Abstract
Triple-negative breast cancer (TNBC) lacks the expressions of estrogen receptor-α, progesterone receptor and human epidermal growth factor receptor-2. The treatment options for TNBC include anthracyclin/taxol based conventional chemotherapy and small molecular inhibitor based targeted therapy. However, the therapeutic efficacy is limited by systemic toxicity and acquired tumor resistance; identification of less toxic testable alternatives is urgently required. Non-toxic nutritional herbs are commonly used in traditional Chinese herbal medicine for general health management and may additionally represent a testable therapeutic alternative for TNBC. The present study examined the growth inhibitory efficacy of the nutritional herb Cornus officinalis (CO) in MDA-MB-231 cells, which represent a cell culture model for TNBC, and identified potential mechanistic leads. In MDA-MB-231 cells, CO induced dose-dependent cytostatic growth arrest [inhibitory concentration (IC)50, 0.1% and IC90, 0.5%], and inhibited anchorage independent colony formation. Mechanistically, CO inhibited G1 to S phase transition leading to G1 arrest and decreased the expression of cyclin D1 and phosphorylated-retinoblastoma proteins. CO additionally altered apoptosis specific BCL-2 associated X protein/B-cell lymphoma-2 expression and upregulated pro-apoptotic caspase-3/7 activity. Collectively, these data provided mechanistic evidence for the efficacy of CO, and validated a mechanism-based approach to prioritize efficacious nutritional herbs as testable alternatives for secondary prevention/treatment of TNBC.
Keywords: triple-negative breast cancer, Chinese herbal medicine, cell culture
Introduction
The American Cancer Society projections for the incidence of invasive breast cancer and mortality estimate 246,660 newly diagnosed invasive breast cancer cases and 40,450 invasive breast cancer related deaths in women in 2018 (1). Therapy resistant invasive breast cancer continues to remain the leading cause of mortality in women in the USA. The triple negative breast cancer (TNBC) represents an aggressive molecular subtype that lacks the expressions of estrogen receptor-α (ER-α), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER-2). TNBC is notable for its resistance to conventional endocrine or HER-2 targeted therapy (2–4). Current treatment options for the TNBC subtype are restricted to anthracyclin, taxol and platinum based conventional chemotherapy or to poly(ADP-ribose) polymerase (PARP), phosphadityl-inositol-3 kinase (PI3K) and mammalian target of rapamycin (m-TOR) selective inhibitor based targeted therapy (4,5). These conventional and targeted treatment options are frequently associated with long-term dose limiting systemic toxicity, de novo or acquired tumor resistance, and emergence of drug resistant cancer stem cells that impact therapeutic efficacy, and thereby, promote drug resistant disease progression (6). These limitations emphasize a need to identify novel, non- toxic treatment options as testable alternatives to existing treatment strategies.
Complementary and alternative approaches utilizing herbal medicines are being extensively used for general health issues and for management of breast cancer. Nutritional herbs represent widely used non-toxic natural substances in traditional Chinese herbal medicine (7–9). Non-toxic natural substances offer a testable alternative as potentially novel approach for secondary prevention/therapy of breast cancer, reduction of therapy associated toxicity and/or for enhanced therapeutic efficacy (8,9). Extracts from mechanistically distinct Chinese nutritional herbs have displayed growth inhibitory effects on cell culture models for Luminal A and the triple-negative molecular subtypes for clinical breast cancer (10–17).
Cornus officinalis (CO) is a nutritional herb in the form of a cherry fruit. It is used as a major ingredient in some Chinese herbal formulations for general health management purposes. Several Cornus species have documented anti-proliferative, anti-oxidant and anti-inflammatory properties. Anthocyanins are among the major bio-active agents (18,19). Additionally, CO extract exhibits growth inhibitory effects on the estrogen receptor positive Luminal A model predominantly via inhibiting estrogen stimulated growth and altering the cellular metabolism of estradiol to favor the generation of anti-proliferative metabolites (12). In an effort to evaluate the utility of CO in the TNBC model, the experiments in the present study were designed to examine the growth inhibitory effects of CO in a cell culture model for TNBC, and to identify potential mechanistic leads and molecular targets for its efficacy.
Materials and methods
Experimental model
The human mammary carcinoma derived ER-α−, PR− and HER-2− MDA-MB-231 cells (20,21) represented the model for TNBC. The MDA-MB-231 cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and were maintained in RPMI medium with L-glutamine and 5% fetal bovine serum (Life Technologies, Grand Island, NY, USA) following the protocol recommended by the vendor.
