Abstract
Background
Liver failure after resection for liver cancer is associated with increased patient mortality. This study aimed to investigate the mechanism of the protective effects of resveratrol, a natural plant-derived compound, on liver injury in a rat model of partial hepatectomy.
Material/Methods
Adult male Sprague-Dawley (SD) rats (n=60) were divided into the sham group (n=20), the liver resection group (n=20), and the liver resection plus resveratrol-treated group (n=20). Liver resection removed 2/3 of the liver resection; resveratrol was given at a dose of 30 mg/kg/day from one week before surgery until death. Liver injury was assessed by serum liver function tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl-transferase (γ-GT) and total bilirubin, histological examination of the rat liver, and liver cell apoptosis using the TUNEL assay. High mobility group box 1 (HMGB1) expression was measured by enzyme-linked immunoassay (ELISA). Sirtuin 1 (SIRT1) and acetylated HMGB1 (Ac-HMGB1) expression were detected by Western blot. Normal human liver cells and HepG2 liver cancer cells were incubated with acetylated HMGB1, and albumin production and ammonia elimination assays were performed.
Results
Resveratrol reduced postoperative liver injury as shown by reduced ALT, AST, γ-GT, and total bilirubin levels, maintained liver structure, and reduced cell apoptosis. Resveratrol treatment reduced the expression and acetylation levels of HMGB1 via the SIRT1 signaling pathway. Resveratrol reversed Ac-HMGB1 induced dysfunction in liver cells cultured in vitro.
Conclusions
Resveratrol reduced liver damage after liver resection in a rat model by upregulating SIRT1 and reducing the acetylation of HMGB1.
MeSH Keywords: Clinical Medicine; HMGB1 Protein; Liver Failure, Acute; Sirtuin 1
Background
Worldwide, hepatocellular carcinoma (HCC), or primary liver cancer, is one of the leading causes of cancer-related death [1–3]. According to the data from International Agency for Research on Cancer, the global incidence rate of liver cancer continues to rise, with more than 222,000 new cases reported in the United States in 2016 [4,5]. Despite recent advances in diagnosis and treatment, liver cancer remains difficult to treat and has a high morbidity rate [6,7]. Patients diagnosed with early-stage liver cancer can be treated by local surgical resection or liver transplantation [7,8]. However, the high recurrence of liver cancer, which is more than 70% at 5 years, remains a major problem following treatment [9–11]. Among the available treatment options, surgical resection is the most effective and provides the best prognosis for long-term survival for patients with liver cancer [12,13]. However, major hepatic resection, defined as the resection of more than four liver segments, may result in postoperative liver failure, which is the major cause of death after liver resection [14]. The liver is highly vascular, which increases the difficulty of surgery [15]. Also, as well as bleeding, hepatic portal vein occlusion can result in ischemia-reperfusion injury resulting in postoperative damage to liver function and distal effects on the lung, kidney and other major organs [16]. Therefore, it remains important to investigate treatments to prevent postoperative liver failure.
Resveratrol, or trans-3,5,4′-trihydroxystilbene, is a compound produced by several plants that counteract environmental stress that has been shown to be a promising agent in the prevention of several human diseases, including cancer [17–19]. Polygonum cuspidatum, which contains a high level of resveratrol, is a plant that has been used for thousands of years in traditional Chinese medicine to protect against inflammation [20,21]. The effects of resveratrol were studied by Jang et al. in 1997, who showed that resveratrol could protect mice against skin cancer in an animal model [22]. Following this initial study, interest in the therapeutic effects of resveratrol gained worldwide attention. There have now been numerous in vivo and in vitro studies that have shown the anti-tumor effects of resveratrol for both cancer initiation and progression [23]. Recently, resveratrol was reported to promote organ recovery after tumor resection. However, the role of resveratrol in liver damage following liver resection remains unclear.
High mobility group box 1 (HMGB1), belonging to high mobility group protein superfamily, is a type of non-histone widely exists in eukaryotic cells [24]. HMGB1 mainly exists in the nucleus under normal circumstance, with the function of maintaining nucleosome structure, regulating DNA repair and transcription through combining with DNA specific sites [25, 26]. It was reported that HMGB1 could mediate immune and inflammatory reaction by activating nuclear factor-κB (NF-κB), and interleukin-1β (IL-1β) signaling pathways, receptor for advanced glycation end product (RAGE)-dependent signaling, and Toll-like receptor 2 (TLR2), and TLR4 [26]. HMGB1 is an important inflammatory mediator that has a critical role in liver disease, including ischemia-reperfusion injury, liver transplantation, viral hepatitis, liver fibrosis, and liver cancer [27–32]. HMGB1 can be released by the injured liver cells to prolong the inflammatory response, which can affect the progression of liver disease [33]. However, the mechanisms underlying HMGB1 synthesis, the receptors for HMGB1, and the intracellular pathway of HMGB1 remain unknown.
