Abstract
Thyroid dysfunction and diabetes mellitus are closely linked. Several studies have documented the increased prevalence of thyroid disorders in patients with diabetes mellitus and vice versa. This review critically discusses the different underlying mechanisms linking type 1 and 2 diabetes and thyroid dysfunction to demonstrate that the association of these two common disorders is unlikely a simple coincidence. We assess the current state of knowledge on the central and peripheral control of thyroid hormone on food intake and glucose and lipid metabolism in target tissues (such as liver, white and brown adipose tissue, pancreatic β cells, and skeletal muscle) to explain the mechanism linking overt and subclinical hypothyroidism to type 2 diabetes and metabolic syndrome. We also elucidate the common susceptibility genes and the pathogenetic mechanisms contributing to the autoimmune mechanism involved in the onset of type 1 diabetes mellitus and autoimmune thyroid disorders. An untreated thyroid dysfunction can impair the metabolic control of diabetic patients, and this association can have important repercussions on the outcome of both of these disorders. Therefore, we offer recommendations for the diagnosis, management, and screening of thyroid disorders in patients with diabetes mellitus, including the treatment of diabetic patients planning a pregnancy. We also discuss the major causes of failure to achieve an optimal management of thyroid dysfunction in diabetic patients and provide recommendations for assessing and treating these disorders during therapy with antidiabetic drugs. An algorithm for a correct approach of these disorders when linked is also provided.
Essential Points
Autoimmune thyroid dysfucntion occurs in 17% to 30% of adults with type 1 diabetes
Thyroid dysfunction is more common in patients with type 2 diabetes than in the general population
Preexisting diabetes mellitus is exacerbated by hyperthyroidism
Insulin treatment should be adjusted in patients with diabetes after the occurrence of thyroid dysfunction
Hyperglycemia should be reevaluated in hyperthyroid subjects after the control of thyroid dysfunction
Liraglutide is not recommended in patients with a personal or family history of medullary thyroid cancer or type 2 multiple endocrine neoplasia
Pioglitazone should not be administered to diabetic patients with clinically active Graves ophthalmopathy
Thyroid dysfunction (TD) and diabetes mellitus (DM) are two of the most frequent chronic endocrine disorders with variable prevalence among different populations.
The prevalence of TD in Europe and the United States is ∼6.6% in adults (1–3); it increases with age and is higher in women than in men. Both hyperthyroidism and hypothyroidism can develop in severe or subclinical forms (4). T3, the active thyroid hormone (TH), exerts a negative feedback at the level of both thyrotrophs in the pituitary and tanycytes in the hypothalamus; it induces a reduction in TRH, as well as TSH secretion in response to adequate tissue levels of TH. Therefore, subclinical thyroid disorders (STDs) are characterized by low or increased serum TSH with TH levels at the upper and lower limits of their reference range, respectively, in subclinical hyperthyroidism (SHyper) and subclinical hypothyroidism (SHypo) (4, 5). STDs are more frequent than overt diseases; they can be asymptomatic and, therefore, undiagnosed and untreated, leading to important adverse events (4, 5).
DM is also a frequent condition in the general population. The global prevalence of this disorder has nearly doubled since 1980, rising from 4.7% to 8.5% in the adult population (6). The National Health and Nutrition Examination Survey (NHANES) III reports that ∼14% of the adult US population suffers from either DM or an impaired fasting glucose levels (7). Data from the Centers for Disease Control and Prevention National Diabetes Fact Sheet estimate that DM may be frequently undiagnosed; ∼35% of the adults >20 years of age and 50% of those >65 years of age in the United States may have prediabetes based on fasting glucose or glycated HbA1c levels (8).
DM and thyroid disease are two closely associated disorders. The NHANES III study reported a higher prevalence of TD in subjects in the United States with diabetes compared with those without diabetes, especially in patients with positive anti-thyroperoxidase (TPO) antibodies (Abs) (3).
