Abstract
Objective
Primary aldosteronism (PA) is a common cause of secondary hypertension, and the long-term effect of excess aldosterone on kidney function is unknown.
Patients and Methods
We used a longitudinal population database from the Taiwan National Health Insurance system and applied a validated algorithm to identify patients with PA diagnosed between 1997 and 2009.
Results
There were 2699 patients with PA recruited, of whom 761 patients with an aldosterone-producing adenoma (APA) were identified. The incidence rate of end-stage renal disease (ESRD) was 3% in patients with PA after targeted treatments and 5.2 years of follow-up, which was comparable to the rate in controls with essential hypertension (EH). However, after taking mortality as a competing risk, we found a significantly lower incidence of ESRD when comparing patients with PA vs EH [subdistribution hazard ratio (sHR), 0.38; P = 0.007] and patients with APA vs EH (sHR 0.55; P = 0.021) after adrenalectomy; however, we did not see similar results in groups with mineralocorticoid receptor antagonist (MRA)‒treated PA vs EH. There was also a significantly lower incidence of mortality in groups with PA and APA who underwent adrenalectomy than among EH controls (P < 0.001).
Conclusion
Regarding incident ESRD, patients with PA were comparable to their EH counterparts after treatment. After adrenalectomy, patients with APA had better long-term outcomes regarding progression to ESRD and mortality than hypertensive controls, but MRA treatments did not significantly affect outcome.
Keywords: primary aldosteronism, ESRD, adrenalectomy, spironolactone, TAIPAI, TSA
Primary aldosteronism (PA; i.e., autonomous aldosterone hypersecretion) is noted in 3.9% of patients with stage 1 hypertension, and the proportion increases to 11.8% in patients with stage 3 hypertension [1]. However, a number of cardiovascular and renal sequelae of PA cannot be entirely attributed to the effects of hypertension alone [2]. The reported risk of cardiovascular events was higher in patients with PA, with proinflammatory mediators and oxidative stress affecting multiple organs, than in otherwise similar patients with essential hypertension (EH) [3].
Prolonged aldosterone excess also causes relative kidney hyperfiltration and reversible intrarenal vascular structural changes, which disguise consequent renal injury, including declining glomerular filtration rate (GFR) and proteinuria [4, 5]. Aldosterone has been reported to induce direct glomerular injury and proteinuria independently of its hemodynamic effects [4, 6], such as a high estimated GFR and albuminuria [7]. Hence, PA is associated with higher rates of renal blood flow [8] and, in a previous study, characterized by partially reversible renal dysfunction in which a dynamic, rather than structural, renal defect was demonstrated in a previous study [9].
Although a long-term follow-up study with limited PA patients showed similar therapeutic effects on kidney function [9], adrenalectomy and mineralocorticoid receptor antagonist (MRA) treatments have different short-term clinical impacts with respect to kidney damage, even with a similar blood pressure‒lowering effect [4, 7, 10]. In fact, an initial temporary worsening of renal function within 1 month of adrenalectomy has been reported [4, 8, 11], and the decline in renal function could be the result of correction of glomerular hyperfiltration via decreased aldosterone excess‒related intrarenal vascular resistance [8] or systemic hemodynamic change [12]. A systemic review supported surgical resection of PA, which can be performed with low morbidity [13]. Adrenalectomy is safe, reverses aldosterone excess, and is effective at normalizing blood pressure and decreasing medication requirements, particularly in younger adults [14]; however, regarding kidney function, the long-term benefit/risk ratio of adrenalectomy compared with MRA treatment is lacking, and additional studies are warranted.
The Taiwan National Health Insurance research database contains records of claims that comprehensively capture information on episodes of care across all hospitals and nearly all health care facilities across Taiwan. Taking advantage of the merits of this database, we examined long-term crucial outcomes of renal events, namely end-stage renal disease (ESRD) and mortality among patients with PA receiving targeted treatment. We scrutinized the effects of different treatment options for PA on ESRD and mortality and subsequently explored the benefits of treatment strategies for reducing long-term ESRD and mortality risk among patients with PA.
1. Materials and Methods
A. Patient Enrollment
This study extracted National Health Insurance (NHI) reimbursement data from 1997 to 2009. The Taiwan government launched the NHI program in 1995. The NHI covers outpatient visits, hospital admissions, prescriptions, and intervention procedures and maintains disease profiles for >99% of the Taiwanese population. The NHI improved access to health care and reduced economic disparity in health care use in the late 1990s. As shown by financial reports from the National Health Insurance Administration (NHIA), the electronic database of NHI claims contains comprehensive information on disease diagnosis and health care use, particularly for cases requiring expensive services. The NHI database has provided research data for various studies on epidemiology, health services research, and clinical medicine [15]. To detect fraud in the NHI, the NHIA routinely audits data submitted by health care institutions [16]. The NHIA has been the only provider to the NHI, and to avoid NHIA rejection of reimbursement claims, physicians in Taiwan usually follow clinical guidelines. Because the gold standard for diagnosis of PA is still AVS which is costly and invasive, Taiwanese physicians follow the clinical practice guidelines of the Society of Aldosteronism—the Taiwan PA consensus [17, 18]—and focus on performing lateralization tests on patients with a high possibility of PA after a confirmation test [18–22].
B. Algorithms for Identifying PA
We used a validated algorithm to detect patients with PA, recruiting only patients aged ≥18 years [International Classification of Diseases Ninth Revision (ICD-9) code: 255.1] [15, 23]. We also found an algorithm with a positive predictive value of 0.9 to ensure the reliability of the sample identified by the algorithm to portray clinical outcomes in patients with PA [15]. We enrolled only patients with PA who had used MRA [belonging to anatomical therapeutic chemical (ATC) class C03D] because our main study aim was to construct a reliable PA sample according to our validation [15, 23]. For comparisons between patients with an aldosterone-producing adenoma (APA) treated with surgery vs MRA, patients with PA and the diagnosis of adrenal tumor (ICD-9 code: 227, 227.0, 239.7) were further classified as having APA. Among patients with PA who underwent adrenalectomy, an adrenal tumor was recorded in 96%. In addition, because patient adherence in taking medication plays a crucial role in successful medical treatment and can affect mortality, we calculated each patient’s medication possession ratio for MRA (belonging to ATC class C03D). Adherence was calculated across all disease-modifying drugs for a 12-month postindex date.
We developed additional algorithms to ascertain comorbid conditions among patients with PA. Except for a couple of morbid conditions requiring more detailed International Classification of Diseases, Ninth Revision, Clinical Modification coding for confirmation, we used only the first three digits of these codes to identify morbid conditions, rather than more digits [15]. This rule also tended to yield a lower rate of type II error in identifying comorbidities. During review of the NHI data, the identification of a specific morbid condition was based on the criterion of at least one inpatient NHI record or three outpatient records with the corresponding disease diagnosis within 1 year before the time of first PA diagnosis; this method has been well validated [15, 24–27].
In this nested propensity, score-matched, case-control study, patients with EH were ascertained from those with the diagnosis of hypertension and received antihypertensive agents (from ATC) after we excluded patients with secondary hypertension. The duration of hypertension was defined as the period from the first prescription of antihypertension agents to the index date.
C. Outcomes
The outcomes of this study were long-term all-cause mortality and ESRD [28]. We used a subsequent selective period of 90 days to define ESRD because all patients receiving dialysis for more than 90 days in Taiwan can apply for dialysis-related catastrophic cards in the NHI program [29]. Patients with catastrophic illness certification who get care for the illness or related conditions within the certificate's validity period do not need to provide a copayment for outpatient or inpatient care.
