Abstract
Background & objectives:
Amino acids are general nutrients having anti-diabetic property. The present study was undertaken to investigate the mechanism of anti-diabetic effects of amino acids in human visceral adipocyte cells in high glucose environment.
Methods:
Experiments were carried out in human visceral adipocytes. Adiponectin (APN) siRNAs were designed using Ambion tools. APN mRNA expression was quantified using real-time polymerase chain reaction, and protein level was studied using ELISA. AMP-activated kinase (AMPK) activity was measured and glucose uptake by 2-deoxyglucose uptake method.
Results:
Amino acids (proline and phenylalanine) exposure to adipocytes significantly (P<0.01) increased APN mRNA by 1.5-folds when compared to control whereas proline increased APN secretion by 10.6-folds (P<0.01), phenylalanine by 12.7-folds (P<0.001) and alanine by 6.3-folds (P<0.01). Free amino acid-induced AMPK activity and glucose uptake were decreased with the transient knockdown of APN.
Interpretation & conclusions:
Antidiabetic effect of the tested amino acids was exhibited by increased glucose uptake through the AMPK pathway by an APN-dependent mechanism in human visceral adipocytes. This should be tested and confirmed in in vivo system. Newer treatment modalities with amino acids which can enhance glucose uptake and APN secretion can be developed as drug for treating both diabetes and obesity.
Keywords: Adiponectin, adiponectin siRNA, amino acids, AMPK activity, anti-diabetic, glucose uptake
Adiponectin (APN) is an adipocyte-derived hormone with anti-diabetic, anti-inflammatory and antiatherogenic properties1. Acute increase in the level of circulating APN triggers a transient decrease in basal glucose level by sensitizing insulin2. In the liver, APN enhances insulin sensitivity, decreases the influx of non-essential fatty acids, increases fatty acid oxidation and reduces hepatic glucose output. In muscle, APN stimulates glucose use and fatty acid oxidation3,4.
APN is decreased in the plasma of type 2 diabetes mellitus (T2DM) associated with insulin resistance (IR). Increase or overexpression of APN has been shown to be associated with increase in the glucose uptake as reported in the cultured skeletal muscle cells5. Levels of APN in the blood are decreased under conditions of obesity, IR and T2DM6,7,8. The plasma APN level is affected by multiple factors, including gender, ageing and lifestyle. Prospective and longitudinal studies9,10 have shown that lower APN levels are associated with a higher incidence of diabetes. Transgenic overexpression of APN in mice leads to improved insulin sensitivity11.
APN activates AMP-activated kinase (AMPK), and this increased phosphorylation of AMPK in turn increases glucose uptake in skeletal muscle cells through glucose transporter type 4 (GLUT4) translocation. This results in switching off the ATP-consuming pathways and switching on ATP-generating pathways resulting in increased glucose uptake and fatty acid oxidation in muscle12. However, little is known about the molecular mechanism involved in APN-mediated glucose uptake in human visceral adipocytes.
APN levels increase when insulin sensitivity improves, which occurs after treatment with insulin-sensitizing drugs13,14. Studies in isolated skeletal muscle or C2C12 myocytes with APN stimulates glucose transport6,15, thereby reduces hyperglycaemia. Molecules such as the agonists of APN can have therapeutic value in T2DM by improving the APN levels thereby increasing the glucose utilization15.
Previous studies from our laboratory showed that amino acid mixture increased the glucose uptake significantly as seen by labelled 2-deoxyglucose uptake and by significant increase in the GLUT4 translocation16. The present study was aimed to investigate if free amino acids (proline, phenylalanine and alanine) can modulate the levels of APN in human visceral adipocyte cells exposed to high glucose environment and in glucose uptake and validating it by silencing the APN mRNA.
Material & Methods
The study was conducted in Biochemistry and Cell Biology department, Vision Research Foundation, Chennai, India, after obtaining permission from Institutional Ethics Committee.
Cell culture: Human visceral preadipocytes (Lonza, USA) were maintained in preadipocyte basal medium (Lonza, USA) containing 2 mM glutamine with 10 per cent foetal bovine serum. When the cell reached 80 per cent confluency, adipocyte-differentiating medium (Lonza, USA) (containing dexamethasone, indomethacin, 3-isobutyl-1-methylxanthine and insulin) was added and left undisturbed for 10 days. Accumulation of lipid droplets was seen from day seven. For further experiments, cells which were 80 per cent differentiated mature adipocytes were used by seeding in 24-well and 12-well plates (10,000/20,000 cells per well). The cells were serum starved for 2 h, and all experiments are carried out in triplicates.
