Abstract
The present study investigated the role of energy loss assessed by vector flow mapping (VFM) in patients with hypertrophic cardiomyopathy (HCM). VFM analysis was performed in 42 patients with HCM and in 40 control subjects, which were matched for age, sex and left ventricular (LV) ejection fraction. The intra-LV and left atrial blood flow were obtained from the apical 3-chamber view, and the energy loss (EL) during the systolic and diastolic phases was calculated. The measurements were averaged over three cardiac cycles and indexed to body surface area. Compared with the controls, the left ventricular energy loss (LVEL)-total value was significantly decreased in patients with HCM during the diastolic phase (P1, P2 and P3; all P<0.05). A tendency for increased systolic LVEL-total values was observed in the patients with HCM compared with the controls (P>0.05). LVEL-base values were decreased in the patients with HCM during P1 and P2 (slow filling time). Compared with the controls, patients with HCM had lower LVEL-mid values during the diastolic phases (P0, P1, P2 and P3; all P<0.05). However, the LVEL-mid value of patients with HCM was higher compared with that of the controls during systolic P5 (P<0.05). LVEL-apex was decreased in patients with HCM during P0, P2 and P3. Compared with the controls, the left atrial energy loss (LAEL) of all three phases in patients with HCM were lower (each P<0.01). The diastolic LVEL values were significantly lower in patients with HCM compared with the controls; however, the systolic LVEL levels tended to be higher in HCM. The LAEL of the reservoir phase, conduit phase and atrial systolic phase were decreased in HCM compared with controls. The present study demonstrated that measurement of EL by VFM is a sensitive method of determining subclinical LV dysfunction in patients with HCM. The value of EL has been considered to be a quantitative parameter for the estimation of the efficiency of intraventricular blood flow.
Keywords: echocardiography, hypertrophic cardiomyopathy, energy loss, vector flow mapping
Introduction
Hypertrophic cardiomyopathy (HCM) occurs at a frequency of at least 1 in 500 in the general population, making it one of the most common inherited heart diseases (1–3). HCM is defined by the presence of increased left ventricular (LV) wall thickness that is not solely explained by abnormal loading conditions (4). The pathology is characterized by asymmetric or concentric myocardial hypertrophy associated with myocardial fiber disarray and fibrosis, leading to global and regional variability, and heterogeneity of systolic and diastolic deformation (5). At least 1/3 of patients with HCM have the non-obstructive form of the disease, with few to no outflow gradients (<30 mm Hg) at rest or with exercise (6).
However, the non-obstructive form of HCM has been less well studied; in particular, data on cardiac function and pathophysiology are ambiguous or inconclusive. Previous studies have demonstrated that patients with HCM with the non-obstructive form of the disease have exertional dyspnea (with preserved systolic function), largely as a result of diastolic dysfunction (6). Furthermore, previous studies have reported that LV diastolic dysfunction is a hallmark of HCM that occurs in the majority of patients (7,8). However, MacIver and Clark (9) suggested that measures of ‘diastolic dysfunction’ were common in HCM, although this may not be the dominant abnormality. It was suggested that the principal abnormality in HCM may be reduced contractile wall stress, even with a normal or increased ejection fraction (9). Blood flow dynamics analysis has emerged as a potential solution, allowing for the measurement of energy loss (EL) generated from blood viscous friction by vector flow mapping (VFM) techniques (10), thereby providing a novel way to assess cardiac function during the early stages of HCM in patients.
The primary aim of the present study was to investigate and analyze the EL by VFM in patients with HCM and control subjects and determine whether the EL may be associated with cardiac systolic and diastolic function in patients with HCM. The secondary aim of the present study was to compare classical echocardiographic parameters and the left atrial (LA) and LV deformation parameters by 2-D speckle tracking echocardiography (STE) in patients with HCM and control subjects, and to determine the impact of global myocardial mass on global longitudinal strain components.
Materials and methods
Study population
The present cross-sectional study included 52 patients diagnosed with the non-obstructive form of HCM and 40 age- and sex-matched healthy controls, who were referred to Qilu Hospital of Shandong University between July 2015 and December 2016. A total of 10 patients were excluded due to hypertension (n=5), irregular arrhythmias (n=1), LV ejection fraction (LVEF) <50% (n=2) and poor acoustic windows (n=2). The following diagnostic criteria for non-obstructive HCM were used, according to the 2014 ESC guidelines on the diagnosis and management of HCM (4): LV hypertrophy with LV wall thickness ≥15 mm for isolated cases and ≥13 mm for familial screening, with asymmetric distribution associated with a nondilated and hyperdynamic chamber in the absence of another cardiac or systemic disease, for example hypertension or aortic stenosis, associated with a preserved LVEF of >50% assessed by 2D echocardiography; and an instantaneous peak Doppler LV outflow tract pressure gradient <30 mm Hg at rest, or during physiological provocation, including the Valsalva maneuver, standing and exercise. All subjects signed an informed consent statement and the study protocol was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University (Jinan, China).
Standard echocardiography
All subjects underwent comprehensive 2D resting echocardiography. Echo-Doppler examinations were completed using a UST-52105 probe (1–5 MHz) on a ProSound F75 ultrasound device (Hitachi Aloka Medical, Ltd., Tokyo, Japan). Echocardiography was performed by experienced sonographers. Subsequently, 2D echocardiographic images of the left ventricle were obtained from the parasternal long-axis and from the three standard LV apical views (4-, 2- and 3-chamber). The following parameters were measured: i) LV end-diastolic diameter (LVEDD; mm) and end-systolic diameter (mm), LV end-diastolic volume (LVEDV; ml) and end-systolic volume (LVESV; ml), LVEF (%) using the Simpson's method, wall thickness, relative wall thickness (RWT), LV mass (LVM) and the LV mass index (LVMI); ii) LA diameter (LAD; mm), LA volume (LAV; ml), LA volume index (LAVI), the passive LAEF (%), the total LAEF (%) and the active LAEF (%); and iii) peak E wave velocity (m/sec), peak A wave velocity (m/sec), E/A, E wave deceleration time (DT; msec) and A wave duration time (Adur; msec) of the mitral inflow wave form measured by pulsed wave Doppler, the mean value of early diastolic velocities (e'; m/sec) from the septal-lateral mitral annulus in the apical 4-chamber view using tissue Doppler imaging and the ratio E/e'.
