Introduction
Early warning of the timing, location and magnitude of harmful algal blooms (HABs) is potentially of great value to coastal zone managers and the aquaculture industry, informing business planning and ensuring the protection of human health. Achieving this goal is, however, far from straightforward as key HAB genera or species exhibit different life cycles and potentially variable toxicity. Variability in local or regional oceanography or hydrography is also be critical to the location and timing of blooms.
High biomass blooms may disrupt wild fisheries or mariculture through physical interference, de-oxygenation or toxicity (Brand et al., 2012). In contrast, low biomass producers of biotoxins are of particular concern to shellfish aquaculture, as there is potential for significant impact on human health through consumption of shellfish that have bio-accumulated toxin within their flesh (Davidson and Bresnan, 2009). HABs may also have a direct impact on humans though aerosol transfer of toxin and disruption to recreational activities on beaches (Fleming et al., 2011).
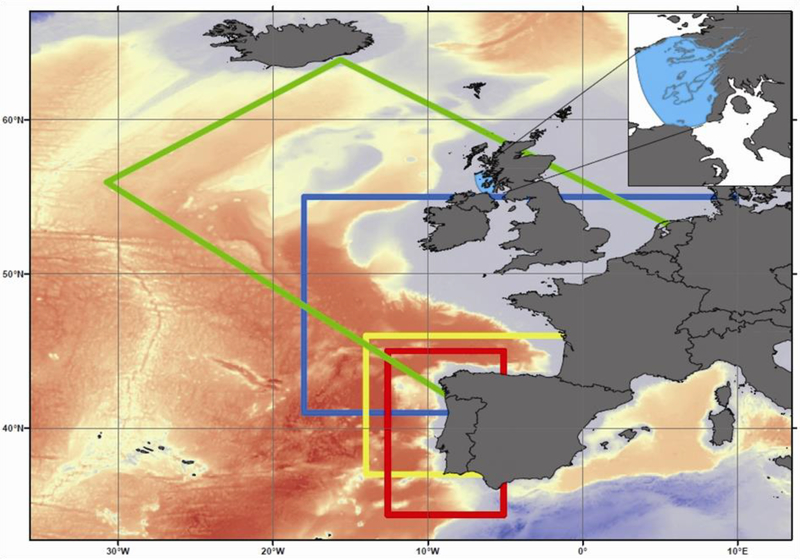
The studies presented in this Special Issue focus on research to develop HAB early warning systems in the Western European waters of Portugal, Spain, France, Ireland and Scotland (Figure 1) as part as part of the EU FP7 programme “Asimuth”. Asimuth sought to produce national or regional forecasting systems that employed expert interpretation to generate an estimated HAB risk. This was approached by combining national monitoring program and satellite remote sensing data streams with improved regional scale physical/biological HAB models.
Figure1:
The Asimuth domain illustrating the seaboard areas for which that models and risk assessments were developed.
In this preface to the Special Issue we outline the main HAB species of concern in Western European waters and the strengths and limitations of different methodologies to provide early warning of their blooms. In the papers that follow we will describe some of the developments in HAB risk assessment that took place within Asimuth in Portugal (papers a,b,c), Spain (papers d,e,f), France (papers g,h,i), Ireland (papers j,k,l), and Scotland (papers m,n,o). In the synthesis paper (Maguire et al.) we summarise the findings of these studies and describe their use in the different national/regional forecasting systems developed during the project.
