Abstract
A significant fraction of internalized transferrin (Tf) concentrates in the endocytic recycling compartment (ERC), which is near the microtubule-organizing center in many cell types. Tf then recycles back to the cell surface. The mechanisms controlling the localization, morphology, and function of the ERC are not fully understood. We examined the relationship of Tf trafficking with microtubules (MTs), specifically the subset of stable, detyrosinated Glu MTs. We found some correlation between the level of stable Glu MTs and the distribution of the ERC; in cells with low levels of Glu MTs concentrated near to the centriole, the ERC was often tightly clustered, whereas in cells with higher levels of Glu MTs throughout the cell, the ERC was more dispersed. The clustered ERC in Chinese hamster ovary cells became dispersed when the level of Glu MTs was increased with taxol treatment. Furthermore, in a temperature-sensitive Chinese hamster ovary cell line (B104-5), the cells had more Glu MTs when the ERC became dispersed at elevated temperature. Microinjecting purified anti-Glu tubulin antibody into B104-5 cells at elevated temperature induced the redistribution of the ERC to a tight cluster. Microinjection of anti-Glu tubulin antibody slowed recycling of Tf to the cell surface without affecting Tf internalization or delivery to the ERC. Similar inhibition of Tf recycling was caused by microinjecting anti-kinesin antibody. These results suggest that stable Glu MTs and kinesin play a role in the organization of the ERC and in facilitating movement of vesicles from the ERC to the cell surface.
INTRODUCTION
Appropriate recycling of membrane proteins and lipids is essential for maintaining the distinct composition of various membranes, for regulating the uptake of nutrients such as glucose and iron, and for the maintenance of cell polarity (Mukherjee et al., 1997). Recycling receptors such as the transferrin receptor (TfR) or the low-density lipoprotein receptor enter cells via coated pits and are then delivered to early sorting endosomes. Many membrane constituents, including recycling receptors, are removed rapidly from the sorting endosomes. In a study of the trafficking of fluorescent lipid analogs in a Chinese hamster ovary (CHO) cell line (Hao and Maxfield, 2000), it was found that ∼40% of internalized C6-NBD-sphingomyelin is returned to the plasma membrane with a t1/2 of 1–2 min. The rest is removed from sorting endosomes at a similar rate (Mayor et al., 1993), but it is delivered to the endocytic recycling compartment (ERC) and then to the cell surface. The ERC is a separate, morphologically and functionally distinct part of the early endosome system that contains recycling receptors but not material targeted to lysosomes for degradation, such as low-density lipoprotein or α2-macroglobulin (Yamashiro et al., 1984; Yamashiro and Maxfield, 1987; McGraw et al., 1993). Electron microscopy studies show that the ERC is made up of mainly narrow-diameter tubules (Hopkins and Trowbridge, 1983; Yamashiro et al., 1984). In CHO cells the ERC is a collection of tubular endosomes concentrated near the microtubule-organizing center (MTOC), and it appears as a compact fluorescent cluster in epifluorescence microscopy.
The localization and organization of the ERC may be functionally important in various cell types. For example, cholesterol is enriched in ERC membranes (Mukherjee et al., 1998) and a high concentration of acyl-coA-cholesterol acyl transferase, the enzyme that esterifies cholesterol, is found in organelles close to the ERC (Khelef et al., 1998, 2000). This proximity may serve to deliver cholesterol to acyl-coA-cholesterol acyl transferase, thus facilitating a rapid response to elevations in plasma membrane cholesterol (Khelef et al., 1998). The localization of the ERC and the trans-Golgi network (TGN) near the MTOC may facilitate transport from endosomes to the trans-Golgi network (Johannes et al., 1997; Ghosh et al., 1998). In polarized epithelia, the organization and localization of elements of recycling compartments may facilitate transcytosis and recycling (Mostov, 1995).
In many cell types, the ERC is concentrated near the MTOC, although the degree of enrichment near the cell center varies among different cell lines. For example, in HEp2 cells the ERC is observed as tubules that are scattered throughout the cytoplasm (Hopkins et al., 1990; Ghosh and Maxfield, 1995). A striking alteration in the morphology of the ERC was observed in a temperature-sensitive mutant CHO cell line, B104-5 (McGraw et al., 1993). At temperatures below 32°C, the ERC had the compact appearance seen in wild-type CHO cells, but at 39°C, the ERC was dispersed throughout the cytoplasm. The dispersal had no measurable effect on the kinetics of transferrin recycling. In both the B104-5 cells and in HEp2 cells the pathway of transferrin recycling is very similar to the pathway in CHO cells except for the distribution of the ERC (McGraw et al., 1993; Ghosh and Maxfield, 1995).
Although both late endosomes and the ERC are concentrated near the cell center, they maintain distinct distributions. The basis for their precise distribution near the cell center is not known. The late endosomes are maintained near the MTOC by the action of the minus-end directed motor, cytoplasmic dynein (Lin and Collins, 1992), and presumably a cytoplasmic dynein also moves recycling membranes toward the MTOC (Burkhardt et al., 1997). To recycle back to the plasma membrane, recycling membranes must reverse direction and move toward the plus-ends of microtubules (MTs), presumably using a kinesin motor. Treatment with microtubule-depolymerizing reagents (e.g., nocodazole) causes dispersion of the ERC, but the kinetics of Tf endocytosis and recycling are unaffected (McGraw et al., 1993). This suggests that translocation on microtubules is not the rate-determining step in recycling from the ERC. However, the normal distribution of the ERC is affected, and it is likely that recycling occurs efficiently and rapidly in nocodazole-treated cells because the recycling membranes never travel far from the plasma membrane.
It seemed possible that the distribution of the ERC might be related to association with a subset of microtubules different from those that are associated with late endosomes and lysosomes. Interphase cells contain a subset of microtubules that are more stable than most microtubules and contain high levels of tubulin that has been modified by removal of the COOH-terminal tyrosine by a carboxypeptidase, leaving a Glu at the COOH terminus (Argarana et al., 1978). These modified microtubules (Glu MTs) can be labeled by antibodies that are selective for the detyrosinated tubulin (Gundersen et al., 1984). Detyrosination is not the cause of the stabilization of MTs but a consequence of MT stability and the fact that the enzyme that retyrosinates tubulin acts only on soluble tubulin (Gundersen et al., 1987; Webster et al., 1987a,b; Khawaja et al., 1988; Bulinski and Gundersen, 1991). It has been found that kinesin binds to Glu MTs with higher affinity than tyrosinated (Tyr) MTs (Liao and Gundersen, 1998). The binding of kinesin would make Glu MTs ideal tracks for the transport of recycling membrane to the plasma membrane.
In this study, we examined the roles of both Glu and Tyr MTs in Tf endocytosis and recycling by fluorescence microscopy and by microinjecting anti-Glu or anti-Tyr tubulin antibodies. We showed that there is a partial correlation between the morphological appearance of the ERC and the level of stable Glu MTs in several cell lines. In CHO cells, we found that the internalization of Tf does not depend on stable Glu MTs, but the overall ERC morphology is affected by the level of stable Glu MTs. A decreased rate of Tf exit from the ERC was found in cells microinjected with function-blocking anti-Glu tubulin antibody or with anti-kinesin antibody but not with anti-Tyr tubulin antibody, indicating that stable Glu MTs and kinesin play a specific role in regulating export from the ERC.
MATERIALS AND METHODS
Cell Culture and Treatment
TRVb-1 cells, a CHO cell line lacking the endogenous TfR and stably expressing the human TfR (McGraw et al., 1987), were grown in bicarbonate-buffered Ham's F-12 medium (Invitrogen, Carlsbad, CA) containing 5% fetal bovine serum (FBS), 100 U/ml penicillin-streptomycin, and 200 μg/ml Geneticin as a selection for the transfected Tf receptors. The cells were grown at 37°C in a humidified atmosphere of 5% CO2. B104-5 cells, a temperature-sensitive mutant cell line isolated from parental TRVb-1 cells, were grown in Ham's F-12 medium containing 5% FBS (McGraw et al. 1993). HeLa cells and African green monkey kidney cells, TC-7, were cultured in DMEM supplemented with 10% FBS medium as described (Gundersen et al., 1984). Taxol and nocodazole (Sigma, St. Louis, MO) were prepared as stock solutions in DMSO and diluted into growth medium so that the final concentration of DMSO did not exceed 0.1% (vol/vol). This concentration of DMSO had no effect on the distribution or levels of Glu or Tyr tubulin and membrane traffic in these cells.
