Abstract
Background
Twin pregnancies are associated with increased perinatal mortality, mainly related to prematurity, but complications during birth may contribute to perinatal loss or morbidity. The option of planned caesarean section to avoid such complications must therefore be considered. On the other hand, randomised trials of other clinical interventions in the birth process to avoid problems related to labour and birth (planned caesarean section for breech, and continuous electronic fetal heart rate monitoring), have shown an unexpected discordance between short‐term perinatal morbidity and long‐term neurological outcome. The risks of caesarean section for the mother in the current and subsequent pregnancies must also be taken into account.
Objectives
To determine the short‐ and long‐term effects on mothers and their babies, of planned caesarean section for twin pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (18 November 2015) and reference lists of retrieved studies.
Selection criteria
Randomised trials comparing a policy of caesarean section with planned vaginal birth for women with twin pregnancy.
Data collection and analysis
Two review authors independently assessed eligibility, quality and extracted data. Data were checked for accuracy. For important outcomes the quality of the evidence was assessed using the GRADE approach.
Main results
We included two trials comparing planned caesarean section versus planned vaginal birth for twin pregnancies.
Most of the data included in the review were from a multicentre trial where 2804 women were randomised in 106 centres in 25 countries. All centres had facilities to perform emergency caesarean section and had anaesthetic, obstetrical, and nursing staff available in the hospital at the time of planned vaginal delivery. In the second trial carried out in Israel, 60 women were randomised. We judged the risk of bias to be low for all categories except performance (high) and outcome assessment bias (unclear).
There was no clear evidence of differences between women randomised to planned caesarean section or planned vaginal birth for maternal death or serious morbidity (risk ratio (RR) 0.86, 95% confidence interval (CI) 0.67 to 1.11; 2844 women; two studies; moderate quality evidence). There was no significant difference between groups for perinatal or neonatal death or serious neonatal morbidity (RR 1.15, 95% CI 0.80 to 1.67; data for 5565 babies, one study, moderate quality evidence). No studies reported childhood disability.
For secondary outcomes there was no clear evidence of differences between groups for perinatal or neonatal mortality (RR 1.41, 95% CI 0.76 to 2.62; 5685 babies; two studies, moderate quality evidence), serious neonatal morbidity (RR 1.03, 95% CI 0.65 to 1.64; 5644 babies; two studies, moderate quality evidence) or any of the other neonatal outcomes reported.
The number of women undergoing caesarean section was reported in both trials. Most women in the planned caesarean group had treatment as planned (90.9% underwent caesarean section), whereas in the planned vaginal birth group 42.9% had caesarean section for at least one twin. For maternal mortality; no events were reported in one trial and two deaths (one in each group) in the other. There were no significant differences between groups for serious maternal morbidity overall (RR 0.86, 95% CI 0.67 to 1.11; 2844 women; two studies) or for different types of short‐term morbidity. There were no significant differences between groups for failure to breastfeed (RR 1.14, 95% CI 0.95 to 1.38; 2570 women, one study; moderate quality evidence) or the number of women with scores greater than 12 on the Edinbugh postnatal depression scale (RR 0.95, 95% CI 0.78 to 1.14; 2570 women, one study; moderate quality evidence).
Authors' conclusions
Data mainly from one large, multicentre study found no clear evidence of benefit from planned caesarean section for term twin pregnancies with leading cephalic presentation. Data on long‐term infant outcomes are awaited. Women should be informed of possible risks and benefits of labour and vaginal birth pertinent to their specific clinical presentation and the current and long‐term effects of caesarean section for both mother and babies. There is insufficient evidence to support the routine use of planned caesarean section for term twin pregnancy with leading cephalic presentation, except in the context of further randomised trials.
Plain language summary
Planned caesarean section for a twin pregnancy
The incidence of twins varies considerably between communities and families and has recently increased because of the number of older mothers and the use of fertility treatments and assisted conception. Infants from a twin pregnancy are at a higher risk of death around the time of birth than are infants from a singleton pregnancy. Some of this is due to a higher risk of preterm birth. The second‐born twin has an increased risk of a poor perinatal outcome compared with the first‐born twin.
A policy of planned vaginal birth for women with a twin pregnancy in a hospital setting is associated with a 30% to 40% rate of emergency caesarean section. When the first twin is born vaginally, there is still a risk of emergency section for the birth of the second twin. It is possible that some of the adverse outcomes may be avoided by appropriately timed delivery by caesarean section but the risks of caesarean section for the mother in the current and subsequent pregnancies must be taken into account.
In this review we included two randomised trials comparing planned caesarean versus planned vaginal birth for twin pregnancies which together included 2864 women. For important outcomes the evidence was assessed as being of moderate quality.
For maternal mortality no events were reported in one trial and two deaths (one in each group) in the other. There was no clear evidence of differences between women randomised to planned caesarean or planned vaginal birth for death or serous illness in either the mothers or babies. No studies reported childhood disability.
The number of women undergoing caesarean section was reported in both trials. Most women in the planned caesarean group had treatment as planned (90.9%), whereas in the planned vaginal birth group 42.9% had caesarean section for at least one twin. There were no significant differences between groups for failure to breastfeed or for postnatal depression.
There is very little clear research evidence to provide guidance on the method of birth for twin pregnancies. The benefits and risks should be made available to women, including short‐term and long‐term consequences for both mother and babies. Future research should aim to provide more clarity on this issue as medical interventions in the birth process should be avoided unless there is reasonable clinical certainty that they will be of long‐term benefit.
Summary of findings
Summary of findings for the main comparison. Planned caesarean section versus planned vaginal birth for women with a twin pregnancy.
| Planned caesarean section versus planned vaginal birth for women with a twin pregnancy | ||||||
| Patient or population: women with a twin pregnancy Setting: hospital settings Intervention: planned caesarean section Comparison: planned vaginal birth | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with planned vaginal birth | Risk with Planned caesarean section | |||||
| Maternal death or serious maternal morbidity | Study population | RR 0.86 (0.67 to 1.11) | 2844 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 83 per 1000 | 71 per 1000 (55 to 92) | |||||
| Moderate | ||||||
| 42 per 1000 | 36 per 1000 (28 to 47) | |||||
| Perinatal or neonatal death or serious neonatal morbidity | Study population | RR 1.15 (0.80 to 1.67) | 5565 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 19 per 1000 | 21 per 1000 (15 to 31) | |||||
| Disability in childhood | Study population | not pooled | 0 (0 study) | No data were available for this outcome | ||
| not pooled | not pooled | |||||
| Perinatal or neonatal death | Study population | RR 1.41 (0.76 to 2.62) | 5685 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 6 per 1000 | 8 per 1000 (5 to 16) | |||||
| Moderate | ||||||
| 3 per 1000 | 4 per 1000 (2 to 8) | |||||
| Serious neonatal morbidity | Study population | RR 1.03 (0.65 to 1.64) | 5644 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 12 per 1000 | 13 per 1000 (8 to 20) | |||||
| Moderate | ||||||
| 6 per 1000 | 6 per 1000 (4 to 10) | |||||
| Longer‐term maternal outcomes: failure to breastfeed | Study population | RR 1.14 (0.95 to 1.38) | 2570 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 136 per 1000 | 155 per 1000 (129 to 188) | |||||
| Longer‐term maternal outcomes: postnatal depression, as defined by trial authors (EPDS > 12) | Study population | RR 0.95 (0.78 to 1.14) | 2570 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 148 per 1000 | 140 per 1000 (115 to 169) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Wide 95% CI crossing the line of no effect
Background
Description of the condition
Twin pregnancy results from one of two distinct biological processes. The more common process is fertilisation of more than one ovum following multiple ovulation. Here the offspring may be of the same gender or not, are genetically no more similar than siblings, and have separate placental circulations and gestational sacs (dizygotic, diamniotic, dichorionic). The less common process is splitting of a single developing embryo. The offspring are of the same gender and genetically identical. The degree of separation depends on the developmental stage at which the separation takes place, and can be anything from separate circulations (monozygotic, dichorionic, diamniotic) to conjoined twins.
The incidence of monozygotic twins is fairly constant, but that of dizygotic twins varies considerably between communities and families, and has recently increased in many well‐resourced health services because of the use of fertility treatments and assisted conception and the increase in the number of older‐aged mothers (Anonymous 2000; Laws 2010; Wilcox 1996). The rate of multiple birth varies from country to country. In Canada, the rate of multiple births increased from 1.9% in 1981 to 2.5% in 1997 (Anonymous 2000; Joseph 2001a). In Australia, the rate in 2008 was 3.1% of all births (Laws 2010).
Over 97% of all multiple pregnancies are twin pregnancies (Joseph 1998). Approximately 40% of twins present as vertex/vertex, 35% as vertex/non‐vertex and the remaining 25% of twins present with the leading baby in a non‐vertex presentation at birth (Blickstein 1987; Grisaru 2000).
Mortality and morbidity for twins versus singletons
Infants from a twin pregnancy are at a higher risk of perinatal/neonatal mortality than infants from a singleton pregnancy (Cheung 2000; Fabre 1988; Ghai 1988; Joseph 2001b; Kiely 1990; Lie 2000). Some of this is due to a higher risk of preterm birth (Joseph 2001b). However, even among twin babies that are greater than 2500 g at birth, there is a higher risk of death than among singletons of the same birthweight. Again, the absolute rates of perinatal mortality and morbidity will vary by population. In Australia, the perinatal death rate for twins for 2008 was 6.8 times higher and for higher multiple births 18.1 times higher than for singleton births (Laws 2010). Kiely 1990 reviewed the data on 16,831 multiple births from the New York City Department of Health's computerised vital records for the period 1978 to 1984. The perinatal mortality rate for twins versus singletons at 2501 to 3000 g was 4.3/1000 versus 3.8/1000 (risk ratio (RR) 1.12) and at 3001 g or more, 7.4/1000 versus 2.2/1000 (RR 3.32), respectively. The intrapartum fetal death rate for twins versus singletons at 2501 to 3000 g was 1.22/1000 versus 0.34/1000 (RR 3.54, 95% confidence interval (CI) 1.82 to 6.88). Other studies have confirmed this higher risk of fetal and neonatal death in twins versus singletons if the pregnancy is at or near term or above 2500 g in birthweight (Cheung 2000; Fabre 1988; Ghai 1988; Lie 2000). Neonatal seizures, respiratory morbidity, and low Apgar scores at one and five minutes have also been shown to be higher for twins versus singleton infants at birthweights greater than 1500 g and greater than 3000 g (Ghai 1988).
