Abstract
Pulmonary hypertension (PH) is a significant complication of sarcoidosis, occurring in ~ 6% to > 20% of cases, and markedly increases mortality among these patients. The clinician should exercise a high index of suspicion for sarcoidosis-associated PH (SAPH) given the nonspecific symptomatology and the limitations of echocardiography in this patient population. The pathophysiology of PH in sarcoidosis is complex and multifactorial. Importantly, there are inherent differences in the pathogenesis of SAPH compared to idiopathic pulmonary arterial hypertension (IPAH), making the optimal management of SAPH controversial. In this section, we review the epidemiology, diagnosis, prognosis, and treatment considerations for SAPH.
Lung transplantation (LT) is a viable therapeutic option for sarcoid patients with severe pulmonary fibrocystic sarcoidosis or SAPH refractory to medical therapy. We discuss the role for LT in patients with sarcoidosis, review the global experience with LT in this population and indications and contraindications to LT.
Keywords: sarcoidosis, pulmonary hypertension, lung transplantation
Introduction
Pulmonary hypertension (PH) is a significant complication of sarcoidosis, occurring in 6 to 74% of patients. 1–9 The presence of PH markedly increases mortality among these patients; this effect is independent of pulmonary function 1,10,11 As will be discussed in detail later, lung transplantation (LT) is a viable therapeutic option for severe pulmonary parenchymal or vascular disease refractory to medical therapy in patients with sarcoidosis12, but has significant morbidity and mortality. In this manuscript, we first discuss in depth sarcoidosis-associated PH (SAPH), including pathogenesis, prevalence, clinical features, and therapy. Finally, we discuss LT (results, indications, and identifying appropriate candidates) for sarcoid patients with end-stage fibrocystic disease or SAPH.
Pathophysiology of PH in Sarcoidosis
The pathophysiology of SAPH is complex and involves multiple potential mechanisms. The World Health Organization (WHO) classifies SAPH in group V, “PH with unclear and/or multifactorial mechanisms” given the considerable overlap with other WHO PH groups.13,14 Obliteration of the pulmonary vasculature by parenchymal fibrosis has been described as a major contributor to SAPH.15 However, a significant proportion of patients with SAPH have normal or near normal lung function and chest radiographs.4,16 A retrospective study from France found that 7 of 22 (32%) patients with sarcoidosis had no radiographic evidence of pulmonary fibrosis at the time of PH diagnosis [mean pulmonary arterial pressure (mPAP) > 25 mmHg] by right heart catheterization (RHC).10 Similarly, another study from a U.S. tertiary center found that 4 of 38 (11%) patients with SAPH had no evidence of parenchymal disease by chest radiography.9 These studies suggest that fibrotic ablation of the pulmonary vascular bed alone may not account for all SAPH.
Granulomatous involvement of the pulmonary vasculature is a common feature of sarcoidosis causing both granulomatous obliteration and angiitis (Figure 1).17–21 An autopsy series of 40 Japanese sarcoidosis patients found granulomatous involvement of the pulmonary vasculature in all cases with frequent occlusion of arterioles and venules.18 The overall extent of granulomatous vascular involvement paralleled parenchymal involvement, but there was a strong predilection for the venous and lymphatic vessels.18 Granulomatous angiitis has been well described in sarcoidosis.17,19 An evaluation of 174 transbronchial biopsies from sarcoid patients found granulomatous angiitis in 72 (41%) with prominence in the veins and venules (89%).17 Another series of 128 open lung biopsies found granulomatous angiitis in 88 (69%) with mostly venous involvement (92%).19
Figure 1A-F–
Vascular findings in pulmonary hypertension associated with sarcoidosis: A) Atheroma (ATH) in large pulmonary artery, a finding in pulmonary hypertension of many causes; B) muscular intrapulmonary artery with medial hypertrophy and intimal hyperplasia also present in pulmonary hypertension of many causes; C) granulomatous arteritis with sarcoid granuloma (arrows) destroying the media of the artery (Art); D) pulmonary veno-occlusive disease, in this case with a granuloma in the wall of the vein (V) as well as generalized fibrosis thickening the vein wall; E and F) marked capillary proliferation in pulmonary capillary hemangiomatosis, a relatively rare finding in the lungs of patients with sarcoidosis and pulmonary hypertension (A trichrome/elastic stain x40, B and C trichrome elastic stain x200, D hematoxylin eosin stain x100, and F reticulin stain x100)
Hypoxic vasoconstriction is a known mechanism of PH in many types of parenchymal lung disease, but the importance of this mechanism in SAPH is not well established. Sulica et al. found no difference in oxygen saturation or need for supplemental oxygen among sarcoid patients with PH (n=31) versus those without PH (n=26).9 In contrast, an evaluation of 363 patients with advanced sarcoidosis listed for LT found that the need for supplemental oxygen was the only independent predictor of PH in a multivariable model.22
Extrinsic compression of pulmonary arteries and veins by fibro-granulomatous involvement of the lymph nodes has been described in numerous case series. 10,18,23 However, a Japanese series of 122 sarcoidosis patients found no difference in mediastinal or hilar lymph node enlargement among those with and without SAPH.4 The significance of this mechanism in the pathogenesis of SAPH is not well defined.
