Abstract
The striatum constitutes the main input structure of the basal ganglia and receives two major excitatory glutamatergic inputs, from the cortex and the thalamus. Excitatory cortico- and thalamostriatal connections innervate the principal neurons of the striatum, the spiny projection neurons (SPNs), which constitute the main cellular input as well as the only output of the striatum. In addition, corticostriatal and thalamostriatal inputs also innervate striatal interneurons. Some of these inputs have been very well studied, for example the thalamic innervation of cholinergic interneurons and the cortical innervation of striatal fast-spiking interneurons, but inputs to most other GABAergic interneurons remain largely unstudied, due in part to the relatively recent identification and characterization of many of these interneurons. In this review, we will discuss and reconcile some older as well as more recent data on the extrinsic excitatory inputs to striatal interneurons. We propose that the traditional feed-forward inhibitory model of the cortical input to the fast-spiking interneuron then inhibiting the SPN, often assumed to be the prototype of the main functional organization of striatal interneurons, is incomplete. We provide evidence that the extrinsic innervation of striatal interneurons is not uniform but shows great cell-type specificity. In addition, we will review data showing that striatal interneurons are themselves interconnected in a highly cell-type-specific manner. These data suggest that the impact of the extrinsic inputs on striatal activity critically depends on synaptic interactions within interneuronal circuitry.
Keywords: connectivity, cortex, glutamate, selective innervation, thalamus
Introduction
The striatum constitutes the main input structure of the basal ganglia. It receives major excitatory projections from the cortex and the thalamus (Kemp & Powell, 1971; Buchwald et al., 1973; Smith et al., 2004). One of the main functions attributed to the striatum is the integration of the massive excitatory corticostriatal and thalamostriatal projections. Essentially, all regions of the cortex project to the striatum in a highly organized manner (Yeterian & Van Hoesen, 1978; Flaherty & Graybiel, 1993; Haber et al., 2006; Haber, 2016; Hintiryan et al., 2016). The corticostriatal system has been the subject of intense investigation and is often considered as the principal excitatory drive of the striatum providing motor and cognitive information to the striatum. The thalamostriatal system is thought to be critical in mediating BG responses to attention-related stimuli and may be engaged in behavioral switching and reinforcement functions (Kimura et al., 2004; Minamimoto et al., 2009; Bradfield et al., 2013; Smith et al., 2014). Although this system originates from several discrete thalamic nuclei, the principal source of thalamostriatal projections arises from the intralaminar nuclei and specifically from the centromedian/parafascicular complex (CM/Pf; (Smith & Parent, 1986b; Berendse & Groenewegen, 1990; Francois et al., 1991; Sadikot et al., 1992; McFarland & Haber, 2000; Smith et al., 2004, 2014).
The striatum is comprised mostly (~95% in rodents) of medium-sized GABAergic spiny projection neurons (SPNs; (Kemp & Powell, 1971; Luk & Sadikot, 2001). They form the major inputs and the only outputs of this structure. The remaining neurons consist of several populations of interneurons that have been classified based on their intrinsic electrophysiological properties, neurochemical and/ or molecular expression profiles, as well as their synaptic connectivity (Smith & Parent, 1986a; Kawaguchi, 1993; Kubota et al., 1993; Kubota & Kawaguchi, 1994; Tepper & Bolam, 2004; Tepper et al., 2010; Tepper & Koós, 2017). There is one population of cholinergic interneurons but several diverse and heterogeneous groups of GABAergic interneurons, that are constantly being updated, as new ones are being discovered and characterized (e.g. Ibanez-Sandoval et al., 2010, 2011; English et al., 2012; Faust et al., 2015; Munoz-Manchado et al., 2016; Garas et al., 2016, 2018).
Thanks to the development of new transgenic mouse models and optogenetic methods, the identification and characterization of striatal GABAergic interneurons, their synaptic connectivity and their differing roles in the function of striatal circuitry is undergoing a very rapid expansion. Until about 10 years ago, only four subtypes of striatal interneurons were identified and well characterized, consisting of one population of cholinergic interneurons (CIN; Kawaguchi, 1993; Kawaguchi et al., 1995), also referred to as TANS because of their spontaneous activity in primates (Kimura et al., 1984; Apicella, 2002) and three populations of GABAergic interneurons comprising parvalbumin-expressing fast-spiking interneurons (FSI), the calretinin-expressing interneurons (CR) and the neuropeptide Y/somatostatin/NOS-expressing low-threshold spike interneuron (NPY-PLTS) (Kawaguchi, 1993; Kawaguchi et al., 1995; Tepper & Bolam, 2004).
Since then, we and others have identified multiple subtypes of non-dopaminergic tyrosine hydroxylase expressing GABAergic interneurons (THINs; Ibanez-Sandoval et al., 2010; Xenias et al., 2015), a second, morphologically, electrophysiologically and neuro-chemically distinct population of NPY-expressing interneurons termed striatal neurogliaform (NGF) interneurons; (Ibanez-Sandoval et al., 2011; English et al., 2012) and at least one other subtype of GABAergic interneuron targeted in the HT3Ra-Cre or 5HT3a-cre mice called the fast adapting interneuron (FAI; Faust et al., 2015; Munoz-Manchado et al., 2016; for recent review see (Tepper & Koós, 2017)).
Understanding how extrinsic inputs are processed by the intrinsic striatal circuitry is essential to understand how these inputs ultimately affect the projection neurons and structures downstream of the striatum. In this review, we will not describe in detail the anatomical or electrophysiological properties of the different striatal GABAergic interneurons subtypes, as these have been reviewed recently elsewhere (Tepper & Koós, 2017). Here, we first review the excitatory cortico- and thalamostriatal inputs to the striatal interneurons. Next, we will describe recent findings on cholinergic input to striatal interneurons. In the last part of the manuscript, we will review new findings with respect to GABAergic interneuron–interneuron interactions.
Glutamatergic input to striatal interneurons
Corticostriatal and thalamostriatal inputs to SPNs have been more extensively studied that any other cell type in the striatum due of course to their large number in comparison with striatal interneurons and to the fact that they represent the only output neurons of the striatum. Recently, the amount of data regarding excitatory input to cholinergic and GABAergic interneurons has significantly increased. Until recently, the classical view regarding GABAergic interneurons’ function was that they were received excitatory input from cortex and thalamus in a non-specific manner and provided feed-forward inhibition to SPNs. Recent findings concerning the cortical and thalamic innervation of the different classes of interneurons listed above force a re-evaluation of this model.
CINs
Cholinergic interneurons receive glutamatergic innervation from both thalamus and cortex. Stimulation of thalamus and cortex can produce monosynaptic excitatory responses in the same CIN (Wilson et al., 1990; Doig et al., 2014). However, both anatomical and physiological studies have shown that the innervation from the thalamus and especially the intralaminar thalamic nuclei is stronger than the relatively weaker cortical innervation of CINs (Meredith & Wouterlood, 1990; Lapper & Bolam, 1992; Ding et al., 2010; Doig et al., 2014; Assous et al., 2017). Electrical stimulation of the parafascicular nucleus modulates acetylcholine release in vivo measured by in vivo microdialysis (Consolo et al., 1996; Zackheim & Abercrombie, 2005; Nanda et al., 2009). Interestingly, the responses observed in those studies are heterogeneous. While Consolo et al. (1996) found an increase in acetylcholine release, for others (Zackheim & Abercrombie, 2005; Nanda et al., 2009), PfN activation (and/or inhibition) seems to induce the opposite effect. In both studies though, thalamic-induced acetylcholine increase in the striatum was observed after infusion of GABAA receptor antagonists in the striatum. Those results, using different manipulations of the PfN, implicate different excitatory and inhibitory components of the CIN response to PfN activation which likely explain the discrepancies. Together with recent data showing that different populations of GABAergic interneurons innervate CINs (English et al., 2012) as well as receive monosynaptic inputs from PfN (Assous et al., 2017), it seems clear that intrastriatal circuitry plays an critical role in the response of CIN to extrinsic glutamatergic inputs.