Some of the higher risk of adverse perinatal outcome in twins compared with singletons may be due to restricted fetal growth which, in turn, may result in a higher risk of adverse events occurring during pregnancy, during labour or during birth. The higher risk may be due to trauma and asphyxia associated with the birth of the second twin. It is possible that some of these adverse outcomes may be avoided by an appropriately timed delivery by caesarean section. The timing of birth for women with a twin pregnancy is the subject of another Cochrane review (Dodd 2014).
In a multicentre randomised controlled trial comparing planned caesarean section and planned vaginal birth for the singleton breech fetus at term, planned caesarean section reduced the risk of perinatal death or serious neonatal morbidity three‐fold (from 5.0% to 1.6%, P less than 0.001) (Hannah 2000). Although some of the deaths in the planned vaginal birth group in this study were due to difficulties associated with the actual birth, some were associated with problems that occurred during labour. Thus, a policy of planned caesarean section may be beneficial for women with a pregnancy at risk of complications during labour because it reduces the exposure of the pregnancy to labour.
How the intervention might work
Evidence of benefits and risks related to planned caesarean section for twins
Outcome for second twin compared with first twin
In a study of 1305 twin pairs at 1500 g or more birthweight delivered between 1988 and 1999 in Nova Scotia, the risk of adverse perinatal outcome (intrapartum fetal death, neonatal death, moderate‐severe respiratory distress syndrome (RDS), asphyxia, trauma and complications of prematurity) was significantly increased for second‐born compared with first‐born twins (RR 2.1, 95% CI 1.4 to 3.1) (Persad 2001b). However, it should be noted that in the Term Breech Trial, short‐term neonatal morbidity was not associated with any long‐term impairment (Whyte 2004).
There is also evidence that the second twin is at greater risk of adverse perinatal outcome, compared to the first twin if delivery is vaginal but the same has not been shown if delivery is by caesarean section. Arnold and colleagues undertook a matched case‐control study of preterm twin pairs (Arnold 1987). The risk of RDS was increased for the second twin compared to the first if delivery was vaginal (odds ratio (OR) 14.2, 95% CI 2.5 to 81.1), but not if delivery was by caesarean section (OR 0.90, 95% CI 0 to 17.8). This could be due to a greater protective effect of vaginal birth for the leading twin.
Outcomes for twins born vaginally versus by caesarean section
Higher rates of adverse perinatal outcome have been reported for the twin pregnancy at or near term if birth is vaginal versus by caesarean section (Barrett 2004). In the Kiely 1990 review, for twins in vertex presentation weighing more than 3000 g at birth, the perinatal mortality rate was 12.3/1000 versus 2.9/1000 (RR 4.22) if birth was vaginal versus caesarean. However, comparisons of actual as opposed to planned method of delivery are subject to considerable bias. In a retrospective study of women with previous caesarean section and a current twin pregnancy, perinatal mortality was more common following trial of labour than repeat caesarean section, but there was no difference in outcome after adjusting for confounding variables (Aaronson 2010).
There is increasing evidence for perinatal benefits related to vaginal birth. In a cross‐sectional study of 6929 inborn infants without congenital anomalies, with gestational ages from 37 to 41 (6/7) weeks with vertex presentation, non‐urgent caesarean delivery under regional anaesthesia compared to vaginal birth under local or no anaesthesia increased the risk of bag and mask ventilation (OR 1.42, 95% CI 1.07 to 1.89) when adjusted for number of gestations, maternal hypertension and birthweight (De Almeida 2010). However, another retrospective study found no significant increase in transient tachypnoea of the newborn associated with increasing rates of elective caesarean section for twin pregnancies over time (Suzuki 2010).
In a retrospective study of twin births at 37 or more weeks' gestation, elective but not emergency caesarean section was associated with an increased risk of transfusion in the neonate (Suzuki 2008).
Outcomes for twins delivered by planned vaginal birth (actual vaginal birth plus emergency caesarean section) versus planned caesarean section
A systematic review of non‐randomised studies that compared the policies of planned vaginal birth and planned caesarean section for the delivery of twins weighing at least 1500 g or reaching at least 32 weeks' gestation (Hogle 2002) found four small studies that were eligible for inclusion in the review (Blickstein 2000; Grisaru 2000; Rabinovici 1987; Wells 1991). A meta‐analysis of the data from the four studies did not find significant differences between the two approaches to delivery in terms of mortality or neonatal morbidity, although low Apgar score at five minutes was reduced with a policy of caesarean section. This finding, however, was confined to twins in which the first twin presented as a breech. A subsequent retrospective comparison of planned vaginal birth versus planned caesarean section for twin pregnancies with a leading breech found no significant differences in perinatal or maternal morbidity, other than an increase in thromboembolic disease in the planned caesarean section group (Sentilhes 2007). Two large retrospective studies of women with previous caesarean section and a current twin pregnancy comparing planned vaginal birth with elective caesarean section (Ford 2006; Varner 2005), found no difference in maternal or perinatal outcome, other than an incidence of uterine rupture of 0.9% in the planned vaginal birth group in one study (Ford 2006).
A comparison of outcomes in two Danish counties with high (57%) and low (28%) caesarean rates for twin pregnancy found no difference in perinatal outcomes (Henriksen 1994).
The previously published versions of this review (Crowther 1996; Hofmeyr 2011) found only one randomised controlled trial of planned caesarean section versus planned vaginal birth for twins, in which 60 pairs of twins were enrolled (Rabinovici 1987). There were no perinatal deaths or cases of serious neonatal morbidity in either group. The sample size was too small to provide evidence of the better approach to delivery.
In low‐resourced settings with limited facilities and personnel, high post‐caesarean section complication rates and uncertain access for the mother to caesarean section facilities in future pregnancies, the benefit to the mother of avoiding caesarean section is greater than in well‐resourced settings.
Outcomes for second twin born by caesarean section following vaginal birth of twin one, compared with caesarean section for both twins
A prospective multicentre cohort study compared the outcome for second twins born by caesarean section following vaginal birth of twin one, compared with caesarean section for both twins. There was no difference in low Apgar scores, low cord blood pH, or neonatal encephalopathy. Neonatal infection was increased in the group with caesarean section following vaginal birth of twin one, but this difference was not statistically significant after logistic regression analysis (Alexander 2008). In a large retrospective study of planned vaginal birth for twin pregnancies, caesarean section for the second twin occurred in 9% of cases and was more common when the presentation of the second twin was non‐vertex, and in pregnancies with other complications, and less common when the vaginal birth of the first twin was operative (Wen 2004a).
Importance of fetal presentation
If the first twin presents as breech, the current trend in well‐resourced health systems, as for singleton breech presentation, is to recommend caesarean section as being safer for the babies. This approach may be influenced by extrapolation of evidence from randomised trials of planned caesarean section for singleton breech presentation (Hofmeyr 2015), though the interpretation of this evidence is the subject of considerable debate. The approach to the delivery of vertex/non‐vertex twins is controversial (ACOG 2002; Barrett 2000; Crowther 1996). For twins presenting vertex/vertex, most clinicians recommend a planned vaginal birth (ACOG 2002; Crowther 1996). However, planned caesarean section may benefit twins in which the first twin is presenting vertex for a number of reasons. As many as 20% of vertex presenting second twins will change presentation spontaneously after the first twin is born (Houlihan 1996). A substantial number of those presenting vertex/vertex will present with serious acute intrapartum problems following the birth of the first twin (for example, conversion to transverse lie, cord prolapse, prolonged interval to delivery of the second twin), which may lead to emergency caesarean section, perinatal death, and neonatal morbidity. Lastly, if there are benefits to avoiding labour, both twins regardless of presentation should benefit.
Maternal outcomes
A policy of planned vaginal birth for women with a twin pregnancy in a hospital setting is associated with a 30% to 40% rate of emergency caesarean section. Among those twins in which the first twin is born vaginally, there is still a risk of emergency caesarean section for the birth of the second twin, ranging from 4% (Suzuki 2009) to 7% (Persad 2001a). In a retrospective case‐control study, the risk of caesarean section for the second twin after vaginal birth of the first was increased in association with a history of infertility therapy, gestational age equal to or greater than 39 weeks, non‐vertex presentation, operative delivery of the first twin and inter‐twin delivery time interval greater than 30 minutes (Suzuki 2009). As the risk of maternal death is highest if delivery is by emergency caesarean section, lowest following a vaginal birth, and intermediate following an elective caesarean section (Hall 1999), increasing numbers of emergency caesarean section may reduce the mortality benefits of planned vaginal births.
A relatively small case‐control study found no increased maternal morbidity for caesarean section for twins compared with singleton pregnancy (Simoes 2007). However, in a large population‐based, matched case‐control study using the United Kingdom Obstetric Surveillance System, the risk of peripartum hysterectomy was increased for women with twin pregnancy (OR 6.30, 95% CI 1.73 to 23.0) (Knight 2008), while another study found twin pregnancy to be a risk factor for post caesarean section wound sepsis (Schneid‐Kofman 2005).
There is growing evidence of an association between vaginal birth and urinary incontinence, particularly if the vaginal birth requires forceps or vacuum extraction (Farrell 2001; Meyer 1998; Wilson 1996). Urinary incontinence identified in the postpartum period has been shown to have long‐lasting effects, with a high risk of urinary incontinence five years later (Viktrup 2001). Use of the supine position for birth has been cited as a possible contributor to incontinence following vaginal birth. Faecal incontinence and incontinence of flatus have been reported to be associated with vaginal birth, particularly if forceps are used, and if there are lacerations involving the anal sphincter (Eason 2002; Zetterström 1999).
In the Term Breech Trial, there was no significant difference in maternal mortality or immediate serious maternal morbidity between planned caesarean section and planned vaginal birth for singleton breech presentation at term (3.9% versus 3.2%, P = 0.35) (Hannah 2002). At three months postpartum, the women in the planned caesarean group reported a lower incidence of urinary incontinence (4.5% versus 7.3%, P = 0.02). Although the incidence of incontinence of flatus was not different between groups, if incontinence of flatus was reported, it was significantly less of a problem in the planned caesarean section group (P = 0.006). At two years, the only significant difference found was less constipation in the planned vaginal birth group (Hofmeyr 2015). However, long‐term risks of caesarean section, particularly risks in subsequent pregnancies such as placenta praevia, placenta accreta, repeat caesarean section and uterine rupture, as well as reduced fertility, have not been evaluated in randomised trials. Thus from a maternal perspective, the relative benefits and risks of planned caesarean section versus planned vaginal birth are not clear‐cut. Any evaluation of risks versus benefits must take into account the likelihood of serious long‐term adverse effects of caesarean section. Data from a large Canadian cohort indicated that even in women with previous caesarean section, maternal mortality was significantly higher with caesarean section than with vaginal birth (Wen 2004b).