Studies have also implicated decreased nitric oxide (NO) and increased endothelin-1 (ET-1) production in the pathogenesis of SAPH. NO is synthesized and released by endothelial cells causing local vasodilation24 and the lack of NO production has been associated with Group I PH (PAH)25, with reported favorable treatment responses to inhaled NO (iNO).15,26 ET-1, also produced by endothelial cells, is a potent vasoconstrictor, smooth muscle mitogen and proinflammatory peptide.27 ET-1 binds to ETA and ETB receptors on endothelial cells causing vasoconstriction, cytokine and growth factor production, and inflammation.28 Several studies have shown increased levels of ET-1 in the urine29, bronchoalveolar lavage30,31, and plasma32 of patients with sarcoidosis compared with controls. Data are limited, but several studies suggest a potential benefit of endothelin receptor blockade in SAPH.33–35
Pulmonary veno-occlusive disease (PVOD) complicating sarcoidosis has been described in several case reports and series.10,36–38 The pathologic hallmark of PVOD is extensive narrowing or occlusion of the pulmonary veins by fibrous tissue.39 Recanalization of occluded vessels occurs with intimal proliferation and thickening, particularly in pulmonary venules and small veins of the interlobular septa.39 Alveolar capillaries may become engorged and tortuous consistent with pulmonary capillary hemangiomatosis (PCH).40 Pulmonary arteries often develop medial hypertrophy and eccentric intimal fibrosis; however, in contrast to PAH, plexiform lesions are typically absent.40 In one SAPH case-series, all explanted lungs obtained at the time of lung transplantation (n=5) demonstrated evidence of PVOD.10
Finally, granulomatous inflammation and fibrosis of the myocardium are common in sarcoidosis and can cause conduction abnormalies, cardiomyopathy, and congestive heart failure. The granulomatous infiltration has a predilection for the basal ventricular septum and left ventricular free wall.41 Cardiac involvement occurs independently of other organs; and as such, the severity of pulmonary disease does not predict cardiac involvement.42 Autopsy series from the U.S. have estimated the prevalence of cardiac involvement at over 25% in patients with sarcoidosis.43,44
Epidemiology and Clinical Features
PH is defined hemodynamically by right heart catheterization (RHC) as a mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg with a pulmonary capillary wedge pressure (PCWP) ≤ 15 mm Hg and pulmonary vascular resistance (PVR) ≥ 3 Wood Units.45 Estimated prevalence rates of PH in sarcoidosis have been highly variable ranging from 6 to 74% 2–9, reflecting the heterogeneous patient populations and diagnostic criteria used in these studies. A study of predominantly African-Americans from a sarcoidosis referral center in Detroit found that 14% had PH based on echocardiographic criteria [estimated right ventricular systolic pressure (RVSP) > 40 mmHg].46 Subsequent multivariable logistic regression showed that lower oxygen saturation during a 6 minute walk test (6MWT) and lower (% predicted) diffusion capacity of carbon monoxide (DLCO) were the only clinical features significantly associated with PH.46 Another study of 246 consecutive Japanese patients from an outpatient sarcoidosis clinic found 6% had PH based on transthoracic echocardiographic (TTE) criteria (estimated RVSP > 40 mmHg).4 Decreased (% predicted) total lung capacity (%TLC) was the only clinical characteristic associated with PH in their multivariable logistic regression analysis.4 All patients with %TLC < 60% had PH; however, many patients with PH had normal %TLC suggesting mechanisms other than parenchymal fibrosis in the pathogenesis of SAPH.4 Maimon et al recently reported 28% of their sarcoid cohort with ‘near normal lung function’ [i.e., forced vital capacity (FVC) > 70%, forced expiratory volume in 1 second (FEV1) > 70%, and DLCO > 60%] at a tertiary center in Israel had concurrent PH (estimated RVSP > 40 mmHg).16 The only clinical characteristic associated with PH in multivariable analysis was a decreased 6 minute walk distance (6MWD).16
Overall, prevalence estimates of SAPH are much higher among patients with advanced fibrocystic disease.3,5,8,9,11,47 Among 363 patients with sarcoidosis listed for lung transplantation in the USA between 1995 to 2002, 74% had PH (mPAP > 25 mmHg) and 36% had severe PH (mPAP > 40 mmHg) by RHC.22 The only clinical characteristic found to be associated with PH in multivariable analysis was the need for supplemental oxygen.22 Similarly, in a study of 106 sarcoid patients evaluated by TTE at a tertiary center; 54 patients (51%) had PH (RVSP ≥ 40 mmHg).9 These studies indicate that PH is common in sarcoidosis with higher prevalence among those with advanced disease. Decreased FVC (% predicted), TLC (% predicted), DLCO (% predicted), 6MWT oxygen saturation, and 6MWD all increase the likelihood of concurrent PH in the setting of sarcoidosis. However, a high index of suspicion is necessary in all sarcoid patients, since PH can occur at any stage of disease and there are no consistent clinical characteristics that predict its presence.
Diagnosis of PH in Sarcoidosis
The diagnosis of PH in sarcoidosis is challenging since the symptoms of SAPH are non-specific. One study found no difference in the presenting symptoms of dyspnea, cough, palpitations and chest pain between sarcoid patients with and without PH.9 Similarly, signs of right heart failure such as elevated jugular venous pressure, S3 or S4 heart sounds, lower extremity edema or right ventricular (RV) heave generally occur in late disease and are not sensitive predictors of PH.9 As discussed above, lung function parameters such as decreased FVC, TLC, DLCO, 6MWT oxygen saturation or distance may be helpful but do not reliably predict SAPH.
RHC remains the gold standard for diagnosing PH (idiopathic or secondary) as it provides the most reliable measurements of pulmonary hemodynamics and cardiac function.48 However, RHC is relatively invasive and expensive which precludes its “routine” use in clinical practice.49 Imaging studies are poor predictors of PH in fibrotic lung diseases including sarcoidosis.16,50 Standard radiography and high-resolution computed tomographic (HRCT) scans of the chest may show enlarged pulmonary arteries with right atrial and RV enlargement (Figure 2). However, these findings, particularly dilated pulmonary arteries, often occur in sarcoid patients without PH. A recent retrospective study from a tertiary care center found no difference in pulmonary artery dilation or RV diameter among sarcoid patients with (n=36) and without (n=91) PH on HRCT.16 Although most patients with SAPH have advanced fibrocystic disease (radiographic stage IV) (Figure 3)4,9, parenchymal destruction is not necessary for PH development and does not correlate with PH severity.9,22,55 A retrospective evaluation of 22 sarcoid patients with PH (i.e., mPAP > 25 mm Hg on RHC) found that 32% had no fibrosis on chest radiography at the time of PH diagnosis.10 Another prospective evaluation of 246 sarcoidosis patients found that CT chest scans were similar in patients with and without PH (i.e., sPAP > 40 mm Hg on TTE), with respect to lymph node enlargement, lung opacification, and thickening of bronchovascular bundles.4
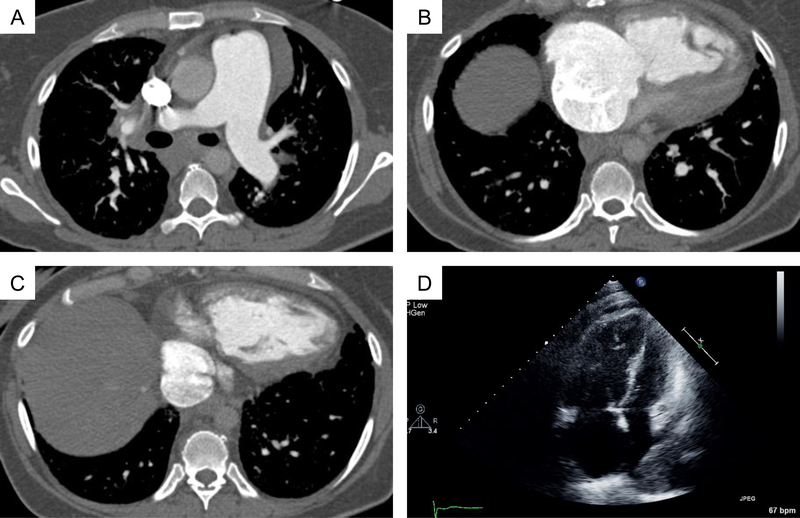
Figure 2. A-C:
44 year old female with biopsy proven sarcoidosis and severe pulmonary hypertension with pulmonary artery pressure 85/45 (mean 54) mmHg. Contrast computed tomographic (CT) images of the chest show pulmonary artery enlargement at 42 mm in maximal axial diameter, severe right atrial and right ventricular enlargement with right ventricular hypertrophy. D: Diastolic apical 4 chamber view on transthoracic echocardiography showing severe right atrial and ventricular enlargement with bowing of the interatrial septum to the left.
