Abstract
The effects of an allelochemical extracted from the culture filtrate of diatom Phaeodactylum tricornutum on the raphidophyte Heterosigma akashiwo were investigated using a series of morphological, physiological and biochemical characters. Growth experiments showed that H. akashiwo was significantly inhibited immediately after exposure to the allelochemical, with many cells rapidly dying and lysing based on microscopic observation. The effects of the allelochemical on the surviving cells were explored using Scanning Electron Microscopy (SEM) and Flow cytometry (FCM), the latter by examination of a suite of physiological parameters (membrane integrity, esterase activity, chlorophyll-a content, membrane potential). The results demonstrate that the membrane of H. akashiwo was attacked by the allelochemical directly, causing cell membrane breakage and loss of integrity. Esterase activity was the most sensitive indicator of the impacts of the allelochemical. Membrane potential and chlorophyll-a content both showed significant decreases following exposure of the Heterosigma cells to high concentrations of the allelochemical for 5 and 6 days. Both were affected, but the membrane potential response was more gradual compared to other effects. The cell size of H. akashiwo did not change compared with the control group. The surviving cells were able to continue to grow and in a few days, re-establish a successful culture, even in the presence of residual allelochemical, suggesting either development of cellular resistance, or the degradation of the chemical.
Keywords: allelochemical, Phaeodactylum tricornutum, Heterosigma akashiwo, Flow cytometry, physiological characters
1. Introduction
Harmful algal blooms (HABs) occur frequently in marine coastal areas and freshwater ecosystems worldwide, causing serious consequences on the environment, aquaculture industries and human health (Anderson, 1997; Horner et al., 1997; Anderson et al., 2012; Dorantes-Aranda et al., 2015). One of the factors thought to be important in phytoplankton competition for resources and community dynamics is allelopathy - the release of secondary metabolites into an organism’s surroundings, thereby affecting the growth or viability of co-occurring organisms (Rice, 1984; Legrand et al., 2003; Roy et al., 2006; Yang et al., 2014).
The production of allelochemicals among dinoflagellates, diatoms, chrysophytes and cyanobacteria has been reported in many marine systems (e.g., Sukenik et al., 2002; Gross, 2003; Legrand et al., 2003; Irfanullah and Moss, 2005; Granéli et al., 2012;). The mechanisms through which allelochemicals are released by phytoplankton and impact on other phytoplankton remain unclear in many marine ecosystems. Allelochemicals influence multiple cell functions including cell division, metabolism, photosynthesis, respiration, and enzyme activity (Duke, 2003; Singh and Thapar, 2003; Belz and Hurle, 2004). For example, the Chlorella vulgaris cell membrane was detached from the cell wall after exposure to N-phenyl-2-naphthylamine (Qian et al., 2009), an allelochemical isolated from root exudates of water hyacinth (Eichhornis crassipes) (Sun et al., 1993). The inhibition of photosynthesis (especially photosystem II) and the inhibition of enzyme activities (e.g., alkaline phosphatase) were also identified as common modes of actions for allelochemicals (Gross et al., 1996; Körner and Nicklisch, 2002; Zhu et al., 2010; Wang et al., 2016 a).. Ethyl 2-methyl acetoacetate (EMA) isolated from Phragmites communis had impacts on respiration and photosynthesis of Microcystis aeruginosa (Li et al., 2007).
One of the challenges in this field of investigation is in characterizing allelochemical effects beyond simple growth rate reductions. In recent years, flow cytometry (FCM) has emerged as a rapid and highly efficient analytical method to measure these types of changes in microalgae (e.g., Xiao et al., 2010; 2011; 2014). The application of FCM provides a convenient diagnostic approach for understanding and quantifying allelopathic interactions (Rioboo et al., 2009). Measurements of enzyme activity (e.g., peroxidases, b-galactosidases, esterases) is another way to assess impacts using direct measurements in microalgae (Peterson and Stauber 1996; Blaise and Ménard 1998; Franklin et al., 2001; Eigemann et al., 2013). Here we use these two methods, together with scanning electron microscopy (SEM) to characterize the morphological, physiological and growth effects of an allelochemical produced by the diatom Phaeodactylum tricornutum on the raphidiphyte Heterosigma akashiwo.
Heterosigma akashiwo is a common, highly successful bloom-forming species responsible for many fish-killing blooms throughout the world, typically in nearly mono-specific blooms at cell densities that lead to the appearance of discolored water (e.g., red tides) (Smayda et al. 1998). In past studies, Phaeodactylum tricornutum had strong negative impacts on the growth of H. akashiwo through allelopathy, and a putative allelochemical (a type of glycinamide compound) was isolated from the filtrate of P. tricornutum (Wang et al., 2016 b), however, details of the growth inhibition and mechanism of action are lacking. In the present study, we document responses of H. akashiwo cells to the allelochemical present in P. tricornutum filtrate. These results provide new insight into the mechanistic effects of allelochemicals in marine ecosystems.