Given that in well‐resourced environments, suicide is becoming an important contributor to maternal mortality, emotional consequences of birth such as depression need to be given importance when weighing up benefits and harms of different approaches to care. Even when emergency caesarean section is performed, some women may gain self‐esteem from having at least attempted to achieve vaginal birth. The sense of control from participation in the decision‐making process may also promote self‐esteem.
Why it is important to do this review
A more detailed account of evidence related to the benefits and risks of caesarean section is given in Lavender 2012.
Given the evidence described above, a systematic review of the information from randomised trials is required to determine the relative benefits and risks of planned caesarean section for twin pregnancy, for the infants and the mother.
Objectives
To assess, from the best available evidence, the effects on mortality and morbidity for mother and babies, of a policy of planned caesarean section versus planned vaginal birth for twin pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
All comparisons of intention to perform caesarean section and intention to birth vaginally, subject to a management protocol, for women with a twin pregnancy at, or before term. Randomised controlled trials, quasi‐randomised trials and cluster‐randomised trials were eligible for inclusion. Cross‐over trials were not eligible for inclusion.
We planned to include studies reported in abstracts provided methods were described in sufficient detail to assess risk of bias.
Types of participants
Women with viable twin pregnancy considered suitable for vaginal birth, excluding women with known serious fetal anomaly.
Types of interventions
Planned caesarean section compared with planned vaginal birth subject to the requirements of a management protocol.
Types of outcome measures
Primary outcomes
Perinatal or neonatal death (excluding fatal anomalies) or serious neonatal morbidity (e.g. severe birth asphyxia, seizures, neonatal encephalopathy, serious birth trauma, severe respiratory distress syndrome (RDS), prolonged neonatal intensive care unit admission; or as defined by trial authors).
Perinatal or infant death (excluding fatal anomalies) or disability in childhood.
Maternal death or serious maternal morbidity (e.g. admission to intensive care unit, septicaemia, organ failure, uterine rupture, hysterectomy, major surgical complication, life‐threatening event, long‐term disability, or as defined by trial authors).
Secondary outcomes
Short‐term perinatal/neonatal morbidity
Perinatal/neonatal death (excluding fatal anomalies)
Serious neonatal morbidity (e.g. seizures, birth asphyxia as defined by trial authors, neonatal encephalopathy, birth trauma)
Apgar score less than seven at five minutes
Apgar score less than four at five minutes
Low cord blood pH as defined by trial authors
High cord blood base deficit as defined by trial authors
Neonatal intensive care unit admission
Neonatal encephalopathy, as defined by trial authors
Birth trauma, as defined by trial authors
Nerve palsy (including brachial plexus injury)
Subdural or intracerebral haemorrhage
Intraventricular haemorrhage: grade III or IV
Cystic periventricular leukomalacia
Septicaemia
Meningitis
Necrotising enterocolitis
Chronic lung disease
Assisted ventilation 24 hours or more
Long‐term infant outcomes
Death (excluding fatal anomalies)
Disability in childhood, as defined by trial authors
Medical problems
Short‐term maternal outcomes
Caesarean section
Regional analgesia
General anaesthesia
Instrumental vaginal delivery
Death
Serious maternal morbidity (e.g. intensive care unit admission, septicaemia, organ failure; or as defined by trial authors)
Intraoperative organ damage
Deep vein thrombosis
Pulmonary embolism
Wound sepsis
Systemic infection
Disseminated intravascular coagulation
Amniotic fluid embolism
Postpartum haemorrhage, as defined by the trial authors
Postpartum anaemia, as defined by trial authors
Blood transfusion
Uterine rupture
Repeat surgery
Hysterectomy
Wound infection
Prolonged hospital stay
Woman not satisfied with care
Longer‐term maternal outcomes (at one to six months)
Breastfeeding failure, as defined by trial authors
Perineal pain
Abdominal pain
Backache or back pain
Any pain
Dyspareunia, as defined by trial authors
Uterovaginal prolapse
Urinary incontinence
Flatus incontinence
Faecal incontinence
Postnatal depression, as defined by trial authors
Postnatal self‐esteem, as defined by trial authors
Postnatal anxiety, as defined by trial authors
Relationship with baby, as defined by trial authors
Relationship with partner, as defined by trial authors
Quality of life
Long‐term maternal outcomes (at more than one year)
Breastfeeding failure, as defined by trial authors
Pperineal pain
Abdominal pain
Backache or back pain
Any pain
Dyspareunia, as defined by trial authors
Uterovaginal prolapse
Urinary incontinence
Flatus incontinence
Faecal incontinence
Infertility
Subsequent pregnancy
Miscarriage or termination of a subsequent pregnancy
Caesarean section in a subsequent pregnancy
Uterine rupture in a subsequent pregnancy
Dysmenorrhoea
Menorrhagia
Postnatal depression, as defined by trial authors
Postnatal self‐esteem, as defined by trial authors
Postnatal anxiety, as defined by trial authors
Quality of life
Relationship with child, as defined by trial authors
Relationship with partner, as defined by trial authors
Health services
Caregiver not satisfied
Cost
We have included outcomes if clinically meaningful; reasonable measures taken to minimise observer bias; missing data insufficient to materially influence conclusions; data available for analysis according to original allocation, irrespective of protocol violations; data available in a format suitable for analysis.
We have reported only outcomes with data available in the analysis tables.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (18 November 2015).
For full search methods used to populate the Cochrane Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeHofmeyr 2011.
For this update, the following methods were used for assessing the five reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
(One of the included studies was carried out by a member of the review team (JB); this author was not involved in data extraction or assessment of risk of bias for this trial.)
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
Blinding participants or staff is not feasible for this type of intervention. We considered that studies were at low risk of bias if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, if more studies are included, we will explore the impact of the level of bias through undertaking Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update the quality of the evidence was assessed using the GRADE approach as outlined in the GRADE Handbook in order to assess the quality of the body of evidence relating to the following outcomes.
Perinatal or neonatal death (excluding fatal anomalies) or serious neonatal morbidity (e.g. severe birth asphyxia, seizures, neonatal encephalopathy, serious birth trauma, severe RDS, prolonged neonatal intensive care unit admission; or as defined by trial authors).
Perinatal or infant death (excluding fatal anomalies).
Maternal death or serious maternal morbidity (e.g. admission to intensive care unit, septicaemia, organ failure, uterine rupture, hysterectomy, major surgical complication, life‐threatening event, long‐term disability, or as defined by trial authors).
Serious neonatal morbidity.
Disability in childhood, as defined by trial authors.
Breastfeeding failure, as defined by trial authors.
Postnatal depression, as defined by trial authors.
We graded evidence for our main comparison: planned caesarean section versus planned vaginal birth.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We planned to use the mean difference if outcomes were measured in the same way between trials or the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We plan to include cluster‐randomised trials in the analyses along with individually‐randomised trials if such trials are identified in future updates. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not applicable for this review.
Other unit of analysis issues
For maternal outcomes, we used the number of mothers as the denominator. For neonatal outcomes, we used the number of babies as the denominator. If we include cluster‐randomised trials in future updates and information on ICC is available, we will use cluster‐trial methods, with each woman regarded as a randomised cluster. In the absence of information on ICC, we will analyse the results of babies as if individually‐randomised, conceding that the width of the confidence interval might be underestimated.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we planned to discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We would have considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
Settings with high (more than 20 per 1000) versus low (less than 20 per 1000) perinatal mortality versus perinatal mortality mixed or unknown.
Leading twin cephalic versus non‐cephalic versus mixed presentation or unknown.
Leading twin cephalic and second twin cephalic versus non‐cephalic versus mixed presentation or unknown.
Gestational age term versus preterm versus mixed or unknown gestational age.
We planned to use all outcomes in subgroup analysis.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014). In future updates, if enough trials are included to make subgroup analysis possible, we will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result. In this version of the review data from only two trials were included and we did not carry out this additional analysis. In future updates we will carry out sensitivity analysis if sufficient data become available.
Results
Description of studies
Results of the search
The search retrieved 13 reports. We included two trials (11 reports), excluded one trial and one report is awaiting classification. See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of studies awaiting classification.
Included studies
We included two trials comparing planned caesarean versus planned vaginal birth for twin pregnancies.
The earlier trial (Rabinovici 1987), involved 60 women with cephalic/non‐cephalic twin pregnancies in labour at 35 or more weeks' gestation who were allocated to vaginal delivery (33) or caesarean section (27). The trial was conducted in Israel.
In the vaginal birth group, two women were delivered by caesarean section, and in four the second twin changed to cephalic presentation. These six women were excluded from the published data analysis. We have included the categorical neonatal outcomes in this review to conform to an intention‐to‐treat analysis. We were not able to include the continuous neonatal variables, and the maternal outcomes for the six excluded women were not reported, so we have not included these outcomes in this review. The reported maternal febrile morbidity (excluding the six women) was increased in the caesarean section group (11/27 versus 3/27).
Most of the data included in the review are from a more recent multicentre trial (Barrett 2013), where 2804 women were randomised in 106 centres in 25 countries. All centres had facilities to perform emergency caesarean section and had anaesthetic, obstetrical, and nursing staff available in the hospital at the time of planned vaginal delivery. Women with a twin pregnancy between 32 weeks’ and 38 weeks six days gestation were recruited to the trial. Inclusion criteria included that the first twin was cephalic, both twins alive and with weight estimated between 1500 g and 4000 g.
In the intervention group, women were randomised to planned lower segment caesarean delivery. If the first twin was delivered vaginally then caesarean section was attempted with the second twin if this was feasible. In the comparison group women were randomised to planned vaginal delivery with induction of labour between 37 weeks five days and 38 weeks six days and women were attended by clinical staff experienced in managing vaginal delivery of twins. The primary outcomes in this study were fetal or neonatal mortality or serious neonatal morbidity and maternal death or serious maternal morbidity before 28 days postpartum. Secondary outcomes included infant or child death or poor neurodevelopmental outcome up to two years, maternal satisfaction with mode of delivery, breastfeeding, quality of life, fatigue or depression.
