Abstract
The aim of the present study was to assess the expression of epithelial-mesenchymal transition biomarkers (E-cadherin and vimentin) and their potential significance as prognostic markers in patients with stage IIIB/IV non-squamous non-small cell lung cancer (NSCLC) enrolled in the INNOVATIONS trial, receiving treatment with either erlotinib/bevacizumab (EB) or cisplatin/gemcitabine/bevacizumab (PGB). The tumor tissues of 104 patients were retrospectively analyzed using immunohistochemistry to assess the expression of E-cadherin and vimentin. The distribution between the treatment arms was 46 patients in the EB-arm and 58 in the PGB-arm. Comparing the treatment arms according to E-cadherin and vimentin expression, the analysis revealed that progression-free survival (PFS) was increased in the PGB treatment group when compared with EB treatment in patients with low expression of E-cadherin [hazard ratio (HR)=0.353; 95% confidence interval (CI) 0.189- 0.658; log-rank P=0.0007] and in those with high expression of vimentin [HR=0.276 (95% CI, 0.115- 0.659), log-rank P=0.0021]. In patients that exhibited high E-cadherin and were negative for vimentin, there was no difference in the PFS between the PGB and EB treatment groups. In conclusion, in non-squamous NSCLC with downregulated E-cadherin and upregulated vimentin, the efficacy of chemotherapy with PGB was superior compared with EB; but the same effect was not observed in patients with high E-cadherin and low vimentin. Although increased PFS was observed in patients with PGB treatment compared with EB treatment in the whole analysis populations, in the subgroup of patients with the mesenchymal phenotype, no prognostic or predictive value of either biomarker could be identified. The potential role of bevacizumab in overcoming chemotherapy resistance in the population with the mesenchymal phenotype has to be further explored.
Keywords: epithelial-mesenchymal transition, E-cadherin, vimentin, non-small cell lung cancer, prognostic biomarkers
Introduction
Metastatic non-small cell lung cancer (NSCLC) is still characterized by poor prognosis in the majority of patients. In recent years, development in molecular subtyping and discovery of predictive biomarkers has led to novel strategies using targeted therapies that have markedly improved survival rates (1–5). Additionally, checkpoint inhibition based on programmed death-ligand 1 expression as a predictive biomarker has been introduced (6). However, for adjuvant and palliative treatment, platinum-doublet chemotherapy remains an important treatment method. The lack of biomarkers that can predict a favorable response to platinum-doublet chemotherapy results in exposure to severe toxicity in many patients without measurable benefit.
Research in recent years increasingly supports the importance of epithelial-to-mesenchymal transition (EMT) in cancer. This process was first described in embryonic development and is characterized by various cellular changes, including the loss of adhesion properties and the development of a mesenchymal phenotype, which promote invasion and metastasis of tumor cells (7,8). Analysis has revealed that EMT markers are associated with survival in patients with NSCLC (9–14). Various agents that inhibit EMT pathways are currently being developed, including inhibitors of transforming growth factor-β, histone deacetylase, focal adhesion kinase and others (15). Recent research has outlined the role of EMT in antitumor immunity, proposing mechanisms of how cancer cells undergoing EMT may contribute to immune escape, resulting in resistance to immunotherapy (16). Additionally, it has been reported that specific anti-cancer therapeutics are able to increase the immunogenicity of dying cancer cells via a mechanism described as immunogenic cell death (ICD) (17).
With respect to EMT-associated markers, an epithelial phenotype is characterized by high expression of E-cadherin and the mesenchymal phenotype by high expression of vimentin, among other markers (18). The function of E-cadherin is associated with tissue integrity and adhesive properties. Changes in tissue integrity and adhesion lead to invasiveness in various cancer types; thus, EMT has an important role in the malignant properties of NSCLC and patient prognosis (13). Vimentin was originally identified in mesenchymal tissue, and has been reported to be associated with cancer progression, patient prognosis and drug resistance (19). Furthermore, vimentin expression was demonstrated to predict the occurrence of metastasis in NSCLC (20); however, studies that have investigated the role vimentin in NSCLC in vivo are rare and the findings are controversial.
In unselected patients, EMT characteristics have been demonstrated to be associated with chemotherapy resistance (21). For epidermal growth factor receptor (EGFR) wild-type patients, the EMT phenotype is potentially be associated with poor response to tyrosine kinase inhibitor (TKI) treatment (22,23). As the current study included two treatment regimens, the influence of EMT in an unselected patient population (with/without chemotherapy and multiple EGFR genotypes) was assessed.
Tumor specimens obtained from the INNOVATIONS trial were used to elucidate the potential impact of EMT on treatment efficacy with and without chemotherapy in conjunction with anti-angiogenic treatment. This trial included patients with inoperable stage IIIB/IV non-squamous NSCLC. At the time of this study, standard treatment for this population was platin-based combination chemotherapy. One of the longest survival times was achieved using the regime of carboplatin-paclitaxel plus bevacizumab, with a median progression-free survival (PFS) of 6 months and a median overall survival (OS) of 12 months (24). The INNOVATIONS study was designed to assess erlotinib/bevacizumab (EB) compared with cisplatin, gemcitabine and bevacizumab (PGB) as a first-line treatment in unselected cisplatin-eligible patients (25).
Patients and methods
Patients
All patients involved in the current study participated in the INNOVATIONS trial. Written informed consent was obtained from all patients prior to the collection and use of all samples. Inclusion criteria and the results have been reported previously (25). The study was approved by the ethics committee of each participating institution and the German regulatory body, the Paul Ehrlich Institute. The study was conducted in accordance with the Declaration of Helsinki (version 1996) and the applicable International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Good Clinical Practice guidelines. The trial was registered on clinicaltrials.gov as NCT00536640 (trial no. EUDRA-CT 2006-004865-32).
