Abstract
Members of the septin family of proteins act as organizational scaffolds in areas of cell division and new growth in a variety of organisms. Herein, we show that in the filamentous fungus Aspergillus nidulans, the septin AspB is important for cellular division, branching, and conidiation both pre- and postmitotically. AspB localizes postmitotically to the septation site with an underlying polarity that is evident as cytokinesis progresses. This localization at the septation site is dependent on actin and occurs before the cross-wall is visible. AspB localizes premitotically as a ring at sites of branching and secondary germ tube emergence. It is the only known branch site marker. In addition, AspB is found at several stages during the development of the asexual reproductive structure, the conidiophore. It localizes transiently to the vesicle/metula and metula/phialide interfaces, and persistently to the phialide/conidiospore interface. A temperature-sensitive mutant of AspB shows phenotypic abnormalities, including irregular septa, high numbers of branches, and immature asexual reproductive structures.
INTRODUCTION
Actively growing cells are faced with two related problems. They must target new material to the appropriate site(s), and they must partition new material such that the resulting cells receive the full complement of cytoplasmic and genetic constituents. Both the targeting and partitioning problems can be solved by establishing organizational cues that can direct vesicle fusion or tether signals that coordinate specific biochemical reactions. Walled organisms, such as fungi and plants, have the added complication of breaking down portions of the existing rigid wall so that new growth can proceed.
Septins, a conserved family of proteins, act as organizational cues, recruiting critical proteins to areas of new growth and cell division (Longtine et al., 1996; Field and Kellogg, 1999; Gladfelter et al., 2001). Septins (CDC3, CDC10, CDC11, and CDC12 gene products) were originally identified as components of the 10-nm filament ring found just inside the plasma membrane at the mother-bud neck in the yeast Saccharomyces cerevisiae (Byers and Goetsch, 1976; Longtine et al., 1996). Temperature-sensitive cdc3, cdc10, cdc11, and cdc12 mutants show delays in mitosis (Carroll et al., 1998; Barral et al., 1999; Longtine et al., 2000) and fail to undergo cytokinesis (Hartwell, 1971), resulting in elongated, multibudded, multinucleate chains of cells. Three other septin genes, SPR3, SPR28, and SEP7, have also been identified in S. cerevisiae. Spr3p and Spr28p play roles in spore development (DeVirgilio et al., 1996; Fares et al., 1996), whereas Sep7p is expressed in vegetative cells and localizes to the mother-bud neck (Carroll et al., 1998; Mino et al., 1998). Members of the septin family have two shared motifs, a GTP-binding P-loop and, with a few exceptions, a coiled-coiled domain that may function in protein–protein interactions.
Septins play several developmental roles in S. cerevisiae. At the bud neck, they help in organizing the septal cell wall by acting as an anchor for Bni4p, which in turn is part of a complex including the chitin synthase responsible for the formation of the bud scar (DeMarini et al., 1997). During spore formation, Spr3p, Spr28p, Cdc3p, and Cdc11p are localized to the leading edge of the prospore sac, where they might influence the deposition of chitosan (DeVirgilio et al., 1996; Fares et al., 1996). Septins also localize several proteins responsible for marking the areas of future bud emergence (Bud3p, Bud4p, and Axl2p), and loss of proper septin function results in a randomization of the budding pattern (Flescher et al., 1993; Chant et al., 1995; Pringle et al., 1995; Halme et al., 1996; Roemer et al., 1996; Sanders and Herskowitz, 1996). Proper septin organization may also help in coordinating morphogenesis and mitosis (Lew, 2000). Several cell cycle-regulating protein kinases localize to the septin ring at the mother-bud neck, and a mitotic delay occurs if the septins fail to organize properly (Carroll et al., 1998; Barral et al., 1999; Shulewitz et al., 1999; Longtine et al., 2000).
Because of distinct differences in the organization and regulation of cell division in budding yeast versus filamentous fungi, Aspergillus nidulans makes a valuable system for the study of septins. In yeast, each mitotic division is followed immediately by cytokinesis. In contrast, an A. nidulans spore undergoes a brief period of isotropic growth, establishes polarity, and develops a germ tube that elongates by tip growth. The nucleus, meanwhile, undergoes several mitotic divisions, and new nuclei move out into the germ tube. Cytokinesis takes place after the third nuclear division by placement of a cross-wall, called a septum, at the basal end of the germ tube (reviewed by Harris et al., 1997; Momany and Taylor, 2000). Subsequent septa are placed such that uniform compartments, each containing three to four nuclei, are formed. In A. nidulans only the tip compartment remains mitotically active. Basal compartments are arrested in interphase unless the compartment forms a branch, in which case mitosis is again initiated (Fiddy and Trinci, 1976; Kaminskyj and Hamer, 1998).
Five septin genes have been found in A. nidulans (Momany et al., 2000). One, aspB (for Aspergillus nidulans septin B), has been shown to be essential (Momany and Hamer, 1997a). In the present study, we show that AspB plays a role in the formation of septa, branches, and asexual reproductive structures.
MATERIALS AND METHODS
Aspergillus Strains and Growth Methods
All strains used in this study are listed in Table 1. Defined minimal medium (Käfer, 1977) with appropriate supplements was used throughout this study. For septation, branch emergence, and secondary germ tube emergence studies, conidia from appropriate strains were grown for 12 h at 37°C. For parasynchronous wave studies, strains were grown for 16 h at 37°C. For AspB localization in sep mutants, conidia from appropriate strains were grown for 12 h at 42°C. For conidiophore localization studies, overnight liquid cultures of A. nidulans strain A850 were grown at 37°C with vigorous shaking. Cultures were collected on miracloth and placed on solid medium to induce conidiation (Miller et al., 1992). Conidiophores were then harvested at 5.0, 5.5, 6.0, 6.5, 7.5, and 8.0 h by scraping in water. For studies in which actin polymerization was inhibited, conidia from appropriate cultures were grown for 10 h at 37°C, transferred to medium containing 1 μg/ml cytochalasin A (CA; Sigma, St. Louis, MO) and grown for 2 h.