D. Statistical Analyses
Student t tests and χ2 tests were used to assess differences between the patients with PA and those with identified EH. We matched patients with PA/APA to patients with EH using a greedy matching algorithm with a caliper width of 0.2 SDs of the log odds of the estimated propensity score. The sampling ratio for patients with PA/patients with EH and for patients with APA/patients with EH was 1:4.
In various subsequent multivariable models for analyzing outcomes, we took into account the propensity score for the PA diagnosis to minimize residual confounding effects in the matching process [23]. To estimate each patient’s propensity score for PA diagnosis, we fit a separate multivariable logistic regression model with factors predicting PA [30]. The caliper distance was 0.25. Multivariable Cox proportional hazard models before and after propensity score matching [23] were applied to estimate hazard ratios (HRs) of study outcomes. The significance level for entry and the significance level for stay were set to 0.15 for conservative purposes. Then, with the aid of substantive knowledge, the best-candidate final Cox model was identified by manually dropping the covariates with P value >0.05 one at a time until all regression coefficients were significantly different from 0.
Cox regression models with time-varying covariates accounted for their influence on risk of ESRD or death. Time-varying covariates took the value 0 before the start of MRA or surgical treatment and could switch to 1 at the start of treatment. Additional adjustments in these models included control for direct effects from age, sex, concomitant medications, and the comorbidities listed in Table 1 (and in an online repository [23]). Regarding factors associated with outcomes in further parametric modeling, we adopted three modeling methods: simple Cox regression, multivariable Cox regression, and competing risk regression. We took mortality as competing risk due to the high mortality rate among elderly patients with ESRD, competing-risk regression using the Fine and Gray model with consideration of the subdistribution hazard ratio (sHR) was also performed to show the risk of progression to ESRD. [31].
Table 1.
Comparison of Characteristics Between Patients With PA and EH for the Whole PA Cohort and for the APA Subgroup Only
| ESRD | Matched EH vs PA | Matched EH vs APA | ||||
|---|---|---|---|---|---|---|
| 1997–2009 | EH (n = 10796) | PA (n = 2699) | P | EH (n = 3044) | APA (n = 761) | P |
| Propensity score | −3.9 ± 1.6 | −3.9 ± 1.6 | 0.993 | −2.5 ± 0.5 | −2.5 ± 0.5 | 0.999 |
| Sex | ||||||
| Female | 5725 (53.0) | 1468 (54.4) | 0.211 | 1763 (57.9) | 432 (56.8) | 0.566 |
| Male | 5071 (47.0) | 1231 (45.6) | 0.211 | 1281 (42.1) | 329 (43.2) | 0.566 |
| Age, y | 51.6 ± 14.7 | 51.6 ± 14.7 | 0.999 | 47.8 ± 13.6 | 48.0 ± 11.5 | 0.382 |
| Urbanization level | ||||||
| Urban | 4959 (45.9) | 1231 (45.6) | 0.954 | 1430 (47.0) | 357 (46.9) | 0.659 |
| Suburban | 2883 (26.7) | 724 (26.8) | 0.954 | 802 (26.4) | 211 (27.7) | 0.659 |
| Rural | 2954 (27.4) | 744 (27.6) | 0.954 | 812 (26.7) | 193 (25.4) | 0.659 |
| Monthly income, n (%) | ||||||
| <USD 640 | 6391 (59.2) | 1621 (60.1) | 0.343 | 1840 (60.5) | 458 (60.2) | 0.988 |
| ≥USD 640 | 3667 (34.0) | 914 (33.9) | 0.343 | 1003 (33.0) | 253 (33.3) | 0.988 |
| Outpatient visits to specialists, n (%) | ||||||
| ≤5 | 882 (8.2) | 241 (8.9) | 0.551 | 298 (9.8) | 72 (9.5) | 0.536 |
| 5–10 | 1185 (11.0) | 301 (11.2) | 0.551 | 351 (11.5) | 76 (10.0) | 0.536 |
| 10–15 | 1534 (14.2) | 368 (13.6) | 0.551 | 473 (15.5) | 113 (14.9) | 0.536 |
| ≥15 | 7195 (66.7) | 1789 (66.3) | 0.551 | 1922 (63.1) | 500 (65.7) | 0.536 |
| Comorbidity, n (%) | ||||||
| Congestive heart failure | 420 (3.9) | 110 (4.1) | 0.658 | 81 (2.7) | 26 (3.4) | 0.270 |
| Cerebrovascular disease | 794 (7.4) | 216 (8.0) | 0.252 | 164 (5.4) | 46 (6.0) | 0.478 |
| CKD | 270 (2.5) | 71 (2.63) | 0.681 | 33 (1.1) | 10 (1.3) | 0.567 |
| COPD | 680 (6.3) | 179 (6.6) | 0.537 | 109 (3.6) | 23 (3.0) | 0.507 |
| Coronary artery disease | 126 (1.2) | 25 (0.9) | 0.308 | 43 (1.4) | 6 (0.8) | 0.209 |
| Dementia | 84 (0.8) | 21 (0.8) | 0.999 | 10 (0.3) | 1 (0.1) | 0.704 |
| Diabetes mellitus | 1447 (13.4) | 398 (14.8) | 0.074 | 328 (10.8) | 83 (10.9) | 0.896 |
| Hemiplegia | 65 (0.6) | 18 (0.7) | 0.680 | 8 (0.3) | 5 (0.7) | 0.153 |
| Liver disease | 657 (6.1) | 148 (5.5) | 0.256 | 149 (4.9) | 35 (4.6) | 0.778 |
| Peptic ulcer | 955 (8.9) | 235 (8.7) | 0.850 | 194 (6.4) | 47 (6.2) | 0.934 |
| Peripheral vascular disease | 55 (0.5) | 10 (0.4) | 0.437 | 15 (0.5) | 2 (0.3) | 0.550 |
| Rheumatoid arthritis | 56 (0.5) | 12 (0.4) | 0.761 | 17 (0.6) | 1 (0.1) | 0.149 |
| Solid tumor | 306 (2.8) | 66 (2.5) | 0.293 | 46 (1.5) | 15 (2.0) | 0.338 |
| SLE | 12 (0.1) | 6 (0.2) | 0.232 | 4 (0.1) | 2 (0.3) | 0.345 |
| Af | 165 (1.5) | 38 (1.4) | 0.724 | 41 (1.4) | 6 (0.8) | 0.271 |
| Dyslipidemia | 1602 (14.8) | 384 (14.2) | 0.430 | 338 (11.1) | 86 (11.3) | 0.898 |
| Parkinson disease | 72 (0.7) | 23 (0.9) | 0.304 | 15 (0.5) | 1 (0.1) | 0.221 |
| Antihypertensive medication, n (%) | ||||||
| Alpha-blocker | 747 (6.9) | 188 (7.0) | 0.932 | 165 (5.4) | 54 (7.1) | 0.082 |
| ACE-I or ARB | 4506 (41.7) | 1116 (41.4) | 0.727 | 1308 (43.0) | 328 (43.1) | 0.967 |
| Beta-blocker | 5145 (47.7) | 1255 (46.5) | 0.291 | 1518 (49.9) | 387 (50.9) | 0.656 |
| CCB | 6731 (62.4) | 1680 (62.3) | 0.929 | 2083 (68.4) | 530 (69.7) | 0.541 |
| Diuretic | 4878 (45.2) | 1213 (44.9) | 0.829 | 1175 (38.6) | 291 (38.2) | 0.868 |
| Other medication, n (%) | ||||||
| Aspirin | 728 (6.7) | 193 (7.2) | 0.443 | 192 (6.3) | 50 (6.6) | 0.803 |
| Clopidogrel | 177 (1.6) | 49 (1.8) | 0.503 | 65 (2.1) | 15 (2.0) | 0.888 |
| Ticlopidine | 112 (1.0) | 30 (1.1) | 0.752 | 21 (0.7) | 3 (0.4) | 0.451 |
| Warfarin | 103 (1.0) | 29 (1.1) | 0.584 | 24 (0.8) | 6 (0.8) | 1.000 |
| PPI | 437 (4.1) | 117 (4.3) | 0.515 | 88 (2.9) | 21 (2.8) | 0.904 |
| H2 blocker | 1076 (10.0) | 271 (10.0) | 0.914 | 260 (8.5) | 64 (8.4) | 0.942 |
| Statin | 961 (8.9) | 244 (9.0) | 0.821 | 222 (7.3) | 53 (7.0) | 0.814 |
| NSAID | 5345 (49.5) | 1333 (49.4) | 0.914 | 1471 (48.3) | 358 (47.0) | 0.543 |
| Steroid | 1006 (9.3) | 277 (10.3) | 0.142 | 238 (7.8) | 57 (7.5) | 0.820 |
| SSRI | 306 (2.8) | 72 (2.7) | 0.696 | 73 (2.4) | 17 (2.2) | 0.894 |
| Nitrate | 28 (0.3) | 9 (0.3) | 0.536 | 8 (0.3) | 0 (0.0) | 0.370 |
| Outcome, n (%) | ||||||
| ESRD | 322 (3.0) | 80 (3.0) | 0.999 | 84 (2.8) | 11 (1.5) | 0.037 |
| Mortality | 1641 (15.2) | 366 (13.6) | 0.032 | 375 (12.3) | 40 (5.1) | <0.001 |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; Af, atrial fibrillation; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; H2, histamine type 2; N, number; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton pump inhibitor; SLE, systemic lupus erythematosus; SSRI, selective serotonin reuptake inhibitor; USD, United States dollar.