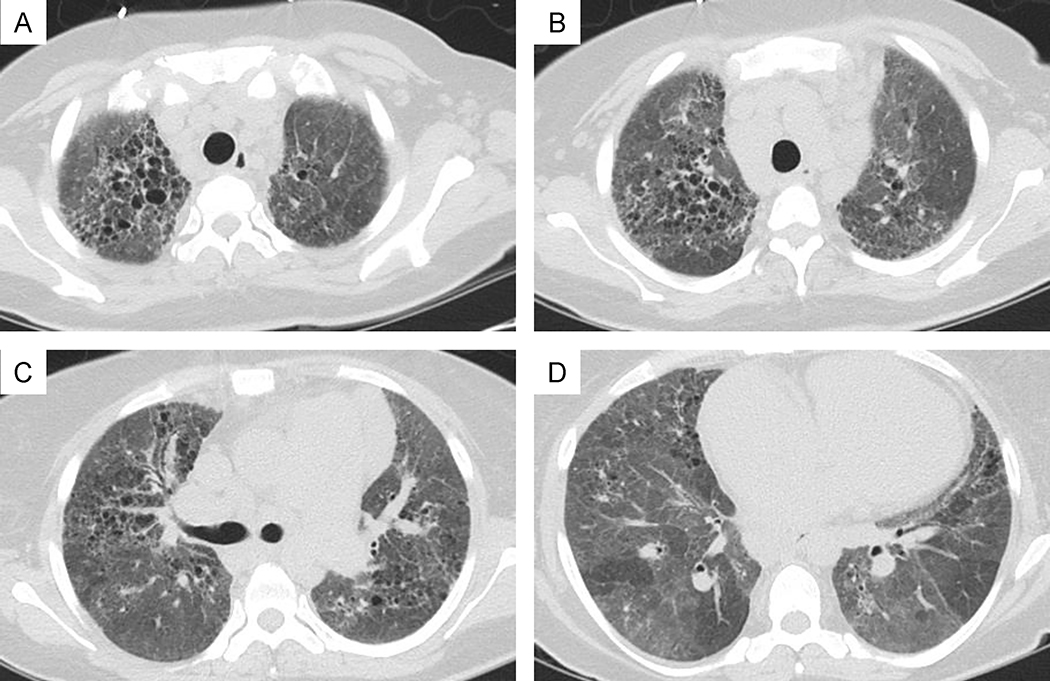
Figure 3: A-D:
High resolution CT of the chest in the same patient shows diffuse upper greater than lower lobe peribronchovascular fine and course reticulation, architectural and pleural parenchymal distortion, traction bronchiectasis and bronchiolectasis, course diffuse ground glass opacification with lobular air-trapping.
Transthoracic echocardiography (TTE) is noninvasive and currently the screening test of choice for PH of all etiologies. However, TTE has important limitations in patients with interstitial lung disease (ILD).51–54 In a study of 106 patients with advanced ILD referred for LT, TTE estimates of systolic pulmonary artery pressure (sPAP) were possible in only 54% of patients.51 The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of TTE for PH (defined as sPAP > 45 mm Hg by RHC) were poor at 85%, 17%, 60%, and 44%, respectively.51 Similarly, TTE-derived RV abnormalities (e.g., RV dilatation, hypertrophy or systolic dysfunction) had a sensitivity, specificity, PPV, and NPV for PH of 76%, 53%, 57%, and 74%, respectively.51 In another study of 61 patients with IPF, estimates of RV systolic pressure (RVSP) were possible in only 33 patients (54%) and the sensitivity, specificity, PPV, NPV for PH (defined as RSVP > 40 mm Hg by RHC) were 76%, 38%, 56%, and 60%, respectively 52. TTE estimates of sPAP can over- or under-estimate the true RVSP and may be unreliable when used in isolation. Nonetheless, TTE remains the screening test of choice for PH and compliments RHC, as it provides additional data on the structure and function of the right and left heart.
As discussed above, several studies have reported associations between PH and physiologic parameters in patients with sarcoidosis, but the results have not been consistent. Parameters that have correlated with PH in sarcoidosis patients include: need for supplemental oxygen 4,22; decreased 6MWD 22,56; desaturation during 6MWT 46; and reduced % DLCO 10,22. Importantly, some studies demonstrated a correlation between % FVC and % FEV1 and PH 4,9, while other studies did not find an association10,22. These studies demonstrate the limited predictive value of any single parameter to accurately predict PH in sarcoidosis.57
Prognosis of PH Complicating Sarcoidosis
Sarcoidosis has a relatively good prognosis with an attributable mortality rate of less than 4%.58,59 Many patients are asymptomatic at presentation and spontaneous remission occurs commonly (50–60%).57,59 Approximately 25% of patients develop interstitial lung disease with 10% progressing to disabling organ failure.60–63 However, the development of SAPH markedly increases mortality. A retrospective French study of 22 sarcoid patients with PH (mPAP > 25 mmHg on RHC), reported 1-, 2- and 5-year survival rates of 84%, 74%, and 59%, respectively. In contrast, 1-, 2-, and 5-year survival in matched sarcoid patients without PH were 100%, 96%, and 96%, respectively (p=0.003).10 A retrospective review of 404 sarcoid patients listed for LT in the USA from 1995 to 2000 reported 111 (27%) deaths awaiting LT.11 Three factors were associated with increased mortality in multivariable analysis: the presence of PH, the amount of supplemental oxygen needed, and African-American race. The mPAP among non-survivors was 41.4 mmHg compared to 31.7 mmHg among survivors (p < 0.001).11 Given the marked increase in mortality, patients with sarcoidosis require close follow-up and vigilance for PH, and the development of PH should prompt consideration for LT 11 (discussed in detail later).
Treatment of PH Complicating Sarcoidosis
Treatment of SAPH remains controversial due to limited available data mostly from case reports and small non-randomized series (Table 1). In theory, anti-inflammatory and immunomodulatory agents have the potential to attenuate PH caused by active granulomatous inflammation in sarcoidosis. A non-randomized study evaluated 12 months of corticosteroid use in 24 patients with radiographic stage II and III sarcoidosis.64 At baseline, RHC demonstrated PH at rest in 3 patients; 18 had exercise-induced PH. At 12 months, chest radiographs and pulmonary function tests (PFTs) improved in 92%, while hemodynamics improved in 50%.64 Another retrospective study evaluated 10 patients with SAPH [stage 0 (n=1); stage II (n=4); stage IV (n=5)] after treatment with high dose prednisone (0.5–1 mg/kg).10 After 3–6 months of treatment, all 5 patients with stage IV disease had unchanged or increased sPAP, whereas 3 of 5 patients with less advanced disease showed mild improvement. Thus, corticosteroids or immunosuppressive agents may be effective in sarcoid patients with active granulomatous inflammation, but may have little benefit in those with severe cystic or fibrotic disease 59,63.