Table 1.
A. nidulans strains
| Strain | Genotype |
|---|---|
| A850a | biA1; ΔargB∷trpCΔB; methG1; veA1; trpC801 |
| ASH51b | sepA1; pyrG |
| AJH44b | sepD5; pyroA4; veA1; pyrG89; wA2 |
| AJM1b | sepG1; pabaA6; pyroA4; chaA1; pyrG89; veA1 |
| AJM68b | sepH1; wA3; veA1; pyrG89; pyroA4 |
| SWJ189c | bimE7; methB3; wA2 |
| A773a | pyrG89; wA3; pyroA4 |
| APW55d | aspB318; ΔargB∷trpCΔB; trpC801 |
Obtained from Fungal Genetics Stock Center, Department of Microbiology, University of Kansas Medical Center, Kansas City, KS 66160-7420.
Obtained from John Hamer, Department of Biological Sciences, Purdue University, West Lafayette, IN.
Obtained from Steve James, Department of Biology, Gettysburg College, Gettysburg, PA.
See text.
Generation of Antibodies and Immunofluorescence
The full-length aspB cDNA was polymerase chain reaction (PCR) amplified from plasmid paspB (Momany and Hamer, 1997a) and fused in-frame to the glutathione S-transferase (GST) tag sequence in the pGEX-KG vector (Guan and Dixon, 1991) to create plasmid pMM17. pMM17 was transformed into Escherichia coli strain XL1-Blue, and the GST-AspB fusion protein was induced and affinity purified using standard protocols. After SDS-PAGE, a single band of the expected 49-kDa size containing ∼200 μg of the fusion protein was excised and injected into rabbits for immunization (Harlow and Lane, 1988). Two additional boosts containing ∼200 μg of fusion protein were required to get sufficient antibody titers as judged by Western blot analysis of total A. nidulans protein.
The 1.5-kb aspB open reading frame was PCR amplified and fused to a six-histidine tag in the Novagen (Madison, WI) pET-28b vector to create plasmid pPW17. pPW17 was transformed into E. coli strain BL21(DE3) and induced with 0.15 mM isopropyl β-d-thiogalactoside for 4 h at 30°C. Supernatant from lysed cells was loaded directly to a 2-ml Ni-NTA column (Amersham Biosciences AB, Uppsala, Sweden), and 1-ml fractions were collected as per standard protocols. Fractions containing a single polypeptide of the appropriate size were electrophoresed on a 10% SDS-PAGE gel and blotted to nitrocellulose. Polyclonal antibodies raised against the GST-AspB fusion were affinity purified against the His-AspB fusion as previously described (Pringle et al., 1989). To test for specificity, the purified antibodies were used to probe Western blots containing 5 mg/lane total protein from A. nidulans strain A850. A goat anti-rabbit secondary antibody conjugated to alkaline phosphatase (Sigma) was used to visualize the primary antibody. A single band of the expected size (49 kDa) was recognized after two rounds of affinity purification (Figure 1d). No band was visible in lanes probed with preimmune serum (our unpublished data).
Figure 1.
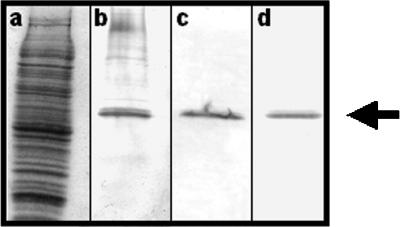
Western analysis of affinity-purified antibodies against AspB. (a) Total protein from A. nidulans strain A850 stained with Coomassie blue. Blots of total protein were incubated with 1:100 dilutions of unpurified immune serum (b), antibodies obtained after one round of affinity purification (c), or antibodies obtained after two rounds of affinity purification (d). A single 49-kDa polypeptide is recognized (arrow).
Fixation and staining of cells were as described previously (Oakley and Osmani, 1993) with an additional 2-h blocking step with 5% dry nonfat milk in buffered phosphate solution before the addition of primary antibody. Secondary goat anti-rabbit conjugated to fluorescein isothiocyanate was obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Actin localization was done using the C4 mouse anti-actin monoclonal antibody (ICN Biomedicals, Aurora, OH) and a Texas Red-labeled sheep anti-mouse secondary (Jackson Immunoresearch Laboratories). For AspB localization to the conidiophore, fresh scrapings from conidiating cultures were added directly to 1 ml of fixer (Oakley and Osmani, 1993). After a 30-min incubation in fixer, conidiophores were harvested by gentle centrifugation (3000 rpm); resuspended in 1 ml of a digestion solution containing 25 mg/ml Novozyme 234 (Sigma), 50% egg white, and 1.2 M sorbitol; and incubated for 2 h with gentle rocking at 37°C. Subsequent steps were as described above except that all solutions were osmotically stabilized with 1.2 M sorbitol.
Generation of Conditional aspB Alleles
A 5-kb EcoRI/PstI genomic fragment was subcloned from plasmid pMM1 (Momany and Hamer, 1997) into pBC KS (Stratagene, La Jolla, CA) to create plasmid pPW20, containing aspB and a 1-kb upstream region. pPW20 was transformed into the E. coli mutator strain XL1-Red (Stratagene). Plasmid DNA was isolated, linearized with EcoRI, and cotransformed with a marker plasmid containing the argB gene into A. nidulans strain A850 by using standard protocols (Yelton et al., 1984). Approximately 4000 Arg+ transformants were replica plated at 30 and 42°C. Fifty-four transformants showing a stable phenotypic defect at 42°C were crossed with A. nidulans strain A773 and progeny were scored. Total DNA was isolated from 12 strains with 1:1 Ts+:Ts− segregation and blotted to nylon membranes. A PCR product containing the aspB open reading frame was used to probe blots and identified one temperature-sensitive transformant with a single copy of aspB. This strain, APW55, was restored to wild-type growth by complementation with the original pPW20 plasmid.