Sirtuin 1 (SIRT1) is a nicotinamide adenine dinucleotide-dependent histone deacetylase that participates in gene transcription, energy metabolism, aging, oxidative stress, and inflammation through deacetylation [34]. Currently, a total of seven mammalian sirtuin homologs have been identified that include SIRT1-7, which are distributed in different regions of the cell. SIRT 2, 6, and 7 are mainly located in the nucleus, SIRT 2 is mainly located in the cytoplasm, SIRT3, 4, and 5 are mainly found in the mitochondria, and SIRT1 is distributed in both the cell cytoplasm and nucleus [35]. The level of SIRT1 in normal liver tissues is very low but has been shown to be overexpressed in liver cancer tissues and cell lines, indicating that SIRT1 may play an important role in liver cancer [36].
Previous studies have shown that the process of translocation of HMGB1 from the cell nucleus to the cytoplasm could be influenced by several post-translational modifications, including acetylation, methylation, and phosphorylation [37,38]. Recently, SIRT1, as a member of NAD-dependent class III histone deacetylase (HDAC), has been reported to regulate the acetylation levels of HMGB1, and to inhibit HMGB1 nuclear translocation and release [39,40]. Therefore, this study aimed to investigate the mechanism of the protective effects of resveratrol, a natural plant-derived compound, on postoperative liver injury in rats, by evaluating HMGB1 acetylation and the role of SIRT1.
Material and Methods
Experimental animals and treatment
A total of 60 male Sprague-Dawley (SD) rats weighing 200–220 g were purchased from the University of Southern California and maintained at 24°C with a 12-hour light/dark cycle, relative humidity of 60%, and free food and water. All the experimental protocols were performed in accordance with the guidelines of the Laboratories Institutional Animal Care and Use Committee of the University of Southern California. The rats were divided into the sham group (n=20), the liver resection group (n=20), and the liver resection plus resveratrol-treated group (n=20). The rats in liver resection group and resveratrol-treated group underwent resection of two-thirds of the liver, and rats in the sham group had surgery without liver resection.
Resveratrol was given to the rats at a dose of 30 mg/kg/day from one week before surgery until death, while control rats received an equivalent volume of saline. The rats fasted on the day before surgery, and all surgery was performed in the morning. The rats were anesthetized by subcutaneous injection of urethane (1.2 g/kg) (Sigma-Aldrich, St Louis, MO, USA) and underwent surgery on the operating table while in the supine position. After opening the abdominal cavity, the left and middle lobes of the liver were exposed to ligate the pedicle, followed by excision and closure of the skin.
Cell culture
Normal human liver cells and HepG2 liver cancer cells were obtained from the Shanghai Institute of Cell Biology (Shanghai, China). All cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a cell incubator with 5% CO2 and 95% air.
Biochemical markers of liver function
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, and γ-glutamyl-transferase (γ-GT) levels in the serum samples from sham, liver resection, and resveratrol-treated groups were determined by commercial kits following the protocols obtained from the manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Blood samples were collected from each group of rats, and serum was separated by centrifuging at 5000 rpm for at least 15 min.
Enzyme-linked immunosorbent assay (ELISA) for measurement of serum levels of high mobility group box 1 (HMGB1)
The relative expression level of HMGB1 in serum samples in the sham, liver resection, and resveratrol-treated groups was evaluated using an ELISA kit obtained (Abcam, Cambridge, MA, USA), according to the manufacturer’s instructions.
Western blot
Lysis buffer was mixed with the serine protease inhibitor phenyl methyl sulfonyl fluoride (PMSF) to a final concentration at 1 mM. A total of 100 mg of fresh liver tissue was added to the lysis buffer and minced at 4°C for 15 min. After centrifuging at 14,000 rpm for 15 min, the supernatant was collected in a new Eppendorf tube. The protein concentration was determined by the bicinchoninic acid (BCA) assay, and the protein was stored at −20°C. Total protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was incubated with a primary rabbit antibody to SIRT1 (1: 10000) (ab32441, Abcam, Cambridge, MA, USA) and a primary rabbit antibody to Ac-HMGB1 (1: 1000) (LS-C413273, Lifespan, Providence, RI, USA) at 4°C overnight and washed with TBS. After incubation in the secondary antibody (1: 1000) at 37°C for 2 h, the membrane was developed using the chemiluminescence method and the band was analyzed using Image Lab software (Bio-Rad, Hercules, CA, USA).
Light microscopy with hematoxylin and eosin (H&E) staining
Rat liver tissue was fixed in 4% paraformaldehyde, dehydrated, and paraffin-embedded. Tissue sections were cut at 4 μm onto glass slides. After dewaxing in xylene and graded alcohols, the rat liver tissue sections were stained with hematoxylin and eosin (H&E) and viewed by light microscopy.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining for cell apoptosis
Rat liver tissue sections were incubated in 3% hydrogen peroxide and methanol for 10 min, and then further incubated with 0.2% Triton X-100 for 5 min. After washing twice in PBS, the tissue sections were treated with 50 μl of TUNEL reagent and incubated at 37°C in the dark for 1 h. After washing three times in PBS, the TUNEL staining for apoptosis was observed under a fluorescence microscope.