Phytoplankton monitoring as early warning
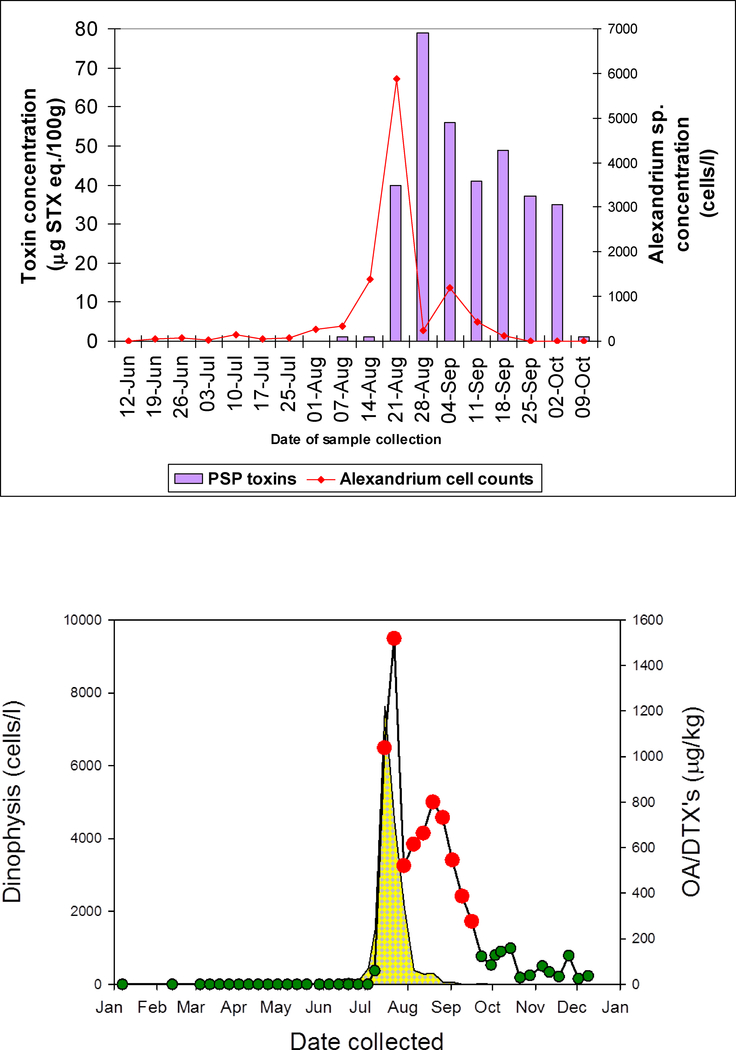
Many countries operate monitoring programmes for the presence of harmful algae. Most frequently these are aimed at ensuring shellfish safety, and in Europe such programmes are a requirement of EU shellfish hygiene directives. In practice, the monitoring methodology used varies by country (Anderson, 1996), but in all cases these time series data sets potentially provide a short-term early warning of risk. Regulators often use HAB data to trigger toxicity testing, i.e. when elevated cell counts of the causative phytoplankton are encountered. Regulatory data are typically made freely available on the internet and hence may provide short-term warning to aquaculturalists of the occurrence of harmful species and the subsequent potential for associated shellfish toxins or fish kills. An example of the applicability of phytoplankton data as an early warning of shellfish toxicity can be seen in Figure 2a that shows a lag between the increase in the concentration of Alexandrium sp. and shellfish toxicity.
Figure 2:
A) An example of early warning of shellfish PSP toxicity based on increased Alexandrium abundance and B) An example of rapid parallel increase in both Dinophysis and DSP shellfish toxins (OA and DTXs) such that no early warning was evident. Both examples are from the Scottish Shetland Isles.
Advective HABs in Asimuth region
Many HAB organisms can be transported significant distances in shelf waters by currents (Farrell et al., 2012; Anderson et al., 2005). In some locations there are known persistent advective mechanisms which result in the transfer of phytoplankton along the coast or from offshore frontal boundaries, providing the potential for regional predictions (e.g., the Maine coastal current system and Alexandrium blooms in the northeastern US; (Anderson et al. ;2005a; McGillicuddy et al. 2011). Knowledge of the Baltic current was especially important in warning Norwegian fish farmers of a major fish killing Chrysochromulina bloom that occurred in 1988 (Dahl et al., 2005). In such cases, there is the potential to give considerable advance warning of where bloom events may occur.
However, for advected blooms the increase in density at an aquaculture site may be more rapid than would be assumed from in situ growth alone, the cells being concentrated by physical as well as biological factors (e.g., convergences, onshore winds, vertical migration.). As the frequency of phytoplankton monitoring is typically no more than weekly, early warning may not always be achievable from phytoplankton time series data. For example Figure 2b shows that the increase in Dinophysis and the associated shellfish toxicity occurred with a lag of less than a week at Seggi bight in the Scottish Shetland Isles in 2013. This rapid increase in toxicity resulted in a shellfish poisoning incident with ~ 50 people suffering the effects of DSP on consuming contaminated shellfish. It is advective HAB blooms that are therefore most difficult to forecast and on which Asimuth concentrated its effort.
Western European HABs
In Western Europe the main shellfish toxin producing phytoplankton are members of the genera Alexandrium, Dinophysis, Pseudo-nitzschia and Azadinium along with the species Gymnodinium catenatum.
Azadinium and its toxin azaspiracid were relatively recently identified (Tillmann et al., 2009), its life cycle remains uncertain, and it small size makes it difficult to monitor by microscopy. All of these factors limit the capacity to develop a robust alert system for this organism and its associated toxins at present.