The aim of this review is to describe the effect of THs on glucose metabolism and assess the current state of knowledge on type 1 and 2 diabetes (T1D and T2D) and coexisting TD, the prevalence of these two associated diseases, and the underlying mechanisms linking these conditions. Both hyperthyroidism and hypothyroidism can impair the metabolic control in patients with diabetes. For this reason, we will discuss the consequences of TD in patients with diabetes and the prognostic implications of these associated comorbidities. Current evidence suggests the necessity of treating TD in patients with DM to improve their prognosis. Therefore, we will offer recommendations for the management of TD in patients with both subclinical and overt thyroid disorders and DM, including the treatment of patients with diabetes who are planning a pregnancy and during pregnancy. To aid clinicians in daily practice, we will provide an algorithm for the evaluation and treatment of TD and DM examined from a global viewpoint. Finally, given the relatively high prevalence of both TD and DM and the serious nature of their prognosis, especially when correlated, we will discuss the need to screen the onset of thyroid disease in patients with diabetes. To this point, we will talk about the controversies regarding the screening program among different guidelines
Methods
Identification of sources
We searched for personal files, MEDLINE articles, meta-analyses, and references of relevant articles and textbooks published from 1977 to 2018, as well as citations from recently published international guidelines.
The following search terms were used: thyroxine, triiodothyronine, hypothyroidism, hyperthyroidism, subclinical thyroid disease, type 1 and type 2 diabetes mellitus, insulin resistance, metabolic syndrome, gestational diabetes, prevalence, incidence, deiodinases, glucose metabolism, lipid metabolism, adipose tissue, appetite regulation, skeletal muscle, screening, morbidity, and mortality.
Methods of evaluation
A critical assessment of the literature was performed. The authors agreed on the criteria for the inclusion or exclusion of the studies considered. Preference was given to high-quality papers, meta-analyses, randomized controlled and longitudinal trials, and studies performed with correct statistical analysis and accurate methods. When identified, limitations in the study design or execution were also discussed.
TD and T1D
Prevalence of TD in patients with T1D
T1D is due to autoimmune β cell destruction, usually leading to absolute insulin deficiency. This disorder is closely associated with autoimmune-induced TD in clinical practice because these endocrine diseases are linked by the same pathophysiological mechanism. They share an autoimmune predisposition, and some genetic factors might contribute to the co-occurrence of autoimmune thyroid disease (AITD) and T1D (9, 10). An increased risk for thyroid autoimmunity has been reported in adults with T1D and late-onset autoimmune-induced diabetes (10–13).
AITD occurs in 17% to 30% of adults with T1D; these patients are at an increased risk of both autoimmune-induced hypothyroidism [Hashimoto thyroiditis, (HT)] as well as hyperthyroidism [Graves disease (GD)] (10–13). T1D patients develop TD at an early age compared with the general population, and therefore autoimmune hypothyroidism is present in 25% of children with T1D (14, 15). Its onset is associated with a more aggressive presentation of TD and poorly controlled diabetes in pediatric patients with T1D (14, 15) [see Table 1 (10–15)].
Table 1.
Prevalence of TD in T1D
| Prevalence | Associated Risk Factors |
|---|---|
| Risk of AITD in adults with T1D | 17%–30% |
| Risk factors for AITD | Female sex, TPO Ab-positive, duration of diabetes |
| Risk of hypothyroidism in children with T1D | 25% |
| Risk factors for hypothyroidism in children | Poorly controlled diabetes |
According to the results of the HUNT study, a population-based study in Nord-Trøndelag, Norway, adult women with T1D have about a twofold higher risk of having hypothyroidism, whereas men with T1D have an approximate fourfold higher risk for developing hypothyroidism, with an increased prevalence in patients with positive TPO Abs (16). Patients with T1D and TPO Ab positivity are 18-fold more likely to develop hypothyroidism compared with type 1 patients with diabetes with TPO negativity during a period of 18 years (17). The onset of TD is frequently associated with duration of diabetes (18).