Excluded studies
We excluded one study reported in a brief abstract (Juan 2014). The study report contained insufficient information to allow assessment of risk of bias and results were not reported. We will reconsider the eligibility of this study if further reports become available.
Risk of bias in included studies
See Figure 1 and Figure 2 for a summary of 'Risk of bias' assessments.
1.
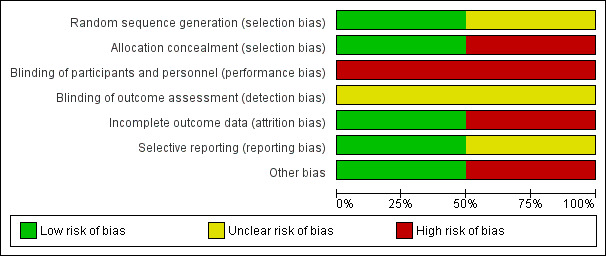
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In the Rabinovici 1987 trial, the allocation procedure was described as being changed randomly by a non‐involved person without prior notice on a time basis. A 20% difference in group sizes was not accounted for (27 versus 33). The possibility of inadequate allocation concealment is therefore high.
In the Barrett 2013 trial, randomisation was by computer with stratification for parity and gestational age using random block sizes. Randomisation was carried out centrally and so allocation concealment was assessed as being at low risk of bias.
Blinding
Blinding was not feasible in either trial and therefore both trials were assessed as being at high risk of performance bias. There was no mention about whether neonatal assessments were blinded in the Rabinovici 1987 study, while in the Barrett 2013 trial there was an attempt to blind assessors for the primary outcomes.
Incomplete outcome data
The Rabinovici 1987 trial was assessed as 'high risk' for attrition bias. Six women allocated to planned vaginal birth were excluded from primary analysis for delivery (this was not in accordance with the protocol) (two caesarean section and four vertex vaginal births). Analysis was not conducted on an intention‐to‐treat basis. However, for the categorical neonatal data we were able to include results of the six women in this review. For these neonatal outcomes attrition bias was assessed as being low.
In the Barrett 2013 study there was very little loss to follow‐up for short‐term outcomes and loss was balanced across groups; this trial was assessed as low risk of bias for this domain.
Selective reporting
No pre‐published protocol was available to check predefined outcome reporting for the Rabinovici 1987 study. A protocol was available for the Barrett 2013 trial and all outcomes appeared to be reported.
Other potential sources of bias
We assessed Rabinovici 1987 as being at 'high risk' for other bias because of a baseline imbalance: caesarean section n = 27 versus vaginal n = 33. In the published report of the Barrett 2013 trial there was no evidence of baseline imbalance and the non‐independence of outcomes for twins was taken into account in the trial analysis, though not in this review [pending ICC data].
Effects of interventions
See: Table 1
Comparison 1: Planned caesarean section versus planned vaginal birth (two studies, 2864 women randomised)
Primary outcomes
Maternal death or serious maternal morbidity: this outcome was reported by both included trials, but in the Rabinovici 1987 trial there were no events, so all estimable data were from the Barrett 2013 trial. There was no clear evidence of differences between women randomised to planned caesarean or planned vaginal birth for maternal death or serous morbidity (risk ratio (RR) 0.86, 95% confidence interval (CI) 0.67 to 1.11; 2844 women; I² = 0%, moderate quality evidence) (Analysis 1.1).
1.1. Analysis.
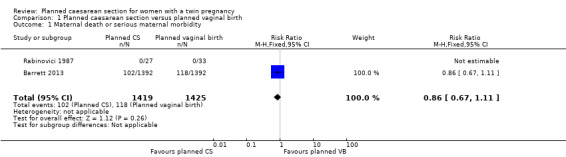
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 1 Maternal death or serious maternal morbidity.
Perinatal or neonatal death or serious neonatal morbidity: there was no significant difference between groups for this outcome reported in the Barrett 2013 trial (RR 1.15, 95% CI 0.80 to 1.67; data for 5565 babies, moderate quality evidence) (Analysis 1.2).
1.2. Analysis.
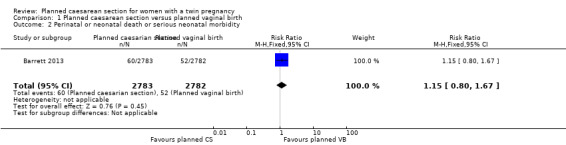
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 2 Perinatal or neonatal death or serious neonatal morbidity.
Perinatal or infant death or childhood disability was not reported. We hope to include data on this outcome in updates if infants from the Barrett 2013 trial are followed up during childhood.
Secondary outcomes
Short‐term Infant outcomes
There was no clear evidence of differences between groups for perinatal or neonatal mortality (RR 1.41, 95% CI 0.76 to 2.62; 5685 babies; two studies, moderate quality evidence) (Analysis 1.4) or serious neonatal morbidity (RR 1.03, 95% CI 0.65 to 1.64; 5644 babies; two studies, moderate quality evidence) (Analysis 1.5).
1.4. Analysis.
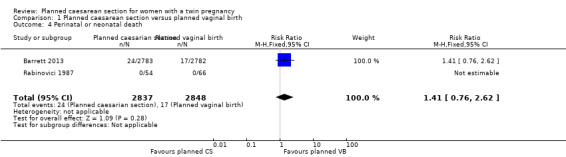
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 4 Perinatal or neonatal death.
1.5. Analysis.
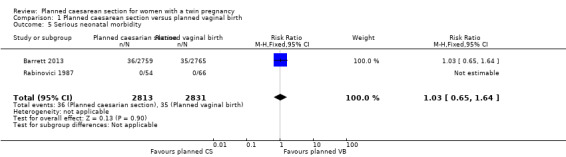
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 5 Serious neonatal morbidity.
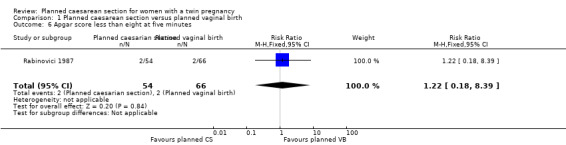
There were few babies with low Apgar scores at one and five minutes and no significant difference between groups (RR 1.22, 95% CI 0.18 to 8.39; 120 babies; one study, and RR 0.29, 95% CI 0.06 to 1.38; 5644 babies; two studies, respectively) (Analysis 1.6; Analysis 1.7).
1.6. Analysis.
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 6 Apgar score less than eight at five minutes.
1.7. Analysis.
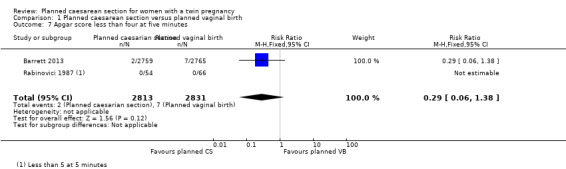
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 7 Apgar score less than four at five minutes.
There was no clear evidence that outcomes differed for women randomised to planned caesarean versus vaginal birth for serious neonatal morbidity including neonatal encephalopathy (reported in the Rabinovici 1987 trial; no events); birth trauma (RR 0.57, 95% CI 0.17 to 1.95; 5644 babies; two studies) Analysis 1.9; nerve palsy (reported in the Rabinovici 1987 trial; no events) Analysis 1.10; subdural or intracerebral haemorrhage (RR 3.01, 95% CI 0.31 to 28.89; 5644 babies; two studies) Analysis 1.11; intraventricular haemorrhage grades III or IV (no events) Analysis 1.12; cystic periventricular leukomalacia (RR 5.01, 95% CI 0.24 to 104.33; 5524 babies; one study) Analysis 1.13; neonatal sepsis up to 72 hours (RR 0.50, 95% CI 0.05 to 5.52; 5524 babies; one study) Analysis 1.14, or necrotising enterocolitis (RR 0.33, 95% CI 0.03 to 3.21; 5524 babies; one study) Analysis 1.15. Event rates for all of these outcomes were low.
1.9. Analysis.
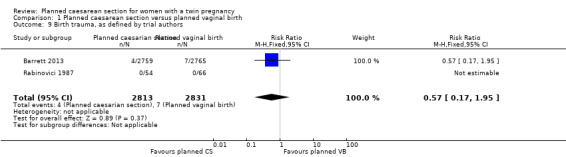
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 9 Birth trauma, as defined by trial authors.
1.10. Analysis.
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 10 Nerve palsy (including brachial plexus injury).
1.11. Analysis.
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 11 Subdural or intracerebral haemorrhage.
1.12. Analysis.
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 12 Intraventricular haemorrhage: grade III or IV.
1.13. Analysis.
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 13 Cystic periventricular leukomalacia.
1.14. Analysis.
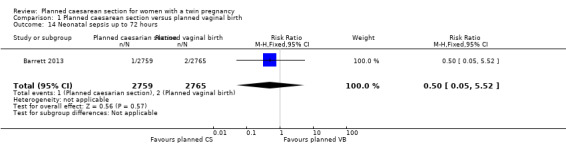
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 14 Neonatal sepsis up to 72 hours.
1.15. Analysis.
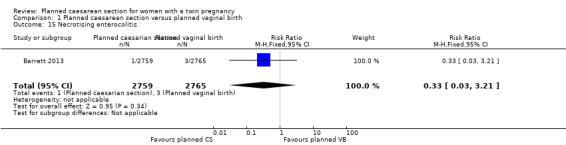
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 15 Necrotising enterocolitis.
The number of babies requiring assisted ventilation for 24 hours or more was reported in the Barrett 2013 trial and there was no strong evidence of any difference between groups (RR 1.59, 95% CI 0.87 to 2.91; 5524 babies), Analysis 1.16.
1.16. Analysis.
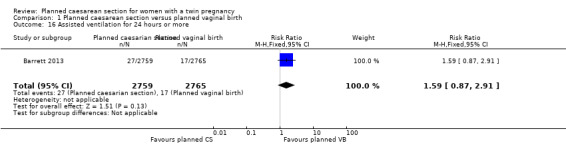
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 16 Assisted ventilation for 24 hours or more.
Short‐term maternal outcomes
The number of women undergoing caesarean section was reported in both trials. Most women in the planned caesarean group had treatment as planned (90.9% underwent caesarean section), whereas in the planned vaginal birth group, 42.9% had caesarean section for at least one twin (Analysis 1.17).