Periodic acid-Schiff (PAS) staining and human dil-acetylated low-density lipoprotein (Dil-Ac-LDL) staining
Treated normal human liver cells and HepG2 liver cancer cells were fixed with 4% paraformaldehyde and incubated with 0.5% Triton for 10 min. Then, cells were stained by PAS and Dil-Ac-LDL (Thermofisher Scientific, Waltham, MA, USA) according to the standard protocols. Images were captured using a confocal microscope (Nikon, Tokyo, Japan).
Albumin production and ammonia elimination
Albumin production in the supernatant of treated normal human liver cells and HepG2 liver cancer cells was detected by the human serum albumin DuoSet enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The ammonia concentration in the culture media was measured using an ammonia assay kit (Megazyme, Bray, Co. Wicklow, Ireland) according to the manufacturer’s protocol.
Statistical analysis
SPSS version 19.0 software was used for data analysis. Measurement data were presented as the mean ± standard deviation (SD). Gauss’s homogeneity test of variance and the variables in a normal distribution were compared by the t-test and one-way analysis of variance (ANOVA). A P-value <0.05 was considered to be statistically significant.
Results
Effects of resveratrol on biochemical markers following liver resection
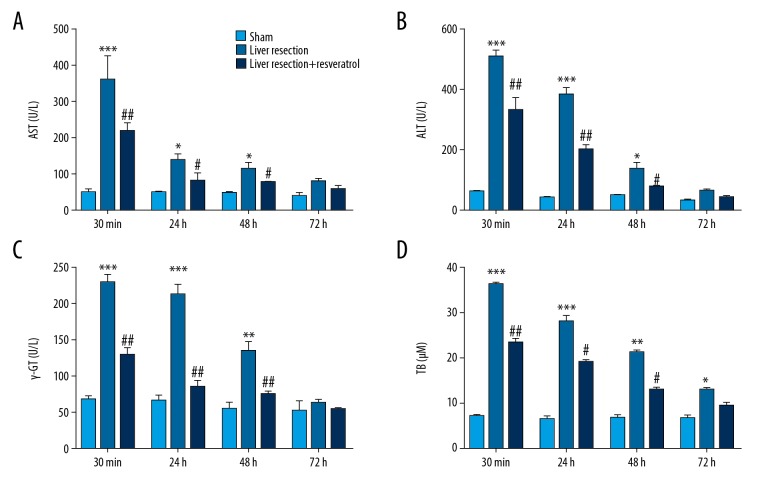
To investigate the effects of resveratrol on liver injury induced by liver resection, four biochemical markers of acute hepatic injury were evaluated in sham, liver resection, and resveratrol groups by commercial reagent kits, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl-transferase (γ-GT) and total bilirubin. There was a significant increase in serum levels of AST, ALT, γ-GT, and total bilirubin levels in the liver resection group compared with the sham group (Figure 1A–1D). In contrast, resveratrol treatment significantly reversed the effects on the upregulation of AST, ALT, and γ-GT induced by liver resection, as well as on the upregulation of total bilirubin that was induced by the loss of liver function (Figure 1A–1D).
Figure 1.
The biochemical markers of early acute hepatic damage were evaluated in the serum samples from rats following liver resection. The following were measured using a commercial kit in the sham, liver resection, and liver resection plus resveratrol-treated groups, respectively, at 30 min, 24 h, 48 h, and 72 h after resection. (A) Level of aspartate transaminase (AST). (B) Level of alanine aminotransferase (ALT). (C) Level of gamma-glutamyl transferase (γ-GT). (D) Level of total bilirubin (TB). * P<0.05, ** P<0.01, *** P<0.001 compared with the sham group; # P<0.05, ## P<0.01, ### P<0.001compared with the liver resection group.
Effects of resveratrol on liver injury by liver resection
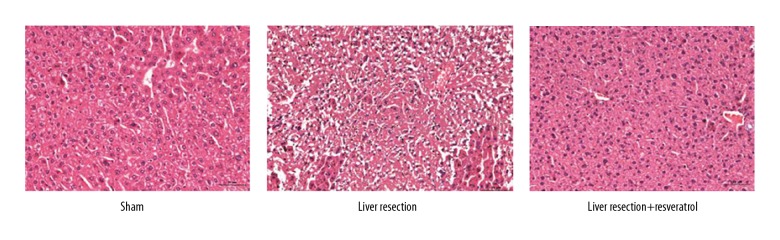
Histology of the rat liver tissue evaluated the effects of resveratrol on liver damage due to liver resection, which showed normal liver parenchyma with clearly defined liver architecture in the sham-treated rat group. In the liver resection group, the liver tissue showed histopathological changes, including disorder of liver architecture, and mononuclear cell infiltrates. Histology of the liver tissues from the rats treated with resveratrol after liver resection showed a significant improvement in the liver morphology (Figure 2).