Association of T1D and AITD
The association of AITD and T1D as two autoimmune-induced endocrine disorders is denominated as autoimmune polyglandular syndrome type 3 variant (APS3). T1D and AITD may also coexist within both the very rare juvenile APS type 1 (encompassing autoimmune hypoparathyroidism and primary hypogonadism) as well as within the APS adult type 2 with Addison disease as the primary endocrine component. However, both in APS1 and APS2, AITD and T1D neither define the diagnosis nor are they the major endocrine components (19, 20). The prevalence of APS3 is ∼1:20,000 (19). It occurs more frequently in women. The male-to-female ratio is 1:3. The incidence of APS3 peaks at ages 20 to 60 years, mostly in the third or fourth decade (20). AITD peaks in the fourth decade for GD or fifth and sixth decade for HT. The simultaneous occurrence of autoimmune-induced hypothyroidism and T1D leads often to hypoglycemia due to decreased insulin requirement and increased insulin sensitivity. Glucose intolerance accompanies autoimmune hyperthyroidism in 50% of patients. In APS3, circulating organ-specific Abs are present in each of the component diseases. Occasionally, Abs will cross-react with more than one gland. Abs usually precede clinical disease; however, in contrast to anti-islet Abs, anti-thyroid Abs can be present for decades without progression to overt disease. Current diagnosis of APS3 involves serological measurement of organ-specific Abs and subsequent functional testing, that is, baseline TSH, FSH, LH, free T4, testosterone, estradiol, fasting morning glucose, and cortisol, an ACTH stimulation test (when adrenal Abs are present), as well as serum Na+, K+, Ca, and blood cell count (19). Management of patients with APS, including their family relatives, is recommended in centers with special expertise in autoimmune endocrine disorders.
Joint susceptibility genes in AITD plus T1D and pathogenic mechanisms contributing to polyglandular autoimmunity
APS3 is a genetically complex and multifactorial syndrome (21). Several genetic loci possibly interact with environmental factors. APS3 is characterized by a complex inheritance pattern. Family and population studies showed that APS3 has a strong genetic background. Whole-genome and candidate gene approaches identified several gene variations, which are present in both T1D and AITD. For APS3, disease susceptibility genes are human leukocyte antigen (HLA) on chromosome 6, protein tyrosine phosphatase nonreceptor type 22 (PTPN22) on chromosome 1, cytotoxic T lymphocyte antigen (CTLA) on chromosome 2, forkhead box P3 (FOXP3) on the X chromosome, and IL-2 receptor α (IL-2Rα) gene region on chromosome 10 (22). These genes are involved in the immune regulation and T cell activation within the immunological synapse (Table 2). Further candidate genes with joint risk for AITD and T1D are the v-erb-b2 erythroblast leukemia viral oncogene homolog 3 (ERBB3) gene on chromosome 12, C-type lectin domain family 16 member A (CLEC16A) on chromosome 16 (involved in pathogen recognition), the proinflammatory cytokine TNF-α gene, major histocompatibility complex (MHC) class I chain-related gene A (MICA), the VNTR (insulin) gene, and the CD40 gene. Thus, T1D and AITD share common susceptibility gene variants, which possibly act pleiotropically as risk factors for the development of autoimmunity in APS3.
Table 2.
Joint Susceptibility Genes for T1D and AITD
| Gene | Chromosome | Function |
|---|---|---|
| Confirmed joint susceptibility genes | ||
| HLA class II genes | 6 | Presents autoantigens to T cells |
| PTPN22 | 1 | Negatively influences T cell receptor signaling pathway |
| CTLA-4 | 2 | Suppresses T cell activation |
| FOXP3 | X | Controls differentiation of regulatory T cells |
| Candidate susceptibility genes | ||
| IL-1RA | 2 | Implicated in pathogenesis of autoimmune diseases |
| IL-4 | 5 | Implicated in Th2 humoral immunity |
| MICA | 6 | NKG2D receptors stimulate NK cells and T cell effector functions |
| TNF-α | 6 | Implicated in pathogenesis of autoimmune diseases |
| Tg | 8 | Represents a major target of the immune response in AITD |
| IL2RA/CD25 | 10 | Impacts production and function of regulatory T cells |
| VNTR (insulin) | 11 | Alters transcription of the insulin gene |
| ERBB3 | 12 | Not defined |
| CLEC16A | 16 | Implicated in pathogen recognition |
| CD40 | 20 | Interacts with CD40 ligand on T cells |
Abbreviations: CD40, B cell–associated molecule CD40; CLEC16A, C-type lectin domain family 16 member A; ERBB3, v-erb-b2 erythroblast leukemia viral oncogene homolog 3; IL-1RA, IL-1 receptor antagonist; IL2RA, IL-2Rα; MICA, MHC class I chain-related gene A; NK, natural killer; NKG2, NK cell group 2 (group of genes that are expressed primarily in NK cells encoding a family of C-type lectins; the NKG2D gene is expressed as a major 1.8-kb and a minor 3.2-kb transcript in NK cell lines and in some T cell lines); Tg, thyroglobulin; VNTR (insulin), insulin gene variable number of an 86-tandem repeat [a penta-allelic 86-bp tandem repeat (VNTR) occurs in intron 2, of which allele 2 (IL1RN*2) is associated with autoimmune conditions].
