Abstract
Breast cancer is the leading cause of female mortality worldwide. Although there are several modern treatments for breast cancer, there is a high rate of recurrence for the majority of treatments; therefore, the search for effective anticancer agents continues. The present study aimed to investigate the anti-breast cancer potential of frullanolide, a compound which is isolated and purified from the Grangea maderaspatana plant, for selected human breast cancer cell lines (MCF-7, MDA-MB-468 and MDA-MB-231). The MTT assay was used to assess cytotoxic activity in breast cancer cell lines of treatment with frullanolide at 1.25, 2.5, 5.0, 10.0 and 20.0 µg/ml. Additionally, the apoptotic induction ability of frullanolide at various concentrations [0.5×, 1× and 2× half maximal inhibitory concentration (IC50)] was investigated by flow cytometry and western blot analysis. Frullanolide exhibited strong anti-breast cancer activity against MDA-MB-468 (IC50, 8.04±2.69 µg/ml) and weak cytotoxicity against the MCF-7 (IC50, 10.74±0.86 µg/ml) and MDA-MB-231 (IC50, 12.36±0.31 µg/ml) cell lines. The IC50 of frullanolide was high in the human normal epithelial breast cell line (MCF-12A) and mouse fibroblast cell line (L-929). Density plot diagrams revealed that frullanolide induced apoptosis in MCF-7, MDA-MB-468 and MDA-MB-231 cells. Notably, a plausible anticancer mechanism was elucidated via cellular apoptosis by p53-independence in the treated MCF-7 cell line and p53-dependence in the treated MDA-MB-468 and MDA-MB-231 cell lines. In conclusion, the present study demonstrated that frullanolide may exert anticancer activity on breast cancer cell lines by inducing apoptosis. Frullanolide offers a possible novel approach to breast cancer therapy.
Keywords: breast cancer cell lines, apoptosis, natural compounds, frullanolide, sesquiterpene lactone
Introduction
Cancer is one of the leading causes of human mortality worldwide, with an estimated 14 million new cancer cases projected for 2030 (1). In women, breast cancer is quickly becoming the leading cause of mortality worldwide, and novel therapeutic avenues are constantly being explored (2). One such line of investigation involves evaluating natural products extracted from plants and endophytic fungi, such as vincristine and vinblastine, which have been demonstrated to exhibit anticancer activities, including the inhibition of breast cancer cell growth (3,4). Studies are continuously being conducted in the search for novel effective and nontoxic anticancer compounds from various medicinal plants.
The genus Grangea belongs to the Compositae (Asteraceae) family and comprises only six species, which are mostly native and distributed throughout Africa, South Asia and Southeast Asia (5,6). G. maderaspatana (L.) Poir. (Phayaa Mutti) is one of the most common medicinal plants used in traditional Thai medicine in various therapeutic approaches, including ingestion of the whole plant to stimulate digestion, reduce pain and inflammation, and regulate menses, while the leaf is used for reducing spasms (7). Although anesthetic, antioxidant, antibacterial and topoisomerase I and II (Top I and II) inhibitory activities have been reported previously for compounds derived from G. maderaspatana (8–12), to the best of our knowledge, no previous studies have assessed whether these compounds possess anticancer activity.
A previous study in our laboratory revealed the presence of sesquiterpene lactones (SLs) in G. maderaspatana (12). SLs are compounds which have several significant cancer-associated implications and are used in targeted therapy against cancer cells, their stem cells and specific signaling pathways (13). Various SLs, including thapsigargin, artemisinin and parthenolide, have been demonstrated to exhibit potent action against certain types of cancer (13). SL extracts, known as eudesmanolides, derived from frullanolide have been demonstrated to possess anticancer activity in oral, non-small cell lung and breast cancer cell lines (12). However, no scientific studies have been conducted concerning frullanolide and its anticancer activities or its cytotoxic mechanisms against breast cancer cells. Therefore, in the present study, the cytotoxic effects of frullanolide and its mode of action on breast cancer cell inhibition were explored.
Materials and methods
Cell cultures
The breast cancer cell lines MCF-7 (HTB-22™), MDA-MB-468 (HTB-132™) and MDA-MB-231 (HTB-26™), and a normal epithelial breast cell line (MCF-12A; CRL-10782™) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA), and maintained at the Department of Biomedical Sciences, Faculty of Medicine, Prince of Songkla University (Hat Yai, Thailand). The mouse fibroblast L-929 (CCL-1™; ATCC) cell line was provided by Professor Teerapol Srichana, Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Prince of Songkla University. The culture conditions of the cell lines followed methods described previously (14). Briefly, MCF-7 cells were grown in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and the MDA-MB-468, MDA-MB-231 and L-929 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc). The cultures were supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), with 100 U/ml of penicillin and streptomycin. The MCF-12A cell line was cultured in DMEM and Ham's F12 medium (GE Healthcare, Chicago, IL, USA), supplemented with 100 ng/ml cholera toxin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 500 ng/ml hydrocortisone (Sigma-Aldrich; Merck KGaA), 0.01 mg/ml bovine insulin (Sigma-Aldrich; Merck KGaA), 20 ng/ml human epidermal growth factor (Invitrogen; Thermo Fisher Scientific, Inc.) and 5% horse serum (Invitrogen; Thermo Fisher Scientific, Inc.). All cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Plant material and isolation
Dried G. maderaspatana (L.) Poir plants were purchased from the Chaokromper Drug Store (Bangkok, Thailand). Their identity was confirmed by Dr Nijsiri Ruangrungsri, Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Chulalongkorn University (Bangkok, Thailand). A voucher specimen (no. 5182) was deposited at the Museum of Natural Medicine, Chulalongkorn University. The dried whole plant materials were ground to coarse powder using a mortar and pestle. The powder was then stored at room temperature (RT) prior to extraction. Crude compounds were extracted with dichloromethane from a powder weight of 1,500 g. The extract was evaporated under vacuum at 55°C, fractionated by silica gel column chromatography (silica gel no. 9385) and then eluted with different gradients of hexane-ethyl acetate 10:0 to 0:10 solvent systems, resulting in 132.2 mg of a compound resembling white needles (Rf 0.85, silica gel CH2Cl2-Acetone 9:1). The structure of the compound was elucidated by 1H, 13C nuclear magnetic resonance spectroscopy and mass spectrometry. Its physical and spectral data were compared with previous reports (9,12). The compound was identified as the sesquiterpene lactone, frullanolide (Fig. 1), with the chemical formula C15H20O2 (parent peak at m/z 232, colorless solid). The compound was stored at −20°C prior to testing with cancer cells.