Recent retrograde rabies tracing has revealed strong monosynaptic innervation of CINs from both cortex and thalamus although cortical inputs tended to make fewer connections (Guo et al., 2015). Both thalamic stimulation and cortical electrical stimulation are able to evoke short-latency spiking that is followed by a pause in firing and a subsequent rebound increase in firing rate in juxtacellular recordings (Doig et al., 2014). Interestingly, with repetitive cortical stimulation, firing probability progressively decreased while it increases after repetitive stimulation from the thalamus (Doig et al., 2014). This is consistent with in vitro slice recording experiments where it was shown that thalamostriatal synapses onto CINs exhibited short-term facilitation which is a factor promoting summation and hence could be responsible for the burst of activity observed in CINs after burst activity of thalamic neurons (Ding et al., 2010). These authors also showed that thalamostriatal stimulation evoked a burst-like response in CINs that triggered a transient depression of corticostriatal EPSCs in SPNs.
This typical pause response, often flanked by periods of bursts in CINs, is observed in vivo following the presentation of a salient stimulus (Aosaki et al., 1994; Graybiel et al., 1994; Matsumoto et al., 2001; Blazquez et al., 2002; Minamimoto & Kimura, 2002). This multiphasic response of CINs depends on normal thalamic innervation as pharmacological blockade of the thalamus abolished the pause and rebound facilitatory responses of TANs in the striatum (Matsumoto et al., 2001). Also, lesion of the parafascicular nucleus has been shown to reduce the firing rate of CINs (Bradfield et al., 2013). These authors also showed that the loss of this connection impairs goal-directed learning after changes in the action-outcome contingencies. It is thus likely that the intralaminar thalamic inputs to the CINs participate in the initial excitation as well as in the pause phase of the response of CINs following the presentation of a salient stimulus (for review, see Goldberg & Reynolds, 2011; Schulz & Reynolds, 2013). Interestingly, we recently found that this connection from the Pf to the CINs was responsible for evoking mono and disynaptic nicotinic EPSPs in NPY-NGF interneurons (Assous et al., 2017). Further, it has been shown that optogenetic stimulation of CINs can trigger dopamine release via activation of presynaptic nicotinic receptors on dopamine terminals (Threlfell et al., 2012). In the same study, similar nicotinic-dependent dopamine release could be elicited through optogenetic activation of thalamostriatal inputs. Those results suggest that in addition to acetylcholine, dopamine may also be important for conveying salience-related signals (Threlfell et al., 2012). The same laboratory has also provided evidence that in addition to thalamic inputs, cortical inputs to CINs can also induce dopamine release by a similar nicotinic mechanism (Kosillo et al., 2016).
In vivo juxtacellular recording and labeling studies show that CINs do not change their firing significantly when cortex switches from slow wave activity to desynchronization (Sharott et al., 2012). However, this study along with others (Wilson et al., 1990; Doig et al., 2014) did show short-latency responses of CINs to cortical stimulation consistent with the connections between cortex and CINs discussed above.
In vivo whole cell recording from a small number of CINs showed that those neurons, similar to FSIs (see below) and SPNs, displayed slow wave oscillations (Reig & Silberberg, 2014). This study also demonstrated that CINs responded to bilateral whisker stimulation, suggesting a role in sensory integration.
Orbitofrontal inputs to CIN are important for animals to track their current state. Recording of CINs in rats performing a behavioral task consisting of several trial blocks referred as ‘state’ which requires the recall of the current state and the learning of changed conditions have shown that dorsomedial but not dorsolateral striatal CINs are essential for the animal to keep track of the current behavioral trial or state. This state information is dependent on orbitofrontal cortex input to CINs (Stalnaker et al., 2016). Those results are consistent with observations showing involvement of CINs in flexible behaviors and in integrating new learning (Ragozzino et al., 2009; Bradfield et al., 2013; Aoki et al., 2015).
Further, it has been shown that CINs exhibit long-term corticostriatal plasticity following tetanic stimulation (Suzuki et al., 2001; Reynolds et al., 2004) or spike timing-dependent plasticity (STDP) protocols (Fino et al., 2008). Interestingly, high-frequency stimulation of the substantia nigra induced persistent potentiation of cortical evoked excitatory responses and also increased the after hyperpolarization potential following the stimulus. Those data obtained in vivo with intracellular recordings provide a possible mechanism that could be involved in the acquisition of the pause response in CINs during learning (Reynolds et al., 2004).
FSI
FSIs receive a substantial innervation from both cortex and thalamus. Anatomical evidence has shown that cortex provides direct and dense innervation to striatal FSI (Lapper et al., 1992; Bennett & Bolam, 1994). Interestingly, in contrast to SPNs, single cortical neurons formed multiple synaptic contacts with individual FSIs (Ramanathan et al., 2002), which likely explains why FSIs seem more sensitive to cortical inputs than SPNs (Parthasarathy & Graybiel, 1997; Mallet et al., 2005). Ramanathan et al. (2002) also demonstrated the convergence of somatosensory and motor cortical areas onto the same FSI, suggesting that sensorimotor integration in the basal ganglia could be mediated at least in part by striatal FSIs.
Anatomical studies have also shown innervation of FSIs from Pf (Rudkin & Sadikot, 1999; Sidibe & Smith, 1999). While those studies reveal a very dense innervation in monkeys, it seems less important than cortical innervation in rats. A recent study compared the modulation of striatal FSIs by thalamostriatal and corticostriatal afferents (Sciamanna et al., 2015). The authors found that similar to corticostriatal and thalamostriatal synapses onto SPNs, corticostriatal synapses onto FSIs exhibit short-term facilitation while in contrast, thalamostriatal synapses exhibit short-term depression. Furthermore, thalamostriatal synapses exhibit more prominent AMPA receptormediated currents than corticostriatal synapses (Sciamanna et al., 2015). We and others have also shown that optogenetic stimulation of terminals from the PfN as well as from cortex was able to induce action potential firing of FSI in mouse striatal slices (Arias-Garcia et al., 2017; Assous et al., 2017).
Mallet et al. (2005, 2006) showed with in vivo juxtacellular recordings and labeling that striatal neurons that exhibit brief action potential waveforms are parvalbumin-positive, consistent with previous in vitro data (Kawaguchi, 1993; Kawaguchi et al., 1995; Koos & Tepper, 1999) and assumptions from in vivo recordings from many others (Berke et al., 2004; Mallet et al., 2005, 2006; Schulz et al., 2011; Lee et al., 2017; O’ Hare et al., 2017). Mallet et al. (2005, 2006) also showed that FSIs respond to cortical stimulation by firing bursts with very short interspike intervals (2–3 ms). Further, cortical desynchronization enhanced FSI activity and facilitated their spike responses to cortical stimulation (Mallet et al., 2005). This was confirmed, using similar techniques in another study by Sharott et al. (2012), where transitioning from slow wave activity to cortical activation resulted in a robust increase in the firing rate of FSIs. Also, these neurons can phase lock their firing to high-frequency cortical oscillations (Berke et al., 2004; van der Meer & Redish, 2009; Sharott et al., 2009, 2012).
Interestingly, spiking to cortical stimulation occurred earlier for FSIs than for projection neurons (Mallet et al., 2005), consistent with their apparent greater sensitivity discussed above. Also, local application of picrotoxin increased spiking of SPNs after cortical stimulation particularly under conditions favoring the activity of FSIs. Those data, together with the powerful inhibition of SPNs by FSIs, put them in a prime position to mediate feed-forward inhibition on SPNs (Koos & Tepper, 1999; Planert et al., 2010; Gittis et al., 2011; Straub et al., 2016; Lee et al., 2017). This also narrows the time window of the excitatory responses of SPNs to cortical stimulation (Mallet et al., 2006). Interestingly, as for CINs, in vivo whole cell recording from a small number of FSIs showed that those neurons displayed slow wave oscillations and responded to bilateral whisker stimulation as well as visual stimulation suggesting a role in sensory integration of those interneurons (Reig & Silberberg, 2014).