Image Acquisition and Adjustment
Microscopic observations were made with a Zeiss (Thornwood, NY) Axioplan microscope. Digital images of immunofluorescence localization were acquired with an Optronics Digital Imaging System (Goleta, CA). Files were imported into Adobe PhotoShop 5.5 (Adobe Systems, Mountain View, CA) for adjustments to brightness and contrast. All images were rotated such that the apical tip was to the right and the basal end was to the left. For parasynchronous wave studies, images of germlings stained with Calcofluor White and Hoechst were converted to grayscale then back to RGB color. Highlights, midtones, and shadows were then adjusted to give a false red coloring to these images.
RESULTS
AspB Localizes to Forming Septa
Septum formation in A. nidulans proceeds by simultaneous actin-mediated invagination of the plasma membrane and deposition of the chitinous primary septum (Momany and Hamer, 1997b). Actin appears very early in septum formation, before the chitin ring appears. To determine whether AspB protein is also present during septum formation, we labeled hyphae with affinity-purified anti-AspB antibodies (Figure 1). Both single and double rings of AspB were present at forming septa (Figure 2).
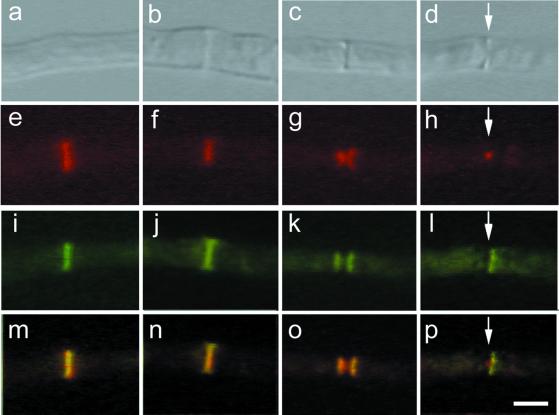
Figure 2.
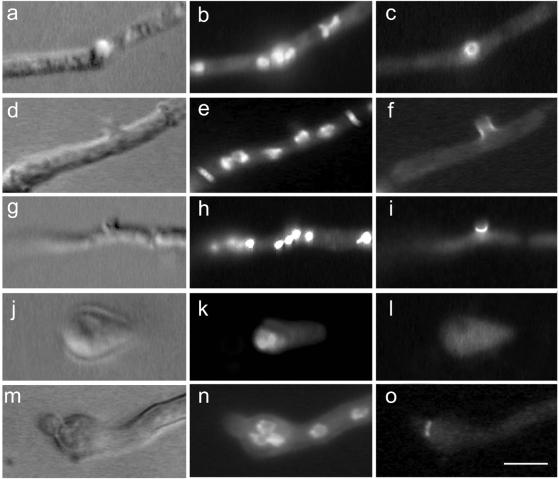
Localization of AspB and actin during septation. Differential interference contrast images (row 1, top); actin localization with Texas Red-conjugated secondary antibody (row 2); AspB localization with fluorescein isothiocyanate-conjugated secondary antibody (row 3); and merger of rows 2 and 3 (row 4). Areas of AspB and actin colocalization appear yellow. Arrows indicate location of septum. (a, e, i, and m) Early stage of septum formation. No cross-wall is visible yet. (b, c, f, g, j, k, n, and o) Intermediate stages of septum formation. (d, h, l, and p) Late stage of septum formation. Bar, 5 μm.
To determine the order of AspB intermediates in septum formation, we double labeled hyphae with antibodies against AspB and F-actin. Based on the known order of actin intermediates during septation (Momany and Hamer, 1997b), we were able to determine the progression of AspB during septum development (Figure 2). 1) A single ring of actin appears, marking the site of septation, before cell wall material is deposited (Figure 2, a and e). A single ring of AspB colocalizes with this early actin ring (Figure 2, i and m). 2) The chitinous septum becomes visible and overlaps with the actin and AspB rings (Figure 2, b, f, j, and n). 3) The invaginating actin ring forms an hourglass-like structure (Figure 2g). The AspB ring does not invaginate, but splits into a double ring flanking the septum (Figure 2o). 4) The actin ring continues to invaginate, eventually contracting to a very small, bright dot. The basal AspB band disappears, leaving a single ring of AspB on the apical side of the septum (Figure 2, d, h, l, and p). Mature septa have no actin or AspB rings associated with them (our unpublished data).
AspB Assembly at Septum Requires Actin and sepA, sepG, and sepH Proteins
To determine whether actin is necessary for AspB localization, we used CA to depolymerize F-actin (Harris et al., 1994). A. nidulans conidia were incubated for 10 h at 37°C then shifted to medium containing 1 μg/ml CA for 2 h. In an untreated control grown for 12 h at 37°C, rings of AspB protein were present in 15% of cells (n = 100). No AspB rings were present after CA treatment (n = 200), suggesting that filamentous actin is required for proper localization of the AspB ring.
To further investigate its relationship to other molecules known to be important in cytokinesis, we localized AspB protein in a group of temperature-sensitive septation (sep) mutants that fail to form septa when grown at restrictive temperature (Harris et al., 1994). A subset of these mutants, sepA, sepD, sepG, and sepH, form septa rapidly on shift from restrictive-to-permissive temperature. Epistasis analysis has shown that sepA, sepD, sepG, and sepH are involved in a linear pathway, with sepH and sepA acting earlier than sepD and sepG (Morrell, 1997).
