Abstract
Background
The processes of mechanical ventilation-induced lung injury (VILI) triggers the release of high-mobility group box 1 (HMGB1), a prominent damage-associated molecular pattern (DAMP) family member, which can cause damage to pulmonary vascular endothelial cells. We aimed to determine whether propofol protected against endothelial cell injury induced by HMGB1 in vitro and in vivo.
Material/Methods
ICR mice (male) were mechanically ventilated for 4 h after anesthetization at both low tidal volume (LVT, 6 ml/kg) and high tidal volume (HVT, 30 ml/kg). A propofol bolus (10 mg/kg) was administered to the animals prior to the onset of ventilation, followed by infusion at 5 mg/(kg·h). We obtained confluent cultures of mouse lung vascular endothelial cells (MLVECs) and then performed cyclic stretching at 20% stretch for 4 h with or without propofol.
Results
HMGB1 reduced the expression of tight junctions between endothelial cells, including VE-cadherin and ZO-1, and increased endothelial permeability, and both were blocked by propofol. We found that MLVECs exhibited mitochondrial oxidative damage by HMGB1, which was successfully suppressed through administration of MnTBAP as well as propofol. Propofol ameliorated HVT-associated lung vascular hyperpermeability and HMGB1 production in vivo. Propofol also inhibited HMBG1 release caused by cyclic stretching in MLVECs in vitro.
Conclusions
Our results prove that the cyto-protective function of propofol protects against lung ventilation-induced dysfunction of the lung endothelial barrier. This function of propofol is mediated through inhibition of HMGB1 release caused by mechanical stretching and mitochondrial oxidative damage triggered by HMGB1.
MeSH Keywords: Acute Lung Injury; HMGB1 Protein; Mitochondria; Oxidative Stress; Propofol; Stress, Mechanical
Background
Mechanical ventilation is unavoidable for regulation of acute lung damage, severe respiratory distress syndrome, and respiratory support therapy during emergency resuscitation. However, excessive lung inflation caused by mechanical ventilation administered at increased tidal volume leads to excessive pulmonary inflation. This inflation then triggers impairment of the blood–gas barrier and enhanced permeability of lungs, which leads to ventilator-induced lung injury (VILI) [1]. This condition is characterized by infiltration of inflammatory cells in the lungs and increased permeability of pulmonary vascular endothelial cells, causing lung edema [2].
Accumulating evidence indicates that mechanical ventilation induces secretion of damage-associated molecular patterns (DAMPs), thereby contributing to lung injury [3]. High mobility group box 1 (HMGB1) is a prominent DAMP family member that is a non-histone chromatin protein exhibiting ubiquitous expression across cell types [4]. After lung injury due to mechanical ventilation or infections, this protein is released outside of cells, thereby functioning like a bioactive liaison molecule [3,5]. Work by Ogawa and colleagues [6] showed that high tidal volume mechanical ventilation triggers the release of HMGB1, finally causing lung injury. Moreover, blocking HMGB1 reduces lung endothelial hyperpermeability, suggesting the influence of HMGB1 on the vascular permeability of lungs [6].
A number of studies have shown that reactive oxygen species (ROS) generation is pivotal in the development of VILI [7,8]. Excess ROS release increases leakage from pulmonary blood vessels and exacerbates pulmonary inflammation and edema [9]. Mitochondria are the main cellular source of ROS, and because of their detrimental effects on mechanical ventilation, mitochondria have recently gained increased attention [8]. Additionally, HMGB1 has been shown to be associated with oxidative stress in diverse diseases [10,11]. As the mitochondria are the main source of ROS, the present study explored whether the effects of HMGB1 on endothelial cells are related to mitochondrial oxidative stress damage.
Since oxidative stress may be a key factor leading to endothelial dysfunction, antioxidant therapy is considered a possible treatment for vascular pathologies. Propofol (2, 6-diisopropylphenol) is an anesthetic chemical administered via the blood and is well known for applications such as sedation and hypnotic [12]. Propofol and free-radical scavengers with phenolic structure, like endogenous antioxidant vitamin E, have antioxidant properties [13]. Propofol easily enters the cellular and subcellular membranes compartments because of its natural lipophilic properties. Many studies have shown that it can protect organs by reducing the release of ROS [14,15]. The present study explored whether propofol suppresses lung vascular endothelial hyperpermeability by HMGB1. We also assessed the protective role of propofol in mitigating mitochondrial oxidative stress damage.
Material and Methods
Reagents and antibodies
Human HMGB1 (recombinant) was from R&D Systems (Minneapolis, USA). Sterile 0.1% BSA/PBS was used to resuspend the protein. Propofol, dextran conjugated with fluorescein isothiocyanate (FITC)(10-kDa), fluorescent probe JC-1, and Evans blue dye were procured from Sigma-Aldrich (St. Louis, MO). MitoSOX was supplied by Molecular Probes (Eugene, OR). VE-Cadherin and GAPDH primary antibodies were supplied by Santa Cruz Biotechnology (Santa Cruz, CA). ZO-1 primary antibodies were obtained from ProteinTech Group (Chicago, IL, USA).
Isolation and characterization of mouse lung vascular endothelial cells
We obtained ICR mice (male) from Shanghai SLAC Laboratory Animal Co. (Shanghai, China). Animals were housed at controlled room temperature with free access to food and water under a natural day/night cycle. Approval for experiments involving mice was obtained from the Experimental Animals Ethics Committee, Shanghai Jiaotong University School of Medicine (XHEC-F-NsFC201-115). We followed a modified protocol previously described to obtain mouse lung vascular endothelial cells (MLVECs) [16]. Mice (3–4 weeks old) were anesthetized using a combination of xylazine (10 mg/kg) and ketamine (70 mg/kg), after which we perfused the right ventricle using cold PBS to drain blood from the lungs. We minced peripheral subpleural lung tissue and grew cells in DMEM culture medium in the presence of 5% CO2 for 60 h at a temperature of 37°C. The growth medium was supplemented with 20% fetal calf serum, 5 mg/ml heparin, 3.7 g/L NaHCO3, 25 mM HEPES, 1 mg/ml hydrocortisone, 5 mg/ml amphotericin, 80 mg/ml of endothelial cell growth supplement obtained from bovine brains, and 0.01% ampicillin/streptomycin. Adherent cells were cultured in basal culture medium after removal of tissue debris. The cobblestone morphological feature and positive staining with specific factor VIII-related antigen (Santa Cruz) helped to identify MLVECs. We observed that most cells (about 90%) stained positive (data not shown). We used MLVEC grown for 3 to 4 passages for our work.
