n=2,061) demonstrated that TYMS, TUBB3 and TOP2A gene expression levels were significantly associated with RFS for ER+ breast cancer. These results suggested that there may be more meaningful tumor-related genes that may be used in RS classification and that the criteria for RS categories may be worth reviewing.
The 21-gene RS test were mainly applied in patients with ER+/HER2−, lymph node metastasis-negative breast cancer. In the future, the scope of application may be expanded to include patients with lymph node metastasis into the analysis to explore the value of the RS assay in these populations.
The 21-gene RS correlated significantly with BMI, pathological type, histological grade, molecular grade and several chemotherapeutic efficacy-related target genes, including TYMS and TOP2A. These results may be used to provide more information compared with clinicopathological indexes and inform treatment plans. The 21-gene RS test may help avoid excessive postoperative adjuvant chemotherapy. Large amounts of data in the Kaplan-Meier plotter database demonstrated that TYMS, TUBB3 and TOP2A gene expression levels were significantly associated with RFS for ER+ breast cancer. Therefore, TYMS, TUBB3 and TOP2A gene expression levels may have prognostic value for ER+ breast cancer.
Acknowledgements
The authors would like to thank the Department of Statistics, The First Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China) for statistical support.
Funding
The study was funded by The National Natural Science Fund of China (grant no. 81702633).
Availability of data and materials
The data generated or analyzed during this study are included in this published article. The Kaplan-Meier used in the present study are available at http://www.kmplot.com.
Authors' contributions
LZ and YR conceived and designed the study. LZ, BW, KW and JH collected the data. LZ, BW and NM analyzed and interpreted the data. CZ, YY and JH assisted in analyzing the data. NM and YY did the follow-up of all patients. LZ, NM, BW, CZ, JH and YY wrote the manuscript. LZ, YR, YY, JH and CZ revised the manuscript. All authors have read and approved the final version of this manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The First Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China). This is a retrospective study, for which formal consent is not required. The research (data analyses) complies with the current laws of the country in which they were performed (The People's Republic of China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Anderson BO, Jakesz R. Breast cancer issues in developing countries: An overview of the Breast Health Global Initiative. World J Surg. 2008;32:2578–2585. doi: 10.1007/s00268-007-9454-z. [DOI] [PubMed] [Google Scholar]
- 3.Zeng H, Zheng R, Zhang S, Zou X, Chen W. Female breast cancer statistics of 2010 in China: Estimates based on data from 145 population-based cancer registries. J Thorac Dis. 2014;6:466–470. doi: 10.3978/j.issn.2072-1439.2014.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Bureau of Statistics of China, corp-author. China Statistics Press; Beijing: 2010. China Statistical Yearbook, 2010. [Google Scholar]
- 5.Salek R, Shahidsales S, Mozafari V. Changing pattern in the clinical presentation of breast cancer in the absence of a screening program over a period of thirty-three years in Iran. Breast. 2016;28:95–99. doi: 10.1016/j.breast.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Somashekhar SP, Sepúlveda MJ, Puglielli S, Norden AD, Shortliffe EH, Rohit Kumar C, Rauthan A, Arun Kumar N, Patil P, Rhee K, et al. Watson for Oncology and breast cancer treatment recommendations: Agreement with an expert multidisciplinary tumor board. Ann Oncol. 2018;29:418–423. doi: 10.1093/annonc/mdx781. [DOI] [PubMed] [Google Scholar]
- 7.Gray J, Druker B. Genomics: The breast cancer landscape. Nature. 2012;486:328–329. doi: 10.1038/486328a. [DOI] [PubMed] [Google Scholar]
- 8.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 9.Kondo M, Hoshi SL, Ishiguro H, Yoshibayashi H, Toi M. Economic evaluation of 21-gene reverse transcriptase-polymerase chain reaction assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer in Japan. Breast Cancer Res Treat. 2008;112:175–187. doi: 10.1007/s10549-007-9842-y. [DOI] [PubMed] [Google Scholar]
- 10.Cronin M, Sangli C, Liu ML, Pho M, Dutta D, Nguyen A, Jeong J, Wu J, Langone KC, Watson D. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;53:1084–1091. doi: 10.1373/clinchem.2006.076497. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol. 2011;29:4273–4278. doi: 10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- 12.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC, Jr, American Society of Clinical Oncology American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 13.Győrffy B, Hatzis C, Sanft T, Hofstatter E, Aktas B, Pusztai L. Multigene prognostic tests in breast cancer: Past, present, future. Breast Cancer Res. 2015;17:11. doi: 10.1186/s13058-015-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 15.Paik S. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. Oncologist. 2007;12:631–635. doi: 10.1634/theoncologist.12-6-631. [DOI] [PubMed] [Google Scholar]
- 16.Wolf I, Ben-Baruch N, Shapira-Frommer R, Rizel S, Goldberg H, Yaal-Hahoshen N, Klein B, Geffen DB, Kaufman B. Association between standard clinical and pathologic characteristics and the 21-gene recurrence score in breast cancer patients: A population-based study. Cancer. 2008;112:731–736. doi: 10.1002/cncr.23225. [DOI] [PubMed] [Google Scholar]