Dose response
For the dose response experiments the non-fractionated aqueous extract of CO was prepared following previously published protocol (12). Briefly, the herb was sequentially boiled in water and centrifuged to concentrate their water soluble components in the final volume of 20 ml. This 100% stock solution of CO was serially diluted in the culture medium to obtain the final concentration range of 1.0, 0.5, 0.1 and 0.05% CO. The cells were treated with the CO extract within this concentration range. The dose response of CO extract was determined by the cell viability assay using the trypan blue dye exclusion test kit (Sigma-Adrich; Merck KGaA, Darmstadt, Germany). The cell viability measurements were conducted for CO treated and control cultures at day 7 post-seeding of 1.0×105 cells. The cell viability was calculated as % viable cells=[1.00-(Number of blue cells ÷ Number of total cells)] ×100. These data were used to identify minimum effective, half maximum, maximum cytostatic and toxic concentrations. The maximum cytostatic concentration was defined by the cell viability equal to or higher than the initial seeding density. The toxic concentration was defined as the cell viability lower than the initial seeding density. The data were expressed as viable cell number (×105), relative to the initial seeding density.
Anchorage independent (AI) growth assay
This assay was performed following the optimized protocol (13,16). The stock solution of agar was prepared by mixing DNA grade agar (Sigma-Adrich; Merck KGaA) with an appropriate volume of 2X RPMI medium (Sigma-Adrich; Merck KGaA) to obtain a 6% agar stock solution. To prepare the basement layer, this stock solution was diluted to 0.6% using the culture medium, dispersed in a 6-well plate and allowed to solidify overnight at 37°C. MDA-MB-231 cell suspension, at a density of 5×105 cells per ml, was prepared in RPMI medium containing 0.33% agar, and this cell suspension was overlaid on the basement layer in the presence or absence of CO. The cultures were incubated at 37°C in a CO2 incubator for 21 days. The AI colonies were stained with 0.005% crystal violet and colony counts were determined at 10X magnification. The data were expressed as AI colony number.
Cell cycle progression
For the analysis of cell cycle progression, 5×104 cells were seeded in T-25 flasks and treated at 24 h. post-seeding with different concentrations of CO for 48 h. The cells were harvested by trypsinization, pelleted at 500 × g, and washed twice with cold phosphate buffered saline pH 7.4 (PBS; Sigma-Adrich; Merck KGaA). The cells were then fixed with cold 70% ethanol, washed with cold PBS, and stained with 50 µg/ml propidium iodide (PI; Sigma-Adrich; Merck KGaA) in PBS, followed by the addition of 10 µg/ml ribonuclease (Sigma-Adrich; Merck KGaA) and incubation for 4 h. in the dark. DNA content was analyzed by flow cytometry using Becton Dickinson FACSCAN Flow Cytometer (BD Biosciences, Research Triangle Park, NC, USA) and analyzed with FACS Express software (De Novo Software, Glendale, CA, USA). The data were expressed as G1:S+G2/M ratio.
Western blot analysis
For the western blot assay, cells were seeded in 10-cm dishes at 70% confluence 1 day before the treatment. Different concentrations of CO were added and incubated for 48 h. in a CO2 incubator at 37°C. Cells were harvested and immediately lysed with radio-immunoprecipitation assay (RIPA) buffer containing protease inhibitors (Sigma-Adrich; Merck KGaA), and centrifuged for 15 min at 10,000 × g. An equal quantity of protein was separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA., USA) and was blocked for 1 h with 5% nonfat dry milk followed by incubation with primary and secondary antibodies. The antibodies against the following proteins were used: β-actin and cyclin D1 (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) and phospho-RB (Ser 780, Cell Signaling Technology, Inc. Danvers, CA, USA). The antibodies were used following the protocol provided by the vendors. The chemo-luminescent signal was developed with ECL-plus reagent (Bio-Rad Laboratories), and detected by autoradiography. The data were expressed as arbitrary scanning unit (ASU).
