Abstract
1,4‐Naphthoquinones are an important class of compounds present in a number of natural products. In this study, a new series of 1,4‐naphthoquinone derivatives were synthesized. All the synthesized compounds were tested for in vitro antimicrobial activity. In this present investigation, two Gram‐positive and five Gram‐negative bacterial strains and one pathogenic yeast strain were used to determine the antibacterial activity. Naphthoquinones tested for its antibacterial potencies, among seven of them displayed better antimicrobial activity against Staphylococcus aureus (S. aureus; 30–70 μg/mL). Some of the tested compounds showed moderate to low antimicrobial activity against Pseudomonas aeruginosa (P. aeruginosa) and Salmonella bongori (S. bongori; 70–150 μg/mL). In addition, most active compounds against S. aureus were evaluated for toxicity to human blood cells using a hemolysis assay. For better understanding, reactive oxygen species (ROS) generation, time‐kill kinetic study, and apoptosis, necrosis responses were investigated for three representative compounds.
Keywords: naphthoquinone derivatives, antimicrobial activity, hemolysis, reactive oxygen species, necrosis
1. Introduction
The World Health Organization listed bacterial infections as a foremost threat to global public health. Based on calculations, multidrug‐resistant bacterial pathogens are responsible for almost 25,000 deaths in Europe every year.1,2 The ESKAPE pathogens such as Enterococcus faecium (E. faecium), Staphylococcus aureus (S. aureus), Klebsiella pneumoniae (K. pneumoniae), Acinetobacter baumannii (A. baumannii), Pseudomonas aeruginosa (P. aeruginosa), and Enterobacter spp. are the most common microorganisms causing life‐threatening infections. Approximately 40 % of hospital‐acquired bacterial infections are induced by these pathogens, and most are resistant to commonly used antibiotics.3 A joint program for development of effective antimicrobial agents has been initiated by 18 European countries and Canada. Therefore, antibacterial agents capable of fighting infections and spread of antibiotic resistance are currently needed.4
Regarding the development of effective antimicrobial agents, naphthoquinones have been the focus of extensive research due to their varied functions and clinical applications.5 A number of natural products contain the naphthoquinone moiety as the core structure. One example is vitamin K, which plays a significant role in bone metabolism, vascular biology, and regulation of blood coagulation in humans.6 1,4‐Naphthoquinones consist of two ketone groups as vital chromophores that can donate or accept electrons (redox) in a number of biological systems. In recent studies, the inhibitory mechanism of 1,4‐naphthoquinone was shown to involve the production of active oxygen species (ROS) by redox cycling, alkylation, or intercalation in the DNA double helix of biomolecules.7,8 1,4‐Naphthoquinones and its related compounds are well‐established for irreversible complexation during ROS generation in proteins, which leads to loss of protein function.9 Due to these properties, 1,4‐naphthoquinone derivatives exhibit biological properties such as antimicrobial,10 antimalarial,11 antitubercular,12 anticancer,13 and trypanocidal14 activities.
Recently, antimicrobial activities of 1,4‐naphthoquinone derivatives have attracted much attention.15,16 Pingaew et al.17 reported the synthesis of novel 1,4‐naphthoquinone‐based sulfonamides and antimicrobial and anticancer potentials (compound I). Cardoso et al.18 performed the synthesis and antibacterial evaluation of sulfonamide‐based lapachone derivatives (compound II). Yıldırım et al.19 reported the synthesis of 2,3‐disubstituted‐aryl‐phenylthio‐1,4‐naphthoquinones as potential antimicrobial agents (compound III). Tandon et al.20,21 described the synthesis and biological evaluation of several new sulfanyl aminonaphthoquinones (compounds IV and V) that displayed important antibacterial activity. Novais et al.22 also reported 1,4‐naphthoquinone‐based sulfanyl derivatives, and their resistance toward Gram‐negative bacteria in biofilms was investigated (compound VI). However, the detailed toxicity and action mechanism for these reported naphthoquinones in humans are unknown to date (Figure 1).
Figure 1.
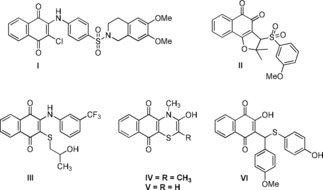
Important nitrogen and sulfur containing naphthoquinones possesses interesting antimicrobial properties.
In earlier reports, we synthesized a panel of 1,4 naphthoquinone‐based derivatives and evaluated their anticancer and antibacterial properties.23, 24, 25, 26 Therefore, in the present study, the synthesis and biological evaluation of a new library of arylamino‐1,4‐naphthoquinone derivatives grafted with S‐cyclohexane and aryl/aliphatic amide moieties as key counterparts was performed. The synthesized compounds (3–5 aa) were examined for in vitro antimicrobial property against a set of Gram‐positive and Gram‐negative bacteria. The toxicity of the selected compounds to human erythrocytes was tested using a hemolysis assay. In addition, the potential antibacterial activities of naphthoquinones using ROS generation, time‐kill kinetic study, apoptosis, and necrosis responses were determined.
2. Results and Discussion
2.1. Chemistry
A new series of 1,4‐naphthoquinone‐based compounds were synthesized, and the synthetic routes for preparation of compounds 3–5 w and 5 x–5 aa are presented in Schemes 1 and 2. The parent molecule 3 was synthesized via the nucleophilic substitution reaction between 2,3‐dichloro‐1,4‐naphthoquinone 1 and 4‐aminophenyl sulfone 2 using our previously reported method.27 Compound 4 was prepared by nucleophilic substitution of an equal molar concentration of compound 3 and cyclohexanethiol in dry acetone. The same equivalent of trimethylamine was used as a base catalyst to move the reaction forward. Under the 2‐h reflux condition, the reaction afforded compound 4 with a 97 % yield. This reaction was also assessed in water and other polar organic solvents such as acetonitrile, methanol, ethanol, and dimethylformamide; however, the reaction was unsuccessful in terms of product yield and formation of close byproducts. From compound 4, the final desired compounds 5 a–w were synthesized; compound 4 was acylated with various lengths of substituted acid chlorides in dry acetone for 30 min. Under the reflux condition, the reaction generated the targeted molecules in moderate to good product yield (65 %–98 %; Scheme 1). Compounds 5 x–y in Scheme 2 were synthesized using our previously reported method.23 Compounds 5 z–5 aa were synthesized via approaches similar to those used in Scheme 1. The products obtained had a moderate to good percentage yield. A decrease in product yield (5 i and 5 l; 67 % and 65 %, respectively) was observed due to the presence of bulky electron donating or electronegative functional groups at the fourth position of the phenyl ring in the molecule.
Scheme 1.
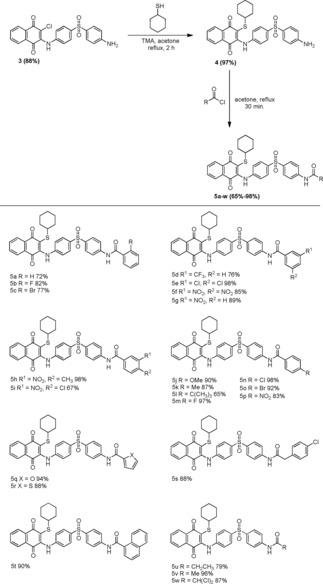
Synthesis and substrate scope of 2‐((4‐((4‐aminophenyl)sulfonyl)phenyl)amino)‐3‐(cyclohexylthio)naphthalene‐1,4‐diones (3–5 w).
Scheme 2.
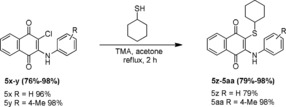
Synthesis of 2‐(cyclohexylthio)‐3‐(phenylamino)naphthalene‐1,4‐diones (5 x–5 aa).
2.2. Biology
2.2.1. In Vitro Antibacterial Activity
Many of the 1,4‐naphthoquinones have been well‐documented for synthetic utility and biologicals properties. However, specific antibacterial targets of arylamino‐1,4‐naphthoquinones remain to be evaluated in a biological study.28, 29, 30
The antibacterial activity of 29 different naphthoquinone derivatives was screened against clinically important bacteria (two strains of Gram‐positive and five strains of Gram‐negative bacteria and one strain of pathogenic yeast) using caspofungin, amphotericin B, chloramphenicol and streptomycin as standard drugs (Table 1). MIC values were calculated using the broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI). The majority of the tested naphthoquinones showed low to good antibacterial activity ranging from 30–300 μg/mL.
Table 1.
In vitro antibacterial activity of 1,4‐naphthoquinone derivatives (3–5 aa) expressed as minimal inhibitory concentration.