Fluorescent Labeling
Human transferrin (Sigma) was iron loaded and purified by Sephacryl S-300 (Amersham Biosciences AB, Uppsala, Sweden) gel-filtration chromatography and conjugated to Cy3 and Alexa488 according to the manufacturer's instruction (Amersham Biosciences AB or Molecular Probes, Eugene, OR, respectively). Bovine serum albumin (BSA) (Sigma) was conjugated to Alexa488 according to the manufacturer's instruction (Molecular Probes). Fluorescent anti-rat or anti-rabbit IgG antibodies were purchased from either Pierce Chemical (Rockford, IL) or Molecular Probes.
Immunofluorescence
TRVb-1, TC-7, or HeLa cells were grown to subconfluence on poly-d-lysine–treated coverslips affixed to holes cut in the bottoms of the tissue culture dishes. For Tf labeling, cells were incubated with 10 μg/ml Cy3-Tf for 1 h at 37°C to reach steady state or for 10 min before fixation. Immediately before fixation, cells were rinsed in medium 1 (150 mM NaCl, 20 mM HEPES, 1 mM CaCl2, 5 mM KCl, and 1 mM MgCl2 pH 7.4) or PBS, fixed in 3.3% paraformaldehyde freshly diluted in PBS for 1 min, and permeabilized for 5 min with −20°C methanol. Glu and Tyr MTs in cells were visualized by indirect fluorescence with rabbit polyclonal antibodies specific for Glu (anti-Glu) (SG) and Tyr (anti-Tyr) (W2) tubulin (Gundersen et al., 1984). For triple labeling cells with Tyr, Glu, and Tf, a rat monoclonal antibody specific for Tyr tubulin (YL1/2) (Kilmartin et al., 1982) was used. In some microinjection experiments, Alexa488- (Molecular Probes), Cy3-, or Cy5-conjugated (Jackson Immunoresearch Laboratories, West Grove, PA) secondary antibodies were used at 1:200 dilution.
Microscopy and Imaging Processing
Wide field fluorescence microscopy was performed on a DMIRB inverted microscope (Leica, Deerfield, IL.) by using a 63× 1.32 numerical aperture plan Apochromat objective. The excitation on the Leica microscope was by a 100-W Hg arc lamp (Leica) with standard fluorescein and rhodamine optics. Images were taken with a cooled charge-coupled device camera (Pentamax 512EFTB frame transfer camera with a 512 × 512 back-thinned EEV; Princeton Instruments, Trenton, NJ). Confocal images were collected on an LSM510 laser scanning confocal unit (Carl Zeiss, Thornwood, NY) attached to an Axiovert 100 M inverted microscope (Carl Zeiss) with a 63× 1.4 numerical aperture plan Apochromat objective (Carl Zeiss). Excitation on the LSM510 laser confocal microscope was with 25-mW Argon laser emitting 488 nm, a 1.0-mW helium/neon laser emitting at 543 nm, and a 5.0-mW helium/neon laser emitting at 633 nm. Emissions were collected using a 505–530-nm band pass filter to collect Alexa488, a 560–615-nm band pass filter to collect Cy3 emission, and a 650-nm long pass filter to collect Cy5. Typically, 0.3–0.5-μm vertical steps were used with axial resolution <1.0 μm. Images were processed using MetaMorph image processing software (Universal Imaging, West Chester, PA). Cross talk of the fluorophores was negligible.
Microinjection
TRVb-1, B104-5, and TC-7 cells were pressure microinjected with affinity-purified (10 mg/ml) anti-Glu (SG), anti-Tyr (W2) rabbit antibodies prepared as described (Gurland and Gundersen, 1995). The anti-kinesin antibody used in this study, HD antibody, was provided by F.K. Gyoeva (Institute of Protein Research, Russian Academy of Science, Moscow, Russia) and has been shown to react with more than one kinesin (Gyoeva and Gelfand, 1991). TRVb-1 and TC-7 cells were pressure microinjected with this anti-kinesin antibody as described (Kreitzer et al., 2000). In some experiments, Alexa488-BSA (0.2 mg/ml) was coinjected to provide a marker for the injected cells. Microinjection was performed as described previously (Gurland and Gundersen, 1995; Mikhailov and Gundersen, 1995). Before injection, antibodies were centrifuged (100,000 × g) for 15 min at 4°C to remove aggregates. We estimated that 5–10% of the cell volume was introduced into injected cells. After microinjection, cells were always rinsed three times in medium 1 before the subsequent procedures (Tf uptake, Tf chase, or fixation). The estimated time between the microinjection of cells and the beginning of the subsequent procedure was 5–10 min. To test the effect on microinjection of anti-Glu and anti-Tyr tubulin antibodies on the distribution of ERC, B104-5 cells were labeled with 10 μg/ml Cy3-Tf for 1 h followed by injection with antibodies. Cells were then fixed, permeabilized, and labeled with Alexa488-conjugated goat anti-rabbit secondary antibody at 1:2000/4000 dilution. To test the effect of microinjected anti-Glu, Tyr tubulin antibodies, or anti-kinesin antibody on uptake of Tf, cells were first injected with antibodies and then washed three times with medium before incubated in 10 μg/ml Cy3-Tf for 10 min at 37°C before fixation. To measure the effect of microinjected anti-Glu or Tyr antibodies and anti-kinesin antibody on Tf recycling, cells were incubated in 10 μg/ml Cy3-Tf for 1 h at 37°C before microinjection. Cells were injected with antibodies in the presence of 10 μg/ml Cy3-Tf. After microinjection, cells were washed three times with medium before chased in growth medium without Cy3-Tf but in the presence of excess unlabeled Tf and desferroxamine for indicated periods of time before fixation. To test the effect of microinjected anti-kinesin antibody on Tf recycling in nocodazole (NZ), cells were incubated in 10 μg/ml Cy3-Tf for 30 min at 37°C followed by a 1-h incubation in 10 μg/ml Cy3-Tf with 20 μM NZ before microinjection. Cells were injected with anti-kinesin antibody in the presence of 10 μg/ml Cy3-Tf and 20 μM NZ. After microinjection, cells were washed three times with medium with 20 μM NZ before chase in growth medium without Cy3-Tf but in the presence of 20 μM NZ, excess unlabeled Tf, and desferroxamine for 1 h.
Image Quantification
All the image analysis was carried out using the Image-1/MetaMorph Imaging System software. To measure the Tf recycling kinetics in control cells or cells microinjected with anti-Glu tubulin antibody, fluorescent images of the same cells were collected at different time points by using wide-field fluorescence microscopy. Before quantifying the amount of fluorescent probes present in cells, the background in the image was removed by subtracting the median pixel intensity determined from a region in the image lacking cells. The mean Cy3-Tf intensity per outlined region (cells) was then measured and plotted using Microsoft Excel. The same method was used to measure cell-associated Tf after treatment with NZ and then with or without microinjection with anti-kinesin antibody. Error bars represent the SD.