Designing of adiponectin siRNA: APN siRNAs were designed using Ambion tool (HSLS, Pittsburgh, USA), and the rules described by Reynolds et al17 for siRNA designing were followed. Two different single-stranded APN siRNAs and a scrambled siRNA were purchased from Merck, USA.
The sequence of APN siRNA and scrambled siRNA were as follows: siRNA 1: 5’AGUGGAGCCAUCAUAGUGGUU 3’; siRNA 2: 5’AUCAUAGUGGUUUUGCUGAUU 3’; and Scrambled siRNA: 5’GUGAUUUACGGGCU AGAGUAU 3’.
Each siRNA was mixed with 4 μl of transfection reagent - ICAfectin (Eurogentec, Germany) and incubated at room temperature for 15 min and then transfected to adipocytes cells.
Standardization of siRNA concentration and time exposure: Designed APN siRNA 1 and 2 were first tested for their efficacy by quantification of APN mRNA by quantitative real-time polymerase chain reaction (PCR) (Applied Biosystems 7300, USA) with SYBR Green chemistry (Eurogentec, Belgium). Time point and concentration of siRNA used were also optimized for further experiments based on quantification of APN mRNA. Transfection was carried for two different time points - 2 and 4 h with two different siRNA concentrations of 10 and 100 nM. RNA was extracted, and real-time PCR was set for mRNA APN to check silencing effect of APN after transfection. Experiments were done in triplicates.
Quantitative real-time polymerase chain reaction: To see whether amino acids have effect on the mRNA expression of APN in adipocytes, mature adipocytes were exposed to 0.5 mM amino acids supplement (proline, phenylalanine and alanine) with and without transient knockdown of APN in high glucose environment of 33 mM glucose with 100 nm insulin for 2 h. RNA extraction was done from adipocytes using TRI reagent (Sigma-Aldrich, USA). One μg of RNA was reverse transcribed by iScript cDNA synthesis kit (Biorad laboratories Inc., USA), and the resultant cDNA was used as the template for amplification of APN18. The primers for human adiponectin were: Forward: 5’- TGGTGAGAAGGGTGAGAA - 3’; reverse: 5’- AGATCTTGGTAAAGCGAATC - 3’. Primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were forward: 5’- GAACATCATCCCTGCCTCTACTG - 3’; reverse: 5’- CGCCTCCTTCACCACCTTC -3’ (Eurogentec, India).
Real-time PCR was performed using SYBR green PCR master mix (Eurogentec, Europe) on ABI 7300 instrument (Applied Biosystems, USA). The values of specific gene were normalized to GAPDH. Quantitative PCRs were carried out in triplicate.
Quantification of APN by ELISA: To see whether amino acids had any effect on the secretion of APN in adipocytes, mature adipocytes were exposed to 0.5 mM amino acids (proline, phenylalanine and alanine) with and without transient knockdown of APN in high glucose environment (33 mM glucose) with 100 nm insulin for 2 h. After exposure, conditioned medium was collected and concentrated by speed vaccum for APN quantification and the steps were followed as described by supplier (R & D Systems, USA).
AMP-activated protein kinase (AMPK) activity: AMPK activity was measured using SAMS peptide activity assay kit as per manufacturer's protocol (Upstate Biotechnology, USA). In brief, 0.5 mM amino acid exposure was given to cells with and without transient knockdown of APN for 2 h. After incubation time, conditioned medium was removed and cells were washed twice with phosphate buffered saline (PBS); 20 μM of SAMS peptide and 5 μci of p32 were used per assay along with AMPK and incubated at 30°C for 15 min and then loaded onto p81 phosphocellulose paper. Paper was washed thrice with 0.75 per cent phosphoric acid and once with acetone. Paper was added to the scintillant, and the radioactivity was measured as disintegration per minute (DPM) in liquid scintillation system (LSS-Beckman 6500, Fullerton, CA, USA).