On the basis of this hazard function, we further simulated and depicted 10-year survival curves for the probability of incident ESRD under different scenarios of treatment with MRA and adrenalectomy among all patients with PA [32, 33]. Specifically, we stratified patients by PA subgroup status including age, sex, urbanization level, monthly income and the comorbidities after targeted treatment and used the mean values of all other covariates for our study patients.
We used R software, version 2.8.1 (Free Software Foundation, Inc., Boston, MA); competing risk analysis was performed with Stata/MP version 12 (Stata Corporation, College Station, TX). A two-sided P value <0.05 was considered statistically significant.
2. Results
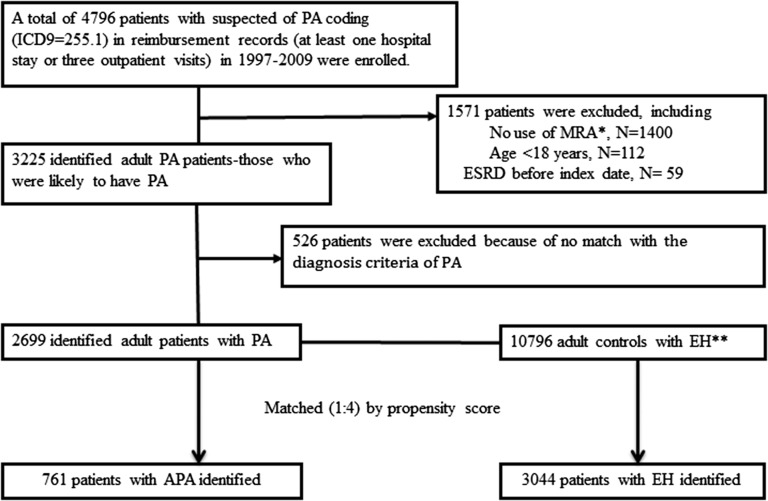
The process of selecting patients for the newly identified PA and matched EH groups is listed in Fig. 1. Among 4796 patients with an initial diagnosis of PA from the NHRI 1997 to 2009 database, 1571 patients were excluded. As shown, 2699 adults had confirmed PA according to the algorithm; among them, 657 adults underwent adrenalectomy and 2042 patients were administered MRA (Fig. 1).
Figure 1.
Flowchart of the participants in the cohort (PA/APA cohort and essential hypertension cohort). *Patients who did not use MRA during the year before or 2 year after the first PA coding. **Secondary hypertension excluded.
A. Comparing Adults With EH and Adults With PA
After propensity score matching during index clinical visits [23], 10,796 patients with EH were identified. The mean age of patients at the time of PA diagnosis was 51.6 years, and the proportion of men was 45.6% (Table 1). Likewise, 761 patients with APA (mean age, 48.0 years, 43.2% male) were matched with 3044 EH controls .
B. Comparing the Risk of ESRD Between Patients With PA/APA and Their EH Matches
The incidence rate of ESRD in the EH control group was 5.8 events/1000 person-years, and the PA group had an incidence rate of 5.5 events/1000 person-years after targeted treatment; the APA group had an incidence rate of 2.3 events/1000 person-years (average, 5.2 ± 3.5 years of follow-up) (Table 2). The competing sHR for developing incident ESRD after targeted treatment was 0.96 (P = 0.730) among the PA cohort, and 0.50 (P = 0.031) among the APA cohort relative to their EH cohort after accounting for mortality in the competing effects.
Table 2.
Incidence and Risks for Outcomes of Interest Between Patients With PA and Their EH Matches for the Whole PA Cohort and the APA Subgroup Only
| ESRD (EH vs PA) | Events | Person-(y) | Incidence Rate[per 1000 Person-(y)] | Events | Person-(y) | Incidence Rate [per 1000 Person-(y)] | Crude Hazard Ratio (95% CI) | P | Adjusted Hazard Ratio (95% CI) | P | Competing Hazard Ratio (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EH | PA | EH vs PA | ||||||||||
| ESRD | 322 | 55,139.4 | 5.8 | 80 | 14,532.3 | 5.5 | 0.96 (0.75, 1.23) | 0.742 | 0.93 (0.73, 1.19) | 0.571 | 0.96 [0.75, 1.22] | 0.730 |
| Mortality | 1641 | 56,429.2 | 29.1 | 366 | 14,751.6 | 24.8 | 0.86 (0.77, 0.96) | 0.009 | 0.86 (0.77, 0.96) | 0.010 | NA | NA |
| ESRD + mortality | 1811 | 55,139.4 | 32.8 | 421 | 14,532.4 | 29.0 | 0.89 (0.80, 0.99) | 0.035 | 0.89 (0.80, 0.99) | 0.031 | NA | NA |
| EH | APA | EH vs APA | ||||||||||
| ESRD | 84 | 17,670.8 | 4.8 | 11 | 4887.5 | 2.3 | 0.49 (0.26, 0.91) | 0.025 | 0.49 (0.26, 0.91) | 0.025 | 0.50 [0.27, 0.94] | 0.031 |
| Mortality | 375 | 18,055.1 | 20.8 | 40 | 4918.8 | 8.1 | 0.40 (0.29, 0.55) | <0.001 | 0.40 (0.29, 0.55) | <0.001 | NA | NA |
| ESRD + mortality | 421 | 17,670.8 | 23.8 | 49 | 4887.5 | 10.0 | 0.43 (0.32, 0.58) | <0.001 | 0.43 (0.32, 0.58) | <0.001 | NA | NA |
Abbreviation: NA; not available.