2. Materials and methods
2.1. Algal culture and isolation of allelochemical from P. tricornutum filtrate
Phaeodactylum tricornutum and H. akashiwo were obtained from the Algal Center of Key Laboratory of Marine Chemistry Theory and Technology, Ocean University of China. The microalgae were cultivated in f/2 medium (Guillard, 1975) prepared using autoclaved seawater (filtered through 0.45 μm Millipore membranes) from Jiaozhou Bay of China. Cultures were grown at (20 ± 1) °C with a 12/12-h light/dark cycle. Illumination was provided by cool white filament lamps at 70 μmol m−2s−1. All glassware was acid-soaked, cleaned with milli-Q water, and autoclaved. Cultures were gently shaken twice manually every day to avoid wall growth and prevent the sedimentation of algae.
A 27-L culture was maintained in a transparent polyethylene container until late exponential phase. The filtrate was obtained by centrifugation (3000 rpm, 15 min). A small number of cells from the pellet were observed under the microscope (Leica DM4000B, Germany) after centrifugation to demonstrate that the cells remained intact. The supernatant was then filtered through a 0.22 μm membrane and the filtrate extracted with ethyl acetate three times in succession. The extracts were pooled and evaporated to dryness using a rotary vacuum evaporator (Beijing Bo Kang Laboratory Instruments Medical Co., Ltd.) under reduced pressure at 40°C. The extract was diluted to 10 mL with DMSO and stored at 4°C.
The ethyl acetate extract from the filtrate of P. tricornutum was purified using HPLC with 99 μL injection volume (repeated 10 times) according to the elution times of chromatographic peaks. Nine isolated fractions were dried under N2 and the residues diluted with 1.0 mL DMSO respectively., The putative allelochemical was obtained from fraction Ⅵ (Wang et al., 2016 b). The isolated allelochemical was dissolved in 1 mL DMSO to the same concentration of crude ethyl acetate extract before the HPLC separation, and this was then used for all bioassays in the present study.
2.2. Sample preparation for SEM
Heterosigma akashiwo was cultured for 4 d with an initial cell density of 1.0×104 cells mL−1. 37 μL DMSO solution was then added into the culture medium of H. akashiwo. After 4 d of exposure to the DMSO, algal cells were collected by centrifugation (1,814.4×g, 10 min) and fixed overnight with 2.5% glutaraldehyde at 4°C. Samples were washed with 0.1 mol l−1 phosphate buffer solution (PBS, pH =7.4) and centrifuged (1,814.4×g, 10 min) three times, then the supernatant was discarded. Algal cells were fixed with 1% osmium tetroxide at 4°C for 1 h, washed in 0.1 mol l−1 PBS (pH=7.4), then centrifuged three times, discarding the supernatant. Algal samples were dehydrated with 30%, 50%, 70%, 80%, 90%, 95% and 100% alcohol solutions for 20 min. Samples were then fixed in tert butyl alcohol and freeze-dried for final SEM (Hitachi, Japan) observation after dehydration.
2.3. Flow cytometric measurements
Flow cytometry was conducted with a BD Accuri C6 flow cytometer (Becton Dickinson, USA) equipped with a blue and red laser (488 nm emission), two light scatter detectors, and four fluorescence detectors with optical filters, including FL1 530/15 nm; FL2 585/20 nm; FL3>670 nm and FL4 675/12.5 nm. The program C Flow Plus from Becton Dickinson was used to collect and analyze signals.
All added concentrations of allelochemical were divided into three dose levels (high, medium, low respectively) in the following experiments (Table 1). When the medium dose of DMSO was added into a 100-mL culture of H. akashiwo as a control, the added concentration was approximate equivalent to the maximum concentration of allelochemical from the filtrate of P. tricornutum. Low and high doses of DMSO solution were equivalent to 0.5 and 3 times the maximum concentration of allelochemical from the P. tricornutum filtrate. High doses of DMSO without allelochemical were added to the culture of H. akashiwo as a control. The growth of H. akashiwo was monitored by counting cell numbers directly using both light microscopy and FCM.
Table 1.
The addition of three levels of allelochemical of P. tricornutum for FCM measurements
| Control | High dose | Medium dose | Low dose | |
|---|---|---|---|---|
| f/2 medium(mL) | 100 | 100 | 100 | 100 |
| Fractions (μL) | 111 DMSO | 111 | 37 | 18.5 |
High dose: About 3 times of the maximum concentration of allelochemical of P. tricornutum.
Medium dose: The approximate maximum concentration of allelochemical of P. tricornutum.
Low dose: About half of the maximum concentration of allelochemical of P. tricornutum.
Control: 111 μL DMSO
Chlorophyll-a content provides information about a cell’s capacity for absorption, transmission and consumption of energy for photosynthesis. One mL of each Heterosigma culture was filtered through a 40 micron mesh to remove large particles that would hinder FCM analysis. The cells were then re-suspended in 1.5 mL centrifuge tubes for FCM analysis. Chlorophyll-a was detected using the FCM’s FL3 detector and mean fluorescence intensity per cell was calculated.