Immunostaining for E-cadherin and vimentin
For immunohistochemical analyses of E-cadherin and vimentin expression in tumor tissues, tissue microarray blocks were cut into 4 µm-thick sections and mounted onto poly-L-lysine glass slides. Tissue sections were deparaffinized with xylene, followed by rehydration with decreasing concentrations of ethanol and immersion in 3% H2O2 for 10 min to reduce endogenous peroxidase activity. Following antigen retrieval in 0.01 M sodium citrate buffer (pH=6.0), the tissue sections were incubated with mouse anti-E-cadherin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA; 1:200) or mouse anti-vimentin (Zymed; Thermo Fisher Scientific, Inc.; 1:400) in PBS overnight in a cold room. The sections were then washed three times with PBS and incubated with the corresponding secondary antibodies for 30 min at 37°C; subsequently, the sections were washed with PBS and incubated for with 3,3′-diaminobenzidine 1 min. The sections were then counterstained with hematoxylin, dehydrated, cleared and permanently mounted with mounting medium. All the procedures were performed at room temperature. Additionally, tissues stained positive for E-cadherin and vimentin were used as positive controls. Sections that were processed by replacing the primary antibody with PBS were used as negative controls.
Microscopic analysis
The degree of immunoreactivity for the proteins was evaluated semi-quantitatively on the basis of staining intensity and the proportion of positive tumor cells. Under a microscope at ×400 magnification, five fields of vision were randomly selected (with no fewer than 200 cells per field). The staining intensity was graded as follows: 0, no staining; 1, light yellow; 2, yellowish brown; and 3, brown. The positive cells were graded according to the percentage of positive cells as follows: 0, no positive tumor cells; 1, ≤10% positive tumor cells; 2, 11–50% positive tumor cells; 3, 51–80% positive tumor cells; and 4, >80% positive tumor cells. The percentage of positive cells and the staining intensity were then multiplied to generate the immunoreactivity score (Remmele and Stegner). For e-cadherin previous studies have used various different cut-offs (26). As there is no standard procedure, we estimated for our study that E-cadherin <8 is already a substantial and not incidental loss. For vimentin the respective literature states that any positive reaction for this marker is a feature of an ongoing EMT since vimentin is not expressed in the normal pulmonary epithelium (27). Based on this, the immunoreactivity was divided into two groups: Low immunoreactivity (a total score of <8) and high immunoreactivity (a total score of ≥8) for E-cadherin; negative immunoreactivity (a total score of 0) and positive immunoreactivity (a total score of >0) for vimentin. Two independent investigators who were blinded to the cases evaluated the immunohistochemical staining. When a discrepancy occurred between the two investigators, a consensus was reached via simultaneous examinations using a double-headed microscope.
Statistical analysis
All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Inc., Cary, NC, USA). Tests were two-sided, P<0.05 was considered to indicate a statistically significant difference. Time-to-event data were analyzed using the Kaplan-Meier method. Distributions between groups were compared by the unstratified log-rank test and also, for the whole groups, by the stratified log-rank test to adjust for the E-cadherin and vimentin expression status, respectively. Cox proportional hazards models were used to identify prognostic ability for PFS by testing each covariate in the model with a Wald χ2 test and estimating hazard ratios (HR) with 95% confidence intervals (CI) based on the Wald tests. Analyses performed in subgroups were not adjusted, whereas variable selection methods were applied to include baseline characteristics (Table I) relevant for the prediction performance of the model of the whole study populations (n=95 patients with E-cadherin expression, 7 observations deleted due to missing values; n=94 patients with vimentin expression, 7 observations deleted due to missing values). Furthermore, an interaction between treatment and expression status (E-cadherin and vimentin, respectively) was considered in the variable selection. Different variable selection methods (forward, backward, stepwise with a critical P-value of 0.15 for entry in and removal from the model) identified the same optimal model (minimal value of the Akaike information criterion of all models in the selection process) containing treatment, age, and Union for International Cancer Control stage for patients with E-cadherin expression. This model was also preferred by forward and stepwise variable selection for patients with vimentin expression. The interaction variable proved not statistically significant and did not meet the critical P-value for model entry in either patient population (with E-cadherin or vimentin expression).
Table I.
Patient characteristics with respect to treatment.
| Treatment arm | ||
|---|---|---|
| Characteristic | Arm A (EB) | Arm B (PGB) |
| Total patients, n | 46 | 58 |
| Age, years (range) | 61.2 (40–85) | 59.8 (41–83) |
| Sex | ||
| Male | 26 (56.5) | 34 (58.6) |
| Female | 20 (43.5) | 24 (41.4) |
| ECOG performance status | ||
| 0 | 25 (54.3) | 30 (51.7) |
| 1 | 19 (41.3) | 27 (46.6) |
| 2 | 2 (4.3) | 1 (1.7) |
| UICC stage | ||
| IIIB | 3 (6.5) | 10 (17.2) |
| IV | 43 (93.5) | 48 (82.8) |
| Histology | ||
| Adenocarcinoma | 40 (87.0) | 54 (93.1) |
| Large cell | 2 (4.3) | 2 (3.4) |
| Not specified | 4 (8.7) | 2 (3.4) |
| Smoking status | 1 (2.2) | 2 (3.4) |
| Missing | ||
| Current smoker | 14 (30.4) | 18 (31.0) |
| Former light smoker | 1 (2.2) | 4 (6.9) |
| Former smoker | 19 (41.3) | 22 (37.9) |
| Never smoked | 11 (23.9) | 12 (20.7) |
| EGFR mutation status | 3 (6.5) | 2 (3.4) |
| Missing | ||
| Mutation | 8 (17.4) | 7 (12.1) |
| Wild type | 35 (76.1) | 49 (84.5) |
| E-cadherin immunoreactivity | 1 (2.2) | 1 (1.7) |
| Missing | ||
| <8 | 24 (52.2) | 30 (51.7) |
| ≥8 | 21 (45.7) | 27 (46.6) |
| Vimentin immunoreactivity | 3 (6.5) | 0 (0) |
| Missing | ||
| 0 | 32 (69.9) | 35 (60.3) |
| >0 | 11 (23.9) | 23 (39.7) |
Data are presented as n, mean (range) or n (%). EB, erlotinib/bevacizumab; PGB, cisplatin/gemcitabine/bevacizumab; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; UICC, Union for International Cancer Control; ECOG, Eastern Cooperative Oncology Group.