Figure 1.
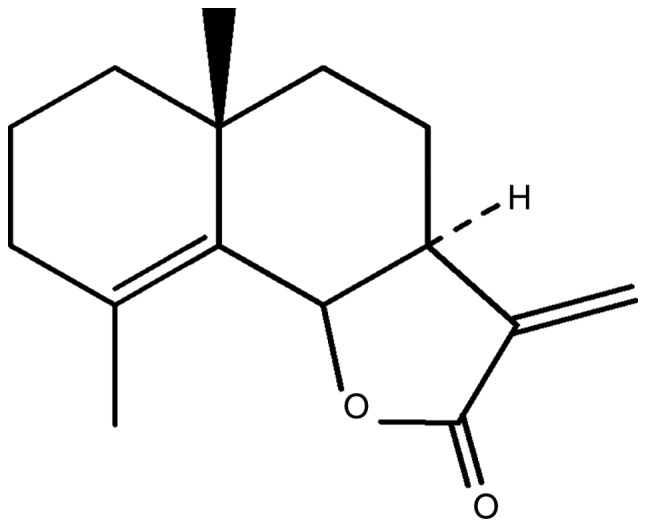
Chemical structure (C15H20O2) of sesquiterpene lactone (frullanolide) isolated from whole plant material of G. maderaspatana (L.) Poir.
MTT assay
All cell lines were seeded in 96-well plates at a density of 2×104 cells/well in 100 µl culture medium/well. The cells were treated with different concentrations of the dimethyl sulfoxide (DMSO)-dissolved frullanolide compound (1.25, 2.5, 5.0, 10.0 and 20.0 µg/ml). After 72 h of incubation at 37°C, 100 µl MTT reagent (0.5 mg/ml) was added to each well and the cultures were incubated for an additional 30 min. The MTT reagent was removed and replaced by DMSO to ensure that solubilization was complete. Absorbance at 570 and 650 nm (reference wavelengths) was measured on a microplate reader. Half-maximal inhibitory concentration (IC50) values were calculated from fitted response curves of the concentration and viability (%). Determination of cytotoxic activity followed the criterion; <5 µg/ml represented highly active; 5–10 µg/ml represented strongly active; and >10 represented weak cytotoxicity (15,16). Normal breast cells (MCF-12A) and L-929 fibroblasts were treated using the aforementioned procedure. Selectivity index (SI) values, indicating selectivity for tested cell lines, were calculated from the ratio of IC50 values of the compounds obtained for normal vs. cancer cells. An SI score >3 represented good selectivity (17). This experiment was performed in triplicate.
Flow cytometry for cell cycle analysis and apoptosis detection
The MCF-7 and MDA-MB-468 cells were seeded at densities of 4×105 cells/well in 12-well plates. The MDA-MB-231 cells were seeded at 3×105 cells/well. The cells were treated for 24 h with three concentrations of frullanolide (0.5×, 1× and 2× IC50). Following removal of the compound, the treated cells were fixed with 70% ethanol at 4°C for 4 h and washed three times with cold PBS. Harvesting and fluorochrome binding of the cells were carried out as described previously (2). For cell cycle analysis, the fixed cells were resuspended in 400 µl propidium iodide (PI; 50 µg/ml) solution/1×106 cells and incubated at RT for 5–10 min in the dark. A total of 5,000 cells were analyzed for each condition with a fluorescence-activated cell sorting (FACS) Calibur flow cytometer (BD Biosciences, San Jose, CA, USA), and histograms of cell population ratios in each phase of the cell cycle were acquired. For apoptosis detection, the apoptotic cells in the treated conditions were stained with Annexin V-fluorescein isothiocyanate (FITC)/PI following the manufacturer's protocol (FITC Annexin V Apoptosis Detection kit I; BD Pharmingen™, BD Biosciences). Dot plot graphs of the apoptotic cell ratios were created. All data were analyzed using WinMDI v.2.9 software (J. Trotter, The Scripps Institute, La Jolla, CA, USA).
Western blot analysis
Untreated cells and cells treated with frullanolide at 0.5× IC50, were harvested at 0, 12, 24 and 48 h. The cells were lysed in radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, Waltham, MA, USA). Protein samples were quantitated by Bradford Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Total proteins (50 µg) of each sample were run separately on a 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane in glycine-methanol buffer at 4°C (100 V, 2 h). The membranes were blocked in 5% low-fat milk in TBS with 0.1% Tween-20 (TBS-T) at RT for 1 h. Subsequently, the blots were incubated at 4°C overnight with primary antibodies; B cell lymphoma 2-associated X protein (Bax; cat no. 5023; 1:1,000 dilution), p21 (cat no. 2947; 1:1,000 dilution), p53 (cat no. 9282; 1:1,000 dilution) and β-actin (cat no. 4967; 1:1,000 dilution), in 1% low-fat milk in TBS-T. All antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The blots were then washed 3 times (5 min each) and incubated with enhanced chemiluminescence (ECL) anti-rabbit immunoglobulin G horseradish peroxidase (cat no. PKNA934; GE Healthcare Life Sciences, Little Chalfont, UK) diluted to 1:5,000 in 1% low-fat milk in TBS-T at RT for 1 h. The protein bands were detected using an ECL chemiluminescent detection kit (Thermo Fisher Scientific, Inc.). Due to the limited amount of frullanolide compound available, each treatment was performed in triplicate. The triplicate samples were pooled prior to protein lysate preparation and western blotting.