Western blot analysis
We used MLVECs or lung tissues to isolate proteins according to standard protocols. Briefly, samples were resuspended in RIPA buffer (Beyotime, Jiangsu, China) with the help of an ultrasonic vibrator and a mechanical homogenizer. We resolved 30 μg of protein on 10% SDS-PAGE and then blotted it to nitrocellulose membranes (Millipore Corp, Bedford, MA). After blocking, we reacted membranes with VE-Cadherin or GAPDH primary antibodies overnight at 4°C, and then performed a procedure using secondary IgG coupled with horseradish peroxidase (Santa Cruz) at room temperature for 1 h. Proteins of interest were detected using an enhanced chemiluminescence Western blotting detection system (Millipore). We estimated band signal strength with a densitometer (Syngene, Braintree, UK) along with Genesnap and Genetools software (Syngene).
FITC-dextran flux
We analyzed cellular permeability of endothelium in vitro by measuring how FTC-dextran diffused across the endothelium [17]. We obtained confluent cultures of MLVECs inside the Transwell inserts (0.4 μM, 12-mm diameter, Corning). We added medium having a final concentration of 1 mg/ml of 10-kDa FITC-dextran into the top Transwell chamber. After 1 h, the amount of dextran (conjugated with FITC) passing through the endothelium into the lower part of the Transwell chamber was estimated by a SynergyTM fluorescence plate reader (Bio-Tek, Winooski, VT, excitation 485/20 nm, emission 528/20 nm).
Flow cytometric analysis to determine expression of mitochondrial superoxide
MitoSOX is a dye that permeates through the cells and specifically binds in mitochondria and displays fluorescence if oxidized by superoxide. MLVECs were exposed to 5 μM MitoSOX for 30 min at 37°C. A FACScan flow cytometer (MACS MiltenyiBiotec) was used for flow cytometry using excitation/emission of 488/625 nm, according to a previously described method [18]. We analyzed the data with the FlowJo software package (Tree Star, San Carlos, CA).
Estimation of ATP concentration
We used a protein extraction solution (Beyotime) to obtain lysates from MLVEC samples. We centrifuged the supernatant at 10 000 g for 10 min, and used it to determine ATP concentration with an ATP bioluminescence assay from Beyotime. The readout from luciferase assay was calculated using an A tube luminometer (Tecan, Austria) to measure the signal.
Finding loss of mitochondrial membrane potential
Fluorescent probe JC-1 was used for detecting mitochondrial membrane potential. JC1 fluoresces green when existing as monomeric form within depolarized mitochondria and gives a red readout in aggregated form indicating polarized mitochondria. We stained MLVECs with 2 μM JC-1 for 15 min. We excited and detected the red and green fluorescent signals at 530/25 nm and 485/20 nm, and at 590/20 nm and 528/20 nm, respectively, through a SynergyTM fluorescent plate reader (Bio-Tek). The degree of mitochondrial membrane potential depolarization was estimated from the ratio of red to green signals. This ratio in turn reflects mitochondrial damage.
MTT assay
We calculated viability of cells using tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay, which is based upon the degree of MTT to formazan reduction (Sigma-Aldrich) catalyzed by live cells.
Immunofluorescent analysis
We used 4% formaldehyde to fix MLVECs for 15 min and permeabilized by 0.1% Triton X-100 for 15 min. We incubated fixed cells in 10% BSA for 1 h, and cells were then reacted with ZO-1 primary antibodies at a dilution of 1: 50 at 37°C for 1.5 h. After washing to remove nonspecific binding, cells were incubated with anti-rabbit IgG-FITC for ZO-1 used in 1: 1000 dilution at 37°C under dark conditions for 1 h. Cells were subjected to 3 PBS washes and exposed to 4′6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) to stain the nuclei. We captured the ICC images using a 50i Nikon fluorescence microscope (Nikon, Melville, NY).
Mechanical ventilation and propofol treatment
After anesthesia, ICR mice (male, 7–9 weeks old) were subjected to tracheotomy and then kept under ventilation using a ventilator (Inspira®, Harvard Apparatus, Boston, MA). The animals were put under ventilation either at low tidal volume [LVT] (6 ml/kg, with 140 breaths/min) or at high tidal volume [HVT]) (30 ml/kg, with 70 breaths/min) for 4 h. For propofol treatment, the animals were infused with the drug at specified amounts just before starting the ventilation. Control animals were subjected to tracheotomy but with spontaneous breathing, and were administered the same saline volume. After experimental procedures, we collected heparinized blood from the arterial line and calculated blood gas using an automated blood gas analyzer (ABL80 FLEX, Radiometer). Table 1 shows that the experimental groups were not significantly different in arterial pH, partial pressure of arterial carbon dioxide (PaCO2), or arterial oxygen partial pressure (PaO2) across experimental cohorts.
Table 1.
Arterial blood gas levels.
| Conrtol Nonventilated |
Propofol Nonventilated |
LVT 6 ml/kg |
HVT 30 ml/kg |
Propofol HVT 30 ml/kg |
|
|---|---|---|---|---|---|
| pH | 7.34±0.03 | 7.30±0.02 | 7.29±0.03 | 7.28±0.03 | 7.31±0.04 |
| PaO2 | 129.2±9.0 | 135.0±5.5 | 135.8±8.4 | 141.6±8.0 | 129.8±4.7 |
| PaCO2 | 38.6±2.3 | 39.1±3.1 | 39.7±3.9 | 36±4.6 | 34.6±3.4 |
Data are presented as mean ±SE. Arterial blood gas levels were obtained from nonventilated mice and mice ventilated at a tidal volume of 6 ml/kg (LVT) or 30 ml/kg (HVT) for 4 h (n=5~6 per group).
Bronchoalveolar lavage
We obtained bronchoalveolar lavage (BAL) fluid through a tracheal catheter in 3 consecutive 1-mL sterile saline aliquots. Protein concentrations and cell counts were estimated as per a previous protocol [19].