THINs
Local striatal stimulation elicits a biphasic response consisting of overlapping glutamatergic EPSPs and GABAA IPSPs in striatal THINs (Ibanez-Sandoval et al., 2010). THINs receive monosynaptic glutamatergic cortical inputs and respond to cortical electrical stimulation with EPSPs that elicit spiking (Ibanez-Sandoval et al., 2010). In a recent study, we also investigated the thalamic input from the PfN to THINs (Assous et al., 2017 and unpublished data). We found that optogenetic stimulation of the PfN evoked large excitatory responses in all THINs which almost always gave rise to an action potential. Those responses were blocked by bath application of AMPA/NMDA antagonists, although in some cases, a small fraction of the excitatory response remained after blocking AMPA/NMDA receptors. This could be due to the involvement of metabotropic glutamate receptors as THINs have been shown to express functional group I mGluR (Partridge et al., 2014).
We also showed that this pathway (along with the feed-forward monosynaptic inhibition of LTS interneurons by THINs discussed below) is involved in the modulation of the prepulse inhibition of the startle reflex, an effect shown to involve a thalamostriatal pathway (Hazlett et al., 2001; Baldan Ramsey et al., 2011; Angelov et al., 2014). Indeed, specific ablation of THINs, using a Cre-dependent diphtheria toxin, induces significant reduction in the prepulse inhibition after presentation of an acoustic startle stimulus (Assous et al., 2017). Our results also demonstrate that this pathway is involved in the disynaptic inhibition observed in LTS interneurons after optogenetic stimulation of the thalamus.
NGF interneurons
In the first description of striatal NGF interneurons (Ibanez-Sandoval et al., 2011), we showed that electrical stimulation of cortex evokes monosynaptic excitatory responses in NGF interneurons. However, unlike LTS interneurons (see below) or FSIs, cortical stimulation could not elicit action potential firing in NGF interneurons, but only subthreshold EPSPs (Ibanez-Sandoval et al., 2011). We obtained similar results following injection of a CAMKII-ChR2 virus in the motor cortex and optogenetic stimulation of cortex (Fig. 1A–D). In this paradigm, spiking could only be elicited in only ~15% of recorded NGF interneurons (Assous et al., 2017). Responses to a train of optogenetic pulses show that corticostriatal synapses onto NGF interneurons are strongly depressing (Assous et al., 2017), Fig. 1D).
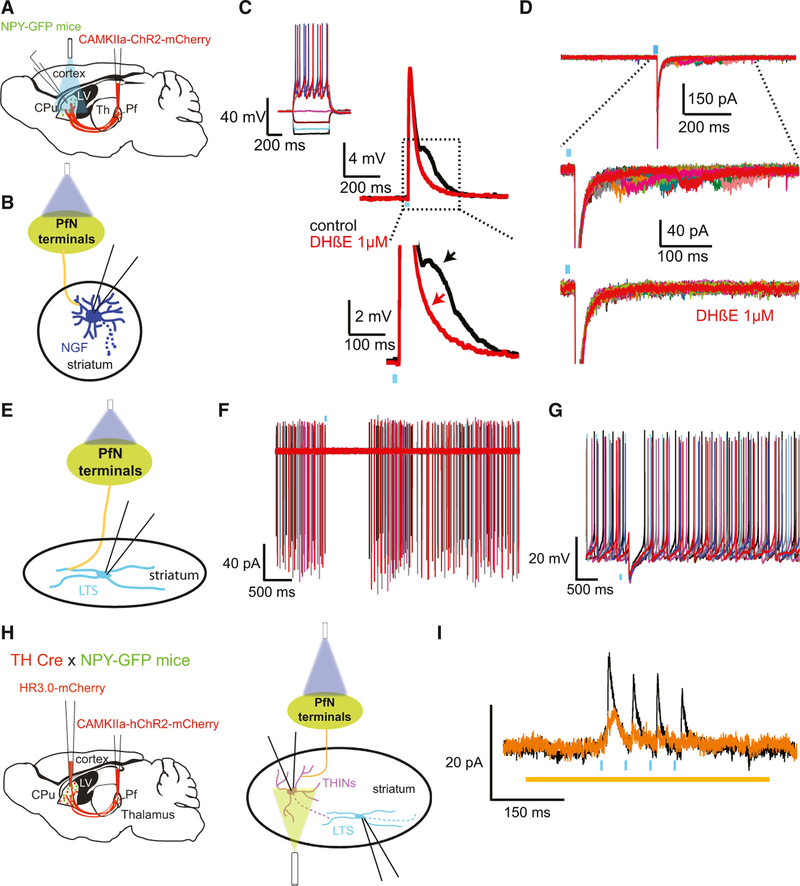
Fig. 1.
Cortical input to LTS and NGF interneurons. (A, B, E) Cartoons depicting the experimental paradigm where an AAV coding for CAMKII-dependent ChR2 was injected in the cortex of an NPY-GFP mouse and whole cell recordings were obtained from the 2 NPY interneuron populations. (B–D) NGF, (E–G) LTS. (C, D) optogenetic cortical stimulation evokes excitatory synaptic responses in both current clamp C and voltage clamp. See text for additional details. (D) The EPSC/Ps can be blocked by bath application of AMPA/NMDA receptor antagonists (CNQX 10 µM and APV 10 µM, respectively). (E–G) Optogenetic cortical stimulation evokes spikes and long-lasting plateau potentials (F). In voltage clamp, the EPSC can be blocked by the same glutamate receptor antagonists. Adapted from Assous et al. (2017), with permission.
In contrast, optogenetic stimulation of thalamostriatal synapses originating from the PfN achieved by the same technique evoked larger EPSPs and action potential firing in ~40% of the recorded NGF interneurons (Fig. 2A–D). Similar to corticostriatal synapses, thalamostriatal synapses onto NGF neurons are also depressing (Assous et al., 2017). Further, in a fraction of NPY-NGF interneurons recorded in the same preparation, we observed that the excitatory responses induced by thalamic stimulation were biphasic (Fig. 2C,D). The first part of the response is due to the monosynaptic glutamatergic innervation from the PfN. On the other hand, the second excitatory response exhibited a significantly longer latency, slower kinetics and variability in its onset latency. The late responses could be blocked by a type II nicotinic receptor antagonist pointing to the role of CIN in the disynaptic activation of NPY-NGF interneurons after optogenetic thalamic stimulation (Assous et al., 2017; Fig. 2C,D).
Fig. 2.
Thalamic innervation of NGF and LTS interneurons. (A, E) Cartoon depicting the experimental paradigm where an AAV coding for CAMKII-dependent ChR2 was injected in the parafascicular nucleus of the thalamus (Pf) of NPY-GFP mice. (C, D) Responses to the thalamic optogenetic stimulation of a typical NGF interneuron (inset). The excitatory response in both current clamp (C) and voltage clamp (D) is biphasic. The second response depends on type II nicotinic receptors as it can be blocked by DHbE (1 µm). (F, G) Responses of an LTS interneuron to optogenetic thalamic stimulation. Most LTS interneurons exhibited a disynaptic inhibition in response to the optogenetic stimulation as illustrated by a pause in their spontaneous firing in cell attach (F) and current clamp (G). H Cartoon depicting the experimental paradigm. In TH-Cre x NPY-GFP mice, an AAV coding for CAMKII-dependent ChR2 was injected into the Pf in combination with a Cre-dependent AAV coding for halorhodopsin virus in the striatum to inhibit THINs. In this preparation, when recording LTS interneurons the disynaptic IPSC induced by thalamic stimulation (black trace) is significantly reduced after inhibition of THINs (orange, I). Adapted from Assous et al. (2017), with permission.