Statistical analysis
The MTT results are presented as the mean ± standard deviation. To evaluate the difference between the cell lines, one-way analysis of variance (ANOVA) with Brown-Forsythe correction was performed, then IC50 values of the cancerous cell lines were compared with MCF-12A cells using one-sided Dunnett's post hoc tests. Statistical analysis was performed with SPSS 20.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Cytotoxic activity of frullanolide
The IC50 values of frullanolide for the breast cancer cell lines MCF-7, MDA-MB-468 and MDA-MB-231 were 10.74±0.86, 8.04±2.69 and 12.36±0.31 µg/ml, respectively. The IC50 value for normal breast cells (MCF-12A) was 28.65±6.57 and 19.07±7.16 µg/ml for the fibroblast L-929 cell line. One-way ANOVA indicated significantly different values among the groups. It was tested whether the compound had lower IC50 values in the cancerous cell lines than when applied to MCF-12A cells. In cancerous cell lines, frullanolide had significantly lower IC50 values compared with MCF-12A, whereas the L-929 fibroblast cell line did not exhibit a significant difference. The results indicated a strong anti-proliferative effect of frullanolide on the breast cancer cell lines, but less of an effect on normal breast and fibroblast cell lines (Table I). The SI, which indicates the safety level of a compound toward normal breast cells, indicated that frullanolide has high cytotoxic activity against MDA-MB-468 (SI=3.56) but was less harmful to normal breast cells.
Table I.
Cytotoxic activity (IC50) of frullanolide purified from whole plant of G. maderaspatana exhibits differing dosage dependence in MCF-7, MDA-MB-468, MDA-MB-231, MCF-12A and L929 cell lines.
| Cell line | IC50a, µg/ml (µM) | Cytotoxic activity | SIb | SI activity |
|---|---|---|---|---|
| MCF-7 | 10.74±0.86c (46.23) | Weak cytotoxicity | 2.67 | Less selectivity |
| MDA-MB-468 | 8.04±2.69c (34.61) | Strongly active | 3.56 | High selectivity |
| MDA-MB-231 | 12.36±0.31c (53.20) | Weak cytotoxicity | 2.32 | Less selectivity |
| MCF-12A | 28.65±6.57 (123.32) | Weak cytotoxicity | None | None |
| L929 | 19.07±7.16 (82.08) | Weak cytotoxicity | None | None |
IC50 values are presented as the mean ± standard deviation.
Activity scores (IC50 µg/ml): <5=highly active; 5–10=strongly active; >10=weak cytotoxicity, considered cytotoxic against human breast cancer MCF-7, MDA-MB-468 and MDA-MB-231 cell lines (15,16)
SI>3=good selectivity (17), SI activity against the epithelial normal breast MCF-12A cell line
P<0.05 for independent IC50 values of cancer cells compared with normal cell line (MCF-12A). IC50, half-maximal inhibitory concentration; SI, selective index.
Cell cycle arrest of frullanolide
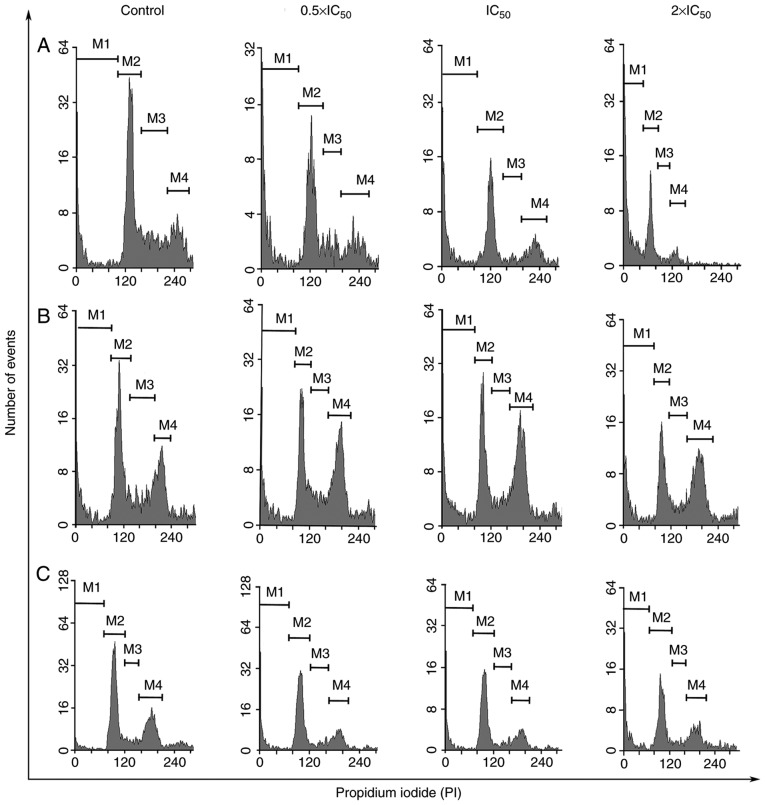
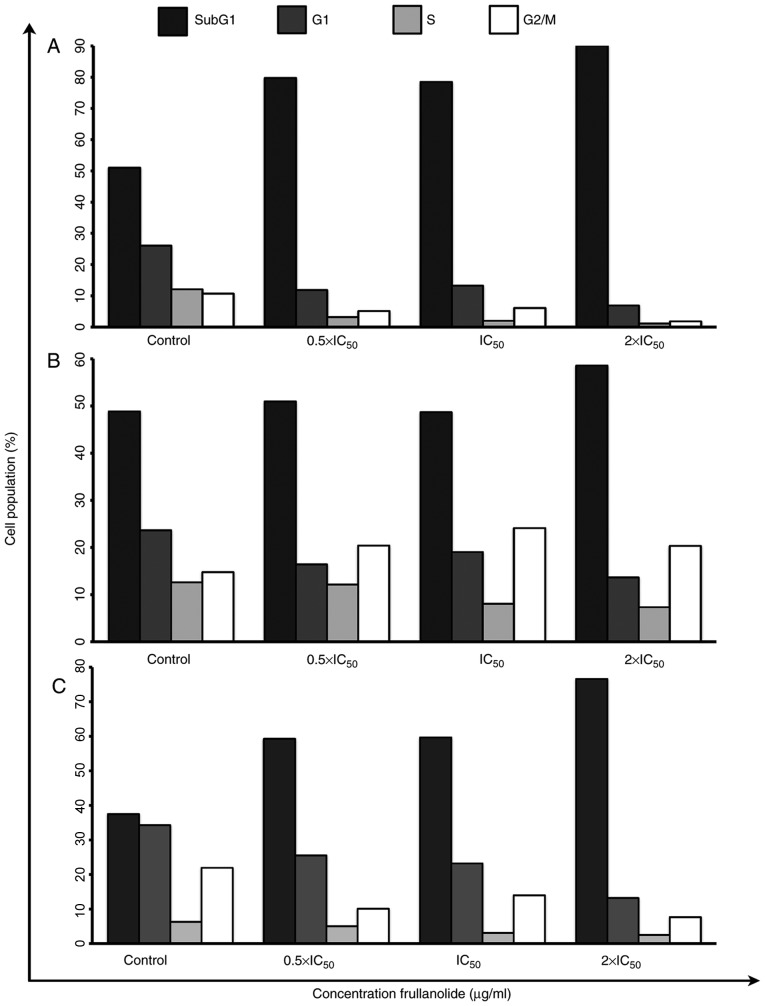
Cell cycle arrest was monitored using flow cytometry (Figs. 2 and 3). Following staining of the nuclei with PI, the FACS analysis revealed that frullanolide induced an increased proportion of SubG1 cell debris for the MCF-7, MDA-MB-468 and MDA-MB-231 cell lines in a dose-dependent manner. The percentage of SubG1 cells in the MCF-7 cell line increased from 79.77 at 0.5×IC50 to 90.10% at 2×IC50, compared with 51.05% in untreated cells (Figs. 2A and 3A). There was also an increase in SubG1 cells in MDA-MB-468, from 50.97 at 0.5×IC50 to 58.60% at 2×IC50 dosage (Figs. 2B and 3B). Additionally, the SubG1 proportion of the MDA-MB-231 cell line increased from 59.31 at 0.5×IC50 to 76.62% at 2×IC50 dose levels, compared with 37.47% in untreated cells (Figs. 2C