Abstract
Malignant glioma is one of the most common types of primary malignancies in the human central nervous system. Temozolomide (TMZ) is the most commonly used drug in clinical therapy of glioma; however, chemoresistance makes glioma difficult to cure and relapse likely. Sirtuin 1 (SIRT1) serves important roles in cell proliferation, differentiation and metabolism, but the role of SIRT1 in human glioma remains largely unexplored. In the present study, SIRT1 expression was assessed in human glioma tissues and cells. RNA interference and SIRT1 inhibitor were used to determine the effect of SIRT1 on glioma growth inhibition and glioma cell chemoresistance in vitro and in vivo. The levels of reactive oxygen species (ROS) in glioma cells were detected with the dihydroethidium probe following SIRT1 inhibition. The results demonstrated that SIRT1 was overexpressed in glioma tissues and cells, and patients with higher SIRT1 expression exhibited poorer prognosis. SIRT1 inhibition inhibited the proliferation of U87 and U251 cells. In addition, SIRT1 knockdown and SIRT1 inhibitor could significantly sensitize glioma cells to TMZ treatment in vitro and in vivo. The expression of Ki67 and p53 was demonstrated to be regulated by SIRT1. Finally, SIRT1 could regulate intracellular ROS generation in TMZ. In summary, SIRT1 was essential for glioma tumorigenesis and glioma cell chemoresistance. SIRT1 inhibition increased the sensitivity of glioma cells for TMZ via the facilitation of intracellular ROS generation, which suggested that SIRT1 may serve as a target for clinical therapy of glioma.
Keywords: glioma, sirtuin 1, proliferation, temozolomide, reactive oxygen species
Introduction
Malignant glioma accounts for ~40% of all intracranial tumors, which makes it the most common primary malignant tumor of the central nervous system (1). Traditionally, gliomas are further categorized as low-grade glioma (I–II) and high-grade glioma (III–IV) according to the World Health Organization glioma grading classification criteria (2). Glioblastoma (GBM) belongs to the highest grade. Patients with malignant glioma exhibit poor prognosis. However, currently there are no well-established methods to inhibit glioma tumor growth. At present, the comprehensive treatments for patients with glioma include surgery, radiotherapy and chemotherapy (3). Temozolomide (TMZ) is a conventional chemotherapy drug for glioma clinical therapy. TMZ has been reported to exhibit therapeutic benefits in prolonging the survival of patients with GBM (4). Although some of the methods show promising results for glioma therapy, the existence of multiple genetic changes in GBM suggests that a single drug is incapable of offering a complete solution to the problem (5,6). Genetic targeted therapy has been reported to be a potential method of resolving the chemotherapy resistance of cancer cells, and specific molecule inhibitors against anomaly genetic targets are now in clinical trials (7–9).
The nicotinamide-adenine dinucleotide (NAD)-dependent protein deacetylase sirtuin 1 (SIRT1) is a key regulator of life span (10–12). The biological function of SIRT1 is linked to cellular stress, apoptosis and metabolism. SIRT1 has been demonstrated to be involved in the tumorigenesis of numerous types of cancer. SIRT1 influences the cell proliferation, differentiation and apoptosis during tumor growth (13–16). SIRT1 is overexpressed in several types of cancer (17–20). However, the biological function of SIRT1 and its regulatory mechanism in human glioma are rarely studied. In the present study, the protective effects of SIRT1 on TMZ-induced toxicity were investigated. The results revealed that SIRT1 was overexpressed in glioma tissues and cell lines. The Kaplan-Meier analysis demonstrated a significant association of SIRT1 expression with the overall survival of patients with glioma. SIRT1 inhibition could markedly sensitize glioma cell to TMZ in vitro and in vivo. SIRT1 could regulate intracellular reactive oxygen species (ROS) generation and the expression of Ki67 and p53 in glioma. In summary, the present study demonstrated that SIRT1 was essential in glioma proliferation and chemoresistance. SIRT1 may serve as a potential therapeutic target for glioma therapy.
Materials and methods
Tissue samples
A total of 29 glioma tissues and 11 normal tissues from sites adjacent to cancer sites were obtained from 24 men and 16 women aged between 35±2.5 and 65±3.2 years old. The tissues were obtained as part of surgery carried out between December 2013 and December 2017 at The First Affiliated Hospital of Shantou University Medical College (Shantou, China). Tissues were stored at −80°C prior to RNA or protein extraction. Informed consent from relevant patients was obtained for tissue collection. The present study was approved by the Ethics Committee of The First Affiliated Hospital of Shantou University Medical College Institutional Review Board (Shantou, China).