| MIC (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compd. | S. aureus | E. coli | P. aeruginosa | S. bongori | K. pneumoniae | E faecalis | E. cloacae | C. albicans |
| 3 | 150±0.23 | >300 | NA | NA | NA | >300 | >300 | >300 |
| 4 | >300 | >300 | 150±0.12 | 300±0.89 | 150±0.81 | 150±0.13 | 150±0.27 | >300 |
| 5 a | 150±0.19 | >300 | NA | NA | >300 | >300 | NA | NA |
| 5 b | 70±0.90 | >300 | >300 | 300±0.71 | >300 | >300 | 300±0.19 | >300 |
| 5 c | 70±0.71 | >300 | >300 | 150±0.09 | 150±0.11 | 150±0.84 | >300 | >300 |
| 5 d | 150±1.10 | >300 | 150±0.21 | 150±0.80 | 150±0.08 | >300 | >300 | >300 |
| 5 e | 150±1.01 | >300 | 300±0.99 | 70±0.10 | 300±0.41 | >300 | >300 | >300 |
| 5 f | 70±0.93 | >300 | >300 | NA | >300 | >300 | >300 | >300 |
| 5 g | 150±0.19 | >300 | 150±1.10 | 150±0.91 | 150±0.14 | >300 | >300 | >300 |
| 5 h | NA | >300 | >300 | >300 | NA | >300 | >300 | NA |
| 5 i | 150±0.70 | 150±0.27 | 150±0.78 | 150±0.83 | 150±0.70 | >300 | 150±0.18 | >300 |
| 5 j | 70±1.20 | >300 | 70±0.81 | >300 | >300 | >300 | >300 | >300 |
| 5 k | NA | >300 | >300 | >300 | >300 | NA | >300 | >300 |
| 5 l | >300 | >300 | >300 | >300 | >300 | >300 | >300 | >300 |
| 5 m | >300 | >300 | >300 | >300 | NA | >300 | >300 | >300 |
| 5 n | 300±0.92 | >300 | >300 | >300 | NA | >300 | >300 | NA |
| 5 o | >300 | >300 | >300 | >300 | >300 | >300 | >300 | >300 |
| 5 p | >300 | >300 | >300 | >300 | >300 | NA | >300 | >300 |
| 5 q | 30±0.74 | >300 | 300±0.28 | 300±0.90 | 300±0.69 | >300 | >300 | >300 |
| 5 r | 150±0.91 | >300 | 300±0.20 | 300±0.82 | 300±0.12 | >300 | >300 | >300 |
| 5 s | 150±0.11 | >300 | >300 | >300 | >300 | NA | >300 | >300 |
| 5 t | 150±0.81 | >300 | >300 | >300 | NA | >300 | >300 | NA |
| 5 u | 150±0.99 | >300 | 150±0.12 | 150±0.97 | 150±0.44 | 150±0.25 | >300 | >300 |
| 5 v | 70±1.51 | >300 | 70±0.80 | >300 | 150±0.10 | >300 | >300 | >300 |
| 5 w | 150±0.85 | >300 | 300±0.11 | 300±1.41 | >300 | >300 | 300±0.55 | >300 |
| 5 x | 300±0.89 | >300 | >300 | >300 | >300 | >300 | >300 | NA |
| 5 y | 70±1.01 | >300 | 300±1.60 | 300±1.30 | 300±0.41 | >300 | >300 | >300 |
| 5 z | >300 | NA | >300 | >300 | NA | >300 | >300 | NA |
| 5 aa | >300 | >300 | NA | >300 | NA | >300 | >300 | >300 |
| Caspofungin | >300 | >300 | >300 | >300 | >300 | >300 | >300 | >300 |
| Amphotericin B | NA | NA | NA | NA | NA | NA | NA | 0.1±0.19 |
| Chloramphenicol | NA | NA | NA | NA | NA | NA | NA | 2±0.76 |
| Streptomycin | 7.8±0.15 | 1.9±1.10 | 7.8±0.71 | 10.2±0.92 | 7.8±0.70 | 7.8±0.91 | 3.2±0.40 | 1.5±0.19 |
Results expressed in μg/mL; Concentration range used: 0.001–2 mg/mL; NA – No Activity; entries in boldface highlight MIC values <70 μg/mL.
Among all the molecules (3–5 aa) studied for antibacterial potency, seven of naphthoquinones such as 5 b, 5 c, 5 f, 5 j, 5 q, 5 v, and 5 y showed the notable activity against S. aureus, with MIC values ranging from 30–70 μg/mL. Specifically, compound 5 q showed potential antibacterial activity against S. aureus, with an MIC of 30 μg/mL. Compounds 5 c, 5 d, 5 g, 5 i, 5 j, 5 u, and 5 v showed moderate antibacterial activity against P. aeruginosa, with MIC values ranging from 70–150 μg/mL. Similar activity (70–150 μg/mL) was observed for compounds 5 c, 5 d, 5 e, 5 g, 5 i, and 5 u against S. bongori. Compared with the parent molecules 3 and 4, the above‐mentioned derivatives exhibited better activity. However, the synthesized naphthoquinones not showed any significant activity against the other bacterial strains such as E. coli, K. pneumoniae, E. faecalis, and E. cloacae, the yeast C. albicans.
Substitution of diaminobenzenesulfone and cyclohexanethiol moieties into compound 1 at the C‐2 and C‐3 positions resulted in improved inhibitory activity. Introduction of an amide group into compound 4 showed a good range of antibacterial activity, although it was mainly contingent on both the structural arrangement of the molecule and the bacterial strain used in the experiment.31
Based on the results obtained, the structure‐activity relationships (SARs) of the synthesized compounds (3–5 aa) were investigated. In summary, S‐cyclohexane, sulfonated arylamine moieties were found to be important for improved antibacterial activity. Aryl amide group on the sulfone nucleus showed encouraging antibacterial activity. Replacement of aryl amide with aliphatic amide moiety showed the weaker antibacterial activity. Electronegative groups (−F and −Br), and electron withdrawing group (NO2) at position 2 and 3 of aryl amide moiety were displayed promising activity. Similarly, furan‐2‐carboxamide moiety also exhibited significant antibacterial activity. Nevertheless, addition at position 4 on aryl amide did not show any antibacterial activity. Hence, the present naphthoquinones established to be exceptional patterns for further improvement as antibacterial candidates.
2.3. Hemolytic Activity in Human Erythrocytes
Toxicity is an important factor when a drug has potential as an antimicrobial therapeutic agent. To verify the toxicity to human erythrocytes, the most active compounds, 5 b, 5 c, 5 f, 5 j, 5 q, 5 v, and 5 y, were evaluated for hemolytic activity; the results are presented in Figure 2. The corresponding MICs were used for all the molecules in this study, except for compound 5 q, in which a 2.33‐fold higher concentration was used. The results showed that all the compounds induced hemolysis in less than 3.4 % of erythrocytes. In addition, vitamin K3 menadione (2‐methyl‐1,4‐naphthoquinone), a well‐known naphthoquinone‐based antimicrobial drug, showed an MIC of 3 μg/mL and induced hemolysis in 3.5 % of erythrocytes (5 μg/mL).32,33 In the present study, all compounds at high dosage (70 μg/mL) damaged approximately only 1–3.4 % of human erythrocytes. The toxicity results indicate that the present compounds are less toxic to human erythrocytes.
Figure 2.
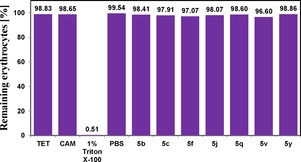
Hemolytic activity of compounds 5 b, 5 c, 5 f, 5 j, 5 q, 5 v, and 5 y. Tetracycline (TET) and chloramphenicol (CAM) were used as standard controls. Triton X‐100 (1 %) and phosphate buffer solution (PBS) were used as positive and negative controls, respectively. The concentration of compound 5 q used 2.33 %×MIC value. The other compounds and antibiotics were tested in their respective MICs.
2.4. Detection of Naphthoquinone‐Generated ROS
Improved ROS level is an important factor for the antibacterial activity of 1,4‐naphthoquinones.34 Naphthoquinones are the redox‐active molecules that induce ROS after diffusion of the bacterial cell membrane. Thus, three highly active representative naphthoquinones (5 b, 5 j, and 5 q) with their respective MIS values were selected for additional investigation of antibacterial activity against S. aureus. The ROS generation induced by naphthoquinone‐treated cells was determined using 2′,7′‐dichlorofluorescein‐diacetate (DCFH‐DA), a non‐fluorescent intracellular ROS probe. During quantification of ROS generation in cells, DCFH‐DA oxidized into highly fluorescent 2′,7′‐dichlorodihydrofluorescein (DCF) by the action of ROS.35 When S. aureus was treated with 5 b, 5 j, or 5 q, the fluorescent DCF dye was changed to DCF+ and showed good fluorescence intensity due to the compound‐induced intracellular ROS formation (Figure 3). A population of DCF+ cells showed higher fluorescence intensity for molecules 5 b and 5 j than 5 q. The compounds 5 b and 5 j exhibited similar fluorescence intensity to the standard antibacterial drug streptomycin. This result indicates that the antibacterial activity of 5 b, 5 j, and 5 q proceeded via oxidative stress of ROS. In addition, the redox poteantial of these compounds were confirmed by cyclic voltammetry study (see Figures S3–S5).
Figure 3.
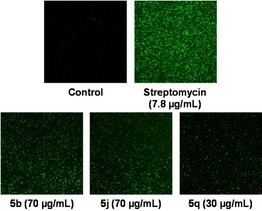
ROS detection of compounds 5 b, 5 j, and 5 q against S. aureus.
2.5. Bactericidal Time‐Kill Kinetic Study
The bactericidal activity of the most active naphthoquinones, 5 b, 5 j, and 5 q, was also investigated using time‐kill assays against S. aureus at the respective MICs (70 μg/mL, 70 μg/mL, and 30 μg/mL). The initial S. aureus count was 106.1 CFU/mL, and the survival of bacteria started to decrease at 4‐h and dropped to 100 CFU/mL after 24‐h. In contrast, compound 5 q displayed superior bactericidal kinetics compared with molecules 5 b and 5 j. The results indicate that all three compounds have time‐dependent bactericidal activity (Figure 4).
Figure 4.
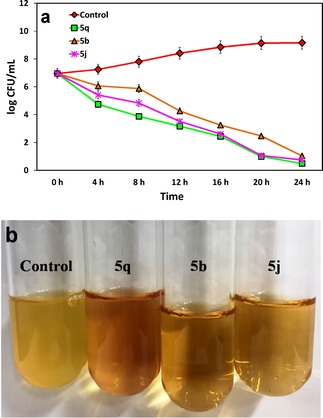
a) Time‐based bactericidal activity of compounds 5 b, 5 j, and 5 q to S. aureus. All the experiments carried out in triplicates and are given the mean ±S.D of CFU/mL. b) Images display S. aureus after 24‐h treatment with compounds 5 b, 5 j, and 5 q.
2.6. Determination of Apoptosis and Necrosis Based on Annexin V‐FITC/PI Assay
Apoptosis is a process of programmed cell death and can be inudced by certain ROS molecules. To investigate the apoptotic mechanism of compounds 5 b, 5 j, and 5 q, Annexin V‐FITC/PI assay was performed. The bacterial cells (S. aureus) were treated with the corresponding MIC of compounds 5 b, 5 j, and 5 q for 24‐h and finally stained with Annexin‐V and PI. The total population of apoptotic cells was measured using flow cytometry (Figure 5). Treatment of bacterial cells with 5 b, 5 j, and 5 q at 70, 70, and 90 μg/mL, respectively, resulted in 84.9, 44.8, and 54.9 % cell death in late apoptosis compared with 3.23 % in control cells. In addition, 5 j resulted in 48.9 % of necrosis cell death whereas, compound 5 q displayed 37.2 % of early apoptosis cell death. Consequently, the apoptotic effect of the compounds follows the order 5 j>5 b>5 q. These results indicate that the three naphthoquinone derivatives induce late apoptosis in S. aureus at fixed concentrations.