Figure 2.
Photomicrographs of the histology of the liver tissue of rats in the sham, liver resection, and liver resection plus resveratrol-treated groups. Histology of the sham, liver resection, and liver resection plus resveratrol-treated rat groups. Hematoxylin and eosin (H&E). Magnification, ×100.
Effects of resveratrol on the rate of cell apoptosis of liver cells after liver resection
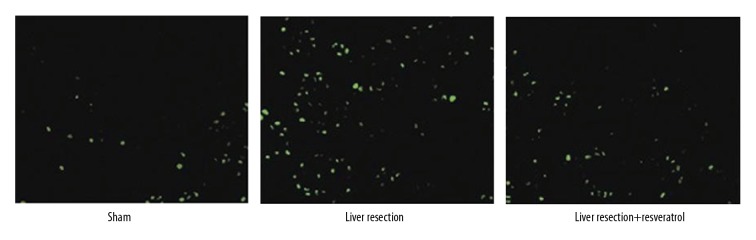
To further evaluate the effects of resveratrol on liver cell apoptosis after resection, TUNEL staining was performed. The results showed that apoptotic liver cells were significantly increased in the liver resection group compared with the sham group. However, resveratrol reduced the increased apoptosis rate induced by liver resection (Figure 3). These results indicated that resveratrol could reduce hepatic injury caused by liver resection in the rat model.
Figure 3.
TUNEL staining for apoptosis in the liver cells of rats in the sham, liver resection, and liver resection plus resveratrol-treated groups. TUNEL staining was performed to analyze cell apoptosis in sham, liver resection, and liver resection plus resveratrol-treated groups, 72 hours after surgery. Magnification, ×100.
Effects of resveratrol on serum expression levels of high mobility group box 1 (HMGB1) in the serum of rats following liver resection
High mobility group box 1 (HMGB1) expression was measured in the rat serum by enzyme-linked immunoassay (ELISA) in the sham, resection, and resveratrol-treated groups at 30 min, 24 h, 48 h, and 72 h after surgery. The results showed that the HMGB1 expression level was significantly increased in the liver resection group compared with the sham group, while treatment with resveratrol significantly reversed the upregulation of HMGB1 induced by liver resection (Figure 4).
Figure 4.
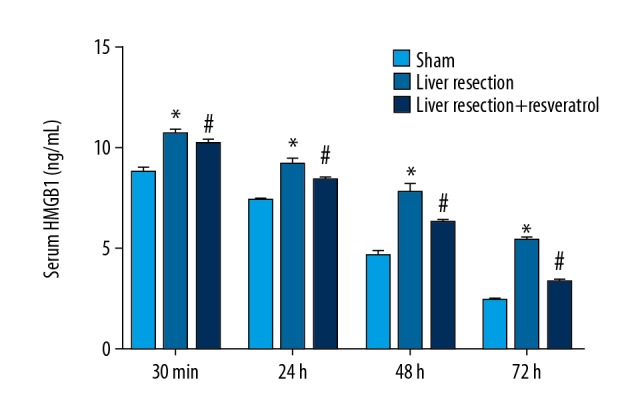
Serum levels of high mobility group box 1 (HMGB1) from sham, liver resection, and liver resection plus resveratrol-treated groups at 30 min, 24 h, 48 h, and 72 h after liver resection. * P<0.05, compared with the sham group; # P<0.05, compared with the liver resection group.
Effects of resveratrol on the expression levels of sirtuin 1 (SIRT1) and acetylated HMGB1 (Ac-HMGB1) in rat liver tissue after liver resection
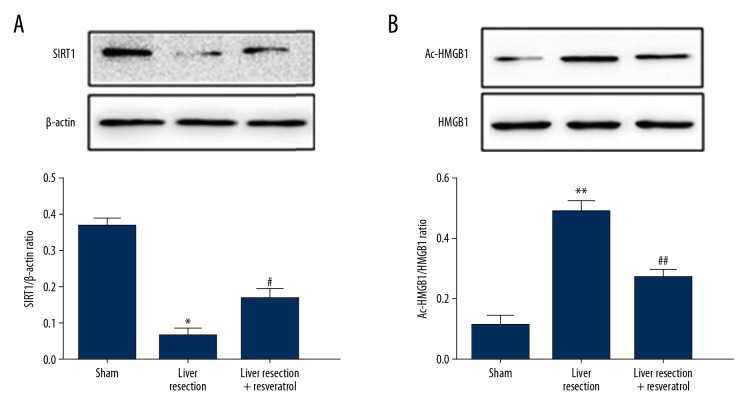
The protein expression levels of sirtuin 1 (SIRT1) and acetylated HMGB1 (Ac-HMGB1) were measured by Western blot in the sham, liver resection, and resveratrol-treated rat groups. Protein expression of SIRT1 was significantly downregulated in the liver resection group compared with the sham group. However, resveratrol treatment significantly reduced the downregulation of SIRT1 induced by liver resection (Figure 5A). Protein expression for Ac-HMGB1 was significantly increased in the liver resection group compared with the sham group, and treatment with resveratrol after surgery reversed the increase in levels of Ac-HMGB1 induced by liver resection (Figure 5B).