Propidium Iodide (PI) was used to verify cell viability as it can combine with DNA and produce pink fluorescence when the algal cell membrane is broken. The cell staining was performed by treating each 0.5 mL algal suspension with 0.455 mL PI (0.14 mg mL−1, working solution dissolved in Mili-Q water) and incubating for 15 min at room temperature. The fluorescence intensity was detected using the FL2 channel of the FCM.
Fluorescein diacetate (FDA) was used to assess esterase activity (Franklin et al., 2001). The cell staining was performed by treating 1mL algal suspensions with 20 μL FDA (0.5 mg mL−1, working solution dissolved in acetone) and incubating for 15 min at room temperature. Green fluorescence was detected using the FL1 channel of the FCM.
3,3’-dihexyloxacarbocyanineine iodide (DiOC6(3)) was used to estimate membrane potential. The cell staining was performed by treating 1 mL algal suspensions with 25 μL DiOC6(3) solution (11.52 μg mL−1, working solution dissolved in DMSO) and incubating for 10 min at room temperature. Fluorescence was measured using the FL1 channel on the FCM.
The forward-angle light scatter signal (FSC) was also measured as an indicator of cell size or cell volume, as the signal intensity is linearly related to the square of the cell diameter or cross sectional area (Cunningham and Buonnacorsi, 1992, Wang et al., 2016 a).
All samples were kept on the ice under dark conditions before the FCM measurements. FCM data were interpreted as the mean fluorescence intensity (MFI).
2.4. Data analysis
To estimate the effect of the isolated allelochemical released by P. tricornutum on H. akashiwo, inhibition rate IR was calculated using Equation. 1 as follows (Sun and Ning, 2005):
| (1) |
where T and C represent the cell density of treatments and control, respectively.
One-way ANOVA analysis was used to test for significant differences in effects among different treatments by SPSS 19. Mean values and standard deviations were calculated from replicates for each treatment (n=3), and the significance level p was set at < 0.05.
3. Results and discussion
3.1. Algal growth inhibition and morphology
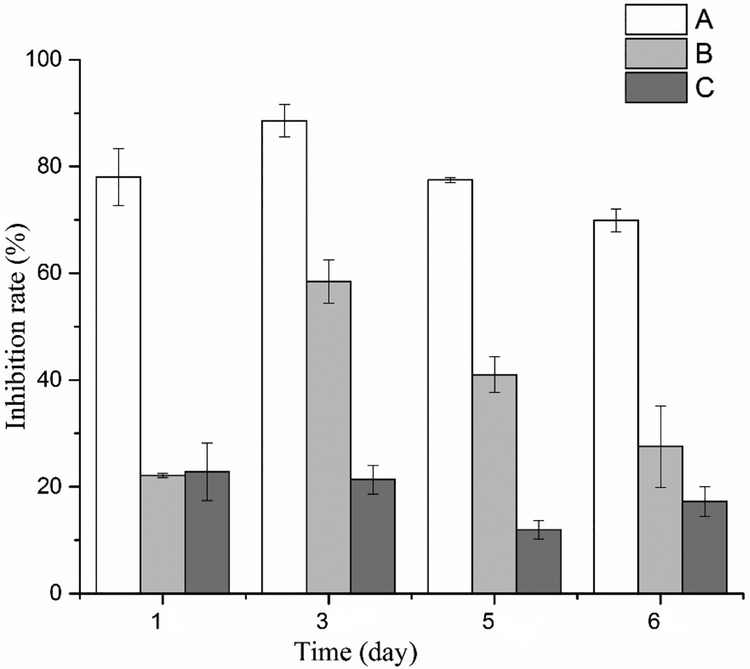
The growth of H. akashiwo was affected by different doses (A: high concentration; B: medium concentration; C: low concentration) of P. tricornutum allelochemical by day 6 or sooner. Cell densities were determined by FCM and direct microscope counting, and the two methods showed a good correlation (R2=0.9806; data not shown). As other physiological characters were also determined by FCM, analyses of the inhibition rates of different treatments were based on FCM measurements (Fig.1).
Figure. 1.
Inhibition rate on H. akashiwo growth during six-day exposure to different concentrations of an allelochemical isolated from P. tricornutum filtrate. A: high dose; B: medium dose; C: low dose. Data are presented as mean ± standard deviation (n=3). Dose concentrations given in Table 1.
3.1.1. Cell mortality
Compared to the control group, H. akashiwo cell density decreased significantly (p < 0.05) as almost 80% of the cells disappeared on day 1 and the percentage of surviving cells remained in the 10–30% range on days 3, 5 and 6 when treated with the highest concentration of the P. tricornutum allelochemical. The inhibition rate was about 20–60% throughout the 6-day culture time for the treatment with the medium concentration. Approximately 20% of the H. akashiwo cells disappeared in low concentration treatment group. The cell density of each treatment thus decreased with increasing concentrations of allelochemical. The allelochemical clearly had a strong disruptive effect on H. akashiwo growth, inducing significant cell mortality and lysis (Fig.1). Inhibition rate is a common parameter used to quantify allopathic effects (e.g., Nakai et al., 1999). Obviously, the acute lethal effect directly killed many H. akashiwo cells and left only a small number of survivors at the high allelochemical concentrations. The FCM analysis then revealed the physiological condition, and future growth of those survivors in each treatment.