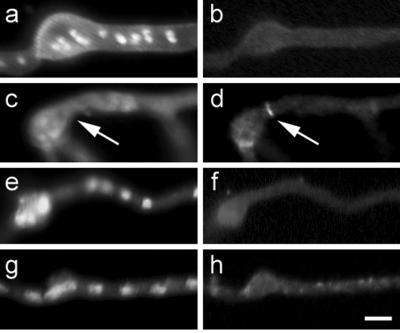
Rings of AspB protein were not seen in sepA, sepG, or sepH at restrictive temperature (Figure 3, a, b, and e–h). However, rings of AspB were seen in sepD strains grown at restrictive temperature (Figure 3, c and d). These rings were present in 6% of the population, and often multiple rings were present in the same germ tube. Thus, it appears that AspB assembles downstream of sepA, sepG, and sepH, but upstream of sepD.
Figure 3.
AspB localization in sep mutants grown at nonpermissive temperature. Chitin and nuclear localization with Calcofluor and Hoechst, respectively (left column). AspB localization (right column). (a and b) sepA. (c and d) sepD. (e and f) sepG. (g and h) sepH. Arrows mark AspB localization in sepD. Bar, 5 μm.
AspB Localization to Septa Is Postmitotic
In S. cerevisiae, septins appear in late G1 phase to mark the point of bud emergence, and persist throughout S, G2, and mitosis until cytokinesis takes place (Longtine et al., 1996). To determine whether AspB assembly at the septum occurs during or after mitosis in A. nidulans, we examined the nuclear state near septin rings. In 150 germlings with AspB single or double rings, all nuclei were in interphase. In the 5% of the population with mitotic nuclei, no AspB rings were present; thus, it appears that AspB assembly does not take place during mitosis.
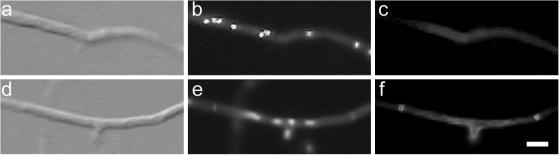
To determine whether AspB ring assembly requires exit from mitosis, AspB was localized in A. nidulans strain SWJ189, which contains the temperature-sensitive bimE7 allele (James et al., 1995). At restrictive temperature, bimE7 nuclei are blocked in the preanaphase stage of mitosis. SWJ189 was grown 10 h at permissive temperature then shifted to fresh medium and grown at restrictive temperature. After 120 min at restrictive temperature, ∼80% of the tip cells examined contained mitotic nuclei, but no AspB rings were visible (n = 100) (Figure 4, a–c). Forty-five minutes after shifting back to permissive temperature, AspB rings were visible in 17% of the hyphae (n = 100) (Figure 4, d–f). Thus, exit from mitosis appears to be needed for AspB assembly.
Figure 4.
Dependence of AspB localization on mitosis. DIC images (left column). Chitin and nuclear staining with Calcofluor and Hoechst (middle column). AspB localization (right column). (a–c) bimE7 mutant grown at 30°C for 10 h and then shifted to 42°C for 2 h. No AspB localization was detected. (d–f) bimE7 mutant grown at 30°C for 10 h, shifted to 42°C for 2 h, and shifted back to 30°C for 45 min. AspB localization was detected at septation sites.
Septation in A. nidulans Occurs in Parasynchronous Wave
Apical cells, which contain up to 30 nuclei, undergo mitosis in a wave that rapidly progresses from the tip to the base of the compartment (Rosenberger and Kessel, 1967; Clutterbuck, 1970). Although it is clear that the wave of nuclear division is followed immediately by a wave of septation, there has been disagreement on the order of septum formation in tip cells. Based on the demonstration by Wolkow et al. (1996) that mitosis triggers septum formation, it seemed likely that the tip-to-base wave of mitosis in the apical cell would be followed by a tip-to-base wave of septum formation. However, earlier work by Clutterbuck (1970) suggested that although the most apical septum formed first, there was no apparent order for formation of those septa further back.
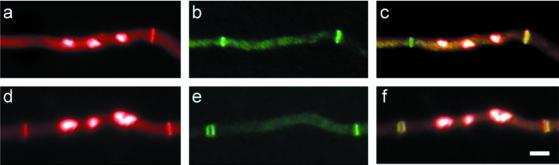
To determine the order of septum formation in tip cells, we examined AspB and chitin rings in 46 A. nidulans apical compartments undergoing multiple septation events. Ninety-five percent of tip compartments showed septum progression from most-to-least mature moving from apex to base (i.e., septa with single AspB rings to the apical side were closer to the cell tip than septa with double AspB rings, septa with double AspB rings were closer to the cell tip than septa with single AspB rings, etc.) (Figure 5). This is the pattern that would be expected if the tip-to-base wave of mitosis triggers a tip-to-base wave of septation.
Figure 5.
Parasynchronous formation of septa in A. nidulans. Chitin and nuclear staining with Calcofluor and Hoechst, respectively (left column). Images were false colored red. AspB localization (middle column). Merged images of left and middle columns (right column). Areas of chitin and AspB colocalization appear yellow. Hyphae are oriented such that the basal end is to left and the apical end is to the right. Bar, 5 μm.
AspB Localizes Premitotically to Branch Points
After compartmentalization by septation, basal hyphal cells enter a resting state with all nuclei arrested in interphase. The nuclear number in these basal compartments averages three to four nuclei. To become mitotically active, these basal compartments must establish a new axis of polarity by the formation of branches. No molecular markers for branching have been identified previously.