T1D and AITD are both organ-specific T cell–mediated diseases. All four confirmed joint susceptibility genes identified for APS3 are involved in the immunological synapse and T cell activation: the HLA-DR molecules present autoantigens to T cells, PTPN22 negatively influences the T cell receptor signaling pathway, CTLA-4 suppresses T cell activation, and FOXP3 regulates the differentiation of regulatory T cells (23).
APS3 is strongly associated with certain alleles of the HLA genes within the MHC (Table 2). HLA class II is a potential gene locus for combined susceptibility to T1D and AITD, as has been shown in whites and Asians (24–33). The gene products of the HLA class II genes are involved in immune reactions. The different HLA class II alleles are characterized by different affinities for peptides. Therefore, some autoantigenic peptides may be recognized by T lymphocyte receptors, whereas others may not (34). Most family studies gave evidence that the haplotype HLA-DR3-DQB1*0201 is the primary haplotype conferring susceptibility to both T1D and AITD within families (29). Here, DR3 seems to be the primary allele conferring risk to both T1D and AITD, whereas DQB1*0201 is less relevant. Many population studies indicate that both HLA haplotypes DR3-DQB1*0201 and DR4-DQB1*0302 contribute to APS3 (24, 35). The HLA-DRB1*03 allele was strongly increased in patients with APS3 (51%) vs both controls [22%, P < 0.0001; relative risk (RR), 2.32; 95% CI, 1.62 to 3.33] and monoglandular autoimmune disease (11%, P < 0.0001). HLA-DRB1*03 was highly prevalent in APS3 patients with early vs late disease onset (P < 0.05). HLA-DRB1*04 allele carriers were more present in APS3 vs controls (53% vs 22%, P < 0.0001; RR, 2.38; 95% CI, 1.68 to 3.38). Furthermore, HLA-DQB1*02 was increased in APS3 vs controls (P < 0.01), whereas HLA-DQB1*06 was decreased (P < 0.001). Thus, HLA-DRB1*03 is a stronger genetic marker in APS3, foremost in those with early disease onset (36).
The different HLA class II alleles show different pocket II structures and different affinities for peptides (37). There are two mechanisms by which HLA class II variants could be involved in the common etiology of T1D and AITD. The first mechanism refers to the structure of the HLA pockets, coded by the HLA class II alleles, and the second mechanism refers to the peptide binding (38, 39). First, two distinct HLA class II molecules (e.g., DQB1 for T1D and DR3 for AITD) with distinct pocket structures are in tight linkage disequilibrium, and thereby they are inherited together and expressed on antigen-presenting cells (APCs) together. Thus, both islet cell peptides and thyroid-derived peptides will fit in these pockets. Second, two distinct HLA class II molecules share a similar HLA class II pocket structure fitting both islet cell peptides as well as thyroid-derived peptides (40). The common pocket structure could also influence the anchoring of the T cell receptor and not the peptide binding.