and 3C). The cell proportions in the G1, S and G2/M phases in the histograms of the MCF-7 treated cells tended to decrease in a dose-dependent manner, from 11.87 to 6.91 (G1), 3.22 to 1.14 (S) and 5.14 to 1.85% (G2/M) (Figs. 2A and 3A). The distribution of MDA-MB-468 cells among the phases also changed in a dose-dependent manner (Fig. 2B). For MDA-MB-468, the number of treated cells in the G1 and S phases gradually decreased until the highest dosage (2×IC50). Conversely, cells appeared to accumulate in the G2/M phase in treated cells (20.43, 24.13 and 20.37% at 0.5×, 1× and 2× IC50, respectively), compared with untreated cells (14.81%; Fig. 3B). Notably, the G1, S and G2/M phases of the MDA-MB-231 cell cycles slightly decreased in a dose-dependent manner from 0.5× to 2×IC50 dose levels (G1, 25.53 to 13.19; S, 5.06 to 2.52; and G2/M, 10.10 to 7.68%) as shown in Figs. 2C and 3C. These results indicated that frullanolide was associated with cell cycle arrest at G2/M in MDA-MB-468 but not in the MCF-7 and MDA-MB-231 cell lines.
Figure 2.
Cell cycle distribution of breast cancer cell lines following frullanolide treatments (0.5×, 1× and 2×IC50) using flow cytometry analysis. Histograms of PI-labelled DNA content of each cell cycle phase in untreated and treated (A) MCF-7, (B) MDA-MB-468 and (C) MDA-MB-231 cells, respectively. M1, M2, M3, M4 in each histogram designate the number of cells in the respective SubG1, G1, S and G2/M phases. IC50, half-maximal inhibitory concentration; PI, propidium iodide.
Figure 3.
Alterations in the number of cells (%) in each stage (SubG1, G1, S and G2/M) in untreated and treated breast cancer cell lines (0.5×, 1× and 2×IC50 of frullanolide). The quantitative data obtained from the Fluorescence Activated Cell Sorter assay represent different cells in each cell division in treated (A) MCF-7, (B) MDA-MB-468 and (C) MDA-MB-231 cells. IC50, half-maximal inhibitory concentration.
Apoptosis induction of frullanolide
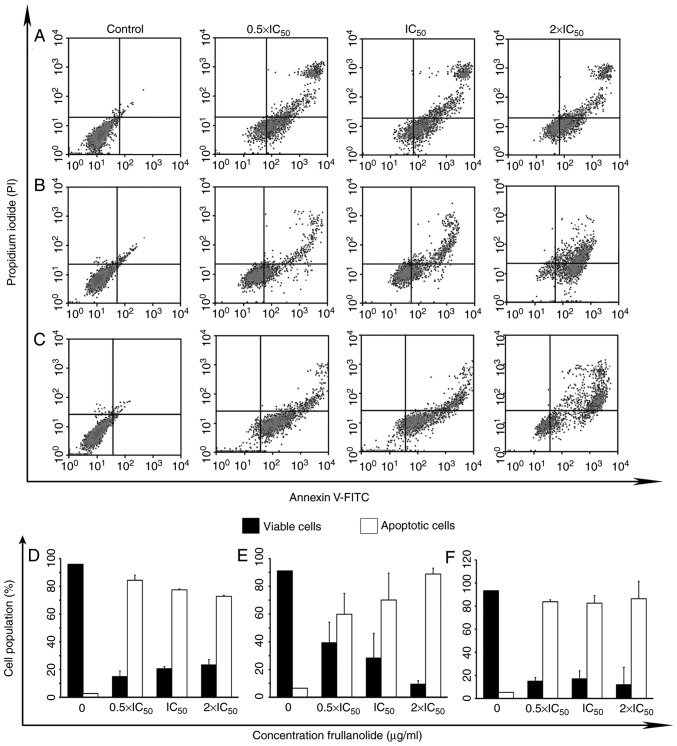
To assess the apoptotic action of frullanolide, Annexin V-FITC/PI was used to stain treated cells. A density plot was created based on the data obtained from the FACS analysis. The plot was divided into four quadrants (Fig. 4A-C), with the lower left quadrant presenting viable cells (Annexin V-negative, PI-negative), the lower right quadrant presenting cells that underwent early apoptosis (Annexin V-positive, PI-negative), the upper right quadrant presenting cells which underwent late apoptosis (Annexin V-positive, PI-positive), and the upper left presenting necrotic cells (Annexin V-negative, PI-positive). After 24 h of treatment of MCF-7 cells, the lowest viable counts along with the highest apoptotic counts (early and late apoptosis) were identified at the 0.5×IC50 dose level (14.97 and 84.38%, respectively; Fig. 4A and D). These results indicated that frullanolide may be effective in MCF-7 cells at different dose levels, particularly at a low dose level (0.5×IC50). Conversely, frullanolide treatment of MDA-MB-468 cells resulted in the lowest number of viable cells (11.96%) and the highest number of apoptotic cells (86.38%) at the highest dosage (2×IC50; Fig. 4B and E). Although no significant differences were observed for the MDA-MB-231 cells regarding the number of viable and apoptotic cells among all treatment conditions, treatment exhibited effective cytotoxic activity compared with the control (Fig. 4C and F). Therefore, the data indicated that frullanolide may induce apoptosis in breast cancer cells at all tested concentrations.