Figure 5.
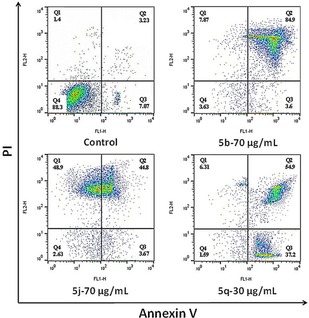
Apoptosis and necrosis determination was carried out by Annexin V‐FITC/PI double staining.
2.7. In Silico Molecular Docking Study
The mechanism binding naphthoquinones with S. aureus was evaluated using a molecular docking approach. Three major compounds, 5 b, 5 j, and 5 q, were used for the study with protein 5XEX (crystal structure of S. aureus PNPase catalytic domain). In silico molecular docking results for the compounds 5 b, 5 j, and 5 q showed molecular interactions with the biologically active site of 5XEX with docking scores of −2.41 kcal/mol, −3.13 kcal/mol, and −3.34 kcal/mol, respectively.
The predicted interactions of compound 5 b show amide NH, two oxygen of the sulfone group and one oxygen in quinone moiety established hydrogen bonds with amino acid residues of LYS A : 251, ARG A : 368, LYS A : 463 and ASP A : 477. In addition, it is also noted, the presence of hydrophobic interactions between the aromatic moieties of the molecule and the non‐polar amino acid residues such as SER A : 403, SER A : 406, ASN A : 404, GLY A : 405, HIS A : 372, ARG A : 84, ARG A : 87, ARG A : 88, ARG A : 368, respectively (Figure 6).
Figure 6.
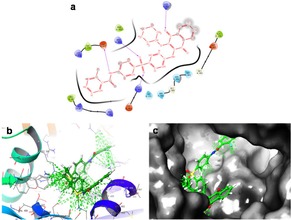
a) 2D ligand interaction of 5 b with the amino acid active site of 5XEX. b) 3D docking of 5 b (S=−2.41 kcal/mol) in the active site of 5XEX. c) Docking packing illustration of 5 b with their suitable binding pockets of 5XEX.
In compound 5 j two oxygen of the sulfone group and one oxygen in quinone moiety forms hydrogen bonds with ARG A : 368, LYS A : 463, LYS A : 251. Further, hydrophobic interactions were also presented between the aryl ketone and non‐polar amino acid residues. Similarly, compound 5 q showed three hydrogen bonding interactions with amide NH, amide oxygen and quinone oxygen. Moreover, π‐π stacking between sulfone phenyl ring and naphthoquinone group with LYS A : 479. Hydrophobic interactions were also observed in aromatic moieties of the molecule (Figures S1–S2).
Based on the results obtained from the docking study, the experimental activity is comparable. The compound 5 q showed strong glide score of −3.34 kcal/mol and better MIC of 30 μg/mL. Besides compounds, 5 b and 5 j showed docking scores of −2.41 kcal/mol, −3.13 kcal/mol with the MIC of 70 μg/mL respectively. Therefore, the order of antibacterial activity following this direction: 5 q>5 j>5 b.
Hence, these docking results indicate present groups of naphthoquinone molecules are active candidates for further clinical development.
3. Conclusions
In conclusion, the present investigation effectively demonstrated the synthesis of desired naphthoquinone derivatives via Michael‐like addition, nucleophilic substitution, and acylation reactions. The in vitro antimicrobial evaluation indicated that insertion of arylamine, cyclohexanethiol, and amide moieties into the 1,4‐naphthoquinone core could be a beneficial approach to enhance the antimicrobial activity. In contrast, at least seven naphthoquinone derivatives showed distinguished antibacterial activity against S. aureus at the collective antibacterial level. In addition, three active compounds against S. aureus were studied for their antibacterial mechanism via ROS generation, time‐kill kinetic assay, apoptosis and necrosis responses. Studied compounds were toxic towards bacteria and non‐toxic to human erythrocytes. Thus, the results from this study may aid in designing of clinically effective naphthoquinone‐based antimicrobial agents for further development.
Experimental Section
Chemistry
Melting points (°C) of all the naphthoquinone derivatives were checked in an open capillary tube using a digital auto melting point apparatus (Stuart, SMP10, Staffordshire, ST15 OSA, UK). All chemicals and solvents were purchased from Sigma‐Aldrich, Alfa Aesar, Korea and used directly without any further purification. Progress of the reactions and purity of the products were checked by thin layer chromatography on a TLC silica gel 60 F254 using the eluting solvent ethyl acetate : hexane (1 : 1). All synthesized compounds were preliminarily characterized by Fourier‐transform infrared spectroscopy analysis (Perkin‐Elmer instrument with a resolution of 0.4 cm−1) using the KBr pellet method. 1H NMR spectroscopy was performed in DMSO‐d6 (400 MHz, Jeol, JNM‐ECZ400s) and 13C NMR spectroscopy was performed in DMSO‐d6 (100 MHz, Jeol, JNM‐ECZ400s) installed at the Center for University‐Wide Research Facilities (CURF) at Chonbuk National University (CBNU). The coupling constants (J value) are reported in Hz. Elemental analysis was carried out in a Flash 2000 Elemental Analyzer (Thermo Fisher Scientific Inc.).
Synthesis of 2‐((4‐((4‐aminophenyl)sulfonyl)phenyl)amino)‐3‐chloronaphthalene‐1,4‐dione (3)
Compound 3 was synthesized using a previously reported method27 with minor modifications. In detail, a mixture of 2,3‐dichloro‐1,4‐naphthoquinone 1 (2.270 g, 10 mmol) and 4‐aminophenyl sulfone 2 (2.048 g, 10 mmol) was added to double distilled deionized water (900 mL) and the mixture was refluxed for 2 h. The reaction mixture was cooled to room temperature. Deep red precipitate that formed was isolated by vacuum filtration, washed with hot water (500 mL) and dried at 50 °C in a hot oven. The obtained solid was purified by column chromatography using a column silica gel 100–200 mesh (ethyl acetate : hexane, 1 : 2) to give compound 3 as deep red crystals (88 %); mp: 239–240 °C; IR (KBr): =552, 697, 1105, 1300, 1560, 1678, 3242, 3364, 3451. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=6.14 (s, 2H), 6.62 (d, J=8.7 Hz, 2H), 7.19 (d, J=8.6 Hz, 2H), 7.54 (d, J=8.7 Hz, 2H), 7.72 (d, J=8.6 Hz, 2H), 7.81 (dt, J=13.6 Hz & J=1.2 Hz, 1H), 7.87 (dt, J=7.4 Hz & J=0.8 Hz, 1H), 8.03 (t, J=5.9 Hz, 2H), 9.52 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=112.9, 119.0, 122.2, 125.9, 126.1, 126.6, 126.7, 129.2, 130.4, 131.6, 133.5, 134.6, 137.0, 142.5, 143.1, 153.4, 176.9, 179.8 ppm. Anal. calcd for C22H15ClN2O4S (438): C, 60.21; H, 3.45; N, 6.38; S, 7.30. Found: C, 60.30; H, 3.36; N, 6.64; S, 7.31; Beilstein test: Cl positive.36
Synthesis of 2‐((4‐((4‐aminophenyl)sulfonyl)phenyl)amino)‐3‐(cyclohexylthio)naphthalene‐1,4‐dione (4)
Compound 3 (2.19 g, 5 mmol) was mixed in a dry acetone (350 mL), and cyclohexanethiol (0.611 mL, 5 mmol) was gradually added to the mixture at room temperature. Trimethylamine (1 equiv. 0.469 mL) was added and the mixture was refluxed for 2 h. The crude mixture was poured into crushed ice and the solid that formed was filtered, washed with hot water (500 mL), dried in a hot oven at 50 °C and purified by silica column chromatography (100–200 mesh, eluting solvents, ethyl acetate : hexane, 1 : 2) to give compound 4, a maroon solid (97 %); mp: 201–202 °C; IR (KBr): =561, 693, 715, 1052, 1113, 1215, 1287, 1340, 1543, 1510, 1667, 3329, 3349, 3459. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.01 (s, 5H), 1.40–1.49 (m, 5H), 2.89 (s, 1H), 6.13 (s, 2H), 6.60 (d, J=8.64 Hz, 2H), 7.11 (d, J=8.48 Hz, 2H), 7.50 (d, J=8.48 Hz, 2H), 7.69 (d, J=8.60 Hz, 2H), 7.78 (t, J=7.40 Hz, 1H), 7.83 (t, J=7.32 Hz, 1H), 7.99 (d, J=7.28 Hz, 2H), 9.36 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.4, 32.6, 44.6, 112.9, 121.0, 121.3, 126.1, 126.3, 126.6, 129.0, 130.8, 132.5, 133.3, 134.3, 135.9, 143.4, 144.8, 153.3, 179.6, 180.8 ppm. Anal. calcd for C28H26N2O4S2 (518): C, 64.84; H, 5.05; N, 5.40; S, 12.36 Found: C, 65.22; H, 5.40; N, 5.73; S, 12.70.