The PTPN22 gene maps on chromosome one location 1p13 (41). This gene encodes the lymphoid tyrosine phosphatase (LYP) protein. Both immature and mature B and T lymphocytes express LYP, which is a negative regulator of signal transduction through the T cell receptor. LYP inhibits the T lymphocyte antigen receptor signaling pathway (42) and binds to protein kinase Csk, thereby limiting the response to antigens (43). LYP associates with the molecular adaptor protein CBL and may be involved in regulating CBL function in the T cell antigen receptor signaling pathway. It binds to Csk, thereby limiting the response to antigens. A single nucleotide polymorphism (SNP) in the PTPN22 gene, a 1858 C→T transition, causing a tryptophan for arginine substitution in the LYP protein (R620W), is associated with T1D, AITD, and vitiligo (44–48). Alternative splicing of this gene results in two transcript variants encoding distinct isoforms of the protein. The minor T allele is associated with T1D and AITD (49–51). This is involved in altered T lymphocyte activation. In Asian patients, a novel SNP in the promoter region of the PTPN22 gene, G1123C, has been associated with T1D and AITD (51). Additional candidate polymorphisms may be also causative (52). In an association study, 310 white subjects with APS3, AITD, or T1D or healthy controls were genotyped for the C1858T polymorphism (53). The PTPN22 1858 minor T allele frequency was strongly increased in patients with APS3 (24%) compared with controls (8.0%, P < 0.001), with patients with AITD only (9%, P < 0.006), or with T1D only (11%, P < 0.028). T allele carriers were also more frequently present in the group with APS3 vs controls (41% vs 14%; OR, 4.35; 95% CI, 2.08 to 9.09), AITD (17%; OR, 3.42; 95% CI, 1.56 to 7.48), and T1D (21%; OR, 2.59; 95% CI, 1.23 to 5.45). Especially in subjects with both HT and T1D, T allele carriers were mostly frequent (50% vs 14%; OR, 6.14; 95% CI, 2.62 to 14.38; P < 0.001). Considering all included patients with AITD, T allele carriers were 29% vs 14.0% in controls (P < 0.008; OR, 2.54; 95% CI, 1.30 to 4.98). Patients carrying the PTPN22 1858 T allele had a twofold increased frequency of the HLA-DRB1*03 allele (65% vs 37%, P < 0.034). Finally, in the first performed genome-wide association study in patients with both T1D and AITD, the PTPN22 gene on chromosome 1 was recognized as joint susceptibility locus with a significantly increased log score (54).
The CTLA-4 gene encodes a negative regulator of T cell activation, which is expressed on the surface of activated T lymphocytes. It is involved in the interaction between T lymphocytes and APCs (55). APCs present to the T lymphocyte receptor an antigenic peptide bound to an HLA class II protein on the cell surface, thus activating T lymphocytes. Furthermore, costimulatory signals on the APC surface interact with receptors (e.g., CTLA-4) on the surface of CD4+ T lymphocytes during antigen presentation. CTLA-4 downregulates T lymphocyte activation (56). A genetic variant that decreases CTLA-4 function and therefore increases T cell activation might promote development of autoimmunity in APS3. CTLA-4 polymorphisms are associated with AITD (45). In contrast, findings are inconsistent with respect to the association of CTLA-4 and T1D, suggesting a weak effect (57–61). A 3′ untranslated region (AT)n microsatellite polymorphism with longer and shorter repeats of AT are related to autoimmunity, whereas longer repeats are associated with decreased inhibitory function of CTLA-4 (62). Longer repeats correlate with a shorter half-life of the CTLA-4 mRNA than do shorter repeats (63). The CTLA-4 AT repeat affects the inhibitory function of CTLA-4 in that the long AT repeat allele is associated with a reduced control of T cell proliferation in patients with GD (62). The causative CTLA-4 gene polymorphism for autoimmunity may be located in the 3′ untranslated region of the CTLA-4 gene. CTLA-4 CT60, another CTLA-4 gene polymorphism, was analyzed in patients with APS3, AITD, T1D, and in healthy controls (53). The CT60 G/G genotype was significantly more common in patients with APS3 than in healthy controls (49% vs 32%; OR, 2.01; 95% CI, 1.07 to 3.77; P = 0.038). The CT60 allele frequencies differed as well between APS3 patients and controls, with the predisposing G allele being increased in APS3 (OR, 1.63; 95% CI, 1.03 to 2.55; P = 0.042). Patients with APS3 did not differ from those with AITD or T1D. Another A/G49 SNP results in a threonine-to-alanine substitution in the signal peptide of the CTLA-4 protein. This leads to a less efficient glycosylation in the endoplasmic reticulum and reduced surface expression of the CTLA-4 protein (64), which negatively affects CTLA-4 function or expression, resulting in increased T cell activation.
The FOXP3 gene modulates the differentiation of regulatory T cells (65). A reduced function, due to genetic variants, could promote the development of autoimmunity in APS3. Both a haplotype consisting of allele 10 of a microsatellite and the T allele of a C/T SNP were related with APS3 (40). Because the microsatellite is located past the zinc finger domain of the FOXP3 gene, it could affect downstream splicing, thereby impeding the function of the gene.