Figure 5.
Expression of sirtuin 1 (SIRT1) and acetylated high mobility group box 1 (Ac-HMGB1) in rat liver tissues. The protein expression of (A) sirtuin 1 (SIRT1) and (B) acetylated high mobility group box 1 (Ac-HMGB1) were assessed by Western blot in the sham, the liver resection, and the liver resection plus resveratrol-treated groups 72 h after surgery. * P<0.05, ** P<0.01, compared with the sham group; # P<0.05, ## P<0.01, compared with the liver resection group.
Effects of resveratrol on Ac-HMGB1-induced dysfunction of rat liver cells
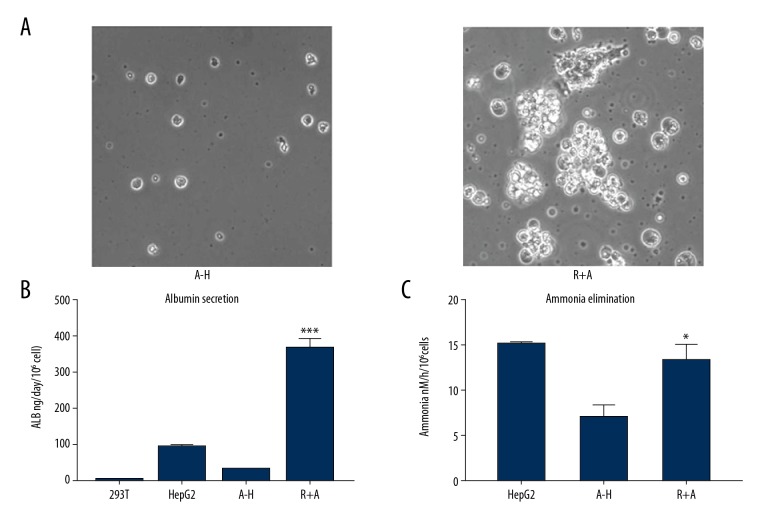
To further investigate the effects of resveratrol, cultured liver cells were treated with Ac-HMGB1 followed by evaluation of their function. Treatment with resveratrol significantly improved the state of the liver cells, reducing the damage induced by Ac-HMGB1 treatment (Figure 6A). To further assess the protective effects of resveratrol on the rat liver cells, albumin production and ammonia elimination were measured in the cultured rat liver cells treated with Ac-HMGB1 alone (A–H) or Ac-HMGB1 and resveratrol (R+A). The results showed that the albumin production was significantly increased in the R+A group compared with the A–H group, in 293T and HepG2 cells (Figure 6B). Ammonia elimination of in vitro cultured liver cells was significantly increased in the R+A group compared with the A–H group (Figure 6C). These results indicated that resveratrol could reverse Ac-HMGB1-induced dysfunction in human liver cells in vitro.
Figure 6.
Effects of resveratrol on acetylated high mobility group box 1 (Ac-HMGB1) induced dysfunction in rat liver cells. (A) Photomicrographs of the in vitro cultured rat liver cells treated with acetylated high mobility group box 1 (Ac-HMGB1) (A–H) or Ac-HMGB1 and resveratrol (R+A). (B) An enzyme-linked immunosorbent assay (ELISA) kit was used to assess the albumin levels of cultured liver cells treated with Ac-HMGB1 or Ac-HMGB1 plus resveratrol. HepG2 and 293T cells were used as the control cells. (C) An ammonia assay kit was used to determine the ammonia elimination of cultured liver cells. HepG2 cells were used as the control. * P<0.05, *** P<0.001.
Discussion
Clinically, liver resection is the most common and effective first-line treatment for liver cancer [12,41]. However, liver resection may cause tissue injury, leading to the activation of inflammatory cells and the release of cytokines and inflammatory mediators [42], which may affect liver structure and function, and lead to liver failure [15,43]. Therefore, there remains a need to develop an effective strategy to overcome the complications associated with partial liver resection. Resveratrol, or trans-3,5,4′-trihydroxystilbene, is found in several dietary sources, including grapes, berries, and peanuts, and is a compound with therapeutic effects on a wide variety of inflammatory disease, in malignancy, infection, and in ischemic injury [23,44,45]. Recently, increasing in vitro and in vivo published evidence has begun to support the effects of resveratrol on liver cancer [46,47]. In the present study, an animal model of partial surgical resection of the liver was created by the removal of two-thirds of the liver in Sprague-Dawley (SD) rats. The effects of resveratrol following partial liver resection were investigated in this animal model.