1.17. Analysis.
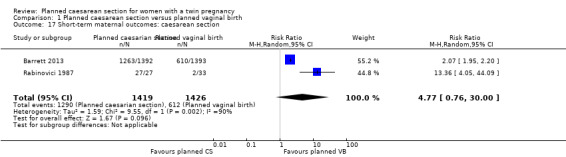
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 17 Short‐term maternal outcomes: caesarean section.
Both trials reported maternal mortality; no events were reported in the Rabinovici 1987 study and two deaths (one in each group) in the Barrett 2013 trial. There were no significant differences between groups for serious maternal morbidity overall (RR 0.86, 95% CI 0.67 to 1.11; 2844 women; two studies) Analysis 1.1, or for different types of short‐term morbidity including thromboembolism requiring anticoagulant therapy (RR 2.50, 95% CI 0.49 to 12.86; 2782 women; one study) Analysis 1.20; wound infection (RR 1.50, 95% CI 0.83 to 2.71; 2782 women; one study) Analysis 1.21; other infection (RR 1.39, 95% CI 0.76 to 2.53; 2782 women; one study) Analysis 1.22; disseminated intravascular coagulation (RR 5.00, 95% CI 0.24 to 104.05; 2782 women; one study) Analysis 1.23; amniotic fluid embolism (RR 3.00, 95% CI 0.12 to 73.58; 2782 women; one study) Analysis 1.24; postpartum haemorrhage (RR 0.78, 95% CI 0.59 to 1.02; 2782 women; one study) Analysis 1.25, or need for a blood transfusion (RR 0.88, 95% CI 0.64 to 1.21; 2782 women; one study) Analysis 1.26.
1.20. Analysis.
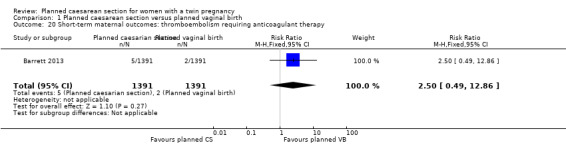
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 20 Short‐term maternal outcomes: thromboembolism requiring anticoagulant therapy.
1.21. Analysis.
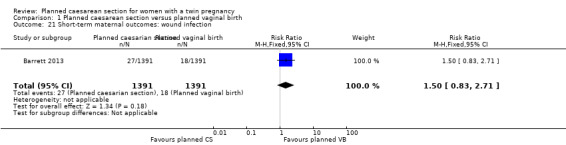
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 21 Short‐term maternal outcomes: wound infection.
1.22. Analysis.
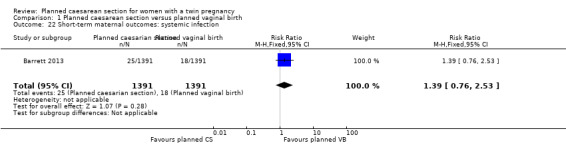
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 22 Short‐term maternal outcomes: systemic infection.
1.23. Analysis.
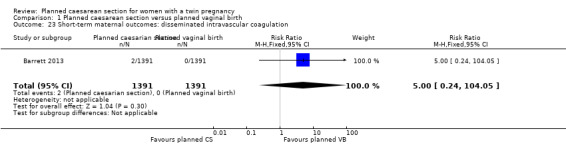
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 23 Short‐term maternal outcomes: disseminated intravascular coagulation.
1.24. Analysis.
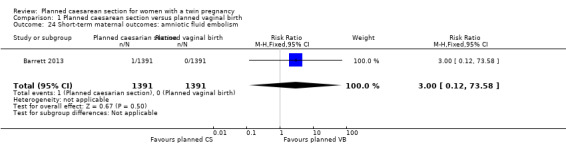
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 24 Short‐term maternal outcomes: amniotic fluid embolism.
1.25. Analysis.
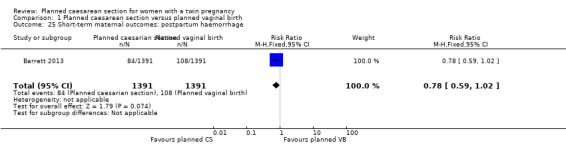
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 25 Short‐term maternal outcomes: postpartum haemorrhage.
1.26. Analysis.
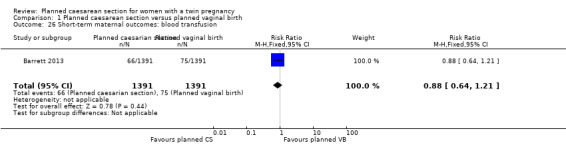
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 26 Short‐term maternal outcomes: blood transfusion.
Longer‐term maternal outcomes
Women in the Barrett 2013 study were followed up in the postpartum period.
Similar numbers of women in the two groups experienced urinary (RR 0.87, 95% CI 0.64 to 1.18), flatus (RR 0.92, 95% CI 0.77 to 1.09) or faecal incontinence (RR 1.02, 95% CI 0.69 to 1.51), (2570 women, one study, Analysis 1.28; Analysis 1.29; Analysis 1.30).
1.28. Analysis.
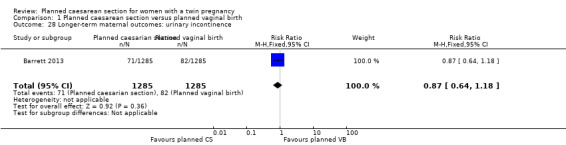
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 28 Longer‐term maternal outcomes: urinary incontinence.
1.29. Analysis.
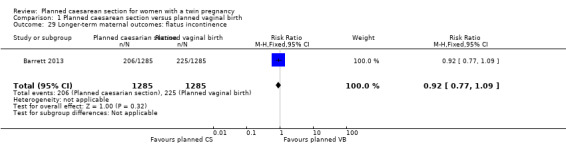
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 29 Longer‐term maternal outcomes: flatus incontinence.
1.30. Analysis.
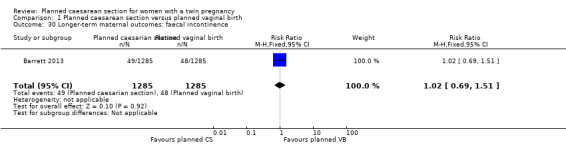
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 30 Longer‐term maternal outcomes: faecal incontinence.
There were no significant differences between groups for failure to breastfeed (RR 1.14, 95% CI 0.95 to 1.38, moderate quality evidence) Analysis 1.27, or the number of women with scores greater than 12 on the Edinbugh postnatal depression scale (RR 0.95, 95% CI 0.78 to 1.14, moderate quality evidence) Analysis 1.31, (2570 women, one study).
1.27. Analysis.
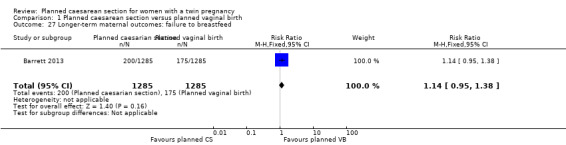
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 27 Longer‐term maternal outcomes: failure to breastfeed.
1.31. Analysis.
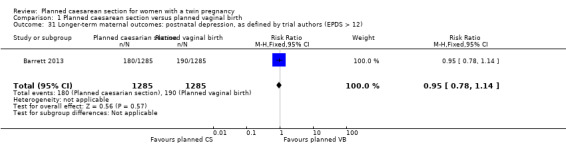
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 31 Longer‐term maternal outcomes: postnatal depression, as defined by trial authors (EPDS > 12).
Discussion
Summary of main results
There were no differences in perinatal or maternal outcomes between the groups for any of the primary or secondary outcomes reported.
Overall completeness and applicability of evidence
The potential effects of the choice of planned mode of delivery in specific clinical circumstances are complex, and extend beyond the easily measurable clinical outcomes, to less obvious consequences such as emotional effects on the mother, her sense of self‐esteem and her relationship with her child or children. Previous large randomised trials of interventions associated with more medical intervention in the birth process, which have included long‐term follow‐up of the children, have shown unexpected discordance between short‐term perinatal morbidity and long‐term neurodevelopmental outcomes. Two examples are planned caesarean section for term breech presentation, and continuous electronic fetal monitoring and scalp blood sampling during labour.
In the Term Breech Trial, in countries with low perinatal mortality rates, there were three perinatal deaths and 26 babies with severe perinatal morbidity of 511 in the planned vaginal birth group, compared with no deaths and severe perinatal morbidity in two of 514 babies following planned caesarean section (Hannah 2000). One would have expected less neurodevelopmental delay at two years in the planned caesarean section group, but the trend (though not statistically significant) was in the opposite direction: 12/457 compared with 7/463 in the planned vaginal birth group (Whyte 2004).
In the Dublin trial of continuous cardiotocography during labour, neonatal seizures occurred in 12 of 6530 babies following continuous electronic monitoring during labour, and 27 of 6554 following intermittent auscultation (Macdonald 1985). There were 14 perinatal deaths in each group. Despite there being far fewer cases of neonatal seizures in the continuous electronic monitoring group, the rate of subsequent cerebral palsy was similar between groups: 12/6527 versus 10/6552 respectively (Grant 1989). Similar comparisons in the Seattle trial of continuous electronic monitoring and scalp blood sampling for preterm labour were: seizures 7/122 versus 7/124 (Luthy 1987); cerebral palsy 16/82 versus 7/91, respectively (Shy 1990).
Because of this consistent trend to more long‐term neurological sequale following medical interventions during childbirth than would be expected on the basis of the short‐term perinatal outcomes, clinical decisions must be based on long‐term rather than short‐term outcomes. As more data relevant to this review become available, it is important that the implications of short‐term perinatal morbidity be interpreted with caution, and the results of long‐term follow‐up be awaited.
As the study of Barrett 2013 was a multicentre trial with a wide geographic distribution, the results should be applicable to a variety of clinical settings. However, caution should be exercised in applying the evidence to settings without ready access to caesarean section, or where there may not be access in subsequent pregnancies.
Quality of the evidence
As 98% to 100% of the data for most outcomes are from one study, the risk of bias for the review approximates that of Barrett 2013. We judged the risk of bias to be low for all categories except performance (high) and outcome assessment bias (unclear).
For outcomes included in the 'Summary of findings' table, we graded the quality of the evidence. For all outcomes where data were available, the evidence was assessed as being of moderate quality, the reason for downgrading was the imprecision of effect estimates.
Potential biases in the review process
One review author (JB) who was lead investigator for Barrett 2013 did not participate in decisions regarding that study.