Table 1:
Summary of Outcomes After PH-Targeted Therapy for SAPH
| Author: | Year | Treatment | n | Major Outcomes | Adverse effects |
|---|---|---|---|---|---|
| SAPH: | |||||
| Preston et al.15 | 2001 | iNO | 8 | ↓ mPAP 18%, ↓ PVR 31%, CO 12% | Ø adverse events |
| Preston et al.15 | 2001 | IV EPO | 6 | Ø mPAP, ↓ PVR 25%, CO 25% | Ø adverse events |
| Fisher et al.67 | 2006 | IV EPO | 7 | ↓ mPAP 21%, ↓ PVR 45%, CO 44%, WHO class 1–2 | ↓ PaO2 in 3/7 with one death |
| Baughman et al.70 | 2009 | Inhaled iloprost | 15 | ↓ mPAP 15%, ↓ PVR 14%, 6MWD 12%, QOL | ↓ PaO2 in 2/15 (mild) |
| Milman et al.5 | 2008 | Sildenafil | 12 | ↓ mPAP 19%, ↓ PVR 48%, CO 36%, Ø 6MWD | Ø adverse events |
| Baughman et al.73 | 2013 | Bosentan in RCT | 35 | ↓ mPAP 11%, ↓ PVR 28%, Ø 6MWD, WHO class or QOL | Ø adverse events |
| Barnett et al.33 | 2009 | IV EPO, sildenafil, bosentan | 22 | ↓ mPAP 20%, ↓ PVR 39%, 6MWD | Ø adverse events |
| Judson et al.74 | 2011 | Ambrisentan | 21 | Ø 6MWD, DLCO, QOL or dyspnea scores | Increased edema and dyspnea |
PH: pulmonary hypertension; SAPH: sarcoidosis associated pulmonary hypertension; iNO: inhaled nitric oxide; IV EPO intravenous epoprostenolol; mPAP = mean pulmonary artery pressure; PVR: mean pulmonary vascular resistance; CO: cardiac output; 6MWD: 6 minute walk distance; PaO2: partial pressure of oxygen; QOL: quality of life; DLCO: diffusion capacity for carbon monoxide; RCT: randomized placebo controlled trial.
The use of PH-specific therapies in SAPH has been extrapolated from studies in Group I PAH. However, there are inherent differences in the pathophysiology of PH between these diseases, and the use PH-targeted therapy in SAPH is controversial. PH-targeted therapy may be ineffective for SAPH as areas of fibrotic or “fixed” remodeling may not respond to pulmonary artery vasodilators. PH-targeted therapy may worsen oxygenation by reversing physiologic hypoxemic vasoconstriction and increasing blood flow to areas with poor ventilation, causing increased shunt or ventilation/perfusion (V/Q) inequality.65,66 In addition, PH-targeted therapy may also cause acute pulmonary edema and hypoxemia through preferential vasodilation of the pulmonary arteries in the setting of downstream venular obliteration by granulomas.67
One non-randomized study treated 8 patients with advanced sarcoidosis (stage III or IV) and concurrent PH (mPAP 55 mm Hg) with vasodilators [i.e., inhaled NO (iNO), intravenous epoprostenol (EPO), or calcium channel blockers (CCB)].15 Short term responses (>20% decrease in PVR) were noted in 7 of 8 receiving iNO, 4 of 6 receiving EPO, and 2 of 5 receiving CCB. Long term iNO was associated with improved 6MWD in all five treated patients; however, five of the eight patients died within 1.5 years of study enrollment. One death was due to catheter-related sepsis but no other significant treatment complications were reported.15 Another retrospective review evaluated treatment with EPO in 7 patients with sarcoidosis [radiologic stages: I (n=1), II (n=1), III (n=1), and IV (n=4)] and PH.67 These patients had severe diffusion impairment (mean DLCO 30% predicted) but only moderate restrictive physiology (mean FVC 59% predicted). Although six of seven patients responded favorably to EPO, defined as >25% reduction in PVR 67, there were significant adverse events due to EPO initiation. One patient died from a cardiac arrest 4 hours after initiation of EPO; two others developed acute respiratory failure due to pulmonary edema (one required mechanical ventilation and the other high flow oxygen supplementation). Both subjects improved with aggressive diuresis and temporary reduction in the EPO dose. Other adverse reactions included systemic hypotension, jaw pain, headache and flushing. After a mean follow-up of 29 months, the five remaining patients were alive on chronic EPO therapy and all had functional improvement by 1 or 2 WHO functional classes.67
Inhaled prostanoids (e.g., iloprost or treprostinil) have the theoretical advantage of drug delivery directly to areas with good ventilation and may provide pulmonary vasodilatation with less shunting compared to parenteral prostanoids.68,69 However, data utilizing inhaled prostanoids in SAPH are scant. An open-label study of 22 patients with SAPH, mostly stage IV sarcoidosis (68%) and moderate PH (mPAP = 33 mm Hg), reported a mild improvement in hemodynamics and quality of life with inhaled iloprost.70 Fifteen patients completed 16 weeks of inhaled iloprost therapy with the following hemodynamic changes: PVR declined by > 20% in 6 patients (40%); mPAP declined > 5 mm Hg in 5 patients (33%). Three patients (20%) improved their 6MW distance by > 30 meters; however, only 1 of these 3 patients showed hemodynamic improvement. Oxygen requirements increased in 2 patients after initiation of therapy and 7 patients withdrew from the study due to intractable cough and difficulty with compliance, but no other significant adverse events were noted.70 Inhaled prostanoids are exceedingly expensive, and given the lack of proven efficacy, have no role as therapy for SAPH.
Oral agents (e.g., ET-1 receptor antagonists and phosphodiesterase inhibitors) have been used in small trials, with anecdotal success. A retrospective study of end-stage sarcoidosis patients referred for LT evaluated the use of the phosphodiesterase inhibitor sildenafil (daily oral dose 75 to 225 mg) in 12 patients with SAPH.5 After a median treatment duration of 4 months (range 1 to 12 months), mPAP decreased by 8 mm Hg; PVR decreased by 5 Wood units; cardiac index increased by 0.6 liters/min/m2; 6MWD was unchanged.5
Bosentan (an ET-1 receptor inhibitor) has been studied in several small series of SAPH 33,34,71, case reports 35,72, and a recent small randomized controlled trial.73 Barnett et al retrospectively evaluated the use of three PH-specific therapies among 22 patients with SAPH at two referral centers 33 where patients received either sildenafil (n=9), bosentan (n=12), or epoprostenol (n=1). After a median of 11 months, WHO functional class had improved by at least one class in 9 (41%), and mean 6MWD increased by 59 meters from baseline (p=0.03). Patients with higher FVC and FEV1 had greater improvement in 6MWD. Among 12 patients who had repeat RHC, mPAP decreased from 49 to 39 mm Hg (p=0.008).33 Transplant-free survival rates at 1- and 3-years were 90% and 64%, respectively, with no adverse events reported.