Reverse transcription-polymerase chain reaction assay for gene expression
The effect of CO on the status of select apoptosis specific gene expression was evaluated using the semi-quantitative Reverse Transcription PCR assay. Gene amplification was monitored for apoptosis specific BAX and BCL-2 genes using gene specific promoters (Cepheid, Inc. Sunnyvale, CA, USA). Briefly, a total of 25 µl of reaction mix was prepared that contained MgCl2 (2 mmol/l), 12.5 µl of 2X Taq PCR Master Mix (Qiagen, Inc., Valencia, CA, USA), 0.25X SYBR dye (Thermo Fisher Scientific, Inc., Waltham, MA, USA), and gene specific primer sets (0.3 µmol/l, provided by the core facility, University of Texas Health Sciences Center, San Antonio, TX, USA). The PCR reaction was set for 40 cycles and the data were compared after normalization with β-actin RNA levels. The data were expressed as ∆∆Ct values for relative gene expression.
Caspase assay
Caspase-3/7 activity in the MDA-MB-231 cells was measured using Caspase-Glo assay kit (Promega Life Sciences, Inc., Madison, WI, USA). Briefly, the cells treated with CO were homogenized by sonication in homogenization buffer (25 mmol/l HEPES, pH 7.5, 5 mmol/l MgCl2, and 1 mmol/l EGTA) and protease inhibitors (all from Sigma-Adrich; Merck KGaA). The homogenate was centrifuged at 6,500 × g at 4°C for 15 min. To 10 µl of the supernatant containing the cellular protein, equal volume of the assay reagent was added and incubated at room temperature for 2 h. The luminescence was measured using Luminometer (Thermo Fisher Scientific, Inc.). The data were expressed as relative luminescence units (RLU).
Statistical analysis
The experiments for dose response, anchorage independent growth, caspase-3/7 activity and relative gene expression profile were conducted in triplicate. The data are expressed as the mean ± standard deviation. Statistically significant differences between the control and multiple treatment groups were assessed by one-way analysis of variance and Dunnett's test as a post hoc test with a threshold of α=0.05, using Microsoft Excel 2013 XLSTAT-Base software.
Results
Growth characteristics of MDA-MB-231 cells
The data presented in Table I summarizes the growth pattern of the experimental model. The MDA-MB-231 cells exhibited a short population doubling time of 15 h and high saturation density, exhibiting a ~33-fold increase relative to the initial seeding density. Additionally, these cells exhibited accelerated cell cycle progression as evidenced by a substantially low G1:S+G2/M ratio of 0.6, relative to a ratio of 2.3 exhibited by non-tumorigenic triple-negative 184-B5 cells (data not shown). Furthermore, unlike the non-tumorigenic 184-B5 cells, these breast carcinoma derived MDA-MB-231 cells exhibited a high number of AI colonies.
Table I.
Status of proliferation end points in a cellular model for triple-negative breast cancer.
| Proliferation end point | Model MDA-MB-231 |
|---|---|
| Doubling time (h)a | 15.0±2.2 |
| Saturation density (×105)b | 32.9±2.3 |
| G1:G2/M ratioc | 0.6±0.3 |
| AI colony numberd | 257±57 |
Determined from exponential growth phase.
Determined at day 7 post-seeding of 1.0×105 cells.
Determined from the flow cytometry-based cell cycle analysis.
Determined at day 21 post-seeding of 5×105 cells.
Mean ± standard deviation, n=3.
Mean ± standard deviation, n=18. AI, anchorage independent.
Dose response of CO
The data presented in Fig. 1A demonstrate a dose-dependent growth inhibition of MDA-MB-231 cells in response to a 7-day treatment with CO. In response to treatment with CO concentrations of 0.05, 0.1 and 1.0%, the viable cell number was reduced to 17.8±1.6×105, 12.5±1.1×105 and 2.5±0.2×105, respectively, relative to 24.8±2.3 ×105 in the control. This dose response experiment identified IC25 as 0.05%, IC50 as 0.1% and IC90 as 0.5% for CO respectively, relative to control. Statistical analysis using ANOVA and Dunnett's test confirmed that control >IC50 and control >IC90 (α=0.05). The concentration of 1% CO exhibited >98% inhibition in viable cell number. In this treatment group the viable cell number was lower than the initial seeding density of 1.0 ×105. The concentration of 1% was therefore considered toxic.
Figure 1.