LTS interneurons
Anatomical evidence first suggested the existence of synaptic contacts between corticostriatal afferents and striatal LTS interneurons (Vuillet et al., 1989). The anatomical evidence regarding thalamic input to these cells is less clear-cut. In monkeys, it has been shown that those interneurons receive direct input from the centromedian thalamic nucleus (Sidibe & Smith, 1999) but another study in rats failed to report any direct input arising from the PfN (Kachidian et al., 1996).
Whole cell recordings have confirmed direct monosynaptic input from the cortex both with electrical (Kawaguchi, 1993; Ibanez-Sandoval et al., 2011) and optogenetic stimulation (Assous et al., 2017; Fig. 1E–G). In contrast to other interneurons as well as SPNs, cortical activation induces spikes and also long-lasting plateau potentials in LTS interneurons (Kawaguchi, 1993; Ibanez-Sandoval et al., 2011; Assous et al., 2017; Fig. 1F). Cortical synapses onto LTS interneurons are strongly depressing, in marked contrast to the short-term facilitation observed in corticostriatal responses onto SPNs and FSIs (Assous et al., 2017; Fig. 1G).
Using juxtacellular recording and labeling in vivo, it has been demonstrated that during cortical slow wave activity NOS+ (LTS) interneurons displayed a heterogeneous firing pattern; some of them exhibited tonic activity, while others were phasically active (Sharott et al., 2012). Interestingly, during cortical activation (which presumably replicates more closely the awake cortical state) the firing pattern of LTS interneurons is phasic and indistinguishable from that of SPNs, which differs from the tonic activity reported or LTS interneurons in slices (Partridge et al., 2009; Ibanez-Sandoval et al., 2011; Beatty et al., 2012; Assous et al., 2017). In this study, LTS interneurons were the only neuronal population reported to reduce their firing rate when transitioning from slow wave activity to cortical activation (Sharott et al., 2012).
Surprisingly, in sharp contrast to NGF interneurons, we found that the vast majority of LTS interneurons did not receive monosynaptic excitatory input from the PfN (Assous et al., 2017). Rather, the most common response of LTS interneurons to PfN optogenetic stimulation was a disynaptic inhibition that resulted from monosynaptic thalamic activation of THINs that then synapsed onto LTS interneurons as discussed above (Assous et al., 2017; Fig. 2E–I).
In cell cell-attached recordings, most LTS interneurons responded to optogenetic stimulation of the thalamus with a relatively long pause followed by a rebound increase of activity (Fig. 2F,G) which, as described above, is also the main response observed in TANs in vivo after thalamic stimulation or following the presentation of a salient stimulus, a behavior known to engage the intralaminar nucleus (See above; Aosaki et al., 1994; Graybiel et al., 1994; Matsumoto et al., 2001; Blazquez et al., 2002; Minamimoto & Kimura, 2002). Those data combined with their similarity in spontaneous tonic firing activity (at least in slice; Beatty et al., 2012; M. Assous & J.M. Tepper, unpublished) suggest that potentially some of the TANs recorded in vivo in the previously described experiments might in fact be LTS as suggested previously (Ibanez-Sandoval et al., 2011; Beatty et al., 2012).
These data reveal an extraordinary specificity in the extrinsic innervation of striatal interneurons from the thalamic PfN (Fig. 3). They also provide evidence that striatal interneurons form an intricate network (also discussed below) and that the role of different GABAergic interneurons is more complex than just receiving excitatory input from cortex/thalamus and relaying feed-forward inhibition to SPNs like the FSI does.
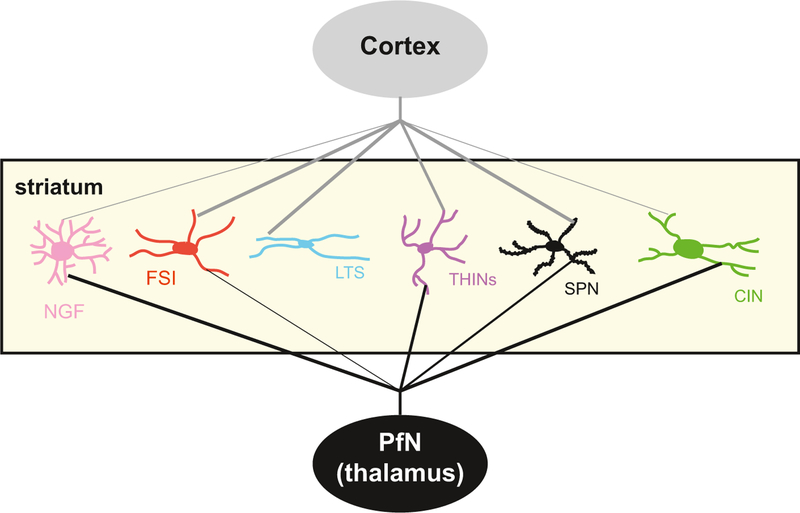
Fig. 3.
Schematic illustrating excitatory cortical and thalamic inputs to striatal neurons. Most striatal neurons receive innervation from both thalamus and cortex (except LTS interneurons that do not receive thalamic input as shown). However, the strength of these inputs differs, as shown by the thickness of the lines. Note the stronger innervation of CINs and NGF interneurons from thalamus (PfN) than from cortex. In contrast, FSIs, LTSs and SPNs receive stronger inputs from the cortex, or exclusively in the case of the LTS interneuron. The ultimate effect of the inputs to different interneurons depends heavily on the intrinsic circuitry formed by the synaptic and electrotonic interconnections of the various interneurons (see text for details). Cortical projections are in gray, thalamic in black.
CR interneurons
Very little is known about the CR-expressing GABAergic interneurons, as there are as yet no Cre-driver lines or fluorescent reporters for the CR gene. However, a recent in vivo study identified multiple subtypes of CR interneurons based on multiple immunofluorescence for CR and secretagogin and other proteins following juxtacellular recording and labeling in anesthetized rats. Simultaneous recordings of cortical activity revealed phase locking of CR units to slow cortical oscillations strongly suggesting, as would be expected based on other striatal GABAergic interneurons, that there is a cortical input to CR interneurons. Further characterization of cortical and thalamic synaptic inputs must await the availability of the appropriate transgenic mouse lines.
Cholinergic regulation of GABAergic interneurons
Striatal CINs have long been known to play a crucial role in striatum acting directly on SPNs via neuromodulatory muscarinic receptors that have been demonstrated to regulate many aspects of striatal functioning (Goldberg et al., 2012). A relatively minor role for presynaptic nicotinic receptors was also recognized, primarily in the context of the regulation of dopamine release (Whiteaker et al., 1995; Wonnacott et al., 2000). However, recent data have shown that that CINs do not solely operate as neuromodulatory neurons but are also part of a fast synaptic circuitry involving nicotinic receptors on striatal GABAergic interneurons. This notion was first suggested by a report that GABAergic IPSCs could be elicited in CINs using extracellular electrical stimulation, or more rarely, by the activation of single CINs. These IPSCs were found to be dependent of the activation of type II nicotinic receptors. The responses were deemed to be recurrent IPSCs as they could be elicited by stimulation of the CINs themselves (Sullivan et al., 2008).
Subsequently, we showed that optogenetic activation of CINs elicits very large, disynaptic recurrent compound GABAergic IPSP/Cs in CINs that are secondary to nicotinic receptor activation. The recurrent IPSC could be separated into biophysically distinct fast and slow components. Using a double transgenic mouse (ChAT-Cre::NPY-GFP), we showed that the GABAA-slow component of the compound GABAergic response elicited in SPNs originated from NGF interneurons (Ibanez-Sandoval et al., 2011; English et al., 2012). However, the identification of the interneuron(s) that mediates the recurrent inhibition in CINs remains uncertain.