Figure 4.
Density plots of frullanolide-induced apoptosis. (A) MCF-7, (B) MDA-MB-468 and (C) MDA-MB-231 cells treated with frullanolide at 0.5×, 1× and 2×IC50. The density plots show four quadrants; the lower left, lower right, upper right and upper left quadrants represent viable, early apoptotic, late apoptotic and necrotic cells, respectively. The percentages of cell populations indicate viable cells (black column) and total apoptotic cells (white column) for the (D) MCF-7, (E) MDA-MB-468 and (F) MDA-MB-231 cell lines. Error bars represent the data obtained from treated breast cancer cells in two independent experiments. FITC, fluorescein isothiocyanate; IC50, half-maximal inhibitory concentration; PI, propidium iodide.
Protein expression by western blot analysis
To elucidate the possible mechanisms of apoptotic induction by frullanolide, the expression levels of three proteins (Bax, p21 and p53) were investigated by western blot analysis at 0.5×IC50, for 12, 24 and 48 h in the three breast cancer cell lines. The overall effect of the frullanolide treatment in these breast cancer cell lines on detected protein expression is presented in Fig. 5. For all cell types treated with 0.5×IC50 frullanolide, Bax protein expression decreased between 12 and 24 h. Its expression was prone to accumulation in treated MCF-7 and MDA-MB-231 cells at 48 h (Fig. 5A, C, J and L). However, expression levels of Bax protein increased at 12 h in treated MDA-MB-468 cells and its expression gradually reduced at 24–48 h (Fig. 5K). Elevated p21 expression was noted at 12 h, and gradually decreased between 24 and 48 h in all three breast cancer cell lines. The p21 expression was upregulated when compared with the controls (Fig. 5G-I). Notably, p53 protein was expressed differentially depending on the cell type. p53 expression was undetectable in MCF-7 cells (Fig. 5A and D), while distinct adverse alterations were identified in the treated MDA-MB-468 and MDA-MB-231 cells (Fig. 5E and F). The p53 protein levels in frullanolide-treated MDA-MB-468 cells increased at 12–24 h, and immediately declined by ~0.80-fold until the end of exposure (24–48 h) when compared with the control group (Fig. 5E). Therefore, it appeared conclusive that the frullanolide mechanism was involved in apoptotic pathways.
Figure 5.
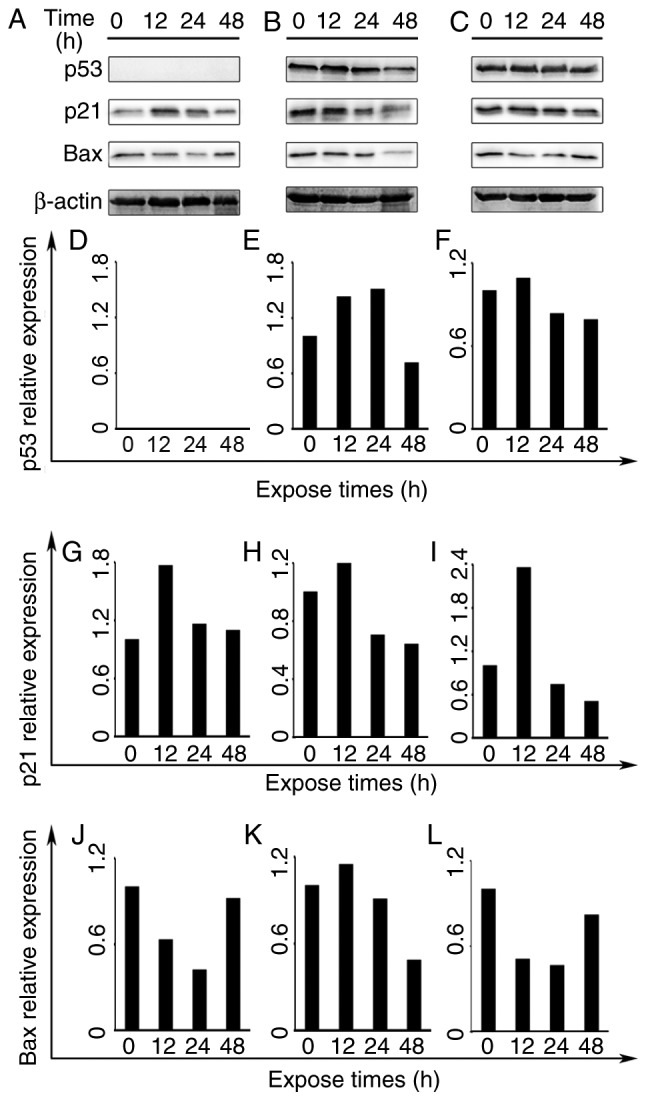
The expression of apoptosis-associated proteins in treated breast cancer cell lines. (A) MCF-7, (B) MDA-MB-468 and (C) MDA-MB-231 cells treated with 0.5× IC50 frullanolide for 12, 24 and 48 h. The bar charts represent the relative expression of (D-F) p53, (G-I) p21 and (J-L) Bax proteins at the indicated times in all frullanolide treated-breast cancer cell lines. Bax, B cell lymphoma 2 associated X protein; IC50, half-maximal inhibitory concentration.