Dose response of CO extract on triple-negative MDA-MB-231 cells. (A) CO induces dose-dependent inhibition of viable cell number. IC25, 0.05%; IC50, 0.1%; and IC90, 0.5%. Results are presented as the mean viable cell number (×105) ± SD. n=3 per treatment group. (B) Inhibition of anchorage independent colony formation. Results are presented as the mean anchorage independent colony number ± SD. n=3 per treatment group. Data were analyzed by ANOVA and Dunnett's test. *P<0.05 vs. control. CO, Cornus officinalis; SD, standard deviation; ANOVA, analysis of variance; IC, inhibitory concentration..
Effect of CO on anchorage independent (AI) growth
The data presented in Fig. 1B summarizes the growth inhibitory effect of CO on AI colony formation of 21 day duration in MDA-MB-231 cells. In response to treatment with CO at the pre-determined IC25, IC50 and IC90 concentrations, the number of AI colonies was reduced to 222±49, 120±27 and 40±9, respectively, relative to 255±57 AI colonies in the control. Statistical analysis using ANOVA and Dunnett's test confirmed that control >IC50 and control >IC90 (α=0.05).
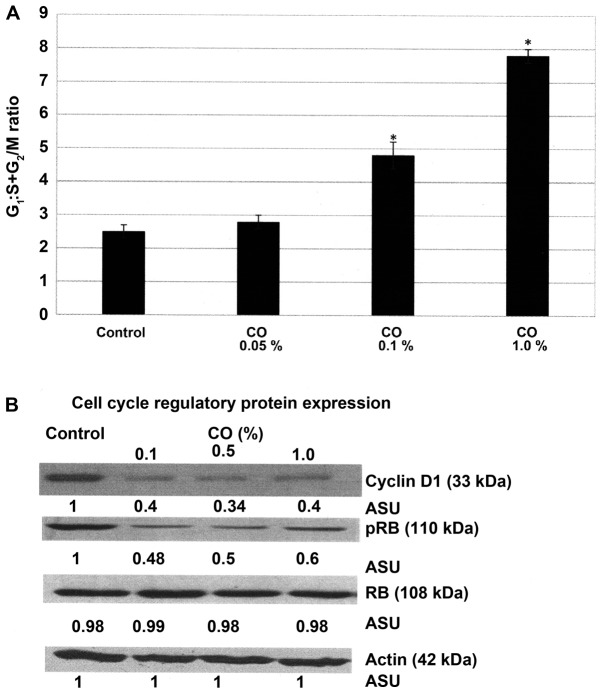
Effect of CO on cell cycle progression
The experiment presented in Fig. 2A examined the effect of CO on the cell cycle progression of MDA-MB-231 cells. The data presented as the G1:S+G2/M ratio demonstrate that a 48 h. treatment with CO was associated with a dose-dependent increase in this ratio. Thus, treatment with 0.1% CO exhibited the G1:S+G2/M ratio of 4.8±0.4, and treatment with 1.0% CO exhibited a ratio of 7.8±0.2, relative to the ratio of 2.5±0.2 exhibited by control. Statistical analysis using ANOVA and Dunnett's test confirmed that control <0.1% CO and control <1.0% CO (α=0.05). These data indicate that CO treatment induced a 92% and a 2.2-fold progressive increase due to G1 arrest and inhibition of the S and G2/M phases of the cell cycle.
Figure 2.
Effect of CO extract on cell cycle progression of MDA-MB-231 cells. (A) Treatment with CO results in a progressive dose-dependent increase in the G1: S+G2/M ratio. Results are expressed as the mean ± standard deviation. n=3 per treatment group. Data were analyzed by analysis of variance and Dunnett's test. (B) Effect of CO extract on the RB signaling pathway. Treatment with CO results in a dose-dependent inhibition of cyclin D1 and p-RB protein expression. *P<0.05 vs. control. CO, Cornus officinalis; RB, retinoblastoma; p, phosphorylated; ASU, arbitrary scanning unit.
The experiment presented in Fig. 2B examines the status of select regulatory proteins in the RB signaling pathway in response to treatment with CO. These data demonstrate that a 48 h. CO treatment resulted in ~60–66% decrease in cyclin D1 expression, and ~40–52% decrease in p-RB expression depending on CO concentration, relative to that in the untreated control cells.