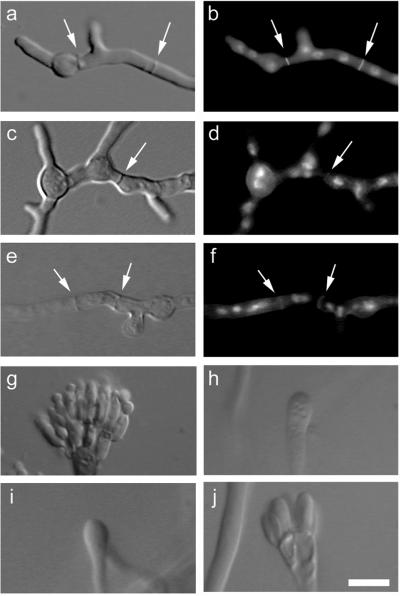
We observed AspB rings in subapical compartments where branching was occurring (Figure 6, a–i). The AspB ring was visible before any change in the profile of the hypha and persisted until the emerging branch was at least 5 μm in length. To determine whether AspB localization to branch points occurs before or after mitosis, we counted nuclear numbers in subapical compartments with AspB label. In 46% of subapical compartments with AspB rings, an odd number of interphase nuclei was present (n = 100) (Figure 6e). Therefore, AspB ring assembly at branch points is premitotic. Because mitosis doubles the number of nuclei, if AspB ring assembly were postmitotic, all subapical compartments with AspB rings should have an even number of nuclei. AspB localization persists at the base of the branch as nearby nuclei enter mitosis (Figure 6h).
Figure 6.
AspB localization to early branches and secondary germ tubes. DIC images (left column). Chitin and nuclear staining with Calcofluor and Hoechst (middle column). AspB localization (right column). (a–c) AspB appears as a ring at the site of branch emergence. (d–f) Side view of emerging branch. AspB localizes as a band at the base of the branch. The odd number of interphase nuclei indicates that localization is premitotic. (g–i) AspB is localized to the branch while the compartment is in mitosis. Compact, bright nuclear staining indicates mitotic nuclei. (j–l) Emerging primary germ tube. No AspB localization is present. (m–o) Emerging secondary germ tube. AspB localizes as a ring at the site of secondary germ tube emergence. Bar, 5 μm.
AspB Localizes to Secondary, but not Primary, Germ Tubes
When A. nidulans conidia break dormancy, they first grow isotropically, maintaining a spherical shape. By the second nuclear division, the young cells establish an axis of polarity and add new cell surface material only at the tip of the emerging germ tube. As early as the third nuclear division, some germlings send out a second germ tube from the conidium, generally 180° from the first (Momany and Taylor, 2000). Cells at the beginning stages of polarity establishment did not show AspB localization at the base of the emerging primary germ tube (n = 150) (Figure 6, j–l). However, a ring structure similar to that seen at branch points was visible at sites of secondary germ tube emergence (Figure 6, m–o).
AspB Localizes to Conidiophores
In A. nidulans, the asexual spores are made by a specialized structure known as the conidiophore (reviewed by Adams et al., 1998). Conidiophore development begins with the partitioning of a thick-walled hyphal cell, called a foot cell, that extends to form an aerial hypha. The tip of the aerial hypha swells to produce a knob-like vesicle. A layer of specialized cells, the metulae, bud from the surface of the vesicle. In turn, conidiogenous cells, the phialides, bud from the tips of the metulae. Phialides produce chains of uninucleate conidia.
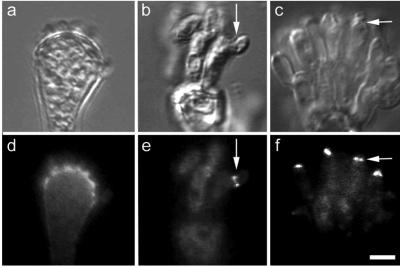
To determine whether AspB might have a role in organizing new growth in asexual reproduction, we induced synchronous conidiophore development, harvested developing conidiophores at several time points, and performed immunolocalization experiments. We observed AspB as a diffuse band at the base of forming metulae (Figure 7d). This band disappeared from the vesicle/metulae interface as the metulae elongated. We also observed rings of AspB at the base of developing phialides (Figure 7e). AspB rings disappeared from the metula/phialide interface as the phialides matured. Finally, we observed AspB at the tips of phialides producing conidiospores. The AspB band at the phialide/conidium interface was present simultaneously in all phialides (Figure 7f).
Figure 7.
AspB localization in the conidiophore. DIC images (top row). AspB localization (bottom row). (a and d) AspB localizes at the vesicle/metulae interface as metulae emerge. (b and e) AspB localizes at the metula/phialide interface. Arrow marks emerging phialide. (c and f) AspB localizes to the phialide/conidiospore interface. Arrow marks emerging conidium. Bar, 5 μm.
AspB Functions in Septation, Branching, and Conidiophore Development
Previous work showed that aspB is an essential gene (Momany and Hamer, 1997a). To determine whether AspB is needed for development of septa, branches, and conidiophores, an A. nidulans strain containing a temperature-sensitive allele of aspB was generated. When grown at restrictive temperature, this strain was able to form septa that were readily visible under differential interference contrast (DIC) imaging but were only faintly visible by staining with Calcofluor White (n = 100) (Figure 8, c–f). Furthermore, the strain containing the mutant allele formed twice the number of branches compared with wild-type hyphae grown at 42°C for 10 h (n = 100) (Figure 8, a and c). Finally, the aspB-318 strain formed abnormal conidiophores that were defective in development. Most mutant conidiophores arrested at the vesicle stage of development (Figure 8, h and i); however, a small portion (16%) was able to form metulae 24 h after exposure to an air interface (n = 100) (Figure 8j). At the same time point, 100% of a wild-type population had produced metulae, phialides, and spores (n = 100) (Figure 8g).