Discussion
SLs, derived from a natural product and a subfamily of terpenoids, have been reported to exhibit numerous potential medicinal properties, including anti-inflammatory, antimicrobial and anticancer actions, in in vitro and in vivo studies, as well as clinical trials (13,18–20). More than 5,000 SLs have been identified from Asteraceae spp. (18), and ~1,500 publications have reported on their anti-inflammatory and anticancer properties (13). In vitro cytotoxicity screening for antitumor agents has been widely employed in various types of cancer cell lines in the search for novel anticancer drugs. A number of potentially active compounds have been identified through a large-scale screening program based on the US National Cancer Institute criteria (21). Purified and crude compounds of one of these anticancer agents were reported to possess strong antitumor activity, with IC50 values of ≤4 and ≤20 µg/ml, respectively (21). Several studies have identified specific SLs and derivatives which exhibit broad-spectrum antitumor activity towards several cancer types, including non-small cell lung cancer, colorectal cancer, leukemia, laryngeal cancer, gynecological cancer and breast cancer (13,22–27). The different anticancer abilities of SLs are associated with their carbocyclic skeleton classification (13,28). Eudesmanolides (6/6-bicyclic compounds) a subgroup of SLs, have been reported in several studies to exhibit anticancer action (29–34). For example, the eudesmane skeleton (santamarine) has been revealed to exhibit potential anticancer properties toward the L1210 murine (IC50=0.41 µg/ml), CCRF-CEM human leukemia (IC50=0.59 µg/ml), KB human nasopharyngeal (IC50=0.16 µg/ml), LS174T human colon carcinoma (IC50=0.92 µg/ml) and MCF-7 breast adenocarcinoma (IC50=0.53 µg/ml) cell lines (29). Li et al (30), studied the efficacy of eudesmane-based santamarine against a number of gynecological cancer cell lines, and revealed that HeLa ovarian cancer and SHIN3 cervical cancer cell line viabilities are decreased by 50% following respective treatments of 2.60 and 3.08 µg/ml santamarine for 48 h, while the HOC-21 and HAC-2 ovarian cancer, and HEC-1 endometrial cancer cell lines had some tolerance (IC50>10 µg/ml) (31). Similarly, the cytotoxic activity of a eudesmanolide compound [15-hydroxy-eudesm-4,11(13)-diene-12-oic acid] is potent toward a panel of human cancer cell lines (PC prostate cancer, HT29 colon cancer, MCF-7 breast cancer and A549 lung cancer) with IC50 of 5.8±0.2, 5.8±0.2, 6.8±0.4 and 69.6±7.1 µM, respectively (31). Five compounds isolated from the flowers of Tanacetum vulgare exhibit degrees of anticancer activity between 15.3 and 60.0 µM against A549 lung cancer cells (32). Notably, a novel 12,8-eudesmanolide extract from Eutypella spp. exhibits cytotoxicity against lymphoma, hepatocarcinoma and myeloid leukemia cell lines (33–34). These and other studies indicate that eudesmanolide-based SLs are potentially strong anticancer compounds for the treatment of various types of cancer, including breast cancer.
In the present study, frullanolide (a eudesmanolide) was assessed by MTT assay, and exhibited potent anti-breast cancer activity in breast cancer cell lines, including MCF-7, MDA-MB-468 and MDA-MB-231. The IC50 values were measured after 72 h. In comparison, the cytotoxicity of frullanolide was strongest for MDA-MB-468, a triple negative breast cancer (TNBC) cell line which lacks estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (35). This TNBC cell line is an important model for breast cancer responsiveness due to the limited choices of chemotherapeutic treatment for this disease (35–37). Notably, in the present study, frullanolide exhibited potent activity against MDA-MB-468 cells, which possess a unique targeted receptor, the epidermal growth factor receptor (EGFR), on the cell membrane (35). This receptor may be involved in the specific interactions with frullanolide, leading to its toxicity in these cells. Using in silico screening, Sawatdichaikul et al (38) reported a list of plant compounds that bind to EGFR. Two promising compounds from the medicinal plants list are exiguaflavanone A and exiguaflavanone B, which are compounds purified from Artemisia indica Willd and Sophora exigua, respectively. These belong to the Asteraceae family, the same family as G. maderaspatana (L.) Poir. The structure of these two compounds has approximately the same carbon skeleton orientation as frullanolide (38). However, further experiments are required to confirm any interactions between frullanolide and EGFR. Additionally, Maldonado et al (27) reported on two novel eudesmanolide structures (C17H25O5 and C17H22O4) extracted from the small genus Kaunia (Asteraceae family) which exhibited strong anti-breast cancer activity toward five breast cancer cell lines; HCC1937 (TNBC), JIMT-1, L56Br-C1, MCF-7 and SK-BR-3. The IC50 values of eudesmanolide C17H25O5 and C17H22O4 ranged between 9.3 and 27.0 µM (L56Br-C1 >SK-BR-3 >JIMT-1>HCC1937>MCF-7) and 3.2–11.0 µM (HCC1937>SK-BR-3>JIMT-1>L56Br-C1>MCF-7) (27). A previous computational structure-based screening method to identify natural compounds that specifically target the mouse double minute 2 homolog protein revealed that, out of the 35 top candidates, 8 eudesmanolide SLs (IJ-1, IJ-3, IJ-5, IJ-6, IJ-9, IJ-11, IH-45 and IH-49) exerted more potent anti-TNBC activity (MDA-MB-231) than anti-non-TNBC (MCF-7) activity at 72 h (39). The IC50 values ranged between 10 and 50 µM (39). In the present study, the anti-breast cancer activity of frullanolide in the MDA-MB-468, MCF-7 and MDA-MB-231 cell lines exhibited similar IC50 levels of 34.61, 46.23 and 53.20 µM, respectively. In comparison, the anti-breast cancer activity of frullanolide in certain cell lines (specifically TNBC) exhibited moderately strong activity, but exhibited high selectivity for breast cancer cells (less harmful to normal breast cells).
To evaluate the mechanism of apoptosis following frullanolide treatment, flow cytometry analysis was selected for measurement of quantitative DNA content (PI staining) and apoptotic cells (double-staining fluorescent PI and annexin V-FITC). Apoptosis-associated protein (p53, p21 and Bax) expression levels were analyzed by western blotting. Notably, apoptotic induction by the appropriate frullanolide treatment occurred in all three breast cancer cell lines, but G2/M arrest only occurred in frullanolide-treated MDA-MB-468 cells. Niculescu et al (40), reported that the level of p21 accumulation serves a role in negatively regulating the G2/M transition. The same study revealed that the expression of p21 is associated with endoreduplication in pRb-negative cells by inhibiting cyclin-dependent kinases leading to G2 arrest (40).