Speckle tracking imaging study
The offline analysis for speckle tracking was performed using a DAS-RS1 workstation (Hitachi-Aloka Medical, Ltd., Tokyo, Japan). LV global longitudinal systolic peak strain (GLS), peak atrial longitudinal strain (PALS) and the atrial strain rate (SR) during systole (SRs), early diastolic (SRe) and atrium contraction (SRa) were obtained from standard long-axis views (4-, 2- and 3-chamber) and were averaged. The LA stiffness index (E/e'/PALS) was calculated (11). Measurements of GLS, PALS and strain rate are presented in Fig. 1.
Figure 1.
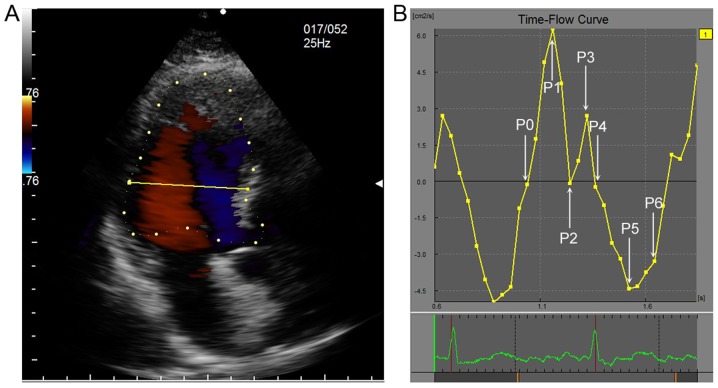
One complete cardiac cycle was divided into 7 timing phases according to time-flow curve. (A) The sample line was located 2 cm below mitral valve annulus. (B) P0 means IVR time, at which time the flow was 0; P1 means rapid filling time, at which time the filling flow was up to maximum; P2 indicated the slow filling phase and the flow was at minimum value; P3 indicated the atrial contraction time of late diastole, at which time the flow was peak value; P4 indicated IVC time, when the flow was at 0; P5 indicated the rapid ejection time, at which the systolic ejection flow reached the peak value; and P6 indicated the slow ejection time. IVR, isovolumic relaxation; IVC, isovolumic contraction.
VFM
Intracardiac flow images were recorded in the apical 3-chamber view. Color flow images were transferred into a VFM workstation (DAS-RS1 5.0; Hitachi-Aloka Medical, Ltd.) for offline analysis. The frame rates were set to the range of 20–25 frames/sec for the subsequent VFM analyses. All images were acquired during three consecutive cardiac cycles. The Nyquist limit for 2D color Doppler imaging was set high enough to mitigate the aliasing phenomenon. A cardiac cycle was selected for the analysis by determining two consecutive QRS complexes as the beginning and ending points. The phases of one cardiac cycle were determined according to a time-flow curve and the opening and closing of the valve with the synchronous ECG. The opening and closing of the mitral valve were defined as the beginning and ending of the diastolic phase, respectively. To determine the ventricular cavity, the endocardial border was manually traced on the first frame. The phases of the cardiac cycle for LVEL analysis are exhibited in Fig. 2. The phases of the cardiac cycle for left atrial energy loss (LAEL) analysis are exhibited in Fig. 3.
Figure 2.
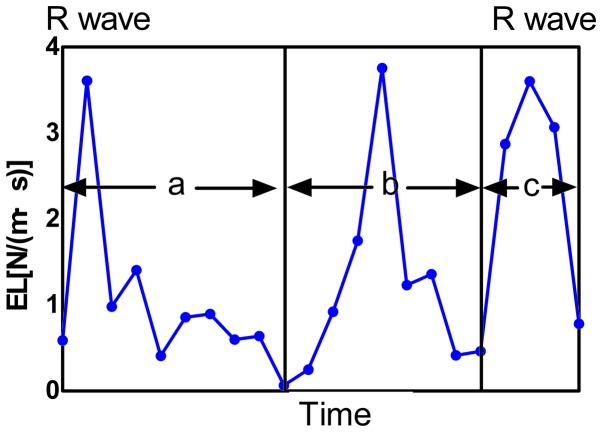
Averaged EL was calculated frame by frame from one R-wave to the next. Changes of LAEL in a complete cardiac cycle were shown. The cardiac cycle was divided into three phases: Ventricular systolic phase (phase a, reservoir phase), early diastolic phase (phase b, conduit phase) and atrial contraction phase (phase c) according to valve opening and closing. EL, energy loss; LAEL, left atrial energy loss.
Figure 3.
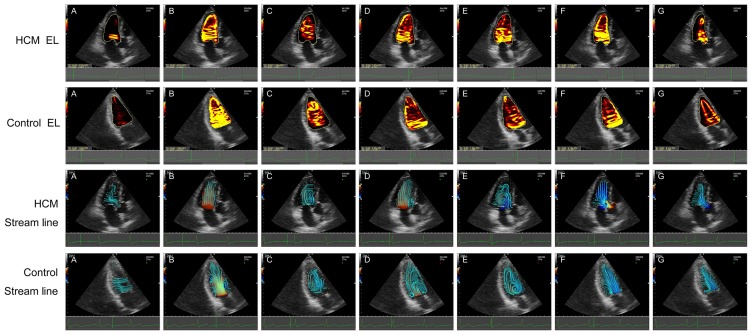
Changes of LVEL and stream line in a complete cycle were shown respectively in HCM group and control group. (A) P0 indicated the IVR time at which time the flow was 0; (B) P1 indicated rapid filling time at which the filling flow time was up to maximum; (C) P2 indicated the slow filling phase where the flow was at minimum value; (D) P3 indicated the atrial contraction time of late diastole at which the flow time was peak value; (E) P4 indicated IVC time when the flow was at 0; (F) P5 indicated the rapid ejection time at which the systolic ejection flow time reached the peak value; and (G) P6 indicated the slow ejection time. LVEL, energy loss of left ventricle; IVR, isovolumic relaxation; IVC, isovolumic contraction; HCM, hypertrophic cardiomyopathy.