Figure 8.
Conditional mutant of aspB. (a, c, and e) DIC images of vegetative hyphae. (b, d, and f) Chitin and nuclear staining of vegetative hyphae with Calcofluor and Hoechst. (a and b) Wild-type A. nidulans grown at 42°C for 10 h. (c–f) A. nidulans strain APW55 (aspB-318) grown at 42°C for 10 h. Note that chitin staining of septa (arrows) appears weaker than in wild type. (g–j) DIC images of conidiophores exposed to an air interface for 24 h. (g) Wild-type conidiophore. (h–j) APW55 conidiophores. Bar, 5 μm.
DISCUSSION
The first clearly defined role for septins was in organization of the division plane at the septum of budding yeast (reviewed by Longtine et al., 1996). Septins from a variety of organisms localize not only to cleavage planes but also to other areas of new cell growth (reviewed by Field and Kellogg, 1999; Trimble, 1999). Our results show that the A. nidulans AspB protein, the first septin studied from a multicellular fungus, localizes both to sites of division and areas of new growth.
AspB Ring at Septation Site Is Polar
Although we expected to find AspB at septation sites, the striking polarity of AspB during septum formation surprised us. Previous work showed that septation in A. nidulans proceeds in three distinct stages. First, a single, faint ring of actin becomes visible. Next, actin thickens into a contracting band with a ring of chitin in its furrow. Finally, actin disappears and a disk of chitin forming the primary septum remains (Momany and Hamer, 1997b). Throughout this process, actin and chitin localization appears symmetrical; there is no obvious difference between the apical face of the septum versus the basal face. In contrast, we found that localization of AspB is asymmetrical late in septum formation (Figures 2 and 5). The apical/basal asymmetry of AspB localization at forming septa suggests that the hypha marks polarity along its length spanning multiple cells, and that AspB either establishes or responds to these polarity cues. A similar septin asymmetry is seen in the unicellular budding yeast S. cerevisiae where certain proteins are localized asymmetrically in a septin-dependent manner (reviewed by Gladfelter et al., 2001). However, our AspB localization studies are the first demonstration of septin polarity in a multicellular organism. Although polar assembly has not been reported for animal septins, it seems likely that at least some animal septins will show a similar apical/basal polarity.
Assembly of AspB at Septation Site Requires Actin, SepA, SepG, and SepH
Previous work has shown that actin is required for the formation of mature septa (Harris et al., 1994; Momany and Hamer, 1997b). To investigate the relationship between actin and AspB, we used the actin-depolymerizing agent cytochalasin A. We observed an absence of AspB rings with drug treatment, suggesting that actin is required for either assembly or stability of the AspB ring. AspB's postmitotic localization suggests that the division site in A. nidulans is organized more like that of Schizosaccharomyces pombe than that of S. cerevisiae. In S. cerevisiae, the septins localize to the site of bud emergence early in the cell cycle and independently of actin. They act not only to organize the cytokinetic machinery but also to localize certain kinases important for the initiation and progression of mitosis (Longtine et al., 1996; Lew, 2000; Gladfelter et al., 2001). In contrast, the S. pombe septins first localize to the presumptive septation site late in anaphase, well after actin has marked this site, and just before the primary septum itself becomes visible (Pringle, personal communication). Interestingly, when grown at restrictive temperature, cells carrying the temperature-sensitive aspB-318 allele make a septum that is readily visible by DIC imaging but stains very faintly with Calcofluor White, suggesting that the septum might not be properly organized.
Our results further suggest that the products of several of the A. nidulans septation (sep) genes are required for AspB ring formation. We performed immunolocalization experiments on temperature-sensitive septation mutants (sepA, sepD, sepG, and sepH) grown at restrictive temperature (Figure 3). We observed the AspB ring in 6% of sepD hyphae, and in none of the other sep mutants. We suspect the low percentage of the sepD population with the AspB ring reflects the dependence of septation upon mitosis and may indicate instability of the early septin ring. Wolkow et al. (1996) showed that the first mitotic division after the germling passes a critical size threshold triggers septation. Other work has shown that the initiation of septation can be separated from the progression of septation (Momany and Hamer, 1997b). Perhaps in the sepD mutant, mitosis triggers the initiation of septation, but the temperature-sensitive defect prevents the progression of septation. If the early AspB single ring is unstable, it might dissociate when septation does not progress. Thus, only the small portion of the population that has recently exited mitosis would be expected to show an AspB ring in sepD cells. Observations of AspB localization in wild-type germlings are consistent with this idea, because only ∼4% show a single AspB ring with no associated chitin ring, presumably representing the cells that have recently exited mitosis and assembled the initial septin ring.
AspB Ring Is an Early Marker for Branch Formation
Nuclei in subapical compartments are arrested in interphase until a branch is formed from the compartment. How sites for branching are chosen and how branching reactivates mitosis are not understood. However, we observed AspB localization at the nascent branch before mitosis occurred (Figure 6e). In fact, we occasionally observed an AspB ring before any change of hyphal profile was visible. AspB localization persisted as the nuclei in the compartment entered mitosis, but was not present in any branches that were longer than 5 μm. The AspB ring is the first molecular marker identified for nascent branches. The presence of AspB at branches before resumption of nuclear division raises the possibility that the septin ring may recruit the cell cycle regulators that reinitiate mitosis in subapical compartments. Future experiments will address this possibility. In addition to this possible role in the control of nuclear division, a role for AspB in organizing new growth at branches is also consistent with our data.