General Procedure for the Preparation of Compounds 5 a–5 w
An equal stoichiometric ratio of compound 4 and various types of acid chlorides were mixed in moisture dried acetone and the reaction mixture was refluxed for 30 min. The obtained crude mixture was poured into crushed ice and the obtained solid was filtered through a vacuum. The solid was dried at 50 °C and purified by column chromatography (eluting solvent, ethyl acetate : hexane, 1 : 2) to obtain pure samples of 5 a–5 w.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)benzamide (5 a)
Violet solid (72 %); mp: 156–157 °C; IR (KBr): =590, 720, 1090, 1122, 1235, 1350, 1523, 1667, 3319, 3451. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.01 (s, 5H), 1.39–1.48 (m, 5H), 2.92 (s, 1H), 7.15 (d, J=8.68 Hz, 2H), 7.53 (t, J=7.68 Hz, 2H), 7.61 (t, J=6.96 Hz, 1H), 7.78–7.81 (m, 3H), 7.84 (d, J=7.04 Hz, 1H), 7.89 (d, J=8.76 Hz, 2H), 7.94 (d, J=7.48 Hz, 2H), 8.00 (t, J=8.52 Hz, 4H), 9.36 (s, 1H), 10.60 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=24.9, 25.2, 32.6, 44.6, 120.0, 120.8, 122.4, 126.0, 126.2, 127.3, 127.7, 128.0, 128.3, 130.8, 131.9, 132.5, 133.3, 133.8, 134.2, 134.3, 135.9, 143.4, 144.2, 144.5, 166.0, 179.5, 180.8 ppm. Anal. calcd for C35H30N2O5S2 (622): C, 67.50; H, 4.86; N, 4.50; S, 10.30 Found: C, 67.12; H, 5.10; N, 4.62; S, 10.65.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐2‐fluorobenzamide (5 b)
Violet solid (82 %); mp: 209–210 °C; IR (KBr): =570, 602, 1035, 1096, 1259, 1556, 1630, 3319, 3423. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.01 (s, 5H), 1.39–1.49 (m, 5H), 2.90 (s, 1H), 7.15 (d, J=8.72 Hz, 2H), 7.34 (q, J=10.56 Hz, 2H), 7.57–7.62 (m, 1H), 7.66 (t, J=8.84 Hz, 1H), 7.77–7.81 (m, 3H), 7.84 (d, J=6.20 Hz, 1H), 7.91 (d, J=3.52 Hz, 4H), 8.00 (d, J=7.76 Hz, 2H), 9.40 (s, 1H), 10.83 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 32.6, 44.6, 116.1, 116.3, 119.6, 120.9, 122.4, 124.6, 124.6, 126.1, 126.3, 127.3, 128.2, 129.8, 130.9, 132.5, 133.4, 133.7, 134.3, 136.2, 143.0, 144.2, 144.4, 158.8 (d, J=247.95 Hz), 163.3, 179.6, 180.9 ppm. 19F NMR (600 MHz, DMSO): δ=−114.4 ppm. Anal. calcd for C35H29FN2O5S2 (640): C, 65.61; H, 4.56; N, 4.37; S, 10.01 Found: C, 65.32; H, 4.70; N, 4.62; S, 10.35.
2‐bromo‐N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)benzamide (5 c)
Maroon solid (77 %); mp: 244–245 °C; IR (KBr): =540, 653, 758, 987, 1024, 1096, 1213, 1358, 1478, 1548, 1661, 3329, 3438. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.39–1.48 (m, 5H), 2.88 (s, 1H), 7.15 (d, J=8.68 Hz, 2H), 7.43 (t, J=7.56 Hz, 1H), 7.50 (t, J=7.36 Hz, 1H), 7.56 (d, J=7.56 Hz, 1H), 7.72 (d, J=7.88 Hz, 1H), 7.78–7.80 (m, 3H), 7.84 (d, J=6.88 Hz, 1H), 7.91 (s, 4H), 8.00 (d, J=7.40 Hz, 2H), 9.41 (s, 1H), 10.91 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 32.6, 44.6, 118.8, 119.5, 120.9, 122.3, 126.1, 126.2, 127.3, 127.7, 128.2, 128.8, 130.8, 131.4, 132.5, 132.7, 133.3, 133.7, 134.3, 136.2, 138.4, 143.0, 144.1, 144.3, 166.2, 179.5, 180.8 ppm. Anal. calcd for C35H29BrN2O5S2 (700): C, 59.91; H, 4.17; N, 3.99; S, 9.14 Found: C, 60.21; H, 4.54; N, 4.32; S, 8.82.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐3‐(trifluoromethyl)benzamide (5 d)
Maroon solid (76 %); mp: 156–157 °C; IR (KBr): =615, 720, 1082, 1115, 1258, 1312, 1658, 3279, 3431. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.01 (s, 5H), 1.40–1.48 (m, 5H), 2.92 (s, 1H), 7.16 (d, J=8.76 Hz, 2H), 7.79–7.85 (m, 5H), 7.93 (t, J=8.84 Hz, 2H), 7.97–7.99 (m, 2H), 8.00–8.02 (m, 3H), 8.24–8.28 (m, 2H), 9.401 (s, 1H), 10.82 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=24.9, 25.3, 32.6, 44.6, 120.3, 120.8, 122.5, 124.3 (d, J=3.33 Hz), 125.2, 126.1, 126.2, 127.4, 128.0, 128.8 (q, J=31.91 Hz), 129.7, 130.8, 131.9, 132.5, 133.4, 133.7, 134.3, 135.1, 136.3, 143.1, 144.3, 144.5, 164.5, 179.5, 180.8 ppm. 19F NMR (600 MHz, DMSO): δ=−61.0 ppm. Anal. calcd for C36H29F3N2O5S2 (690): C, 62.60; H, 4.23; N, 4.06; S, 9.28 Found: C, 62.81; H, 4.51; N, 3.80; S, 9.13.
3,5‐dichloro‐N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)benzamide (5 e)
Maroon solid (98 %); mp: 265–266 °C; IR (KBr): =696, 1038, 1209, 1251, 1663, 3179, 3429. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.39–1.47 (m, 5H), 2.92 (s, 1H), 7.15 (d, J=8.80 Hz, 2H), 7.78–7.80 (m, 3H), 7.83 (dd, J=7.86 Hz & J=1.52 Hz, 1H), 7.86 (t, J=1.84 Hz, 1H), 7.91 (d, J=9.04 Hz, 2H), 7.96 (t, J=2.24 Hz, 3H), 7.99 (dd, J=7.34 Hz & J=1.48 Hz, 3H), 9.39 (s, 1H), 10.74 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 32.6, 44.6, 120.2, 120.8, 122.5, 126.1, 126.2, 126.5, 126.6, 127.4, 128.0, 130.8, 132.5, 133.4, 133.6, 134.6, 136.4, 137.4, 142.9, 144.3, 144.4, 163.1, 179.5, 180.8 ppm. Anal. calcd for C35H28Cl2N2O5S2 (691): C, 60.78; H, 4.08; N, 4.05; S, 9.27 Found: C, 60.42; H, 4.45; N, 4.02; S, 9.20. Beilstein test: Cl positive.36
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐3,5‐dinitrobenzamide (5 f)
Violet solid (85 %); mp: 278–279 °C; IR (KBr): =609, 721, 887, 982, 1009, 1118, 1251, 1358, 1581, 1660, 3125, 3449. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.01 (s, 5H), 1.39–1.47 (m, 5H), 2.91 (s, 1H), 7.16 (d, J=8.68 Hz, 2H), 7.77–7.83 (m, 4H), 7.96 (t, J=4.84 Hz, 3H), 8.00 (t, J=8.88 Hz, 3H), 9.00 (t, J=1.88 Hz, 1H), 9.14 (d, J=1.92 Hz, 2H), 9.39 (s, 1H), 11.15 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 32.6, 44.6, 120.6, 120.8, 121.4, 122.6, 126.1, 126.2, 127.4, 128.1, 130.8, 132.5, 133.4, 133.5, 134.3, 136.8, 136.9, 142.6, 144.3, 144.4, 148.0, 161.8, 179.5, 180.8 ppm. Anal. calcd for C35H28N4O9S2 (712): C, 58.98; H, 3.96; N, 7.86; S, 9.00 Found: C, 58.61; H, 4.25; N, 7.50; S, 9.27.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐3‐nitrobenzamide (5 g)
Blue violet solid (89 %); mp: 165–166 °C; IR (KBr): =598, 612, 890, 1102, 1237, 1599, 1665, 3115, 3441. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.01 (s, 5H), 1.39–1.47 (m, 5H), 2.91 (s, 1H), 7.15 (d, J=8.68 Hz, 2H), 7.79–7.83 (m, 4H), 7.85 (d, J=7.80 Hz, 1H), 7.93 (d, J=8.80 Hz, 2H), 8.00 (t, J=9.12 Hz, 4H), 8.38 (d, J=7.84 Hz, 1H), 8.44 (d, J=8.28 Hz, 1H), 8.77 (s, 1H), 9.40 (s, 1H), 10.92 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=24.9, 25.3, 32.6, 44.6, 120.3, 120.8, 122.5, 126.1, 126.2, 126.5, 127.4, 128.1, 130.2, 130.8, 132.5, 133.4, 133.6, 134.3, 134.3, 135.6, 136.4, 143.0, 144.3, 144.4, 147.6, 163.8, 179.5, 180.8 ppm. Anal. calcd for C35H29N3O7S2 (667): C, 62.95; H, 4.38; N, 6.29; S, 9.60 Found: C, 63.23; H, 4.20; N, 5.90; S, 9.62.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐4‐methyl‐3‐nitrobenzamide (5 h)
Maroon solid (98 %); mp: 268–269 °C; IR (KBr): =912, 1189, 1217, 1348, 1492, 1572, 1662, 3105, 3402. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.01 (s, 5H), 1.39–1.48 (m, 5H), 2.59 (s, 3H), 2.92 (s, 1H), 7.15 (d, J=8.64 Hz, 2H), 7.68 (d, J=8.08 Hz, 1H), 7.79 (d, J=8.64 Hz, 3H), 7.83 (d, J=7.16 Hz, 1H), 7.91 (d, J=8.80 Hz, 2H), 7.99 (s, 3H), 8.01 (d, J=3.28 Hz, 1H), 8.19 (d, J=8.12 Hz, 1H), 8.55 (s, 1H), 9.36 (s, 1H), 10.78 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=19.5, 24.9, 25.3, 32.6, 44.6, 120.2, 120.8, 122.5, 123.6, 126.0, 126.2, 127.3, 128.0, 130.8, 132.1, 132.5, 133.0, 133.1, 133.3, 133.7, 134.3, 136.3, 136.6, 143.0, 144.3, 144.5, 148.7, 163.6, 179.5, 180.8 ppm. Anal. calcd for C36H31N3O7S2 (681): C, 63.42; H, 4.58; N, 6.16; S, 9.41 Found: C, 63.11; H, 4.67; N, 6.15; S, 9.37.