Agreements and disagreements with other studies or reviews
The findings of this review are consistent with those of previous non‐randomised studies and reviews which found little evidence of differences between caesarean section and vaginal birth for twin pregnancy (Ford 2006; Henriksen 1994; Hogle 2002, Sentilhes 2007; Varner 2005).
Authors' conclusions
Implications for practice.
Data mainly from one large, multicentre study found no clear evidence of benefit from planned caesarean section for term twin pregnancies with leading cephalic presentation. Data on long‐term infant outcomes are awaited. Women should be informed of possible risks during labour and vaginal birth pertinent to their specific clinical presentation, as well as the beneficial perinatal effects of labour and vaginal birth, and the current and long‐term effects of caesarean section for both mother and babies. Medical interventions in the birth process such as caesarean section should be avoided unless there is reasonable clinical certainty that they will be of long‐term benefit. There is insufficient evidence to support the routine use of planned caesarean section for term twin pregnancy with leading cephalic presentation, except in the context of further randomised trials.
Implications for research.
Data on long‐term infant outcomes from Barrett 2013 are awaited. In view of the wide confidence intervals for several key outcomes, further randomised trials would be justified. Trials are also needed to assess the usefulness of planned caesarean section for preterm twin pregnancy.
What's new
| Date | Event | Description |
|---|---|---|
| 9 May 2019 | Amended | Edited Declarations of interest |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 12, 2011
| Date | Event | Description |
|---|---|---|
| 18 November 2015 | New search has been performed | Search updated and one new study added. We assessed the quality of the evidence using the GRADE approach and a 'Summary of findings' table has been added. |
| 18 November 2015 | New citation required but conclusions have not changed | New data added for one new study (Barrett 2013), previously in ongoing. Conclusions remain the same. |
Acknowledgements
For this update, Therese Dowswell (TD) (Cochrane Pregnancy and Childbirth Group) provided assistance in data extraction and entry and in grading the evidence for the 'Summary of findings' table. TD is supported by the NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Stephen Milan for assistance with data extraction and compiling a previous version of the review; Sonja Henderson and the Cochrane Pregnancy and Childbirth Group team for administrative support.
Data and analyses
Comparison 1. Planned caesarean section versus planned vaginal birth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death or serious maternal morbidity | 2 | 2844 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.11] |
| 2 Perinatal or neonatal death or serious neonatal morbidity | 1 | 5565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.67] |
| 3 Perinatal or infant death or disability in childhood | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Perinatal or neonatal death | 2 | 5685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.76, 2.62] |
| 5 Serious neonatal morbidity | 2 | 5644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.64] |
| 6 Apgar score less than eight at five minutes | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.18, 8.39] |
| 7 Apgar score less than four at five minutes | 2 | 5644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.38] |
| 8 Neonatal encephalopathy, as defined by trial authors | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Birth trauma, as defined by trial authors | 2 | 5644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.17, 1.95] |
| 10 Nerve palsy (including brachial plexus injury) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Subdural or intracerebral haemorrhage | 2 | 5644 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [0.31, 28.89] |
| 12 Intraventricular haemorrhage: grade III or IV | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Cystic periventricular leukomalacia | 1 | 5524 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.01 [0.24, 104.33] |
| 14 Neonatal sepsis up to 72 hours | 1 | 5524 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.52] |
| 15 Necrotising enterocolitis | 1 | 5524 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.21] |
| 16 Assisted ventilation for 24 hours or more | 1 | 5524 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.87, 2.91] |
| 17 Short‐term maternal outcomes: caesarean section | 2 | 2845 | Risk Ratio (M‐H, Random, 95% CI) | 4.77 [0.76, 30.00] |
| 18 Short‐term maternal outcomes: mortality | 2 | 2844 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.97] |
| 19 Short‐term maternal outcomes: serious maternal morbidity | 2 | 2842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.11] |
| 20 Short‐term maternal outcomes: thromboembolism requiring anticoagulant therapy | 1 | 2782 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.86] |
| 21 Short‐term maternal outcomes: wound infection | 1 | 2782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.83, 2.71] |
| 22 Short‐term maternal outcomes: systemic infection | 1 | 2782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.76, 2.53] |
| 23 Short‐term maternal outcomes: disseminated intravascular coagulation | 1 | 2782 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 104.05] |
| 24 Short‐term maternal outcomes: amniotic fluid embolism | 1 | 2782 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.58] |
| 25 Short‐term maternal outcomes: postpartum haemorrhage | 1 | 2782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.02] |
| 26 Short‐term maternal outcomes: blood transfusion | 1 | 2782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 27 Longer‐term maternal outcomes: failure to breastfeed | 1 | 2570 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.95, 1.38] |
| 28 Longer‐term maternal outcomes: urinary incontinence | 1 | 2570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.64, 1.18] |
| 29 Longer‐term maternal outcomes: flatus incontinence | 1 | 2570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.77, 1.09] |
| 30 Longer‐term maternal outcomes: faecal incontinence | 1 | 2570 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.69, 1.51] |
| 31 Longer‐term maternal outcomes: postnatal depression, as defined by trial authors (EPDS > 12) | 1 | 2570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.78, 1.14] |
1.8. Analysis.
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 8 Neonatal encephalopathy, as defined by trial authors.
1.18. Analysis.
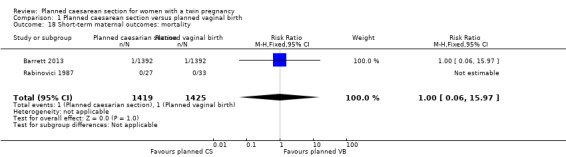
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 18 Short‐term maternal outcomes: mortality.
1.19. Analysis.
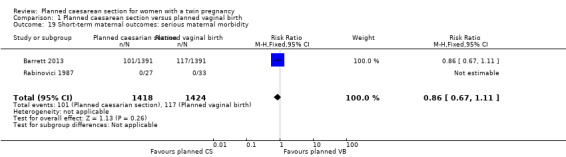
Comparison 1 Planned caesarean section versus planned vaginal birth, Outcome 19 Short‐term maternal outcomes: serious maternal morbidity.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrett 2013.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: recruitment between Dec 2003‐April 2011, 2804 women randomised in 106 centres in 25 countries. Participating centres assessed fetal growth and well‐being with the use of ultrasonography at least every 4 weeks and with the use of non‐stress or biophysical profile tests twice weekly if needed; were prepared to perform a CS within 30 minutes if necessary; and had anaesthetic, obstetrical, and nursing staff available in the hospital at the time of planned vaginal delivery. Inclusion criteria: women with twin pregnancy between 32 weeks’ and 38 weeks 6 days gestation. First twin cephalic, both twins alive and weight estimated between 1500 g and 4000 g confirmed by ultrasound. Exclusion: monoamniotic twins, fetal reduction at 13 or more weeks, lethal anomaly, contraindication to VB or previous participation in the twin birth study. |
|
| Interventions | Experimental intervention: 1398 women (2795 fetuses) randomised to planned lower segment caesarean delivery. If the first twin delivered vaginally in this group, then CS was attempted with the second twin if this was feasible. Control/comparison intervention: 1406 women (2812 fetuses) randomised to planned vaginal delivery with induction of labour between 37 weeks 5 days and 38 weeks 6 days. (Use of continuous electronic monitoring, epidural and oxytocin at the discretion of the obstetrician. Women were attended by obstetricians experienced in managing vaginal delivery of twins.) |
|
| Outcomes | Primary outcome: fetal or neonatal mortality or serious neonatal morbidity. (Neonatal morbidity 0‐27 days) Serious morbidity defined. Maternal death or serious maternal morbidity before 28 days postpartum (defined). Secondary outcomes, infant or child death or poor neurodevelopmental outcome up to 2 years. Maternal satisfaction with mode of delivery, breastfeeding, quality of life, fatigue or depression. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally‐controlled randomisation by computer with stratification for parity and gestational age using random block sizes. |
| Allocation concealment (selection bias) | Low risk | See above. Centralised randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of women and staff is not feasible for this type of intervention. Lack of blinding could possibly lead to changes in management that might affect outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was an attempt to blind assessors for the primary outcome. It was not clear if this was successful. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was very limited loss to follow‐up for the primary outcomes (2783/ 2795 fetuses included in analysis of primary outcome in the intervention group and 2782/2812 in the control group). Loss balanced in intervention and control groups. There was some further loss to follow‐up for longer‐term maternal outcomes. ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol available as supplementary information and all outcomes appeared to be reported. |
| Other bias | Low risk | There was no evidence of baseline imbalance. The analysis took account of correlation between outcomes for twins. |
Rabinovici 1987.
| Methods | 'Randomized control trial.' | |
| Participants | Inclusion criteria: twin pregnancy, induced or spontaneous labour, both alive. 1st vertex, 2nd breech or transverse, 35‐42 weeks estimated gestational age, no known fetal anomaly, no signs of acute placental insufficiency or abruption, normal amniotic fluid volume, normal FHR testing, no maternal or obstetric indications for a specific route of delivery, cervix < 7 cm dilated. 60 women randomised. | |
| Interventions | Experimental intervention: planned lower segment CS, preferably with epidural analgesia, but dependent on preference of anaesthetist. Control/comparison intervention: planned VB following evaluation of labour progress using ‘Friedman curve’; continuous electronic fetal monitoring of both babies; lie of 2nd twin confirmed clinically or with ultrasound; if breech, assisted breech delivery planned; of fetal distress or poor progress despite oxytocin, total breech extraction done; artificial rupture of second sac as late as possible; if second twin in oblique or transverse lie, internal version and complete breech extraction under general analgesia or epidural analgesia if in place; routine episiotomy. |
|
| Outcomes | Method of delivery; birthweight, Apgar scores, neonatal and maternal complications. | |
| Notes | Setting: Chaim Sheba Medical Centre labour ward. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation “changed randomly by a non‐involved person without prior notice on a time basis”. 20% difference in group sizes not accounted for (27 vs 33). |
| Allocation concealment (selection bias) | High risk | See above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned whether neonatal assessments blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 women allocated to planned VB excluded from primary analysis for delivery not according to protocol (2 CS and 4 vertex VBs). Analysis was not conducted on an ITT basis. Only categorical neonatal data have been included in the review as data given separately for the 6 excluded women and could be added to the primary data, thus low risk of bias for these outcomes. |
| Selective reporting (reporting bias) | Unclear risk | No pre‐published protocol available to check predefined outcome reporting. |
| Other bias | High risk | Baseline imbalance: CS n = 27 vs vaginal n = 33. |
CS: caesarean section FHR: fetal heart rate ITT: intention‐to‐treat VB: vaginal birth vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Juan 2014 | No numerical data given in abstract. Might be included when full report available. |
Characteristics of studies awaiting assessment [ordered by study ID]
Dera 2009.
| Methods | 'Randomized.' |
| Participants | Women with twin pregnancy 32 to 38 weeks 6 days. |
| Interventions | Planned CS vs planned VB. |
| Outcomes | No data given in published abstract. |
| Notes | Full report awaited. |
CS: caesarean section VB: vaginal birth vs: versus
Differences between protocol and review
The methods section has been updated to reflect the Pregnancy and Childbirth Group's updated methods text and the latest Cochrane Handbook (Higgins 2011). A 'Summary of findings' table has been incorporated for this update and the quality of the evidence assessed using the GRADE approach.