The EL was automatically calculated following the selection of the region of interest. From the velocity vector fields of the intra-atrial blood flow, EL was calculated for each frame of the cine loop image. EL was defined as follows:
where µ indicates the blood viscosity coefficient, which was set as 0.004 Pa/sec. The total EL and averaged EL were calculated. The measurements were averaged over three cardiac cycles and indexed to body surface area (BSA).
Statistical analyses
All continuous variables are presented as the mean ± standard deviation. For the comparison of data, Student's t-tests were used to compare continuous data between the HCM and control groups. χ2 analysis was used to compare categorical variables between the two groups. One-way analysis of variance was performed, followed by multiple comparisons using the least significant difference test or Tamhane's T2 test. To assess the correlation between parameters, Pearson's or Spearman's correlation analysis was used. All statistical analyses were performed using SPSS software 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Baseline clinical characteristics
Baseline clinical characteristics of all the participants are summarized in Table I. The results revealed that the body mass index and BSAs of patients with HCM were significantly increased compared with those of the control group (25.67±3.96 kg/m2 vs. 23.58±2.94 kg/m2, P=0.026; 1.79±0.18 m2 vs. 1.64±0.15 m2, P=0.002), whereas the age and sex distribution, heart rate, cholesterol, triglyceride, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol of the two groups were similar. The systolic blood pressure of the patients with HCM was significantly increased compared with the control group (132±13 mmHg vs. 115±12 mmHg; P=0.02), although approximately within the normal range according to the HBP diagnostic criteria (12). The baseline characteristics of HCM patients were similar to those in the control group.
Table I.
Clinical parameters in patients with HCM and control subjects.
| Parameters | HCM (n=42) | Control (n=40) | P-value |
|---|---|---|---|
| Age (years) | 53.74±12.98 | 50.56±12.93 | 0.319 |
| Sex (M/F) | 20/11 | 16/20 | 0.081 |
| Heart rate | 68.52±10.49 | 68.52±10.49 | 0.198 |
| BMI (kg/m2) | 25.67±3.96 | 23.58±2.94 | 0.026 |
| BSA (m2) | 1.79±0.18 | 1.64±0.15 | 0.002 |
| SBP (mmHg) | 132±13 | 115±12 | 0.028 |
| DBP (mmHg) | 87±10 | 74±9 | 0.051 |
| Cho (mmol/l) | 4.227±1.132 | 4.650±0.878 | 0.163 |
| TG (mmol/l) | 1.402±0.654 | 1.459±0.416 | 0.7154 |
| LDL-C (mmol/l) | 2.685±1.063 | 2.683±0.914 | 0.993 |
| HDL-C (mmol/l) | 1.127±0.203 | 1.301±0.379 | 0.124 |
Values were shown as the mean ± standard deviation. HCM, hypertrophic cardiomyopathy; BMI, body mass index; BSA, body surface area; SBP, systolic blood pressure; DBP, diastolic blood pressure; Cho, cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
The 2D classical and STE echocardiographic parameters of the left ventricle are summarized in Table II. In general, patients with HCM had a greater LV wall thickness including left ventricular posterior wall, interventricular septum and RWT, and a higher ventricular mass (LVM and LVMI). No significant differences were observed in the LVEDD and LVEF in the patients with HCM compared with the control group. However, LVEDV and LVESV were significantly reduced compared with the control group (65.07±19.87 vs. 78.78±15.08 and 21.28±8.78 vs. 27.06±5.57, respectively; both P=0.002). The E/e' and EDT were significantly higher in the HCM group compared with the controls (10.71±3.88 vs. 6.59±1.46; P<0.001 and 221.57±105.11 vs. 169.5±27.92; P=0.011, respectively), whereas E, A, the E/A ratio and Adur were not significantly different between the HCM and control groups. Notably, the GLS was significantly lower in the HCM group compared with the control group (−11.06±0.41 vs. −15.69±0.42; P<0.001). These results suggest that patients with HCM had hypertrophic ventricular walls and smaller cardiac cavities, whereas impaired systolic function (GLS) and diastolic function (EDT) had already occurred when the traditional systolic function indicator LVEF and diastolic function indicator E/e' were still within normal range.
Table II.
Two-dimensional classical and speckle tracking echocardiography echocardiographic parameters of the left ventricle in control subjects and patients with HCM.
| Parameters | HCM (n=42) | Control (n=40) | P-value |
|---|---|---|---|
| RWT | 0.48±0.15 | 0.33±0.04 | <0.001 |
| LVM (g) | 293.97±101.55 | 119.15±24.90 | <0.001 |
| LVMI (g/m2) | 156.46±61.43 | 71.53±12.36 | <0.001 |
| LVPW (mm) | 10.74±3.02 | 7.42±0.91 | <0.001 |
| IVS (mm) | 19.21±4.07 | 8.94±1.58 | <0.001 |
| LVEDD (mm) | 46.18±5.81 | 45.11±3.24 | 0.371 |
| LVEDV (ml) | 65.07±19.87 | 78.78±15.08 | 0.002 |
| LVESV (ml) | 21.28±8.78 | 27.06±5.57 | 0.002 |
| LVEF | 0.63±0.18 | 0.65±0.04 | 0.458 |
| E (cm/sec) | 72.44±17.16 | 77.89±14.69 | 0.165 |
| A (cm/sec) | 66.28±27.91 | 72.19±18.74 | 0.321 |
| E/A | 1.27±0.59 | 1.14±0.36 | 0.291 |
| E/e' | 10.71±3.883 | 6.586±1.462 | <0.001 |
| EDT (msec) | 221.57±105.11 | 169.5±27.92 | 0.011 |
| Adur (msec) | 168.13±30.44 | 153±40.84 | 0.373 |
| GLS (%) | −11.06±0.406 | −15.69±0.424 | <0.001 |
Values were shown as the mean ± standard deviation. RWT, relative wall thickness; LVM, left ventricular mass; LVMI, left ventricular mass index; LVPW, left ventricular posterior wall; IVS, interventricular septum; LVEDD, left ventricular end of diastolic diameter; LVEDV, left ventricular end of diastolic volume; LVESV, left ventricular end of systolic volume; LVEF, left ventricular ejection fraction; EDT, E wave deceleration time, Adur, A wave duration time; GLS, global longitudinal systolic strain; STE, speckle tracking echocardiography; HCM, hypertrophic cardiomyopathy.