C. Comparing the Risk of ESRD and Mortality Between Patients With PA/APA and Their EH Matches
Table 3 shows that the risks of mortality (adjusted HR, 0.22; P < 0.001), ESRD (sHR, 0.38; P = 0.007), and ESRD with mortality (adjusted HR, 0.26; P < 0.001) among patients with PA who underwent adrenalectomy were all significantly decreased compared with those of EH controls. In an additional analysis, patients with APA who underwent adrenalectomy had decreased incidences of ESRD (sHR, 0.55; P = 0.021), mortality (adjusted HR, 0.31; P < 0.001), and ESRD with mortality (adjusted HR, 0.35; P < 0.001) compared with EH controls. However, patients with PA who underwent MRA treatment had a risk of incident ESRD similar to that of EH controls (sHR, 1.08; P = 0.570). Similarly, there was no significant difference in the risk of incident ESRD between APA and EH groups when MRA treatment was used (sHR, 0.39; P = 0.100) (Table 3).
Table 3.
Comparison of Risks of ESRD and Death Between Patients With PA and Their EH Matches for the Whole PA Cohort and the APA Subgroup Only by Targeted Treatments
| Adrenalectomy | P | MRA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Therapeutic Option | Crude Hazard Ratio (95% CI) | P | Adjusted Hazard Ratio (95% CI) | P | Competing Hazard Ratio (95% CI) | Crude Hazard Ratio (95% CI) | P | Adjusted Hazard Ratio (95% CI) | P | Competing Hazard Ratio (95% CI) | P | |
| EH vs PA | ||||||||||||
| ESRD | 0.46 (0.23, 0.92) | 0.028 | 0.46 (0.23, 0.93) | 0.031 | 0.38 (0.19, 0.76) | 0.007 | 1.09 (0.85, 1.41) | 0.492 | 0.93 (0.72, 1.20) | 0.567 | 1.08 (0.83, 1.39) | 0.570 |
| Mortality | 0.22 (0.15, 0.34) | <0.001 | 0.22 (0.15, 0.34) | <0.001 | NA | NA | 1.05 (0.94, 1.18) | 0.381 | 1.05 (0.94, 1.18) | 0.380 | NA | NA |
| ESRD + mortality | 0.26 (0.18, 0.38) | <0.001 | 0.26 [0.18, 0.37) | <0.001 | NA | NA | 1.08 (0.97, 1.20) | 0.175 | 1.08 (0.97, 1.20) | 0.178 | NA | NA |
| EH vs APA | ||||||||||||
| ESRD | 0.53 (0.26, 1.11) | 0.092 | 0.43 (0.21, 0.89) | 0.024 | 0.55 (0.27,0.89] | 0.021 | 0.39 (0.12, 1.25) | 0.113 | 0.31 (0.10, 1.03) | 0.080 | 0.39 (0.12, 1.22) | 0.100 |
| Mortality | 0.30 (0.19, 0.46) | <0.001 | 0.31 (0.20, 0.47) | <0.001 | NA | NA | 0.67 (0.42, 1.07) | 0.095 | 0.61 (0.38, 0.99) | 0.045 | NA | NA |
| ESRD + mortality | 0.34 (0.23, 0.50) | <0.001 | 0.35 (0.24, 0.52) | <0.001 | NA | NA | 0.65 (0.42, 1.00) | 0.051 | 0.61 (0.39, 0.94) | 0.027 | NA | NA |
Abbreviation: NA, not available.
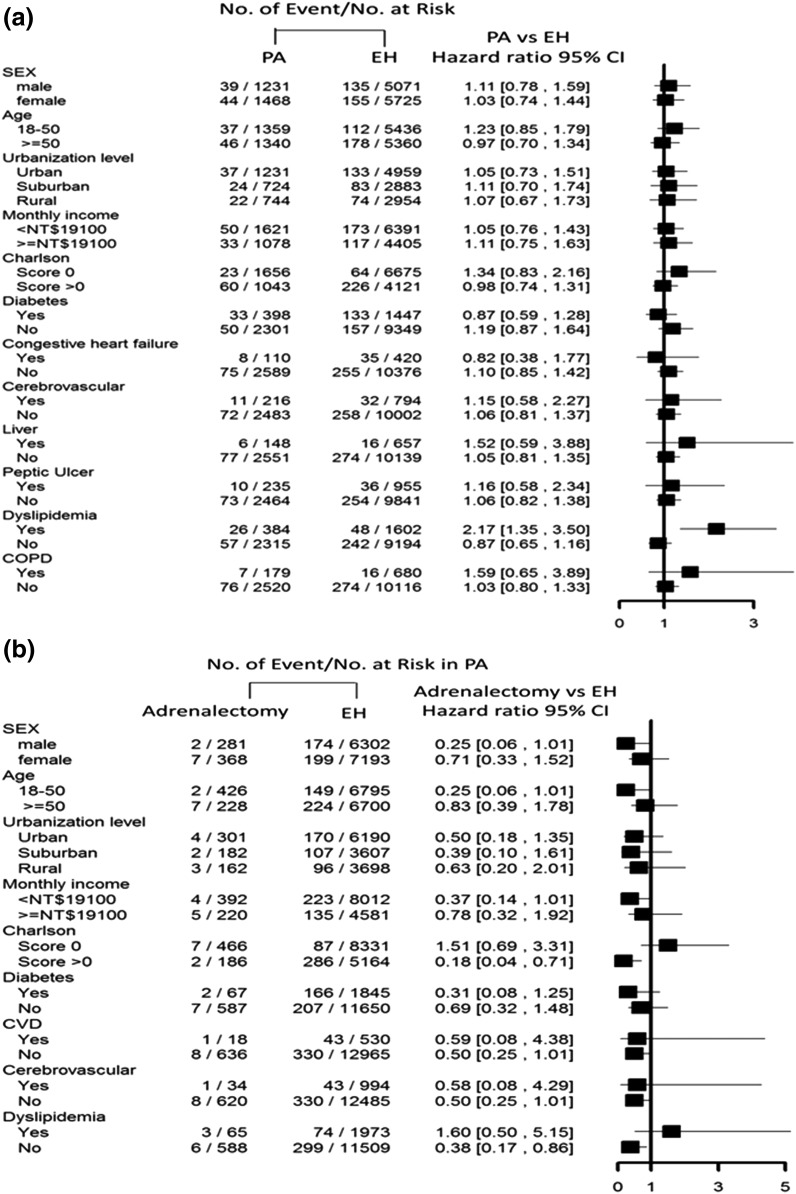
In the subgroup analysis, there were no significant differences in incident ESRD among patients with PA after targeted treatment vs patients with EH (Fig. 2a). In terms of undergoing adrenalectomy, there was a significantly lower incidence of ESRD among patients with a Charlson score >0 (HR, 0.18; 95% CI: 0.04 to 0.71) (Fig. 2b)
Figure 2.
(a) Risk of incident ESRD between patients with PA and EH controls and (b) between patients with PA and adrenalectomy and their EH controls by participant characteristics. CVD, cardiovascular disease, NT$, New Taiwan dollar.