While the potent paralytic shellfish poisoning (PSP) causative genera Alexandrium is present in Western Europe, in contrast to locations such as the Gulf of Maine in which large scale advection of A. fundyense occurs annually (see below), its impact in Europe is most often within semi-enclosed regions of restricted exchange. Examples include A. minutum in the Penze estuary Northern France (Chapelle et al., 2010), A catanella in the and Tau Lagoon Southern France (Collos et al., 2007), A. minutum in Cork Harbour Ireland (Touzet et al., 2007) and A. tamarense in the fjordic sea lochs of Scotland (Touzet et al., 2010). Blooms typically appear when conditions are suitable to promote excystment of cells from the sediment. While the (typically) localized appearance of this organism in Europe offers the potential of some degree of early warning of shellfish toxicity based on cell counts (e.g. Figure 1), it also indicates Alexandrium is best modelled at a local scale. Such an approach is feasible in, for example Ireland, where PSP only occurs in one location, Cork Harbour (Ní Rathaille and Raine, 2011), but is more difficult to resource in regions where Alexandrium blooms are more spatially and temporally variable.
The remaining major Western European biotoxic HAB organisms (Dinophysis, Pseudo-nitzschia, Gynmodinium catenautm) have the potential to be advected to/along the coastline and hence require the larger physical/biological approach taken within Asimuth. These species either do not have a cyst or spore stage, or if they do (e.g., G. catenatum), that resting state has a very short mandatory dormancy that allows rapid recolonization of the water column. Localized cyst or spore seedbeds are thus less important in the dynamics of these HABs than persistent planktonic populations of these species. Similarly the ichthyotoxic Karenia mikimotoi (another non-cyst-former) is apparently increasingly prevalent in western European waters having been observed at bloom densities frequently in recent years with offshore development and advective transport being evident (Bresnan et al., 2010).
Remote sensing
In offshore environments, where advective HABs often initiate, difficulties in obtaining regular samples means that potentially harmful phytoplankton cannot be directly monitored in a routine, easy or cost effective manner. Remote sensing offers a potential solution to this, and may be conducted either by satellite or through the deployment of in situ sensors of HAB species or their toxins. However, a number of factors limit the effectiveness of this approach.
Satellite earth observation of ocean colour provides an estimate of chlorophyll concentration in near surface waters, although the availability of information can be limited by cloud cover. The reliance on total chlorophyll means that the information is most valuable for high biomass organisms, with applications including red tides in Ariake Sound Japan (Ishizaka et al., 2006). In general some degree of ground truthing is required to verify the composition of a remotely detected bloom. Development of algorithms for the specific detection and classification of harmful species is on-going, an example being the Florida red tide organism Karenia brevis bloom index (Amin et al., 2009). A similar approach has been taken in Europe, based on parameters such as absorption, total backscatter, and water-leaving radiance for the dinoflagellate Kareina mikimotoi, but further development will be required to make such systems operational (Shutler et al., 2012), and application to other less easily characterised HAB organisms will be challenging. Notwithstanding these difficulties, remote sensing provides the only practical method of initiating mathematical models of advective HAB species.
In situ sensors overcome some of the limitations of satellite systems, but lack spatial resolution, and their deployment often comes at significant cost. However, in certain situations such an approach has addressed the need for species-specific identification and enumeration. K. brevis produces a pigment, gyroxanthin-diester, that is sufficiently distinct to be biomarker within the Gulf of Mexico region (Richardson and Pinckney, 2004). Instruments have therefore been developed allowing the quantification of this pigment from research vessels (Kirkpatrick et al., 2003) and by means of autonomous underwater vehicles (AUVs). This approach has great potential for monitoring HAB organisms that have unique pigmentations, but for the majority of HAB species that are not so readily characterised, alternative approaches to automated detection are needed.
A more broadly applicable in situ approach utilizes species- or strain-specific molecular “probes” that can label HAB cells of interest so they can be detected visually, electronically, or chemically. Probes and assays of multiple types are now available for many of the HAB species, with molecular counting methods being routinely employed in major research programs, e.g. Anderson et al. (2005b). Application in monitoring programmes, while not unknown (Rhodes et al., 2001), is less prevalent due to issues of probe specificity, cost, and the difficulty of using fixatives in the field.
While still somewhat developmental, at least in terms of their routine application, these cell detection technologies open the door to an era where remote, sub-surface, near real-time detection of specific HAB taxa can be envisioned. An early example of such technology is the Environmental Sampling Processor (ESP); (Scholin et al., 2009) that autonomously collects discrete water samples and automates application of molecular probes to identify specific organisms.
Modelling methodology
A range of mathematical model based approaches have been applied to the problem of HAB prediction. They fall within the broad categories of conceptual, empirical and statistical. Examples of each of these are presented below.