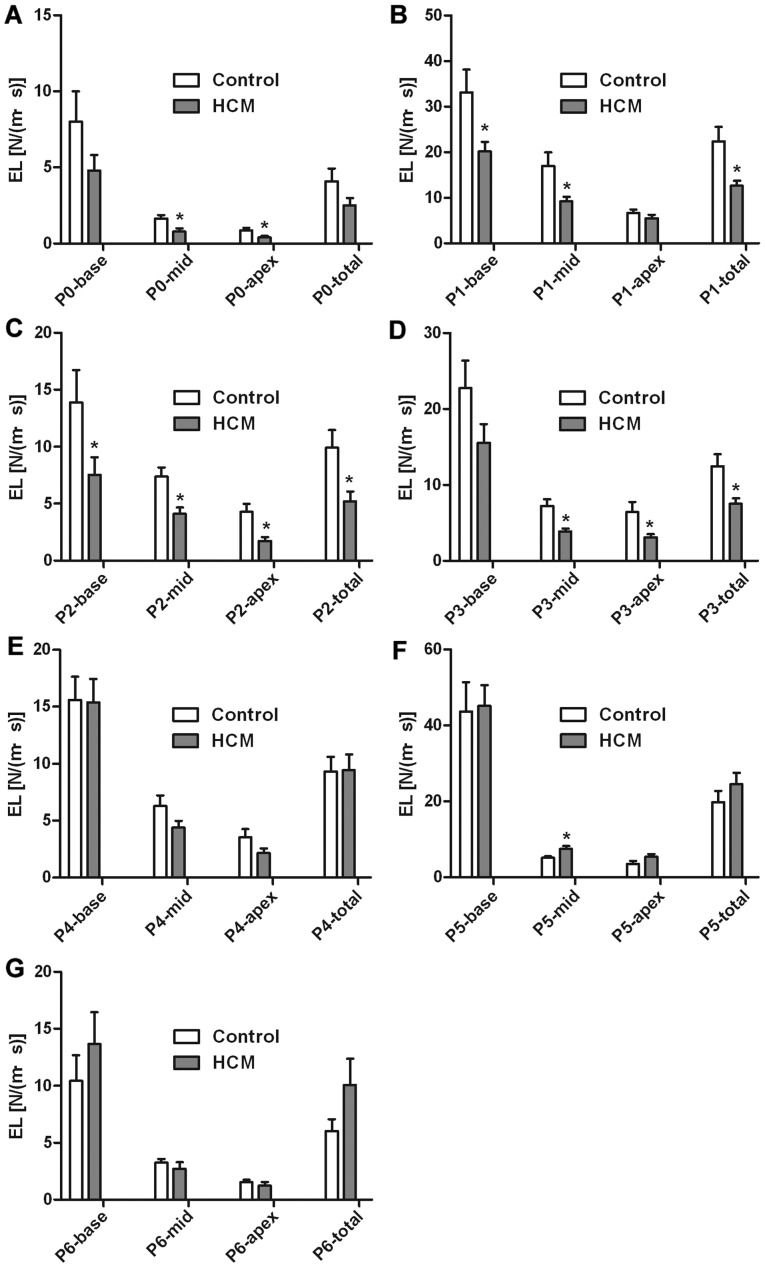
Alterations in dissipative EL values were noted between the HCM and the control group, as summarized in Figs. 4 and 5. Importantly, the streamline of the left ventricle in the control group was more intensive and regular compared with the HCM group (Fig. 3B, F and G), and the vortex size was smaller in patients with HCM (Fig. 3C-E). Secondly, EL in all phases gradually decreased from the base to the middle and apex in the HCM and control groups. The EL-total of P1 (rapid filling time) and P5 (rapid ejection time) were higher compared with the other phases. Thirdly, compared with the controls, the EL-total was significantly decreased in patients with HCM during the diastolic phase (P1, P2 and P3; all P<0.05). However, a tendency for increased systolic EL-total values referring to P4, P5 and P6 in patients with HCM compared with controls was observed (P>0.05). The EL-base during P1 and P2 were significantly decreased in patients with HCM (both P<0.05). Significant differences were observed in the EL-mid between the two groups. Compared with the controls, the EL-mid values of patients with HCM were significantly lower during the diastolic phases (P0, P1, P2 and P3; all P<0.05). However, the EL-mid values of patients with HCM were significantly higher compared with those of the controls during systolic P5 (P<0.05). The EL-apex was significantly decreased in patients with HCM during diastole phase including P0, P2 and P3 (all P<0.05). These results suggest that in patients with HCM, the change of diastolic EL was more significant than that of systolic EL, and therefore myocardial diastolic function may be more vulnerable in patients with HCM.
Figure 4.
Comparison of LVEL of different timing between HCM and control. (A) P0 indicated the IVR time; (B) P1 indicated the rapid filling time; (C) P2 indicated the slow filling phase; (D) P3 indicated the atrial contraction time of late diastole; (E) P4 indicated the IVC time; (F) P5 indicated rapid ejection time; and (G) P6 indicated the slow ejection time. Compared with controls, EL-total was significantly decreased in patients with HCM during the diastolic phase (P1, P2 and P3; all P<0.05). The systolic EL-total in patients with HCM tended to be larger compared with the control (P>0.05). EL-base was decreased in patients with HCM during P1 and P2 (slow filling time). Compared with the controls, the EL-mid of patients with HCM were lower during the diastolic phases (P0, P1, P2 and P3; all P<0.05). However, the EL-mid of patients with HCM was higher than that of the controls during systolic P5 (P<0.05). EL-apex was decreased in patients with HCM during P0, P2 and P3. *P<0.05 vs. control. LVEL, energy loss of left ventricle; EL, energy loss; HCM, hypertrophic cardiomyopathy; LVEL, energy loss of left ventricle; IVR, isovolumic relaxation; IVC, isovolumic contraction; N/(m·s), Newton/(meter·second).