Cell lines and lentiviral transfection
U251 and U87MG cell lines were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). A172, T98 and HEB normal glial cell lines were purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China). Short tandem repeat (STR) profiling was used for cancer cell line testing. The STR testing result revealed that the U87 cell line used in the present study matched the American Type Culture Collection (ATCC) U87MG cell line. Although the ATCC U87MG cell line is suspected to not be the original GBM cell line from the University of Uppsala, which was established in 1968, the ATCC U87MG cell line was derived from the central nervous system, perhaps from another unknown patient with glioma (21). Therefore, the ATCC U87MG cell line was appropriate for use in the present study. All cancer cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc. Waltham, MA, USA), which was supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin and 1% streptomycin (Gibco; Thermo Fisher Scientific, Inc.). Cells were cultured at 37°C in a humidified atmosphere with 5% CO2. A total of 1×106 cells were transiently transfected with the SIRT1 short hairpin RNA (shSIRT1) lentivirus using the plent-U6-Puro lentiviral vector kit according to the manufacturer's protocol (Thermo Fisher Scientific, Inc.). Subsequently, 6 µg/ml polybrene (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to enhance the transfection efficiency. Following 24 h transfection, the suspension was replaced by normal DMEM. U251-shSIRT1 and U87-shSIRT1 cells were then screened using puromycin (2 µg/ml). After 7 days screening, the stable U251-shSIRT1 and U87-shSIRT1 cell lines were used for subsequent experiments.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
For the RT-qPCR assay, total mRNA was extracted from tumor tissues and cells using the Total RNA Extraction reagent (Nanjing Sunshine Biotech Co., Ltd., Nanjing, China). Subsequently, mRNA was reverse transcribed to cDNA using the Reverse TransScript kit (Takara Bio, Inc., Otsu, Japan). The qPCR assay was performed using the SYBR® Premix Ex Taq™ II kit (Takara Bio, Inc.). All procedures were performed according to the manufacturer's protocols. The RT-qPCR assay was performed using the Bio-Rad iQ5 Real-Time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the following protocol: Initial activation step for 10 min at 95°C pre-denaturation, followed by 40 cycles of amplification, with 30 sec denaturation at 95°C, 10 sec annealing at 60°C and 60 sec extension at 72°C. The primer sequences used were as follows: SIRT1 forward, 5′-ATCTGACTTTGCTCCCCTTAACC-3′, and reverse, 5′-GGGCCCTGGTTGCAAGA-3′; β-actin was used as an internal reference, and the primer sequences of β-actin were forward, 5′-CAATGACCCCTTCATTGACC-3′, and reverse, 5′-GACAAGCTTCCCGTTCTCAG-3. The relative gene expression was quantified using the 2−ΔΔCq method (22).
Western blot analysis
Tissues and cells were lysed with radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Shanghai, China), followed by determination of the total protein concentration by bicinchoninic acid protein assay (Beyotime Institute of Biotechnology). Total protein samples (20 µg/lane) were loaded and proteins with different molecular weights were separated on a 10% SDS-PAGE gel. Proteins were transferred onto polyvinylidene difluoride membranes following electrophoresis. The membranes were blocked for 1 h at room temperature in 5% non-fat milk. Subsequently, the membranes were incubated with primary antibodies at 4°C overnight. The primary antibodies were anti-SIRT1 (1:2,000 dilution; cat. no. ab110304; Abcam, Cambridge, UK), anti-Ki67 antibody (1:2,000 dilution; cat. no. ab15580; Abcam), anti-p53 antibody (1:2,000; cat. no. ab131442; Abcam) and mouse anti-β-actin antibody (1:2,000; cat. no. ab8226; Abcam). Following incubation, the membranes were washed with TBS containing 0.1% Tween-20, and incubated with horseradish peroxidase-conjugated goat anti-mouse (1:4,000 dilution; cat. no. A0216; Beyotime Institute of Biotechnology) an/d goat anti-rabbit (1:4,000 dilution; cat. no. A0208; Beyotime Institute of Biotechnology) secondary antibodies at room temperature for 1 h. Subsequently, membranes were soaked in enhanced chemiluminescence kit (cat. no. P0018; Beyotime Institute of Biotechnology) for 1 min and visualized under the chemiluminescence detection system (ChemiDocXRS; Bio-Rad Laboratories, Inc.).
Cell proliferation assay
Cell proliferation was detected via water soluble tetrazolium salts 1 (WST-1) assay. Cells (2,000/well) were seeded in 96-well plates, following culturing for 0, 24, 48 and 72 h, respectively. A total of 10 µl WST-1 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to each well and incubated for 30 min. Subsequently, absorbance at 490 nm was detected using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA), according to the manufacturer's protocols. All assays were repeated at least three times.
TMZ toxicity assay
The SIRT1-specific inhibitor selisistat (EX527) was purchased from Selleck Chemicals (Houston, TX, USA). Briefly, 200 nM EX527 was added to glioma cell culture medium. Glioma cells were further cultured at 37°C for 24, 48 and 72 h. U87 and U251 cells were exposed to TMZ to induce cell death. In order to define TMZ toxicity, different concentrations of TMZ (0, 50, 100, 150 and 200 µM) were added to 2×106 U87 and U251 cells for 24 h, prior to detect cell viability with WST-1 assay. The results allowed determining 100 µM as the working concentration for TMZ. Cells were then incubated with 100 µM TMZ at 37°C for 0, 24, 48 and 72 h. Subsequently, all the cells treated with EX527 and TMZ were incubated in WST-1 following the manufacturer's protocol. TMZ (200 µM) was used in the time dependent cell viability assay. All assays were repeated at least three times.