3.1.2. Cell Size and Morphology
Changes in cell size were explored using the ratio of the mean FSC signal in the treated cultures to that of the controls. No significant change of cell size was observed in the treated cells (p<0.05; Fig.2). In a similar finding, the size of Microcystis aeruginosa did not change when exposed to the allelochemical ferulic acid (FA) at concnetratiosn less than 0.7 mM (Wang et al., 2016 a).
Figure. 2.
Influence of different concentrations of allelochemical isolated from P. tricornutum filtrate on the size of H. akashiwo cells during six-days of exposure. A: high dose; B: medium dose; C: low dose. D: Control. Dose concentrations given in Table 1. Data are presented as mean ± standard deviation (n=3). Similar lowercase letters indicate no significant difference (p < 0.05).
SEM images were used to observe the effect of the allelochemical on the morphology of. H. akashiwo cells exposed to the medium dose of DMSO solution for 4 days. The healthy cells of H. akashiwo in the control group were intact and agglomerated as shown in Fig.3A. There is no rigid cell wall around the H. akashiwo cell, as the outermost layer of the cell is a naked membrane, which may facilitate agglomeration during the process of sample preparation prior to SEM observation (Guo, 1994). In the treated cells, the outer membrane of H. akashiwo was damaged, with multiple holes of different sizes and shapes apparent on many cells (Fig.3B). Some cells were in very bad condition, and would likely die with such damage (Fig.3C, D, E). The effect on the membrane was presumably caused by the allelochemical isolated from P. tricornutum filtrate. Further examination of this effect was explored using probes for membrane integrity and other physiological parameters.
Figure. 3.
SEM micrographs of H. akashiwo cells. A: Control group; B, C, D, E, cells treated with the allelochemical isolated from P. tricornutum filtrate.
3.2. Effects on photosynthetic activities
The mean in vivo chlorophyll-a fluorescence of H. akashiwo cells was measured after exposure to different concentrations of the P. tricornutum allelochemical. There was no apparent change in the low and medium dosage treatments compared to the control group (Fig.4). The chlorophyll-a content decreased by 10% after 1 and 3 days of exposure to the high concentration of allelochemicl, and by 20% after days 5 and 6.
Figure. 4.
Influence of six-day exposure to different concentrations of allelochemical isolated from P. tricornutum filtrate on the chlorophyll-a content of H. akashiwo cells. A: high dose; B: medium dose; C: low dose. D: Control. Dose concentrations given in Table 1. Data are presented as mean ± standard deviation (n=3). Similar lowercase letters indicate no significant difference (p < 0.05).
Photosynthesis is the central physiological process for primary producers in marine systems, and thus has been widely reported as an important target of allelochemicals (Kӧrner and Nicklisch, 2002). Li et al (2007) found the allelochemical EMA produced by P. communis decreased the content of chlorophyll-a for Microcystis aeruginosa. The decrease in chlorophyll-a content in the present study confirmed inhibition of photosynthetic activity of H. akashiwo cells by the allelochemical extracted from P. tricornutum filtrate, although the effects were only observed with the highest exposures.
3.3. Influence on membrane integrity and potential
Intact cell membranes are necessary for maintaining normal cellular functions. Damaged cell membranes will thus affect cell survival and growth. Cell membrane integrity was quantified as the percentage of viable cells in the different concentrations of allelochemical treatments revealed by PI-staining. Vital dyes such as PI are normally excluded from the inside of healthy cells, but freely cross the membrane and enter the cell to stain internal components (like DNA) if the membranes are damaged. After a short-term exposure (day 3), the percentage of cells with intact membranes in different treatments was lower than the control group (p < 0.05). After longer duration exposures (days 5 and 6), membrane integrity of the medium and low concentration treatments was not significantly different from the control group. However, high allelochemical exposures decreased the percentage of intact H. akashiwo cells by about 10–18% (p < 0.05). The percentage of viable H. akashiwo cells was about 3–5% lower after short-term allelochemical exposure to the low and medium concentration treatments (day 3), with the effect disappearing on days 5 and 6. This transient effect presumably reflects the growth and division of surviving cells (Fig.5), and perhaps also demonstrates that the survivors had some inherited resistance to the allelochemical or that the chemical had degraded.
Figure. 5.
Influence of six-day exposure to different concentrations of allelochemical isolated from P. tricornutum filtrate on the membrane integrity of H. akashiwo cells. A: high dose; B: medium dose; C: low dose. D: Control. Dose concentrations given in Table 1. Data are presented as mean ± standard deviation (n=3). Similar lowercase letters indicate no significant difference (p < 0.05).