There are more than 20 types of transaminase that are the important indicators for liver function. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl-transferase (γ-GT), and total bilirubin are the most commonly used clinical indicators for the evaluation of liver function [48,49]. The alteration of ALT, AST, total bilirubin, and γ-GT following liver injury may be associated with trauma and changes in intraoperative blood flow and have an important role in the evaluation of liver function following surgery [50]. The findings of the present study indicated that the downregulation of ALT and AST, and the upregulation of total bilirubin, and γ-GT induced by surgical resection could be abolished by resveratrol treatment in the rat model. Damaged to liver tissue and increased cell apoptosis caused by surgical resection could also be reversed by resveratrol treatment, which supported the protective effects of resveratrol on the liver in the rat model.
A previously published study showed that resveratrol treatment was protective against liver injury due to sepsis, through suppression of nucleocytoplasmic translocation of high mobility group box 1 (HMGB1) by upregulating sirtuin 1 (SIRT1) expression [40]. HMGB1 is a protein that is actively secreted by activated monocytes and macrophages, or passively released by necrotic or injured cells [25,51]. HMGB1 can bind with DNA as part of the structure of chromatin in the cells, promoting specific DNA target protein assembly [52]. Post-translational modification, such as acetylation, phosphorylation, and methylation can affect the subcellular localization of HMGB1 [53]. HMGB1 acetylation plays an important role in the regulation of its secretion and release [54]. A recent study found that liver ischemia-reperfusion can significantly inhibit the activity of HDAC1, HDAC4, and HDAC5, thereby facilitating HMGB1 acetylation and release [55]. In macrophages, PARP-1 inhibits HMGB1 nuclear translocation and release by regulating HMGB1 acetylation [54]. The translocation of HMGB1 from the cytoplasm to the nucleus was found to be increased in acute liver failure, and suppression of HMGB1 levels has been shown to reduce organ damage caused by sepsis and systemic inflammation [56–58]. Also, overexpression of HMGB1 in hepatocytes significantly increased the risk of liver injury caused by sepsis [40]. In the present study, the expression and acetylation levels of HMGB1 in the liver resection group in the rat model were significantly increased when compared with the sham group, and resveratrol could abolish the overexpression and hyperacetylation of HMGB1 induced by liver resection.
SIRT1 has previously been shown to participate in multiple physiological processes, including gene transcription, energy metabolism, and inflammation [38,59,60]. Recently, studies have confirmed that SIRT1 expression induced by inflammation can promote HMGB1 acetylation, and accelerate its nuclear translocation and release [61,62]. SIRT1 has also been shown to mediate the suppression of nucleocytoplasmic translocation of HMGB1 and the protective effects of resveratrol on liver injury induced by sepsis [40]. In the present study, downregulation of SIRT1 in liver tissues induced by surgical resection was abolished by resveratrol treatment. Furthermore, the protective effects of resveratrol were evaluated in vitro in cultured liver cells, which were treated with acetylated HMGB1 (Ac-HMGB1). The results showed that the dysregulation of albumin secretion and impaired elimination of ammonia by liver cells cultured in vitro caused by Ac-HMGB1 could be reversed by resveratrol.
Conclusions
The findings from this study in a rat model of liver damage following partial liver resection, supported by in vitro studies, indicated that the natural compound, resveratrol, might have a protective role by inhibiting the expression and acetylation of high mobility group box 1 (HMGB1) by upregulating sirtuin 1 (SIRT1). The findings from this preliminary study in a rat model require support by further in vivo studies on the effects of resveratrol on liver injury caused by surgery. However, the findings indicate that resveratrol might be a compound that merits further investigation for its potential role in protecting the liver from injury following partial surgical resection.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Gravitz L. Liver cancer. Nature. 2014;516:S1. doi: 10.1038/516S1a. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Han KH, Gores G, et al. Liver cancer: Approaching a personalized care. J Hepatol. 2015;62:S144–56. doi: 10.1016/j.jhep.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut. 2014;63:844–55. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 7.Liu CY, Chen KF, Chen PJ. Treatment of liver cancer. Cold Spring Harb Perspect Med. 2015;5:a021535. doi: 10.1101/cshperspect.a021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525–35. doi: 10.1038/nrclinonc.2014.122. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 10.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–82. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology. 2012;55:476–82. doi: 10.1002/hep.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–55. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 13.Cheung TT, Poon RT, Yuen WK, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A single-center experience. Ann Surg. 2013;257:506–11. doi: 10.1097/SLA.0b013e31827b947a. [DOI] [PubMed] [Google Scholar]