Lung wet-to-dry weight ratio
The ratio of lung wet-to-dry (W/D) weight was used to evaluate formation of edema in pulmonary tissues. After the experiment, we weighed pulmonary tissues immediately after removing them (wet weight). The tissues were then dried in an 80°C oven for 48 h, and the dry weight was estimated. We calculated the ratio of pulmonary wet to dry weight.
Extravasation assay of Evans blue dye
We calculated capillary permeability of lungs in animals by Evans blue dye extravasation, by a method previously published [20]. We introduced 30 mg/kg Evans blue dye into the internal jugular vein of animals 1 h prior to euthanasia. During the experiment, we collected heparinized blood through cardiac puncture, followed by PBS perfusion of the right ventricle to eliminate pulmonary intravascular dye. Lung tissue was isolated and pulverized in PBS. We extracted Evans blue using 2 volumes of formamide (Sigma-Aldrich) followed by overnight incubation at 60°C, followed by centrifuging at 5000 g for 30 min at 4°C. The absorption of Evans blue dye in the lung supernatants and plasma was measured at 620 nm and corrected for heme component as follows: A620 (corrected)=A620−(1.426×A740+0.030). We thus calculated the Evans blue index as the ratio of the amount of dye in the lungs to the concentration of plasma dye.
Lung histopathologic examination
Lung tissues were fixed with 10% formalin and embedded in paraffin for histopathological analysis. Hematoxylin and eosin (HE) staining was performed on 4-μm sections of the embedded tissue. The slides were scored by 2 blinded pathologists. The criteria for lung inflammation enumeration was done as previously described [21]. The scores given represent following extent of damage: 0 for normal tissue; 1 for minimal inflammatory alteration; 2 for mild to moderate inflammatory variation (but no obvious damage to the lung architecture); 3 for moderate inflammatory injury (marked by alveolar septae thickening); 4 for moderate to severe inflammatory damage (including nodule or patches of pneumonitis which affected the normal architecture); and 5 for grave inflammatory trauma showing complete deletion of the field of observation.
Real-time RT-PCR
We isolated cellular RNA of the MLVECs or lung tissue and converted them to cDNA. qPCR was performed by SYBRGreen dye chemistry (F Hoffmann-La Roche Ltd, Basel, Switzerland) and using the MiniOpticon™ Real-Time PCR Detection System (BioRad, Hercules, CA). We designed the primers using cDNA sequences in GeneBank. Each reaction consisted of diluted cDNA (2.0 μl), each paired primer (0.2 μmol/L), 1 U Taq DNA polymerase (Qiagen, Beijing, China), and 200 μmol/L deoxynucleotide triphosphates in PCR buffer (1×). qPCR conditions were previously standardized prior to obtain linear relationships between initial RNA concentration and PCR product. We used 58–61°C annealing temperature and amplification for 40 cycles. The melt curve was set from 60°C to 95°C. β-actin was used for internal reference. The primer sequences for HMGB1 and β-actin used were: HMGB1 forward, 5′-TGGCAAAGGCTGACAAGGCTC-3′, COX2 reverse, 5′-GGATGCTCGCCTTTGATTTTGG-3′; β-actin forward, 5′-CTGTATGCCTCTGGTCGTAC-3′, β-actin reverse, 5′-TGATGTCACGCACGATTTCC-3′. We employed the comparative Ct (threshold cycle) method with arithmetic formulae (2−ΔΔCt) to determine the relative quantitation of gene expression for target and housekeeping genes. Target gene expressions were estimated relative to the internal reference gene.
Estimation of mitochondrial DNA (mtDNA) by qPCR
We used a commercial kit (Beyotime) to extract cellular genomic DNA. The mtDNA content was estimated by qPCR. We used DNA primers to measure cytochrome oxidase 2 (COX2) and uncoupling protein 2 (UCP2) for mtDNA and nucleic DNA, respectively. We used the following primers for UCP2 and COX2: COX2 forward, 5′-TTTTCAGGCTTCACCCTAGATGA-3′, COX2 reverse, 5′-GAAGAATGTTATGTTATGTTTACTCCTA-3′; UCP2 forward, 5′-GCGACCAGCCCATTGTAGA-3′, UCP2 reverse, 5′-GCGTTCTGGGTACCATCCTAAC-3′. mtDNA content was obtained from the ratio of COX2 to UCP2 expression.
Measurement of HMGB1
We estimated the content of HMGB1 in BAL fluids and homogenates obtained from lung tissue or cell culture supernatant through a commercially available ELISA kit (Novateinbio, Cambridge, MA) following the manufacturer’s instructions.
Cyclic stretch
We obtained confluent cultures of MLVECs on Bioflex® culture plates coated with Collagen I, and then performed cyclic stretch at 20% stretch for 4 h using the Flexcell® FX-5000 Tension System as per a previously published protocol [5]. We set up the cyclic stretch of 30 cycles/min (0.5 Hz) frequency, alternating between 1 cycle of stretch and 1 cycle of relaxation. Control cells were cultured in the same 37°C incubator with 5% CO2.
Statistical analysis
We represented data as means ±S.E.M. Comparisons among all experimental groups were done using one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test. A p value <0.05 was set as statistically significant.
Results
Propofol attenuates HMGB1-induced downregulation of cell adherence junction molecule and endothelial hyperpermeability
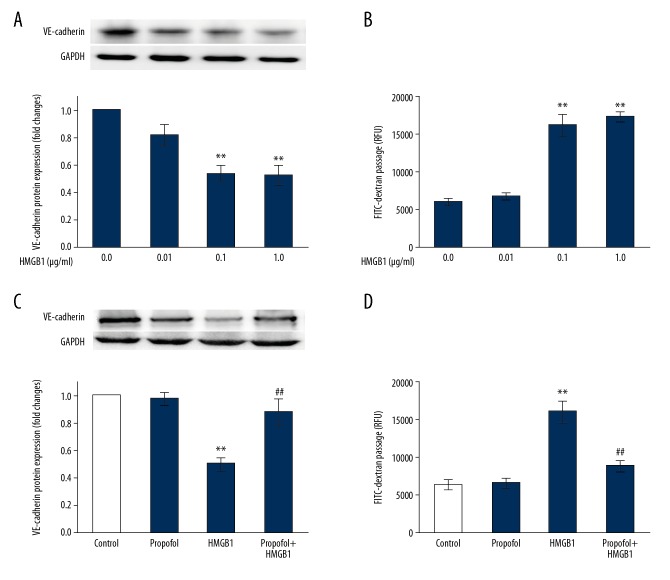
Endothelial cellular junctions regulate permeability to plasma constituents as well as circulating cells. The integrity of junctions is based on the structure possessed by junction proteins and molecules, as well their functions [22]. We began by exploring if cell adherence junction molecule vascular endothelial (VE)-cadherin expression is changed by HMGB1. As shown in Figure 1A, following treatment for 24 h, the expression of VE-cadherin protein decreased significantly with the increasing HMGB1 concentrations (0.1 μg/ml−1 μg/ml).