Using a different double transgenic optogenetic strategy (ChAT-Chr2::HT3Ra-Cre), we showed that the large IPSCs elicited in SPNs by activation of cholinergic axons could be reduced in amplitude or almost completely blocked by simultaneous optogenetic inhibition of the 5HT3a receptor expressing striatal interneurons (Faust et al., 2016). These experiments show that most or perhaps all of the fast IPSCs in SPNs triggered by cholinergic stimulation originate from local interneurons (but see also (Nelson et al., 2014)).
In addition, we showed that not only NGF interneurons (Fig. 4B) but also FAIs (Fig. 4C) receive large suprathreshold nicotinic EPSPs, suggesting the involvement of the FAIs in the fast IPSC component observed in SPNs. However, the IPSP measured in SPNs after stimulation of FAIs is in some respects different from the fast IPSC component elicited optogenetically. Indeed, DHßE can fully block the disynaptic inhibition seen in SPNs but fails to block EPSPs or prevent firing of action potentials in most FAIs. Additionally, the low initial release probability and strong facilitation of the FAI to SPN synapse suggest that little inhibition is provided by FAIs during the first spike in a train, which would occur when the fast IPSC is observed in SPNs (Faust et al., 2015). Therefore, it remains unclear whether these cells are responsible for the fast IPSC.
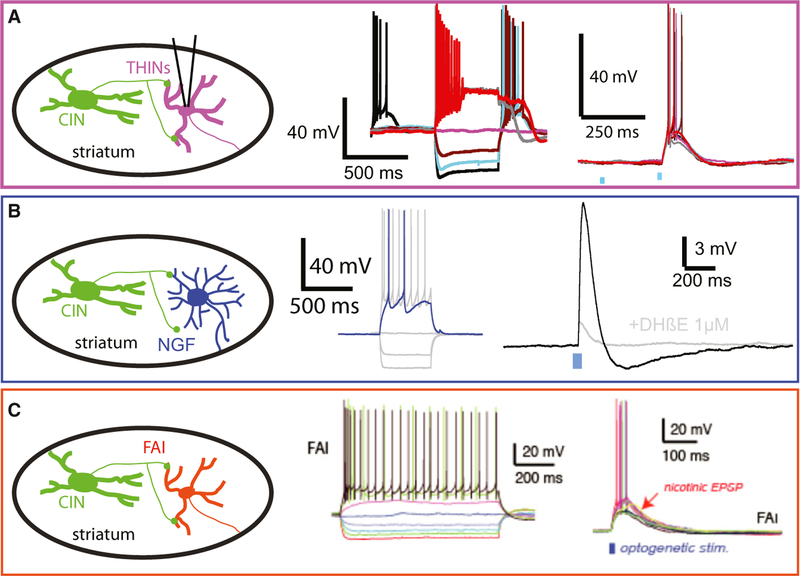
Fig. 4.
Nicotinic responses elicited by optogenetic simulation of cholinergic axons in ChAT-ChR2 mice ex vivo in four types of identified GABAergic interneurons, (A) THIN. (B) NGF. (C) FAI. Note the large amplitude, suprathreshold EPSPs. Panel C is adapted from Faust et al. (2015), with permission.
It has recently been demonstrated that THINs also express functional nicotinic receptors (Luo et al., 2013; Ibanez-Sandoval et al., 2015). Local application of a cholinergic agonist, carbachol, induces depolarization and action potential firing. The source(s) of the ACh that activates these nicotinic receptors and whether these excitatory nicotinic responses can be induced by stimulation of intrinsic and/or extrinsic (Dautan et al., 2014) cholinergic neurons remains somewhat unclear. We have recently found using double transgenic ChAT ChR2::TH-Cre mice that type I THINs respond with large EPSPs and fire action potentials after local optogenetic stimulation of cholinergic neurons (M. Assous & J.M. Tepper, unpublished; Fig. 4A).
It has been recently shown that cholinergic neurons located in the brainstem provide a direct innervation of the striatal complex (Dautan et al., 2014). Using ChAT-Cre transgenic rats, the authors selectively labeled cholinergic neurons in different areas of the pedunculopontine and laterodorsal tegmental nuclei. They showed that cholinergic neurons topographically innervate wide areas of the striatal complex forming principally asymmetric synapses with dendritic shafts and spines. At present, the synaptic targets of those cholinergic axons have not been identified, but it is possible that at least part of the nicotinic responses that we observed in many GABAergic interneurons (Fig. 4) might arise from brainstem nuclei (Dautan et al., 2014).
Interneuron–Interneurons interactions
The classical view on GABAergic interneuron function has been that they operate as independent, parallel, feed-forward inhibitory elements, each providing temporally or otherwise specialized inhibitory inputs to SPNs (Koos et al., 2004; Gittis & Kreitzer, 2012). While this perspective is likely true for some interneuron populations such as the FSIs that only target SPNs (Koos & Tepper, 1999; Gittis et al., 2010; Planert et al., 2010; Szydlowski et al., 2013; Garas et al., 2016) in addition to interacting with each other via chemical and electrical synapses (Koos & Tepper, 1999; Szydlowski et al., 2013) it is clearly not true for all of the other striatal GABAergic interneurons. For example, we have identified a novel GABAergic interneuron that contacts other GABAergic interneurons, but does not synapse onto SPNs (M. Assous & J.M. Tepper, unpublished).
As discussed above, there is good evidence that other interneuron populations interact with each other in different and cell-type-specific ways. CINs innervate at least 3 other GABAergic interneurons: NGF (English et al., 2012; Faust et al., 2015; Assous et al., 2017), FAI (Faust et al., 2015) and THINs (M. Assous & J.M. Tepper, unpublished; Fig. 4), but not FSIs. All those inputs comprise fast nicotinic receptor signaling for the most part, although presynaptic muscarinic modulation of some of these interneurons has also been observed (Koos & Tepper, 2002; M. Assous & J.M. Tepper, unpublished). Those connections are highly cell type specific as they exhibit different nicotinic receptor pharmacology, and there is a lack of cholinergic synaptic innervation of some GABAergic interneurons (FSI and LTS, English et al., 2012).
Conversely, it has also been shown that CINs receive GABAergic innervation from several populations of striatal interneurons. One is the unidentified recurrent interneuron mentioned above. Other intrastriatal GABAergic inputs to CINs originating from several identified interneurons have been reported by others and us. LTS interneurons and THINs provide GABAA-mediated innervation to CINs (Holley et al., 2015; Straub et al., 2016), and we showed that NGF interneurons provide an atypical GABAA-slow innervation onto CINs and SPNs (Ibanez-Sandoval et al., 2011; English et al., 2012). Both NGF and THINs receive suprathreshold excitatory innervation from the thalamus (Assous et al., 2017). In this context, the potential role of those interneurons in the pause response of CINs as well as in the GABAergic-mediated decrease in striatal acetylcholine levels (Zackheim & Abercrombie, 2005; Nanda et al., 2009) observed after thalamic stimulation would be interesting to investigate.
We also found that THINs form highly cell-type-specific connections. In addition to inhibiting CINs (Fig. 5), we also described that THINs strongly inhibited LTS interneurons (Assous et al., 2017; Fig. 5). This pathway is at the center of the disynaptic inhibition observed in the majority of LTS interneurons after optogenetic thalamic stimulation and mediates the thalamostriatal-dependent modulation of prepulse inhibition of the startle reflex (Assous et al., 2017). In contrast, using the same optogenetic methods, we found that THINs do not innervate significantly FSI or NGF interneurons, here again highlighting the specificity in interneuron–interneuron connections (Assous et al., 2017; Figs 5 and 6).
Fig. 5.