Many allelochemicals have been found to reduce algal cell membrane integrity, thereby leading to the leakage of cell constituents (e.g., proteins, nucleic acids and inorganic ions), enhancing proton influx (Johnston et al., 2003; Campos et al., 2009) and finally causing catastrophic cell membrane damage. EMA was found to oxidize the major fatty acids of cyanobacterial cell membranes and cause leakage of Ca2+, K+ and Mg2+ (Hong et al., 2008). Likewise, when FA was added to a culture of M. aeruginosa, the percentage of damaged cells increased with increasing concentration (Wang et al., 2016 a). No intact cells were found when M. aeruginosa was exposed to 3.47 mM FA at 96 h. Based on evidence from SEM and the membrane integrity dyes used here, it is clear that damage of cell membranes is one reason for the inhibitory effects of the P. tricornutum allelochemical on H. akashiwo.
The fluorescence of DiOC6(3) was also used to assess the change of membrane potential due to the allelochemical. This measures the difference in potential between the interior and exterior of a biological cell. The mean fluorescence intensity of DIOC6(3) is shown in Fig.6. It did not respond rapidly to the different doses of allelochemical treatments used here. The mean fluorescence intensity of DIOC6(3) showed no difference from the control group after 3 d of exposure, but did eventually decrease by 40% on day 5 for the three treatment groups. The inhibitory effect became even more evident with high allelochemical concentration on day 6, with fluorescence suppressed by 70% compared to the control. However, the DIOC6(3) intensity increased and showed no difference from the control group on day 6 at medium and low concentrations of the allelochemical. This result was not in accordance with the rapid change in membrane potential observed for M. aeruginosa exposed to FA after only 8 h, highlighting the dependence of the effect on the sensitivity of the target cell and the nature of the allelochemical (Wang et al., 2016 a). More studies of the effects of allelochemicals on membrane potential should reveal more details of the mechanisms of the inhibitory response.
Figure. 6.
Influence of six-day exposure to different concentrations of allelochemical isolated from P. tricornutum filtrate on the membrane potential of H. akashiwo cells. A: high dose; B: medium dose; C: low dose. D: Control. Dose concentrations given in Table 1. Data are presented as mean ± standard deviation (n=3). Similar lowercase letters indicate no significant difference (p < 0.05).
3.4. Inhibition on esterase activity
Esterase is a type of hydrolase enzyme that exists in many organisms and plays an important role in many biological functions. An esterase activity assay using fluorescein diacetate (FDA) has been proposed as a rapid endpoint to evaluate the toxicity of environmental pollutants on algal species (Regel et al., 2002; Hadjoudja et al., 2009). As shown in Fig.7, FDA fluorescence was significantly inhibited by allelochemical exposure even with the lowest concentration treatment and in the earliest stages of the experiment (days 1 and 3), following a dose-dependent pattern The fluorescence of FDA for the three treatments all showed a pronounced decrease of 35–50% compared to the control group on day 1. It increased subsequently, but still remained lower than the control group, reaching a 10–20% decrease on day 3. However, esterase activity of each treatment group showed no difference compared to the control group on days 5 and 6. This suggests that H. akashiwo was vulnerable during the initial period of exposure, but that subsequent divisions of the surviving cells led to a population that had physiologically adapted to the allelochemical through time, perhaps though some type of resistance among daughter cells. As above, it may be that the allelochemical lost some of its potency through degradation.
Figure. 7.
Influence of six-day exposure to different concentrations of allelochemical isolated from P. tricornutum filtrate on the esterase activity of H. akashiwo cells.. A: high dose; B: medium dose; C: low dose. D: Control. Dose concentrations given in Table 1. Data are presented as mean ± standard deviation (n=3). Similar lowercase letters indicate no significant difference (p < 0.05).
Esterase activity of H. akashiwo cell was the most sensitive and rapid response to the allelochemical of P. tricornutum compared to the other physiological measures. Correlation analysis showed that the growth of H. akashiwo had a positive relationship with the integrity of the cell membrane (r =0.812, p< 0.001), and the activity of esterase also exhibited a positive correlation with the integrity of the cell membrane, especially with the medium and high concentrations treatments (r=0.746, p<0.05;r=0.791, p<0.05).
Allelopathy is clearly an important factor in competition among phytoplankton in marine ecosystems (Legrand et al., 2003), but the exact nature of compounds involved in the inhibition process and the inhibitory mechanisms are still unclear in most cases. The allelopathic effect of Prymnesium parvum, which can produce toxins with haemolytic, ichthyotoxic and cytotoxic properties, caused changes in the plankton community structure, resulting in a decrease in both chlorophyll a and carbon uptake (Fistarol et al., 2003). Alexandrium tamarense also produces potent allelochemicals comprising a suite of large non-proteinaceous and probably non-polysaccharide compounds between 7 kDa and 15 kDa with lytic activity against a wide variety of marine microorganisms (Ma et al., 2011). Allelopathy is clearly an important factor in competition among phytoplankton in marine ecosystems (e.g., Legrand et al., 2003), however, the exact nature of compounds involved in the inhibition process and the inhibitory mechanisms are still unclear. The allelopathic effect of Prymnesium parvum, which produces toxins with haemolytic, ichthyotoxic and cytotoxic properties, caused changes in the plankton community structure, resulting in a decrease in both chlorophyll a and carbon uptake (Fistarol et al., 2003). Alexandrium tamarense also produces potent allelochemicals comprising a suite of large non-proteinaceous and probably non-polysaccharide compounds between 7 kDa and 15 kDa with lytic activity against a wide variety of marine microorganisms (Ma et al., 2011).