4‐chloro‐N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐3‐nitrobenzamide (5 i)
Light maroon solid (67 %); mp: 169–170 °C; IR (KBr): =698, 751, 890, 1101, 1216, 1309, 1412, 1508, 1598, 1660, 3009, 3389. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.01 (s, 5H), 1.39–1.49 (m, 5H), 2.91 (s, 1H), 7.15 (d, J=7.68 Hz, 2H), 7.78–7.85 (m, 3H), 7.93 (d, J=8.00 Hz, 2H), 7.96–8.00 (m, 6H), 8.24 (d, J=8.56 Hz, 1H), 8.62 (s, 1H), 9.39 (s, 1H), 10.88 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 32.6, 44.7, 120.3, 120.9, 122.5, 125.0, 126.1, 126.3, 127.4, 128.1, 128.5, 130.8, 132.0, 132.5, 132.9, 133.4, 133.6, 134.2, 134.3, 136.6, 142.8, 144.3, 144.4, 147.3, 163.0, 179.6, 180.9 ppm. Anal. calcd for C35H28ClN3O7S2 (701): C, 59.87; H, 4.02; N, 5.98; S, 9.13 Found: C, 59.80; H, 4.32; N, 6.02; S, 8.98. Beilstein test: Cl positive.36
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐4‐methoxybenzamide (5 j)
Blue violet solid (90 %); mp: 250–251 °C; IR (KBr): =718, 887, 925, 1108, 1251, 1392, 1582, 1668, 3142, 3392. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.38–1.47 (m, 5H), 2.37 (s, 3H), 2.91 (s, 1H), 7.15 (d, J=8.60 Hz, 2H), 7.33 (d, J=8.00 Hz, 2H), 7.76–7.82 (m, 4H), 7.87 (t, J=9.68 Hz, 4H), 8.00 (t, J=8.64 Hz, 4H), 8.39 (s, 1H), 10.52 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=21.0, 25.0, 25.3, 32.6, 44.6, 120.0, 120.9, 122.4, 126.1, 126.3, 127.3, 127.8, 128.0, 128.9, 130.8, 131.4, 132.5, 133.4, 133.8, 134.3, 135.8, 142.1. 143.6, 144.2, 144.5, 165.8, 179.6, 180.8 ppm. Anal. calcd for C36H32N2O6S2 (652): C, 66.24; H, 4.94; N, 4.29; S, 9.82 Found: C, 66.09; H, 4.89; N, 3.97; S, 9.80.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐4‐methylbenzamide (5 k)
Blue violet solid (87 %); mp: 256–257 °C; IR (KBr): =852, 918, 1119, 1282, 1352, 1480, 1501, 1648, 3109, 3402. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.38–1.47 (m, 5H), 2.91 (s, 1H), 3.83 (s, 3H), 7.06 (d, J=7.40 Hz, 2H), 7.15 (d, J=7.68 Hz, 2H), 7.76–7.84 (m, 4H), 7.88 (d, J=7.76 Hz, 2H), 7.95 (d, J=7.52 Hz, 2H), 7.98–8.01 (m, 4H), 9.39 (s, 1H), 10.45 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 32.6, 44.6, 55.4, 113.6, 119.9, 120.9, 122.3, 126.1, 126.2, 126.2, 127.3, 128.0, 129.8, 130.8, 132.5, 133.4, 133.9, 134.3, 135.6, 143.7, 144.2, 144.5, 162.2, 165.3, 179.5, 180.8 ppm. Anal. calcd for C36H32N2O5S2 (636): C, 67.90; H, 5.07; N, 4.40; S, 10.07 Found: C, 67.52; H, 4.98; N, 4.30; S, 10.22.
4‐(tert‐butyl)‐N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)benzamide (5 l)
Blue violet solid (65 %); mp: 163–164 °C; IR (KBr): =781, 889, 11029, 1351, 1668, 3325, 3402. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.29 (s, 9H), 1.38–1.47 (m, 5H), 2.91 (s, 1H), 7.15 (d, J=8.68 Hz, 2H), 7.54 (d, J=8.40 Hz, 2H), 7.78–7.84 (m, 4H), 7.87 (d, J=5.32 Hz, 2H), 7.89 (d, J=5.68 Hz, 2H), 7.98–8.02 (m, 4H), 9.39 (s, 1H), 10.54 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 30.8, 32.6, 34.6, 44.6, 119.9, 120.9, 122.4, 125.2, 126.1, 126.2, 127.3, 127.6, 128.0, 130.8, 131.6, 132.5, 133.3, 133.9, 134.3, 135.7, 143.6, 144.2, 144.5, 154.9, 165.9, 179.5, 180.8 ppm. Anal. calcd for C39H38N2O5S2 (678): C, 69.00; H, 5.64; N, 4.13; S, 9.45 Found: C, 69.08; H, 5.45; N, 4.39; S, 9.42.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐4‐fluorobenzamide (5 m)
Blue violet solid (97 %); mp: 242–243 °C; IR (KBr): =682, 714, 882, 1108, 1251, 1327, 1482, 1509, 1661, 3314, 3342. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.38–1.47 (m, 5H), 2.91 (s, 1H), 7.15 (d, J=8.76 Hz, 2H), 7.37 (t, J=8.88 Hz, 2H), 7.78–7.80 (m, 3H), 7.83 (dd, J=7.36 Hz & J=1.40 Hz, 1H), 7.90 (d, J=8.88 Hz, 2H), 7.98–8.04 (m, 6H), 9.39 (s, 1H), 10.63 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 32.6, 44.6, 115.3, 115.5, 120.1, 120.9, 122.4, 126.1, 126.2, 127.4, 128.0, 130.5, 130.6, 132.5, 133.4, 133.8, 134.3, 136.0, 143.4, 144.2, 144.5, 164.2 (d, J=248.53 Hz), 164.9, 179.5, 180.8 ppm. 19F NMR (600 MHz, DMSO): δ=−107.8 ppm. Anal. calcd for C35H29FN2O5S2 (640): C, 65.61; H, 4.56; N, 4.37; S, 10.01 Found: C, 65.25; H, 4.62; N, 4.30; S, 10.00.
4‐chloro‐N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)benzamide (5 n)
Blue violet solid (98 %); mp: 229–230 °C; IR (KBr): =664, 712, 882, 941, 1108, 1201, 1352, 1482, 1527, 1662, 3142, 3340. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.38–1.47 (m, 5H), 2.91 (s, 1H), 7.15 (d, J=8.52 Hz, 2H), 7.55 (d, J=8.56 Hz, 1H), 7.61 (d, J=8.56 Hz, 2H), 7.78–7.84 (m, 4H), 7.91 (t, J=3.68 Hz, 2H), 7.95 (d, J=6.76 Hz, 1H), 7.98 (d, J=1.84 Hz, 3H), 8.00 (d, J=1.84 Hz, 1H), 9.39 (s, 1H), 10.67 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.5, 25.9, 33.1, 45.2, 120.7, 121.4, 126.6, 126.8, 127.9, 128.6, 129.0, 129.2, 130.3, 131.6, 133.1, 133.5, 133.9, 134.3, 134.9, 136.6, 137.4, 143.8, 144.8, 145.0, 165.5, 180.1, 181.4 ppm. Anal. calcd for C35H29ClN2O5S2 (657): C, 63.96; H, 4.45; N, 4.26; S, 9.76 Found: C, 64.05; H, 4.32; N, 4.27; S, 9.91. Beilstein test: Cl positive.36
4‐bromo‐N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)benzamide (5 o)
Blue violet solid (92 %); mp: 241–242 °C; IR (KBr): =754, 821, 994, 1108, 1251, 1415, 1598, 1665, 3214, 3418. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.38–1.47 (m, 5H), 2.91 (s, 1H), 7.15 (d, J=8.64 Hz, 2H), 7.75 (d, J=8.56 Hz, 2H), 7.78–7.84 (m, 4H), 7.90 (d, J=9.92 Hz, 4H), 7.99 (d, J=8.08 Hz, 4H), 9.39 (s, 1H), 10.66 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 32.6, 44.6, 120.1, 120.8, 122.4, 125.8, 126.1, 126.2, 127.3, 128.0, 129.9, 130.8, 131.4, 132.5, 133.3, 133.4, 133.7, 134.3, 136.1, 143.3, 144.2, 144.5, 165.0, 179.5, 180.8 ppm. Anal. calcd for C35H29BrN2O5S2 (701): C, 59.91; H, 4.17; N, 3.99; S, 9.14 Found: C, 59.97; H, 4.32; N, 4.15; S, 8.99.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐4‐nitrobenzamide (5 p)
Blue violet solid (83 %); mp: 169–170 °C; IR (KBr): =754, 821, 994, 1108, 1251, 1415, 1598, 1665, 3214, 3418. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.38–1.47 (m, 5H), 2.91 (s, 1H), 7.15 (d, J=8.60 Hz, 2H), 7.76–7.83 (m, 4H), 7.92 (d, J=8.72 Hz, 2H), 7.99 (t, J=8.68 Hz, 4H), 8.16 (d, J=8.60 Hz, 2H), 8.36 (d, J=8.56 Hz, 2H), 9.39 (s, 1H), 10.91 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 32.6, 44.6, 120.3, 120.8, 122.5, 123.5, 126.1, 126.2, 127.4, 128.1, 129.3, 130.8, 132.5, 133.4, 133.6, 134.3, 136.5, 139.9, 143.0, 144.3, 144.4, 149.3, 164.4, 179.5, 180.8 ppm. Anal. calcd for C35H29N3O7S2 (667): C, 62.95; H, 4.38; N, 6.29; S, 9.60 Found: C, 62.89; H, 4.39; N, 6.48; S, 9.92.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)furan‐2‐carboxamide (5 q)
Blue violet solid (94 %); mp: 212–213 °C; IR (KBr): =653, 714, 833, 1011, 1254, 1385, 1522, 1662, 3145, 3421. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.38–1.48 (m, 5H), 2.90 (s, 1H), 6.71 (dd, J=3.48 Hz & J=1.76 Hz, 1H), 7.14 (d, J=8.76 Hz, 2H), 7.38 (d, J=3.56 Hz, 1H), 7.77–7.81 (m, 3H), 7.83 (dd, J=7.40 Hz & J=1.32 Hz, 1H), 7.87 (d, J=8.84 Hz, 2H), 7.95–8.00 (m, 5H), 9.36 (s, 1H), 10.53 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=24.9, 25.2, 32.5, 44.6, 112.2, 115.6, 120.0, 120.8, 122.4, 126.0, 126.2, 127.3, 127.9, 130.8, 132.5, 133.3, 133.7, 134.3, 136.0, 142.9, 144.2, 144.4, 146.1, 146.8, 156.3, 179.5, 180.8 ppm. Anal. calcd for C33H28N2O6S2 (612): C, 64.69; H, 4.61; N, 4.57; S, 10.47 Found: C, 64.38; H, 4.65; N, 4.58; S, 10.20.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)thiophene‐2‐carboxamide (5 r)
Blue violet solid (88 %); mp: 224–225 °C; IR (KBr): =915, 1105, 1248, 1355, 1508, 1650, 3140, 3411. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.38–1.46 (m, 5H), 2.90 (s, 1H), 7.15 (d, J=8.68 Hz, 2H), 7.23 (t, J=4.60 Hz, 1H), 7.78–7.84 (m, 4H), 7.89 (d, J=8.68 Hz, 3H), 7.95–8.00 (m, 4H), 8.05 (d, J=3.72 Hz, 1H), 9.39 (s, 1H), 10.56 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=24.9, 25.3, 32.6, 44.6, 120.0, 120.9, 122.4, 126.1, 126.2, 127.4, 128.1, 128.1, 129.9, 130.8, 132.5, 132.7, 133.4, 133.7, 134.3, 135.9, 139.1, 143.1, 144.2, 144.4, 160.2, 179.5, 180.8 ppm. Anal. calcd for C33H28N2O5S3 (628): C, 63.04; H, 4.49; N, 4.46; S, 15.30 Found: C, 63.01; H, 4.54; N, 4.80; S, 15.13.