RESULTS
Distribution of ERC Correlates with Level of Stable Glu MTs in Different Cell Types
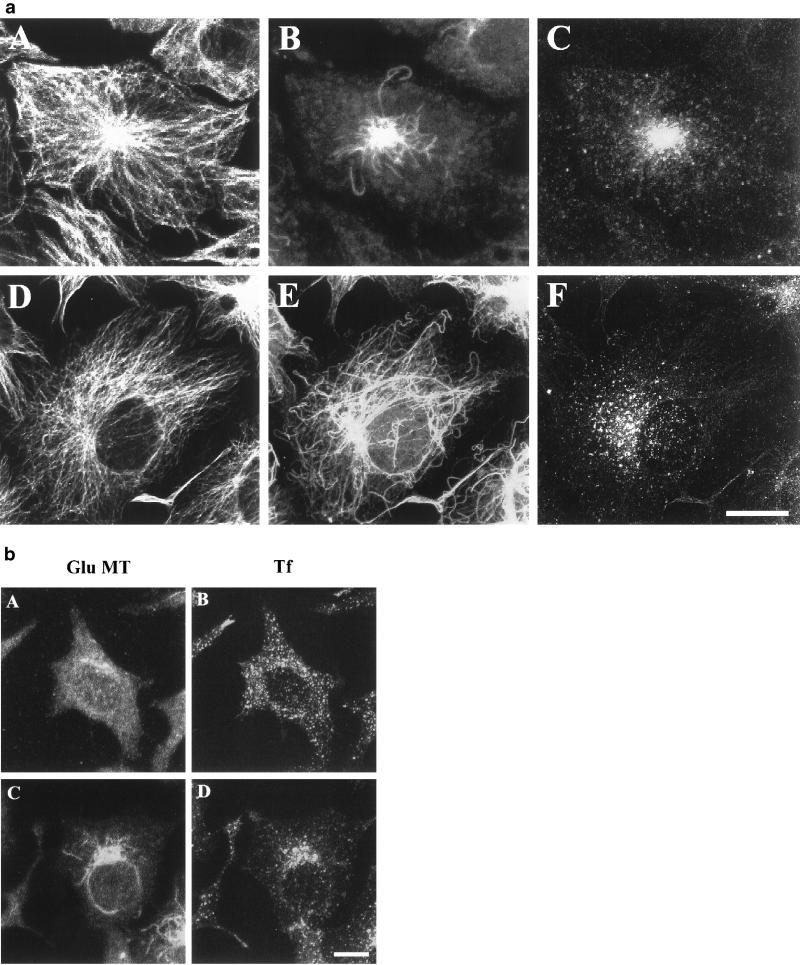
We examined the relationship between the distribution of the ERC with MTs in TRVb-1 and TC-7 cells, a cell type previously used to study the role of Glu MTs (Gurland and Gundersen, 1993). Cells were incubated with Cy3-Tf for 1 h at 37°C to label the ERC. The cells were then fixed, permeabilized, and immunostained for MTs. The fixation and permeabilization methods used were a compromise between retaining the immunostaining of MTs and maintaining the labeled Tf. This results in some granularity in MT staining, perhaps due to steric blocking by membrane vesicle proteins. The methods used retain a high fraction of labeled Tf in a distribution essentially indistinguishable from living cells. As illustrated in Figure 1a, the ERC is concentrated near the MTOC of TRVb-1 cells (Figure 1a, A–C). TRVb-1 cells have relatively low levels of stable Glu MTs concentrated in the perinuclear region of the cells (Figure 1a, B). When the images of Cy3-Tf–labeled ERC (Figure 1a, C) were superimposed with Glu MTs (Figure 1a, B), colocalization was evident. When similar analysis was performed on TC-7 cells (Figure 1a, D–F), the distribution of the ERC was not as tightly concentrated at the MTOC as in TRVb-1 cells. Concomitantly, a more extended network of stable Glu MTs was observed in TC-7 cells (Figure 1a, E). A similar correlation was also observed in B104-5 cells (Figure 3), COS-7, and normal rat kidney cells in which a dispersed ERC correlated with an extended network of Glu MTs (our unpublished data). In A549 cells, a human carcinoma epithelial lung cell line, the expression of Glu MTs was highly variable within the population of cells, and the ERC was more dispersed in cells expressing high levels of Glu MTs (our unpublished data). However, in HEp2 cells, a human carcinoma cells in which Tf-containing endosomes have been shown to be widely dispersed (Hopkins et al., 1990; Ghosh and Maxfield, 1995), no Glu MTs were detected (our unpublished data). Interestingly, when the distribution of ERC and Glu MTs were examined in HeLa cells, the expression of Glu MTs was also highly variable within the population of cells. In some HeLa cells, as observed in HEp2 cells, almost no Glu MTs were detected (Figure 1b, A), and the ERC was widely dispersed throughout the cytoplasm (Figure 1b, B). However, in some other HeLa cells, we observed detectable Glu MTs, and in these cells the distribution of ERC was largely correlated with the network of Glu MTs (Figure 1b, C and D). This morphological survey suggests that there is a general, but imperfect, correlation between the overall distribution of ERC and the amount and distribution of stable Glu MTs. We carried out a series of studies to determine more precisely what role stable Glu MTs play in the distribution and function of the ERC.
Figure 1.
Correlation of ERC distribution with a subset, detyrosinated Glu MTs in different cell lines. a, TRVb-1 (A–C) and TC-7 (D–F) cells were incubated with Cy3-Tf (C and F) for 1 h at 37°C to reach steady state before fixation. Cells were then fixed, permeabilized, and stained with rat monoclonal anti-Tyr tubulin (A and D) and rabbit polyclonal anti-Glu tubulin (B and E) antibodies followed by incubation with Alexa488-conjugated goat anti-rat and Cy5-conjugated goat anti-rabbit secondary antibodies. Projections of confocal z-section images are shown. b, HeLa cells were incubated with Cy3-Tf for 1 h at 37°C to reach steady state before fixation. Cells were permeabilized and stained with rabbit polyclonal anti-Glu tubulin (A and C) antibody followed by incubation with Alexa488-conjugated goat anti-rabbit antibody. Projections of confocal images were shown. Bar, 10 μm.
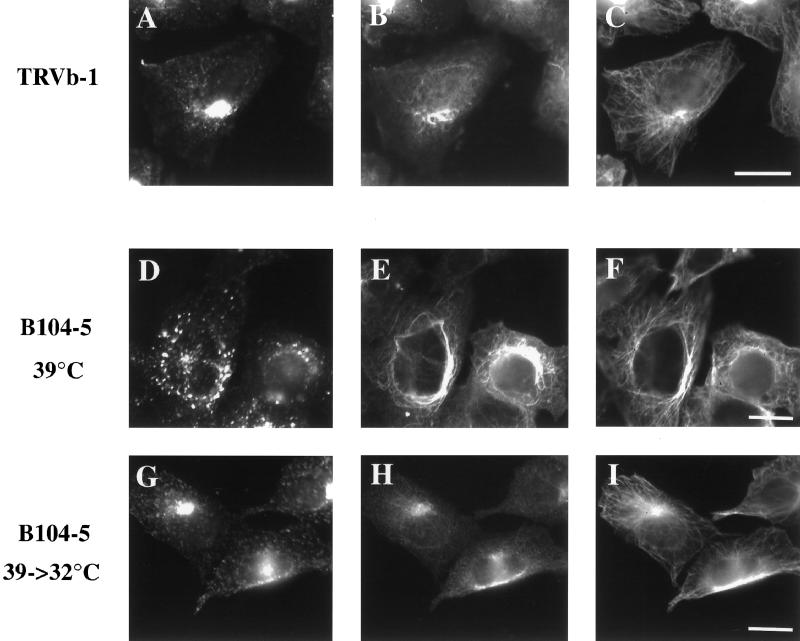
Figure 3.
Distribution of ERC, stable Glu MTs, and Tyr MTs at permissive and elevated temperatures in B104-5 cells. (A–C) TRVb-1 cells were incubated with Cy3-Tf (A) for 1 h at 37°C before fixation. (D–F) B104-5 cells were grown at permissive temperature (32°C) before shifting to nonpermissive temperature (39°C) for 4 h. Cells were then incubated with Cy3-Tf (D) for 1 h at 39°C (D–F) or returned to 32°C for 4 h (G–I) in the presence of Cy3-Tf (G). After fixation, all cells were permeabilized and stained with polyclonal rabbit anti-Glu tubulin antibody followed with Cy5-goat anti-rabbit secondary antibody (B, E, and H) and rat monoclonal followed with Alexa488-anti-rat secondary antibody (C, F, and I). Bar, 10 μm.