Contributions of authors
CA Crowther prepared a previous version of this review, limited to twin pregnancies with non‐vertex second twin (Caesarean delivery for the second twin ‐ seeCrowther 1996), and contributed to the development of this protocol and review.
JF Barrett reviewed the topic, prepared a first draft of the background, and contributed to the protocol and review development.
GJ Hofmeyr prepared the first draft of the protocol and of the review, and is the guarantor of the review.
Sources of support
Internal sources
(GJH) Effective Care Research Unit, University of the Witwatersrand, University of Fort Hare, Eastern Cape Department of Health, South Africa.
(CC) ARCH: Australian Research Centre for Health of Women and Babies, Robinson Research Institute, The University of Adelaide, Australia.
External sources
(GJH) HRP‐UNDP/UNFPA/WHO/World Bank Special Programme in Human Reproduction, Geneva, Switzerland.
(CC) National Health and Medical Research Council, Australia Funding for the PCG Australian and New Zealand Satellite, Australia.
National Institute for Health Research (NIHR), UKNIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines, UK.
Declarations of interest
JF Barrett is principal investigator for Barrett 2013. He did not participate in decisions relating to that study.
Justus Hofmeyr receives royalties from UpToDate for chapters related to breech pregnancy, delivery of a baby in breech presentation and external cephalic version. UpToDate is an electronic publication by Wolters Kluwer to disseminate evidence‐based medicine (such as Cochrane reviews).
Edited (no change to conclusions)
References
References to studies included in this review
Barrett 2013 {published data only}
- Barrett J. Evidence based intra‐partum management of twin pregnancy. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):19. [Google Scholar]
- Barrett J, Aztalos E, Willan A, Joseph K, Armson BA, Hutton E, et al. The Twin Birth Study: a multicenter RCT of planned cesarean section (CS) and planned vaginal birth (VB) for twin pregnancies 320 to 386/7 weeks. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S4‐5. [Google Scholar]
- Barrett J, Hutton E, Twin birth study group. Twin birth study (TBS). Perinatal Society of Australia and New Zealand 7th Annual Congress; 2003 March 9‐12; Tasmania, Australia. 2003:P162.
- Barrett JF, Hannah ME, Hutton EK, Willan AR, Allen AC, Armson BA, et al. A randomized trial of planned cesarean or vaginal delivery for twin pregnancy. New England Journal of Medicine 2013;369(14):1295‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton E, Barrett J. The twin birth study: a randomised controlled trial of planned vaginal or planned caesarean birth for twin pregnancies. International Confederation of Midwives 30th Triennial Congress. Midwives: Improving Women’s Health; 2014 June 1‐4; Prague, Czech Republic. 2014:C158.
- Hutton EK, Hannah ME, Ross S, Asztalos EV, Willan AR, Allen AC, et al. Maternal 3 month outcomes after planned cesarean (CS) vs planned vaginal birth (VB) for twin pregnancies: The Twin Birth Study (TBS). Reproductive Sciences (Thousand Oaks, Calif.) 2014;21(3 Suppl 1):284A. [Google Scholar]
- Hutton EK, Hannah ME, Ross S, Joseph KS, Ohlsson A, Asztalos EV, et al. Maternal outcomes at 3 months after planned caesarean section versus planned vaginal birth for twin pregnancies in the Twin Birth Study: a randomised controlled trial. BJOG: an International Journal of Obstetrics and Gynaecology 2015;122(12):1653‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoff K, Mason D, Mergler S, Sanchez J, Barrett JFR, Asztalos E. Processes implemented to ensure successful follow‐up during ongoing recruitment. Clinical Trials (London, England) 2011;8(4):503. [Google Scholar]
- McLeod L, Barrett J, Hewson S, Hannah ME. Women's views regarding participation in a proposed randomized controlled trial of twin delivery. JOGC: Journal of Obstetrics and Gynaecology Canada 2004;26(6):575‐9. [DOI] [PubMed] [Google Scholar]
Rabinovici 1987 {published data only}
- Rabinovici J, Barkai G, Reichman B, Serr DM, Mashiach S. Randomized management of the second non‐vertex twin: vaginal delivery or cesarean section. American Journal of Obstetrics and Gynecology 1987;156:52‐6. [DOI] [PubMed] [Google Scholar]
- Rabinovici J, Barkai G, Reichman B, Serr DM, Mashiach S. The randomized management of the second non‐vertex twin: vaginal delivery or cesarean section?. Proceedings of 11th European Congress of Perinatal Medicine; 1988 April 10‐13; Rome, Italy. 1988.
References to studies excluded from this review
Juan 2014 {published data only}
- Juan DH, Min C. Study on the route of delivery and pregnancy outcome in twin pregnancy. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(Suppl 1):435. [Google Scholar]
References to studies awaiting assessment
Dera 2009 {published data only}
- Dera A, Breborowicz GH, Szczapa‐Krenz H. Natural delivery is safe: outcome differences by mode of delivery by time. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(Suppl 1):43‐4. [Google Scholar]
Additional references
Aaronson 2010
- Aaronson D, Harlev A, Sheiner E, Levy A. Trial of labor after cesarean section in twin pregnancies: maternal and neonatal safety. Journal of Maternal‐Fetal & Neonatal Medicine 2010;23(6):550‐4. [DOI] [PubMed] [Google Scholar]
ACOG 2002
- ACOG. Educational bulletin. Special problems of multiple gestation. 1998 No. 253. Compendium of Selected Publications. American College of Obstetricians and Gynecologists, 2002:222‐32. [Google Scholar]
Alexander 2008
- Alexander JM, Leveno KJ, Rouse D, Landon MB, Gilbert SA, Spong CY, et al. Cesarean delivery for the second twin. Obstetrics & Gynecology 2008;112(4):748‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2000
- Anonymous. Health Canada. Canadian Perinatal Health Report. Ottawa: Minister of Public Health Works and Government Services Canada, 2000. [Google Scholar]
Arnold 1987
- Arnold C, McLean F, Kramer M, Usher R. Respiratory distress syndrome in second‐born versus first‐born twins: a matched case‐control analysis. New England Journal of Medicine 1987;317:1121‐5. [DOI] [PubMed] [Google Scholar]
Barrett 2000
- Barrett J, Bocking A. The SOGC consensus statement on management of twin pregnancies (part I). Journal of Obstetrics and Gynaecology Canada: JOGC 2000;7:519‐29. [Google Scholar]
Barrett 2004
- Barrett JF. Delivery of the term twin. Best Practice & Research. Clinical Obstetrics & Gynaecology 2004;18(4):625‐30. [DOI] [PubMed] [Google Scholar]
Blickstein 1987
- Blickstein I, Schwartz‐Shoham, Lancet M, Borenstein R. Vaginal delivery of the second twin in breech presentation. Obstetrics & Gynecology 1987;69:774‐6. [PubMed] [Google Scholar]
Blickstein 2000
- Blickstein I, Goldman RD, Kupferminc M. Delivery of breech first twins: a multicenter retrospective study. Obstetrics & Gynecology 2000;95:37‐42. [DOI] [PubMed] [Google Scholar]
Cheung 2000
- Cheung YB, Yip P, Karlberg J. Mortality of twins and singletons by gestational age: a varying‐coefficient approach. American Journal of Epidemiology 2000;152:1107‐16. [DOI] [PubMed] [Google Scholar]
De Almeida 2010
- Almeida MF, Guinsburg R, Costa JO, Anchieta LM, Freire LM, Campos D Jr. Non‐urgent caesarean delivery increases the need for ventilation at birth in term newborn infants. Archives of Disease in Childhood Fetal & Neonatal Edition 2010;95(5):F326‐F330. [DOI] [PubMed] [Google Scholar]
Dodd 2014
- Dodd JM, Deussen AR, Grivell RM, Crowther CA. Elective birth at 37 weeks’ gestation for women with an uncomplicated twin pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD003582.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eason 2002
- Eason E, Labrecque M, Marcoux S, Mondor M. Anal incontinence after childbirth. Canadian Medical Association Journal 2002;166:326‐30. [PMC free article] [PubMed] [Google Scholar]
Fabre 1988
- Fabre E, Agüero R, Augustin JL, Pérez‐Hiraldo MP, Bescos JL. Perinatal mortality in twin pregnancy: an analysis of birth weight‐specific mortality rates and adjusted mortality rates for birth weight distributions. Journal of Perinatal Medicine 1988;16:85‐91. [DOI] [PubMed] [Google Scholar]
Farrell 2001
- Farrell SA, Allen VM, Baskett TF. Parturition and urinary incontinence in primiparas. Obstetrics & Gynecology 2001;97:350‐6. [DOI] [PubMed] [Google Scholar]
Ford 2006
- Ford AA, Bateman BT, Simpson LL. Vaginal birth after cesarean delivery in twin gestations: a large, nationwide sample of deliveries. American Journal of Obstetrics & Gynecology 2006;195(4):1138‐42. [DOI] [PubMed] [Google Scholar]
Ghai 1988
- Ghai V, Vidyasagar D. Morbidity and mortality factors in twins, an epidemiologic approach. Clinics in Perinatology 1988;15:123‐40. [PubMed] [Google Scholar]
Grant 1989
- Grant A, O'Brien N, Joy MT, Hennessy E, MacDonald D. Cerebral palsy among children born during the Dublin randomised trial of intrapartum monitoring. Lancet 1989;8674:1233‐6. [DOI] [PubMed] [Google Scholar]
Grisaru 2000