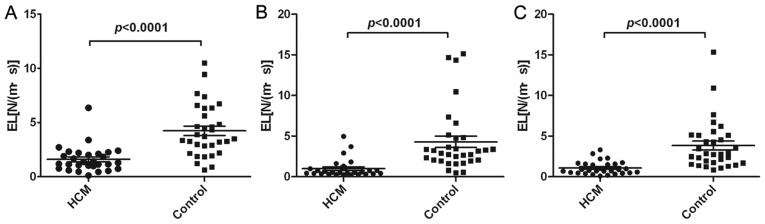
Figure 5.
Comparison of LAEL of different timing between HCM and control. (A) The comparison of LAEL of ventricular systole phase (reservoir phase), (B) the comparison of LAEL of early diastolic phase (conduit phase) and (C) the comparison of LAEL of atrial contraction phase. LAEL, left atrial energy loss; N/(m·s), Newton/(meter·second).
Echocardiographic parameters of the left atrium
The 2D classical and STE echocardiographic parameters of the left atrium are summarized in Table III. Briefly, there were significant differences in LA diameter and volume (P<0.001). The LAD, LAV and LAVI were significantly higher in patients with HCM, whereas the PALS, measured at the end of the reservoir phase, was significantly decreased in the patients with HCM compared with the controls (P<0.001). The absolute values of mean strain rate (mSR) at systolic (mSRs), early diastolic (mSRe) and at atrial contraction (mSRa) were all significantly decreased in patients with HCM (P<0.001).
Table III.
Two-dimensional classical and speckle tracking echocardiography echocardiographic parameters of LA in control subjects and patients with HCM.
| Parameters | HCM (n=42) | Control (n=40) | P-value |
|---|---|---|---|
| LAD (mm) | 40.16±5.66 | 30.47±5.03 | <0.001 |
| LAV (ml) | 63.93±25.44 | 40.86±11.57 | <0.001 |
| LAVI (ml/m2) | 35.31±13.50 | 24.64±6.86 | <0.001 |
| LAPEF | 0.3152±0.1463 | 0.3440±0.0978 | 0.321 |
| LAAEF | 0.3493±0.1098 | 0.3240±0.0842 | 0.282 |
| LATEF | 56.52±7.61 | 55.00±6.83 | 0.412 |
| PALS (%) | 22.22±1.411 | 37.47±1.840 | <0.001 |
| mSRs (s−1) | 1.212±0.08 | 1.78±0.07 | <0.001 |
| mSRe (s−1) | −0.9772±0.066 | −2.095±0.176 | <0.001 |
| mSRa (s−1) | −1.009±0.093 | −1.946±0.117 | <0.001 |
| Stiffness index | 0.5609±0.317 | 0.2706±0.090 | <0.001 |
Values were indicated as the mean ± standard deviation. LAD, left atrial diameter; LAV, left atrial volume; LAVI, left atrial volume index; LAPEF, passive left atrial emptying fraction; LAAEF, active left atrial emptying fraction; LATEF, total left atrial emptying fraction; PALS, peak atrial longitudinal strain; mSRs, mean strain rate at systolic; mSRe, mean strain rate at early diastolic; mSRa, mean strain rate at atrial contraction.
LA physiology during every cardiac cycle consists of three different phases that modulate LV filling, termed the reservoir phase, the conduit phase and the atrial systolic phase (13,14). To analyze LA function, the measurements of LAEL were divided into these three phases. LAEL reached its peak values during LV systole, early diastole and atrial contraction (Fig. 2). Compared with the controls, the LAEL of all three phases in patients with HCM were significantly decreased (P<0.0001; Fig. 5). In patients with HCM, the LA function during the reservoir, conduit and atrial systolic phases was all impaired.
Analysis of correlation
Correlation analysis between specific parameters measured in the current study was examined (data not shown). Notably, the GLS did not correlate with LVEF, E/e', LA stiffness index and LVEL, although it did exhibit a significant correlation with the PALS (r=−0.373; P=0.011) and the LAEL of the early diastolic phase (r=−0.348; P=0.032). The diastolic LVEL during P1 phase correlated with E/e' (r=0.682; P<0.001) and the LA stiffness index (r=0.474; P=0.002). The systolic LVEL during P5 and P6 phase correlated with the PALS (r=0.430, P=0.004; r=0.387 P=0.009, respectively). The LAEL during LV systole and atrial contraction phase exhibited a significant correlation with the PALS (r=0.367, P=0.025; r=0.503, P=0.003, respectively) and LA stiffness index (r=−0.439, P=0.009; r=−0.317, P=0.047, respectively). These results indicated that the diastolic and systolic LVEL, as well as LAEL, were in accordance with traditional echocardiographic parameters in reflecting diastolic and systolic dysfunction.
Discussion
The present study investigated phasic alterations in the strength of EL in patients with HCM as a novel method of quantification of LV and LA function. For patients with early-stage HCM, radial contractile function (EF or fractional shortening) is typically normal or increased (15). The results of the present study demonstrated that during the early stages of HCM, the LV diastolic EL and the LV GLS were reduced, while E/e', although increased compared with the control group, remained <14. However, there was no significant alteration in LVEF. This suggested that LV systolic and diastolic dysfunction had already occurred during the early stages, while LVEF and E/e' were in the normal range. The present results also indicated that during the whole cardiac cycle, including the LV ejection, early diastolic and late diastolic stages, LAEL and LA systolic peak strain were reduced, indicating that atrial mechanical functioning was impaired.
Clinically, hypertrophy and disarray of the myocardial fibers are the principal deformities in patients with HCM, and these may result in impaired kinetic characteristics (16). Early detection and interventions for LV dysfunction are essential for patients with HCM. Color Doppler flow imaging and tissue doppler imaging (TDI) have been widely used to assess subclinical LV dysfunction in patients with HCM (17,18). Speckle-tracking strain is a more advanced method compared with TDI, since it is angle-independent and more sensitive in its capturing of myocardial damage (19,20). The newly-developed VFM, based on color Doppler imaging, with the use of a continuity equation and STE, has been demonstrated to be useful in the detection of subclinical LV dysfunction in patients with infarctions, end-stage renal diseases and aortic regurgitation diseases (21). The dissipative EL is derived from the velocity vector field of intraventricular blood flow and is considered to reflect the efficiency of blood flow, and thus may be an indicator of LV function (10).