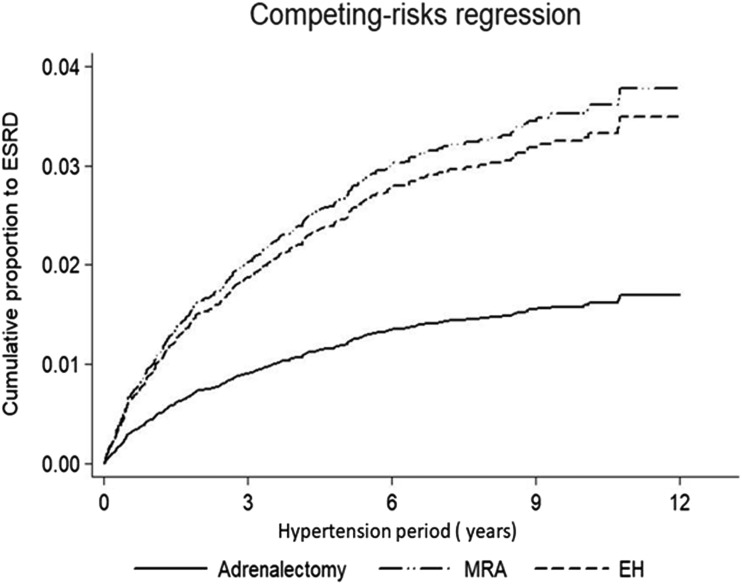
Using the Cox regression model, we further assessed incident ESRD for patients with PA who were given MRA or adrenalectomy, with EH as the control and mortality as a competing risk (Fig. 3). A longer period of hypertension led to a similar cumulative proportion of ESRD for those given MRA (P = 0.100); however, there was a smaller cumulative proportion of ESRD for those who underwent adrenalectomy (P = 0.021) compared with patients with EH.
Figure 3.
Cox regression model comparing incident ESRD in patients with adrenalectomy (P = 0.021), MRA (P = 0.100) treatment, and EH during the follow-up period with mortality taken as a competing risk.
D. Comparison of the Effect of Adrenalectomy on the Long-Term Risk of Incident ESRD Under the Framework of a Subgroup Analysis
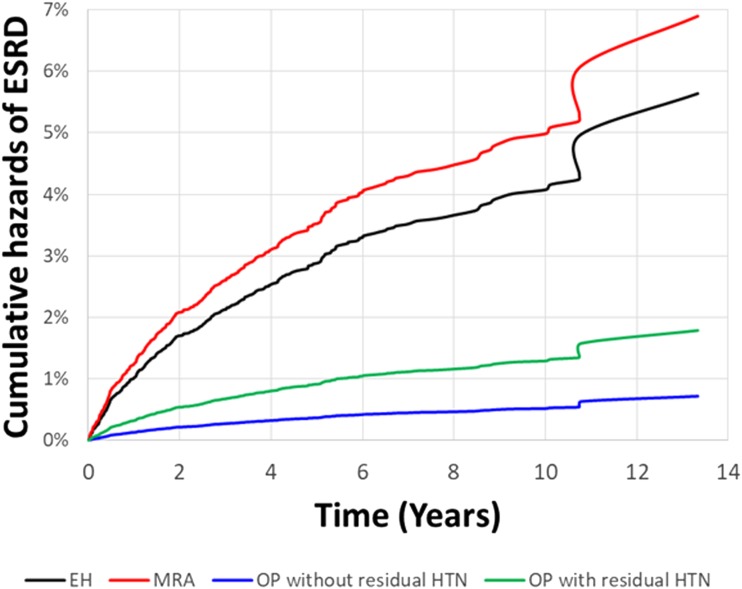
To investigate the consistency of the beneficial effect of adrenalectomy among different groups of PA patients, we conducted a subgroup analysis with respect to baseline comorbidity that further adjusted for age and sex. Our simulation results (Fig. 4) showed the beneficial effect of adrenalectomy among different treatments in patients with PA for the entire period (estimated 10 years) after index diagnosis. We found that adrenalectomy was consistently associated with a much lower long-term risk of ESRD, especially in those without residual hypertension after adrenalectomy. Patients with PA who received MRA had the highest incidence of ESRD.
Figure 4.
Future 10-y probability of freedom from ESRD events. The simulation curves were based on different scenarios of targeted treatment with MRA and adrenalectomy stratified by subsequent blood pressure. The incident ESRD was lower among patients with PA who underwent adrenalectomy, especially those without residual hypertension after adrenalectomy during follow-up. IHA, idiopathic hyperaldosteronism; OP.
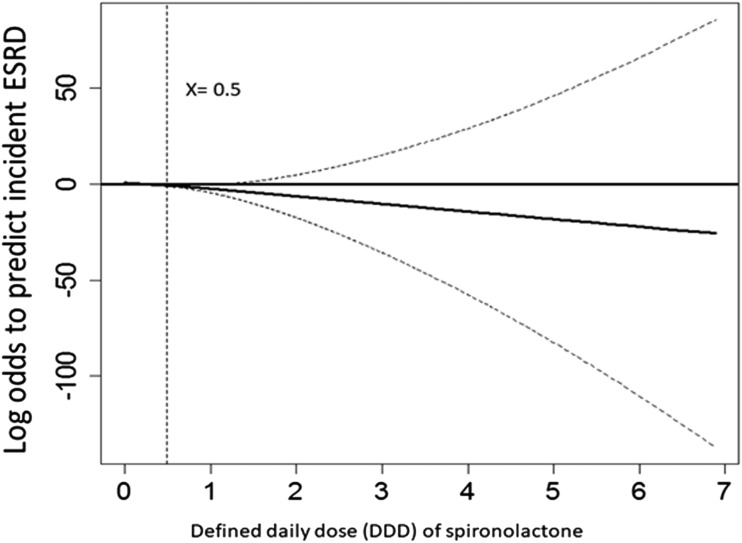
E. Dose-Response Relationship Between Spironolactone and the Probability of Developing Incident ESRD
We further evaluated the dose-response relationship between MRA and ESRD. Targeting MRA users, we found the HR was 0.82 (95% CI, 0.67 to 0.96; P < 0.001) per defined daily dose (DDD) of MRA after adjustment for age, comorbidities, and influential drugs (c statistic 0.85; adjusted R2, 0.31). However, the GAM plot showed that the dose-response curve was nonlinear (Fig. 5). One-half to one DDD of MRA was sufficient to prevent the initiation of long-term dialysis. This favorable effect increased in a dose-response manner up to one DDD, at which point a ceiling effect was reached.
Figure 5.
The dose-response relationship between spironolactone and the probability of developing incident ESRD using generalized additive modeling. The defined daily dose (DDD) of MRA = 0.5 is equal to a daily dose of 37.5 mg.
3. Discussion
A. Main Findings
Regarding incident ESRD, patients with PA were comparable to those with EH after targeted treatment. We further demonstrated significantly lower incidences of ESRD and mortality in patients with PA who underwent adrenalectomy than in EH controls; no differences were seen in ESRD and mortality rates in patients with PA receiving MRA management compared with EH controls. According to our plot of 10-year probability of freedom from dialysis, prolonged duration of hypertension will increase the episode of incident ESRD. Our results show that patients with PA have a decreased incidence of ESRD after undergoing adrenalectomy, implying the importance of early diagnosis of APA and advising adrenalectomy for patients with APA and acceptable surgical risk.
To the best of our knowledge, the incidence of ESRD among patients with PA has not been reported. Our report details information regarding the association between different kinds of targeted PA treatments and the incidence of ESRD (i.e., 3% as EH, with an average 5.2 years of follow-up). Coincidentally, in one retrospective study of 11,912 veterans with hypertension who were followed up for an average of 9.8 years, the estimated cumulative ESRD rate was 2.8% [34].