Conceptual models
Conceptual models of HAB dynamics are of great value in providing a first order evaluation of HAB likelihood. Crucial to the formulation of such models is an understanding of the growth characteristics and life cycle of the organism of interest. Typically this will be determined from observation and laboratory study and for some organisms is relatively advanced. However, the diversity of behaviours of different species of key genera and uncertainties related to processes such as mixotrophy often prevent a robust model from being formulated.
A detailed understanding of the hydrography of the area under investigation, including adjacent waters that influence the localized flows is also required (Gowen et al., 2012). To achieve this understanding moored instruments and survey cruises are needed.
In some (eutrophied) regions another potentially important feature in the application of conceptual models is documentation of the nutrient environment that the HAB will occupy (Glibert et al., 2005) but see Gowen et al. (2012). This can be a particular problem, as while survey cruises provide large-scale snapshots of the nutrient fields, these change constantly and are quickly out of date, while “climatological” or long term average nutrient fields lack the necessary resolution.
Empirically based numerical HAB prediction models
Glibert et al. (2010) review many numerical modelling developments related to HABs. It is therefore not our intention to further review such HAB modelling studies here, but rather to identify a range of modelling methodologies and their strengths and weaknesses within an alert system through a range of examples below.
Risk assessment style models
Such models are typically relatively simple computationally, distilling the relationship between a HAB and its environment to a few key parameters or thresholds. Geographical areas that have well-defined and understood seasonal weather patterns and physical oceanography are most amenable to this approach. An early example for the rias of Galicia (NW Spain) was presented by (Fraga et al., 1988) with the relaxation of upwelling due to a change in wind direction being demonstrated to result in the transport of offshore populations of Gymnodinium catenatum into the rias.
A further example relates to blooms of Dinophysis and resulting shellfish toxicity in the bays of southwest of Ireland (Raine et al., 2010). A wind-driven two-layer oscillatory flow exchanges a substantial proportion of the bays’ volumes, and HAB events arise with the associated transport of harmful populations into them. Development of this approach is presented by Dabrowski et al. (this issue).
Ecosystem based models
These coupled physical/biological models are typically Eulerian in nature, calculating biological variables at fixed locations in space, with these variables being subject to physical (advection, diffusion) and biological processes (growth, mortality etc). Prediction is achieved by solving equations on a grid lattice.
Many recent developments in this field of modelling have related to the prediction of climate change on phytoplankton aggregated as single or multiple functional groups. Their ability to simulate HABs was reviewed by (Allen et al., 2008) with particular reference to high biomass blooms in eutrophic coastal waters. While such models were found to have some skill in predicting blooms it is clear that models that take phytoplankton to the functional group level only rarely have the capacity to make accurate forecasts of even high biomass HABs. However, some studies have extended this approach to specifically parameterise and simulate particular organisms of interest (e.g., high biomass) within an ecosystem model framework, two examples of which are presented below.
Vanhoutte-Brunier et al. (2008) modelled the 2003 Karenia mikimotoi bloom in the western English Channel by coupling a hydrodynamic/sediment model and a pelagic biochemical model that uses a Nutrient–Phytoplankton–Zooplankton–Detritus (NPZD) structure. The phytoplankton component of this model (previously categorized simply as diatoms, dinoflagellates and nanoflagellates) was amended with a specific representation of K. mikimotoi. Similarly, the model of (Lacroix et al., 2007) couples a 3D hydrodynamic model with a biogeochemical model to simulate the transport and seasonal dynamics of inorganic and organic carbon, nutrients, phytoplankton, bacterioplankton and zooplankton. In this case a specific representation of the nuisance colonial prynnesiophyte Phaeocystis was included.
Individual based models
Alternative and promising computational modelling approaches combine a physical model with an individual based model (IBM) that considers only the specific organism of interest. IBM models calculate biological variables while following individual (or meta-) particles in space. Model (virtual) particles each represent a cohort (typically billions) of real cells. Particles, that may hold biological properties, are subject to advection and diffusion and their position is tracked around the model domain. Example operational IBM based models are presented in the “coupled observational modelling systems” section below.
Statistical models
Statistically based models synthesise the measured relationships between phytoplankton abundance and potentially causative environmental conditions. A number of recent examples of this approach relate to Pseudo-nitzschia with Anderson et al. (2009) applying linear hindcasting to determine the environmental conditions associated with Pseudo-nitzschia spp. blooms in the Santa Barbara Channel, and both (Lane et al., 2009) and (Anderson et al., 2010) using logistic regression, to hindcast of Pseudo-nitzschia blooms in Monterey Bay and Chesapeake Bay respectively.