- 14.Reddy SK, Barbas AS, Turley RS, et al. A standard definition of major hepatectomy: Resection of four or more liver segments. HPB (Oxford) 2011;13:494–502. doi: 10.1111/j.1477-2574.2011.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav K, Shrikhande S, Goel M. Post hepatectomy liver failure: Concept of management. J Gastrointest Cancer. 2014;45:405–13. doi: 10.1007/s12029-014-9646-3. [DOI] [PubMed] [Google Scholar]
- 16.Grimes N, Devlin J, Dunne DF, et al. The role of hepatectomy in the management of metastatic gastric adenocarcinoma: A systematic review. Surg Oncol. 2014;23:177–85. doi: 10.1016/j.suronc.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Dhar S, Kumar A, Rimando AM, et al. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget. 2015;6:27214–26. doi: 10.18632/oncotarget.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprouse AA, Herbert BS. Resveratrol augments paclitaxel treatment in MDA-MB-231 and paclitaxel-resistant MDA-MB-231 breast cancer cells. Anticancer Res. 2014;34:5363–74. [PubMed] [Google Scholar]
- 19.Ji Q, Liu X, Fu X, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/beta-catenin signal pathway. PLoS One. 2013;8:e78700. doi: 10.1371/journal.pone.0078700. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Vastano BC, Chen Y, Zhu N, et al. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J Agric Food Chem. 2000;48:253–56. doi: 10.1021/jf9909196. [DOI] [PubMed] [Google Scholar]
- 21.Burns J, Yokota T, Ashihara H, et al. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–40. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 22.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 23.Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: Focus on in vivo evidence. Endocr Relat Cancer. 2014;21:R209–25. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Guan X, Zuo X, et al. The role of high mobility group box 1 (HMGB1) in the pathogenesis of kidney diseases. Acta Pharmaceutica Sinica B. 2016;6:183–88. doi: 10.1016/j.apsb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinotti S, Patrone M, Ranzato E. Emerging roles for HMGB1 protein in immunity, inflammation, and cancer. Immunotargets Ther. 2015;4:101–9. doi: 10.2147/ITT.S58064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubaniewicz A. Microbial and human heat shock proteins as ‘danger signals’ in sarcoidosis. Human immunol. 2013;74:1550–58. doi: 10.1016/j.humimm.2013.08.275. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Kubota S, Nagaya M, et al. The role of HMGB-1 on the development of necrosis during hepatic ischemia and hepatic ischemia/reperfusion injury in mice. J Surg Res. 2005;124:59–66. doi: 10.1016/j.jss.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Ilmakunnas M, Tukiainen EM, Rouhiainen A, et al. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver Transpl. 2008;14:1517–25. doi: 10.1002/lt.21573. [DOI] [PubMed] [Google Scholar]
- 30.Jung JH, Park JH, Jee MH, et al. Hepatitis C virus infection is blocked by HMGB1 released from virus-infected cells. J Virol. 2011;85:9359–68. doi: 10.1128/JVI.00682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kao YH, Jawan B, Goto S, et al. High-mobility group box 1 protein activates hepatic stellate cells in vitro. Transplant Proc. 2008;40:2704–5. doi: 10.1016/j.transproceed.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 32.Yan W, Chang Y, Liang X, et al. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55:1863–75. doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabo G, Mandrekar P, Dolganiuc A. Innate immune response and hepatic inflammation. Semin Liver Dis. 2007;27:339–50. doi: 10.1055/s-2007-991511. [DOI] [PubMed] [Google Scholar]
- 34.Ogiku M, Kono H, Hara M, et al. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia-reperfusion in rats. J Pharmacol Exp Ther. 2011;339:93–98. doi: 10.1124/jpet.111.182592. [DOI] [PubMed] [Google Scholar]
- 35.Pulla VK, Battu MB, Alvala M, et al. Can targeting SIRT-1 to treat type 2 diabetes be a good strategy? A review. Expert Opin Ther Targets. 2012;16:819–32. doi: 10.1517/14728222.2012.703656. [DOI] [PubMed] [Google Scholar]
- 36.Portmann S, Fahrner R, Lechleiter A, et al. Antitumor effect of SIRT1 inhibition in human HCC tumor models in vitro and in vivo. Mol Cancer Ther. 2013;12:499–508. doi: 10.1158/1535-7163.MCT-12-0700. [DOI] [PubMed] [Google Scholar]