The IL-2Rα/CD25 gene impacts production and function of regulatory T cells actively suppressing autoreactive T cells in the periphery (66). Polymorphisms in the CD25 gene region might affect the function of regulatory T cells, and thereby could influence the development of the autoimmune diseases T1D and AITD (67). The CLEC16A gene contains a C-type lectin domain, and the encoded protein is detected in immune cells (68). It is implicated in pathogen recognition and might predispose for immune-mediated diseases.
The proinflammatory cytokine TNFα gene is located within the class III region of the MHC between HLA-B loci of class I and HLA-D loci of class II. It encodes the proinflammatory cytokine TNFα. The uncommon A allele of the TNFα −308 SNP is associated with increased transcription and production of the TNFα protein, which has been implicated in the pathogenesis of autoimmune diseases (69, 70). The putative association between a polymorphism of the TNFα −308 and APS3 was analyzed (71). The TNFα −308*A allele occurred more frequently in patients (0.27) than in controls (0.16, P = 0.008). Also, TNFα −308*A carriers were more frequent in patients than controls (48% vs 31%; OR, 1.89; 95% CI, 1.19 to 3.00). The frequency of the AA genotype was increased in APS3 (P = 0.014). APS3 patients with AITD and the TNFα −308 AA genotype showed the highest prevalence of thyroid autoantibodies. Finally, HLA-DRB1*03 and TNFα −308*A alleles were strongly associated in patients with APS3 (88%, P < 0.00001). Collectively, these findings indicate similar immunogenetics of T1D and AITD.
Genetic mitochondrial diabetes
DM has been reported in mitochondrial diseases caused by autosomal recessive mutations in the nuclear genes POLG, RRM2B, OPA1, and MPV17 (72). Mitochondrial dysfunction can lead to type 1 or type 2 DM. The average age of onset of this disorder is 38 years for the common m.3243A>G mutation, and 40 to 56 years for other mutations. Individuals with m.3243A>G and DM have combined insulin deficiency and insulin resistance and a high risk of progression of their dysfunction. Genetic mitochondrial disease should be suspected in patients with endocrine dysfunction (DM, ovarian failure, adrenal insufficiency, and hypoparathyroidism) and associated with multisystem disease. However, tyroid dysfunction has been infrequently reported in mitochondrial disease. A polyendocrinopathy including DM, adrenal insufficiency, and hypothyroidism was reported in twins with a heterozygous POLG mutation p.G517V27 (72).
Maturity‐onset diabetes and association between hepatocyte nuclear factor-1α and thyroid cancer
Maturity-onset diabetes of the young (MODY) is a monogenic form of DM characterized by autosomal dominant inheritance and early age of onset (<25 years) (73). Mutations in the hepatocyte nuclear factor (HNF)-1α or HNF-1β genes are responsible for MODY type 3 and MODY type 5, respectively (73). Interestingly, HNF-1α may also influence carcinogenesis. HNF-1β is expressed in papillary cancer cell lines with high human nicotinamide N-methyltransferase gene expression (74). It is not expressed in other papillary, follicular, and Hürthle cancer cell lines and in primary cultures of normal thyroid cells and benign thyroid conditions. Moreover, both HNF-1α mRNA and protein have been detected in anaplastic thyroid cancer cell lines, suggesting a potential role of HNF-1α in more aggressive forms of thyroid cancer (75). Further studies on the function of HNF-1α and HNF-1β in thyroid cancer cells should be performed to individualize molecular targeted therapy in the future.