2‐(4‐chlorophenyl)‐N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)acetamide (5 s)
Maroon solid (88 %); mp: 147–148 °C; IR (KBr): =911, 1189, 1345, 1508, 1612, 1660, 3142, 3400. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=0.99 (s, 5H), 1.37–1.44 (m, 5H), 2.88 (s, 1H), 3.68 (s, 2H), 7.13 (d, J=8.76 Hz, 2H), 7.34 (q, J=8.48 Hz, 4H), 7.74 (s, 1H), 7.77 (d, J=8.52 Hz, 3H), 7.81 (t, J=2.12 Hz, 1H), 7.83 (s, 2H), 7.85 (d, J=1.52 Hz, 1H), 7.98 (d, J=1.32 Hz, 1H), 8.00 (d, J=1.24 Hz, 1H), 9.35 (s, 1H), 10.56 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=24.9, 25.2, 32.5, 42.3, 44.6, 119.0, 120.8, 122.3, 126.0, 126.2, 127.2, 128.1, 128.1, 130.8, 130.9, 131.3, 132.5, 133.3, 133.8, 134.3, 134.3, 135.6, 143.2, 144.1, 144.4, 169.4, 179.5, 180.8 ppm. Anal. calcd for C36H31ClN2O5S2 (671): C, 64.42; H, 4.66; N, 4.17; S, 9.55 Found: C, 64.20; H, 4.61; N, 4.47; S, 9.60. Beilstein test: Cl positive.36
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)‐1‐naphthamide (5 t)
Maroon solid (90 %); mp: 242–243 °C; IR (KBr): =618, 722, 920, 1109, 1251, 1366, 1501, 1660, 3142, 3409. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.02 (s, 5H), 1.40–1.49 (m, 5H), 2.91 (s, 1H), 7.16 (d, J=8.56 Hz, 2H), 7.57–7.63 (m, 3H), 7.75–7.83 (m, 5H), 7.93 (d, J=8.60 Hz, 2H), 8.01 (t, J=8.72 Hz, 5H), 8.09 (d, J=8.24 Hz, 1H), 8.14 (dd, J=6.20 Hz & J=3.44 Hz, 1H), 9.41 (s, 1H), 10.99 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.0, 25.3, 32.6, 44.6, 119.7, 120.9, 122.3, 124.8, 124.9, 125.7, 126.1, 126.3, 126.4, 127.1, 127.3, 128.2, 128.3, 129.4, 130.5, 130.8, 132.5, 133.1, 133.4, 133.9, 133.9, 134.3, 136.0, 143.5, 144.2, 144.5, 167.7, 179.6, 180.9 ppm. Anal. calcd for C39H32N2O5S2 (672): C, 69.62; H, 4.79; N, 4.16; S, 9.53 Found: C, 69.50; H, 4.71; N, 4.18; S, 9.60.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)propionamide (5 u)
Blue violet solid (79 %); mp: 153–154 °C; IR (KBr): =930, 1108, 1251, 1420, 1588, 1662, 3112, 3425. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.00 (s, 5H), 1.06 (t, J=7.48 Hz, 3H), 1.39–1.47 (m, 5H), 2.34 (q, J=7.60 Hz, 2H), 2.90 (s, 1H), 7.13 (d, J=8.72 Hz, 2H), 7.76 (d, J=8.60 Hz, 3H), 7.80 (d, J=10.28 Hz, 4H), 7.83–7.85 (m, 1H), 7.99 (dd, J=7.50 Hz & J=1.0 Hz, 2H), 9.35 (s, 1H), 10.25 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=9.3, 24.9, 25.2, 29.5, 32.5, 44.6, 118.7, 120.8, 122.3, 126.0, 126.2, 127.2, 128.1, 130.8, 132.5, 133.3, 133.9, 134.3, 135.1, 143.5, 144.1, 144.5, 172.6, 179.5, 180.8 ppm. Anal. calcd for C31H30N2O5S2 (574): C, 64.79; H, 5.26; N, 4.87; S, 11.16 Found: C, 64.53; H, 5.23; N, 4.98; S, 11.51.
N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)acetamide (5 v)
Blue violet solid (96 %); mp: 159–160 °C; IR (KBr): =1129, 1358, 1471, 1556, 1658, 3142, 3408. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=0.99 (s, 5H), 1.39–1.46 (m, 5H), 2.06 (s, 3H), 2.89 (s, 1H), 7.14 (d, J=8.64 Hz, 2H), 7.76 (d, J=8.72 Hz, 5H), 7.82 (d, J=8.64 Hz, 3H), 7.99 (d, J=7.36 Hz, 2H), 9.38 (s, 1H), 10.34 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=24.6, 25.5, 25.9, 33.1, 45.2, 119.3, 121.4, 122.9, 126.6, 126.8, 127.8, 128.7, 131.4, 133.0, 133.9, 134.4, 134.9, 135.8, 144.0, 144.7, 145.0, 169.6, 180.1, 181.4 ppm. Anal. calcd for C30H28N2O5S2 (560): C, 64.26; H, 5.03; N, 5.00; S, 11.44 Found: C, 64.49; H, 5.01; N, 5.24; S, 11.80.
2,2‐dichloro‐N‐(4‐((4‐((3‐(cyclohexylthio)‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2‐yl)amino)phenyl)sulfonyl)phenyl)acetamide (5 w)
Maroon solid (87 %); mp: 154–155 °C; IR (KBr): =971, 1125, 1389, 1582, 1668, 3125, 3420. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=0.99 (s, 5H), 1.38–1.47 (m, 5H), 2.89 (s, 1H), 6.59 (s, 1H), 7.14 (d, J=8.72 Hz, 2H), 7.76–7.79 (m, 5H), 7.84 (d, J=7.28 Hz, 1H), 7.91 (d, J=8.80 Hz, 2H), 8.00 (dd, J=7.28 Hz & J=1.30 Hz, 2H), 9.37 (s, 1H), 11.02 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=24.9, 25.2, 32.5, 44.6, 67.0, 119.9, 120.8, 122.5, 126.0, 126.2, 127.3, 128.3, 130.8, 132.5, 133.3, 133.4, 134.3, 137.1, 141.7, 144.3, 144.3, 162.2, 179.5, 180.8 ppm. Anal. calcd for C30H26Cl2N2O5S2 (629): C, 57.23; H, 4.16; N, 4.45; S, 10.19 Found: C, 57.42; H, 4.53; N, 4.81; S, 10.15. Beilstein test: Cl positive.36
General Procedure for the Synthesis of Compounds 5 x–5 y
2,3‐Dichloro‐1,4‐naphthoquinone 1 (2.270 g, 10 mmol) and aryl amines 2 a–b (10 mmol) were added to double distilled water (800 mL) and the reaction mixture was refluxed for 2–3 h. The mixture was cooled to room temperature and the precipitates formed were isolated by vacuum filtration, washed with hot water (500 mL) and dried at 50 °C. The resulting solid product was purified by column chromatography using column silica gel 100–200 mesh (ethyl acetate : hexane 1 : 4) to give compounds 5 x–5 y.
2‐chloro‐3‐(phenylamino)naphthalene‐1,4‐dione (5 x)
Deep maroon solid (96 %); mp: 222–223 °C; IR (KBr): =718, 852, 1225, 1298, 1335, 1440, 1549, 1615, 1672, 3250, 3334. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=7.11‐7.14 (m, 3H), 7.31 (t, J=7.72 Hz, 2H), 7.79 (dt, J=7.56 Hz & J=1.04 Hz, 1H), 7.85 (dt, J=7.44 Hz & J=1.12 Hz, 1H), 8.02 (d, J=7.60 Hz, 2H), 9.29 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=114.2, 123.8, 124.2, 125.8, 126.2, 127.7, 130.0, 131.8, 132.9, 134.5, 138.6, 142.9, 176.4, 179.8 ppm. Anal. calcd for C16H10ClNO2 (283): C, 67.74; H, 3.55; N, 4.94 Found: C, 67.42; H, 3.51; N, 4.88; Beilstein test: Cl positive.36
2‐chloro‐3‐(p‐tolylamino)naphthalene‐1,4‐dione (5 y)
Light maroon solid (98 %); mp: 205–206 °C; IR (KBr): =718, 817, 1019, 1241, 1287, 1330, 1496, 1517, 1562, 1597, 1676, 3225. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=2.28 (s, 3H), 7.02 (d, J=8.2 Hz, 2H), 7.11 (t, J=8.2 Hz, 2H), 7.78 (dt, J=7.4 Hz & J=1.1 Hz, 1H), 7.86 (dt, J=7.4 Hz & J=1.3 Hz, 1H), 8.02 (dd, J=7.5 Hz & J=1.4 Hz, 2H), 9.26 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=20.6, 113.2, 124.1, 126.0, 126.5, 128.4, 130.2, 132.0, 133.1, 133.7, 134.8, 136.1, 143.2, 176.6, 180.1 ppm. Anal. calcd for C17H12ClNO2 (297): C, 68.58; H, 4.06; N, 4.70. Found: C, 68.85; H, 3.97; N, 5.07; Beilstein test: Cl positive.36
General Procedure for the Synthesis of Compounds 5 z–5 aa
Compounds 5 x–5 y (5 mmol) was dissolved in dry acetone (300 mL), and cyclohexanethiol (0.611 mL, 5 mmol) was gradually added to the reaction mixture with constant stirring at room temperature. Trimethylamine (1 equiv. 0.469 mL) was added and the mixture was refluxed for 2 h. The resulted mixture was poured into crushed ice and the solid formed was filtered, washed with hot water (500 mL), dried at 50 °C and purified by column chromatography (ethyl acetate : hexane, 1 : 4) to give the pure compounds 5 z–5 aa.