Allelopathy has the potential to be used as the basis of an effective control or bloom mitigation strategy to inhibit algal growth in natural blooms, particularly those that are harmful and where bloom suppression has benefits to society or to ecosystems. There is, however, a need for further study to advance this concept and evaluate its logistical feasibility and environmental suitability. Until recently, the most successful application of the use of naturally-produced chemicals in harmful algal bloom (HAB) control involves allelochemicals released from barley straw as a bloom suppression strategy for freshwater HABs (Sellner et al. 2013). Phenolic compounds in barley straw are thought to be the main inhibitor of algal growth (Xiao et al. 2013; Huang et al. 2015; Terlizzi et al. 2002). Iredale et al. (2012) showed that microbial degradation of the barley straw releases hydrogen peroxide as well as inhibitory products from the lignin. Unfortunately, barley straw would have limited use against HABs in coastal marine environments due to the continual exchange of seawater with tides, etc., and the relatively limited number of algal species that are sensitive (Terlizzi et al. 2002; Hagström et al. 2010).
Hu and Hong (2008) reviewed the potential application of allelopathy from aquatic plants (macroalgae) on microalgae, and Shao et al. (2013) reviewed the use of several other biologically-derived substances that have negative impacts on algal (mainly phenols, quinones, alkaloids, organic acids, amino acids, and terpenes). Those authors also discussed the reasons progress in the use of allelochemicals in HAB control has been slow, including difficulties in obtaining sufficient material, relatively low sensitivity of targeted HAB species, ecological and public health concerns, and the potential release of toxins as a result of cell lysis during the treatment. Yet another major concern is that the extrapolation from laboratory studies to the natural field applications is hindered by the complexity of predator-prey and general plankton community dynamics (Mayali and Azam 2004). Clearly, the practical application of allelochemicals in bloom control needs further study and careful field evaluation.
4. Conclusions
Heterosigma akashiwo growth was significantly suppressed by the allelochemical contained in the ethyl acetate extract of P. tricornutum. Many H. akashiwo cells rapidly died and disappeared from the medium, as observed by the light microscope and the FCM. A possible mechanism for this effect was revealed by SEM imagery, which showed numerous holes with different shapes and sizes on the outer cell. FCM was applied with vital stains to examine physiological parameters in the surviving cells. Although those cells were still intact, or semi-intact, the FCM analyses could reveal the nature of the damage that had been experienced. The allelochemical released by P. tricornutum was found to influence H. akashiwo mainly by decreasing the esterase activity and the integrity of the cell membrane, thereby releasing cytoplasm and other cellular constituents. Esterase activity was the most useful and sensitive parameter to evaluate the influence of the P. tricornutum allelochemical on H. akashiwo. Membrane potential and the content of chlorophyll-a were also affected, but the membrane potential response increased through time, in contrast to other parameters, which generally followed an opposite trend. More studies of the mechanisms underlying the response of membrane potential are needed.
In summary, a Phaeodactylum allelochemical caused catastrophic damage to exposed Heterosigma cells, leading to cell lysis and death, but surviving cells were also impacted, showing effects that reflect damage to membrane integrity and some biochemical properties such as esterase activity. Nevertheless, surviving cells can continue to grow and in a few days, re-establish a successful culture, even in the presence of residual allelochemical, suggesting either development of cellular resistance, or the biodegradation of the chemical.
Acknowledgements
The authors wish to thank the National Programme on Global Change and Air-Sea Interaction (Grant No. GASI-03-01-02-01); the National Key Research and Development Program [Grant No. 2016YFC1402101]; the assessment of nanomaterials on biological and ecological effects in the coastal area (Grant No. 201505034).