ROS determination assay
A total of 1×106 cells were cultured in 6-well plates. Prior to ROS detection, cells were washed twice with PBS. Subsequently, cells were incubated with 5 µM dihydroethidium (DHE; Vigorous Biotechnology Beijing Co., Ltd., Beijing, China) in PBS for 30 min at 37°C in the dark. Following incubation, the cells were washed twice with PBS and fixed in 4% paraformaldehyde for 30 min at room temperature. Following two washes with PBS, images were captured by fluorescence microscopy (×40 magnification). At least five images were captured for each group. Relative fluorescence intensity was calculated by ImageJ software 7.0 (National Institutes of Health, Bethesda, MD, USA).
Animal studies
A total of 20 nude mice (age, 4 weeks) were purchased from Vital River Laboratories Co., Ltd. (Beijing, China). All mice (22±1.5 g) were housed at a controlled temperature (22–28°C) and humidity (60%) under a 12-h light/dark cycle. Mice had free access to food and water. The stably transfected negative control (NC) and shSIRT1 lentivirus U87 cells (2×106 for each side) were suspended in PBS and implanted subcutaneously into male Balb/c nude mice. A total of 10 mice received NC lentivirus and 10 received shSIRT1 lentivirus (subcutaneous injection). Treatment with 200 nM EX527 was performed for all mice, whereas treatment with 100 µM TMZ was performed for the 10 normal mice. The length (a) and width (b) of the tumors were monitored every 2 days. After 30 days of monitoring, all nude mice were sacrificed. Tumor volume (V) was calculated using the formula V=ab2/2. Animal experiments conducted in the present study were approved by the Ethics Committee of The First Affiliated Hospital of Shantou University Medical College Institutional Review Board.
Statistical analysis
All the data were expressed as the means ± standard error of the mean from at least three independent experiments. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used for data analysis. Statistical comparisons among different groups were performed by one-way analysis of variance, followed by Newman-Keuls comparison test. Kaplan-Meier method was used for the survival analysis, and Cox regression analysis was used for the univariate and multivariate analyses. P<0.05 was considered to indicate a statistically significant difference.
Results
SIRT1 expression is upregulated in glioma tissues and cell lines
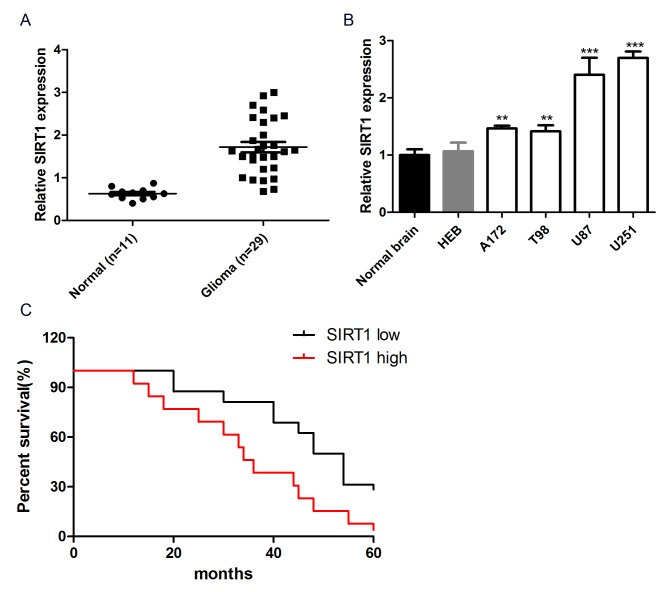
To investigate the role of SIRT1 in glioma cell chemotherapeutic agent resistance, the expression level of SIRT1 in glioma tissues and cells was detected. The results revealed that the mRNA expression level of SIRT1 was significantly increased in 29 glioma tissues compared with 11 normal brain tissues (Fig. 1A). In addition, SIRT1 exhibited significantly higher expression in glioma cell lines (A172, T98, U87 and U251) than normal human brain glial cell lines (HEB) and normal brain tissue (Fig. 1B). These data reflected SIRT1 upregulation in glioma tissues and cell lines. Kaplan-Meier analysis was performed to study the association between SIRT1 expression and the prognosis of patients with glioma. Patients were divided into SIRT1 high and SIRT1 low groups, based on the mean expression levels of SIRT1 in all samples. The Kaplan-Meier survival curve revealed that patients with glioma with high SIRT1 expression had a shorter overall survival than those with low SIRT1 expression (P<0.05; Fig. 1C), indicating that SIRT1 expression was associated with the prognosis of patients with glioma. Overall, SIRT1 exhibited high expression in glioma tissues and cells and was associated with the prognosis of patients with glioma.
Figure 1.