Other statistical approaches sometimes applied include bayesian analysis that allows multiple possible predictive factors and relationships to be assessed to determine which models are the most plausible given the observed data. Hamilton et al., (2009) used this technique to study Lyngbya majuscula, a nuisance cyanophyte in Deception Bay, a small embayment near Brisbane, Australia. Finally, (Davidson et al., 2009) employed path analysis, a form of multiple regression analysis that can be used to determine the most probable relationships among a set of variables to analyse, in an attempt to determine which variables were governing the major 2006 K. mikimotoi bloom in Scottish waters.
While potentially providing insight into bloom dynamics, the difficulty in obtaining real time information on the predictive environmental conditions often makes these models difficult to apply in forecast mode.
Coupled Observational Modelling Systems
Operational coupled observational/modelling HAB prediction systems, while few in number, do exist. Two well-developed examples of this approach relate to in the Gulf of Maine and Gulf of Florida and reply on the IBM approach outlined above.
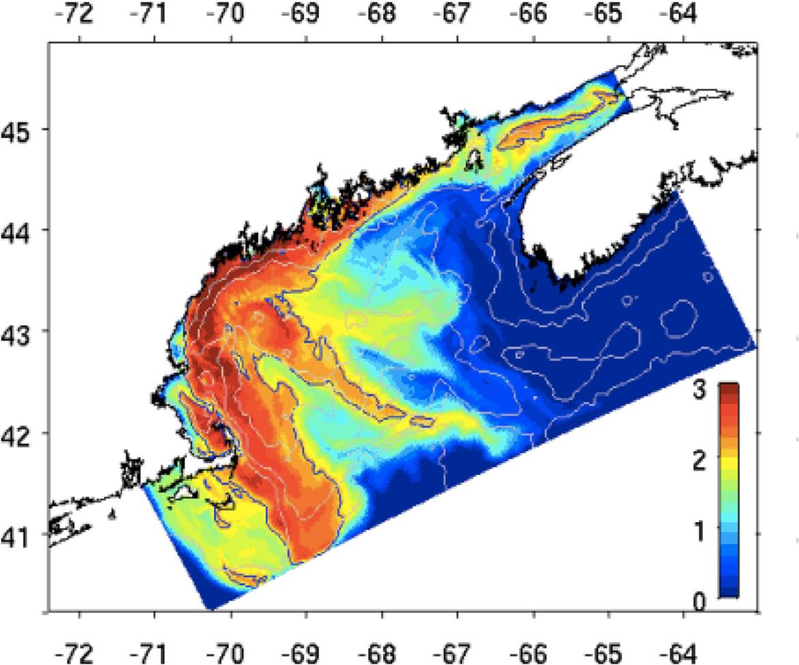
Coastal waters of the northeastern United States are subject to annually recurring PSP events caused by Alexandrium fundyense blooms that are transported in a generally southwesterly direction along the coast by coastal current advection, with wind driven forcing being capable of moving surface waters, and their associated HAB populations, both onshore and offshore. These competing transport mechanisms make prediction of the timing and magnitude of any coastal bloom difficult to determine by observation alone. A coupled physical/biological model that includes a representation of both a resting cyst and a vegetative cell (Anderson, 1998) is therefore employed to predict the development and transport of the bloom (McGillicuddy et al. 2005). The model comprises a Regional Ocean Modeling System (ROMS) approach with a coupled biological sub-model that represents the timing and rates of cyst germination and cell growth. The model is initialised based on detailed knowledge of the distribution of A. fundayense cysts in the region prior to each growth season. This information is gathered by ship based surveys (Anderson et al. in press). The model has been successfully used for hindcasts (Li et al., 2009) and is now being used to issue weekly nowcasts and forecasts, and even seasonal or annual forecasts (McGillicuddy et al. 2011), Figure 3.
Figure 3:
Example, predicted (modelled) Alexandrium fundyense distribution in the Gulf of Maine.
A second successful example is the NOAA harmful algal bloom operational forecast (HAB-OFS) that operates in Florida and Texas in the US (Stumpf et al., 2008). This system relies on a number of the approached discussed above including satellite imagery, field observations and mathematical models. It also incorporates public health reports and buoy data to predict blooms and their movement. It is, however, limited to mono-specific high biomass blooms that are detectable from satellites using ocean colour algorithms, and the actual forecasts are based on passive particle transport. A related forecast effort is now being used for cyanobacterial blooms in Lake Erie (Wynne et al. 2011)
Conclusion
A range of methods and technologies are available to provide HAB early warning. However, risk assessment based on any one of these is fraught with uncertainties. In Western Europe the building blocks of multi-parameter warning systems exist, with a relatively few organisms of concern, well-developed shellfish and plankton monitoring programmes, the availability of satellite based remote sensing data, and (in most regions) pre-existing hydrodynamics models. The scientific literature also includes a number of biologically based models of the HAB organisms of concern suitable for coupling to hydrodynamic models. In-situ HAB sensors are few, but moored instruments for physical and chemical recording provide data with which to constrain the physical component of models.