Underlying Mechanisms of the Association Between TD and Type 2 DM
Prevalence of TD in patients with T2D
T2D is due to a progressive loss of β cell insulin secretion commonly on the background of insulin resistance (76). In 2013 it was estimated that ∼382 million people had DM, of whom 90% to 95% had T2D (76). According to the World Health Organization, the prevalence of DM is expected to increase to 592 million by 2035, developing in ∼7.8% to 8.8% of adults (77) with an epidemic risk of T2D in populations such as China, Oceania, South and Central Asia, Latin America, and the Middle East (78–80). This increasing prevalence of T2D worldwide is probably due to unhealthy lifestyles and the increasing aging population. Insulin resistance, defined as the inability of insulin to increase glucose uptake and utilization in peripheral tissues (muscle, adipose tissue and liver), is a very early event in the pathogenesis of T2D, inducing β cell dysfunction (81, 82). Insulin resistance and the underlying metabolic abnormalities (overnutrition, obesity, and poor physical exercise and inactivity) can be present for years before the onset of hyperglycemia and the clinical diagnosis of T2D. During its early stages, β cells compensate for insulin resistance by increasing insulin secretion to ensure an appropriate glucose uptake and metabolism in peripheral tissues. However, β cells are unable to support persistent hyperinsulinemia and, subsequently, postprandial hyperglycemia can develop with the onset of overt T2D in adults. Insulin resistance can occur as part of a cluster of cardiovascular and metabolic abnormalities commonly identified as metabolic syndrome (MetS) (83). This disorder is recognized as an independent risk factor for T2D and cardiovascular disease (CVD), leading to the development of hypertension and accelerated atherosclerosis or polycystic ovarian syndrome, in relationship to the age of the patients and the genetic background (84, 85). TD is more common in patients with T2D than in the general population and can adversely influence the metabolic control. Goiter has been recognized as a risk factor for TD in patients with DM, as observed in nondiabetics (86), and parity is a risk factor for TD in women with diabetes (87). The overall prevalence of TD in patients with DM in studies from Europe and Saudi Arabia ranges from 4% to 20% (80, 88).
A few studies have prospectively investigated the relationship between TD and the incidence of diabetes (89–91). Two Danish register-based studies have reported conflicting results (89, 91). A nationwide registry study reported an increased risk of DM in individuals with hyperthyroidism (89), whereas two other studies reported an increased risk of DM in patients with hypothyroidism (90, 91).
Hyperthyroidism and T2D
Prevalence and risk of progression
The prevalence of hyperthyroidism in patients with diabetes is higher than in the general population (92); it was found in 4.4% of adult patients with T2D (92), while SHyper was present in ∼2% to 4% of T2 patients with diabetes (93, 94). New diagnosis of SHyper in patients with T2D was higher in females than in males (4.3% vs 3.5%), and the relative risk was significantly increased in females only (94). Advanced age and the presence of goiter are significantly and independently correlated with the presence of SHyper in the population with diabetes, suggesting that toxic multinodular goiter is a more frequent cause of hyperthyroidism than GD (95). The presence of T2D does not predict the incidence of hyperthyroidism in the elderly population with diabetes (96).
Hypothyroidism and T2D
Prevalence of hypothyroidism in T2D
TH deficiency is unlikely to be a coincidence in patients with T2D because the prevalence of hypothyroidism is higher in patients with diabetes than in the general population. Subclinical and overt hypothyroidism are the most common form of TD in T2D and MetS (97, 98). The prevalence of hypothyroidism in T2D ranges from 6% to 20% in epidemiologic studies across different ethnic groups (Table 3) (99–104). This wide range could reflect differences in age, sex, and iodine intake in the populations surveyed. Female sex, older age, obesity, TPO Ab positivity, and hospitalization are associated with an increased risk of developing hypothyroidism in T2D (97, 99–102). A significant increased risk of hypothyroidism was observed in patients with T2D >65 years of age with an OR of 4.2 and a clear difference between males and females (OR, 4.82 vs 2.60), patients with obesity and without obesity (OR, 2.56 vs 3.11), and presence or absence of thyroid autoantibodies (OR, 4.26 vs 2.93) (100).
Table 3.
Prevalence of TD in T2D
| Prevalence | Associated Risk Factors |
|---|---|
| Risk of hypothyroidism in adults with T2D | 6%–20% |
| Risk factors for hypothyroidism | Female sex, TPO Ab+, advanced age, hospitalization |
| Risk of hypothyroidism in T2D >65 years of age | OR, 4.82 males vs 2.60 females |
| OR, 2.56 obese vs 3.11 nonobese | |
| OR, 4.26 TPO+ vs 2.93 TPO− | |
| Risk of SHypo in T2D | 10.2% |
| Prevalence of subclinical hypothyroidsm in adults with T2D vs healthy controls* | 1.93-Fold increased risk (95% CI, 1.66–2.24) |
A large longitudinal study from Australia in women with T2D reported that SHypo is a common finding in T2D (97). It was the prevalent form of TH deficiency in females with diabetes (103, 104) and patients with positive TPO Abs (96, 100–102). In line with these results, a meta-analysis on 36 articles confirmed a higher pooled prevalence of SHypo in patients with T2D when compared with healthy controls (1.93-fold increased risk; 95% CI, 1.66 to 2.24) (103). It was associated with an increased risk of diabetic microvascular complications (104).