SIRT1 expression is upregulated in glioma tissues and cell lines. (A) The expression of SIRT1 was detected in human glioma tissues and adjacent non-tumor tissues using RT-qPCR. (B) The expression of SIRT1 in U87, U251, A172, T98 and HEB cell lines and normal brain tissue was detected using RT-qPCR. (C) The Kaplan-Meier survival curve of patients with glioma was created based on the expression levels of SIRT1 protein. **P<0.001 and ***P<0.001 compared with normal brain tissues. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; SIRT1, sirtuin 1.
SIRT1 knockdown inhibits glioma cell proliferation
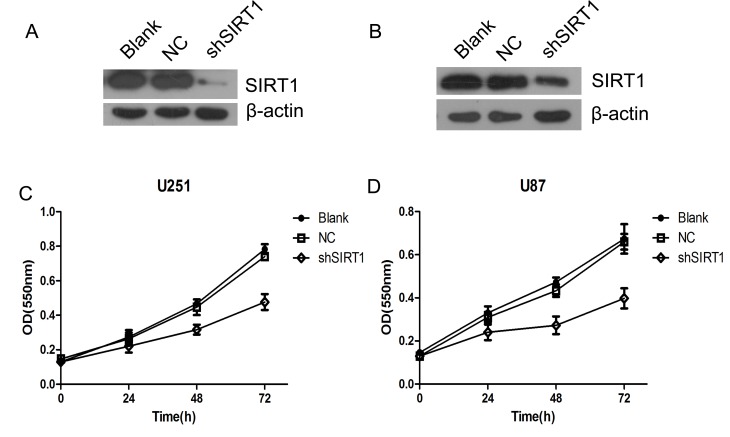
To investigate the biological function of SIRT1 in glioma growth, SIRT1 was silenced using two short hairpin RNAs in U251MG (Fig. 2A) and U87MG (Fig. 2B) cells. The proliferation of U87 and U251 cells were detected by WST-1 cell viability assay. The results revealed that SIRT1 knockdown significantly inhibited glioma cell proliferation in U87 and U251 cells (Fig. 2C and D). This suggested that SIRT1 is essential for glioma cell proliferation.
Figure 2.
SIRT1 knockdown inhibits glioma cell proliferation. (A) SIRT1 was silenced by shRNA in U251 cells. (B) SIRT1 was silenced by shRNA in U87 cells. (C) Growth curve of U87 cells as detected by WST-1 assay. (D) Growth curve of U251 cells as detected by WST-1 assay. NC, negative control; OD, optical density; shRNA, short hairpin RNA; shSIRT1, short hairpin RNA sirtuin 1; SIRT1, sirtuin 1; WST-1, water soluble tetrazolium salts 1. All determination assays were carried out in triplicate.
TMZ downregulates the expression of SIRT1 in glioma cells
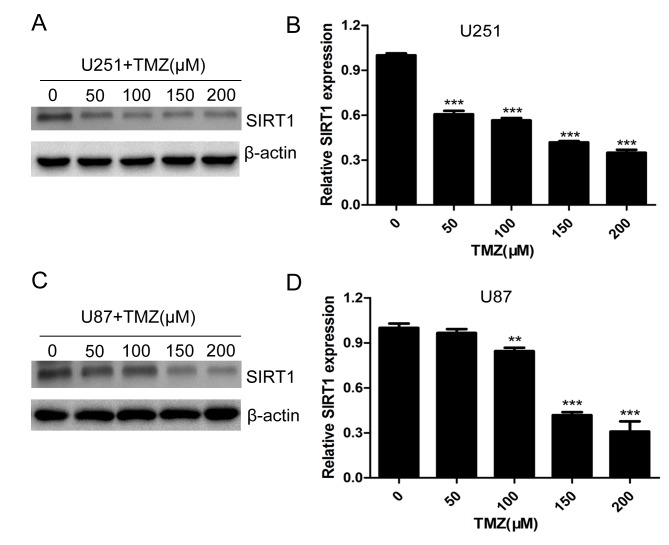
Temozolomide is one of the most important drugs used in clinical therapy of glioma (23,24). To investigate the role of SIRT1 in glioma cell chemotherapeutic agent resistance, U251 and U87 cells were treated with different concentrations of TMZ. The protein and mRNA levels of SIRT1 were significantly decreased with increasing concentrations of TMZ in U251 (Fig. 3A and B) and U87 (Fig. 3C and D) cells, indicating that SIRT1 expression was regulated by TMZ treatment.
Figure 3.
TMZ downregulates SIRT1 expression in glioma cells. (A) The expression of SIRT1 in U251 cells with TMZ treatment was detected by western blotting. (B) RT-qPCR detection of SIRT1 expression in U251 cells treated with different concentrations of TMZ. (C) The expression of SIRT1 in U87 cells treated with TMZ was detected by western blotting. (D) RT-qPCR detection of SIRT1 expression in U87 cells treated with different concentrations of TMZ. **P<0.001 and ***P<0.001 compared with the control group. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; SIRT1, sirtuin 1; TMZ, temozolomide.