A-1. Kidney function in aldosteronism
Persistent hypertension and electrolyte imbalance are not the only effects of PA; a proinflammatory effect causing end-organ damage has also been observed [3, 15]. The glomerular hemodynamic situation in PA also occurs in intraglomerular hypertension and excessive proximal tubular sodium reabsorption, which may trigger tubuloglomerular feedback and glomerular hyperfiltration via mineralocorticoid receptor activation in macula densa cells [35]. These data do not necessarily contradict the findings of our current study. Sodium retention‒induced hypertension results in an augmented GFR and subsequent pressure natriuresis and nephropathy [36], despite the potential initial adaptive value of resetting of tubuloglomerular feedback in offsetting the renal sodium-retaining actions of aldosterone for tubular injuries [10]. Moreover, long-term excessive aldosterone concentrations have caused enduring hypertension due to increased arterial stiffness [37]. Growing evidence supports the idea that the nonhemodynamic actions of aldosterone are responsible for small- and medium-sized arterial injuries and nephropathy [6], as well as interstitial and renal vascular damage [38].
The hypertensive duration of PA is associated with heavy proteinuria and an increased renal resistance index [4]. The activation of mineralocorticoid receptors injures podocytes and disrupts the glomerular filtration barrier, leading to proteinuria and the progression of chronic kidney disease [39]. These findings raise the ultimate issues of early diagnosis and targeted treatment of PA with respect to kidney function.
A-2. Outcome in patients with PA/APA who underwent adrenalectomy or MRA treatment
Previous reports with a limited number of patients with PA showed that the subsequent rate of decline in glomerular filtration was comparable in PA and EH, whereas albuminuria did not progress in the remainder of the follow-up period [9]. Adrenalectomy not only corrects high blood pressure and biochemical parameters but also reverses adverse vascular changes in patients with APA [40]. This finding is also supported by the observation in a recent study that patients with PA who underwent adrenalectomy had reduced risk of mortality and lower blood pressure levels than their matched EH controls [33].
In our analysis, the long-term solid outcome of ESRD rate decreased after surgical intervention compared with MRA treatment alone in patients with EH or PA, although decreased short-term kidney function was found in patients with APA after adrenalectomy [4]. Improvements in renal function include microalbuminuria due to the resolution of glomerular hyperfiltration in PA after adrenalectomy [9, 41]. Our results suggest that patients with APA who respond to adrenalectomy demonstrate better outcomes in 10-year probability of incident ESRD than those receiving MRA alone (Fig. 3) Furthermore, in patients with PA who respond to adrenalectomy, the absence of residual hypertension is less likely to lead to ESRD.
MRA treatment may induce an increase in aldosterone level and subsequently trigger a vicious cycle that leads to the insufficient effect of prescribed MRA on blocking mineralocorticoid receptors activated by the high plasma aldosterone level. On the other hand, it is likely that glucocorticoid cosecretion exists in patients with PA and at least partially contributes to associated adverse metabolic risks, including insulin resistance, type 2 diabetes mellitus, and osteoporosis [42–45]. In recent studies, adrenalectomy in patients with APA decreased glucocorticoid secretion, restored osteoporosis, attenuated adverse metabolic risks, and improved quality of life [42, 44–46], findings attributed to decreased glucocorticoid levels in addition to mineralocorticoid excess. The previously mentioned studies indicate that glucocorticoid cosecretion in PA may relate to systemic effects, which could further explain the benefits of adrenalectomy relative to MRA treatment.
Our study also revealed the importance of performing a dose-response analysis of MRA use, incident ESRD, and mortality. Further concerns about MRA prescription, such as drug adherence with an inadequate dose (Fig. 4), also exist. Our additional analysis of the DDD of MRA on ESRD incidence also showed that only patients with PA who were exposed to a dose-dependent DDD >0.5 (dose equal to 37.5 mg of spironolactone) experienced decreased long-term ESRD. Dysmenorrhea and gynecomastia, presenting as adverse effects of antiandrogens, can result from spironolactone treatment, particularly in a dose-dependent relationship [47, 48]. These phenomena suggest that the usual doses of MRA in clinical practice may be too low; this problem warrants more attention.
A-3. Hypertensive duration and outcome in patients with PA
Our findings support the notion that adrenalectomy attenuates incident ESRD risk more than in patients with PA receiving MRA and in patients with EH. Patients with PA benefit from decreased blood pressure levels and use of fewer antihypertensive drugs after surgical management [49]. Hypertension in patients with PA can decrease within 1 year after surgery; sometimes it resolves generally or improves between 1 and 6 months after adrenalectomy [50]. As demonstrated by a prospective study, proteinuria and cystatin C (a marker of kidney function) dropped off soon after adrenalectomy but still failed to significantly decrease 1 year after the initiation of MRA treatment [4]. Given that adrenalectomy eliminates the source of aldosterone hypersecretion on the cardiovascular system, cerebrovascular system, and kidneys, it has been fully delineated that surgery successfully improves electrolyte levels and normalizes hypertension [51, 52]. According to the Primary Aldosteronism Surgical Outcome study, rates of partial to complete clinical success ranged from 37% to 47% [53]. Regarding to the incidence of ESRD, our study established the evidence that adrenalectomy decreased the risk in patients with major comorbidity, and the risk could be reduced up to 82% in those Charlsons score >0. Our result disclosed the great benefit of patients with PA and major comorbidities who receive adrenalectomy when compared with patients with EH. (Fig. 2).
A-4. Strengths and limitations
This study has some limitations. This was a retrospective cohort study, and some biochemical data could not be obtained. Because of characteristics of the NHI database of Taiwan, several potential confounders were lacking, including blood pressure, detailed renal function, proteinuria, and serum potassium, aldosterone, and renin levels, which are associated with response to treatment. This indeed limited our study, and further studies are needed to measure these effects. In addition, we focused on progression to ESRD after two different therapies as the renal outcome, rather than on developing or worsening of chronic kidney disease. Therefore, the absence of data such as estimated glomerular filtration rate(eGFR) and proteinuria level did not affect the renal outcome, as the registration of ESRD in the NHI database in Taiwan is credible and officially certified [54]. Moreover, we could not identify patients with PA and bilateral adenomas or unilateral hyperplasia, as the difference between them was ambiguous and always confused by micronodules. In fact, the incidence of the two subgroups is relatively low, and our results were robust across the different models, strengthening our conclusions. The diagnostic reliability of PA and its comorbidities was validated in detail in our cohort. Moreover, our analysis considered time-varying covariates, including MRA treatment and cumulative doses of MRA as a dose-dependent covariate in the model, ensuring that patients were at risk only when they had used these drugs.
Of note, the data retrieved and examined in this study came from health insurance registrations, for which diagnostic tests were not controlled as ideally as some single or multicenter clinical studies could have easily achieved. Nevertheless, these are real-life data with completed medical records that allowed us to scrutinize some large-scale, long-term follow-up outcomes in patients with PA among a population of 23 million people, according to ICD-9 diagnostic and current procedural terminology(CPT) coding. We believe this big data approach offers important insights into the understanding of PA, which has not been examined and could not be offered with smaller-scale multicenter studies. Further prospective trials are warranted to confirm the significantly beneficial effect of adrenalectomy on incident ESRD among patients with PA.
4. Conclusion
Although patients with PA had an incidence of ESRD compatible with that of patients with EH after targeted treatment, our results highlight the importance of early diagnosis of PA and appropriate adrenalectomy for patients who are suitable for surgery to lower the risk of long-term incident ESRD and improve survival. MRA management did not provide comparable renal function and survival advantages. Further prospective studies are necessary to confirm our results.
Acknowledgments
We thank Mr. Eric B. Chueh for English editing; the staff of the Eighth Core Lab, Department of Medical Research, National Taiwan University Hospital for technical support during the study. We appreciate and thank the members of the Taiwan Clinical Trial Consortium, TCTC (grant MOST 106-2321-B-182-002, 105-2314-B-002-045). We express our sincere gratitude to the staff of the Taiwan Clinical Trial Consortium, TCTC. Some of the authors are employed by two organizations financially supporting the study: National Taiwan University Hospital and National Health Research Institutes. The other funding organization, National Science Council, played no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; and in the preparation, review, and approval of the manuscript.