Most recently, Baughman et al evaluated bosentan in a small randomized, double-blind, placebo-controlled trial.73 Thirty-five SAPH patients from five sites were randomized 2:1 to bosentan (n=23) or placebo (n=12), for 16 weeks. Patients were excluded for severe airway obstruction (FEV1/FVC <35% predicted), NYHA functional class IV status, left ventricular ejection fraction <35%, cardiac index < 2.0 liters/min/m2, and/or right atrial pressure > 15 mm Hg. Approximately half of the patients randomized to both groups had FVC < 60% predicted with Scadding Stage IV chest radiographs. After 16-weeks of therapy, there was a significant decrease in mPAP for those treated with bosentan [mean decrease 4 mm Hg (from 36 to 32 mm Hg)] compared with placebo [mean increase 1 mm Hg (from 30 to 31 mm Hg)], p<0.05. PVR decreased by 1.7 Wood units (from 6.1 to 4.4 Wood units) in the bosentan group compared with a 0.2 Wood unit increase (from 4.0 to 4.2 Wood units) in the placebo group, p>0.05. However, there was no improvement in functional class, quality of life, or 6MWD associated with bosentan therapy. No significant adverse events were reported.73
Ambrisentan (an ET-1 receptor antagonist) was evaluated in an open label prospective trial of 21 patients with SAPH recruited from two outpatient clinics74. Patients received ambrisentan 5 mg per day for 4 weeks, then 10 mg per day for 20 weeks. Exclusion criteria included: FVC < 40% predicted, 6MWD < 150 meters, WHO functional class IV, and left ventricular systolic dysfunction. The mean FVC, FEV1, and DLCO of those enrolled were: 62%, 59% and 33% (% predicted), respectively. After 24 weeks of treatment, there was no significant change in 6MWD, DLCO, quality of life, or dyspnea scores. However, this study was poorly powered with 11 (52%) patients discontinuing the study. Eight patients discontinued ambrisentan due to medical reasons (increased edema or dyspnea), and 3 discontinued for social reasons. Only 10 patients completed 24 weeks of therapy.74
In a recent retrospective review of 24 patients with SAPH, median survival without lung transplantation (LT) was 5.3 years 75. Eleven patients were treated with pulmonary vasodilators. More patients who died or underwent LT had moderate or severe pulmonary fibrosis (67% vs 15%), RV dysfunction (80% vs 8%), and were in WHO class IV (67% vs 31%). Mortality rates were not statistically different between patients treated with pulmonary vasodilators (54.5%) compared to untreated patients (38%, p =0.44). By Cox regression survival model, lower body surface area, RV dysfunction, and presence of moderate or severe fibrosis were predictors of worse outcomes, but treatment with pulmonary vasodilators was not predictive of mortality 75. Among 11 patients treated with pulmonary vasodilators, 6MWT improved and BNP improved during follow-up; there was a trend towards improvement in hemodynamics.
In summary, the role of PH-targeted therapy for SAPH remains controversial. Data from available studies are confounded by small sample size, lack of suitable controls, differences in the study methodology, heterogeneous patient populations, duration of treatment, and follow-up. Furthermore, the multifactorial nature of PH in sarcoidosis is problematic since PH may be caused by parenchymal fibrosis, granulomatous obliteration, and/or angitis of small vessels, hypoxic vasoconstriction, extrinsic compression of pulmonary vasculature, PVOD, and left heart failure. Inclusion of these diverse patients into a single study may add significant variability to the treatment response. We believe that a subset of patients with PH-sarcoidosis may benefit from PH-targeted therapy, but additional studies are required to determine optimal agents, appropriate recipients, and indications for therapy in this patient population.
Conclusion
PH is common among patients with advanced fibrocytic sarcoidosis, but can occur at any stage with no consistent clinical characteristics or functional parameters to predict its presence. TTE remains the screening test of choice, but a high index of suspicion is necessary given the often insidious onset and limited predictive value of any single modality. The development of PH in sarcoidosis markedly increases mortality and should prompt consideration for lung transplantation. Treatment of SAPH remains controversial due to limited data from mostly case reports and small non-randomized series. Due to the unique pathogenesis of PH in sarcoidosis, the extrapolation of studies from other WHO PH groups should be done with caution. Randomized clinical trials are necessary to determine the optimal agents and indications for therapy among patients with SAPH.
Lung transplantation for sarcoidosis
Lung transplantation (single or bilateral) is a viable therapeutic option for patients with end-stage pulmonary parenchymal sarcoidosis or SAPH 76. The International Society for Heart and Lung Transplantation (ISHLT) Registry reported that 954 patients with sarcoidosis had LT between January 1995 and June 2012 (representing 2.5 % of LTs performed globally within that time frame) 77. Among the 954 LT recipients, 265 had single lung transplants (SLT) (1.9% of all transplants) and 689 had bilateral lung transplants (BLT) (2.9% of all lung transplants).
When should patients be referred for lung transplantation?
Lung transplantation is reserved for patients with severe, chronic progressive pulmonary parenchymal or vascular disease who have failed medical therapy12. Which patients should be considered for LT is complex. Indications and contraindications for LT for sarcoidosis are generally similar to other indications 78. However, LT is not an appropriate use of resources for patients with severe extrapulmonary sarcoidosis that is disabling or life-threatening.
Assessing prognosis of sarcoid patients
In early publications, mortality rates among sarcoid patients awaiting LT were high (27–53%) 8,11,47. These high mortality rates on the waiting list likely reflect advanced disease and delayed referral for LT. However, deciding when to refer sarcoid patients for LT is difficult, as reliable models predicting mortality do not exist. Severe dysfunction on pulmonary function tests (PFTs) is associated with increased mortality among sarcoid patients 79, but no clear cutoff point for any pulmonary functional parameter predicts outcome. A retrospective review of the United Network for Organ Sharing (UNOS) database from 1995 to 2000 identified 405 patients with sarcoidosis awaiting LT in the United States; 111 (27.4%) died while awaiting LT 11. However, PFTs did not correlate with mortality. Mean % predicted FVC among survivors was 43.1% compared to 41.3% among nonsurvivors (p = 0.14). Mean % predicted FEV1 values were similar among survivors (35.8%) compared to nonsurvivors (36.7%, p = 0.48) 11. Three factors were associated with increased mortality while on the LT waiting list: 1) the presence of PH; 2) the amount of supplemental oxygen needed: 3) African-American race 11. The mPAP of survivors was 31.7 mm Hg compared to 41.4 mm Hg among nonsurvivors (p < 0.01). Mean supplemental 02 requirements were 2.2 versus 2.9 L/min among survivors and nonsurvivors, respectively (p < 0.001). In a single center study of 43 patients with sarcoidosis awaiting LT, three factors were associated with a higher mortality by univariate analysis (i.e., mPAP ≥ 35 mmHg; cardiac index < 2 L/min/m2; RAP ≥ 15 mm Hg) 47. By multivariate analysis, mRAP > 15 mm Hg was the only independent prognostic variable [5.2 fold odds ratio (OR) for mortality]. The presence of PAH is an ominous sign in sarcoidosis, and warrants referral for LT as well as consideration for aggressive medical therapy.