SIRT1 inhibition increases TMZ sensitivity of glioma cells
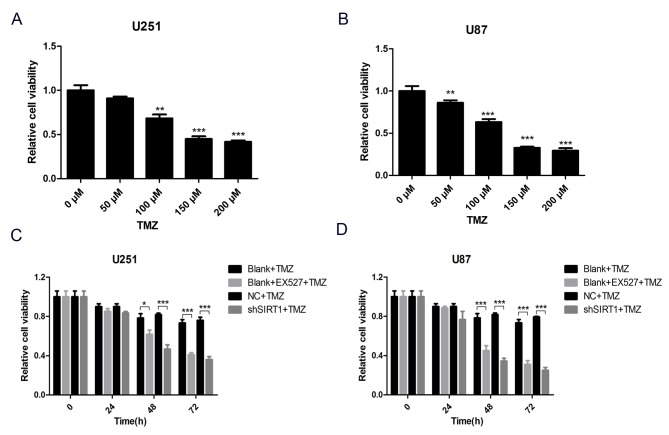
To comprehensively evaluate the association between SIRT1 and glioma cell TMZ sensitivity, a SIRT1 specific inhibitor, EX527, was used to inhibit SIRT1 protein activity in U251 and U87 cells. Subsequently, cells were treated with different concentrations of TMZ for 24 h and cell viability was assessed by WST-1 assay. The results demonstrated that cell viability decreased with TMZ treatment in U251 and U87 cells (Fig. 4A and B). To avoid any extra toxicity TMZ may induce, 100 µM was selected as the working concentration of TMZ in subsequent tests. The result of the WST-1 assay demonstrated that SIRT1 activity inhibition markedly decreased viability of U251 and U87 cells treated with TMZ, as did SIRT1 knockdown (Fig. 4C and D). These results demonstrated that SIRT1 was indispensable for glioma cell TMZ sensitivity.
Figure 4.
SIRT1 knockdown and SIRT1 inhibitor increase glioma cell TMZ sensitivity. (A) The viability of U251 cells treated with different concentrations of TMZ was detected by WST-1 assay. (B) The viability of U87 cells treated with different concentrations of TMZ was detected by WST-1 assay. (C) The viability of U251 cells treated with TMZ or SIRT1 inhibitor was detected by WST-1 assay. (D) The viability of U87 cells treated with TMZ or SIRT1 inhibitor was detected by WST-1 assay. *P<0.05, **P<0.001 and ***P<0.001 compared with the relative untreated group. EX527, selisistat, SIRT1 inhibitor; NC, negative control; shSIRT1, short hairpin RNA SIRT1; SIRT1, sirtuin 1; TMZ, temozolomide; WST-1, water soluble tetrazolium salts 1.
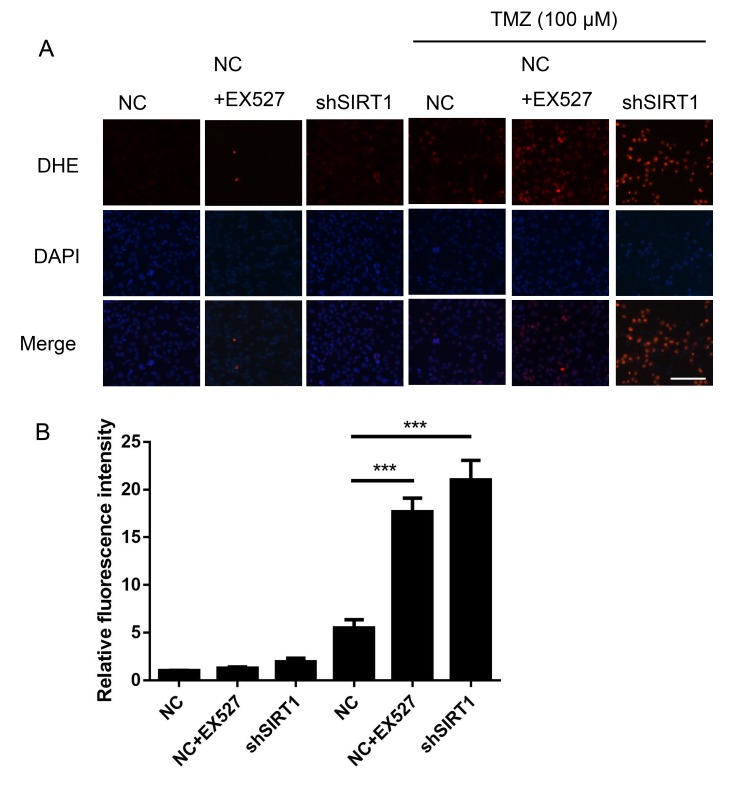
SIRT1 inhibition increases ROS generation in glioma
TMZ has been reported to increase ROS production (3), suggesting that ROS are a key factor in TMZ mediated glioma clinical therapy. DHE staining was performed to assess the association between SIRT1 and intracellular ROS generation in glioma. As shown in Fig. 5, shSIRT1 significantly increased the ROS level in U251 cells treated with TMZ, by ~4-fold compared with only TMZ treatment. Simultaneously, EX527 increased the ROS level in U251 cells (Fig. 5). The results suggested that SIRT1 was an important regulator for glioma cell ROS generation.