Glucose uptake: To see amino acid augment glucose uptake through APN, deoxyglucose uptake was done in high glucose condition with and without transient knockdown of APN. Mature adipocytes in adipocyte growth medium were serum starved for 2 h and subjected to the experimental conditions; 0.5 mM amino acid with and without APN knockdown with 33 mM glucose was added along with the 14C-2-deoxyglucose (2 μci/ml) as a tracer for the glucose uptake in the presence of 100 nM insulin. After 10 min of exposure at 37°C, the medium was removed and the reaction was arrested by adding ice-cold PBS immediately; the cells were washed thrice with PBS and lysed using lysis buffer (0.1% SDS in 0.2 N NaOH) and read in a liquid scintillation counter (Beckman-6500, Fullerton, CA, USA)16. Deoxyglucose uptake was expressed as DPM per milligram protein. Protein concentrations of whole cell lysate were determined using the bicinchoninic acid method16(Thermoscientific, USA).
Statistical analysis: All values were expressed as mean±standard error of mean. Unpaired Student's t-test was used to analyze the significance.
Results
Optimization of siRNA concentration and time: APN mRNA silencing effect of the two designed siRNA with varying time period and concentration showed that 10 nm of si1 at 2 and 4 h after transfection showed 69 and 56 per cent decrease in APN mRNA, whereas si2 did not show significant decrease. Scrambled si did not show any decrease in APN mRNA. GAPDH was used as housekeeping gene to normalize APN expression. Since si1 at 10 nm concentration after 2 h of transfection showed maximum decrease in APN mRNA, it was chosen for the further experiments (Fig. 1).
Fig. 1.
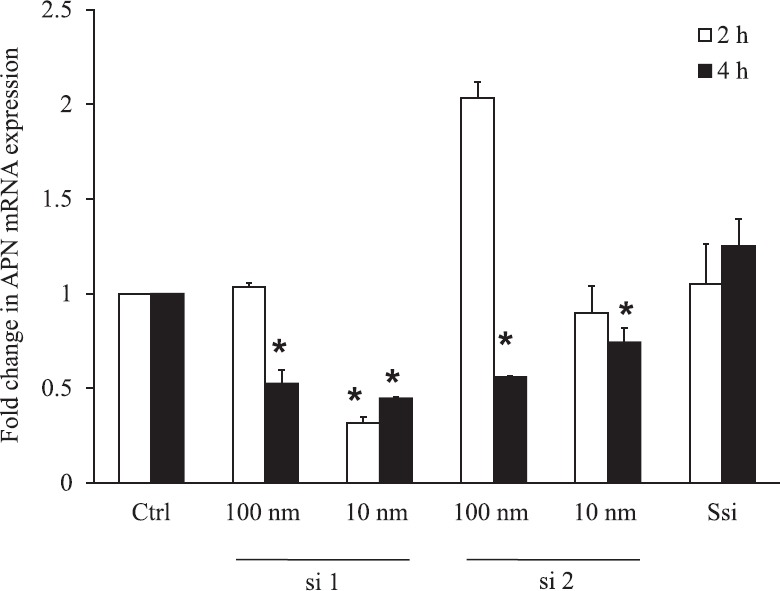
Optimization of adiponectin (APN) siRNA quantitative real-time PCR for APN mRNA. Adiponectin si1 10 nm at 2 (P≤0.001) and 4 h (P≤0.001) significantly decreased APN mRNA by 69 and 56 per cent whereas APN si2 100 nm (P≤0.001) and 10 nm (P<0.05) at 4 h decreased expression by 50 and 30 per cent. The maximum decrease of adiponectin mRNA was observed in APN si1 at 10 nm concentration for 2 h. *Significance (P<0.05) compared to control without siRNA. Ssi, Scrambled SiRNA.
Amino acids increase APN mRNA and protein expression: Conditioned-medium RNA of the mature adipocytes which were exposed to 0.5 mM amino acids (proline, phenylalanine and alanine) with and without transient knockdown of APN (si1 10 nm) in high glucose environment (33 mM glucose) along with 100 nm insulin for two hours was used to quantify the APN mRNA and protein expression.
Exposure of adipocytes to amino acids (proline and phenylalanine) significantly (P<0.01) increased APN mRNA by 1.5-folds when compared to control. Alanine did not show increase in APN mRNA. APN si1 10 nm decreased the APN mRNA significantly in control (P<0.001) and in the presence of amino acid proline (P<0.001) and phenylalanine (P<0.01) when compared to cells not treated with APN si1 (Fig. 2A).
Fig. 2.