Results of LT in sarcoidosis
Survival for sarcoidosis patients undergoing LT is generally similar to other indications. The ISHLT Registry from January 1995 to June 2012 cited the following conditional median survival rates for patients surviving to 1 year: sarcoidosis (8.5 years); interstitial lung diseases (7.0 years); cystic fibrosis (10.5 years); idiopathic PAH (10.0 years); COPD (6.9 years) 77. Although survival rates are better with BLT compared to SLT (all indications), data are skewed by heterogeneous patient populations, differences in comorbidities and age, and other confounding factors. 77. Data regarding the risks/benefits of either SLT or BLT for sarcoidosis are lacking. However, BLT is preferred when bilateral bronchiectasis or mycetomas 80 are present. Many centers consider mycetomas to contraindicate LT, but some sarcoid patients with mycetomas have been transplanted successfully 81,82. However, in one study of 9 patients with aspergillomas (6 had sarcoidosis) who underwent LT, median survival was worse in mycetoma patients (16 months) compared to 56.7 months in LT recipients without mycetomas (p=0.003) 82. Persistent aspergillus infection has been noted post LT 82 and aggressive antifungal prophylaxis in the pre and post-LT period is essential. Most programs perform BLT among sarcoid patients with PAH, but this is based on center preference as randomized studies have not been done.
Shorr et al retrospectively reviewed UNOS data from 1995 to 2000; 30-day post-LT survival was 83% among 133 sarcoid patients compared to 91% among LT recipients for other conditions (p=0.002) 83. The adjusted odds ratio (OR) for mortality among sarcoid patients was 1.45 but this was not statistically significant (p=0.18). After controlling for health insurance and other conditions, patients with sarcoidosis were no more likely to die than persons undergoing LT for other conditions 83. Significant predictors of mortality included combined heart-lung transplant, need for mechanical ventilation, treatment in an intensive care unit (ICU) at time of LT, pre-LT FEV1, need for supplemental oxygen, and donor age. African-American recipients had nearly 50% higher mortality (adjusted OR, 1.49; p = 0.045). Recipients receiving organs from African-Americans had nearly 50% higher mortality (adjusted OR, 1.44; p = 0.008) 83. The impact of race was independent of health insurance status. Interestingly, previous studies cited worse outcomes among African-Americans receiving renal 84 or hepatic transplants 85.
Among sarcoid patients receiving SLT, complications in the native remaining lung may include: recurrent infections, bronchiectasis, pneumothorax, or mycetomas 59. Additionally, recurrent non-necrotizing granulomata have been noted in the lung allograft in up to one third of sarcoid patients 86,76, but are usually not associated with symptoms. A few cases of clinically significant recurrences have have been reported 87,76,88. In one case, a LT recipient was retransplanted for recurrent sarcoidosis that was progressive and refractory to therapy 76.
References
- 1.Cordova FC, D’Alonzo G. Sarcoidosis-associated pulmonary hypertension. Current opinion in pulmonary medicine. September 2013;19(5):531–537. [DOI] [PubMed] [Google Scholar]
- 2.Battesti JP, Georges R, Basset F, Saumon G. Chronic cor pulmonale in pulmonary sarcoidosis. Thorax. February 1978;33(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluskowski J, Hawrylkiewicz I, Zych D, Wojtczak A, Zielinski J. Pulmonary haemodynamics at rest and during exercise in patients with sarcoidosis. Respiration. 1984;46(1):26–32. [DOI] [PubMed] [Google Scholar]
- 4.Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. May 2006;129(5):1246–1252. [DOI] [PubMed] [Google Scholar]
- 5.Milman N, Burton CM, Iversen M, Videbaek R, Jensen CV, Carlsen J. Pulmonary hypertension in end-stage pulmonary sarcoidosis: therapeutic effect of sildenafil? The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. March 2008;27(3):329–334. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell DN, Scadding JG. Sarcoidosis. The American review of respiratory disease. December 1974;110(6):774–802. [DOI] [PubMed] [Google Scholar]
- 7.Rizzato G, Pezzano A, Sala G, et al. Right heart impairment in sarcoidosis: haemodynamic and echocardiographic study. Eur J Respir Dis. February 1983;64(2):121–128. [PubMed] [Google Scholar]
- 8.Shorr AF, Davies DB, Nathan SD. Outcomes for patients with sarcoidosis awaiting lung transplantation. Chest. July 2002;122(1):233–238. [DOI] [PubMed] [Google Scholar]
- 9.Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest. September 2005;128(3):1483–1489. [DOI] [PubMed] [Google Scholar]
- 10.Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. January 2006;61(1):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest. September 2003;124(3):922–928. [PubMed] [Google Scholar]
- 12.Shah L Lung transplantation in sarcoidosis. Seminars in respiratory and critical care medicine. February 2007;28(1):134–140. [DOI] [PubMed] [Google Scholar]
- 13.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. October 2009;30(20):2493–2537. [DOI] [PubMed] [Google Scholar]
- 14.Lahm T, Chakinala MM. World health organization group 5 pulmonary hypertension. Clinics in chest medicine. December 2013;34(4):753–778. [DOI] [PubMed] [Google Scholar]
- 15.Preston IR, Klinger JR, Landzberg MJ, Houtchens J, Nelson D, Hill NS. Vasoresponsiveness of sarcoidosis-associated pulmonary hypertension. Chest. September 2001;120(3):866–872. [DOI] [PubMed] [Google Scholar]
- 16.Maimon N, Salz L, Shershevsky Y, Matveychuk A, Guber A, Shitrit D. Sarcoidosis-associated pulmonary hypertension in patients with near-normal lung function. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. March 2013;17(3):406–411. [DOI] [PubMed] [Google Scholar]
- 17.Takemura T, Matsui Y, Oritsu M, et al. Pulmonary vascular involvement in sarcoidosis: granulomatous angiitis and microangiopathy in transbronchial lung biopsies. Virchows Archiv. A, Pathological anatomy and histopathology. 1991;418(4):361–368. [DOI] [PubMed] [Google Scholar]
- 18.Takemura T, Matsui Y, Saiki S, Mikami R. Pulmonary vascular involvement in sarcoidosis: a report of 40 autopsy cases. Hum Pathol. November 1992;23(11):1216–1223. [DOI] [PubMed] [Google Scholar]
- 19.Rosen Y, Moon S, Huang CT, Gourin A, Lyons HA. Granulomatous pulmonary angiitis in sarcoidosis. Archives of pathology & laboratory medicine. April 1977;101(4):170–174. [PubMed] [Google Scholar]