Figure 1.
Propofol attenuates HMGB1-induced downregulation of cell adherence junction molecule and hyperpermeability of endothelium. (A, B) Primary cultured MLVECs were cultured in presence of HMGB1 at the doses of 0.01 g/ml−1 g/ml for 24 h. VE-cadherin protein expression (A) were estimated by Western blot, and permeability of endothelial cells (B) were determined by flux analysis using FITC-dextran (n=4). (C, D) MLVECs were incubated using HMGB1 (0.1 μg/ml) in presence or absence of propofol (100 μM) for 24 h. VE-cadherin protein level (C) was estimated using Western blot, and permeability of endothelium (D) were calculated by flux analysis using FITC-dextran (n=4). Results are represented as mean ±SEM. * p<0.05, ** p<0.01 vs. Control; ## p<0.01 vs. HMGB1 (0.1 μg/ml).
To analyze the integrity of endothelial cells, we selected 10-kDa FITC-dextran to detect its permeability to the monolayer endothelium. Figure 1B shows endothelial that cellular permeability increased dose-dependently after HMGB1 (0.1 μg/ml−1 μg/ml) treatment for 24 h. To determine if the increased permeability in endothelial cells caused by HMGB1 is due to cell viability, MTT method was used to detect the percentage of viable cells after HMGB1 (0.01 μg/ml−1 μg/ml) treatment for 24 h, and we found no significant change (data not shown).
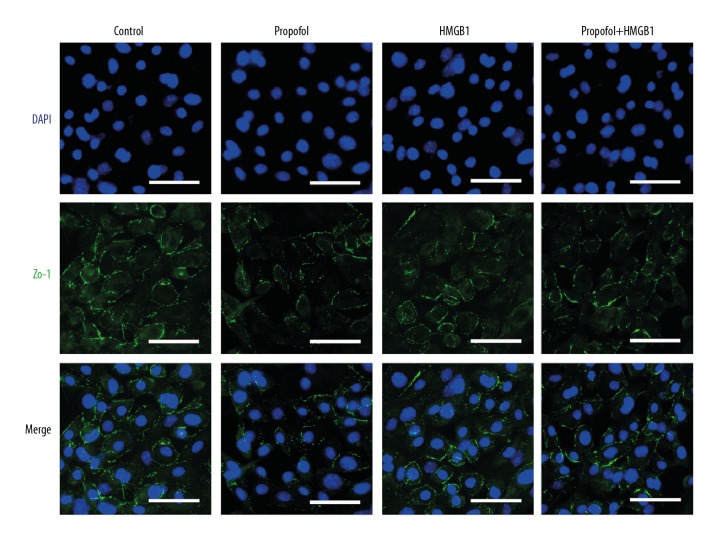
Next, we treated the cells with propofol in combination with HMGB1 and found that the drug (100 μM) reversed HMGB1-induced downregulation of cell adherence junction molecule VE-cadherin as well as endothelial hyperpermeability (Figure 1C, 1D). Meanwhile, immunocytochemistry assay showed that HMGB1 (0.1 μg/ml) administration reduced the cell-to-cell junction protein ZO-1 expression in the primary cultured MLVECs (Figure 2). Propofol also reestablished ZO-1 localization in the endothelial cellular junctions (Figure 2). Propofol treatment alone did not influence the permeability of endothelium or expression of the cell adherence junction molecule.
Figure 2.
Effect of propofol or MnTBAP on ZO-1 (a tight junction protein) disruption in primary cultured MLVECs by HMGB1. Primary cultured MLVECs were incubated in presence of HMGB1 and/or without propofol (100 μM) or MnTBAP (100 μM) for 24 h. Cells were isolated and stained with ZO-1 fluorescein isothiocyanate (FITC)-labeled antibodies (green) as well as DAPI (blue nuclear stain). Original magnification used was ×400. Scale bars correspond to 50 μm.
Mitochondrial oxidative damage participates in t HMGB1-induced downregulation of cell adherence junction molecule and endothelial hyperpermeability
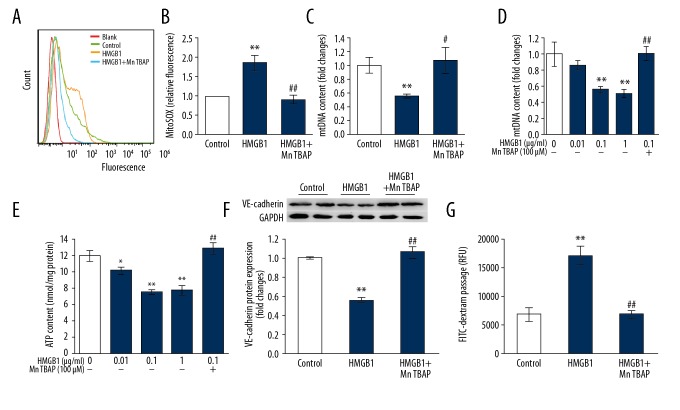
The generation of mitochondrial reactive oxygen species is detected by a specific detection probe (MitoSOX) which localizes in mitochondria only. We found that HMGB1 (0.1 μg/ml) treatment of endothelial cells resulted in a significant enhancement in mitochondrial superoxide synthesis (Figure 3A, 3B) and a significant reduction in DNA content of mitochondria (Figure 3C). Mitochondrial membrane potential (Figure 3D) and ATP content (Figure 3E) were also dose-dependently decreased after HMGB1 (0.01 μg/ml−1 μg/ml)) treatment. These results indicate mitochondrial oxidative damage is catalyzed by HMGB1.