The goal of the Asimuth was therefore, for the first time, to produce a coherent HAB early warning system for the western European seaboard. Given that the HABs of primary concern differ from country to country, as do the format of national regulatory monitoring programmes and the hydrodynamic models available, a fully integrated region-wide alert system was not practicable, nor desirable. Neither did we seek to directly recreate those alert systems that are operational elsewhere, as again, the organisms, hydrography and available infrastructure differ too much. Rather, national systems were formulated based on local or regional needs. These were designed, however, to be compatible, allowing allow easy transfer of information and approaches from one region to another.
Acknowledgements
The Asimuth project was supported by the EU FP7 Programme, Space Theme, Grant Agreement No.: 261860. Support for DMA was provided by the Woods Hole Center for Oceans and Human Health, National Science Foundation Grant (OCE-1314642) and National Institute of Environmental Health Sciences Grant (1-P01-ES021923–01).
References
- Allen JI, Smyth TJ, Siddorn JR, Holt M, 2008. How well can we forecast high biomass algal bloom events in a eutrophic coastal sea? Harmful Algae 8, 70–76. [Google Scholar]
- Amin R, Zhou J, Gilerson A, Gross B, Moshart F, Ahmed S, 2009. Novel Optical Techniques for Detecting and Classifying Toxic Dinoflagellate Karenia Brevis Blooms Using Satellite Imagery. Opt. Express 17, 9126–9144. [DOI] [PubMed] [Google Scholar]
- Anderson C, Siegel D, Kudela R, Brzezinski M, 2009. Empirical models of toxigenic Pseudo-nitzschia blooms: Potential use as a remote detection tool in the Santa Barbara Channel. Harmful Algae 8, 478–492. [Google Scholar]
- Anderson CR, Sapiano MRP, Prasad MBK, Long W, Tango PJ, Brown CW, Murtugudde R, 2010. Predicting potentially toxigenic Pseudo-nitzschia blooms in the Chesapeake Bay. J. Mar. Syst 83, 127–140. [Google Scholar]
- Anderson DM, Keafer BA, Kleindinst JL, McGillicuddy DJ Jr., Martin JL, Norton K, Pilskaln CH, Smith JL, Sherwood CR, Butman B Alexandrium fundyense cysts in the Gulf of Maine: Long-term time series of abundance and distribution, and linkages to past and future blooms, in press. 10.1016/j.dsr2.2013.10.002 [DOI] [PMC free article] [PubMed]
- Anderson D, Townsend DW, McGillicuddy DJ, JT T, 2005. The Ecology and Oceanography of Toxic Alexandrium fundyense Blooms in the Gulf of Maine. Deep Sea Res. Part II Top. Stud. Oceanogr 52, 2365–2876. [Google Scholar]
- Anderson DM, 1998. Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions, in: Anderson DM, Cembella AD, Hallegraeff G (Eds.), The Physiological Ecology of Harmful Algal Blooms. Springer-Verlag, Heidelberg, pp. 29–48. [Google Scholar]
- Anderson DM, Kulis DM, Keafer B. a., Gribble KE, Marin R, Scholin C. a., 2005a. Identification and enumeration of Alexandrium spp. from the Gulf of Maine using molecular probes. Deep Sea Res. Part II Top. Stud. Oceanogr 52, 2467–2490. [Google Scholar]
- Anderson DM, Stock C. a., Keafer B. a., Bronzino Nelson A, Thompson B, McGillicuddy DJ, Keller M, Matrai P. a., Martin J, 2005b. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep Sea Res. Part II Top. Stud. Oceanogr 52, 2522–2542. [Google Scholar]
- Anderson P, 1996. Design and Implementation of some Harmful Algal Monitoring Systems.