AspB Ring Is Seen at Some Areas of New Growth and not at Others
The emergence of the initial germ tube from the conidium differs from that of subsequent germ tubes in both shape and mitotic state. Primary germ tubes emerge after the first mitotic division. These germ tubes give the swollen conidiospore a pointed, pear-shaped appearance (Figure 6j). This tapered look disappears as the germ tube lengthens. The secondary germ tubes, however, appear to be similar to branch points, with a narrower point of emergence (Figure 6m). That AspB rings localize to secondary, but not primary, germ tubes may reflect an underlying difference of organization between these two events. One possible explanation is that a preexisting signal is present in the conidium and directs the growth of the primary germ tube. This does not, however, mean that septins do not play a role in primary germ tube emergence, because there are at least four other septins in A. nidulans, and any one of these could be involved in the initial event.
AspB Is Involved in Proper Organization of Conidiophore
In S. cerevisiae, the site of bud emergence is circumscribed by the septin ring late in G1 phase (Longtine et al., 1996). New growth emerges exclusively within the confines of this ring as the daughter cell starts to form. Later, after mitosis, the septins act to anchor the components that will ultimately synthesize the cell wall material that separates the mother and daughter cells (DeMarini et al., 1997; Gladfelter at al., 2001). In A. nidulans condiophores, it is possible that AspB may play an analogous role by circumscribing the site of metula emergence from the vesicle layer and phialide emergence from the metula layer. This would explain the transient AspB localization to these two interfaces and is supported by the observation that the aspB-318 strain is unable to form the metula layer properly when grown at restrictive temperature. When conidia are formed at the tip of the phialide cells, new growth is followed immediately by cytokinesis. AspB at the tip of the phialide could be delimiting the site of conidium emergence, organizing the cytokinetic machinery, or both. It is not clear whether the simultaneous localization of AspB at the tips of the phialides reflects persistent localization or the rapid reformation of a new “bud site” as mature conidia are displaced.
CONCLUSION
The dynamic aspect of AspB assembly in both premitotic and postmitotic areas may in part be explained if different AspB binding partners are present at different stages of development. Our results are consistent with a model in which septins act as an organizational scaffold that recruits other proteins to specific sites.
ACKNOWLEDGMENTS
We thank Steve James (Gettysburg College) and John Hamer (Paradigm Genetics) for strains, and Gretel Guest, Brain Shaw, and John Zhao for critical reading of this manuscript. This work was supported by National Science Foundation grant 9904629 to M.M.. P.J.W. was supported by the National Institutes of Health training grant in molecular and cellular mycology to University of Georgia (AI07373).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–06-0312. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–06-0312
REFERENCES
- Adams TH, Weiser JK, Yu JH. Asexual sporulation in Aspergillus nidulans. Microbiol Mol Biol Rev. 1998;62:35–54. doi: 10.1128/mmbr.62.1.35-54.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Parra M, Bidlingmaier S, Snyder M. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 1999;13:176–187. doi: 10.1101/gad.13.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CW, Altman R, Schieltz D, Yates JR, Kellogg D. The septins are required for the mitosis-specific activation of the Gin4 kinase. J Cell Biol. 1998;143:709–717. doi: 10.1083/jcb.143.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J, Mischke M, Mitchell E, Herskowitz I, Pringle JR. Role of Bud3p in producing the axial budding pattern of yeast. J Cell Biol. 1995;129:767–778. doi: 10.1083/jcb.129.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck AJ. Synchronous nuclear division and septation in Aspergillus nidulans. J Gen Microbiol. 1970;60:133–135. doi: 10.1099/00221287-60-1-133. [DOI] [PubMed] [Google Scholar]
- DeMarini DJ, Adams AEM, Fares H, De Virgilio C, Valle G, Chuang JS, Pringle JR. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiaecell wall. J Cell Biol. 1997;139:75–93. doi: 10.1083/jcb.139.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVirgilio C, DeMarini DJ, Pringle JR. SPR28, a sixth member of the septin gene family in Saccharomyces cerevisiaethat is expressed specifically in sporulating cells. Microbiology. 1996;142:2897–2905. doi: 10.1099/13500872-142-10-2897. [DOI] [PubMed] [Google Scholar]
- Fares H, Goetsch L, Pringle JR. Identification of a developmentally regulated septin and involvement of the septins in spore formation in Saccharomyces cerevisiae. J Cell Biol. 1996;132:399–411. doi: 10.1083/jcb.132.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddy C, Trinci APJ. Mitosis, septation, branching and the duplication cycle in Aspergillus nidulans. J Gen Microbiol. 1976;97:169–184. doi: 10.1099/00221287-97-2-169. [DOI] [PubMed] [Google Scholar]
- Field C, Kellogg D. Septins: cytoskeletal polymers or signaling GTPases? Trends Cell Biol. 1999;9:387–394. doi: 10.1016/s0962-8924(99)01632-3. [DOI] [PubMed] [Google Scholar]
- Flescher EG, Madden K, Snyder M. Components required for cytokinesis are important for bud site selection in yeast. J Cell Biol. 1993;122:373–386. doi: 10.1083/jcb.122.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter, A.S., Pringle, J.R., and Lew, D.J. (2001). The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. (in press). [DOI] [PubMed]