Figure 3.
Mitochondrial oxidative injury participates in HMGB1-induced inhibition of cell adherence junction molecule and endothelial hyperpermeability. (A, B) MitoSOX staining was used to detected mitochondrial superoxide expression followed by analysis using flow cytometry. Both the flow cytometric representative histograms (A) and the results (B) are shown (n=3). (C–G) MLVECs were exposed to HMGB1 (0.1μg/ml) in presence or absence of MnTBAP (100 μM) for 24 h, after which cells were isolated for mtDNA estimation (C), diagnosis of loss of membrane potential of mitochondria (D), and ATP generation (E) (n=4). VE-cadherin protein expression (F) were calculated by Western blot, and permeability of endothelium (G) were estimated by analysis of FITC-dextran flux (n=4). Results are represented as mean ±SEM. * p<0.05, ** p<0.01 vs. Control; # p<0.05, ## p<0.01 vs. HMGB1 (0.1 μg/ml).
We further sought to understand if mitochondrial oxidative damage influenced HMGB1-mediated endothelial cell injury and whether a novel mimetic for mitochondrial superoxide dismutase 2 (SOD2) (MnTBAP) inhibited synthesis of mitochondria superoxide. MnTBAP (100 μM) was observed to almost completely block HMGB1-regulated mitochondrial superoxide synthesis and mitochondrial oxidative damage (Figure 3A–3E). Meanwhile, MnTBAP (100 μM) notably prevented VE-cadherin suppression by HMGB1 (Figure 3F) as well as endothelial hyperpermeability (Figure 3G) in MLVECs. In addition, MnTBAP (100 μM) salvaged ZO-1 presence in the endothelial cellular junctions (Figure 2). Thus, these findings predict the contribution of oxidative damage of mitochondria to HMGB1-induced suppression of cell adherence junction molecule and hyperpermeability of endothelium.
Propofol weakens mitochondrial oxidative damage catalyzed by HMGB1
To demonstrate whether the protective influence of propofol on endothelial cells is achieved by mitigating mitochondrial oxidative damage, we examined the changes of mitochondrial oxidative damage after propofol was combined with HMGB1 treatment of cells. As shown in Figure 4, propofol (100 μM) significantly attenuated mitochondrial superoxide release induced by HMGB1 and significantly reversed mitochondrial DNA content induced by HMGB1 in MLVECs (p<0.01 versus HMGB1, Figure 4A, 4B). The increased ATP content and mitochondrial membrane potential showed the molecule (100 μM) could reverse HMGB1-induced mitochondrial oxidative damage (Figure 4C, 4D).
Figure 4.
Propofol inhibits HMGB1-induced mitochondrial oxidative damage. (A, B) Primary cultured MLVECs were cultured using HMGB1 (0.1 μg/ml) in presence or absence of propofol (100 μM) for 24 h. A, MitoSOX staining was used to detect levels of mitochondrial superoxide followed by analysis using flow cytometry (n=3). B, Cells were isolated to estimate mtDNA (n=4). (C, D) MLVECs were incubated using HMGB1 (0.1 μg/ml) in presence or absence of propofol (100μM) for 24 h (n=4). Cells were isolated for estimating level of ATP production (C) and loss of mitochondrial membrane potential (D). Results are expressed as mean ±SEM. ** p<0.01 vs. Control; # p<0.05, ## p<0.01 vs. HMGB1 (0.1 μg/ml).
Taken together, our data show that it might be protective against endothelial cell injury from HMGB1 through suppressing oxidative damage of mitochondria. We nest explored whether its administration improved ventilation-triggered vascular hyperpermeability of lungs in vivo.
Propofol administration overcomes lung vascular hyperpermeability caused by ventilation of high tidal volume
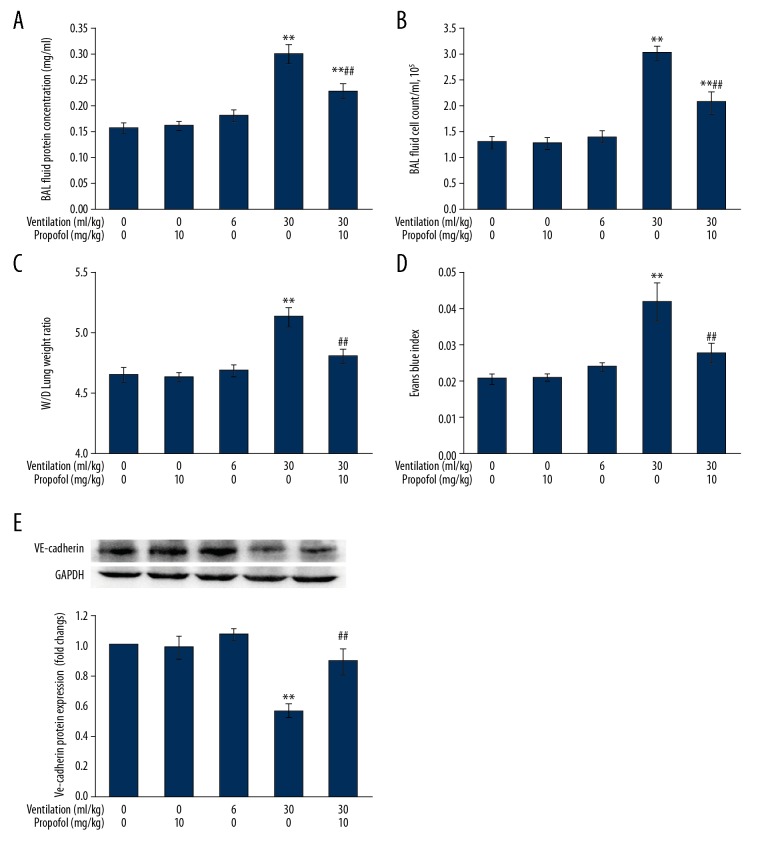
Figure 5A, 5B shows that animals subjected to HVT (30 ml/kg) displayed relevant lung injury as proven from protein content surge and higher cell count present in BAL fluid (p<0.01 compared to control cohort), which was significantly and dose-dependent suppressed by propofol treatment. Propofol (10 mg/kg) and LVT (6 ml/kg) had no influence on protein amount and cell count in BAL fluid.