Low Concentration of Taxol Causes Redistribution of ERC in Cells
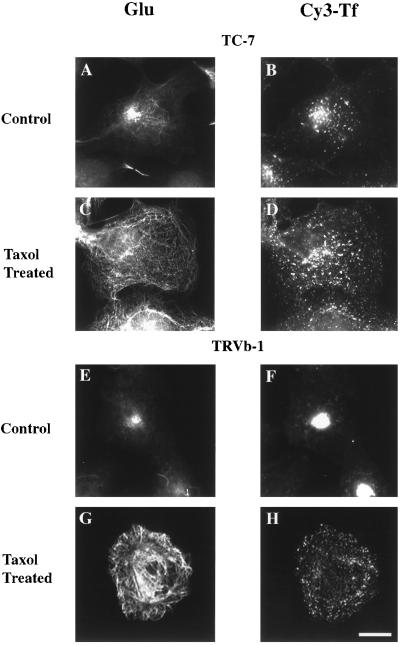
To further test whether differences in the distribution of the ERC are due to the different levels of stable MTs, we treated both TC-7 and TRVb-1 cells with low concentrations of taxol. This inhibits the rapid turnover of dynamic MTs and results in an increased level of stabilized Glu MTs (Gundersen et al., 1987). As shown in Figure 2, an increased level of Glu-MTs was evident in cells treated with 1 μM taxol (Figure 2, C and G). Compared with the ERC distribution in untreated cells (Figure 2, B and F), more dispersed Tf-containing vesicles were seen in cells treated with taxol (Figure 2, D and H). In both cell types internalized Tf was found in scattered vesicles throughout the cell after taxol treatment. We found that the amount of Tyr MTs was decreased in these taxol-treated cells, but the overall distribution of Tyr MTs was not grossly affected (our unpublished data). These results show that within a single cell line the redistribution of the ERC correlates with an increased level of stable Glu MTs induced by taxol treatment in cells.
Figure 2.
Effect of taxol (1 μM) on ERC. TC-7 (A–D) cells were untreated (A and B) or treated with 1 μM taxol for 1 h (C and D) and incubated with Cy3-Tf for 10 min. TRVb-1 (E–H) cells were untreated (E and F) or treated with 1 μM taxol for 4 h (G and H) and then incubated with Cy3-Tf for 10 min. Cells were then fixed, permeabilized, and stained with rabbit polyclonal anti-Glu tubulin antibody followed by Cy5-conjugated goat anti-rabbit secondary antibody (A, C, E, and G). Cy3-Tf labeling is shown in B, D, F, and H. Bar, 10 μm.
Alteration of ERC Morphology in Mutant TRVb-1 Cell Line (B104-5) Correlates with Increased Level of Glu MTs
The preceding results indicate that there is a correlation between the levels of stable Glu MTs and the appearance of the ERC and suggest that stable Glu MTs might play a role in recycling membrane localization or transport. To further examine this hypothesis, we used a previously isolated, temperature-sensitive mutant cell line, B104-5, which has a striking temperature-induced alteration in the morphology of the ERC (McGraw et al., 1993). At the restrictive temperature a dispersed ERC is observed that reverts to the wild-type distribution at lower temperatures. These characteristics allowed us to directly investigate the correlation between stable Glu MTs and the distribution of the ERC without drug treatment. As shown in Figure 3, at 39°C, B104-5 cells exhibited a dispersed ERC labeled with Cy3-Tf (Figure 3D) as shown previously (McGraw et al., 1993). Concomitantly, at the higher temperature an elevated level of Glu MTs was detected in the cells (Figure 3E). When the temperature was lowered to 32°C for 4 h (Figure 3, G–I), the morphology of the ERC (Figure 3G) became similar to that seen in the parental cells (Figure 3A). Lowering the temperature in B104-5 cells also returned the level of Glu MTs to a level comparable to that of the parental cells (Figure 3, B and H). The recovery of ERC localization began after shifting cells to 32°C for 1 h (our unpublished data). Although it is unclear what mechanism regulates the levels of the Glu MTs in B104-5 cells, it is clear that the temperature switch induces concomitant changes in the distribution of the ERC and the level of Glu MTs. Although we did not detect an obviously increased level of Tyr MTs in B104-5 cells at the higher temperature, we noticed that the appearance of the overall Tyr MTs was changed to some degree. It appears that the Tyr MTs have a tendency to wrap around the nucleus, as do the Glu MTs, in the B104-5 cells at elevated temperature.
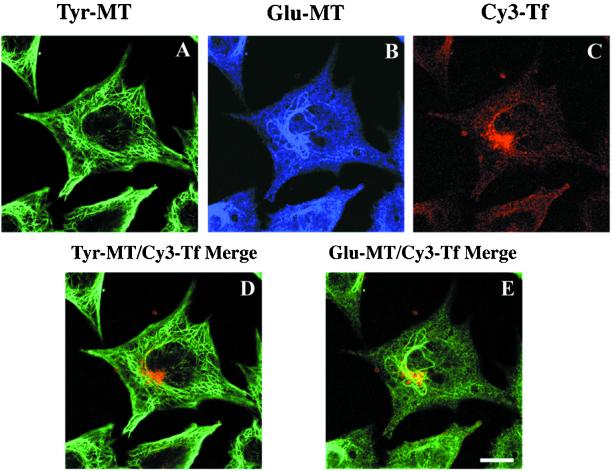
The elevated level of Glu MTs in B104-5 cells and the dispersed ERC localization at the restrictive temperature permit a better examination of the association of the ERC with Glu MTs and Tyr MTs. We triple labeled B104-5 cells with monoclonal anti-Tyr tubulin antibody, polyclonal anti-Glu tubulin antibody, and Cy3-Tf, and cells were examined using confocal microscopy. Single confocal planes were collected to analyze the degree of colocalization of the ERC with Glu MTs and with Tyr MTs. As shown in Figure 4, a strong correlation of the ERC position with Glu MTs is evident from the yellow staining in superimposed images. In all cells examined, the region enriched in ERC was associated with stable Glu MTs. In contrast, only limited colocalization of the ERC with Tyr MTs was observed. Results from these experiments further establish the association of the ERC with stable Glu MTs.
Figure 4.
Confocal microscopy analysis of B104-5 cells triple labeled with Cy3-Tf, anti-Tyr, and anti-Glu tubulin antibodies. B104-5 cells were shifted to 39°C for 4 h before incubation with Cy3-Tf for an additional hour at 39°C. Cells were then fixed, permeabilized, and stained with rat monoclonal anti-Tyr tubulin and rabbit polyclonal anti-Glu tubulin antibodies followed with Alexa488-conjugated goat anti-rat and Cy5-conjugated goat anti-rabbit secondary antibodies. Confocal images of triple-labeled cells were taken as z-series. In A–C, confocal images of Tyr MTs, Glu MTs, and Tf at a single focal plane. In bottom panel, confocal images from the same focal plane, shown in the top panel, were superimposed. For a direct comparison, Cy3-Tf (red) was superimposed either with Tyr-MT (green) (D) or Glu-MT (also green, pseudocolored from Cy5 labeling shown in the top panel) (E). Bar, 10 μm.