Financial Support: This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sector.
Disclosure Summary: The authors declare no conflict of interest.
Glossary
Abbreviations:
- APA
aldosterone-producing adenoma
- DDD
defined daily dose
- EH
essential hypertension
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- HR
hazard ratio
- ICD-9
International Classification of Diseases Ninth Revision
- MRA
mineralocorticoid receptor antagonist
- NHI
National Health Insurance
- NHIA
National Health Insurance Administration
- PA
primary aldosteronism
- sHR
subdistribution hazard ratio
References and Notes
- 1. Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811–1820. [DOI] [PubMed] [Google Scholar]
- 2. Valsan D, Burhan U, Teehan G. Resistant hypertension. Adv Exp Med Biol. 2017;956:181–189. [DOI] [PubMed] [Google Scholar]
- 3. Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62(2):331–336. [DOI] [PubMed] [Google Scholar]
- 4. Wu VC, Kuo CC, Wang SM, Liu KL, Huang KH, Lin YH, Chu TS, Chang HW, Lin CY, Tsai CT, Lin LY, Chueh SC, Kao TW, Chen YM, Chiang WC, Tsai TJ, Ho YL, Lin SL, Wang WJ, Wu KD; TAIPAI Study Group. Primary aldosteronism: changes in cystatin C-based kidney filtration, proteinuria, and renal duplex indices with treatment. J Hypertens. 2011;29(9):1778–1786. [DOI] [PubMed] [Google Scholar]
- 5. Halimi JM, Mimran A. Albuminuria in untreated patients with primary aldosteronism or essential hypertension. J Hypertens. 1995;13(12 Pt 2):1801–1802. [PubMed] [Google Scholar]
- 6. Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004;66(1):1–9. [DOI] [PubMed] [Google Scholar]
- 7. Kuo CC, Wu VC, Tsai CW, Wu KD; Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group). Relative kidney hyperfiltration in primary aldosteronism: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011;12(2):113–122. [DOI] [PubMed] [Google Scholar]
- 8. Sechi LA, Di Fabio A, Bazzocchi M, Uzzau A, Catena C. Intrarenal hemodynamics in primary aldosteronism before and after treatment. J Clin Endocrinol Metab. 2009;94(4):1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Catena C. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295(22):2638–2645. [DOI] [PubMed] [Google Scholar]
- 10. Wu VC, Yang SY, Lin JW, Cheng BW, Kuo CC, Tsai CT, Chu TS, Huang KH, Wang SM, Lin YH, Chiang CK, Chang HW, Lin CY, Lin LY, Chiu JS, Hu FC, Chueh SC, Ho YL, Liu KL, Lin SL, Yen RF, Wu KD; TAIPAI Study Group. Kidney impairment in primary aldosteronism. Clin Chim Acta. 2011;412(15–16):1319–1325. [DOI] [PubMed] [Google Scholar]
- 11. Dustan HP, Corcoran AC, Page IH. Renal function in primary aldosteronism. J Clin Invest. 1956;35(12):1357–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navar LG. Physiology: hemodynamics, endothelial function, renin-angiotensin-aldosterone system, sympathetic nervous system. J Am Soc Hypertens. 2014;8(7):519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muth A, Ragnarsson O, Johannsson G, Wängberg B. Systematic review of surgery and outcomes in patients with primary aldosteronism. Br J Surg. 2015;102(4):307–317. [DOI] [PubMed] [Google Scholar]
- 14. Steichen O, Lorthioir A, Zinzindohoue F, Plouin PF, Amar L. Outcomes of drug-based and surgical treatments for primary aldosteronism. Adv Chronic Kidney Dis. 2015;22(3):196–203. [DOI] [PubMed] [Google Scholar]
- 15. Wu VC, Hu YH, Wu CH, Kao CC, Wang CY, Yang WS, Lee HH, Chang YS, Lin YH, Wang SM, Chen L, Wu KD; TAIPAI Study Group. Administrative data on diagnosis and mineralocorticoid receptor antagonist prescription identified patients with primary aldosteronism in Taiwan. J Clin Epidemiol. 2014;67(10):1139–1149. [DOI] [PubMed] [Google Scholar]
- 16. Wu PC, Wu CJ, Lin CJ, Pan CF, Chen CY, Huang TM, Wu CH, Lin SL, Chen YM, Chen L, Wu VC; NSARF GroupKidney Consortium. Pentoxifylline decreases dialysis risk in patients with advanced chronic kidney disease. Clin Pharmacol Ther. 2015;98(4):442–449. [DOI] [PubMed] [Google Scholar]
- 17. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF Jr, Montori VM; Endocrine Society. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266–3281. [DOI] [PubMed] [Google Scholar]
- 18. Wu VC, Hu YH, Er LK, Yen RF, Chang CH, Chang YL, Lu CC, Chang CC, Lin JH, Lin YH, Wang TD, Wang CY, Tu ST, Jeff Chueh SC, Chang CC, Tseng FY, Wu KD; TAIPAI Group. Case detection and diagnosis of primary aldosteronism: the consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2017;116(12):993–1005. [DOI] [PubMed] [Google Scholar]
- 19. Wu VC, Lo SC, Chen YL, Huang PH, Tsai CT, Liang CJ, Kuo CC, Kuo YS, Lee BC, Wu EL, Lin YH, Sun YY, Lin SL, Chen JW, Lin SJ, Wu KD; TAIPAI Study Group. Endothelial progenitor cells in primary aldosteronism: a biomarker of severity for aldosterone vasculopathy and prognosis. J Clin Endocrinol Metab. 2011;96(10):3175–3183. [DOI] [PubMed] [Google Scholar]
- 20. Wu VC, Huang KH, Peng KY, Tsai YC, Wu CH, Wang SM, Yang SY, Lin LY, Chang CC, Lin YH, Lin SL, Chu TS, Wu KD. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep. 2015;5(1):11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu VC, Chueh SC, Chang HW, Lin LY, Liu KL, Lin YH, Ho YL, Lin WC, Wang SM, Huang KH, Hung KY, Kao TW, Lin SL, Yen RF, Chen YM, Hsieh BS, Wu KD; TAIPAI Study Group. Association of kidney function with residual hypertension after treatment of aldosterone-producing adenoma. Am J Kidney Dis. 2009;54(4):665–673. [DOI] [PubMed] [Google Scholar]
- 22. Wu VC, Chang HW, Liu KL, Lin YH, Chueh SC, Lin WC, Ho YL, Huang JW, Chiang CK, Yang SY, Chen YM, Wang SM, Huang KH, Hsieh BS, Wu KD; TAIPAI Study Group. Primary aldosteronism: diagnostic accuracy of the losartan and captopril tests. Am J Hypertens. 2009;22(8):821–827. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y-Y, Lin, Y-HH, Huang W-C, Chueh E, Chen L, Yang S-Y, Lin P-C, Lin L-Y, Lin Y-H, Wu V-C, Chu T-S, Wu KD. Data from: Adrenalectomy improves the long-term risk of end-stage renal disease and mortality of primary aldosteronism. figshare 2019. Deposited 25 February 2019 https://figshare.com/s/7b33324f216cdb755240. [DOI] [PMC free article] [PubMed]
- 24. Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, Chu TS, Chiang WC, Wu KD, Tsai PR, Chen L, Ko WJ; NSARF Group. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25(3):595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang WJ, Chao CT, Huang YC, Wang CY, Chang CH, Huang TM, Lai CF, Huang HY, Shiao CC, Chu TS, Chen YM, Wu VC, Ko WJ, Wu KD;National Taiwan University Study Group on Acute Renal Failure. The impact of acute kidney injury with temporary dialysis on the risk of fracture. J Bone Min Res. 2014;29(3):676–684. [DOI] [PubMed] [Google Scholar]