Figure 5.
Propofol treatment suppresses pulmonary vascular hyperpermeability associated with ventilation at increased tidal volume. Animals were put on LVT (6 ml/kg) or HVT (30 ml/kg) ventilation for 4 h. Propofol (10 mg/kg) was infused just prior to the onset of ventilation, followed by infusion at 5 mg/(kg·h). Concentrations of protein (A) and cell count (B) were analyzed in BAL fluid. Lung W/D weight ratio (C) and Evans blue index (D) were estimated as the pulmonary edema index and vascular permeability. VE-cadherin protein expression (E) was assessed by Western blot analysis (n=7). Results are presented as mean ±SEM. ** p<0.01 vs. Control; ## p<0.01 vs. 30 ml/kg ventilation.
Protection by propofol against ventilator-mediated vascular leak was further tested from estimation of W/D lung weight ratio, Evans blue index, and levels of VE-cadherin. Figure 5C–5E shows that HVT caused a higher W/D lung weight ratio and Evans blue permeability, and also reduced synthesis of VE-cadherin (p<0.01 vs. control group), which was remarkably different from the drug administration (p<0.01 vs. HVT group).
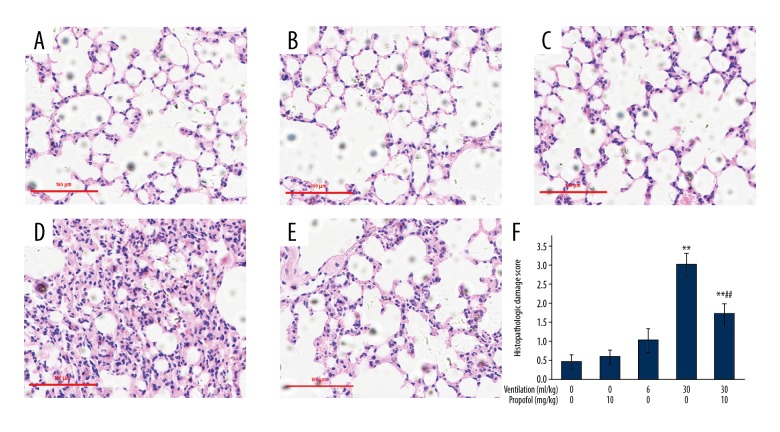
Mouse pulmonary tissue section histopathologic analysis (Figure 6) revealed HVT-induced diffused interstitial edema, thickening of alveoli, and significant reduction in air spaces of alveoli, as well as presence of leukocytes in lungs and a high value for histopathologic damage (p<0.01 vs. control group). These effects were significantly altered by propofol treatment (Figure 6E, 6F; p<0.01 vs. HVT group). Our findings indicate that propofol suppresses ventilator-induced lung vascular hyperpermeability in vivo.
Figure 6.
Propofol use improves pulmonary histopathological alterations caused by ventilation at increased tidal volume. Animals were put on LVT (6 ml/kg) or HVT (30 ml/kg) ventilation for 4 h. Propofol (10 mg/kg) was injected prior to the onset of ventilation, followed by infusion at 5 mg/(kg·h). The left lower lung was surgically isolated for histopathological analysis by HE staining. (A) Control; (B) Propofol treatment; (C) 6 ml/kg Ventilation; (D) 30ml/kg Ventilation; (E) 30ml/kg Ventilation along with propofol treatment. Original magnification, was kept at ×200. Scale bars correspond to 100 μm. (F) The extent of pulmonary injury was scored. Results are presented as mean ±SEM (n=7). ** p<0.01 vs. Control group; ## p<0.01 vs. 30 ml/kg ventilation group.
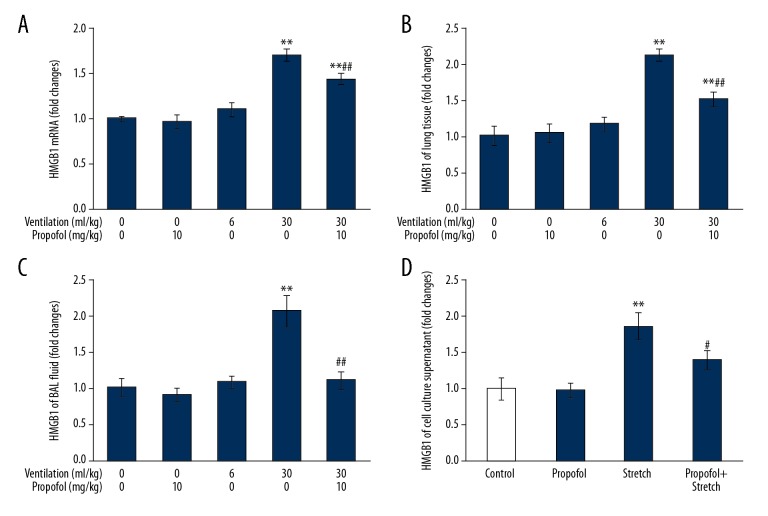
Propofol inhibits HMGB1 production in vivo and in vitro caused by mechanical stress
As shown in Figure 7, in the HVT group, HMGB1 RNA levels in lung tissues and HMGB1 protein in both lung tissues and BAL fluid were significantly higher than that in the control group (p<0.01). In addition, propofol administration inhibited ventilator-induced production of HMGB1 in pulmonary tissues and release in BAL fluid. (Figure 7A–7C). Next, we examined whether it had any effect in vitro. As expected, propofol treatment reduced HMGB1 content in cell culture supernatant upon exposure to cyclic stretch for 4 h (Figure 7D).
Figure 7.
Propofol suppresses HMGB1 production by mechanical stretch in vivo and in vitro. Animals were put on LVT (6 ml/kg) or HVT (30 ml/kg) ventilation for 4 h. Propofol (10 mg/kg) was introduced prior to onset of ventilation, followed by infusion at 5 mg/(kg·h). HMGB1 mRNA expression (A) in mouse pulmonary tissue was determined by qPCR. HMGB1 protein expression in mouse pulmonary tissues (B) and BAL fluid (C) was estimated by ELISA assay (n=7). Primary cultured MLVECs were exposed to cyclic stretch in presence or absence of propofol (100 μM) for 4 h. HMGB1 protein levels in cell culture supernatant (D) were determined by ELISA assay (n=4). Results are displayed as mean ±SEM. ** p<0.01 vs. Control; ## p<0.01 vs. 30 ml/kg ventilation; # p<0.05 compared to cyclic stretch.