Role of Glu MT and Tyr MT in ERC Distribution
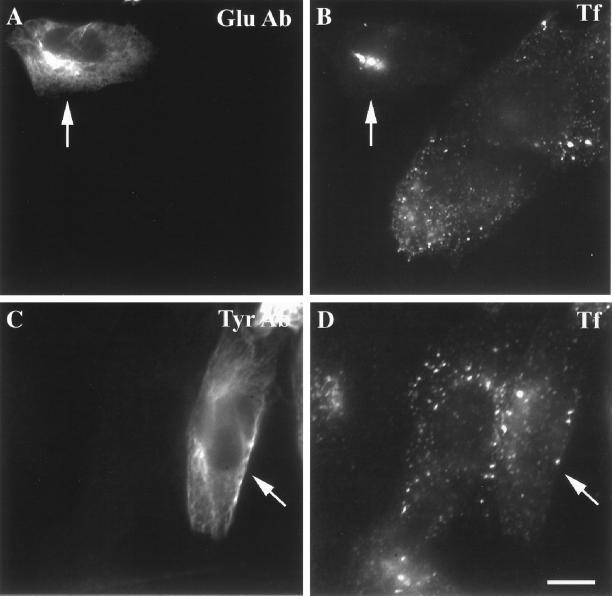
To directly examine the specific role of Glu MTs and Tyr MTs in the ERC distribution, we microinjected function-blocking anti-Glu tubulin or anti-Tyr tubulin antibodies. This allowed us to assay the specific function of these classes of MTs in membrane traffic. Previously, cells microinjected with the anti-Glu tubulin antibody were shown to have collapsed intermediate filaments with no effect on the overall interphase MTs, including the Glu MTs (Gurland and Gundersen, 1995). B104-5 cells, grown at 39°C for 4 h were incubated with Cy3-Tf for an additional hour at 39°C, followed by microinjection with anti-Glu tubulin antibody (Figure 5, top) or anti-Tyr tubulin antibody (Figure 5, bottom). As shown in Figure 5, cells injected with anti-Glu tubulin antibody had a Tf-labeled ERC that was condensed near the MTOC compared with the surrounding uninjected cells that have the characteristically dispersed ERC. On the other hand, no consistent changes were observed in the ERC morphology in anti-Tyr tubulin antibody-injected cells. In some cells there was a decrease in the amount of Tf in the ERC, but this was variable. It is important to point out that in the anti-Glu or anti-Tyr tubulin antibody-injected cells, only a subset of microtubules became labeled without masking the whole microtubule array or grossly perturbing MT function (also see Gurland and Gundersen, 1995). In addition, with both low and high anti-Tyr tubulin antibody-injected cells, we did not observe significant bundling of MTs.
Figure 5.
Effect of microinjected anti-Glu tubulin antibody on the ERC distribution in B104-5 cells. B104-5 cells were shifted to 39°C for 4 h followed by incubation with Cy3-Tf (B and D) for 1 h at 39°C before microinjection of anti-Glu tubulin antibody (A and B) or anti-Tyr tubulin antibody (C and D). After microinjection, as described in MATERIALS AND METHODS, cells were rinsed three times in medium 1 (5 min) before fixation and permeabilization. Injected anti-Glu and anti-Tyr tubulin antibodies to Glu MTs and Tyr MTs were detected with Alexa488-conjugated goat anti-rabbit secondary antibody. Arrows indicate the microinjected cells. Bar, 10 μm.
Role of Glu MT and Tyr MT in Microtubule-based Tf Movement
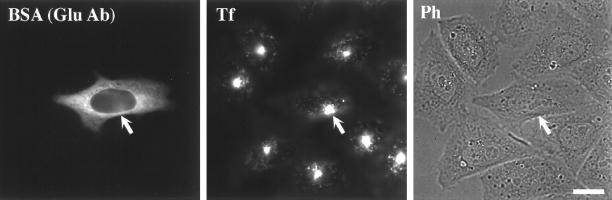
To examine the role of Glu MTs in the transport of Tf to the cell center, TRVb-1 cells were microinjected with anti-Glu tubulin antibody, and then they were incubated with Cy3-Tf for 10 min to see whether antibodies to Glu-MT affected the transport of Tf. Cells microinjected with anti-Glu tubulin antibody could internalize Cy3-Tf and concentrate it in the paranuclear region the same as the surrounding uninjected cells (Figure 6). Identical results were obtained in cells injected with high or low amounts of anti-Glu tubulin antibody (by fluorescence intensity of Alexa488-BSA or MT labeling). Similar experiments were conducted with anti-Tyr tubulin-specific antibody. Approximately 50% of the cells injected with the highest amount of anti-Tyr tubulin antibody displayed an apparent difference in Cy3-Tf labeling such that these cells showed no accumulation of Tf in the central ERC after 10 min uptake compared with surrounding uninjected cells (our unpublished data). Cells injected with lower amounts of anti-Tyr tubulin antibody had no discernible effect.
Figure 6.
Effect of microinjected anti-Glu antibody on internalization of Cy3-Tf to the ERC. TRVb-1 cells were injected with anti-Glu tubulin antibody. As described in MATERIALS AND METHODS, after microinjection, cells were rinsed three times in medium 1 (5 min). Cells were then incubated with Cy3-Tf for 10 min in medium followed with washes in medium 1 for three times before fixation. Alexa488-BSA was included in the injection solution to mark microinjected cells. Arrows indicate the microinjected cells. Bar, 10 μm.
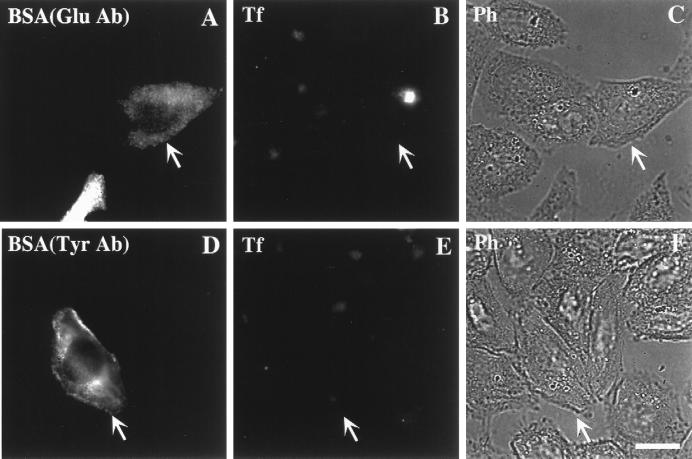
Next, we investigated whether stable Glu-MTs play a functional role in export from the ERC to the plasma membrane. TRVb-1 cells were incubated with Cy3-Tf for 1 h before microinjection of the anti-Glu or anti-Tyr tubulin antibodies (still in the presence of Cy3-Tf). After injection, cells were chased in the absence of Cy3-Tf. As shown in Figure 7, only cells microinjected with anti-Glu tubulin antibody retained Cy3-Tf in the perinuclear ERC after a 45-min chase (Figure 7, A–C, arrows), whereas Cy3-Tf in the surrounding uninjected cells was nearly completely chased out. Under the same conditions, cells microinjected with anti-Tyr tubulin antibody at all injection levels showed no difference in Cy3-Tf recycling compared to the surrounding uninjected cells (Figure 7, D–F).
Figure 7.
Effect of microinjected anti-Glu and anti-Tyr tubulin antibodies on Tf recycling. TRVb-1 cells were incubated with Cy3-Tf for 1 h. Cells were then microinjected either with anti-Glu tubulin antibody (A–C) or with anti-Tyr tubulin antibody (D–F) in the presence of Cy3-Tf followed by a chase in the absence of Cy3-Tf but with excess unlabeled Tf and desferroxamine for 45 min before fixation. As described in MATERIALS AND METHODS, after microinjection, cells were rinsed three times in medium 1 (5 min) before Tf chase. Alexa488-BSA was included in the injection solution to mark microinjected cells. Arrows indicate the microinjected cells. Bar, 10 μm.
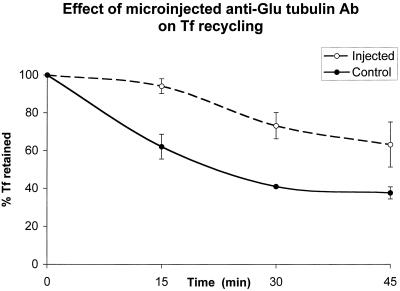
A quantitative analysis of the effects of microinjecting anti-Glu or anti-Tyr tubulin antibodies is summarized in Table 1. We found that ∼60% of all anti-Glu tubulin antibody-injected cells exhibited Tf retention determined by fluorescence microscopy after a 45–60-min chase (Table 1). To further analyze the role of Glu MTs in Tf recycling, quantitative kinetic analysis of the effect of microinjected anti-Glu tubulin antibody in Tf recycling was performed. As shown in Figure 8, a significant reduction in the rate of Tf exit from the ERC was detected in cells injected with anti-Glu tubulin antibody compared with control cells. The amount of antibody injected into individual cells would be expected to affect the response. Although we have not tried to quantify this effect, it appeared that there was greater Tf retention in cells injected with higher amounts of anti-Glu tubulin antibody. When cells were chased for longer period of times (e.g., 4 h), a complete release of internalized Cy3-Tf was observed in anti-Glu tubulin-injected cells (our unpublished data). We conclude that the Glu MTs play a specific role in facilitating transport from the ERC to the cell surface.