- 20.Carrington CB. Structure and function in sarcoidosis. Annals of the New York Academy of Sciences. 1976;278:265–283. [DOI] [PubMed] [Google Scholar]
- 21.Rosen Y Pathology of sarcoidosis. Seminars in respiratory and critical care medicine. February 2007;28(1):36–52. [DOI] [PubMed] [Google Scholar]
- 22.Shorr AF, Helman DL, Davies DB, Nathan SD. Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. The European respiratory journal. May 2005;25(5):783–788. [DOI] [PubMed] [Google Scholar]
- 23.Damuth TE, Bower JS, Cho K, Dantzker DR. Major pulmonary artery stenosis causing pulmonary hypertension in sarcoidosis. Chest. December 1980;78(6):888–891. [DOI] [PubMed] [Google Scholar]
- 24.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. American journal of respiratory and critical care medicine. February 1994;149(2 Pt 1):538–551. [DOI] [PubMed] [Google Scholar]
- 25.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. The New England journal of medicine. July 27 1995;333(4):214–221. [DOI] [PubMed] [Google Scholar]
- 26.Milman N, Svendsen CB, Iversen M, Videbaek R, Carlsen J. Sarcoidosis-associated pulmonary hypertension: acute vasoresponsiveness to inhaled nitric oxide and the relation to long-term effect of sildenafil. The clinical respiratory journal. October 2009;3(4):207–213. [DOI] [PubMed] [Google Scholar]
- 27.Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovascular research. February 1 2004;61(2):227–237. [DOI] [PubMed] [Google Scholar]
- 28.Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. The New England journal of medicine. June 17 1993;328(24):1732–1739. [DOI] [PubMed] [Google Scholar]
- 29.Sofia M, Mormile M, Faraone S, Alifano M, Carratu P, Carratu L. Endothelin-1 excretion in urine in active pulmonary sarcoidosis and in other interstitial lung diseases. Sarcoidosis. September 1995;12(2):118–123. [PubMed] [Google Scholar]
- 30.Reichenberger F, Schauer J, Kellner K, Sack U, Stiehl P, Winkler J. Different expression of endothelin in the bronchoalveolar lavage in patients with pulmonary diseases. Lung. 2001;179(3):163–174. [DOI] [PubMed] [Google Scholar]
- 31.Terashita K, Kato S, Sata M, Inoue S, Nakamura H, Tomoike H. Increased endothelin-1 levels of BAL fluid in patients with pulmonary sarcoidosis. Respirology. March 2006;11(2):145–151. [DOI] [PubMed] [Google Scholar]
- 32.Letizia C, Danese A, Reale MG, et al. Plasma levels of endothelin-1 increase in patients with sarcoidosis and fall after disease remission. Panminerva medica. December 2001;43(4):257–261. [PubMed] [Google Scholar]
- 33.Barnett CF, Bonura EJ, Nathan SD, et al. Treatment of sarcoidosis-associated pulmonary hypertension. A two-center experience. Chest. June 2009;135(6):1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baughman RP, Engel PJ, Meyer CA, Barrett AB, Lower EE. Pulmonary hypertension in sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG / World Association of Sarcoidosis and Other Granulomatous Disorders. June 2006;23(2):108–116. [PubMed] [Google Scholar]
- 35.Foley RJ, Metersky ML. Successful treatment of sarcoidosis-associated pulmonary hypertension with bosentan. Respiration. 2008;75(2):211–214. [DOI] [PubMed] [Google Scholar]
- 36.Hoffstein V, Ranganathan N, Mullen JB. Sarcoidosis simulating pulmonary veno-occlusive disease. The American review of respiratory disease. October 1986;134(4):809–811. [DOI] [PubMed] [Google Scholar]
- 37.Jones RM, Dawson A, Jenkins GH, Nicholson AG, Hansell DM, Harrison NK. Sarcoidosis-related pulmonary veno-occlusive disease presenting with recurrent haemoptysis. The European respiratory journal. August 2009;34(2):517–520. [DOI] [PubMed] [Google Scholar]
- 38.Portier F, Lerebours-Pigeonniere G, Thiberville L, et al. [Sarcoidosis simulating a pulmonary veno-occlusive disease]. Revue des maladies respiratoires. 1991;8(1):101–102. [PubMed] [Google Scholar]
- 39.Wagenvoort CA, Wagenvoort N. The pathology of pulmonary veno-occlusive disease. Virchows Archiv. A, Pathological anatomy and histology. 1974;364(1):69–79. [DOI] [PubMed] [Google Scholar]
- 40.Montani D, O’Callaghan DS, Savale L, et al. Pulmonary veno-occlusive disease: recent progress and current challenges. Respiratory medicine. July 2010;104 Suppl 1:S23–32. [DOI] [PubMed] [Google Scholar]
- 41.Roberts WC, McAllister HA Jr., Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). The American journal of medicine. July 1977;63(1):86–108. [DOI] [PubMed] [Google Scholar]
- 42.Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. June 2008;133(6):1426–1435. [DOI] [PubMed] [Google Scholar]
- 43.Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Archives of pathology & laboratory medicine. February 1995;119(2):167–172. [PubMed] [Google Scholar]
- 44.Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. March 1994;11(1):26–31. [PubMed] [Google Scholar]
- 45.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. June 30 2009;54(1 Suppl):S43–54. [DOI] [PubMed] [Google Scholar]
- 46.Bourbonnais JM, Samavati L. Clinical predictors of pulmonary hypertension in sarcoidosis. The European respiratory journal. August 2008;32(2):296–302. [DOI] [PubMed] [Google Scholar]
- 47.Arcasoy SM, Christie JD, Pochettino A, et al. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest. September 2001;120(3):873–880. [DOI] [PubMed] [Google Scholar]
- 48.Saggar R, Aboulhosn J, Belperio JA, Zisman DA, Lynch JP 3rd. Diagnosis and hemodynamic assessment of pulmonary arterial hypertension. Seminars in respiratory and critical care medicine. August 2009;30(4):399–410. [DOI] [PubMed] [Google Scholar]
- 49.Hoeper MM, Lee SH, Voswinckel R, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. December 19 2006;48(12):2546–2552. [DOI] [PubMed] [Google Scholar]
- 50.Devaraj A, Wells AU, Meister MG, Corte TJ, Hansell DM. The effect of diffuse pulmonary fibrosis on the reliability of CT signs of pulmonary hypertension. Radiology. December 2008;249(3):1042–1049. [DOI] [PubMed] [Google Scholar]
- 51.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. American journal of respiratory and critical care medicine. March 1 2003;167(5):735–740. [DOI] [PubMed] [Google Scholar]
- 52.Nathan SD, Shlobin OA, Barnett SD, et al. Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respiratory medicine. September 2008;102(9):1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zisman DA, Ross DJ, Belperio JA, et al. Prediction of pulmonary hypertension in idiopathic pulmonary fibrosis. Respiratory medicine. October 2007;101(10):2153–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. May 2011;139(5):988–993. [DOI] [PubMed] [Google Scholar]