2‐(cyclohexylthio)‐3‐(phenylamino)naphthalene‐1,4‐dione (5 z)
Deep blue violet solid (79 %); mp: 150–151 °C; IR (KBr): =918, 1258, 1339, 1451, 1668, 3145, 3428. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.07 (d, J=5.92 Hz, 5H), 1.43–1.54 (m, 5H), 2.95 (s, 1H), 7.04–7.07 (m, 3H), 7.27 (t, J=7.76 Hz, 2H), 7.77 (dt, J=7.40 Hz & J=1.04 Hz, 1H), 7.83 (dt, J=7.40 Hz & J=1.12 Hz, 1H), 7.99 (d, J=7.64 Hz, 2H), 9.02 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=25.1, 25.2, 32.5, 44.5, 115.8, 122.6, 123.4, 125.9, 126.1, 127.8, 130.7, 132.8, 132.9, 134.4, 139.4, 146.6, 180.1, 180.3 ppm. Anal. calcd for C22H21NO2S (363): C, 72.70; H, 5.82; N, 3.85; S, 8.82 Found: C, 72.41; H, 5.98; N, 3.91; S, 8.53.
2‐(cyclohexylthio)‐3‐(p‐tolylamino)naphthalene‐1,4‐dione (5 aa)
Black solid (98 %); mp: 118–119 °C; IR (KBr): =754, 812, 1108, 1282, 1458, 1552, 1662, 3142, 3418. 1H NMR (400 MHz, DMSO‐d6, 25 °C, TMS): δ=1.07 (d, J=6.76 Hz, 5H), 1.43–1.54 (m, 5H), 2.26 (s, 3H), 2.95 (s, 1H), 6.96 (d, J=8.24 Hz, 2H), 7.07 (d, J=8.20 Hz, 2H), 7.75 (dt, J=7.52 Hz & J=1.08 Hz, 1H), 7.81 (dt, J=7.40 Hz & J=1.24 Hz, 1H), 7.97 (t, J=7.36 Hz, 2H), 8.97 (s, 1H) ppm. 13C NMR (100 MHz, DMSO‐d6): δ=20.5, 25.2, 25.3, 32.5, 44.6, 114.7, 122.8, 125.9, 126.1, 128.3, 130.7, 132.6, 132.8, 134.4, 137.0, 147.0, 180.1, 180.2 ppm. Anal. calcd for C23H23NO2S (377): C, 73.18; H, 6.14; N, 3.71; S, 8.49 Found: C, 73.01; H, 6.48; N, 3.95; S, 8.13.
Biology
In vitro Antibacterial Activity
The bacterial strains used including S. aureus (ATCC 6538), E. faecalis (PCM 2673), E. coli (ATCC 8739), S. bongori (PCM 2552), E. cloacae (PCM 2569), K. pneumoniae (PCM1), P. aeruginosa (PCM 2562), and yeast C. albicans (ATCC10231) were collected from the Department of Molecular Biology, The John Paul II Catholic University of Lublin, Poland.
The in vitro antimicrobial study was performed using the microbroth dilution method against tested organisms, followed by a previously described method.37,38 The bacterial culture was inoculated in Mueller Hinton Broth medium (Biocorp, Warsaw, Poland) and incubated at 37 °C and with vigorous shaking (200 rpm) for 24‐h. Bacterial cell suspensions at initial inoculums of 5×105 in Mueller‐Hinton liquid medium were exposed to the examined compounds at relevant concentrations (range, 0.001–2 mg/mL) for 24‐h at 37 °C. Simultaneously, the standard antibiotics caspofungin, amphotericin B, chloramphenicol and streptomycin (as a positive control) were tested against the bacterial pathogens. The MIC was defined as the lowest concentration of the compound that inhibited the visible growth of the microorganism. All the experiments were conducted in triplicate.
The MICs of the tested compounds were determined based on the broth dilution method as recommended by CLSI, with some minor modifications.39 The final concentrations of naphthoquinones and antibiotics ranged from 2000 μg/mL–0.7 μg/mL. The microtiter plate wells contained 100 μL of each dilution. Next, 20 μL of yeast inoculum (OD530‐nm=1–5×103) was added to each well, and 100 μL of uninoculated medium was included as a sterility control (blank). The plates were incubated at 37 °C for 48‐h. The MIC was defined as the lowest concentration of disinfectant that inhibited visible microorganism growth. All the experiments were performed in triplicate.
Hemolytic Assay
Human blood samples were provided voluntarily by the author of the manuscript (age 38 years, male). The blood was collected in sterile tubes containing citrate dextrose solution as an anticoagulant. To separate erythrocytes from plasma, the samples were centrifuged at 500×g for 10 min at 4 °C, and the supernatant was removed. Next, the erythrocytes were resuspended in PBS buffer (10 mM phosphate, pH 7.5; 150 mM NaCl) and centrifuged at 500×g for 10 min at 4 °C. The washing procedure was repeated until a transparent supernatant was obtained. After washing, the erythrocytes were resuspended in PBS buffer at a final concentration of 2 %. Simultaneously, the compounds were prepared at the MIC concentration (70 μg/mL) in a final volume of 50 μL DMSO. The prepared compounds were mixed with 450 μL of the 2 % erythrocyte suspension and incubated for 1‐h at 37 °C. Next, the samples were centrifuged at 5000×g for 10 min, and absorbance was measured at a wavelength of 415 nm.1
ROS detection
The generation of ROS was investigated using the fluorescent dye DCFH‐DA.40 S. aureus was cultivated, thoroughly washed with PBS buffer, and resuspended in PBS buffer (pH 7.2). Bacterial cells were stained for 30 min with 10 μM DCFH‐DA at 37 °C under dark conditions. Next, the cells were washed with water until the unreacted DCFH‐DA was removed. DCFH‐DA‐treated bacterial cells were incubated with compounds 5 b, 5 j, and 5 q (70 μg/mL, 70 μg/mL, and 30 μg/mL, respectively) for 4‐h. Lastly, ROS generation in samples was examined under a fluorescence microscope (LSM 510 META‐Carl Zeiss, Jena, Germany) The excitation‐emission wavelengths were fixed at 488 nm and 535 nm, respectively.41
Bactericidal Time‐Kill Kinetic Study
The bacteria S. aureus were cultured overnight and diluted to 1 : 10,000 in MHB medium. The cells were incubated at 37 °C and aerated at 225 rpm for 2 h. The compounds 5 b, 5 j, and 5 q were treated with bacteria at their respective MIC in culture tubes at 37 °C and 225 rpm. At regular intervals, 100 μL of bacterial solution was placed in a 96‐well plate, centrifuged for 3 min, and resuspended in 100 μL of phosphate buffer (1×PBS). Serially diluted bacterial suspensions were incubated and maintained on MHA plates at 37 °C overnight. Finally, colonies were calculated, and CFU per mL was counted. All the experiments were performed in triplicate.42
Determination of Apoptosis and Necrosis on Annexin V‐FITC/PI Assay
The amount of dead S. aureus cells after treatment with compounds 5 b, 5 j, and 5 q was determined based on FITC‐Annexin V/PI using a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). The compounds at their respective MICs were added to a bacterial solution (1×107) for 12‐h. After incubation, the bacterial solution was centrifuged and washed with PBS buffer (pH 7.2) to remove the excess bacterial media. The collected cell pellet was resuspended in Annexin V binding buffer. The staining procedure was conducted according to the manufacturer's instructions. Lastly, the stained cells were examined using a BD FACSCalibur flow cytometer. The obtained data were extracted using FlowJo 10.0.7 software (Treestar Inc, Ashland, OR, US).35
In silico Molecular Docking Study
To better understand the binding modes of the prepared naphthoquinone analogues (5 b, 5 j, and 5 q), a docking model was created using the crystal structure of the S. aureus PNPase catalytic domain (PDB ID: 5XEX).43 Prior to molecular docking, the crystal structure of S. aureus receptor was prepared using the protein preparation wizard in Schrödinger Maestro (version 8.5, Schrödinger, LLC, New York, 2010). The crystal structure was downloaded from the protein data bank (PDB), and the chemical structures of the compounds were sketched using ChemDraw professional 15.1. Docking was accomplished using an Extra Precision (XP) mode docking protocol. XP GS score was used to evaluate the docking output, and PyMOL was employed to visually examine the binding mode.44
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was supported by grants from the Medical Research Center Program (NRF‐2017R1A5A2015061) through the National Research Foundation (NRF), which is funded by the Korean government (MSIP). This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20184030202210).
P. Ravichandiran, M. Masłyk, S. Sheet, M. Janeczko, D. Premnath, A. R. Kim, B.-H. Park, M.-K. Han, D. J. Yoo, ChemistryOpen 2019, 8, 589.
Contributor Information
Dr. Palanisamy Ravichandiran, Email: ravichandru55@gmail.com.
Prof. Dong Jin Yoo, Email: djyoo@jbnu.ac.kr.