- 17.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, et al. Breast Cancer Intergroup of North America Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Albain KS, André F, Bergh J, et al. Panel members Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsunoda Y, Suzuki K, Tsunoda A, Takimoto M, Kusano M. Evaluation of 5-fluorouracil related genes in breast cancer to predict the effect of adjuvant therapy with oral fluorouracil derivatives. Oncol Rep. 2010;23:771–777. [PubMed] [Google Scholar]
- 21.Yu Z, Sun J, Zhen J, Zhang Q, Yang Q. Thymidylate synthase predicts for clinical outcome in invasive breast cancer. Histol Histopathol. 2005;20:871–878. doi: 10.14670/HH-20.871. [DOI] [PubMed] [Google Scholar]
- 22.Metro G, Zheng Z, Fabi A, Schell M, Antoniani B, Mottolese M, Monteiro AN, Vici P, Lara Rivera S, Boulware D, et al. In situ protein expression of RRM1, ERCC1, and BRCA1 in metastatic breast cancer patients treated with gemcitabine-based chemotherapy. Cancer Invest. 2010;28:172–180. doi: 10.3109/07357900903095722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galmarini CM, Treilleux I, Cardoso F, Bernard-Marty C, Durbecq V, Gancberg D, Bissery MC, Paesmans M, Larsimont D, Piccart MJ, et al. Class III beta-tubulin isotype predicts response in advanced breast cancer patients randomly treated either with single-agent doxorubicin or docetaxel. Clin Cancer Res. 2008;14:4511–4516. doi: 10.1158/1078-0432.CCR-07-4741. [DOI] [PubMed] [Google Scholar]
- 24.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braybrooke JP, Levitt NC, Joel S, Davis T, Madhusudan S, Turley H, Wilner S, Harris AL, Talbot DC. Pharmacokinetic study of cisplatin and infusional etoposide phosphate in advanced breast cancer with correlation of response to topoisomerase IIalpha expression. Clin Cancer Res. 2003;9:4682–4688. [PubMed] [Google Scholar]
- 26.Lánczky A, Nagy Á, Bottai G, Munkácsy G, Szabó A, Santarpia L, Győrffy B. miRpower: A web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res Treat. 2016;160:439–446. doi: 10.1007/s10549-016-4013-7. [DOI] [PubMed] [Google Scholar]
- 27.Orucevic A, Bell JL, McNabb AP, Heidel RE. Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat. 2017;163:51–61. doi: 10.1007/s10549-017-4170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stemmer SM, Klang SH, Ben-Baruch N, Geffen DB, Steiner M, Soussan-Gutman L, Merling S, Svedman C, Rizel S, Lieberman N. The impact of the 21-gene Recurrence Score assay on clinical decision-making in node-positive (up to 3 positive nodes) estrogen receptor-positive breast cancer patients. Breast Cancer Res Treat. 2013;140:83–92. doi: 10.1007/s10549-013-2603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stemmer SM, Steiner M, Rizel S, Soussan-Gutman L, Ben-Baruch N, Bareket-Samish A, Geffen DB, Nisenbaum B, Isaacs K, Fried G, et al. Clinical outcomes in patients with node-negative breast cancer treated based on the recurrence score results: Evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017;3:33. doi: 10.1038/s41523-017-0033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stemmer SM, Steiner M, Rizel S, Geffen DB, Nisenbaum B, Peretz T, Soussan-Gutman L, Bareket-Samish A, Isaacs K, Rosengarten O, et al. Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the Recurrence Score results: Evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017;3:32. doi: 10.1038/s41523-017-0033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MH, Han W, Lee JE, Kim KS, Park H, Kim J, Bae SY, Shin HJ, Lee JW, Lee ES. The clinical impact of 21-gene recurrence score on treatment decisions for patients with hormone receptor-positive early breast cancer in Korea. Cancer Res Treat. 2015;47:208–214. doi: 10.4143/crt.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holt S, Bertelli G, Humphreys I, Valentine W, Durrani S, Pudney D, Rolles M, Moe M, Khawaja S, Sharaiha Y, et al. A decision impact, decision conflict and economic assessment of routine Oncotype DX testing of 146 women with node-negative or pNImi, ER-positive breast cancer in the U.K. Br J Cancer. 2013;108:2250–2258. doi: 10.1038/bjc.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo SS, Mumby PB, Norton J, Rychlik K, Smerage J, Kash J, Chew HK, Gaynor ER, Hayes DF, Epstein A, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 34.Martínez Del Prado P, Alvarez-López I, Domínguez-Fernández S, Plazaola A, Ibarrondo O, Galve-Calvo E, Ancizar-Lizarraga N, Gutierrez-Toribio M, Lahuerta-Martínez A, Mar J. Clinical and economic impact of the 21-gene recurrence score assay in adjuvant therapy decision making in patients with early-stage breast cancer: Pooled analysis in 4 Basque Country university hospitals. Clinicoecon Outcomes Res. 2018;10:189–199. doi: 10.2147/CEOR.S146095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dieci MV, Guarneri V, Giarratano T, Mion M, Tortora G, De Rossi C, Gori S, Oliani C, Merlini L, Pasini F, et al. First prospective nulticenter Italian study on the impact of the 21-gene recurrence score in adjuvant clinical decisions for patients with ER positive/HER2 negative breast cancer. Oncologist. 2018;23:297–305. doi: 10.1634/theoncologist.2017-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamauchi H, Nakagawa C, Takei H, Chao C, Yoshizawa C, Yagata H, Yoshida A, Hayashi N, Hell S, Nakamura S. Prospective study of the effect of the 21-gene assay on adjuvant clinical decision-making in Japanese women with estrogen receptor-positive, node-negative, and node-positive breast cancer. Clin Breast Cancer. 2014;14:191–197. doi: 10.1016/j.clbc.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Eiermann W, Rezai M, Kümmel S, Kühn T, Warm M, Friedrichs K, Schneeweiss A, Markmann S, Eggemann H, Hilfrich J, et al. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol. 2013;24:618–624. doi: 10.1093/annonc/mds512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated or analyzed during this study are included in this published article. The Kaplan-Meier used in the present study are available at http://www.kmplot.com.