- 55.Emirgil C, Sobol BJ, Herbert WH, Trout K. The lesser circulation in pulmonary fibrosis secondary to sarcoidosis and its relationship to respiratory function. Chest. October 1971;60(4):371–378. [DOI] [PubMed] [Google Scholar]
- 56.Baughman RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest. July 2007;132(1):207–213. [DOI] [PubMed] [Google Scholar]
- 57.Macfarlane JT. Prognosis in sarcoidosis. Br Med J (Clin Res Ed). May 26 1984;288(6430):1557–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. The New England journal of medicine. November 22 2007;357(21):2153–2165. [DOI] [PubMed] [Google Scholar]
- 59.Lynch JP 3rd, Kazerooni EA, Gay SE. Pulmonary sarcoidosis. Clinics in chest medicine. December 1997;18(4):755–785. [DOI] [PubMed] [Google Scholar]
- 60.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. American journal of respiratory and critical care medicine. August 1999;160(2):736–755. [DOI] [PubMed] [Google Scholar]
- 61.Baughman RP. Pulmonary sarcoidosis. Clinics in chest medicine. September 2004;25(3):521–530, vi. [DOI] [PubMed] [Google Scholar]
- 62.Patterson KC, Strek ME. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Annals of the American Thoracic Society. August 2013;10(4):362–370. [DOI] [PubMed] [Google Scholar]
- 63.Lynch JP 3rd, Ma YL, Koss MN, White ES. Pulmonary sarcoidosis. Seminars in respiratory and critical care medicine. February 2007;28(1):53–74. [DOI] [PubMed] [Google Scholar]
- 64.Gluskowski J, Hawrylkiewicz I, Zych D, Zielinski J. Effects of corticosteroid treatment on pulmonary haemodynamics in patients with sarcoidosis. The European respiratory journal. April 1990;3(4):403–407. [PubMed] [Google Scholar]
- 65.Pitsiou G, Papakosta D, Bouros D. Pulmonary hypertension in idiopathic pulmonary fibrosis: a review. Respiration. 2011;82(3):294–304. [DOI] [PubMed] [Google Scholar]
- 66.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. September 2007;132(3):998–1006. [DOI] [PubMed] [Google Scholar]
- 67.Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. November 2006;130(5):1481–1488. [DOI] [PubMed] [Google Scholar]
- 68.Olschewski H, Ghofrani HA, Walmrath D, et al. Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. American journal of respiratory and critical care medicine. August 1999;160(2):600–607. [DOI] [PubMed] [Google Scholar]
- 69.Olschewski H Inhaled iloprost for the treatment of pulmonary hypertension. Eur Respir Rev. March 2009;18(111):29–34. [DOI] [PubMed] [Google Scholar]
- 70.Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG / World Association of Sarcoidosis and Other Granulomatous Disorders. July 2009;26(2):110–120. [PubMed] [Google Scholar]
- 71.Baughman RP. Pulmonary hypertension associated with sarcoidosis. Arthritis Res Ther. 2007;9 Suppl 2:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma S, Kashour T, Philipp R. Secondary pulmonary arterial hypertension: treated with endothelin receptor blockade. Tex Heart Inst J. 2005;32(3):405–410. [PMC free article] [PubMed] [Google Scholar]
- 73.Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis associated pulmonary hypertension: A double-blind placebo controlled randomized trial. Chest. October 31 2013. [DOI] [PubMed] [Google Scholar]
- 74.Judson MA, Highland KB, Kwon S, et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG / World Association of Sarcoidosis and Other Granulomatous Disorders. October 2011;28(2):139–145. [PubMed] [Google Scholar]
- 75.Dobarro D, Schreiber BE, Handler C, Beynon H, Denton CP, Coghlan JG. Clinical characteristics, haemodynamics and treatment of pulmonary hypertension in sarcoidosis in a single centre, and meta-analysis of the published data. The American journal of cardiology. January 15 2013;111(2):278–285. [DOI] [PubMed] [Google Scholar]
- 76.Walker S, Mikhail G, Banner N, et al. Medium term results of lung transplantation for end stage pulmonary sarcoidosis. Thorax. April 1998;53(4):281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report--2013; focus theme: age. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. October 2013;32(10):965–978. [DOI] [PubMed] [Google Scholar]
- 78.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. July 2006;25(7):745–755. [DOI] [PubMed] [Google Scholar]
- 79.Baughman RP, Winget DB, Bowen EH, Lower EE. Predicting respiratory failure in sarcoidosis patients. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG / World Association of Sarcoidosis and Other Granulomatous Disorders. September 1997;14(2):154–158. [PubMed] [Google Scholar]
- 80.Wollschlager C, Khan F. Aspergillomas complicating sarcoidosis. A prospective study in 100 patients. Chest. October 1984;86(4):585–588. [DOI] [PubMed] [Google Scholar]
- 81.Patterson GA. Clinical-pathologic conference in general thoracic surgery: bilateral lung transplantation for sarcoidosis with aspergilloma. The Journal of thoracic and cardiovascular surgery. July 2002;124(1):171–175. [DOI] [PubMed] [Google Scholar]
- 82.Hadjiliadis D, Sporn TA, Perfect JR, Tapson VF, Davis RD, Palmer SM. Outcome of lung transplantation in patients with mycetomas. Chest. January 2002;121(1):128–134. [DOI] [PubMed] [Google Scholar]
- 83.Shorr AF, Helman DL, Davies DB, Nathan SD. Sarcoidosis, race, and short-term outcomes following lung transplantation. Chest. March 2004;125(3):990–996. [DOI] [PubMed] [Google Scholar]
- 84.Young CJ, Gaston RS. African Americans and renal transplantation: disproportionate need, limited access, and impaired outcomes. The American journal of the medical sciences. February 2002;323(2):94–99. [DOI] [PubMed] [Google Scholar]
- 85.Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of orthotopic liver transplantation: a cohort study. Lancet. January 26 2002;359(9303):287–293. [DOI] [PubMed] [Google Scholar]
- 86.Collins J, Hartman MJ, Warner TF, et al. Frequency and CT findings of recurrent disease after lung transplantation. Radiology. May 2001;219(2):503–509. [DOI] [PubMed] [Google Scholar]
- 87.Martinez FJ, Orens JB, Deeb M, Brunsting LA, Flint A, Lynch JP, 3rd. Recurrence of sarcoidosis following bilateral allogeneic lung transplantation. Chest. November 1994;106(5):1597–1599. [DOI] [PubMed] [Google Scholar]
- 88.Bjortuft O, Foerster A, Boe J, Geiran O. Single lung transplantation as treatment for end-stage pulmonary sarcoidosis: recurrence of sarcoidosis in two different lung allografts in one patient. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. Jan-Feb 1994;13(1 Pt 1):24–29. [PubMed] [Google Scholar]