Results
Patient characteristics
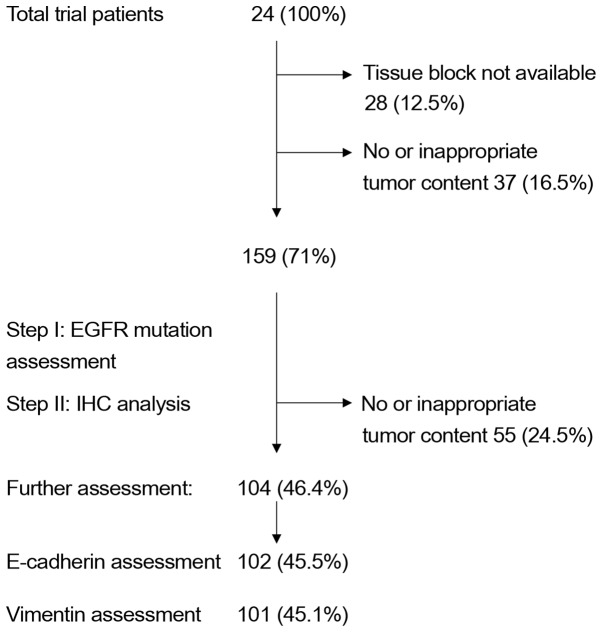
Of the 224 patients randomly assigned to one of the two treatment arms (EB or PGB) in the trial, 102 were immunohistochemically evaluated for E-cadherin expression and 101 patients for vimentin expression. In total, 104 patient samples were assessed (99 providing enough material for E-cadherin and vimentin staining, 3 for E-cadherin only and 2 for vimentin only; Table I). This drop in sample number was due to the decision to initially analyze all specimens for EGFR mutations, as reported earlier (25), and then to proceed to EMT assessment, leaving less material for further testing, due to unavailable tissue samples or no tumor tissue left in the paraffin blocks (Fig. 1).
Figure 1.
INNOVATION trial. Flow diagram of the steps associated with tissue procurement and IHC analysis. EGFR, epidermal growth factor receptor; IHC, immunohistochemistry.
Survival analysis
In the 102 patients analyzed for E-cadherin expression (high or low) the comparison of treatment arms revealed a significant difference in PFS [HR=0.449 (95% CI, 0.292–0.691), log-rank P=0.0002; PGB vs. EB treatment], and with no significant difference in OS [HR=0.686 (95% CI, 0.429–1.095), log-rank P=0.1114; data not shown]. Very similar results were obtained in the corresponding analyses for the 101 patients analyzed for vimentin expression (positive or negative): PFS [HR=0.494 (95% CI, 0.319–0.764) PGB vs. EB treatment, log-rank P=0.0012]; OS [HR=0.684 (95% CI, 0.426–1.098), log-rank P=0.1131].
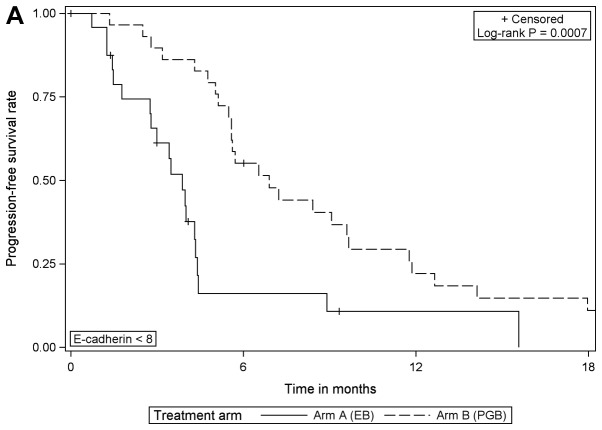
Survival analysis and E-cadherin expression
Comparing the treatment arms according to E-cadherin expression, the analysis revealed that in patients with low expression of E-cadherin, PFS was significantly increased when treated with PGB compared with EB [HR=0.353 (95% CI, 0.189–0.658), log-rank P=0.0007]; but the same effect was not detected in those with high expression of E-cadherin (Table II; Fig. 2). Furthermore, the comparison of OS between treatment arms for patients with low E-cadherin expression revealed borderline statistical significance [HR=0.530 (95% CI, 0.275–1.023), log-rank P=0.0546; EB arm median, 9.1 months; PGB arm median, 12.0 months; data not shown].
Table II.
Progression-free survival with respect to treatment and E-cadherin immunoreactivity.
| Comparison | E-cadherin immunoreactivity <8 | E-cadherin immunoreactivity ≥8 | Log-rank P-value | HRa (95% CI): E-cadherin immunoreactivity ≥8 vs. <8 | Wald chi-square P-valuea |
|---|---|---|---|---|---|
| EB arm: Median PFS in months | 3.9 | 3.0 | 0.7415 | 1.113 (0.588–2.109) | 0.7423 |
| PGB arm: Median PFS in months | 6.9 | 5.6 | 0.3924 | 1.275 (0.729–2.227) | 0.3942 |
| Log-rank P-value | 0.0007 | 0.0626 | – | – | – |
| HRa (95% CI): PGB vs. EB | 0.353 (0.189–0.658) | 0.567 (0.309–1.040) | – | – | – |
| Wald chi-square P-valuea | 0.0011 | 0.0667 | – | – | – |
Univariate Cox model. HR, hazard ratio; CI, confidence interval; EB, erlotinib/bevacizumab; PGB, cisplatin/gemcitabine/bevacizumab.