Discussion
The adverse effects of mechanical ventilation can induce biological trauma and release a variety of molecules such as cytokines, chemokines, and endogenous DAMPs [2]. HMGB1, which is one of the best characterized DAMPs, contributes to the increased pulmonary vascular permeability during VILI [6]. Human and animal studies observed elevated levels of HMGB1 after mechanical ventilation [6,23,24]. Abraham et al. [25] reported that intratracheal introduction of HMGB1 resulted in pulmonary edema and lung injury in mice, while anti-HMGB1 antibody reduced the increase in vascular permeability associated with mechanical ventilation [6]. However, the mechanisms by which HMGB1 induces excessive permeability of lung endothelial cells remain largely unknown. The protein caused cytoskeletal rearrangement and barrier disruption in lung endothelial cells, as proven by previous work [26,27]. The present study demonstrates that HMGB1 induces downregulation of cell adherence junction molecules and increased lung endothelial permeability, and propofol can alleviate this effect caused by HMGB1.
We also found that HMGB1 causes mitochondrial dysfunction, including increased mitochondrial superoxide release, as well as decreased potential of mitochondrial membrane, mitochondrial DNA content, and ATP generation. MnTBAP, a mitochondrial SOD2 mimetic, can reverse HMGB1-triggered mitochondrial oxidative damage and endothelial injury. These results indicate that mitochondrial dysfunction, especially excessive production of mitochondrial superoxide, can cause HMGB1-induced pulmonary endothelial barrier malfunction.
Although many studies have reported the advantages of propofol, its therapeutic role with respect to VILI has been unknown. Our work is the first to show that propofol significantly suppresses pulmonary vascular endothelial cell injury caused by mechanical ventilation in a VILI murine system. Lung tissue is especially susceptible to oxidative damage due to its different structure and function [28]. Oxidative stress is involved in pulmonary edema, especially during mechanical ventilation-induced lung injury [1,29]. Excessive ROS production oxidizes different biomolecules, causing oxidative damage to different cellular structures such as mitochondria [30]. Propofol, an intravenous anesthetic agent, can exert anti-oxidation effects such as the endogenous antioxidant vitamin E [13]. It has been found that the drug can reduce hepatic I/R injury and mitochondrial dysfunction by inhibiting HIF-1α [31]. In addition, in a rat model of acute lung injury caused by LPS, propofol protected rats and human alveolar epithelial cells against HMGB1 expression [32]. In accord with such reports, our in vitro work proved that it almost absolutely altered HMGB1-triggered downregulation of cell adherence junction molecule and endothelial hyperpermeability via reducing the release of mitochondrial ROS and mitigating damage due to oxidative stress of mitochondria in primary cultures of murine lung vascular endothelial cells.
Our study shows that propofol inhibits HMGB1 production in endothelial cells induced through mechanical stress, as well as reduced mitochondrial oxidative damage caused by HMGB1. There may be several signaling pathways involved. Recent research shows that the Nrf2/HO-1 pathway plays an important role in inhibiting secretion and production of HMGB1. For example, glycyrrhizin decreases HMGB1 secretion via p38/Nrf2-dependent activation of HO-1 in RAW 264.7 cells activated by lipopolysaccharides and in endotoxemic mice [33]. In addition, Zaouali et al. [34] demonstrated decreases in SIRT1 with HMGB1 and increased autophagy in steatotic livers, contributing to increased tolerance to cold IRI. Moreover, Lu et al. [35] report that the JAK/STAT1 pathway to be required for LPS- or interferon-induced nuclear translocation of HMGB1. However, the detailed mechanisms require more studies for correct delineation.
It should be pointed out that high tidal volume ventilation has been abandoned in clinical practice at present. The reason for the use of high tidal volume VT 30 ml/kg is based on the following 2 considerations. First, although normal tidal volume can also cause lung injury, after all, the damage is mild, which is not conducive to the study of acute pathogenesis. Second, most of the lung parenchyma of ARDS patients have lost ventilation function, and normally ventilated lung tissue accounts for less than one-third. Small tidal volume ventilation (VT: 6 mL/kg) is commonly used in these patients, and the actual ventilation capacity of normal lung tissue will be more than 20 mL/kg [36].
Conclusions
In summary, our current work demonstrated that HMGB1 could trigger ROS generation in mitochondria and mitochondrial malfunction, thus increasing lung vascular endothelial permeability. We explored possible advantages of propofol in suppressing the ventilator-induced pulmonary vascular edema and leakage caused by ventilation. Propofol protects the pulmonary endothelial barrier through suppression of secretion of HMGB1 and mitochondrial oxidative injury from HMGB1.
Footnotes
Source of support: The study was supported by the Shanghai Sailing Program (No. 16YF1407300)
Conflict of interest
None.