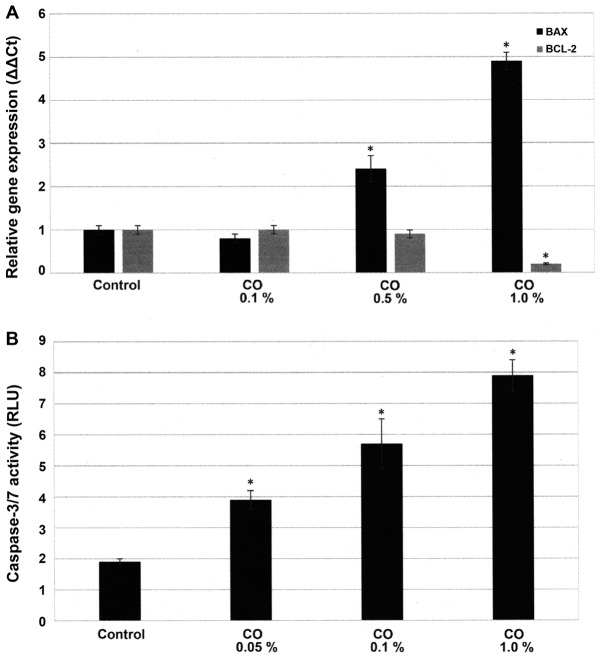
Effect of CO on cellular apoptosis
The experiment presented in Fig. 3A examines the status of apoptosis specific BAX and BCL-2 gene expressions. These data presented as ∆∆Ct values demonstrate that in response to a 48 h. treatment with 0.5% CO and 1.0% CO the expression of pro-apoptotic BAX gene was 2.5±0.2 and 4.9±0.5, while that of anti-apoptotic BCL-2 gene was 0.9±0.1 and 0.2±0.1, relative to untreated control. Statistical analysis using ANOVA and Dunnett's test confirmed that control <0.5% CO and control <1.0% CO for BAX expression (α=0.05), and control >1.0% CO for BCL-2 expression (α=0.05). Thus, these data demonstrate that in response to treatment with CO the expression of pro-apoptotic BAX gene exhibited a ~2.5- and ~2.9-fold increase, while that of anti-apoptotic BCL-2 gene exhibited a ~10 and ~80% decrease, relative to the untreated control.
Figure 3.
Effect of CO extract on apoptosis. (A) Treatment with CO extract results in upregulated expression of BAX gene and downregulated expression of BCL-2 gene. Results are expressed as mean δδ Ct ± SD. n=3 per treatment group. (B) Treatment with CO results in a dose-dependent upregulation of caspase-3/7 activity. Results are expressed as the mean RLU ± SD. n=3 per treatment group. Data were analyzed by ANOVA and Dunnett's test. *P<0.05 vs. respective control. CO, Cornus officinalis; BAX, BCL-2 associated X protein; BCL-2, B-cell lymphoma-2; RLU, relative luminescent unit; ANOVA, analysis of variance; SD, standard deviation.
The experiment presented in Fig. 3B examined the effect of a 48 h. treatment with CO on pro-apoptotic caspase-3/7 activity. The data presented as RLU demonstrate that treatment with 0.1% CO resulted in RLU value of 5.7±0.3, and treatment with 1.0% CO resulted in RLU value of 7.9±0.5, relative to RLU value of 1.9±0.1 for the control. Statistical analysis using ANOVA and Dunnett's test confirmed that control <0.1% CO and control <1.0% CO (α=0.05). Thus, treatment with CO induced a 2.0- and 3.1-fold increase in caspase-3/7 activity.
Discussion
Approximately 10–20% of clinical breast cancers lack the expressions of ER-α, PR and HER-2 and are classified as TNBC (2,4,22,23). The treatment option for the TNBC molecular subtype is predominantly restricted to conventional chemotherapy or to pathway selective small molecule based targeted therapy (3–5). Long-term therapeutic efficacy of these treatment options is compromised predominantly due to acquired tumor resistance and emergence of drug resistant cancer stem cell population (6,22–24). These limitations emphasize a need for identification of non-toxic effective alternatives as testable therapeutic options.
Human tissue derived cell culture models offer valuable mechanistic approaches to identify clinically translatable leads to the inhibitory efficacy of a number of nutritional herbs against the Luminal A and TNBC models (10–17). Compared to the non-tumorigenic triple-negative 184-B5 cells, the present TNBC model exhibits loss of homeostatic growth control and persistent cancer risk as evidenced by hyper-proliferation, accelerated cell cycle progression and increased AI colony formation. In the present TNBC model several nutritional herbs induce cytostatic growth arrest and/or cellular apoptosis and inhibit anchorage independent colony formation, thereby re-establishing cellular homeostasis and reducing cancer risk. At the mechanistic levels, growth inhibitory efficacy of these herbs involve RB, BAX/BCL-2, caspase, and RAS-RAF-MEK-ERK mediated signaling pathways (15–17).