- Grisaru D, Fuchs S, Kupferminc MJ, Har‐Toov J, Niv J, Lessing JB. Outcome of 306 twin deliveries according to first twin presentation and method of delivery. American Journal of Perinatology 2000;17:303‐7. [DOI] [PubMed] [Google Scholar]
Hall 1999
- Hall MH, Bewley S. Maternal mortality and mode of delivery. Lancet 1999;354:776. [DOI] [PubMed] [Google Scholar]
Hannah 2000
- Hannah ME, Hannah WJ, Hewson S, Hodnett E, Saigal S, Willan A, et al. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Lancet 2000;356:1375‐83. [DOI] [PubMed] [Google Scholar]
Hannah 2002
- Hannah ME, Hannah WJ, Hodnett E, Chalmers B, Kung R, Willan A, et al. Outcomes at 3 months postpartum for women enrolled in the multicenter international randomized Term Breech Trial of planned cesarean section and planned vaginal birth for breech presentation at term. JAMA 2002;287:1822‐31. [DOI] [PubMed] [Google Scholar]
Henriksen 1994
- Henriksen TB, Sperling L, Hedegaard M, Ulrichsen H, Ovlisen B, Secher NJ. Cesarean section in twin pregnancies in two Danish counties with different cesarean section rates. Acta Obstetricia et Gynecologica Scandinavica 1994;73(2):123‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2015
- Hofmeyr GJ, Hannah M, Lawrie TA. Planned caesarean section for term breech delivery. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD000166.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogle 2002
- Hogle K, Hutton E, McBrien KA, Barrett J, Hannah ME. Cesarean delivery for twins: a systematic review and meta‐analysis. American Journal of Obstetrics and Gynecology 2002;188:220‐7. [DOI] [PubMed] [Google Scholar]
Houlihan 1996
- Houlihan C, Knuppel RA. Intrapartum management of multiple gestations. Complicated labor and delivery II. Clinics in Perinatology 1996;23:91‐116. [PubMed] [Google Scholar]
Joseph 1998
- Joseph KS, Kramer MS, Marcoux S, Ohlsson A, Wen SW, Allen A, et al. Determinants of preterm birth rates in Canada from 1981 through 1983 and from 1992 through 1994. New England Journal of Medicine 1998;339:1434‐9. [DOI] [PubMed] [Google Scholar]
Joseph 2001a
- Joseph KS, Marcoux S, Ohlsson A, Liu S, Allen AC, Kramer MS, et al. Changes in stillbirth and infant mortality associated with increases in preterm birth among twins. Pediatrics 2001;108:1055‐61. [DOI] [PubMed] [Google Scholar]
Joseph 2001b
- Joseph KS, Allen AC, Dodds L, Vincer MJ, Armson BA. Causes and consequences of recent increases in preterm birth among twins. Obstetrics & Gynecology 2001;98:57‐64. [DOI] [PubMed] [Google Scholar]
Kiely 1990
- Kiely JL. The epidemiology of perinatal mortality in multiple births. Bulletin of the New York Academy of Medicine 1990;66:618‐37. [PMC free article] [PubMed] [Google Scholar]
Knight 2008
- Knight M, Kurinczuk JJ, Spark P, Brocklehurst P. Cesarean delivery and peripartum hysterectomy. Obstetrics & Gynecology 2008;111(1):97‐105. [DOI] [PubMed] [Google Scholar]
Lavender 2012
- Lavender T, Hofmeyr GJ, Neilson JP, Kingdon C, Gyte GML. Caesarean section for non‐medical reasons at term. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD004660.pub3] [DOI] [PubMed] [Google Scholar]
Laws 2010
- Laws PJ, Li Z, Sullivan EA. Australia's mothers and babies 2008. AIHW Perinatal Statistic Unit. Canberra, 2010:Perinatal Statistic Series no. 24. Cat. no PER 50.
Lie 2000
- Lie RT. Invited commentary: intersecting perinatal mortality curves by gestational age ‐ are appearances deceiving?. American Journal of Epidemiology 2000;152:1117‐9. [DOI] [PubMed] [Google Scholar]
Luthy 1987
- Luthy DA, Shy KK, Belle G, Larson EB, Hughes JP, Benedetti TJ, et al. A randomized trial of electronic fetal monitoring in preterm labor. Obstetrics & Gynecology 1987;69:687‐95. [PubMed] [Google Scholar]
Macdonald 1985
- Macdonald D, Grant A, Sheridan‐Pereira M, Boylan P, Chalmers I. The Dublin randomized controlled trial of intrapartum fetal heart rate monitoring. American Journal of Obstetrics and Gynecology 1985;152:524‐39. [DOI] [PubMed] [Google Scholar]
Meyer 1998
- Meyer S, Schreyer A, Grandi P, Hohlfeld P. The effects of birth on urinary continence mechanisms and other pelvic‐floor characteristics. Obstetrics & Gynecology 1998;92:613‐8. [DOI] [PubMed] [Google Scholar]
Persad 2001a
- Persad V, Baskett T, O'Connell CM, Scott HM. Combined vaginal‐cesarean delivery of twin pregnancies. Obstetrics & Gynecology 2001;98:1032‐7. [DOI] [PubMed] [Google Scholar]
Persad 2001b
- Persad V, Young D, Armson A, Joseph KS, Baskett T. Determinants of perinatal morbidity and death among the second of twins. American Journal of Obstetrics and Gynecology 2001;184:S188. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schneid‐Kofman 2005
- Schneid‐Kofman N, Sheiner E, Levy A, Holcberg G. Risk factors for wound infection following cesarean deliveries. International Journal of Gynecology & Obstetrics 2005;90(1):10‐5. [DOI] [PubMed] [Google Scholar]
Sentilhes 2007
- Sentilhes L, Goffinet F, Talbot A, Diguet A, Verspyck E, Cabrol D, et al. Attempted vaginal versus planned cesarean delivery in 195 breech first twin pregnancies. Acta Obstetricia et Gynecologica Scandinavica 2007;86(1):55‐60. [DOI] [PubMed] [Google Scholar]
Shy 1990
- Shy KK, Luthy DA, Bennett FC, Whitfield M, Larson EB, Belle G, et al. Effects of electronic fetal heart rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. New England Journal of Medicine 1990;322(9):588‐93. [DOI] [PubMed] [Google Scholar]
Simoes 2007
- Simoes T, Aboim L, Costa A, Ambrosio A, Alves S, Blickstein I. Puerperal complications following elective Cesarean sections for twin pregnancies. Journal of Perinatal Medicine 2007;35(2):104‐7. [DOI] [PubMed] [Google Scholar]
Suzuki 2008
- Suzuki S, Inde Y, Igarashi M, Miyake H. Elective cesarean as a risk factor for transfusion after delivery of twins. Journal of Nippon Medical School: Nihon Ika Daigahu Zasshi 2008;75(4):247‐9. [DOI] [PubMed] [Google Scholar]
Suzuki 2009
- Suzuki S. Risk factors for emergency cesarean delivery of the second twin after vaginal delivery of the first twin. Journal of Obstetrics & Gynaecology Research 2009;35(3):467‐71. [DOI] [PubMed] [Google Scholar]
Suzuki 2010
- Suzuki S, Yamashita E, Inde Y, Hiraizumi Y, Satomi M. Increased rate of elective cesarean delivery and neonatal respiratory disorders in twin pregnancies. Journal of Nippon Medical School: Nihon Ika Daigahu Zasshi 2010;77(2):93‐6. [DOI] [PubMed] [Google Scholar]
Varner 2005
- Varner MW, Leindecker S, Spong CY, Moawad AH, Hauth JC, Landon MB, et al. The Maternal‐Fetal Medicine Unit cesarean registry: trial of labor with a twin gestation. American Journal of Obstetrics and Gynecology 2005;193(1):135‐40. [DOI] [PubMed] [Google Scholar]
Viktrup 2001
- Viktrup L, Lose G. The risk of stress incontinence 5 years after first delivery. American Journal of Obstetrics and Gynecology 2001;185:82‐7. [DOI] [PubMed] [Google Scholar]
Wells 1991
- Wells SR, Thorp JM, Bowes WA. Management of the nonvertex second twin. Surgery, Gynecology and Obstetrics 1991;172:383‐5. [PubMed] [Google Scholar]
Wen 2004a
- Wen SW, Fung KF, Oppenheimer L, Demissie K, Yang Q, Walker M. Occurrence and predictors of cesarean delivery for the second twin after vaginal delivery of the first twin. Obstetrics & Gynecology 2004;103(3):413‐9. [DOI] [PubMed] [Google Scholar]
Wen 2004b
- Wen SW, Rusen ID, Walker M, Listen R, Kramer MS, Baskett T, et al. Comparison of maternal mortality and morbidity between trial of labour and elective cesarean section among women with previous caesarean delivery. American Journal of Obstetrics and Gynecology 2004;191:1263‐9. [DOI] [PubMed] [Google Scholar]
Whyte 2004
- Whyte H, Hannah ME, Saigal S, Hannah WJ, Hewson S, Amankwah K, et al. Outcomes of children at 2 years after planned cesarean birth versus planned vaginal birth for breech presentation at term: the International Randomized Term Breech Trial. American Journal of Obstetrics and Gynecology 2004;191:864‐71. [DOI] [PubMed] [Google Scholar]
Wilcox 1996
- Wilcox LS, Kiely JL, Melvin CL, Martin MC. Assisted reproductive technologies: estimates of their contribution to multiple births and newborn hospital days in the United States. Fertility and Sterility 1996;65:361‐6. [DOI] [PubMed] [Google Scholar]
Wilson 1996
- Wilson PD, Herbison RM, Herbison GP. Obstetric practice and the prevalence of urinary incontinence three months after delivery. British Journal of Obstetrics and Gynaecology 1996;103:154‐61. [DOI] [PubMed] [Google Scholar]
Zetterström 1999
- Zetterström JP, López A, Anzén B, Dolk A, Norman M, Mellgren A. Anal incontinence after vaginal delivery: a prospective study in primiparous women. British Journal of Obstetrics and Gynaecology 1999;106:324‐30. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Crowther 1996
- Crowther CA. Caesarean delivery for the second twin. Cochrane Database of Systematic Reviews 1996, Issue 1. [DOI: 10.1002/14651858.CD000047] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2011
- Hofmeyr GJ, Barrett JF, Crowther CA. Planned caesarean section for women with a twin pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD006553.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