Figure 5.
SIRT1 inhibition increases the generation of reactive oxygen species in glioma. (A) The reactive oxygen species level of U251 cells was detected by DHE staining. Scale bar, 200 µm. (B) Statistical analysis of the DHE fluorescence intensity. ***P<0.001. DHE, dihydroergotamine; EX527, selisistat, SIRT1 inhibitor; NC, negative control; shSIRT1, short hairpin RNA SIRT1; SIRT1, sirtuin 1; TMZ, temozolomide.
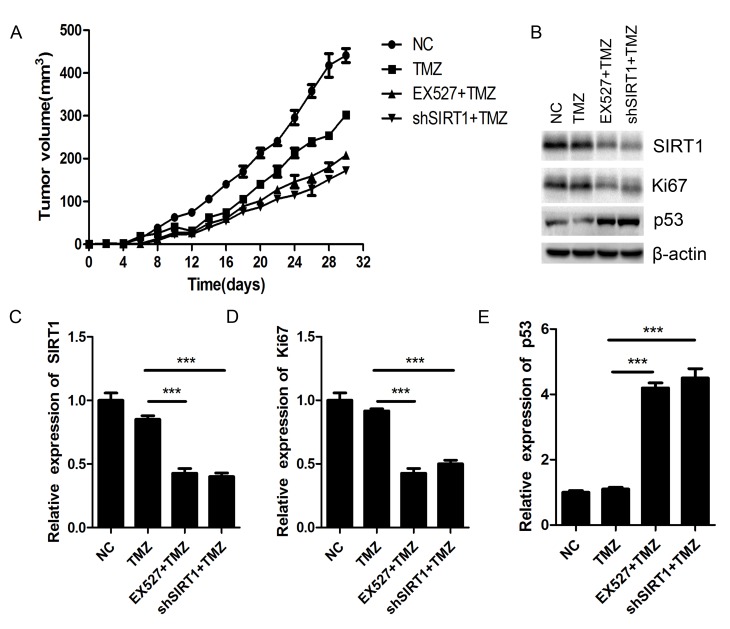
SIRT1 inhibition sensitizes glioma to TMZ in vivo
Nude mice xenograft was performed to investigate the biological effect of SIRT1 on glioma chemosensitivity in vivo. The SIRT1 knockdown U87MG cells and the NC U87MG cells were implanted into nude mice subcutaneously. After 30 days of TMZ (100 µM) treatment, the tumor volumes of shSIRT1 U87MG cells-implanted mice were smaller than those of NC U87MG cells-implanted mice. Simultaneously, EX527 treatment exhibited similar inhibitory effects on glioma tumor growth as shSIRT1 (Fig. 6A). Both shSIRT1 and EX527 treatment significantly decreased SIRT1 expression in the mice (Fig. 6B and C). The protein level of Ki67 greatly decreased in SIRT1 knockdown and EX527 treated tumors compared with the NC tumors (Fig. 6B and D), while p53 expression was markedly increased in SIRT1 knockdown and EX527 treated mice tumors (Fig. 6B and E). As Ki67 is closely associated with cell proliferation and p53 is the most important tumor suppressor, SIRT1 may regulate glioma proliferation by regulating the expression of Ki67 and p53.
Figure 6.
SIRT1 inhibition sensitizes glioma tumors to TMZ in vivo. (A) Determination of nude mice tumor growth. Tumor volume was calculated every 2 days following injection. (B) The expression of SIRT1, Ki67 and p53 in nude mice was detected by western blotting. (C) The relative expression level of SIRT1 in nude mice. (D) The relative expression level of Ki67 in nude mice. (E) The relative expression level of p53 in nude mice. ***P<0.001. EX527, selisistat, SIRT1 inhibitor; NC, negative control; shSIRT1, short hairpin RNA SIRT1; SIRT1, sirtuin 1; TMZ, temozolomide.
Discussion
During past decades, malignant glioma has been one of the most common primary malignant tumors of the brain. Although techniques for the clinical therapy of glioma have developed in previous years, the prognosis of patients with glioma remains poor (25). The high incidence and low survival rate of patients with glioma prompts exploration of novel therapeutic strategies. Molecularly targeted therapy is considered to be a potential approach. The identification of novel molecular targets is currently being investigated extensively (26).
SIRT1 is a NAD-dependent protein deacetylase involved in numerous pivotal biological processes, including energy metabolism, DNA repair and mitochondrial homeostasis (27). These biological processes are crucial for cell growth, differentiation, migration and survival, which makes SIRT1 a key regulator in numerous diseases (28). At present, the role of SIRT1 in cancer has been preliminarily studied, and SIRT1 has been reported as an oncogene in a number of cases (29,30). In the present study, tissue analysis demonstrated that SIRT1 was overexpressed in glioma tissues compared with non-tumor tissues. Additionally, SIRT1 was overexpressed in glioma cell lines compared with normal brain cells. The Kaplan-Meier survival analysis demonstrated that high expression levels of SIRT1 were associated with poor prognosis. These findings demonstrated that SIRT1 may serve a vital role in glioma tumorigenesis.