Figure 2.
Progression-free survival according to the treatment arm for (A) E-cadherin expression <8 and (B) E-cadherin ≥8. EB, erlotinib/bevacizumab; PGB, cisplatin/gemcitabine/bevacizumab.
Survival analysis and vimentin expression
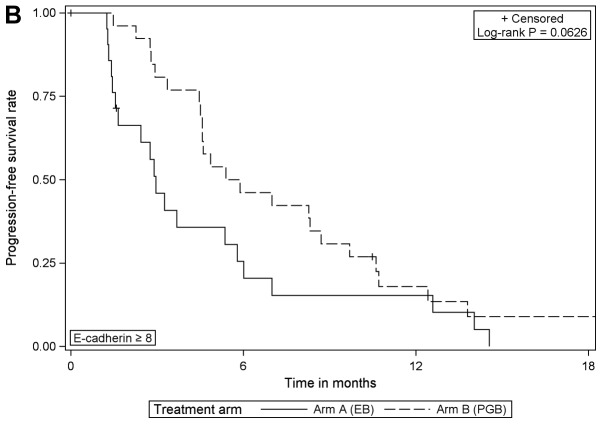
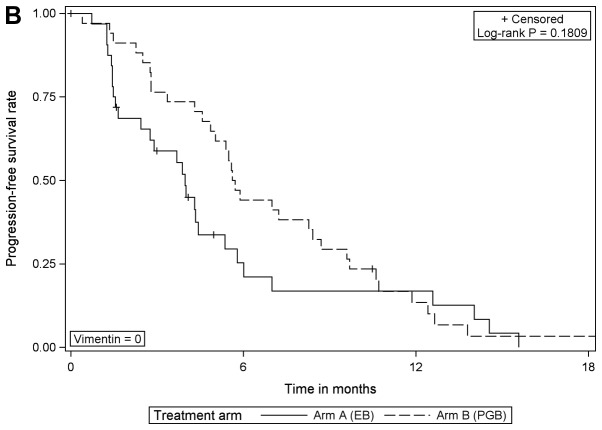
When comparing the treatment arms according to vimentin expression, the analysis revealed that PFS was significantly improved in vimentin-positive patients that received PGB treatment compared with EB [HR=0.276 (95% CI, 0.115–0.659), log-rank P=0.0021]; however, there was no difference in those that were negative for vimentin (Table III; Fig. 3). In patients with negative or with positive vimentin expression there were no statistically significant differences in OS when comparing the different treatment arms (data not shown).
Table III.
Progression-free survival with respect to treatment and vimentin immunoreactivity.
| Comparison | Vimentin immunoreactivity=0 | Vimentin immunoreactivity >0 | Log-rank P-value | HRa (95% CI): Vimentin immunoreactivity >0 vs. 0 | Wald chi-square: P-valuea |
|---|---|---|---|---|---|
| EB arm: Median PFS in months | 4.0 | 3.0 | 0.4594 | 1.336 (0.616–2.899) | 0.4630 |
| PGB arm: Median PFS in months | 5.7 | 6.9 | 0.0811 | 0.598 (0.334–1.072) | 0.0845 |
| Log-rank P-value | 0.1809 | 0.0021 | – | – | – |
| HRa (95% CI): PGB vs. EB | 0.703 (0.417–1.183) | 0.276 (0.115–0.659) | – | – | – |
| Wald chi-square P-valuea | 0.1841 | 0.0038 | – | – | – |
Univariate Cox model. PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; EB, erlotinib/bevacizumab; PGB, cisplatin/gemcitabine/bevacizumab.
Figure 3.
Progression-free survival according to the treatment arm for (A) vimentin expression=0 and (B) vimentin expression >0. EB, erlotinib/bevacizumab; PGB, cisplatin/gemcitabine/bevacizumab.
The impact of treatment on PFS all patients with E-cadherin and vimentin expression was assessed via log-rank tests comparing PFS in both treatment groups with and without adjustment for the expression status (unstratified log-rank tests, P=0.0002 E-cadherin group, P=0.0012 vimentin group; stratified log-rank tests, P=0.0003 adjusted for E-cadherin expression, P=0.0117 adjusted for vimentin expression), and in multivariable analyses of PFS (Tables IV and V). The results of interaction analyses between treatment and expression status (E-cadherin and vimentin), which were performed in the variable selection process, were not statistically significant (Tables IV and V).
Table IV.