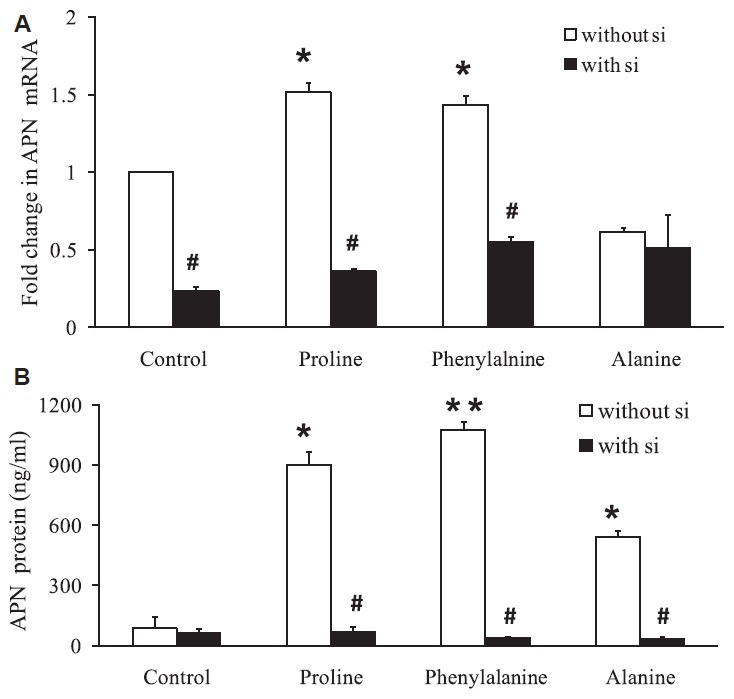
Amino acids increase adiponectin mRNA expression and protein levels. (A) Quantitative PCR for adiponectin (APN) mRNA after amino acid exposure in cultured adipocytes with and without transient knockdown of adiponectin si1 10 nm. (B) Quantification of adiponectin by ELISA showed increase by 4-13-folds after amino acid exposure whereas the adiponectin si1 showed a decreased protein expression even in the presence of amino acid. *P<0.05 compared to cells treated with amino acid and without amino acids. P<0.05 compared to cells treated with and without siRNA in the presence of amino acids.
APN protein expression was increased to 4-13-folds after amino acid exposure to the adipocytes cells when compared to control. Proline increased APN secretion by 10.6-folds (P<0.01), phenylalanine increased the APN secretion by 12.7-folds (P<0.001), alanine by 6.3-folds (P<0.01) and APN si1 10 nm decreased APN protein expression in control as well as in the presence of amino acid proline (P<0.05), phenylalanine (P<0.001) and alanine (P<0.05) when compared to cells not treated with APN si1 (Fig. 2B).
APN knockdown decreased amino acid-induced AMPK activity and glucose uptake: Amino acids (proline, phenylalanine and alanine) were able to increase the AMPK activity but could not attain significance. Transient knockdown of APN in the presence of amino acid proline (P<0.05), phenylalanine (P<0.05) and alanine (P<0.05) decreased AMPK activity when compared to cells not treated with APN si1. Transient knockdown of APN by siRNA decreased amino acid-induced AMPK activity significantly (Fig. 3A).
Fig. 3.
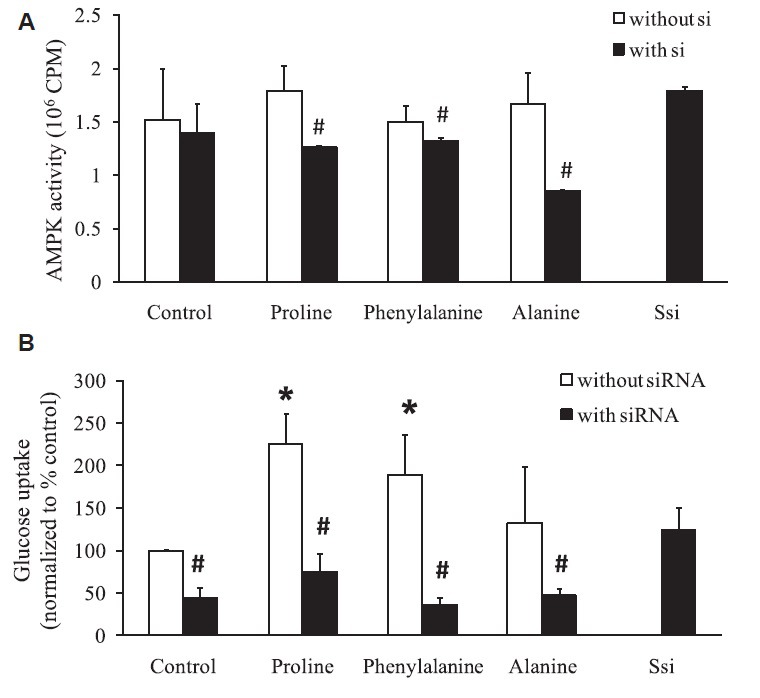
Amino acids mediate glucose uptake through adiponectin (A) AMPK activity was measured using SAMS peptide activity after amino acids exposure in cultured adipocytes with and without transient knockdown of adiponectin. (B) Glucose uptake was increased in the presence of amino acids whereas glucose entry into the cells was significantly decreased after silencing the adiponectin. *P<0.05 compared to cells treated with amino acid and without amino acids. #P<0.05 compared to cells treated with and without siRNA in the presence of amino acids.