In the present study, MDA-MB-231 cells in response to treatment with CO exhibited dose-dependent growth inhibitory effects as evidenced by cytostatic growth arrest, and reduction in AI colony formation. The data generated from dose response experiments identified the effective range of 0.05–0.5% for CO in the present model, suggesting potent growth inhibitory efficacy at relatively low doses of CO. Additionally, the cytostatic growth arrest induced by CO was associated with a progressive increase in the G1:S+G2/M ratio, indicating an effect of CO on cell cycle progression.
The data from the experiment designed to examine the effect of CO on the RB pathway clearly demonstrate that CO inhibited the expression of cyclin D1 and of phosphorylated RB (p-RB) in a dose-dependent manner. The tumor suppressor retinoblastoma (RB) gene has a well-documented function in the regulation of cell cycle progression. The tumor suppressive function of RB via the cyclin D-CDK4/6-pRB pathway has been documented to be frequently compromised in therapy resistant basal-like and triple-negative molecular subtypes of clinical breast cancer and therefore, may represent a therapeutic target (25–27). Additionally, small molecule inhibitors of CDK4/CDK6 in combination with aromatase inhibitors or with selective estrogen receptor modulators have documented clinical efficacy in hormone receptor positive metastatic breast cancer (28,29). Specific small molecule inhibitors of CDK4/CDK6 may also represent viable treatment options for TNBC (30,31). In the present study, the dose-dependent decrease in cyclin D1 and pRB expression suggests involvement of the RB pathway in the efficacy of CO. In this context it is noteworthy that kinase-dependent site specific phosphorylation of RB represents one of the major post-translational modification that is critical for its tumor suppressive function via inactivation of cell cycle progression and promotion of cellular apoptosis (25,26). It is also noteworthy that another nutritional herb Dipsacus asperoides exhibits inhibition of CDK4 and CDK6 expression in the MDA-MB-231 model for TNBC (17). Additionally, recent evidence on human TNBC samples and on patient derived TNBC xenograft models suggests that expression of EGFR, it's recently identified partner MT4-MMP and RB is strongly associated with the efficacy of a combination of EGFR and CDK4/6 based targeted therapy (32). Thus, the present data on inhibition of cyclin D1 and pRB provide mechanistic leads that the RB pathway might represent a molecular target for the efficacy of CO in the present model system.
The experiment designed to examine the effect of CO on cellular apoptosis demonstrated that treatment with CO produced a progressive increase in pro-apoptotic BAX expression, while that of anti-apoptotic BCL-2 was decreased. Additionally, this CO-mediated modulation in the BAX/BCL-2 pathway positively correlated with a dose-dependent increase in the pro-apoptotic caspase-3/7 activity. It is well established that the intrinsic mitochondrial apoptotic pathway involves altered membrane permeability, cytochrome-c release, and apoptosome mediated activation of caspase-9 and subsequently of caspase-3/7 (33,34). In the present TNBC model, treatment with CO has identified pro-apoptotic mechanistic leads for the efficacy of CO (16). Thus collectively, these data on cellular apoptosis support the evidence that induction of cellular apoptosis by CO may be via a caspase-dependent mechanism. This suggestive mechanistic lead indicates involvement of the intrinsic apoptotic cascade where expression of caspase-3/7 activity represents a late-occurring event. Investigating additional molecular pathways relevant to intrinsic apoptosis cascade represents future research directions that are focused to examine the specific effect of CO on cellular apoptosis and to investigate mechanisms and molecular targets responsible for pro-apoptotic efficacy of CO.
Traditional Chinese herbal medicine utilizes combination of several herbs for herbal formulations that are boiled in water to prepare herbal tea for consumption by the patients. Thus, non-fractionated aqueous extract of CO used in the present study simulates clinical administration of herbal formulations. In this context it is conceivable that individual constitutive herbs in the formulations may contain multiple bio-active agents with distinct growth modulatory roles. Although in the present study, non-fractionated aqueous extract from CO has exhibited potent growth inhibitory effects via potential mechanistic leads for its efficacy, little evidence is available regarding the identity of active agent(s) responsible for these effects. However, it is noteworthy that anthocyanins, representing major bio-active agents, may in part be responsible for the cellular and biochemical effects of CO (18,19).