- Guan K, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Halme A, Michelitch M, Mitchell EL, Chant J. Bud10p directs axial cell polarization in budding yeast and resembles a transmembrane receptor. Curr Biol. 1996;6:570–579. doi: 10.1016/s0960-9822(02)00543-2. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Harris SD, Hamer L, Sharpless KE, Hamer JE. The Aspergillus nidulans sepAgene encodes an FH 1/2 protein involved in cytokinesis and the maintenance of cellular polarity. EMBO J. 1997;16:3474–3483. doi: 10.1093/emboj/16.12.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SD, Morrell JL, Hamer JE. Identification and characterization of Aspergillus nidulansmutants defective in cytokinesis. Genetics. 1994;136:517–532. doi: 10.1093/genetics/136.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- James SW, Mirabito PM, Scacheri PC, Morris NR. The Aspergillus nidulans bimE(blocked in mitosis) gene encodes multiple cell-cycle functions involved in mitotic checkpoint control and mitosis. J Cell Sci. 1995;108:3485–3499. doi: 10.1242/jcs.108.11.3485. [DOI] [PubMed] [Google Scholar]
- Käfer E. Meiotic and mitotic recombination in Aspergillusand its chromosomal aberrations. Adv Genet. 1977;19:33–131. doi: 10.1016/s0065-2660(08)60245-x. [DOI] [PubMed] [Google Scholar]
- Kaminskyj SG, Hamer JE. hyp loci control cell pattern formation in vegetative mycelium of Aspergillus nidulans. Genetics. 1998;148:669–680. doi: 10.1093/genetics/148.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ. Cell-cycle checkpoints that ensure coordination between nuclear and cytoplasmic events in Saccharomyces cerevisiae. Curr Opin Genet Dev. 2000;10:47–53. doi: 10.1016/s0959-437x(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C, Pringle JR. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/s0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Theesfeld CL, McMillan JN, Weaver E, Pringle JR, Lew DJ. Septin-dependent assembly of a cell cycle-regulatory module in Sacharomyces cerevisiae. Mol Cell Biol. 2000;20:4049–4061. doi: 10.1128/mcb.20.11.4049-4061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KY, Wu J, Miller BL. StuA is required for cell pattern formation in Aspergillus. Genes Dev. 1992;6:1770–1782. doi: 10.1101/gad.6.9.1770. [DOI] [PubMed] [Google Scholar]
- Mino A, Tanaka K, Kamei T, Umikawa M, Fujiwara T, Takai Y. Shs1p: a novel member of septin that interacts with Spa2p, involved in polarized growth in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1998;251:732–736. doi: 10.1006/bbrc.1998.9541. [DOI] [PubMed] [Google Scholar]
- Momany M, Hamer JE. The Aspergillus nidulansseptin encoding gene. aspB is essential for growth. Fungal Genet Biol. 1997a;21:92–100. doi: 10.1006/fgbi.1997.0967. [DOI] [PubMed] [Google Scholar]
- Momany M, Hamer JE. Relationship of actin, microtubules, and crosswall synthesis during septation in Aspergillus nidulans. Cell Motil Cytoskeleton. 1997b;38:373–384. doi: 10.1002/(SICI)1097-0169(1997)38:4<373::AID-CM7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Momany M, Taylor I. Landmarks in the early duplication cycles of Aspergillus fumugatus and Aspergillus nidulans: polarity, germ tube emergence and septation. Microbiology. 2000;146:3279–3284. doi: 10.1099/00221287-146-12-3279. [DOI] [PubMed] [Google Scholar]
- Momany M, Zhao J, Lindsey R, Westfall PJ. Characterization of the Aspergillus nidulans septin (asp) gene family. Genetics. 2000;157:969–977. doi: 10.1093/genetics/157.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell J. A molecular genetic analysis of septation in Aspergillus nidulans. Ph.D. Thesis. West Lafayette, IN: Purdue University; 1997. [Google Scholar]
- Oakley BR, Osmani SA. In: Cell cycle analysis using the filamentous fungus Aspergillus nidulans. In The Cell Cycle: A Practical Approch. Fantes P, Brooks R, editors. Oxford, UK: Oxford University Press; 1993. pp. 127–142. [Google Scholar]
- Pringle JR, Bi E, Harkins HA, Zahner JE, DeVirgilio C, Chant J, Corrado K, Fares H. Establishment of cell polarity in yeast. Cold Spring Harbor Symp Quant Biol. 1995;60:729–744. doi: 10.1101/sqb.1995.060.01.079. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Preston RA, Adams AEM, Stearns T, Drubin DG, Haarer BK, Jones EW. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Roemer T, Madden K, Chang J, Snyder M. Selection of axial growth sites in yeast requires Axl2p, a novel plasma membrane glycoprotein. Genes Dev. 1996;10:777–793. doi: 10.1101/gad.10.7.777. [DOI] [PubMed] [Google Scholar]
- Rosenberger RF, Kessel M. Synchrony of nuclear replication in individual hyphae of Aspergillus nidulans. J Bacteriol. 1967;94:1464–1469. doi: 10.1128/jb.94.5.1464-1469.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SL, Herskowitz I. The BUD4 protein of yeast, required for axial budding, is localized to the mother/bud neck in a cell cycle-dependent manner. J Cell Biol. 1996;134:413–427. doi: 10.1083/jcb.134.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulewitz MJ, Inouye CJ, Thorner J. Hsl1 localizes to a septin ring and serves as an adaptor in regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:7123–7137. doi: 10.1128/mcb.19.10.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble WS. Septins: a highly conserved family of membrane-associated GTPases with functions in cell division and beyond. J Membr Biol. 1999;169:75–81. doi: 10.1007/s002329900519. [DOI] [PubMed] [Google Scholar]
- Wolkow TD, Harris SD, Hamer JE. Cytokinesis in Aspergillus nidulansis controlled by cell size, nuclear positioning and mitosis. J Cell Sci. 1996;109:2179–2188. doi: 10.1242/jcs.109.8.2179. [DOI] [PubMed] [Google Scholar]
- Yelton MM, Hamer JE, Timberlake WE. Transformation of Aspergillus nidulans by using a trpCplasmid. Proc Natl Acad Sci USA. 1984;81:1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]