Early detection and interventions for LV dysfunction are essential for patients with HCM. In the present study, patients with HCM were observed to have thicker ventricular walls and larger ventricular masses, despite a smaller ventricular cavity. The GLS was significantly reduced in patients with HCM, despite normal LV systolic function, as assessed with LVEF, suggesting the presence of a global subclinical systolic dysfunction. Similar results have been observed in a number of studies (4,5,22,23). The present findings revealed that the diastolic EL was significantly decreased in patients with HCM compared with controls. The significantly decreased diastolic EL suggested that myocardial diastolic function appeared to be more vulnerable in patients with HCM, which was in accordance with previous studies (24–26). Furthermore, the positive correlation between diastolic LVEL and the traditional diastolic parameter E/e' suggested a connection between diastolic LVEL and diastolic function. Although only EL-mid at systolic P5 in HCM was significantly higher compared with the control, a tendency towards increased systolic EL was observed in patients with HCM. In the present study, blood flow velocity decreased from base to apex, thus EL decreased from base to apex.
Blood flow dynamics in the left ventricle feature the formation of vortices, which are associated with the smooth redirection of flow from the inlet to the outflow tract (27,28). A recent study indicated that vortex-ring formation reflects diastolic function and overall cardiac health (29). The vortex of the blood flow is formed during early diastole, is sustained throughout the diastolic phase and the blood stream is subsequently directed to the outflow tract in a laminar flow during the systolic phase (27,30,31). The instability of the vortex also readily leads to a loss of coherence, turbulence and the breaking up of the flow into small, irregular vortex structures (27). Stugaard et al (21) reported that diastolic EL increased in aortic regurgitation (AR), proportional to its severity. A group from China observed that systolic and diastolic EL were increased in patients with diabetes mellitus with normal LVEF, and that uncontrolled blood glucose levels may lead to increased systolic and diastolic EL in the left ventricle (10). The present study suggested that LVEL is sensitive in terms of the subclinical LV dysfunction of patients with HCM, including systolic and diastolic function. As patients with HCM had smaller ventricular cavities, stiffer ventricular walls and elevated filling pressures, the diastolic filling flow from atrium to ventricle has a slower velocity, a smaller vortex and a decreased EL (32). During the systolic phase, the decreased longitudinal deformation with compensation of increased radial deformation maintains a normal or supernormal LVEF (4,8,33), which results in increased systolic EL in the ventricle.
To the best of our knowledge, the LA physiology during every cardiac cycle consists of three different phases that modulate LV filling: The reservoir phase, the conduit phase and the atrial systolic phase (13,14). When the mitral valve opens, the LA cavity is in direct contact with the LV cavity; therefore, any alterations in LA structure, function and hemodynamics may represent the average LV filling pressure history.
The present study found that LA size and volume were significantly increased in patients with HCM. Previous studies have suggested that the E/e' ratio is an instant measurement of LV filling pressure, and the LA volume index reflects the cumulative effect of the filling pressures over time (20). The reduced PALS, mSRs, mSRe and mSRa measured by STE were also similar to previous studies (14,20,34,35). In the present study, LA reservoir function (PALS and mSRs) and conduit function (mSRe) were impaired in patients with HCM, without an increase in LA pump function (mSRa), which is an important compensatory mechanism that facilitates LA filling during aging (36). The LA stiffness index was elevated in patients with HCM, although not in the control group, which indicated that atrial fibrosis and stiffness may be present in patients with HCM with existing diastolic dysfunction of the LV, as heart failure, arterial hypertension and atrial fibrillation was observed (20,37). LA function progressively decreases in patients with HCM, and LA longitudinal strain has been recognized as a useful parameter to predict LA dysfunction and elevated LV filling pressure (20,35).
The current study revealed that the EL in the reservoir, conduit and atrial systolic phases was decreased in HCM compared with the controls. The extent of active, passive and conduit filling by the atrium is significantly influenced by the compliance of the left ventricle (14). Structural atrial remodeling represents an additional morpho-functional correlation with LA strain, and a close correlation exists between PALS and left atrium myocardial fibrosis (14). The present study revealed that the LAEL positively correlated with the PALS and negatively correlated with the stiffness index. It was observed that decreased PALS indicated LA pressure rose to maintain adequate LV filling in response to increased LV stiffness or non-compliance, as previously observed in patients with HCM (38) and increased LA pressure causes elevated atrial wall tension, which leads to chamber dilatation and stretching of the atrial myocardium (39). LA wall stiffness and fibrosis, as evaluated by PALS, may lead to LA dysfunction and a reduction in EL throughout the reservoir, conduit and atrial systolic phases. A negative impact of diabetes on LA function was previously demonstrated via assessment of phasic LAEL by VFM, and even in the presence of a normal left atrium size, the EL during the reservoir and conduit phases decreased, but increased in the atrial systolic phase (40). In patients with various degrees of chronic AR, diastolic EL increases with the severity of AR. In this situation, EL produced by inefficient turbulent flow may be a burden to the heart (21).
Of note, there are a number of limitations to the present cross-sectional study. The principal limitation of the study is the relatively small sample size. Future studies with larger sample sizes and increased long-term follow-up times are required to confirm the prognostic value of EL in HCM. Furthermore, the current software (DAS-RS1 5.0 workstation) was used for LV analysis to study LA strain and strain rate. A certain degree of inaccuracy may have arisen due to technical reasons.
The present study demonstrated the functional and structural alterations, in addition to hemodynamic alterations, in patients with the non-obstructive form of HCM with preserved EF. The results suggested that, although EF and E/e' may be normal, patients with HCM may have systolic and diastolic dysfunction. Furthermore, the present findings revealed that VFM combined with 2D STE may be a useful tool for the detection of LA and LV subclinical dysfunction in patients with HCM. VFM and the derived dissipative EL may provide a promising novel method to quantify heart function. However, prospective studies are required to confirm the present findings and evaluate the usefulness of EL as a prognostic marker of HCM.
Acknowledgements
Not applicable.