In conclusion, the present study outcome validates a relevant cell culture model for the triple-negative molecular subtype of clinical breast cancer, and offers a facile experimental approach to prioritize efficacious natural substances as testable alternatives for endocrine therapy resistant breast cancer.
Acknowledgements
Part of the data included in the present study has been previously presented at the San Antonio Breast Cancer Symposium, December 2015. (https://cancerres.aacrjournals.org/content/76/4_Supplement/P3-09-04) (16).
Funding
Principal funding support for this research was provided by the philanthropic contributions to the American Foundation for Chinese Medicine by the Randall and Barbara Smith Foundation, and the Sophie Stenbeck Family Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
NTT conceived the study design, formulated the experimental protocols and prepared the manuscript. HBN performed the experiments, and organized and analyzed the data. GYCW selected the nutritional herb for the present study, and contributed to the data interpretation and preparation of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.American Cancer Society, corp-author. Facts & Figures. American Cancer Society Inc.; Atlanta, GA: 2017. [Google Scholar]
- 2.Sørlie T, Perou CM, Tibshirany R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baselga J, Swain SM. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 4.Dinh P, Satirou C, Piccart MJ. The evaluation of treatment strategies: Aiming at the target. Breast. 2007;16(Suppl 2):S10–S16. doi: 10.1016/j.breast.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Anders CK, Winer EP, Ford JM, Dent R, Silver DP, Siedge GW, Carey LA. Poly(ADP-ribose) polymerase inhibition: ‘Targeted’ therapy for triple-negative breast cancer. Clin Cancer Res. 2010;16:4702–4710. doi: 10.1158/1078-0432.CCR-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean M, Fojo T, Bates S. Tumor stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 7.Tindle HA, Davis RB, Phillips RS, Eisenburg DM. Trends in the use of complementary and alternative medicine by US adults: 1997–2002. Altern Ther Health Med. 2005;11:42–49. [PubMed] [Google Scholar]
- 8.Molassiotis A, Scott JA, Kearney N, Pud D, Magri M, Selvekerova S, Bruyns I, Fernadez-Ortega P, Panteli V, Margulies A, et al. Complementary and alternative medicine use in breast cancer patients in Europe. Support Care Cancer. 2006;14:260–267. doi: 10.1007/s00520-005-0883-7. [DOI] [PubMed] [Google Scholar]
- 9.Helyer LK, Chin S, Chui BK, Fitzgerald B, Verma S, Rakovitch E, Dranitsaris G, Clemons M. The use of complementary and alternative medicines among patients with locally advanced breast cancer - a descriptive study. BMC Cancer. 2006;6:39–46. doi: 10.1186/1471-2407-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee B, Telang N, Wong GY. Growth inhibition of estrogen receptor positive human breast cancer cells by Taheebo from the inner bark of Tabebuia avellanedae tree. Int J Mol Med. 2009;24:253–260. doi: 10.3892/ijmm_00000228. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Sepkovic DW, Bradlow HL, Telang NT, Wong GY. Lycium barbarum inhibits growth of estrogen receptor positive human breast cancer cells by favorably altering estradiol metabolism. Nutr Cancer. 2009;61:408–414. doi: 10.1080/01635580802585952. [DOI] [PubMed] [Google Scholar]
- 12.Telang NT, Li G, Sepkovic DW, Bradlow HL, Wong GY. Anti-proliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor-positive clinical breast cancer. Mol Med Rep. 2012;5:22–28. doi: 10.3892/mmr.2011.617. [DOI] [PubMed] [Google Scholar]
- 13.Telang N, Li G, Sepkovic D, Bradlow HL, Wong GY. Comparative efficacy of extracts from Lycium barbarum bark and fruit on estrogen receptor positive human mammary carcinoma MCF-7 cells. Nutr Cancer. 2014;66:278–284. doi: 10.1080/01635581.2014.864776. [DOI] [PubMed] [Google Scholar]