- 26. Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One. 2014;9(12):e112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu VC, Wu PC, Wu CH, Huang TM, Chang CH, Tsai PR, Ko WJ, Chen L, Wang CY, Chu TS, Wu KD; National Taiwan University Study Group on Acute Renal Failure (NSARF) Group. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–242. [DOI] [PubMed] [Google Scholar]
- 29. Wu VC, Shiao CC, Chang CH, Huang TM, Lai CF, Lin MC, Chiang WC, Chu TS, Wu KD, Ko WJ, Wang CY, Wang SM, Chen L. Long-term outcomes after dialysis-requiring acute kidney injury. BioMed Res Int. 2014;2014:365186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin YF, Ko WJ, Chu TS, Chen YS, Wu VC, Chen YM, Wu MS, Chen YW, Tsai CW, Shiao CC, Li WY, Hu FC, Tsai PR, Tsai TJ, Wu KD; NSARF Study Group. The 90-day mortality and the subsequent renal recovery in critically ill surgical patients requiring acute renal replacement therapy. Am J Surg. 2009;198(3):325–332. [DOI] [PubMed] [Google Scholar]
- 31. Uchino S, Doig GS, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Nacedo E, Gibney N, Tolwani A, Ronco C, Kellum JA; Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators. Diuretics and mortality in acute renal failure. Crit Care Med. 2004;32(8):1669–1677. [DOI] [PubMed] [Google Scholar]
- 32. Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev. 2015;24(10):1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee BC, Chang CC, Liu KL, Chang YC, Wu VC, Huang KH. Evaluation of right adrenal vein anatomy by Dyna computed tomography in patients with primary aldosteronism. Sci Rep. 2016;6(1):28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu VC, Wang SM, Chang CH, Hu YH, Lin LY, Lin YH, Chueh SC, Chen L, Wu KD. Long term outcome of aldosteronism after target treatments [published correction appears in Sci Rep. 2017;7:45249] Sci Rep. 2016;6(1):32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perry HM Jr, Miller JP, Fornoff JR, Baty JD, Sambhi MP, Rutan G, Moskowitz DW, Carmody SE. Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension. 1995;25(4):587–594. [DOI] [PubMed] [Google Scholar]
- 36. Fu Y, Hall JE, Lu D, Lin L, Manning RD Jr, Cheng L, Gomez-Sanchez CE, Juncos LA, Liu R. Aldosterone blunts tubuloglomerular feedback by activating macula densa mineralocorticoid receptors. Hypertension. 2012;59(3):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ribstein J, Du Cailar G, Fesler P, Mimran A. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol. 2005;16(5):1320–1325. [DOI] [PubMed] [Google Scholar]
- 38. Strauch B, Petrák O, Zelinka T, Wichterle D, Holaj R, Kasalický M, Safarík L, Rosa J, Widimský J Jr. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens. 2008;21(10):1086–1092. [DOI] [PubMed] [Google Scholar]
- 39. Lam EY, Funder JW, Nikolic-Paterson DJ, Fuller PJ, Young MJ. Mineralocorticoid receptor blockade but not steroid withdrawal reverses renal fibrosis in deoxycorticosterone/salt rats. Endocrinology. 2006;147(7):3623–3629. [DOI] [PubMed] [Google Scholar]
- 40. Zhu C, Huang S, Yuan Y, Ding G, Chen R, Liu B, Yang T, Zhang A. Mitochondrial dysfunction mediates aldosterone-induced podocyte damage: a therapeutic target of PPARγ. Am J Pathol. 2011;178(5):2020–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chang CH, Hu YH, Tsai YC, Wu CH, Wang SM, Lin LY, Lin YH, Satoh F, Wu KD, Wu VC. Arterial stiffness and blood pressure improvement in aldosterone-producing adenoma harboring KCNJ5 mutations after adrenalectomy. Oncotarget. 2017;8(18):29984–29995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sechi LA, Colussi G, Di Fabio A, Catena C. Cardiovascular and renal damage in primary aldosteronism: outcomes after treatment. Am J Hypertens. 2010;23(12):1253–1260. [DOI] [PubMed] [Google Scholar]
- 43. Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, Feuchtinger A, Chortis V, Gilligan LC, Ludwig P, Riester A, Asbach E, Hughes BA, O’Neil DM, Bidlingmaier M, Tomlinson JW, Hassan-Smith ZK, Rees DA, Adolf C, Hahner S, Quinkler M, Dekkers T, Deinum J, Biehl M, Keevil BG, Shackleton CH, Deeks JJ, Walch AK, Beuschlein F, Reincke M. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2(8):93136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91(2):454–459. [DOI] [PubMed] [Google Scholar]
- 45. Wu VC, Chang CH, Wang CY. , Lin YH, Kao TW, Lin PC, Chu TS, Chang YS, Chen L, Wu KD, Chueh SJ Risk of fracture in primary aldosteronism: a population-based cohort study. J Bone Min Res. 2017;32(4):743–752. [DOI] [PubMed] [Google Scholar]
- 46. Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, Wu KD, Yang WS; TAIPAI Study Group. Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017;35(8):1698–1708. [DOI] [PubMed] [Google Scholar]
- 47. Sukor N, Kogovsek C, Gordon RD, Robson D, Stowasser M. Improved quality of life, blood pressure, and biochemical status following laparoscopic adrenalectomy for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2010;95(3):1360–1364. [DOI] [PubMed] [Google Scholar]
- 48. Jeunemaitre X, Chatellier G, Kreft-Jais C, Charru A, DeVries C, Plouin PF, Corvol P, Menard J. Efficacy and tolerance of spironolactone in essential hypertension. Am J Cardiol. 1987;60(10):820–825. [DOI] [PubMed] [Google Scholar]
- 49. Karagiannis A, Tziomalos K, Papageorgiou A, Kakafika AI, Pagourelias ED, Anagnostis P, Athyros VG, Mikhailidis DP. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother. 2008;9(4):509–515. [DOI] [PubMed] [Google Scholar]
- 50. van der Linden P, Steichen O, Zinzindohoué F, Plouin PF. Blood pressure and medication changes following adrenalectomy for unilateral primary aldosteronism: a follow-up study. J Hypertens. 2012;30(4):761–769. [DOI] [PubMed] [Google Scholar]
- 51. Carey RM. Primary aldosteronism. J Surg Oncol. 2012;106(5):575–579. [DOI] [PubMed] [Google Scholar]
- 52. Carter Y, Roy M, Sippel RS, Chen H. Persistent hypertension after adrenalectomy for an aldosterone-producing adenoma: weight as a critical prognostic factor for aldosterone’s lasting effect on the cardiac and vascular systems. J Surg Res. 2012;177(2):241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF Jr, Gomez-Sanchez CE, Funder JW, Reincke M; Primary Aldosteronism Surgery Outcome (PASO) Investigators. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nan-Ping Y, Yi-Hui L, Chi-Yu C, Jin-Chyr H, I-Liang Y, Nien-Tzu C, Chien-Lung C. Comparisons of medical utilizations and categorical diagnoses of emergency visits between the elderly with catastrophic illness certificates and those without. BMC Health Serv Res. 2013;13(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]