Table 1.
Effect of Tf recycling in cells microinjected with antibodies specifically against Glu tubulin and Tyr tubulin peptides
| % of cells showing Cy3-Tf retention in the ERC | |
|---|---|
| Anti-Try antibody (W2 injected) | 12 (n = 203) |
| Anti-Glu antibody (SG injected) | 59 (n = 197) |
| Control (no injection) | 5 (n = 327) |
Data are the percentage of the control or the microinjected cells (n) exhibiting retention of Cy3-Tf in ERC after 1-h chase. TRVb-1 cells were incubated with Cy3-Tf for 1 h before the microinjection of the anti-Tyr or anti-Glu antibodies as described in MATERIALS AND METHODS. Cells were then washed and chased in the absence of Cy3-Tf but in the presence of excess unlabeled Tf and desferroxamine for 1 h.
Figure 8.
Kinetic analysis of the effect of microinjected anti-Glu tubulin antibody on Tf recycling. TRVb-1 cells were incubated with Cy3-Tf for 1 h. Cells were then microinjected with anti-Glu tubulin antibody followed by a chase in the absence of Cy3-Tf but with excess unlabeled Tf and desferroxamine for the indicated times before fixation. Alexa488-BSA was included in the injection solution to mark microinjected cells. As described in the MATERIALS AND METHODS section, after microinjection, cells were rinsed three times in medium 1 (5 min) before Tf chase. Images of cells (both injected and uninjected) were collected at the indicated time points, and the fluorescence power of Cy3-Tf per cell was measured. Results of a representative example of all the experiments are shown. Error bars represent the SD and 100% represents the fluorescence power of Cy3-Tf at 0 min chase after the initial pause labeling of Cy3-Tf.
Effect of Microinjected Anti-Kinesin Antibody in Microtubule-based Tf Movement
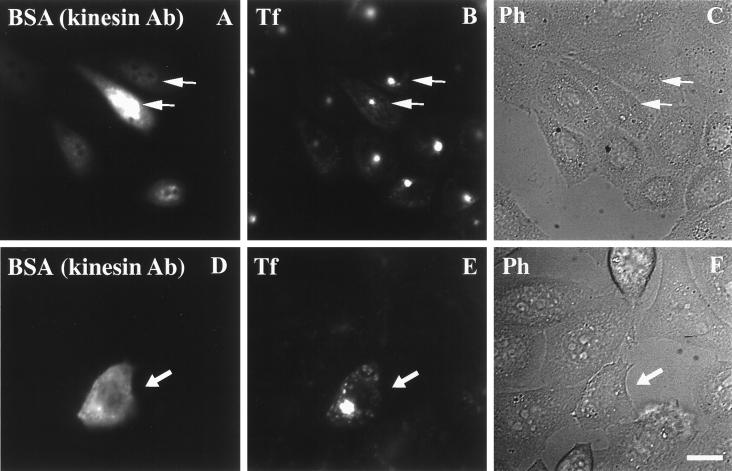
We compared the effects of anti-Glu tubulin antibody injection with the effects of anti-kinesin antibody injection. First, we verified that anti-kinesin antibody did not affect the transport of Tf to the cell center. TRVb-1 cells were microinjected with anti-kinesin antibody, and then they were incubated with Cy3-Tf for 10 min. Cells microinjected with anti-kinesin antibody could internalize Cy3-Tf and concentrate it in the juxtanuclear region the same as the surrounding uninjected cells (Figure 9, A–C, arrows). We next tested the effect of microinjected anti-kinesin antibody on the transport of Tf from the ERC to the plasma membrane, and we found that cells injected with anti-kinesin antibody retained Cy3-Tf in the ERC after a 60-min chase (Figure 9, D–F, arrows). A quantitative analysis of the effects of injecting anti-kinesin antibody is summarized in Table 2.
Figure 9.
Effect of microinjected anti-kinesin antibody on Tf internalization and recycling. (A–C) TRVb-1 cells were injected with anti-kinesin antibody and rinsed three times in medium 1 (5 min) followed by incubation with Cy3-Tf for 10 min. Cells were then washed in medium 1 three times before fixation. (D–F) TRVb-1 cells were incubated with Cy3-Tf for 1 h and then injected with anti-kinesin antibody and rinsed three times in medium 1 (5 min) followed by an additional hour chase in the absence of Cy3-Tf but with excess unlabeled Tf and desferroxamine before fixation. Alexa488-BSA was included in the injection solution to mark microinjected cells. Arrows indicate the microinjected cells. Bar, 10 μm.
Table 2.
Effect of Tf recycling in cells microinjected with anti-kinesin antibody
| % of cells showing Cy3-Tf retention in the ERC | |
|---|---|
| Anti-kinesin antibody (injected) | 95 (n = 74) |
| Control (no injection) | 4 (n = 103) |
Data are the percentage of the control or the microinjected cells (n) exhibiting retention of Cy3-Tf in ERC after 1-h chase. TRVb-1 cells were incubated with Cy3-Tf for 1 h before the microinjection of the anti-kinesin antibody as described in MATERIALS AND METHODS. Cells were then washed and chased in the absence of Cy3-Tf but in the presence of excess unlabeled Tf and desferroxamine for 1 h.
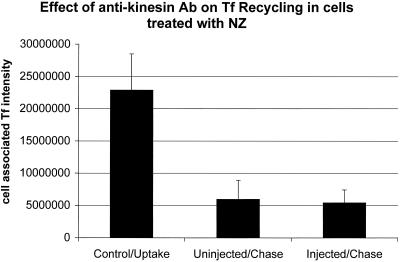
To investigate the possibility of any non-MT–related effect of the microinjected anti-kinesin antibody, we tested whether the microinjected anti-kinesin antibody had any effect on transferrin recycling in cells treated with NZ to disrupt MTs. TRVb-1 cells were incubated with Cy3-transferrin for 0.5 h followed by incubation with 20 μM NZ for 1 h in the presence of Tf to label all the endocytic compartments in cells. Cells were microinjected with anti-kinesin antibody in the presence of NZ and Tf. After microinjection, cells were chased for an hour in the absence of Tf but in the present of 20 μM NZ. Under this condition, as shown in Figure 10, no differences in the extent of transferrin recycling were observed between cells injected with anti-kinesin antibody compared with control uninjected cells. This ruled out the possibility of non-MT–related effects from microinjection of the anti-kinesin antibody on Tf recycling.
Figure 10.
Effect of microinjected anti-kinesin antibody on Tf recycling under NZ treatment. TRVb-1 cells were incubated with Cy3-transferrin for 0.5 h followed by incubation with 20 μM NZ for 1 h in the presence of Tf. Cells were then microinjected with anti-kinesin antibody in the presence of NZ and Tf. After microinjection, cells were rinsed three times in medium 1 with NZ (5 min) and chased for 1 h in the presence of 20 μM NZ and in the absence of Cy3-Tf but with excess unlabeled Tf and desferroxamine before fixation. Images of cells (both injected and uninjected) were collected at time points before chase but after uptake of Tf (control/uptake), after 1 h chase in cells with no injection of anti-kinesin antibody (uninjected/chase) or in cells injected with the anti-kinesin antibody (injected/chase). Fluorescence power of Cy3-Tf per cell was measured. A total of 85 cells was injected. Error bars represent the SD.