References
- Anderson DM, 1997. Turning back the harmful red tide. Nature 388, 513–514. [Google Scholar]
- Anderson DM, Cembella AD, Hallegraeff GM, 2012. Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci 4, pp.143–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz RG, Hurle K, 2004. A novel laboratory screening bioassay for crop seedling allelopathy. J. Chem. Ecol 30(1), 175–198. [DOI] [PubMed] [Google Scholar]
- Blaise C, Ménard L, 1998. A micro-algal solid-phase test to assess the toxic potential of freshwater sediments. Water. Qual. Res. J. Can 33(1), 133–151. [Google Scholar]
- Campos FM, Couto JA, Figueiredo AR, Tóth IV, Rangel AO, Hogg TA, 2009. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food. Microbiol 135(2), 144–151. [DOI] [PubMed] [Google Scholar]
- Cunningham A, Buonnacorsi GA, 1992. Narrow-angle forward light scattering from individual algal cells: implications for size and shape discrimination in flow cytometry. J. Plankton Res 14(2), 223–234. [Google Scholar]
- Dorantes-Aranda JJ, Seger A, Mardones JI, Nichols PD, Hallegraeff GM, 2015. Progress in Understanding Algal Bloom-Mediated Fish Kills: The Role of Superoxide Radicals, Phycotoxins and Fatty Acids. PLoS ONE 10(7), e0133549. doi: 10.1371/journal.pone.0133549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke SO, 2003. Ecophysiological aspects of allelopathy. Planta 217(4), 529–539. [DOI] [PubMed] [Google Scholar]
- Eigemann F, Hilt S, Schmitt-Jansen M, 2013. Flow cytometry as a diagnostic tool for the effects of polyphenolic allelochemicals on phytoplankton. Aquat. Bot 104(1), 5–14. [Google Scholar]
- Fistarol GO, Legrand C, Granéli E, 2003. Allelopathic effect of Prymnesium parvum on a natural plankton community. Mar Ecol Prog Ser 255(8), 115–125. [Google Scholar]
- Franklin NM, Stauber JL, Lim RP, 2001. Development of flow cytometry‐based algal bioassays for assessing toxicity of copper in natural waters. Environ. Toxicol. Chemistry 20(1), 160–170. [PubMed] [Google Scholar]
- Granéli E, Edvardsen B, Roelke DL, Hagström JA, 2012. The ecophysiology and bloom dynamics of Prymnesium, spp. Harmful Algae 14(SI), 260–270. [Google Scholar]
- Gross EM, 2003. Allelopathy of aquatic autotrophs. Crit. Rev. Plant Sci 22, 313–339. [Google Scholar]
- Gross EM, Meyer H, Schilling G, 1996. Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum. Phytochemistry 41(1), 133–138. [Google Scholar]
- Guillard RRL, 1975. Culture of phytoplankton for feeding marine invertebrates[M]// Culture of Marine Invertebrate Animals. Springer; US: 29–60. [Google Scholar]
- Guo Y,1994. Studies on Heterosigma akashiwo (HaDa) HaDa in the Dalian Bight, Liaoning, China, J. Oceanologia Et Limnologia Sinica 25, 211–215. [Google Scholar]
- Hadjoudja S, Vignoles C, Deluchat V, Lenaina JF, Le-Jeunea AH, Baudua M, 2009. Short term copper toxicity on Microcystis aeruginosa and Chlorella vulgaris using flow cytometry. Aquat. Toxicol 94(4), 255–264. [DOI] [PubMed] [Google Scholar]
- Hilt S, Gross EM, 2008. Can allelopathically active submerged macrophytes stabilise clear-water states in shallow lakes? Basic. Appl. Ecol 9(4), 422–432. [Google Scholar]
- Hong Y, Hu HY, Li FM, 2008. Growth and physiological responses of freshwater green alga Selenastrum capricornutum, to allelochemical ethyl 2-methyl acetoacetate (EMA) under different initial algal densities. Pestic. Biochem. Phys 90(3), 203–212. [Google Scholar]
- Horner RA, Garrison DL, Plumley FG, 1997. Harmful algal blooms and red tide problems on the U.S. west coast, J. Limnol. Oceanogr 42, 1076–1088. [Google Scholar]
- Irfanullah MH, Moss B, 2005. Allelopathy of filamentous green algae. Hydrobiologia 543, 169–179. [Google Scholar]
- Johnston MD, Hanlon GW, Denyer SP, Lambert RJW, 2003. Membrane damage to bacteria caused by single and combined biocides. J. Appl. Microbiol 94(6), 1015–1023. [DOI] [PubMed] [Google Scholar]
- Körner S, Nicklisch A, 2002. Allelopathic growth inhibition of selected phytoplankton species by submerged macrophytes1. J. Phycol 38(5), 862–871. [Google Scholar]
- Legrand C, Rengefors K, Fistarol GO, Granéli E, 2003. Allelopathy in phytoplankton - biochemical, ecological and evolutionary aspects, Phycologia 42(4), 406–419. [Google Scholar]
- Li FM, Hu HY, Chong YX, M YJ, G MT, 2007. Influence of EMA isolated from Phragmites communis on physiological characters of Microcystis aeruginosa. China Environmental Science 27(3), 377–381. [Google Scholar]