Similar results were observed in glucose uptake. Amino acids - proline (P<0.01) and phenylalanine (P<0.01) showed a significant increase in glucose uptake where transient knockdown of APN decreased glucose uptake in control (P<0.01) as well as in the presence of amino acid proline (P<0.01), phenylalanine (P<0.001) and alanine (P<0.05) when compared to cells not treated with si1 (Fig. 3B).
Thus, free amino acids - proline, phenylalanine and alanine modulated the level of APN thereby increasing AMPK activity and in turn glucose uptake in a high glucose environment. This amino acid-induced increase in AMPK activity and glucose uptake were decreased with the transient knockdown of APN indicating that amino acid by improving the APN levels increased the glucose uptake.
Discussion
APN is reported to have antidiabetic, antiobesity and anti-atherogenic properties. Administration of physiological dose of APN in lipoatrophic diabetic mice and murine T2DM reversed IR19 indicating the crucial and direct role of APN in IR. Identification of small molecules for replenishment of APN will have therapeutic value in diseases such as T2DM and MS.
Amino acids are general nutrients which are non-pharmacological and non-toxic. Amino acids remove the excess glucose in the blood20 and also upregulate IR system21. Amino acids are proven to be antidiabetic and anticataractogenic in diabetic rats22. Oral supplementation of amino acid decreased postprandial blood glucose in T2DM patients23. In addition, beneficial effects of amino acids were studied in CHO-K1 cells in which amino acids mixture increased glucose uptake and GLUT4 translocation when the cells were exposed to high glucose environment16.
Blümer et al24 has reported that branched-chain amino acid mixture can stimulate the APN production and that amino acids act as substrates for protein synthesis and thus for APN synthesis. Glycine has already been reported to induce the mRNA of APN in cultured 3T3-L1 cells25. In the present study, proline, phenylalanine, alanine and amino acids of specific mixture improved APN mRNA as well as secretion in cultured human adipocytes about 4-10-folds.
Decreased APN levels are reported in obesity, IR and type 2 diabetes6 whereas increase or overexpression of APN increases the glucose uptake in the cultured skeletal muscles cells. APN stimulates AMPK activation which in turn increases glucose uptake in muscles and decreases gluconeogenesis in liver5. To further confirm the mechanism, APN mRNA was targeted by siRNA. AMPK activity and glucose uptake were measured in the presence of amino acid with and without siRNA. Amino acid increased the AMPK activity and glucose uptake, but this amino acid-induced increase was affected by the transient knockdown of APN. Meng et al26 reported that when 3T3 L1 cells were infected with APN siRNA expression vectors, glucose transport was decreased to a great extent.
Our study had certain limitations. Even though amino acids are general nutrients, metabolomic studies have revealed a positive correlation of branched-chain amino acids and phenylalanine27,28 in the incidence of T2DM. This could be considered as a major concern for therapeutic usage of amino acids; however, metabolomics data analysis was mostly of observational studies which explain only association; in vivo experiments should be done to confirm the therapeutic potential of these amino acids.
In conclusion, the present study showed that amino acid increased APN an insulin-sensitizing hormone and thereby increases AMPK and glucose uptake. These free amino acids should be tested in vivo for their ability to increase APN and their antidiabetic effect. Amino acids can have a therapeutic intervention in IR and T2DM by improving APN levels as seen in cultured adipocyte cells.
Footnotes
Financial support & sponsorship: Authors acknowledge the Indian Council of Medical Research, New Delhi, for providing the research grant (ICMR - 5/4/6/6/09/NCD II).
Conflicts of Interest: None.