Currently, TMZ is the most efficient chemotherapy agent for clinical therapy of GBM. It can extend the median survival rate by 12–15 months (31). However, long-term TMZ treatment may induce cancer cell resistance. Damage to glioma cells, induced by TMZ, can be repaired by O6-Methylguanine-DNA methyltransferase (MGMT), thus inducing cancer cell resistance, while methylation of the MGMT promoter leads to an increase in TMZ sensitivity (32). Additional molecular mechanisms of TMZ resistance in glioma cells remain unknown. In the present study, SIRT1 knockdown or SIRT1 activity inhibition greatly sensitized glioma cells to TMZ treatment in vitro and in vivo, indicating that SIRT1 was an important regulator of TMZ resistance in glioma cells. Notably, the glioma cell lines treated with TMZ exhibited MGMT promoter hypermethylation status (33). An intracellular ROS detection assay suggested that SIRT1 knockdown or treatment with SIRT1 inhibitor significantly increased glioma cell ROS level following treatment with TMZ, indicating that SIRT1 was closely associated with intracellular ROS generation.
In conclusion, SIRT1 was demonstrated to be overexpressed in glioma tissues and cells and SIRT1 may regulate glioma TMZ sensitivity by regulating intracellular ROS generation. The present study illuminated the role and regulatory mechanism of SIRT1 in glioma tumor growth and chemotherapy resistance. SIRT1 may serve as a novel promising therapeutic target for clinical therapy of glioma in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YMX, HWC and RL conceived and designed the experiments. HWC, RL, ZWZ, JHH and YMX developed the methodology. HWC, RL, ZHZ and QTW performed the experiments. HWC, RL, ZHZ and QTW performed the analysis and interpretation of the data. HWC and RL wrote, reviewed and revised the manuscript. YMX supported study supervision. All authors have read and approved the manuscript.
Ethics approval and consent to participate
Informed consent from relevant patients was obtained for all tissue sample obtaining procedures. The tissue sample obtaining procedures were approved by the ethics committee of The First Affiliated Hospital of Shantou University Medical College Institutional Review Board (Shantou, China). Animal experiments conducted in the present study were approved by the Ethics Committee of First Affiliated Hospital of Shantou University Medical College Institutional Review Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF, Agarwalla PK, Chheda MG, Campos B, Wang A, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26:756–784. doi: 10.1101/gad.187922.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classifcation of tumours of the central nervous system. Acta Neuro Pathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: From molecular pathology to targeted treatment. Annu Rev Pathol. 2014;9:1–25. doi: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 5.Liao AY, Shi RR, Jiang YL, Tian S, Li P, Song F, Qu Y, Li J, Yun H, Yang X. SDF-1/CXCR4 axis regulates cell cycle progression and epithelial-mesenchymal transition via up-regulation of survivin in glioblastoma. Mol Neurobiol. 2016;53:210–215. doi: 10.1007/s12035-014-9006-0. [DOI] [PubMed] [Google Scholar]
- 6.Altieri R, Fontanella M, Agnoletti A, Pnciani PP, Spena G, Crobeddu E, Pilloni G, Tardivo V, Lanotte M, Zenga F, et al. Role of nitric oxide in glioblastoma therapy: Another step to resolve the terrible puzzle. Transl Med UniSa. 2014;12:54–59. [PMC free article] [PubMed] [Google Scholar]
- 7.De Paepe A, Vandeneede N, Strens D, Specenier P. The economics of the treatment and follow-up of patients with glioblastoma. Value Health. 2015;18:A448. doi: 10.1016/j.jval.2015.09.1122. [DOI] [Google Scholar]
- 8.Lv B, Yang X, Lv S, Wang L, Fan K, Shi R, Wang F, Song H, Ma X, Tan X, et al. CXCR4 signaling induced epithelial-mesenchymal transition by PI3K/AKT and ERK pathways in glioblastoma. Mol Neurobiol. 2015;52:1263–1268. doi: 10.1007/s12035-014-8935-y. [DOI] [PubMed] [Google Scholar]
- 9.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 10.Ford J, Jiang M, Milner J. Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res. 2005;65:10457–10463. doi: 10.1158/0008-5472.CAN-05-1923. [DOI] [PubMed] [Google Scholar]
- 11.Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD(+)-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci USA. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 13.Saunders LR, Verdin E. Sirtuins: Critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- 14.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer-a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 15.Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Choi HN, Bae JS, Jamiyandorj U, Noh SJ, Park HS, Jang KY, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression and role of SIRT1 in hepatocellular carcinoma. Oncol Rep. 2011;26:503–510. doi: 10.3892/or.2011.1301. [DOI] [PubMed] [Google Scholar]