Powerful and selective connectivity of THINs with other interneurons. Top left: THINs transfected with ChR2-EYFP spiking after a blue light pulse Top right: Schematic representing specificity of the connectivity of THINs. Panels from top to bottom: LTS interneurons receive a strong inhibitory input from THINs, while NGF and FSI do not. CINs receive inhibitory input from THINs. Adapted from Assous et al. (2017), with permission.
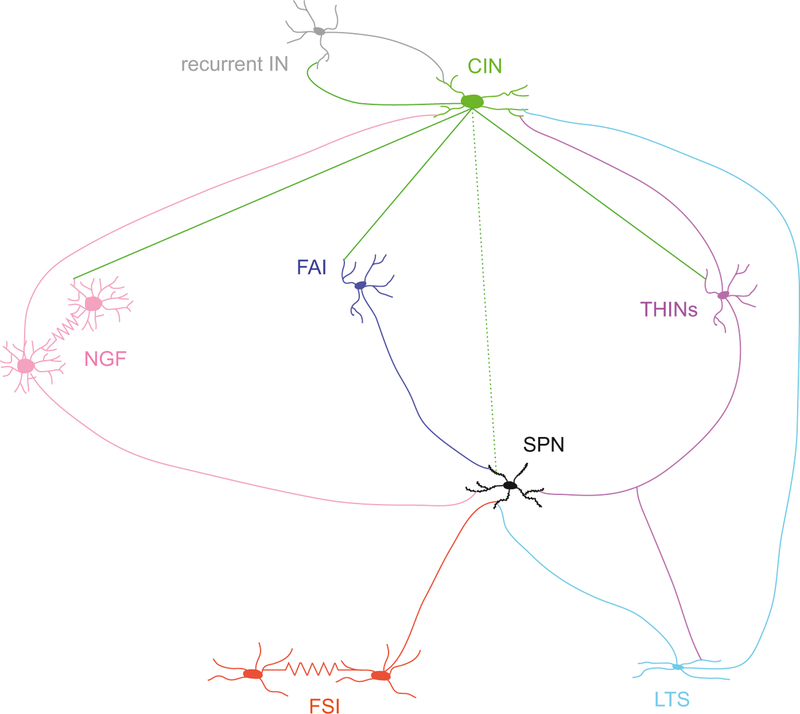
Fig. 6.
Schematic illustrating interneurons connectivity. Note the complexity of the circuit where in addition to connecting SPNs, several functional interneuron–interneuron synaptic connections have been recently discovered. There is also one interneuronal circuit whose presence has been suggested involving a recurrent IN (in gray) targeting CINs. The dotted line linking CIN and SPNs represent muscarinic neuromodulation while the solid lines emanating from the CIN indicate nicotinic synapses.
Conclusions
Excitatory inputs originating from cortex and thalamus onto the striatum are essential for striatal function and a large variety of behaviors. Besides innervating the SPNs, these glutamatergic inputs also innervate most striatal interneurons (summarized in Fig. 3). Traditionally, the function of cortical and thalamic input to striatal GABAergic interneurons was considered to exert feed-forward inhibition on SPNs and by this mechanism regulate precisely their spike timing. In this review, we showed that even if this view is valid to some extent (for FSIs for example), it is grossly incomplete. Indeed, there is now growing evidence showing that the extrinsic innervation of striatal interneurons is not uniform but very specific (Fig. 4). Some interneurons receive predominantly (or only in the case of the LTS interneuron) input from one source or the other. Excitatory inputs to striatal interneurons also exhibit various short-term and long-term plasticities, which may provide them with different functions. We are also accumulating increasing amounts of data showing that striatal interneurons are themselves synaptically and electronically interconnected with great specificity and selectivity. This suggests that the impact of extrinsic inputs on striatal activity critically depends on synaptic interactions within the interneuronal circuitry. Finally, although we focused here on extrinsic glutamatergic input originating from the cortex and the thalamus, similar specificity in the innervation of striatal interneurons would presumably also exist for the other sources of innervation to the striatum.
Acknowledgements
This work was supported by NS034865 to JMT.
Abbreviations
- CIN
Cholinergic interneurons
- CM/Pf
Centromedian/parafascicular complex
- CR
Calretinin-expressing interneurons
- FAI
Fast adapting interneuron
- FSI
Fast-spiking interneurons
- LTS
Low-threshold spike interneuron
- mGluR
Metabotropic glutamate receptors
- NGF
Neurogliaform
- SPNs
Spiny projection neurons
- STDP
Spike timing-dependent plasticity
- THINs
Tyrosine hydroxylase expressing interneurons
Footnotes
Edited by Yoland Smith. Reviewed by Jérôme Baufreton, University of Bordeaux, France; and Andrew Sharott, University of Oxford, UK
All peer review communications can be found with the online version of the article.
Conflict of interest
The authors declare no conflict of interest.
References
- Angelov SD, Dietrich C, Krauss JK & Schwabe K (2014) Effect of deep brain stimulation in rats selectively bred for reduced prepulse inhibition. Brain Stimul, 7, 595–602. [DOI] [PubMed] [Google Scholar]
- Aoki S, Liu AW, Zucca A, Zucca S & Wickens JR (2015) Role of striatal cholinergic interneurons in set-shifting in the rat. J. Neurosci, 35, 9424–9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM & Kimura M (1994) Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J. Neurosci, 14, 3969–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P (2002) Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur. J. Neurosci, 16, 2017–2026. [DOI] [PubMed] [Google Scholar]
- Arias-Garcia MA, Tapia D, Laville JA, Calderon VM, Ramiro-Cortes Y, Bargas J & Galarraga E (2017) Functional comparison of corticostriatal and thalamostriatal postsynaptic responses in striatal neurons of the mouse. Brain Struct. Funct, 223, 1229–1253. [DOI] [PubMed] [Google Scholar]
- Assous M, Kaminer J, Shah F, Garg A, Koos T & Tepper JM (2017) Differential processing of thalamic information via distinct striatal interneuron circuits. Nat. Commun, 8, 15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldan Ramsey LC, Xu M, Wood N & Pittenger C (2011) Lesions of the dorsomedial striatum disrupt prepulse inhibition. Neuroscience, 180, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty JA, Sullivan MA, Morikawa H & Wilson CJ (2012) Complex autonomous firing patterns of striatal low-threshold spike interneurons. J. Neurophysiol, 108, 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD & Bolam JP (1994) Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience, 62, 707–719. [DOI] [PubMed] [Google Scholar]
- Berendse HW & Groenewegen HJ (1990) Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J. Comp. Neurol, 299, 187–228. [DOI] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J & Eichenbaum HB (2004) Oscillatory entrainment of striatal neurons in freely moving rats. Neuron, 43, 883–896. [DOI] [PubMed] [Google Scholar]
- Blazquez PM, Fujii N, Kojima J & Graybiel AM (2002) A network representation of response probability in the striatum. Neuron, 33, 973–982. [DOI] [PubMed] [Google Scholar]
- Bradfield LA, Bertran-Gonzalez J, Chieng B & Balleine BW (2013) The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron, 79, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald NA, Price DD, Vernon L & Hull CD (1973) Caudate intracellular response to thalamic and cortical inputs. Exp. Neurol, 38, 311–323. [DOI] [PubMed] [Google Scholar]
- Consolo S, Baldi G, Giorgi S & Nannini L (1996) The cerebral cortex and parafascicular thalamic nucleus facilitate in vivo acetylcholine release in the rat striatum through distinct glutamate receptor subtypes. Eur. J. Neurosci, 8, 2702–2710. [DOI] [PubMed] [Google Scholar]
- Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T & Mena-Segovia J (2014) A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J. Neurosci, 34, 4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA & Surmeier DJ (2010) Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron, 67, 294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig NM, Magill PJ, Apicella P, Bolam JP & Sharott A (2014) Cortical and thalamic excitation mediate the multiphasic responses of striatal cholinergic interneurons to motivationally salient stimuli. J. Neurosci, 34, 3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, Tepper JM & Koos T (2012) GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat. Neurosci, 15, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust TW, Assous M, Shah F, Tepper JM & Koos T (2015) Novel fast adapting interneurons mediate cholinergic-induced fast GABAA inhibitory postsynaptic currents in striatal spiny neurons. Eur. J. Neurosci, 42, 1764–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust TW, Assous M, Tepper JM & Koos T (2016) Neostriatal GABAergic interneurons mediate cholinergic inhibition of spiny projection neurons. J. Neurosci, 36, 9505–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Deniau JM & Venance L (2008) Cell-specific spike-timing-dependent plasticity in GABAergic and cholinergic interneurons in corticostriatal rat brain slices. J. Physiol, 586, 265–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty AW & Graybiel AM (1993) Output architecture of the primate putamen. J. Neurosci, 13, 3222–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois C, Percheron G, Parent A, Sadikot AF, Fenelon G & Yelnik J (1991) Topography of the projection from the central complex of the thalamus to the sensorimotor striatal territory in monkeys. J. Comp. Neurol, 305, 17–34. [DOI] [PubMed] [Google Scholar]