- Brand LE, Campbell L, Bresnan E, 2012. Karenia: The biology and ecology of a toxic genus. Harmful Algae 14, 156–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnan E, Fernand L, Davidson K, Edwards ME, MiIlligan SM, Gowan RG, Silke J, S K, Raine R, 2010. Climate Change impacts on Harmful Algal Blooms (HABs), in: MCCIP Annual Report Card 2010–11, MCCIP Science Review. 10pp. [Google Scholar]
- Chapelle A, Labry C, Sourisseau M, Lebreton C, Youenou A, Crassous P, Pelagos ID, Gkss F, 2010. Alexadnrium minutum growth controlled by phosphorous: An applied model. J. Mar. Syst 83, 181–191. [Google Scholar]
- Collos Y, Vaquer A, Laabir M, Abadie E, Laugier T, Pastoureaud A, Souchu P, 2007. Contribution of several nitrogen sources to growth of Alexandrium catenella during blooms in Thau lagoon, southern France. Harmful Algae 6, 781–789. [Google Scholar]
- Dahl E, Bagøien E, Edvardsen B, Stenseth NC, 2005. The dynamics of Chrysochromulina species in the Skagerrak in relation to environmental conditions. J. Sea Res 54, 15–24. [Google Scholar]
- Davidson K, Bresnan E, 2009. Shellfish toxicity in UK waters: a threat to human health? Environ. Health 8 Suppl 1, S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson K, Gowen RJ, Tett P, Bresnan E, Harrison PJ, Mckinney A, Milligan S, Mills DK, Silke J, Crooks A, 2012. Harmful algal blooms : How strong is the evidence that nutrient ratios and forms influence their occurrence ? Estuar. Coast. Mar. Sci 115, 399–413. [Google Scholar]
- Davidson K, Miller P, Wilding TA, Shutler J, Bresnan E, Kennington K, Swan S, 2009. A large and prolonged bloom of Karenia mikimotoi in Scottish waters in 2006. Harmful Algae 8, 349–361. [Google Scholar]
- Farrell H, Gentien P, Fernand L, Lunven M, Reguera B, González-Gil S, Raine R, 2012. Scales characterising a high density thin layer of Dinophysis acuta Ehrenberg and its transport within a coastal jet. Harmful Algae 15, 36–46. [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Walsh CJ, Nierenberg K, Clark J, Reich A, Hollenbeck J, Benson J, Cheng YS, Naar J, Pierce R, Bourdelais AJ, Abraham WM, Kirkpatrick G, Zaias J, Wanner A, Mendes E, Shalat S, Hoagland P, Stephan W, Bean J, Watkins S, Clarke T, Byrne M, Baden DG, 2011. Review of Florida Red Tide and Human Health Effects. Harmful Algae 10, 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga S, Anderson D, Bravo I, 1988. Influence of upwelling relaxation on dinoflagellates and shellfish toxicity in Ria de Vigo, Spain. Estuarine, Coast Shelf Sci. 27, 349–361. [Google Scholar]
- Glibert PM, Allen JI, Bouwman a. F., Brown CW, Flynn KJ, Lewitus AJ, Madden CJ, 2010. Modeling of HABs and eutrophication: Status, advances, challenges. J. Mar. Syst 83, 262–275. [Google Scholar]
- Glibert PM, Seitzinger SP, Heil CA, Burkholder JM, Parrow MW, Codispoti LA, Kelly V, 2005. Eutrophication in the Global Proliferation of Harmful Algal Blooms The Role of Oceanography 18, 198–209. [Google Scholar]
- Goffredi SK, Jones WJ, Scholin C. a, Marin R, Vrijenhoek RC, 2006. Molecular detection of marine invertebrate larvae. Mar. Biotechnol. (NY). 8, 149–60. [DOI] [PubMed] [Google Scholar]
- Gowen RJ, Mckinney A, Tett P, Bresnan E, Davidson K, Harrison PJ, Milligan S, Mills DK, Silke J, Crooks AM, 2012. Anthropogenic nutrient enrichment and blooms of harmful phytoplankton. Oceanogr. Mar. Biol 50, 65–126. [Google Scholar]
- Hamilton G, McVinish R, Mengersen K, 2009. Bayesian model averaging for harmful algal bloom prediction. Ecol. Appl 19, 1805–1814. [DOI] [PubMed] [Google Scholar]
- Ishizaka J, Kitaura Y, Touke Y, Sasaki H, Tanaka A, H M, T S, 2006. Satellite detection of red tide in Ariake Sound 1998–2201. J Oceanogr. 62, 37–45. [Google Scholar]
- Kirkpatrick GJ, Millie D, Moline MA, Schofield O, 2000. Optical discrimination of a phytoplankton species in natural mixed populations. Limnol. Oceanogr 45, 467–471. [Google Scholar]
- Kirkpatrick GJ, Orrico C, Moline MA, Oliver M, Schofield O, 2003. Continuous hyperspectral absorption measurements of coloured dissolved organic material in aquatic systems. Appl. Opt 42, 6564–6568. [DOI] [PubMed] [Google Scholar]