- 17.Huffman DM, Grizzle WE, Bamman MM, Kim JS, Eltoum IA, Elgavish A, Nagy TR. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67:6612–6618. doi: 10.1158/0008-5472.CAN-07-0085. [DOI] [PubMed] [Google Scholar]
- 18.Jang KY, Kim KS, Hwang SH, Kwon KS, Kim KR, Park HS, Park BH, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression and prognostic significance of SIRT1 in ovarian epithelial tumours. Pathology. 2009;41:366–371. doi: 10.1080/00313020902884451. [DOI] [PubMed] [Google Scholar]
- 19.Jang KY, Hwang SH, Kwon KS, Kim KR, Choi HN, Lee NR, Kwak JY, Park BH, Park HS, Chung MJ, et al. SIRT1 expression is associated with poor prognosis of diffuse large B-cell lymphoma. Am J Surg Pathol. 2008;32:1523–1531. doi: 10.1097/PAS.0b013e31816b6478. [DOI] [PubMed] [Google Scholar]
- 20.Qu Y, Zhang J, Wu S, Li B, Liu S, Cheng J. SIRT1 promotes proliferation and inhibits apoptosis of human malignant glioma cell lines. Neurosci Lett. 2012;525:168–172. doi: 10.1016/j.neulet.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Allen M, Bjerke M, Edlund H, Nelander S, Westermark B. Origin of the U87MG glioma cell line: Good news and bad news. Sci Transl Med. 2016;8:354re3. doi: 10.1126/scitranslmed.aaf6853. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Valero I, Saiz-Ladera C, Torres S, Hernández-Tiedra S, García-Taboada E, Rodríguez-Fornés F, Barba M, Dávila D, Salvador-Tormo N, Guzmán M, et al. Targeting glioma initiating cells with a combined therapy of cannabinoids and temozolomide. Biochem Pharmacol. 2018;157:266–274. doi: 10.1016/j.bcp.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita AS, da Costa Rosa M, Borodovsky A, Festuccia WT, Chan T, Riggins GJ. Demethylation and epigenetic modification with 5-Azacytidine reduces IDH1 mutant glioma growth in combination with Temozolomide. Neuro Oncol. 2018 doi: 10.1093/neuonc/noy146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa PM, Cardoso AL, Nóbrega C, Pereira de Almeida LF, Bruce JN, Canoll P, Pedroso de Lima MC. MicroRNA-21 silencing enhances the cytotoxic effect of the antiangiogenic drug sunitinib in glioblastoma. Hum Mol Genet. 2013;22:904–918. doi: 10.1093/hmg/dds496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung AL, Maxwell R, Theodros D, Belcaid Z, Mathios D, Luksik AS, Kim E, Wu A, Xia Y, Garzon-Muvdi T, et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology. 2018;7:e1466769. doi: 10.1080/2162402X.2018.1466769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan Y, Dong CL, Patel J, Duan C, Wang X, Wu X, Cao Y, Pu L, Lu D, Shen T, Li J. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell Physiol Biochem. 2015;35:1116–1124. doi: 10.1159/000373937. [DOI] [PubMed] [Google Scholar]
- 28.Chen HC, Lu Q, Fei XF, Shen L, Jiang D, Dai D. miR-22 inhibits the proliferation, motility, and invasion of human glioblastoma cells by directly targeting SIRT1. Tumor Biol. 2016;37:6761–6768. doi: 10.1007/s13277-015-4575-8. [DOI] [PubMed] [Google Scholar]
- 29.Zhou YF, Zhou Z, Zhang W, Hu X, Wei H, Peng J, Jiang S. SIRT1 inhibits adipogenesis and promotes myogenic differentiation in C3H10T1/2 pluripotent cells by regulating Wnt signaling. Cell Biosci. 2015;5:61. doi: 10.1186/s13578-015-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shuang T, Wang M, Zhou Y, Shi C. Over-expression of Sirt1 contributes to chemoresistance and indicates poor prognosis in serous epithelial ovarian cancer (EOC) Med Oncol. 2015;32:260. doi: 10.1007/s12032-015-0706-8. [DOI] [PubMed] [Google Scholar]
- 31.Linz U. Commentary on Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial (Lancet Oncol. 2009;10:459-466) Cancer. 2010;116:1844–1846. doi: 10.1002/cncr.24950. [DOI] [PubMed] [Google Scholar]
- 32.Gaspar N, Marshall L, Perryman L, Bax DA, Little SE, Viana-Pereira M, Sharp SY, Vassal G, Pearson AD, Reis RM, et al. MGMT-independent temozolomide resistance in pediatric glioblastoma cells associated with a PI3-kinase-mediated HOX/stem cell gene signature. Cancer Res. 2010;70:9243–9252. doi: 10.1158/0008-5472.CAN-10-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyko IV, Nakada M, Sabit H, Teng L, Furuyama N, Hayashi Y, Kawakami K, Minamoto T, Fedulau AS, Hamada J. Glycogen synthase kinase 3β inhibition sensitizes human glioblastoma cells to temozolomide by affecting O6-methylguanine DNA methyltransferase promoter methylation via c-Myc signaling. Carcinogenesis. 2013;34:2206–2217. doi: 10.1093/carcin/bgt182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.