References
- 1. Janeczko M., Demchuk O. M., Strzelecka D., Kubiński K., Masłyk M., Eur. J. Med. Chem. 2016, 124, 1019–1025. [DOI] [PubMed] [Google Scholar]
- 2. Ma S., Ma S., ChemMedChem 2012, 7, 1161–1172. [DOI] [PubMed] [Google Scholar]
- 3. Bionda N., Stawikowski M., Stawikowska R., Cudic M., López-Vallejo F., Treitl D., Medina-Franco J., Cudic P., ChemMedChem 2012, 7, 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roca I., Akova M., Baquero F., Carlet J., Cavaleri M., Coenen S., Cohen J., Findlay D., Gyssens I., Heure O. E., Kahlmeter G., Kruse H., Laxminarayan R., Liébana E., López-Cerero L., MacGowan A., Martins M., Rodríguez-Baño J., Rolain J. M., Segovia C., Sigauque B., Tacconelli E., Wellington E., Vila J., New Microbes New Infect. 2015, 6, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rho Y. S., Kim S. Y., Kim W. J., Yun Y. K., Sin H. S., Yoo D. J., Synth. Commun. 2004, 34, 3497–3511. [Google Scholar]
- 6. Vermeer C., Schurgers L. J., Hematol. Oncol. Clin. North Am. 2000, 14, 339–353. [DOI] [PubMed] [Google Scholar]
- 7. Medina-Silva R., Barros M. P., Galhardo R. S., Netto L. E., Colepicolo P., Menck C. F., Res. Microbiol. 2006, 157, 275–281. [DOI] [PubMed] [Google Scholar]
- 8. Karkare S., Chung T. T., Collin F., Mitchenall L. A., McKay A. R., Greive S. J., Meyer J. J., Lall N., Maxwell A., J. Biol. Chem. 2013, 288, 5149–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dias F. R. F., Novais J. S., Devillart T. A. d. N. S., da Silva W. A., Ferreira M. O., Loureiro R. D. S., Campos V. R., Ferreira V. F., de Souza M. C. B. V., Castro H. C., Cunha A. C., Eur. J. Med. Chem. 2018, 156, 1–12. [DOI] [PubMed] [Google Scholar]
- 10. Novais J. S., Campos V. R., Silva A. C. J. A., de Souza M. C. B. V., Ferreira V. F., Keller V. G. L., Ferreira M. O., Dias F. R. F., Vitorino M. I., Sathler P. C., Santana M. V., Resende J. A. L. C., Castro H. C., Cunha A. C., RSC Adv. 2017, 7, 18311–18320. [Google Scholar]
- 11. Lanfranchi D. A., Cesar-Rodo E., Bertrand B., Huang H. H., Day L., Johann L., Elhabiri M., Becker K., Williams D. L., Davioud-Charvet E., Org. Biomol. Chem. 2012, 10, 6375–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pullella G. A., Wild D. A., Nealon G. L., Elyashberg M., Piggott M. J., J. Org. Chem. 2017, 82, 7287–7299. [DOI] [PubMed] [Google Scholar]
- 13.K. Li, B. Wang, L. Zheng, K. Yang, Y. Li, M. Hu, D. He, Bioorg. Med. Chem. Lett 2017.
- 14. Lara L. S., Moreira C. S., Calvet C. M., Lechuga G. C., Souza R. S., Bourguignon S. C., Ferreira V. F., Rocha D., Pereira M. C. S., Eur. J. Med. Chem. 2018, 144, 572–581. [DOI] [PubMed] [Google Scholar]
- 15. Ravichandiran P., Premnath D., Vasanthkumar S., J. Chem. Biol. 2014, 7, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sreelatha T., Kandhasamy S., Dinesh R., Shruthy S., Shweta S., Mukesh D., Karunagaran D., Balaji R., Mathivanan N., Perumal P. T., Bioorg. Med. Chem. Lett. 2014, 24, 3647–3651. [DOI] [PubMed] [Google Scholar]
- 17. Pingaew R., Prachayasittikul V., Worachartcheewan A., Nantasenamat C., Prachayasittikul S., Ruchirawat S., Prachayasittikul V., Eur. J. Med. Chem. 2015, 103, 446–459. [DOI] [PubMed] [Google Scholar]
- 18.M. F. Cardoso, A. T. Gomes, C. D. Moreira, M. M. Simoes, M. G. Neves, D. R. da Rocha, F. C. da Silva, C. Moreirinha, A. Almeida, V. F. Ferreira, J. A. Cavaleiro, Molecules (Basel, Switzerland) 2017, 22. [DOI] [PMC free article] [PubMed]
- 19. Yıldırım H., Bayrak N., Tuyun A. F., Kara E. M., Çelik B. Ö., Gupta G. K., RSC Adv. 2017, 7, 25753–25764. [Google Scholar]
- 20. Tandon V. K., Maurya H. K., Yadav D. B., Tripathi A., Kumar M., Shukla P. K., Bioorg. Med. Chem. Lett. 2006, 16, 5883–5887. [DOI] [PubMed] [Google Scholar]
- 21. Tandon V. K., Maurya H. K., Verma M. K., Kumar R., Shukla P. K., Eur. J. Med. Chem. 2010, 45, 2418–2426. [DOI] [PubMed] [Google Scholar]
- 22. Novais J. S., Moreira C. S., Silva A. C. J. A., Loureiro R. S., Sá Figueiredo A. M., Ferreira V. F., Castro H. C., da Rocha D. R., Microb. Pathog. 2018, 118, 105–114. [DOI] [PubMed] [Google Scholar]
- 23. Ravichandiran P., Subramaniyan S. A., Kim S.-Y., Kim J.-S., Park B.-H., Shim K. S., Yoo D. J., ChemMedChem 2019, 14, 532–544. [DOI] [PubMed] [Google Scholar]
- 24. Ravichandiran P., Jegan A., Premnath D., Periasamy V. S., Vasanthkumar S., Med. Chem. Res. 2015, 24, 197–208. [DOI] [PubMed] [Google Scholar]
- 25. Ravichandiran P., Athinarayanan J., Premnath D., Periasamy V. S., Alshatwi A. A., Vasanthkumar S., Spectrochim. Acta Part A 2015, 139, 477–487. [DOI] [PubMed] [Google Scholar]
- 26. Ravichandiran P., Premnath D., Vasanthkumar S., Front. Chem. Sci. Eng. 2015, 9, 46–56. [Google Scholar]
- 27. Ravichandiran P., Kannan R., Ramasubbu A., Muthusubramanian S., Samuel V. K., J. Saudi Chem. Soc. 2016, 20, S93–S99. [Google Scholar]
- 28. Katritzky A. R., Huang L., Sakhuja R., Synthesis 2010, 2010, 2011–2016. [Google Scholar]
- 29. Tandon V. K., Yadav D. B., Singh R. V., Chaturvedi A. K., Shukla P. K., Bioorg. Med. Chem. Lett. 2005, 15, 5324–5328. [DOI] [PubMed] [Google Scholar]
- 30. Kim Y. S., Park S. Y., Lee H. J., Suh M. E., Schollmeyer D., Lee C. O., Bioorg. Med. Chem. 2003, 11, 1709–1714. [DOI] [PubMed] [Google Scholar]
- 31. Chang J., Zhang S.-J., Jiang Y.-W., Xu L., Yu J.-M., Zhou W.-J., Sun X., ChemMedChem 2013, 8, 976–984. [DOI] [PubMed] [Google Scholar]
- 32. Schlievert P. M., Merriman J. A., Salgado-Pabon W., Mueller E. A., Spaulding A. R., Vu B. G., Chuang-Smith O. N., Kohler P. L., Kirby J. R., Antimicrob. Agents Chemother. 2013, 57, 5432–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qadri S. M., Eberhard M., Mahmud H., Foller M., Lang F., Eur. J. Pharmacol. 2009, 623, 10–13. [DOI] [PubMed] [Google Scholar]
- 34. Li K., Wang B., Zheng L., Yang K., Li Y., Hu M., He D., Bioorg. Med. Chem. Lett. 2018, 28, 273–277. [DOI] [PubMed] [Google Scholar]
- 35. Sheet S., Vinothkannan M., Balasubramaniam S., Subramaniyan S. A., Acharya S., Lee Y. S., ACS Omega 2018, 3, 14551–14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Becker H., Berger W., Domschke G., Fanghänel E., Faust J., Fischer M., Gentz F., Gewald K., Gluch R., Mayer R., Müller K., Pavel D., Schmidt H., Schollberg K., Schwetlick K., Zeppenfeld G., in Organicum (Eds.: H. Becker, W. Berger, G. Domschke, E. Fanghänel, J. Faust, M. Fischer, F. Gentz, K. Gewald, R. Gluch, R. Mayer, K. Müller, D. Pavel, H. Schmidt, K. Schollberg, K. Schwetlick, G. Zeppenfeld), Pergamon, 1973, pp. 607–664. [Google Scholar]
- 37. Janeczko M., Kazimierczuk Z., Orzeszko A., Niewiadomy A., Szyszka E. K. Ryszard, Masłyk M., Pol. J. Microbiol. 2016, 65, 359–364. [DOI] [PubMed] [Google Scholar]
- 38. Janeczko M., Masłyk M., Kubiński K., Golczyk H., Yeast 2017, 34, 253–265. [DOI] [PubMed] [Google Scholar]
- 39.B. D. Alexander, Clinical and Laboratory Standards Institute, Wayne, PA 2017.
- 40. Eruslanov E., Kusmartsev S., Methods Mol. Biol. 2010, 594, 57–72. [DOI] [PubMed] [Google Scholar]
- 41. Koontz J., Kontrogianni-Konstantopoulos A., J. Biol. Chem. 2014, 289, 3468–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chu W. C., Bai P. Y., Yang Z. Q., Cui D. Y., Hua Y. G., Yang Y., Yang Q. Q., Zhang E., Qin S., Eur. J. Med. Chem. 2018, 143, 905–921. [DOI] [PubMed] [Google Scholar]
- 43. Wang X., Wang C., Wu M., Tian T., Cheng T., Zhang X., Zang J., FEBS Lett. 2017, 591, 3523–3535. [DOI] [PubMed] [Google Scholar]
- 44. Friesner R. A., Murphy R. B., Repasky M. P., Frye L. L., Greenwood J. R., Halgren T. A., Sanschagrin P. C., Mainz D. T., J. Med. Chem. 2006, 49, 6177–6196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