- Garas FN, Kormann E, Doig NM, Vinciati F, Nakamura KC, Dorst MC, Smith Y, Magill PJ et al. (2016) Secretagogin expression delineates functionally-specialized populations of striatal parvalbumin-containing interneurons. Elife, 5, e16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garas FN, Shah RS, Vinciati F, Smith Y, Magill PJ & Sharott A (2018) Structural and molecular heterogeneity of calretinin-expressing interneurons in the rodent and primate striatum. J. Comp. Neurol, 526, 877–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH & Kreitzer AC (2012) Striatal microcircuitry and movement disorders. Trends Neurosci, 35, 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ & Kreitzer AC (2010) Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J. Neurosci, 30, 2223–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Leventhal DK, Fensterheim BA, Pettibone JR, Berke JD & Kreitzer AC (2011) Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. J. Neurosci, 31, 15727–15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA & Reynolds JN (2011) Spontaneous firing and evoked pauses in the tonically active cholinergic interneurons of the striatum. Neuroscience, 198, 27–43. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Ding JB & Surmeier DJ (2012) Muscarinic modulation of striatal function and circuitry. In Fryer A, Christopoulos A & Nathanson N (Eds), Muscarinic Receptors. Handbook of Experimental Pharmacology, vol 208 Springer, Berlin, Heidelberg, pp. 223–241. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW & Kimura M (1994) The basal ganglia and adaptive motor control. Science, 265, 1826–1831. [DOI] [PubMed] [Google Scholar]
- Guo Q, Wang D, He X, Feng Q, Lin R, Xu F, Fu L & Luo M (2015) Whole-brain mapping of inputs to projection neurons and cholinergic interneurons in the dorsal striatum. PLoS One, 10, e0123381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2016) Corticostriatal circuitry. Dialogues Clin. Neurosci, 18, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P & Calzavara R (2006) Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci, 26, 8368–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Tang CY, Fleischman MB, Wei TC, Byne W & Haznedar MM (2001) Thalamic activation during an attention-to-prepulse startle modification paradigm: a functional MRI study. Biol. Psychiat, 50, 281–291. [DOI] [PubMed] [Google Scholar]
- Hintiryan HF, Foster NN, Bowman I, Bay M, Song MY, Gou L, Yamashita S, Bienkowski MS et al. (2016) The mouse cortico-striatal projectome. Nat. Neurosci, 19, 1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley SM, Joshi PR, Parievsky A, Galvan L, Chen JY, Fisher YE, Huynh MN, Cepeda C et al. (2015) Enhanced GABAergic inputs contribute to functional alterations of cholinergic interneurons in the R6/2 mouse model of Huntington’s disease. eNeuro, 2 10.1523/ENEURO.0008-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Tecuapetla F, Unal B, Shah F, Koos T & Tepper JM (2010) Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J. Neurosci, 30, 6999–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Tecuapetla F, Unal B, Shah F, Koos T & Tepper JM (2011) A novel functionally distinct subtype of striatal neuropeptide Y interneuron. J. Neurosci, 31, 16757–16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Xenias HS, Tepper JM & Koos T (2015) Dopaminergic and cholinergic modulation of striatal tyrosine hydroxylase interneurons. Neuropharmacology, 95, 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachidian P, Vuillet J, Nieoullon A, Lafaille G & Kerkerian-Le Goff L (1996) Striatal neuropeptide Y neurones are not a target for thalamic afferent fibres. Neuroreport, 7, 1665–1669. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y (1993) Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J. Neurosci, 13, 4908–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ & Emson PC (1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci, 18, 527–535. [DOI] [PubMed] [Google Scholar]
- Kemp JM & Powell TP (1971) The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos. T. Roy. Soc. B, 262, 429–439. [DOI] [PubMed] [Google Scholar]
- Kimura M, Rajkowski J & Evarts E (1984) Tonically discharging putamen neurons exhibit set-dependent responses. Proc. Natl. Acad. Sci. USA, 81, 4998–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Minamimoto T, Matsumoto N & Hori Y (2004) Monitoring and switching of cortico-basal ganglia loop functions by the thalamo-striatal system. Neurosci. Res, 48, 355–360. [DOI] [PubMed] [Google Scholar]
- Koos T & Tepper JM (1999) Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci, 2, 467–472. [DOI] [PubMed] [Google Scholar]
- Koos T & Tepper JM (2002) Dual cholinergic control of fast-spiking interneurons in the neostriatum. J. Neurosci, 22, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM & Wilson CJ (2004) Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J. Neurosci, 24, 7916–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosillo P, Zhang YF, Threlfell S & Cragg SJ (2016) Cortical control of striatal dopamine transmission via striatal cholinergic interneurons. Cereb. Cortex, 26, 4160–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y & Kawaguchi Y (1994) Three classes of GABAergic interneurons in neocortex and neostriatum. Jpn. J. Physiol, 44(Suppl. 2), S145–S148. [PubMed] [Google Scholar]
- Kubota Y, Mikawa S & Kawaguchi Y (1993) Neostriatal GABAergic interneurones contain NOS, calretinin or parvalbumin. Neuroreport, 5, 205–208. [DOI] [PubMed] [Google Scholar]
- Lapper SR & Bolam JP (1992) Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience, 51, 533–545. [DOI] [PubMed] [Google Scholar]
- Lapper SR, Smith Y, Sadikot AF, Parent A & Bolam JP (1992) Cortical input to parvalbumin-immunoreactive neurones in the putamen of the squirrel monkey. Brain Res, 580, 215–224. [DOI] [PubMed] [Google Scholar]
- Lee K, Holley SM, Shobe JL, Chong NC, Cepeda C, Levine MS & Masmanidis SC (2017) Parvalbumin interneurons modulate striatal output and enhance performance during associative learning. Neuron, 93 1451–1463 e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC & Sadikot AF (2001) GABA promotes survival but not proliferation of parvalbumin-immunoreactive interneurons in rodent neostriatum: an in vivo study with stereology. Neuroscience, 104, 93–103. [DOI] [PubMed] [Google Scholar]
- Luo R, Janssen MJ, Partridge JG & Vicini S (2013) Direct and GABA-mediated indirect effects of nicotinic ACh receptor agonists on striatal neurones. J. Physiol, 591, 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S & Gonon F (2005) Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J. Neurosci, 25, 3857–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C & Gonon F (2006) Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J. Neurosci, 26, 3875–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM & Kimura M (2001) Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J. Neurophysiol, 85, 960–976. [DOI] [PubMed] [Google Scholar]
- McFarland NR & Haber SN (2000) Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J. Neurosci, 20, 3798–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer MA & Redish AD (2009) Low and high gamma oscillations in rat ventral striatum have distinct relationships to behavior, reward, and spiking activity on a learned spatial decision task. Front. Integr. Neurosci, 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE & Wouterlood FG (1990) Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J. Comp. Neurol, 296, 204–221. [DOI] [PubMed] [Google Scholar]
- Minamimoto T & Kimura M (2002) Participation of the thalamic CM-Pf complex in attentional orienting. J. Neurophysiol, 87, 3090–3101. [DOI] [PubMed] [Google Scholar]
- Minamimoto T, Hori Y & Kimura M (2009) Roles of the thalamic CM-PF complex-Basal ganglia circuit in externally driven rebias of action. Brain Res. Bull, 78, 75–79. [DOI] [PubMed] [Google Scholar]