The findings of the present study supported the observation of cell cycle arrest in MDA-MB-468, in that an increase of p21 expression after 12 h of treatment was observed. The expression of p21 also induces the apoptotic pathway in p53-dependent and -independent pathways (41). Overall, after 12 h of treatment, apoptosis was induced by increasing the expression of p21 and Bax at various time points in the MDA-MB-468, MDA-MB-231 and MCF-7 cell lines.
In the present study, p53 expression in treated MCF-7 cells was not detectable due to its short half-life, caused by proteasomal degradation (42,43). This effect may be regulated by p53-independent p21 activation molecules, including transforming growth factor β, tumor necrosis factor α, histone deacetylase inhibitors, interferon γ and interleukin 6 (41).
In conclusion, the results of the present study suggested that the apoptotic pathway was involved in frullanolide-induced cell death via p21 induction and p53-independent pathways in the MCF-7 cell line and p53-dependent pathways in the MDA-MB-468 and MDA-MB-231 breast cancer cell lines. Further experiments are required to clarify the molecular mechanism involved in the anti-breast cancer activity of frullanolide.
Acknowledgements
The authors would like to thank Mr. David Patterson (International Affairs Office, Faculty of Medicine, Prince of Songkla University, Hat Yai, Thailand) for English proofreading.
Funding
The present study was financially supported by grants from the Faculty of Medicine, Prince of Songkla University (grant no. REC57-0162-04-2) and Prince of Songkla University Funding (grant no. MED560604S).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
SC performed the experiments, acquired and analyzed the data, and was a major contributor in the manuscript writing. PG was responsible for the cytotoxic activity assay and interpreted the data. TS provided the protocol and guidance for FACS analysis. SS extracted and purified the frullanolide compound. RB performed the western blotting. KK designed all the experiments, analyzed the data and was a major contributor in editing the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Stewart BW, Wild CP, editors. World cancer Report 2014 Lyon. International Agency for Research on Cancer (IARC) 2014:pp16–53. [Google Scholar]
- 2.Srisawat T, Sukpondma Y, Chimplee S, Kanokwiroon K, Tedasen A, Graidist P. Extracts from vatica diospyroides type SS fruit show low dose activity against MDA-MB-468 breast cancer cell line via apoptotic action. Biomed Res Int. 2014;2014:479602. doi: 10.1155/2014/479602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noble RL. The discovery of the vinca alkaloids-chemotherapeutic agents against cancer. Biochem Cell Bio. 1990;68:1344–1351. doi: 10.1139/o90-197. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Patil D, Rajamohanan PR, Ahmad A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS One. 2013;8:e71805. doi: 10.1371/journal.pone.0071805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel V, Shukla S, Patel S. Free radical scavenging activity of Grangea maderaspatana Poir. Pharmacogn Mag. 2009;5:381–387. doi: 10.4103/0973-1296.58156. [DOI] [Google Scholar]
- 6.Rao VM, Damu GLV, Sudhakar D, Rao CV. Two new bio-active flavones from Grangea maderaspatana (Artemisia maderaspatana) Asian J Chem. 2009;21:1552–1558. [Google Scholar]
- 7.Chaturvedi D. Research Signpost; Kerala, India: 2011. Sesquiterpene lactones: Structural diversity and their biological activities. In: Tiwari VK and Mishra BB (eds.): Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry; pp. 313–334. [Google Scholar]
- 8.Ahmed M, Islam MM, Hossain CF, Khan OF. A preliminary study on the analgesic activity of Grangea maderaspatana. Fitoterapia. 2011;72:553–554. doi: 10.1016/S0367-326X(00)00326-9. [DOI] [PubMed] [Google Scholar]
- 9.Ruangrungsi N, Kasiwong S, Likhitwitayawuid K. Constituents of Grangea maderaspatana a new Eudesmanolide. J Nat Prod. 1989;52:130–134. doi: 10.1021/np50061a016. [DOI] [Google Scholar]
- 10.Sangmalee S, Laorpaksa A, Sukrong S. A topoisomerase II poison screen of ethnomedicinal Thai plants using a yeast cell-based assay. J Ethnopharmacol. 2012;142:432–437. doi: 10.1016/j.jep.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Singh D, Mathela CS, Pande V, Panwar A. Antioxidant and antimicrobial activity of Grangea maderaspatana (L.) Poir. Extract. JDDT. 2013;1:46–52. [Google Scholar]
- 12.Uppatanpreecha P. Dissertation; Bangkok, Chulalongkorn University: 2009. Topoisomerase I inhibitory activity from Thai medicinal plants in yeast cell-based assay. [Google Scholar]
- 13.Ghantous A, Gali-Muhtasib H, Vuorela H, Saliba NA, Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov Today. 2010;15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Srisawat T, Chumkaew P, Heed-Chim W, Sukpondma Y, Kanokwiroon K. Phytochemical screening and cytotoxicity of crude extracts of Vatica diospyroides Symington type LS. Trop J Pharm Res. 2013;12:71–76. [Google Scholar]
- 15.Alitheen NB, Mashitoh AR, Yeap SK, Shuhaimi M, Manaf AA, Nordin L. Cytotoxic effect of damnacanthal, nordamnacanthal, zerumbone and betulinic acid isolated from Malaysian plant sources. Int Food Res J. 2010;17:711–719. [Google Scholar]
- 16.Wibowo A, Ahmat N, Hamzah AS, Sufian AS, Ismail NH, Ahmad R, Jaafar FM, Takayama H. Malaysianol A, a new trimer resveratrol oligomer from the stem bark of Dryobalanops aromatica. Fitoterapia. 2011;82:676–681. doi: 10.1016/j.fitote.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Bézivin C, Tomasi S, Lohézic-Le Dévéhat F, Boustie J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine. 2003;10:499–503. doi: 10.1078/094471103322331458. [DOI] [PubMed] [Google Scholar]