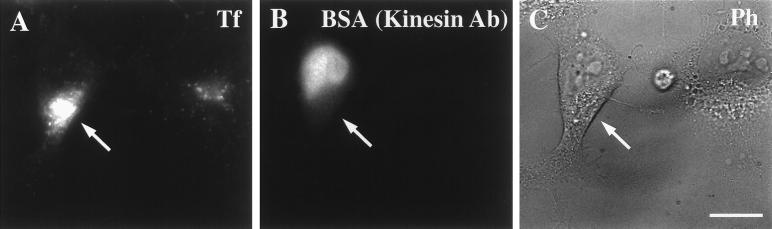
Microinjection of Anti-Kinesin Antibody Collapses the ERC and Slows Tf Recycling in TC-7 Cells
As shown in Figure 5, cells injected with anti-Glu tubulin antibody had a Tf-labeled ERC that was condensed near the MTOC. We also showed that microinjecting both anti-Glu tubulin and anti-kinesin antibodies (but not anti-Tyr tubulin antibody) had a profound effect on the recycling of Tf from the ERC (Figures 7–9). To see whether microinjection of anti-kinesin antibody would also cause the collapse of the ERC, we microinjected anti-kinesin antibody into TC-7 cells. The cells were incubated with Cy3-Tf for 1 h before the microinjection of anti-kinesin antibody. After microinjection, the cells were chased in the absence of Cy3-Tf but in the presence of unlabeled Tf for 1 h. As shown in Figure 11, slowed Tf recycling was observed along with the collapse of the ERC in anti-kinesin antibody-injected cells (Figure 11, arrows). In the surrounding uninjected cells, most of Cy3-Tf was chased out after an hour of Tf chase. These results again demonstrate the involvement of Glu MTs and kinesin in Tf recycling.
Figure 11.
Effect of microinjected anti-kinesin antibody on the ERC distribution and Tf recycling in TC-7 cells. TC-7 cells were incubated with Cy3-Tf for 1 h before the microinjection of anti-kinesin antibody. As described in MATERIALS AND METHODS, after microinjection, cells were rinsed three times in medium 1 (5 min) and chased in the absence of Cy3-Tf but in the presence of unlabeled Tf and desferroxamine for 1 h before fixation. The Tf is shown in A and the injected cells are identified by fluorescent BSA that was coinjected with the anti-kinesin antibody (B). In the injected cells (arrows), slowed Tf recycling was observed along with the collapse of the ERC. In the surrounding uninjected cells, most Cy3-Tf was chased out after an hour. Bar, 10 μm.
DISCUSSION
In this study, we investigated the role of stable Glu MTs in the distribution and transport of recycling membranes. Several organelles, including the Golgi apparatus, late endosomes, and in many cells the ERC are preferentially found near to the MTOC. However, these organelles also maintain distinct distributions, which suggests that concentration near the center of the cell by minus-end directed MT motors is only partially responsible for their localization. As opposed to late endosomes, most of the membrane constituents of the ERC must be transported back to the cell surface, and this suggests that these membranes might need to associate with plus-end directed motors such as kinesins. It has been shown that kinesins have a high affinity for stable Glu MTs (Liao and Gundersen, 1998), and this led us to investigate whether the Glu MTs played a special role in the organization of the ERC or in the movement of membrane organelles from the ERC to the plasma membrane. It has been observed previously that the overall distribution of the ERC varies among different cell types, and the association with stable MTs is the first proposed explanation for this difference.
Several lines of evidence presented here support the hypothesis that ERC membranes and transport intermediates involved in movement to the plasma membrane associate preferentially with stable Glu MTs. First, there was a general, but imperfect, correlation between the amount and distribution of Glu MTs and the degree of dispersal of the ERC in various cell lines. Second, treatment with low concentrations of taxol increases the amount of stable Glu MTs in cells and also increases the dispersal of the ERC. Comparison of the distribution of recycling Tf with Tyr MTs and Glu MTs by confocal microscopy shows a much closer association between the ERC and the Glu MTs. All of these data support an association of the ERC with stable Glu MTs. Significantly, in B104-5 cells the dispersal of the ERC at elevated temperature correlated very well with an increase in the amount of Glu MTs and the more extensive distribution of these stable MTs throughout the cytoplasm. As an exception to the general correlation, a highly dispersed distribution of the ERC was observed in HEp2 cells without detectable Glu MTs. A similar highly dispersed distribution of the ERC was seen in a subpopulation of HeLa cells that lacked detectable Glu MTs (Figure 1b, A and B). The HEp2 cells recycle Tf with overall kinetics similar to the recycling in TRVb1 cells (Ghosh and Maxfield, 1995). This provides evidence that the accumulation of the detyrosinated tubulin is not required for translocation of recycling membrane components.
The association of the ERC with Glu MTs could be due to association with plus-end or minus-end directed transport, or it could be unassociated with transport. For example, the stable MTs could be providing a scaffold for membrane-cytoskeletal linkages. The antibody microinjection experiments provide valuable information about the functional role of Glu MTs. Microinjection with function-blocking anti-Glu tubulin antibodies does not prevent delivery of Tf to a pericentriolar distribution, but it does slow the recycling of Tf to the cell surface. These data argue against a role for stable Glu MTs in minus-end directed transport of Tf, but they strongly support a role for stable Glu MTs in plus-end directed transport of recycling membrane. The effects seen upon microinjection of anti-Glu tubulin antibodies were very similar to the effects of injecting anti-kinesin antibodies. This suggests that both Glu MTs and kinesin are involved in the transport away from the pericentriolar region.
The correlation between levels of Glu MTs and the dispersal of the ERC is somewhat complex. We observed three types of ERC morphology associated with Glu MTs. First, a very tight ERC was observed in TRVb-1 cells in which a very tightly focused network of Glu MTs was observed. Second, in several cell lines with higher levels of Glu MTs, we observed a more dispersed ERC. However, a very highly dispersed ERC was observed in HEp2 cells, which lack Glu MTs, and in a subpopulation of HeLa cells with no detectable Glu MTs. Because of their heterogeneity in levels of Glu MTs, the HeLa cells are particularly informative. In HeLa cells with low levels of Glu MTs, the transferrin is tightly clustered around the MTOC (Figure 1b, C and D). However, in the HeLa cells with no detectable Glu MTs and in the HEp2 cells, the ERC is not centered around the MTOC at all (Figure 1b, A and B). A consistent explanation of these findings would be that ERC membranes have a preference to associate with stable Glu MTs. If the distribution of Glu MTs is compact near the center of the cell, ERC membranes are also mainly in the cell center. In cells with Glu MTs the initial outward movement will start on these stable MTs, and antibody to Glu MTs or kinesin will prevent the recycling membranes from moving on the Glu MTs. If there are no Glu MTs, the recycling membranes must distribute along the Tyr MTs, and there is no preferential central localization of the recycling membrane in such cells.
Although Glu MTs are associated with plus-end transport of recycling membrane constituents, the precise role of these MTs in recycling remains uncertain. First, recycling takes place in cells treated with nocodazole, which have very low levels of any MTs, at the same rate as in control cells (McGraw et al., 1993). This indicates that microtubules are not necessary for the last steps of vesicle movement to the plasma membrane. Furthermore, the dispersal of the ERC in B104-5 cells does not affect the rate of Tf recycling (McGraw et al., 1993). These data suggest that the rate-determining step in Tf recycling is not absolutely dependent upon any MTs. The effects of antibodies to Glu MTs on Tf recycling suggest that Glu MTs are required to deliver recycling membrane out to the cell periphery where an MT-independent process may carry out the last transport steps. In nocodazole-treated cells, Tf never moves far from the cell surface, so the plus-end motility would not be required. At present, we cannot rule out the possibility that noncentrosomal MT minus ends may play a role in the transport of recycling membrane. However, it is very difficult to assess the role of such MTs at present because no marker is available to label the minus end of MTs specifically.
Although the post-translational modification of stable MTs by carboxypeptidase has been known for several years, the functional roles of this modification remain uncertain. A role for Glu MTs and kinesin in the organization of intermediate filaments has been demonstrated (Kreitzer et al., 1999), but it has not been clear whether Glu MTs have different roles in membrane traffic than dynamic Tyr MTs. The data presented in this article show that Glu MTs have a special role in movement of recycling membrane from the cell center to the periphery. Glu MTs have a preferential orientation toward the front of migrating fibroblasts (Cook et al., 1998), and recycling receptors are also preferentially delivered near the front of motile cells (Kupfer et al., 1985, 1987). Our data indicate that the vectorial delivery may be a consequence of the distribution of the Glu MTs. Stable MTs may also play a role in other types of oriented recycling such as the basolateral versus apical recycling in polarized epithelia.