Multivariable analysis of PFS with respect to treatment adjusted for UICC stage, age, E-cadherin immunoreactivity and interactions between treatment and E-cadherin immunoreactivity.
| A, PFS of treatment adjusted for UICC stage, and age (AIC=605.819) | ||
|---|---|---|
| Variable | HR (95% CI) | Wald chi-square P-value |
| PGB vs. EB | 0.413 (0.263–0.651) | 0.0001 |
| UICC stage IV vs. IIIB | 0.522 (0.269–1.014) | 0.0551 |
| Age (per year) | 0.980 (0.956–1.005) | 0.1114 |
| B, PFS of treatment adjusted for E-cadherin immunoreactivity and interaction between treatment and E-cadherin immunoreactivity (AIC=610.410) | ||
| Variable | HR (95% CI) | Wald chi-square P-value |
| PGB vs. EB | E-cadherin immunoreactivity <8: PGB vs. EB: 0.385 (0.205–0.722) | 0.0029 |
| E-cadherin immunoreactivity ≥8: PGB vs. EB: 0.524 (0.283–0.969) | ||
| E-cadherin immunoreactivity: ≥8 vs. <8 | EB arm: E-cadherin immunoreactivity ≥8 vs. <8: 0.904 (0.474–1.722) | 0.7583 |
| PGB arm: E-cadherin immunoreactivity ≥8 vs. <8: 1.230 (0.688–2.198) | ||
| E-cadherin immunoreactivity × treatment | – | 0.4870 |
PFS, progression-free survival; HR, hazard ratio; EB, erlotinib/bevacizumab; PGB, cisplatin/gemcitabine/bevacizumab; AIC, Akaike Information Criterion; UICC, Union for International Cancer Control.
Table V.
Multivariable analysis of progression-free survival with respect to treatment adjusted for UICC stage, age, Vimentin immunoreactivity, and interactions between treatment and Vimentin immunoreactivity.
| A, PFS of treatment adjusted for UICC stage and age (AIC=592.325) | ||
|---|---|---|
| Variable | HR (95% CI) | Wald chi-square P-value |
| PGB vs. EB | 0.458 (0.289–0.725) | 0.0009 |
| UICC stage IV vs. IIIB | 0.532 (0.274–1.034) | 0.0626 |
| Age (per year) | 0.979 (0.954–1.004) | 0.0951 |
| B, PFS of treatment adjusted for Vimentin immunoreactivity, and interactions between treatment and Vimentin immunoreactivity (AIC=594.103) | ||
| Variable | HR (95% CI) | Wald chi-square P-value |
| PGB vs. EB | Vimentin immunoreactivity=0: PGB vs. EB: 0.649 (0.382–1.105) | 0.1115 |
| Vimentin immunoreactivity >0: PGB vs. EB: 0.287 (0.123–0.668) | ||
| Vimentin immunoreactivity ≥8 vs. <8 | EB arm: Vimentin immunoreactivity >0 vs. 0: 1.337 (0.618–2.895) | 0.4610 |
| PGB arm: Vimentin immunoreactivity >0 vs. 0: 0.591 (0.321–1.087) | ||
| Vimentin immunoreactivity × treatment | – | 0.1072 |
PFS, progression-free survival; HR, hazard ratio; EB, erlotinib/bevacizumab; PGB, cisplatin/gemcitabine/bevacizumab; AIC, Akaike Information Criterion; UICC, Union for International Cancer Control.
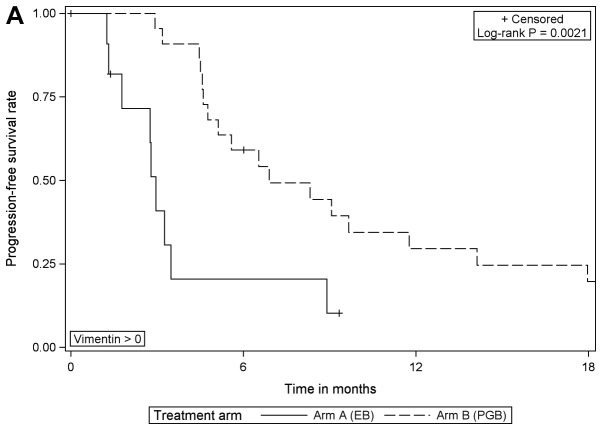
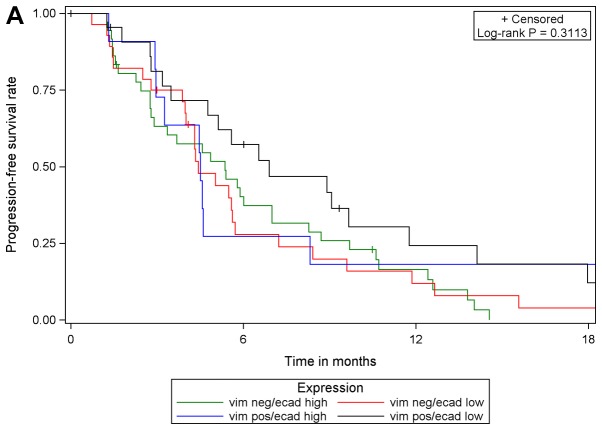
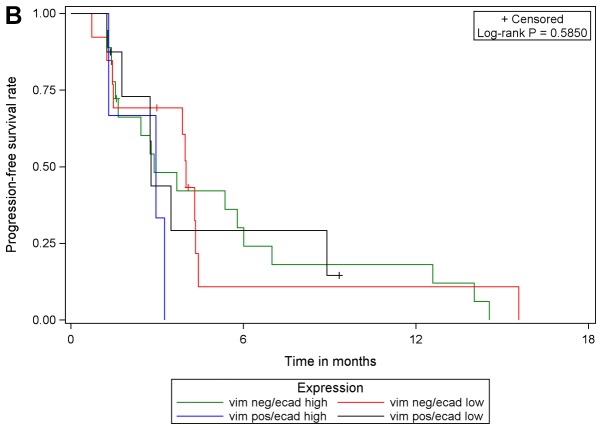
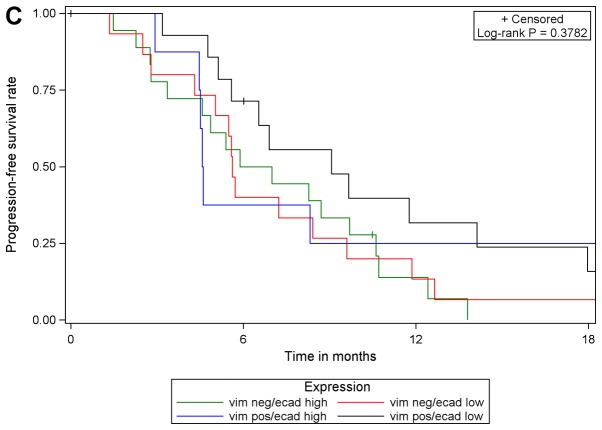
Although PGB treatment increased PFS compared with EB treatment in analysis of the whole study population [102 patients with E-cadherin expression (high or low) and 101 patients with vimentin expression (high or low)] and in subgroups of patients with low E-cadherin expression or high vimentin expression, no prognostic or predictive value of either biomarker expression was identified. In multivariable Cox regression analyses of PFS with different covariates, only the different treatment regimens had a statistically significant effect on PFS. The effect of either biomarker expression, their interaction with treatment or one another was statistically significant. These results were supported by comparing PFS in the four subgroups with respect to biomarker expression: E-cadherin low/vimentin negative, E-cadherin low/vimentin positive, E-cadherin high/vimentin negative, E-cadherin high/vimentin positive. The results of log-rank tests were not statistically significant when comparing patients with valid measurements of E-cadherin and vimentin (n=99) among the four sub-groups, irrespective of treatment and within each treatment arm (EB, n=42; PGB, n=57; Fig. 4).