Funding
The present study was supported by research grants from the Natural Science Foundation of Shandong Province (grant no. ZR2014HQ037), the National Natural Science Foundation of China (grant nos. 81600633, 81570400, 81470560, 81670411, 81471036, 81400285 and 81702194), the Key Research and Development Program of Shandong Province (grant no. 2017GSF18156), the Medicine and Health Science Technology Development Program of Shandong province (grant no. 2016WS0091), the Shandong Provincial Natural Science Foundation of China (grant no. ZR2015CL012) and the Research Program of Qilu Hospital of Shandong University (grant no. 2017QLQN37).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YC, WZ and YS drafted the manuscript. MiZ, LL, MeZ and GJ collected and analyzed the data. ML, YZ and XS statistically analyzed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The current study was approved by the Ethics Committee of Qilu Hospital of Shandong University (Jinan, China). Written informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ingles J, Burns C, Bagnall RD, Lam L, Yeates L, Sarina T, Puranik R, Briffa T, Atherton JJ, Driscoll T, Semsarian C. Nonfamilial hypertrophic cardiomyopathy: Prevalence, natural history, and clinical implications. Circ Cardiovasc Genet. 2017;10(pii):e001620. doi: 10.1161/CIRCGENETICS.116.001620. [DOI] [PubMed] [Google Scholar]
- 2.Jacoby DL, DePasquale EC, McKenna WJ. Hypertrophic cardiomyopathy: Diagnosis, risk stratification and treatment. CMAJ. 2013;185:127–134. doi: 10.1503/cmaj.120138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houston BA, Stevens GR. Hypertrophic cardiomyopathy: A review. Clin Med Insights Cardiol. 2015;8(Suppl 1):S53–S65. doi: 10.4137/CMC.S15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Authors/Task Force members. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the diagnosis and management of hypertrophic cardiomyopathy of the European society of cardiology (ESC) Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 5.Voilliot D, Huttin O, Hammache N, Filippetti L, Vaugrenard T, Aliot E, Sadoul N, Juillière Y, Selton-Suty C. Impact of Global and segmental hypertrophy on two-dimensional strain derived from three-dimensional echocardiography in hypertrophic cardiomyopathy: Comparison with healthy subjects. J Am Soc Echocardiogr. 2015;28:1093–1102. doi: 10.1016/j.echo.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet (London, England) 2013;381:242–255. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 7.Kalra A, Harris KM, Maron BA, Maron MS, Garberich RF, Haas TS, Lesser JR, Maron BJ. Relation of Doppler tissue imaging parameters with heart failure progression in hypertrophic cardiomyopathy. Am J Cardiol. 2016;117:1808–1814. doi: 10.1016/j.amjcard.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Saccheri MC, Cianciulli TF, Lax JA, Guerra JE, Redruello HJ, Weich Glogier FL, Gagliardi JA, Dorelle AN, Prezioso HA, Vidal LA. Impaired myocardial function in hypertrophic cardiomyopathy. Echocardiography. 2009;26:657–664. doi: 10.1111/j.1540-8175.2008.00871.x. [DOI] [PubMed] [Google Scholar]
- 9.MacIver DH, Clark AL. Contractile dysfunction in sarcomeric hypertrophic cardiomyopathy. J Card Fail. 2016;22:731–737. doi: 10.1016/j.cardfail.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T, Itatani K, Inuzuka R, Shimizu N, Shindo T, Hirata Y, Miyaji K. Dissipative energy loss within the left ventricle detected by vector flow mapping in children: Normal values and effects of age and heart rate. J Cardiol. 2015;66:403–410. doi: 10.1016/j.jjcc.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–15. doi: 10.1161/CIRCIMAGING.108.813071. [DOI] [PubMed] [Google Scholar]
- 12.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 13.Cameli M, Mandoli GE, Mondillo S. Left atrium: The last bulwark before overt heart failure. Heart Fail Rev. 2017;22:123–131. doi: 10.1007/s10741-016-9589-9. [DOI] [PubMed] [Google Scholar]
- 14.Cameli M, Ciccone MM, Maiello M, Modesti PA, Muiesan ML, Scicchitano P, Novo S, Palmiero P, Saba PS, Pedrinelli R, Gruppo di Studio Ipertensione, Prevenzione e Riabilitazione, Società Italiana di Cardiologia Speckle tracking analysis: A new tool for left atrial function analysis in systemic hypertension: An overview. J Cardiovasc Med (Hagerstown) 2016;17:339–343. doi: 10.2459/JCM.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 15.Xu HY, Chen J, Yang ZG, Li R, Shi K, Zhang Q, Liu X, Xie LJ, Jiang L, Guo YK. Early marker of regional left ventricular deformation in patients with hypertrophic cardiomyopathy evaluated by MRI tissue tracking: The effects of myocardial hypertrophy and fibrosis. J Magn Reson Imaging. 2017;46:1368–1376. doi: 10.1002/jmri.25681. [DOI] [PubMed] [Google Scholar]
- 16.Ozawa K, Funabashi N, Takaoka H, Kamata T, Kanaeda A, Saito M, Nomura F, Kobayashi Y. Characteristic myocardial strain identified in hypertrophic cardiomyopathy subjects with preserved left ventricular ejection fraction using a novel multi-layer transthoracic echocardiography technique. Int J Cardiol. 2015;184:237–243. doi: 10.1016/j.ijcard.2015.01.070. [DOI] [PubMed] [Google Scholar]
- 17.Kato TS, Noda A, Izawa H, Yamada A, Obata K, Nagata K, Iwase M, Murohara T, Yokota M. Discrimination of nonobstructive hypertrophic cardiomyopathy from hypertensive left ventricular hypertrophy on the basis of strain rate imaging by tissue Doppler ultrasonography. Circulation. 2004;110:3808–3814. doi: 10.1161/01.CIR.0000150334.69355.00. [DOI] [PubMed] [Google Scholar]
- 18.Oki T, Mishiro Y, Yamada H, Onose Y, Matsuoka M, Wakatsuki T, Tabata T, Ito S. Detection of left ventricular regional relaxation abnormalities and asynchrony in patients with hypertrophic cardiomyopathy with the use of tissue Doppler imaging. Am Heart J. 2000;139:497–502. doi: 10.1016/S0002-8703(00)90094-2. [DOI] [PubMed] [Google Scholar]