- 14.Telang N, Li G, Katdare M, Sepkovic D, Bradlow L, Wong G. Inhibitory effects of Chinese nutritional herbs in isogenic breast carcinoma cells with modulated estrogen receptor function. Oncol Lett. 2016;12:3949–3957. doi: 10.3892/ol.2016.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telang NT, Nair HB, Wong GY. Efficacy of Tabebuia avellanedae extract on a cell culture model for triple negative breast cancer. Cancer Res. 2014;74(Suppl) SABCS, P5-14-02. [Google Scholar]
- 16.Telang N, Nair HB, Wong GY. Effect of Cornus officinalis (CO) on a model for triple negative breast cancer. Cancer Res. 2015;75(Suppl) SABCS, P3-09-04. [Google Scholar]
- 17.Telang N, Nair HB, Wong GY. Efficacy of Dipsacus asperoides (DA) in a model for triple negative breast cancer. Cancer Res. 2017;77(Suppl 4) doi: 10.1158/1538-7445.SABCS16-P4-13-04. P4-13-04-P4-13-04. [DOI] [Google Scholar]
- 18.Seeram NP, Schutzki R, Chandra A, Nair MG. Characterization, quantification, and bioactivities of anthocyanins in Cornus species. J Agric Food Chem. 2002;50:2519–2523. doi: 10.1021/jf0115903. [DOI] [PubMed] [Google Scholar]
- 19.Chang JS, Chiang LC, Hsu FF, Lin CC. Chemoprevention against hepatocellular carcinoma by Cornus officinalis in vitro. Am J Chin Med. 2004;32:717–725. doi: 10.1142/S0192415X04002296. [DOI] [PubMed] [Google Scholar]
- 20.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subik K, Lee JF, Baxter L, Strzepak T, Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG, Tang P. The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer (Auckl) 2010;4:35–41. [PMC free article] [PubMed] [Google Scholar]
- 22.Hudis CA, Gianni L. Triple-negative breast cancer: An unmet medical need. Oncologist. 2011;16(Suppl 1):S1–S11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 23.Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, Blayney DW, Niland JC, Winer EP, Weeks JC. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Telang N. Putative cancer-initiating stem cells in cell culture models for molecular subtypes of clinical breast cancer. Oncol Lett. 2015;10:3840–3846. doi: 10.3892/ol.2015.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox LA, Chen G, Lee EY. Tumor suppressor genes and their role in breast cancer. Breast Cancer Res Treat. 1994;32:19–38. doi: 10.1007/BF00666203. [DOI] [PubMed] [Google Scholar]
- 26.Burkhart DL, Sage J. Cellular mechanisms of tumor suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosco EE, Knudson ES. RB in breast cancer: At the crossroads of tumorigenesis and treatment. Cell Cycle. 2007;6:667–671. doi: 10.4161/cc.6.6.3988. [DOI] [PubMed] [Google Scholar]
- 28.Sherr CJ, Roberts JM. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 29.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 30.Alves CL, Elias D, Lyng M, Bak M, Kirkegaard T, Lykkesfeldt AE, Ditzel HJ. High CDK6 protects cells from fulvestrant-mediated apoptosis and is a predictor of resistance to fulvestrant in estrogen receptor-positive metastatic breast cancer. Clin Cancer Res. 2016;22:5514–5526. doi: 10.1158/1078-0432.CCR-15-1984. [DOI] [PubMed] [Google Scholar]
- 31.VanArsdale T, Boshoff C, Arndt KT, Abraham RT. Molecular pathways: Targeting the cyclin D-CDK4/6 axis for cancer treatment. Clin Cancer Res. 2015;21:2905–2910. doi: 10.1158/1078-0432.CCR-14-0816. [DOI] [PubMed] [Google Scholar]
- 32.Foidart P, Yip C, Radermacher J, Blacher S, Lienard M, Montero-Ruiz L, Maquoi E, Montaudon E, Château-Joubert S, Collignon J, et al. Expression of MT4-MMP, EGFR, and RB in triple-negative breast cancer strongly sensitizes tumors to erlotinib and palbociclib combination therapy. Clin Cancer Res. 2018 Nov 30; doi: 10.1158/1078-0432.CCR-18-1880. (Epub ahead of print). doi: 10.1158/1078–0432.CCR-18-1880. [DOI] [PubMed] [Google Scholar]
- 33.Tait SW, Green DR. Mitochondrion and cell death: Outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 34.Ichim G, Tait SW. A fate worse than death: Apoptosis as an oncogenic process. Nat Rev Cancer. 2016;16:539–548. doi: 10.1038/nrc.2016.58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.