- Ma H, Krock B, Tillmann U, Muck A, Wielsch N, Svatoš A, Cembella A, 2011. Isolation of activity and partial characterization of large non-proteinaceous lytic allelochemicals produced by the marine dinoflagellate Alexandrium tamarense. Harmful Algae 11(6), 65–72. [Google Scholar]
- Nakai S, Inoue Y, Hosomi M, Murakami A, 1999. Growth inhibition of blue-green algae by allelopathic effects of macrophytes. Water. Sci. Technol 39(8), 47–53. [Google Scholar]
- Peterson SM, Stauber JL, 1996. New Algal Enzyme Bioassay for the Rapid Assessment of Aquatic Toxicity. B. Environ. Contam. Tox 56(5), 750–757. [DOI] [PubMed] [Google Scholar]
- Qian HF, Xu XY, Chen W, J H, J YX, L WP, F ZW, 2009. Allelochemical stress causes oxidative damage and inhibition of photosynthesis in Chlorella vulgaris. Chemosphere 75(3), 368–75. [DOI] [PubMed] [Google Scholar]
- Regel RH, Ferris JM, Ganf GG, Brookes JD, 2002. Algal esterase activity as a biomeasure of environmental degradation in a freshwater creek. Aquat. Toxicol 59(3), 209–223. [DOI] [PubMed] [Google Scholar]
- Rice EL, 1984. Allelopathy. 2nd edn Academic Press, London: 422 pp. [Google Scholar]
- Rioboo C, O’Connor JE, Prado R, Herrero C, Cid A, 2009. Cell proliferation alterations in Chlorella cells under stress conditions. Aquat. Toxicol 94(3), 229–237. [DOI] [PubMed] [Google Scholar]
- Roy S, Alam S, Chattopadhyay J, 2006. Competing effects of toxin-producing phytoplankton on overall plankton populations in the Bay of Bengal Bull, J. Math Biol 68, 2303–2320. [DOI] [PubMed] [Google Scholar]
- Singh NB, Thapar R, 2003. Allelopathic influence of Cannabis sativa on growth and metabolism of Parthenium hysterophorus. Allelopathy. J 12(1), 61–70. [Google Scholar]
- Smayda TJ, Anderson DM, Cembella AD, Hallegraeff GM, 1998. Ecophysiology and bloom dynamics of Heterosigma akashiwo (Raphidophyceae). Nato. Asi. Series. G. Ecological. Sciences 41, pp.113–132. [Google Scholar]
- Sukenik A, Eshkol R, Livne A, Hadas O, Rom M, Tchernov D, Vardi A, Kaplan A, 2002. Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (cyanobacteria): A novel allelopathic mechanism. Limnol. Oceanogr 47(6), 1656–1663. [Google Scholar]
- Sun J, Ning XR, 2005. Marine phytoplankton specific growth rate. J. Adv in Earth Sci.20: 939–945. [Google Scholar]
- Sun WH, Yu SW, Yang SY, Zhao PW, Yu ZW, Wu HM, Huang SY, Tang CS, 1993. Allelochemicals from root exudates of water hyacinth (Eichhornis crassipes). Physiol. Mol. Biol. Pla 19, 92–96. [Google Scholar]
- Wang R, Hua M, Yu Y, Zhang M, Xian QM, Yin DQ, 2016a. Evaluating the effects of allelochemical ferulic acid on Microcystis aeruginosa by pulse-amplitude-modulated (PAM) fluorometry and flow cytometry. Chemosphere 147, 264–271. [DOI] [PubMed] [Google Scholar]
- Wang R, Wang JT, Xue QN, Tan LJ, Cai J, Wang HY, 2016b. Preliminary analysis of allelochemicals produced by the diatom Phaeodactylum tricornutum. Chemosphere 165, 298–303. [DOI] [PubMed] [Google Scholar]
- Xi X, Chen YX, Liang XQ, Lou LP, 2010. Effects of Tibetan hulless barley on bloom-forming cyanobacterium (Microcystis aeruginosa) measured by different physiological and morphologic parameters. Chemosphere 81(9), 1118–1123. [DOI] [PubMed] [Google Scholar]
- Xiao X, 2012. Allelopathic Inhibition of Cvanobacteria by Barley Straw and Its Mechanism [dissertation]. Zhejiang University. [Google Scholar]
- Xiao X, Han ZY, Chen YX, Liang XQ, Li H, Qian YC, 2011. Optimization of FDA-PI method using flow cytometry to measure metabolic activity of the cyanobacteria, Microcystis aeruginosa. Phys. Chem. Earth Parts A/B/C 36(9–11), 424–429. [Google Scholar]
- Xiao X, Huang H, Ge Z, Rounge TB, Shi J, 2014. A pair of chiral flavonolignans as novel anti-cyanobacterial allelochemicals derived from barley straw (Hordeum vulgare): characterization and comparison. Environ. Microbiol 16(5), 1238–1251. [DOI] [PubMed] [Google Scholar]
- Yang J, Deng XR, Xian QM, Li AM, 2014. Allelopathic effect of Microcystis aeruginosa on Microcystis wesenbergii: microcystin-LR as a potential allelochemicals. Hydrobiologia 727, 65–73. [Google Scholar]
- Zhu J, Liu B, Wang J, Gao Y, Wu Z, 2010. Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquat. Toxicol 98(2), 196–203. [DOI] [PubMed] [Google Scholar]