- 19.Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Støylen A, Ihlen H, Lima JA, Smiseth OA, Slørdahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: Validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 20.Cameli M, Mandoli GE, Loiacono F, Dini FL, Henein M, Mondillo S. Left atrial strain: A new parameter for assessment of left ventricular filling pressure. Heart Fail Rev. 2016;21:65–76. doi: 10.1007/s10741-015-9520-9. [DOI] [PubMed] [Google Scholar]
- 21.Stugaard M, Koriyama H, Katsuki K, Masuda K, Asanuma T, Takeda Y, Sakata Y, Itatani K, Nakatani S. Energy loss in the left ventricle obtained by vector flow mapping as a new quantitative measure of severity of aortic regurgitation: A combined experimental and clinical study. Eur Heart J Cardiovasc Imaging. 2015;16:723–730. doi: 10.1093/ehjci/jev035. [DOI] [PubMed] [Google Scholar]
- 22.Serri K, Reant P, Lafitte M, Berhouet M, Le Bouffos V, Roudaut R, Lafitte S. Global and regional myocardial function quantification by two-dimensional strain: Application in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1175–1181. doi: 10.1016/j.jacc.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 23.Urbano-Moral JA, Rowin EJ, Maron MS, Crean A, Pandian NG. Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2014;7:11–19. doi: 10.1161/CIRCIMAGING.113.000842. [DOI] [PubMed] [Google Scholar]
- 24.Maron BJ, Spirito P, Green KJ, Wesley YE, Bonow RO, Arce J. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1987;10:733–742. doi: 10.1016/S0735-1097(87)80264-4. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Sun D, Yang J. Diastolic dysfunction of hypertrophic cardiomyopathy genotype-positive subjects without hypertrophy is detected by tissue Doppler imaging: A systematic review and Meta-analysis. J Ultrasound Med. 2017;36:2093–2103. doi: 10.1002/jum.14250. [DOI] [PubMed] [Google Scholar]
- 26.Ho CY. Hypertrophic cardiomyopathy: Preclinical and early phenotype. J Cardiovasc Transl Res. 2009;2:462–470. doi: 10.1007/s12265-009-9124-7. [DOI] [PubMed] [Google Scholar]
- 27.Pedrizzetti G, La Canna G, Alfieri O, Tonti G. The vortex-an early predictor of cardiovascular outcome? Nat Rev Cardiol. 2014;11:545–553. doi: 10.1038/nrcardio.2014.75. [DOI] [PubMed] [Google Scholar]
- 28.Kilner PJ, Yang GZ, Wilkes AJ, Mohiaddin RH, Firmin DN, Yacoub MH. Asymmetric redirection of flow through the heart. Nature. 2000;404:759–761. doi: 10.1038/35008075. [DOI] [PubMed] [Google Scholar]
- 29.Gharib M, Rambod E, Kheradvar A, Sahn DJ, Dabiri JO. Optimal vortex formation as an index of cardiac health. Proc Natl Acad Sci USA. 2006;103:6305–6308. doi: 10.1073/pnas.0600520103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borazjani I, Westerdale J, McMahon EM, Rajaraman PK, Heys JJ, Belohlavek M. Left ventricular flow analysis: Recent advances in numerical methods and applications in cardiac ultrasound. Comput Math Methods Med. 2013;2013:395081. doi: 10.1155/2013/395081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abe H, Caracciolo G, Kheradvar A, Pedrizzetti G, Khandheria BK, Narula J, Sengupta PP. Contrast echocardiography for assessing left ventricular vortex strength in heart failure: A prospective cohort study. Eur Heart J Cardiovasc Imaging. 2013;14:1049–1060. doi: 10.1093/ehjci/jet049. [DOI] [PubMed] [Google Scholar]
- 32.Maron BJ. Hypertrophic cardiomyopathy: A systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 33.Butz T, van Buuren F, Mellwig KP, Langer C, Plehn G, Meissner A, Trappe HJ, Horstkotte D, Faber L. Two-dimensional strain analysis of the global and regional myocardial function for the differentiation of pathologic and physiologic left ventricular hypertrophy: A study in athletes and in patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2011;27:91–100. doi: 10.1007/s10554-010-9665-5. [DOI] [PubMed] [Google Scholar]
- 34.Aly MF, Brouwer WP, Kleijn SA, van Rossum AC, Kamp O. Three-dimensional speckle tracking echocardiography for the preclinical diagnosis of hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2014;30:523–533. doi: 10.1007/s10554-014-0364-5. [DOI] [PubMed] [Google Scholar]
- 35.Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, D'Ascenzi F, Focardi M, Favilli R, Pierli C, et al. Correlation of left atrial strain and Doppler measurements with invasive measurement of left ventricular End-diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography. 2016;33:398–405. doi: 10.1111/echo.13094. [DOI] [PubMed] [Google Scholar]
- 36.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.CIR.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 37.Cameli M, Mandoli GE, Loiacono F, Sparla S, Iardino E, Mondillo S. Left atrial strain: A useful index in atrial fibrillation. Int J Cardiol. 2016;220:208–213. doi: 10.1016/j.ijcard.2016.06.197. [DOI] [PubMed] [Google Scholar]
- 38.Paraskevaidis IA, Panou F, Papadopoulos C, Farmakis D, Parissis J, Ikonomidis I, Rigopoulos A, Iliodromitis EK, Th Kremastinos D. Evaluation of left atrial longitudinal function in patients with hypertrophic cardiomyopathy: A tissue Doppler imaging and two-dimensional strain study. Heart. 2009;95:483–489. doi: 10.1136/hrt.2008.146548. [DOI] [PubMed] [Google Scholar]
- 39.Pavlopoulos H, Nihoyannopoulos P. Left atrial size: A structural expression of abnormal left ventricular segmental relaxation evaluated by strain echocardiography. Eur J Echocardiogr. 2009;10:865–871. doi: 10.1093/ejechocard/jep093. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Hou D, Ma R, Ding G, Yin L, Zhang M. Early detection of left atrial energy loss and mechanics abnormalities in diabetic patients with normal left atrial size: A study combining vector flow mapping and tissue tracking echocardiography. Med Sci Monit. 2016;22:958–968. doi: 10.12659/MSM.897385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.