Figure 4.
Progression-free survival with respect to E-cadherin/vimentin expression in (A) the total population, (B) in patients in treatment arm A (erlotinib/bevacizumab) and (C) in patients in treatment arm B (cisplatin/gemcitabine/bevacizumab). vim, vimentin; ecad, E-cadherin; pos, positive; neg, negative.
E-cadherin and vimentin association with EGFR or smoking status
Fisher's exact test was performed to determine whether the proportion of patients with different E-cadherin/vimentin expression varies with different EGFR status and smoking histories. There was no association between the biomarker expression and specific EGFR or smoking status (E-cadherin low/vimentin positive; data not shown).
Discussion
In this study, the expression of the EMT-associated markers E-cadherin and vimentin in NSCLC was semi-quantitated using immunostaining, and their value in predicting clinical outcomes in patients from the INNOVATIONS trial was evaluated. There is evidence indicating that the loss of E-cadherin predicts a poor response to treatment with EGFR inhibitors, including TKIs (erlotinib and gefitinib) and monoclonal antibodies (cetuximab) (28,29). In the study published by Ren et al (28) the epithelial phenotype was associated with a significantly higher objective response rate, longer PFS and longer OS in the wild-type EGFR subgroup. Contradictory to these findings, in the current study, there was no difference in the outcome of patients treated with EB when categorized according to epithelial phenotype (high E-cadherin or negative vimentin).
Previous studies have reported that including bevacizumab with erlotinib treatment is beneficial for patients harboring activating EGFR mutations (30,31), with relevance at least in molecularly defined subgroups (32). In the EGFR wild-type population there is currently no defined biomarker identified to predict whether EGFR TKI treatment will be beneficial. Evaluation of EMT markers in the present study addressed this question, as one of the treatment arms included the TKI erlotinib in combination with bevacizumab, without cytostatic chemotherapy. However, limitations of the present study are the non-predefined exclusion of EGFR-mutated patients and the restriction of analysis to patients with samples available for EMT assessment, resulting in reduced patient numbers. Furthermore, reflecting on histological subtype and expression of EMT markers, variations have been described, with lower E-cadherin expression in squamous cell carcinoma compared with adenocarcinoma (33).
Regarding the potential prognostic impact of EMT markers, negative or low E-cadherin expression has been associated with poor prognosis in patients with NSCLC undergoing resection (11,14); however, results are variable, potentially due to heterogeneous study conditions (use of different antibodies, immunohistochemical staining techniques and scoring systems). Thus, the clinical significance of E-cadherin expression in NSCLC remains controversial. In the present study, no prognostic impact of E-cadherin or vimentin was identified in EB-treated patients.
In an EGFR wild-type population, Garassino et al (34) reported that cytostatic treatment with docetaxel significantly improves PFS compared with EGFR TKI treatment in a second-line setting. From further trials (35,36), it has been established that the addition of vascular endothelial growth factor receptor (VEGFR) inhibition to docetaxel treatment (ramucirumab or nintedanib, respectively) improves survival in previously treated patients with advanced NSCLC.
In the current analysis, treatment with chemotherapy and bevacizumab (PGB) was superior to EB treatment in patients displaying a mesenchymal phenotype (low E-cadherin or high vimentin), but not in those with an epithelial phenotype (high E-cadherin or low vimentin). These results are contradictory to previously published data, which stated that EMT facilitates drug-resistance (21). According to the findings of the present study, it may be hypothesized that patients with a mesenchymal phenotype in particular would benefit from VEGFR inhibition (bevacizumab) in addition to combination chemotherapy (cisplatin/gemcitabine). Unfortunately, the trial did not include a comparison of cytostatic treatment with and without anti-VEGFR treatment, or even an arm containing anti-VEGFR treatment alone. This impedes more precise conclusions and should be performed in further research.
As EMT can be reversible, the role of bevacizumab as implemented in the present trial setting, or anti-angiogenesis in general, should be considered more thoroughly. In vitro data have demonstrated that VEGFR activation mediates EMT (37,38). Li et al (39) reported that the inhibitory effect of vandetanib (inhibitor of VEGFR and EGFR tyrosine kinase activities) were associated with EMT, leading to a loss of E-cadherin and gain of vimentin expression. Pretreatment with vandetanib increased cisplatin sensitivity of the tumor cells suggesting that blocking the effects of the growth factors associated with EMT may reverse chemotherapy resistance. In addition to this pathway, the concept of ICD has been introduced. Certain anticancer therapeutics may have the potential to induce ICD, maximizing antitumor immunity through this mechanism to increase the effect of a treatment (17). As none of the agents used in the INNOVATIONS trial have specifically been described as ICD-inducers previously, further research is required to explore possible associations with the findings of the current study.