- Lacroix G, Ruddick K, Park Y, Gypens N, Lancelot C, 2007. Validation of the 3D biogeochemical model MIRO&CO with field nutrient and phytoplankton data and MERIS-derived surface chlorophyll a images. J. Mar. Syst 64, 66–88. [Google Scholar]
- Lane J, Raimondi P, Kudela R, 2009. Development of a logistic regression model for the prediction of toxigenic Pseudo-nitzschia blooms in Monterey Bay, California. Mar. Ecol. Prog. Ser 383, 37–51. [Google Scholar]
- Li Y, He R, McGillicuddy DJ, Anderson DM, Keafer B. a., 2009. Investigation of the 2006 Alexandrium fundyense bloom in the Gulf of Maine: In-situ observations and numerical modeling. Cont. Shelf Res 29, 2069–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy DJ, Anderson DM, Lynch DR and Townsend DW. 2005. Mechanisms regulating the large-scale seasonal fluctuations in Alexandrium fundyense populations in the Gulf of Maine: results from a physical-biological model. Deep-Sea Res. II, 52: 2698–2714. [Google Scholar]
- McGillicuddy DJ, Townsend DW, He R, Keafer BA, Kleindinst JL, Li Y, Manning JP, Mountain DG, Thomas MA, and Anderson DM. 2011. Suppression of the 2010 Alexandrium fundyense bloom by changes in physical, biological, and chemical properties of the Gulf of Maine. Limnol. Oceanogr 56(6): 2411–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ní Rathaille A, Raine R, 2011. Seasonality in the excystment of Alexandrium minutum and Alexandrium tamarense in Irish coastal waters. Harmful Algae 10, 629–635. [Google Scholar]
- Raine R, McDermott G, Silke J, Lyons K, Nolan G, Cusack C, 2010. A simple short range model for the prediction of harmful algal events in the bays of southwestern Ireland. J. Mar. Syst 83, 150–157. [Google Scholar]
- Rhodes LL, Scholin CA, Tyrell J, Adamson J, Todd K, 2001. The integrations of DNA probes into New Zealand’s routine phytoplankton monitoring programmes, in: Hallegraeff G, Blackburn S, Bolch C, RJ L (Eds.), Harmful Algal Blooms 2001. Intervovernmental Oceanographic Organisation of UNESCO, pp. 429–432. [Google Scholar]
- Richardson TL, Pinckney JL, 2004. Monitoring of the toxic dinoflagellate Karenia brevis using gyroxanthin-based detection methods. J. Appl. Phycol 16, 315–328. [Google Scholar]
- Scholin CA, Doucette G, Jensen S, Roman B, Pargett D Iii, R.M., Preston C, Jones W, Feldman J, Everlove C, Harris A, Alvarado N, Massion E, Birch J, Greenfield D, 2009. Remote sensing of marine microbes, small invertebrates, harmful algae and biotoxins using the Environmental Sample Processor (ESP). Oceanography 22, 158–167. [Google Scholar]
- Shutler JD, Davidson K, Miller PI, Swan SC, Grant MG, Bresnan E, 2012. An adaptive approach to detect high-biomass algal blooms from EO chlorophyll- a data in support of harmful algal bloom monitoring. Remote Sens. Lett 3, 101–110. [Google Scholar]
- Stumpf RP, Litaker RW, Lanerolle L, Tester P. a., 2008. Hydrodynamic accumulation of Karenia off the west coast of Florida. Cont. Shelf Res 28, 189–213. [Google Scholar]
- Tillmann U, Elbrächter M, Krock B, John U, Cembella A, 2009. Azadinium spinosum gen. et sp. nov. (Dinophyceae) identified as a primary producer of azaspiracid toxins. Eur. J. Phycol 44, 63–79. [Google Scholar]
- Touzet N, Davidson K, Pete R, Flanagan K, McCoy GR, Amzil Z, Maher M, Chapelle A, Raine R, 2010. Co-occurrence of the West European (Gr.III) and North American (Gr.I) ribotypes of Alexandrium tamarense (Dinophyceae) in Shetland, Scotland. Protist 161, 370–84. [DOI] [PubMed] [Google Scholar]
- Touzet N, Franco JM, Raine R, 2007. Characterization of nontoxic and toxin-producing strains of Alexandrium minutum (Dinophyceae) in Irish coastal waters. Appl. Environ. Microbiol 73, 3333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte-Brunier A, Fernand L, Ménesguen A, Lyons S, Gohin F, Cugier P, 2008. Modelling the Karenia mikimotoi bloom that occurred in the western English Channel during summer 2003. Ecol. Modell 210, 351–376. [Google Scholar]
- Estimating cyanobacterial bloom transport by coupling remotely sensed imagery and a hydrodynamic model 2011. Wynne TT, Stumpf RP, Tomlinson MC, Schwab DJ, Watabayashi GY, and Christensen JD. Ecological Applications 2011. 21:7, 2709–2721 [DOI] [PubMed] [Google Scholar]