References
- 1.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–8. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 2.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 4.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–33. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 5.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: More than just another fat cell hormone? Diabetes Care. 2003;26:2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 7.Tong HV, Luu NK, Son HA, Hoan NV, Hung TT, Velavan TP, et al. Adiponectin and pro-inflammatory cytokines are modulated in Vietnamese patients with type 2 diabetes mellitus. J Diabetes Investig. 2017;8:295–305. doi: 10.1111/jdi.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleidi S, Issa A, Bustanji H, Khalil M, Bustanji Y. Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm J. 2015;23:250–6. doi: 10.1016/j.jsps.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yatagai T, Nagasaka S, Taniguchi A, Fukushima M, Nakamura T, Kuroe A, et al. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52:1274–8. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 10.Krakoff J, Funahashi T, Stehouwer CD, Schalkwijk CG, Tanaka S, Matsuzawa Y, et al. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care. 2003;26:1745–51. doi: 10.2337/diacare.26.6.1745. [DOI] [PubMed] [Google Scholar]
- 11.Otabe S, Yuan X, Fukutani T, Wada N, Hashinaga T, Nakayama H, et al. Overexpression of human adiponectin in transgenic mice results in suppression of fat accumulation and prevention of premature death by high-calorie diet. Am J Physiol Endocrinol Metab. 2007;293:E210–8. doi: 10.1152/ajpendo.00645.2006. [DOI] [PubMed] [Google Scholar]
- 12.Hardie DG. AMP-activated protein kinase: A key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36:28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- 13.Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 14.Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB, et al. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo . Biochem Biophys Res Commun. 2004;314:580–5. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 15.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CC, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: Acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–13. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvi R, Bhuvanasundar R, Saijyothi AV, Sulochana KN, Angayarkanni N. Amino acids potentiate insulin signaling in CHO-K1 at high glucose conditions. Arch Med Res. 2012;43:173–82. doi: 10.1016/j.arcmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–30. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 18.Piñeiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–9. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 20.Ramakrishnan S, Sulochana KN, Punitham R, Arunagiri K. Free alanine, aspartic acid, or glutamic acid reduce the glycation of human lens proteins. Glycoconj J. 1996;13:519–23. doi: 10.1007/BF00731438. [DOI] [PubMed] [Google Scholar]
- 21.Sulochana KN, Rajesh M, Ramakrishnan S. Insulin receptor tyrosine kinase activity in monocytes of type 2 diabetes mellitus patients receiving oral L-lysine. Indian J Biochem Biophys. 2001;38:331–4. [PubMed] [Google Scholar]
- 22.Sulochana KN, Punitham R, Ramakrishnan S. Beneficial effect of lysine and amino acids on cataractogenesis in experimental diabetes through possible antiglycation of lens proteins. Exp Eye Res. 1998;67:597–601. doi: 10.1006/exer.1998.0547. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan Sulochana K, Lakshmi S, Punitham R, Arokiasamy T, Sukumar B, Ramakrishnan S, et al. Effect of oral supplementation of free amino acids in type 2 diabetic patients – A pilot clinical trial. Med Sci Monit. 2002;8:CR131–7. [PubMed] [Google Scholar]
- 24.Blümer RM, van Roomen CP, Meijer AJ, Houben-Weerts JH, Sauerwein HP, Dubbelhuis PF, et al. Regulation of adiponectin secretion by insulin and amino acids in 3T3-L1 adipocytes. Metabolism. 2008;57:1655–62. doi: 10.1016/j.metabol.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Macedo R, Sanchez-Muñoz F, Almanza-Perez JC, Duran-Reyes G, Alarcon-Aguilar F, Cruz M, et al. Glycine increases mRNA adiponectin and diminishes pro-inflammatory adipokines expression in 3T3-L1 cells. Eur J Pharmacol. 2008;587:317–21. doi: 10.1016/j.ejphar.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 26.Meng FP, Wang CB, Li Liangqiong, Po Hang. Effect of adiponectin siRNA on the glucose transport in 3T3 L1 adipocytes. Chin J Pathophysiol. 2008;24:2142–6. [Google Scholar]
- 27.Guasch-Ferré M, Hruby A, Toledo E, Clish CB, Martínez-González MA, Salas-Salvadó J, et al. Metabolomics in prediabetes and diabetes: A systematic review and meta-analysis. Diabetes Care. 2016;39:833–46. doi: 10.2337/dc15-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost HG, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62:639–48. doi: 10.2337/db12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]