To better understand the effect of bevacizumab, it may be worthwhile to design trials with anti-angiogenic treatment in conjunction with chemotherapy to assess the predictive impact of EMT markers, or even to consider stratification according to those. Furthermore, the increasing evidence for EMT-mediated tumor immune escape supports the rationale for the development of EMT-blockers to increase the response to checkpoint inhibitor immunotherapy (16).
In conclusion, even though with the inherent limitations outlined, the present study raises interest regarding the therapeutic relevance of EMT markers, the mesenchymal phenotype and the impact of anti-angiogenic treatment. Targeting EMT may offer important perspectives for the therapeutic approaches in advanced lung cancer.
Acknowledgements
Not applicable.
Funding
The INNOVATIONS trial was supported by Roche Diagnostics GmbH (Mannheim, Germany), who also provided erlotinib and bevacizumab, with an unrestricted grant (grant no. ML20262) to our sponsor ‘ABC Aktion Bronchialkarzinom e.V.’ group (Study ID, ABC-2006-NSCLC-01) as well as a grant for conducting the study and the analyses of the associated biomarker.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MV, PC, PAS and MT designed the study. MV, PC and PAS analyzed the probes (immunohistochemical examination). MV, PC and MT interpreted the results. PN and AR performed the statistical analysis. NR, JRF, SA, CK, MS, MW and MT enrolled the patients, performed the diagnostic procedures and treatments, and assisted with the implementation and execution of the trial. All authors read, reviewed and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the study are appropriately investigated and resolved.
Ethics approval and consent to participate
The present study was approved by the ethics committee of each participating institution and the German body, The Paul Ehrlich Institute. The study was conducted in accordance with the Declaration of Helsinki (version 1996) and the applicable International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Good Clinical Practice guidelines. It was registered on clinicaltrials.gov as NCT00536640 (EUDRA-CT 2006-004865-32). Written informed consent was obtained from all patients prior to the collection and use of all samples.
Patient consent for publication
Not applicable.
Competing interests
MT received an honorarium (grant) from Roche Diagnostics GmbH. All other authors declare no competing interests.
References
- 1.Duruisseaux M, Besse B, Cadranel J, Pérol M, Mennecier B, Bigay-Game L, Descourt R, Dansin E, Audigier-Valette C, Moreau L, et al. Overall survival with crizotinib and next generation ALK-inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINAKL): A French nationwide cohort retrospective study. Oncotarget. 2017;8:21903–21917. doi: 10.18632/oncotarget.15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, Giannone V, D'Amelio AM, Jr, Zhang P, Mookerjee B, Johnson BE. Dabrafenib plus trametinib in patients with previously untreated BRAF V600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 3.Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shea M, Costa DB, Rangachari D. Management of advanced non-small cell lung cancers with known mutations or rearrangements: Latest evidence and treatment approaches. Ther Adv Respir Dis. 2016;10:113–129. doi: 10.1177/1753465815617871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus Chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 7.Kupferman ME, Jiffar T, El-Naggar A, Yilmaz T, Zhou G, Xie T, Feng L, Wang J, Holsinger FC, Yu D, Myers JN. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene. 2010;29:2047–2059. doi: 10.1038/onc.2009.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdsal CA, Damsky CH, Pedersen RA. The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development. 1993;118:829–844. doi: 10.1242/dev.118.3.829. [DOI] [PubMed] [Google Scholar]
- 9.Lee YC, Wu CT, Chen CS, Chang YL. E-cadherin expression in surgically-resected non-small cell lung cancers-a clinicopathological study. Thorac Cardiovasc Surg. 2000;48:294–299. doi: 10.1055/s-2000-7885. [DOI] [PubMed] [Google Scholar]
- 10.Kase S, Sugio K, Yamazaki K, Okamoto T, Yano T, Sugimachi K. Expression of E-cadherin and ß-catenin in human non-small cell lung cancer and the clinical significance. Clin Cancer Res. 2000;6:4789–4796. [PubMed] [Google Scholar]
- 11.Choi YS, Shim YM, Kim SH, Son DS, Lee HS, Kim GY, Han J, Kim J. Prognostic significance of E-cadherin and ß-catenin in resected stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2003;24:441–449. doi: 10.1016/S1010-7940(03)00308-7. [DOI] [PubMed] [Google Scholar]
- 12.Richardon F, Young GD, Sennello R, Wolf J, Argast GM, Mercado P, Davies A, Epstein PM, Wacker B. The evaluation of E-cadherin and vimentin as biomarkers of clinical outcomes among patients with non-small cell lung cancer treated with erlotinib as second- and third-line therapy. Anticancer Res. 2012;32:537–552. [PubMed] [Google Scholar]
- 13.Soltermann A, Tischler V, Arbogast S, Braun J, Probst-Hensch N, Weder W, Moch H, Kristiansen G. Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res. 2008;14:7430–7437. doi: 10.1158/1078-0432.CCR-08-0935. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Liu J, Yue D, Gao L, Wang D, Zhang H, Wang C. Clinical significance or E-cadherin, ß-catenin, vimentin and S100A4 expression in completely resected squamous cell lung carcinoma. J Clin Pathol. 2013;66:937–945. doi: 10.1136/jclinpath-2013-201467. [DOI] [PubMed] [Google Scholar]