- 37.Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–97. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 38.Zeng W, Shan W, Gao L, et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci Rep. 2015;5:16013. doi: 10.1038/srep16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabadi MM, Xavier S, Vasko R, et al. High-mobility group box 1 is a novel deacetylation target of Sirtuin1. Kidney Int. 2015;87:95–108. doi: 10.1038/ki.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu W, Lu Y, Yao J, et al. Novel role of resveratrol: Suppression of high-mobility group protein box 1 nucleocytoplasmic translocation by the upregulation of sirtuin 1 in sepsis-induced liver injury. Shock. 2014;42:440–47. doi: 10.1097/SHK.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 41.Wang JH, Kuo YH, Wang CC, et al. Surgical resection improves the survival of selected hepatocellular carcinoma patients in Barcelona clinic liver cancer stage C. Dig Liver Dis. 2013;45:510–15. doi: 10.1016/j.dld.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Doussot A, Nardin C, Takaki H, et al. Liver resection and ablation for metastatic melanoma: A single center experience. J Surg Oncol. 2015;111:962–68. doi: 10.1002/jso.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin S, Fu Q, Wuyun G, Wuyun T. Management of post-hepatectomy complications. World J Gastroenterol. 2013;19:7983–91. doi: 10.3748/wjg.v19.i44.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abba Y, Hassim H, Hamzah H, Noordin MM. Antiviral activity of resveratrol against human and animal viruses. Adv Virol. 2015;2015 doi: 10.1155/2015/184241. 184241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiner GM, Ducruet AF. Resveratrol and pharmacological potentiation in ischemic stroke. Neurosurgery. 2014;74:N17–18. doi: 10.1227/01.neu.0000450234.14363.07. [DOI] [PubMed] [Google Scholar]
- 46.Delmas D, Jannin B, Cherkaoui Malki M, Latruffe N. Inhibitory effect of resveratrol on the proliferation of human and rat hepatic derived cell lines. Oncol Rep. 2000;7:847–52. doi: 10.3892/or.7.4.847. [DOI] [PubMed] [Google Scholar]
- 47.Faghihzadeh F, Hekmatdoost A, Adibi P. Resveratrol and liver: A systematic review. J Res Med Sci. 2015;20:797–810. doi: 10.4103/1735-1995.168405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang W, Wang Y, Jiang X, et al. Protective effect of flavonoids from Ziziphus jujuba cv. Jinsixiaozao against acetaminophen-induced liver injury by inhibiting oxidative stress and inflammation in mice. Molecules. 2017;22(10) doi: 10.3390/molecules22101781. pii: E1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen R, Wang J, Tang S, et al. Role of polymorphic bile salt export pump (BSEP, ABCB11) transporters in anti-tuberculosis drug-induced liver injury in a Chinese cohort. Sci Rep. 2016;6:27750. doi: 10.1038/srep27750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin YH, Ko JS, Kim GS, et al. Impact of hepatic macrovesicular and microvesicular steatosis on the postoperative liver functions after right hepatectomy in living donors. Transplant Proc. 2012;44:512–15. doi: 10.1016/j.transproceed.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Fang P, Pan HC, Lin SL, et al. HMGB1 contributes to regeneration after spinal cord injury in adult zebrafish. Mol Neurobiol. 2014;49:472–83. doi: 10.1007/s12035-013-8533-4. [DOI] [PubMed] [Google Scholar]
- 52.Magna M, Pisetsky DS. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol Med. 2014;20:138–46. doi: 10.2119/molmed.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richard SA, Jiang Y, Xiang LH, et al. Post-translational modifications of high mobility group box 1 and cancer. Am J Transl Res. 2017;9:5181–96. [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Z, Li L, Chen L, et al. PARP-1 mediates LPS-induced HMGB1 release by macrophages through regulation of HMGB1 acetylation. J Immunol. 2014;193:6114–23. doi: 10.4049/jimmunol.1400359. [DOI] [PubMed] [Google Scholar]
- 55.Zou JY, Crews FT. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS One. 2014;9:e87915. doi: 10.1371/journal.pone.0087915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou RR, Zhao SS, Zou MX, et al. HMGB1 cytoplasmic translocation in patients with acute liver failure. BMC Gastroenterol. 2011;11:21. doi: 10.1186/1471-230X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Zhu S, Zhou R, et al. Therapeutic potential of HMGB1-targeting agents in sepsis. Expert Rev Mol Med. 2008;10:e32. doi: 10.1017/S1462399408000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu QY, Yao YM. Inflammatory response and immune regulation of high mobility group box-1 protein in treatment of sepsis. World J Emerg Med. 2010;1:93–98. [PMC free article] [PubMed] [Google Scholar]
- 59.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35:146–54. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vachharajani VT, Liu T, Brown CM, et al. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J Leukoc Biol. 2014;96:785–96. doi: 10.1189/jlb.3MA0114-034RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alisi A, Nobili V, Ceccarelli S, et al. Plasma high mobility group box 1 protein reflects fibrosis in pediatric nonalcoholic fatty liver disease. Expert Rev Mol Diagn. 2014;14:763–71. doi: 10.1586/14737159.2014.928205. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Wang LK, Wang LW, et al. Cisplatin protects against acute liver failure by inhibiting nuclear HMGB1 release. Int J Mol Sci. 2013;14:11224–37. doi: 10.3390/ijms140611224. [DOI] [PMC free article] [PubMed] [Google Scholar]