- 18.Chadwick M, Trewin H, Gawthrop F, Wagstaff CF. Sesquiterpenoid lactones: Benefits to plants and people. Int J Mol Sci. 2013;14:12780–12805. doi: 10.3390/ijms140612780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreuger MR, Grootjans S, Biavatti MW, Vandenabeele P, D'Herde K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: Focus on parthenolide. Anticancer Drugs. 2012;23:883–896. doi: 10.1097/CAD.0b013e328356cad9. [DOI] [PubMed] [Google Scholar]
- 20.Matejić J, Šarac Z, Ranđelović V. Pharmacological activity of sesquiterpene lactones. Biotechnol Biotechnol Equip. 2010;24(Suppl 1):S95–S100. doi: 10.1080/13102818.2010.10817819. [DOI] [Google Scholar]
- 21.Cordell GA, Kinghorn D, Pezzuto JM. Separation, structure elucidation, and bioassay of cytotoxic natural products. Colegate SM, Molyneux RJ, editors. Bioactive natural products, Boca raton, CRC press. 1993:195–216. [Google Scholar]
- 22.Wu C, Chen F, Rushing JW, Wang X, Kim HJ, Huang G, Haley-Zitlin V, He G. Antiproliferative activities of parthenolide and golden feverfew extract against three human cancer cell lines. J Med Food. 2006;9:55–61. doi: 10.1089/jmf.2006.9.55. [DOI] [PubMed] [Google Scholar]
- 23.van Haaften C, Duke CC, Weerheim AM, Smit NP, van Haard PM, Darroudi F, Trimbos BJ. Potent cytotoxic effects of Calomeria amaranthoides on ovarian cancers. J Exp Clin Cancer Res. 2011;30:1–6. doi: 10.1186/1756-9966-30-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischedick JT, Pesic M, Podolski-Renic A, Bankovicc J, de Vosd RCH, Perić M, Todorovićg S, Tanicc N. Cytotoxic activity of sesquiterpene lactones from Inula britannica on human cancer cell lines. Phytochem Lett. 2012;6:246–252. doi: 10.1016/j.phytol.2013.02.006. [DOI] [Google Scholar]
- 25.Kabeer FA, Sreedevi GB, Nair MS, Rajalekshmi DS, Gopalakrishnan LP, Kunjuraman S, Prathapan R. Antineoplastic effects of deoxyelephantopin, a sesquiterpene lactone from Elephantopus scaber, on lung adenocarcinoma (A549) cells. J Integr Med. 2013;11:269–277. doi: 10.3736/jintegrmed2013040. [DOI] [PubMed] [Google Scholar]
- 26.Costantino VV, Mansilla SF, Speroni J, Amaya C, Cuello-Carrión D, Ciocca DR, Priestap HA, Barbieri MA, Gottifredi V, Lopez LA. The sesquiterpene lactone dehydroleucodine triggers senescence and apoptosis in association with accumulation of DNA damage markers. PLoS One. 2013;8:e53168. doi: 10.1371/journal.pone.0053168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maldonado EM, Svensson D, Oredsson SM, Sterner O. Cytotoxic sesquiterpene lactones from Kauna lasiophthalma Griseb. Sci Pharm. 2014;82:147–160. doi: 10.3797/scipharm.1310-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babaeia G, Aliarab A, Abroon S, Rasmi Y, Gholizadeh-Ghaleh Aziz S. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed Pharmacother. 2018;106:239–246. doi: 10.1016/j.biopha.2018.06.131. [DOI] [PubMed] [Google Scholar]
- 29.Ma G, Chong L, Li Z, Cheung AH, Tattersall MH. Anticancer activities of sesquiterpene lactones from Cyathocline purpurea in vitro. Cancer Chemother Pharmacol. 143-152. 2009;64 doi: 10.1007/s00280-008-0863-y. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Ni ZY, Zhuc MC, Dong M, Wang SW, Shi QW, Zhang ML, Wang YF, Huo CH, Kiyota H, Cong B. Antitumour activities of sesquiterpene lactones from Inula helenium and Inula japonica. Z Naturforsch C. 2012;67:375–380. doi: 10.5560/ZNC.2012.67c0375. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoud AA, AlFredan MA, El-Sayed WM. Isolation, characterization and anticancer activity of seven compounds from the aerial parts of Conyza triloba. Int J Pharmacognosy and Phytochem Res. 2016;8:2071–2079. [Google Scholar]
- 32.Rosselli S, Bruno M, Raimondo FM, Spadaro V, Varol M, Koparal AT, Maggio A. Cytotoxic effect of eudesmanolides isolated from flowers of Tanacetum vulgare ssp. Siculum. Molecules. 2012;17:8186–8195. doi: 10.3390/molecules17078186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivero A, Quintana J, Eiroa JL, Lo´pez M, Triana J, Bermejo J, Este´vez F. Potent induction of apoptosis by germacranolide sesquiterpene lactones on human myeloid leukemia cells. Eur J Pharmacol. 2003;482:77–84. doi: 10.1016/j.ejphar.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Wang Y, Wu AA, Zhang L, Hu Z, Huang H, Xu Q, Deng X. New 12,8-eudesmanolides from Eutypella sp. 1–15. J Antibiot (Tokyo) 2017;70:1029–1032. doi: 10.1038/ja.2017.89. [DOI] [PubMed] [Google Scholar]
- 35.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudisa CA, Gianni L. Triple-negative breast cancer: An unmet medical need. Oncologist. 2011;16(Suppl 1):S1–S11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 37.Kandula M, Ch KK, Ys AR. Molecular mechanism and targeted therapy options of triple-negative (ER, PgR, HER−2/neu) breast cancer: Review. World J Oncol. 2013;4:137–141. doi: 10.4021/wjon681e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawatdichaikul O, Hannongbua S, Sangma C, Wolschann P, Choowongkomon K. In silico screening of epidermal growth factor receptor (EGFR) in the tyrosine kinase domain through a medicinal plant compound database. J Mol Model. 2012;18:1241–1254. doi: 10.1007/s00894-011-1135-z. [DOI] [PubMed] [Google Scholar]
- 39.Qin JJ, Wang W, Voruganti S, Wang H, Zhang WD, Zhang R. Identification of a new class of natural product MDM2 inhibitor: In vitro and in vivo anti-breast cancer activities and target validation. Oncotarget. 2015;6:2623–2640. doi: 10.18632/oncotarget.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niculescu III AB, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21Cip1/Waf1 at both the G1/S and the G2/M cell cycle transitions: pRb ss a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/MCB.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gartel ALand Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;8:639–649. [PubMed] [Google Scholar]
- 42.Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1:1001–1008. [PubMed] [Google Scholar]
- 43.Tsvetkov P, Reuven N, Shaul Y. Ubiquitin-independent p53 proteasomal degradation. Cell Death Differ. 2010;17:103–108. doi: 10.1038/cdd.2009.67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.