It is unclear whether the association of ERC membranes with stable Glu MTs is due to their stability or to the post-translational removal of the C-terminal Tyr. It is possible that kinesin or other protein(s) binds with higher affinity to polymerized tubulin with a C-terminal Glu (Liao and Gundersen, 1998). On the other hand, the stability of Glu MTs is thought to be due to the binding of proteins at the end of the MT (Infante et al., 2000; Palazzo et al., 2000) and this stability leads to the accumulation of C-terminal Glu through the progressive action of the carboxypeptidase (Argarana et al., 1978). The proteins that promote MT stability could also facilitate binding of ERC membranes independently of the identity of the C-terminal residue.
In this article, we have presented evidence for a special role for stable Glu MTs in the organization and function of the ERC. A specialized association with these MTs may help to organize the precise localization of recycling membranes within the central region of the cell, which is also enriched in several other organelles that are moved there by minus-end microtubule motors. The association with stable Glu MTs may also play an important role in targeted delivery of recycling membranes toward certain parts of the cell, such as the front of migrating cells.
ACKNOWLEDGMENTS
We are grateful to Dr. Geri Kreitzer (Weill Medical College of Cornell University) for suggestions and help with microinjection. We thank Dr. Timothy McGraw for helpful suggestions, Dr. Lynda Pierini and Lee Cohen-Gould for advice in confocal microscopy, and Thwe T. Soe for technical assistance. Anti-kinesin antibody was generously provided by F.K. Gyoeva. We thank Dr. Sushmita Mukherjee and Bob Vasquez for critical reading of the manuscript and Mingming Hao for helping to prepare the manuscript. This work was supported by grants from the National Institutes of Health to F.R.M. (DK-27083) and G.G.G. (GM-42026).
Abbreviations used:
- ERC
endocytic recycling compartment
- Tf
human transferrin
- TfR
human transferrin receptor
- MTOC
microtubule-organizing center
- MTs
microtubules
- NZ
nocodazole
- CHO
Chinese hamster ovary
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01-05-0224. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–05-0224.
REFERENCES
- Argarana CE, Barra HS, Caputto R. Release of [14C]tyrosine from tubulinyl-[14C]tyrosine by brain extract. Separation of a carboxypeptidase from tubulin-tyrosine ligase. Mol Cell Biochem. 1978;19:17–21. doi: 10.1007/BF00231230. [DOI] [PubMed] [Google Scholar]
- Bulinski JC, Gundersen GG. Stabilization of post-translational modification of microtubules during cellular morphogenesis. Bioessays. 1991;13:285–293. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook TA, Nagasaki T, Gundersen GG. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J Cell Biol. 1998;141:175–185. doi: 10.1083/jcb.141.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh RN, Mallet WG, Soe TT, McGraw TE, Maxfield FR. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J Cell Biol. 1998;142:923–936. doi: 10.1083/jcb.142.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh RN, Maxfield FR. Evidence for nonvectorial, retrograde transferrin trafficking in the early endosomes of HEp2 cells. J Cell Biol. 1995;128:549–561. doi: 10.1083/jcb.128.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Khawaja S, Bulinski JC. Postpolymerization detyrosination of alpha-tubulin: a mechanism for subcellular differentiation of microtubules. J Cell Biol. 1987;105:251–264. doi: 10.1083/jcb.105.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurland G, Gundersen GG. Protein phosphatase inhibitors induce the selective breakdown of stable microtubules in fibroblasts and epithelial cells. Proc Natl Acad Sci USA. 1993;90:8827–8831. doi: 10.1073/pnas.90.19.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurland G, Gundersen GG. Stable, detyrosinated microtubules function to localize vimentin intermediate filaments in fibroblasts. J Cell Biol. 1995;131:1275–1290. doi: 10.1083/jcb.131.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoeva FK, Gelfand VI. Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature. 1991;353:445–448. doi: 10.1038/353445a0. [DOI] [PubMed] [Google Scholar]
- Hao M, Maxfield FR. Characterization of rapid membrane internalization and recycling. J Biol Chem. 2000;275:15279–15286. doi: 10.1074/jbc.275.20.15279. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990;46:335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante AS, Stein M, Zhai Y, Borisy G, Gundersen GG. Detyrosinated (Glu) Microtubules are stabilized by an ATP-sensitive plus-end cap. J Cell Sci. 2000;113:3907–3919. doi: 10.1242/jcs.113.22.3907. [DOI] [PubMed] [Google Scholar]
- Johannes L, Tenza D, Antony C, Goud B. Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J Biol Chem. 1997;272:19554–19561. doi: 10.1074/jbc.272.31.19554. [DOI] [PubMed] [Google Scholar]
- Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988;106:141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelef N, Buton X, Beatini N, Wang H, Meiner V, Chang TY, Farese RV, Jr, Maxfield FR, Tabas I. Immunolocalization of acyl-coenzyme A: cholesterol O-acyltransferase in macrophages. J Biol Chem. 1998;273:11218–11224. doi: 10.1074/jbc.273.18.11218. [DOI] [PubMed] [Google Scholar]
- Khelef N, Soe TT, Quehenberger O, Beatini N, Tabas I, Maxfield FR. Enrichment of acyl coenzyme A: cholesterol O-acyltransferase near trans-Golgi network and endocytic recycling compartment. Arterioscler Thromb Vasc Biol. 2000;20:1079–5642. doi: 10.1161/01.atv.20.7.1769. [DOI] [PubMed] [Google Scholar]
- Kilmartin JV, Wright B, Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982;93:576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer G, Liao G, Gundersen GG. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo via a kinesin-dependent mechanism. Mol Biol Cell. 1999;10:1105–1118. doi: 10.1091/mbc.10.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer G, Marmorstein A, Okamoto P, Vallee R, Rodriguez-Boulan E. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nat Cell Biol. 2000;2:125–127. doi: 10.1038/35000081. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Dennert G, Singer SJ. The reorientation of the Golgi apparatus and the microtubule-organizing center in the cytotoxic effector cell is a prerequisite in the lysis of bound target cells. J Mol Cell Immunol. 1985;2:37–49. [PubMed] [Google Scholar]
- Kupfer A, Swain SL, Singer SJ. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987;165:1565–1580. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G, Gundersen GG. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J Biol Chem. 1998;273:9797–9803. doi: 10.1074/jbc.273.16.9797. [DOI] [PubMed] [Google Scholar]
- Lin SX, Collins CA. Immunolocalization of cytoplasmic dynein to lysosomes in cultured cells. J Cell Sci. 1992;101:125–137. doi: 10.1242/jcs.101.1.125. [DOI] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw TE, Dunn KW, Maxfield FR. Isolation of a temperature-sensitive variant Chinese hamster ovary cell line with a morphologically altered endocytic recycling compartment. J Cell Physiol. 1993;155:579–594. doi: 10.1002/jcp.1041550316. [DOI] [PubMed] [Google Scholar]
- McGraw TE, Greenfield L, Maxfield FR. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J Cell Biol. 1987;105:207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov AV, Gundersen GG. Centripetal transport of microtubules in motile cells. Cell Motil Cytoskeleton. 1995;32:173–186. doi: 10.1002/cm.970320303. [DOI] [PubMed] [Google Scholar]
- Mostov KE. Regulation of protein traffic in polarized epithelial cells. Histol Histopathol. 1995;10:423–431. [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Zha X, Tabas I, Maxfield FR. Cholesterol distribution in living cells: fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys J. 1998;75:1915–1925. doi: 10.1016/S0006-3495(98)77632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo AF, Cook TA, Alberts AS, Gundersen GG. mDia regulates the formation, and orientation of stable microtubules. submitted. 2000. [DOI] [PubMed] [Google Scholar]
- Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Assembly and turnover of detyrosinated tubulin in vivo. J Cell Biol. 1987a;105:265–276. doi: 10.1083/jcb.105.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci USA. 1987b;84:9040–9044. doi: 10.1073/pnas.84.24.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro DJ, Maxfield FR. Acidification of morphologically distinct endosomes in mutant and wild-type Chinese hamster ovary cells. J Cell Biol. 1987;105:2723–2733. doi: 10.1083/jcb.105.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]