- 15.Voon DC, Huang RY, Jackson RA, Thiery JP. The EMT spectrum and therapeutic opportunities. Mol Oncol. 2017;11:878–891. doi: 10.1002/1878-0261.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thierry JP, Chouaib S. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11:824–846. doi: 10.1002/1878-0261.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gark AD, Dudek-Peric A, Romanio E, Agostinis P. Immunogenic cell death. Int J Dev Biol. 2015;59:131–140. doi: 10.1387/ijdb.150061pa. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Shames DS, Hasegawa Y. Emerging evidence of epithelial-to-mesenchymal transition in lung carcinogenesis. Respirology. 2012;17:1048–1059. doi: 10.1111/j.1440-1843.2012.02173.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhihua Y, Zhang X, Luo Y, Li S, Huang L, Li Z, Li P, Chen G. Prognostic values of vimentin expression and its clinicopathological significance in non-small cell lung cancer: A meta-analysis of observational studies with 4118 cases. PLoS One. 2016;11:e0163162. doi: 10.1371/journal.pone.0163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dauphin M, Barbe C, Lemaire S, Nawrocki-Raby B, Lagonotte E, Delepine G, Birembaut P, Gilles C, Polette M. Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas. Lung Cancer. 2013;81:117–122. doi: 10.1016/j.lungcan.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Sui H, Zhu L, Deng W, Li Q. Epithelial-mesenchymal transition and drug resistance: Role, molecular mechanisms and therapeutic strategies. Oncol Res Treat. 2014;37:584–589. doi: 10.1159/000367802. [DOI] [PubMed] [Google Scholar]
- 22.Yauch RL, Januario T, Eberhard DA, Cavet G, Zhu W, Fu L, Pham TQ, Soriano R, Stinson J, Seshagiri S, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;11:8686–8698. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- 23.Witta SE, Gemmill RM, Hirsch FR, Coldren CD, Hedman K, Ravdel L, Helfrich B, Dziadziuszko R, Chan DC, Sugita M, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66:944–950. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- 24.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 25.Thomas M, Fischer J, Andreas S, Kortsik C, Grah C, Serke M, von Eiff M, Witt C, Kollmeier J, Müller E, et al. Erlotinib and bevacizumab versus cisplatin, gemcitabine and bevacizumab in unselected nonsquamous nonsmall cell lung cancer. Eur Respir J. 2015;46:219–229. doi: 10.1183/09031936.00229014. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Liu H, Ding M, Liu J, Zhan P, Fu X, Gan L. The impact of E-cadherin expression on non-small cell lung cancer survival: A meta-analysis. Mol Biol Rep. 2012;39:9621–9628. doi: 10.1007/s11033-012-1827-1. [DOI] [PubMed] [Google Scholar]
- 27.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren S, Su C, Wang Z, Li J, Fan L, Li B, Li X, Zhao C, Wu C, Hou L, et al. Epithelial phenotype as a predictive marker for response to EGFR-TKIs in non-small cell lung cancer patients with wild-type EGFR. Int J Cancer. 2014;135:2962–2971. doi: 10.1002/ijc.28925. [DOI] [PubMed] [Google Scholar]
- 29.Nikolova DA, Asangani IA, Nelson LD, Hughes DP, Siwak DR, Mills GB, Harms A, Buchholz E, Pilz LR, Manegold C, Allgayer H. Cetuximab attenuates metastasis and u-PAR expression in non-small cell lung cancer: u-PAR and E-cadherin are novel biomarkers of cetuximab sensitivity. Cancer Res. 2009;69:2461–2470. doi: 10.1158/0008-5472.CAN-08-3236. [DOI] [PubMed] [Google Scholar]
- 30.Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, Vlahovic G, Soh CH, O'Connor P, Hainsworth J. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): A double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377:1846–1854. doi: 10.1016/S0140-6736(11)60545-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosorni Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): An open-label, randomized, mutlicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 32.Rosell R, Dafni U, Felip E, Curioni-Fontecedro A, Gautschi O, Peters S, Massuti B, Palmero R, Aix SP, Carcereny E, et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): An international, multicentre, single-arm, phase 2 trial. Lancet Respir Med. 2017;5:435–444. doi: 10.1016/S2213-2600(17)30129-7. [DOI] [PubMed] [Google Scholar]
- 33.Prudkin L, Liu DD, Ozburn NC, Sun M, Behrens C, Tang X, Brown KC, Bekele BN, Moran C, Wistuba II. Epithelial-to-mesenchymal transition in the development and progression of adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol. 2009;22:668–678. doi: 10.1038/modpathol.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garassino MC, Martelli O, Broggini M, Farina G, Veronese S, Rulli E, Bianchi F, Bettini A, Longo F, Moscetti L, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): A randomized controlled trial. Lancet Oncol. 2013;14:981–988. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 35.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomized phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 36.Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomized controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 37.Fantozzi A, Gruber DC, Pisarsky L, Heck C, Kunita A, Yilmaz M, Meyer-Schaller N, Cornille K, Hopfer U, Bentires-Alj M, Christofori G. VEGF-mediated angiogenesis links EMT-induced cancer stemness to tumor initiation. Cancer Res. 2014;74:1566–1575. doi: 10.1158/0008-5472.CAN-13-1641. [DOI] [PubMed] [Google Scholar]
- 38.Yi ZY, Feng LJ, Xiang Z, Yao H. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in hepatocellular carcinoma cells. J Invest Surg. 2011;24:67–76. doi: 10.3109/08941939.2010.542272. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Yang X, Su LJ, Flaig TW. VEGFR and EGFR inhibition increases epithelial cellular characteristics and chemotherapy sensitivity in mesenchymal bladder cancer cell. Oncol Rep. 2010;24:1019–1028. doi: 10.3892/or.2010.1019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.