References
- 1.Vadász I, Brochard L. Update in acute lung injury and mechanical ventilation 2011. Am J Respir Crit Care Med. 2012;186:17–23. doi: 10.1164/rccm.201203-0582UP. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Xu CF, Liu YJ, et al. Salidroside attenuates ventilation induced lung injury via SIRT1-dependent inhibition of NLRP3 Inflammasome. Cell Physiol Biochem. 2017;42:34–43. doi: 10.1159/000477112. [DOI] [PubMed] [Google Scholar]
- 3.Kuipers MT, van der Poll T, Schultz MJ, Wieland CW. Bench-to-bedside review: Damage-associated molecular patterns in the onset of ventilator-induced lung injury. Crit Care. 2011;15:235. doi: 10.1186/cc10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi JH, Seo GS, Cheon JH, Lee SH. Isoliquiritigenin inhibits TNF-α-induced release of high-mobility group box 1 through activation of HDAC in human intestinal epithelial HT-29 cells. Eur J Pharmacol. 2017;796:101–9. doi: 10.1016/j.ejphar.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Dong WW, Liu YJ, Lv Z, et al. Lung endothelial barrier protection by resveratrol involves inhibition of HMGB1 release and HMGB1-induced mitochondrial oxidative damage via an Nrf2-dependent mechanism. Free Radic Biol Med. 2015;88:404–16. doi: 10.1016/j.freeradbiomed.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa EN, Ishizaka A, Tasaka S, et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 2006;174:400–7. doi: 10.1164/rccm.200605-699OC. [DOI] [PubMed] [Google Scholar]
- 7.Davidovich N, DiPaolo BC, Lawrence GG, et al. Cyclic stretch–induced oxidative stress increases pulmonary alveolar epithelial permeability. Am J Respir Cell Mol Biol. 2013;49:156–64. doi: 10.1165/rcmb.2012-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker JC. Mitochondrial damage pathways in ventilator induced lung injury (VILI): An update. J Lung Health Dis. 2018;2:18–22. [PMC free article] [PubMed] [Google Scholar]
- 9.Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal. 2009;11:1651–67. doi: 10.1089/ars.2008.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang D, Kang R, Zeh HJ, III, Lotze MTL. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14:1315–35. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, Tang D, Kang R. Oxidative stress-mediated HMGB1 biology. Front Physiol. 2015;6:93. doi: 10.3389/fphys.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Wu Q, You L, et al. Propofol attenuates pancreatic cancer malignant potential via inhibition of NMDA receptor. Eur J Pharmacol. 2017;795:150–59. doi: 10.1016/j.ejphar.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Braz MG, Braz LG, Freire CMM, et al. Isoflurane and propofol contribute to increasing the antioxidant status of patients during minor elective surgery: A randomized clinical study. Medicine (Baltimore) 2015;94(31):e1266. doi: 10.1097/MD.0000000000001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu HT, Tseng YT, Hsu YY, et al. Propofol attenuates lipopolysaccharide-induced reactive oxygen species production through activation of Nrf2/GSH and suppression of NADPH oxidase in human alveolar epithelial cells. Inflammation. 2015;38:415–23. doi: 10.1007/s10753-014-0046-4. [DOI] [PubMed] [Google Scholar]
- 15.Yu W, Gao D, Jin W, et al. Propofol prevents oxidative stress by decreasing the ischemic accumulation of succinate in focal cerebral ischemia – reperfusion injury. Neurochem Res. 2018;43:420–29. doi: 10.1007/s11064-017-2437-z. [DOI] [PubMed] [Google Scholar]
- 16.Xiang M, Shi X, Li Y, et al. Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J Immunol. 2011;187:4809–17. doi: 10.4049/jimmunol.1102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem. 2009;284:25602–11. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai DF, Johnson SC, Villarin JJ, et al. Mitochondrial oxidative stress mediates angiotensin II–induced cardiac hypertrophy and Gαq overexpression – induced heart failure. Circ Res. 2011;108:837–46. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birukova AA, Fu P, Chatchavalvanich S, et al. Polar head groups are important for barrier-protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007;292:L924–35. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- 20.Tager AM, LaCamera P, Shea BS, et al. The lysophosphatidic acid receptor LPA 1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Wei J, You X, et al. Inhibition of hydrogen sulfide generation contributes to lung injury after experimental orthotopic lung transplantation. J Surg Res. 2013;182:e25–33. doi: 10.1016/j.jss.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–22. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 23.Peltz ED, Moore EE, Eckels PC, et al. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock (Augusta, Ga) 2009;32:17. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Souza ABF, Chírico MTT, Cartelle CT, et al. High-fat diet increases HMGB1 expression and promotes lung inflammation in mice subjected to mechanical ventilation. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/7457054. 7457054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham E, Arcaroli J, Carmody A, et al. Cutting edge: HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–54. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 26.Wolfson RK, Chiang ET, Garcia JGN. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res. 2011;81:189–97. doi: 10.1016/j.mvr.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luan Z, Hu B, Wu L, et al. Unfractionated heparin alleviates human lung endothelial barrier dysfunction induced by high mobility group box 1 through regulation of P38-GSK3β-snail signaling pathway. Cell Physiol Biochem. 2018;46:1907–18. doi: 10.1159/000489375. [DOI] [PubMed] [Google Scholar]
- 28.Langen RCJ, Korn SH, Wouters EFM. ROS in the local and systemic pathogenesis of COPD. Free Radic Biol Med. 2003;35:226–35. doi: 10.1016/s0891-5849(03)00316-2. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Gross C, Desai AA, et al. Endothelial cell signaling and ventilator-induced lung injury: Molecular mechanisms, genomic analyses, and therapeutic targets. Am J Physiol Lung Cell Mol Physiol. 2017;312:L452. doi: 10.1152/ajplung.00231.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhat AH, Dar KB, Anees S, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother. 2015;74:101–10. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Bellanti F, Mirabella L, Mitarotonda D, et al. Propofol but not sevoflurane prevents mitochondrial dysfunction and oxidative stress by limiting HIF-1α activation in hepatic ischemia/reperfusion injury. Free Radic Biol Med. 2016;96:323–33. doi: 10.1016/j.freeradbiomed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Liu C, Wang G. Propofol protects rats and human alveolar epithelial cells against lipopolysaccharide-induced acute lung injury via inhibiting HMGB1 expression. Inflammation. 2016;39:1004–16. doi: 10.1007/s10753-016-0330-6. [DOI] [PubMed] [Google Scholar]
- 33.Kim YM, Kim HJ, Chang KC. Glycyrrhizin reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and endotoxemic mice by p38/Nrf2-dependent induction of HO-1. Int Immunopharmacol. 2015;26:112–18. doi: 10.1016/j.intimp.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Zaouali MA, Panisello A, Lopez A, et al. Cross-talk between Sirtuin 1 and high-mobility box 1 in steatotic liver graft preservation. Transplant Proc. 2017;49:765–69. doi: 10.1016/j.transproceed.2017.01.071. [DOI] [PubMed] [Google Scholar]
- 35.Lu B, Antoine DJ, Kwan K, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci USA. 2014;111:3068–73. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank JA, Matthay MA. Science review: Mechanisms of ventilator-induced injury. Crit Care. 2003;7:233–41. doi: 10.1186/cc1829. [DOI] [PMC free article] [PubMed] [Google Scholar]