- Munoz-Manchado AB, Foldi C, Szydlowski S, Sjulson L, Farries M, Wilson C, Silberberg G & Hjerling-Leffler J (2016) Novel striatal GABAergic interneuron populations labeled in the 5HT3a(EGFP) mouse. Cereb. Cortex, 26, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda B, Galvan A, Smith Y & Wichmann T (2009) Effects of stimulation of the centromedian nucleus of the thalamus on the activity of striatal cells in awake rhesus monkeys. Eur. J. Neurosci, 29, 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP & Kreitzer AC (2014) Striatal cholinergic interneurons Drive GABA release from dopamine terminals. Neuron, 82, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’ Hare JK, Li H, Kim N, Gaidis E, Ade K, Beck J, Yin H & Calakos N (2017) Striatal fast-spiking interneurons selectively modulate circuit output and are required for habitual behavior. Elife, 6 10.7554/eLife.26231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy HB & Graybiel AM (1997) Cortically driven immediateearly gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J. Neurosci, 17, 2477–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Janssen MJ, Chou DY, Abe K, Zukowska Z & Vicini S (2009) Excitatory and inhibitory synapses in neuropeptide Y-expressing striatal interneurons. J. Neurophysiol, 102, 3038–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Lewin AE, Yasko JR & Vicini S (2014) Contrasting actions of group I metabotropic glutamate receptors in distinct mouse striatal neurones. J. Physiol, 592, 2721–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planert H, Szydlowski SN, Hjorth JJ, Grillner S & Silberberg G (2010) Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. J. Neurosci, 30, 3499–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA & Rozman S (2009) Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol. Learn. Mem, 91, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S, Hanley JJ, Deniau JM & Bolam JP (2002) Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J. Neurosci, 22, 8158–8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig R & Silberberg G (2014) Multisensory integration in the mouse striatum. Neuron, 83, 1200–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI & Wickens JR (2004) Modulation of an after hyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J. Neurosci, 24, 9870–9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkin TM & Sadikot AF (1999) Thalamic input to parvalbumin-immunoreactive GABAergic interneurons: organization in normal striatum and effect of neonatal decortication. Neuroscience, 88, 1165–1175. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Smith Y & Bolam JP (1992) Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. J. Comp. Neurol, 320, 228–242. [DOI] [PubMed] [Google Scholar]
- Schulz JM & Reynolds JN (2013) Pause and rebound: sensory control of cholinergic signaling in the striatum. Trends Neurosci, 36, 41–50. [DOI] [PubMed] [Google Scholar]
- Schulz JM, Pitcher TL, Savanthrapadian S, Wickens JR, Oswald MJ & Reynolds JN (2011) Enhanced high-frequency membrane potential fluctuations control spike output in striatal fast-spiking interneurones in vivo. J. Physiol, 589, 4365–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciamanna G, Ponterio G, Mandolesi G, Bonsi P & Pisani A (2015) Optogenetic stimulation reveals distinct modulatory properties of thalamostriatal vs corticostriatal glutamatergic inputs to fast-spiking interneurons. Sci. Rep, 5, 16742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharott A, Moll CK, Engler G, Denker M, Grun S & Engel AK (2009) Different subtypes of striatal neurons are selectively modulated by cortical oscillations. J. Neurosci, 29, 4571–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharott A, Doig NM, Mallet N & Magill PJ (2012) Relationships between the firing of identified striatal interneurons and spontaneous and driven cortical activities in vivo. J. Neurosci, 32, 13221–13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidibe M & Smith Y (1999) Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience, 89, 1189–1208. [DOI] [PubMed] [Google Scholar]
- Smith Y & Parent A (1986a) Neuropeptide Y-immunoreactive neurons in the striatum of cat and monkey: morphological characteristics, intrinsic organization and co-localization with somatostatin. Brain Res, 372, 241–252. [DOI] [PubMed] [Google Scholar]
- Smith Y & Parent A (1986b) Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus). Neuroscience, 18, 347–371. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF & Sidibe M (2004) The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci, 27, 520–527. [DOI] [PubMed] [Google Scholar]
- Smith Y, Galvan A, Ellender TJ, Doig N, Villalba RM, Huerta-Ocampo I, Wichmann T & Bolam JP (2014) The thalamostriatal system in normal and diseased states. Front. Syst. Neurosci, 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Berg B, Aujla N & Schoenbaum G (2016) Cholinergic interneurons use orbitofrontal input to track beliefs about current state. J. Neurosci, 36, 6242–6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Saulnier JL, Begue A, Feng DD, Huang KW & Sabatini BL (2016) Principles of synaptic organization of GABAergic interneurons in the striatum. Neuron, 92, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Chen H & Morikawa H (2008) Recurrent inhibitory network among striatal cholinergic interneurons. J. Neurosci, 28, 8682–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Miura M, Nishimura K & Aosaki T (2001) Dopamine-dependent synaptic plasticity in the striatal cholinergic interneurons. J. Neurosci, 21, 6492–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowski SN, Pollak Dorocic I, Planert H, Carlen M, Meletis K & Silberberg G (2013) Target selectivity of feedforward inhibition by striatal fast-spiking interneurons. J. Neurosci, 33, 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM & Bolam JP (2004) Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol, 14, 685–692. [DOI] [PubMed] [Google Scholar]
- Tepper JM & Koós T (2017). Striatal GABAergic interneurons. In Steiner H & Tseng K (Eds), Handbook of Basal Ganglia Structure and Function 2nd Edition, Handbook of Behavioral Neuroscience, Vol 24 Academic Press, Cambridge, MA, pp. 157–178. [Google Scholar]
- Tepper JM, Tecuapetla F, Koos T & Ibanez-Sandoval O (2010) Heterogeneity and diversity of striatal GABAergic interneurons. Front. Neuroanat, 4, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K & Cragg SJ (2012) Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron, 75, 58–64. [DOI] [PubMed] [Google Scholar]
- Vuillet J, Kerkerian L, Salin P & Nieoullon A (1989) Ultrastructural features of NPY-containing neurons in the rat striatum. Brain Res, 477, 241–251. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Garcha HS, Wonnacott S & Stolerman IP (1995) Locomotor activation and dopamine release produced by nicotine and isoare-colone in rats. Brit. J. Pharmacol, 116, 2097–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT & Kitai ST (1990) Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J. Neurosci, 10, 508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L & Jones IW (2000) Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur. J. Pharmacol, 393, 51–58. [DOI] [PubMed] [Google Scholar]
- Xenias HS, Ibanez-Sandoval O, Koos T & Tepper JM (2015) Are striatal tyrosine hydroxylase interneurons dopaminergic? J. Neurosci, 35, 6584–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH & Van Hoesen GW (1978) Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res, 139, 43–63. [DOI] [PubMed] [Google Scholar]
- Zackheim J & Abercrombie ED (2005) Thalamic regulation of striatal acetylcholine efflux is both direct and indirect and qualitatively altered in the dopamine-depleted striatum. Neuroscience, 131, 423–436. [DOI] [PubMed] [Google Scholar]