Changes in TSH and/or TH in longitudinal studies and incidence of diabetes
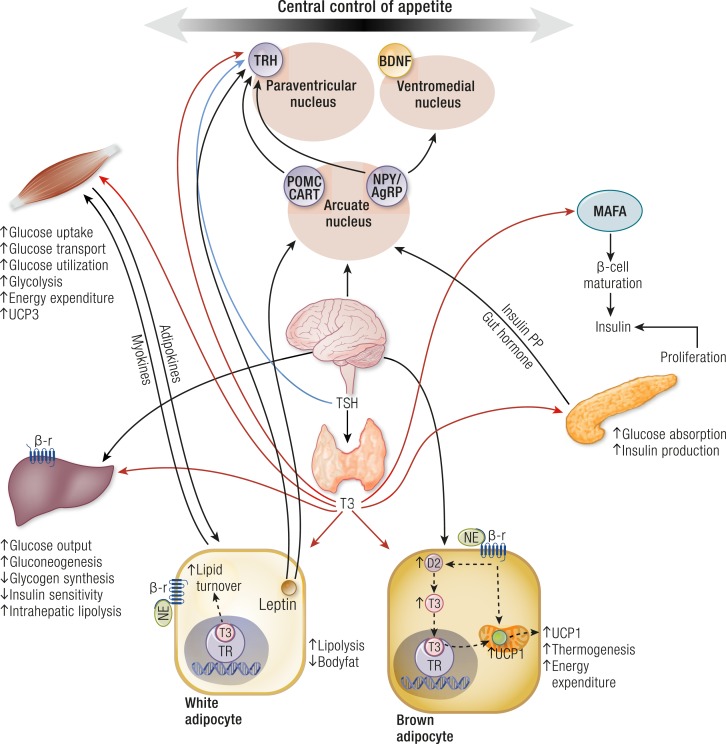
Serum TSH was positively associated with hyperglycemia and insulin resistance in euthyroid subjects in several studies (105–107). TSH may directly affect metabolic parameters and stimulate leptin secretion in human adipose tissue (108–111). It exerts an important role in hepatic glucose metabolism with stimulative effects on hepatic glucose production in vivo and in vitro (112, 113). TSH increases the expression of glucose 6-phosphate and phosphoenolpyruvate carboxykinase (PEPCK) at the mRNA level in a mouse liver (112, 113). Moreover, TSH reduces insulin secretion and its synthesis from pancreatic β cells and consequently increases serum blood glucose levels (108–110). Leptin is an important neuroendocrine regulator of the hypothalamic–pituitary–thyroid (HPT) axis; it acts directly by regulating TRH gene expression in the paraventricular nucleus (PVN) and indirectly by regulating TRH via effects in the arcuate nucleus (ARC) (108–115) (Fig. 1). Leptin levels correlate with TSH levels and are elevated in patients with hypothyroidism (108–110). Moreover, leptin levels are elevated in many patients with diabetes and might stimulate the synthesis of TSH by affecting the HPT axis via Janus activating kinase (JAK)-2/signal transducer and activator of transcription (STAT)3 factor (114, 115).
Figure 1.
Central and peripheral regulation of TH on food intake, glucose and lipid metabolism in target organs such as liver, white and brown adipose tissue, pancreatic β cells, and skeletal muscle. AgRP, agouti-related protein; BDNF, brain-derived neurotrophic factor; CART, cocaine- and amphetamine-regulated transcript; D2, deiodinase type 2; NE, norepinephrine; NPY, neuropeptide Y; POMC, proopiomelanocortin; PP, pancreatic polypeptide; SNS, sympathetic nervous system; α-MSH, α-melanocyte–stimulating hormone; β-r, β-receptor.
Some prospective studies have investigated whether changes in serum TSH and/or THs were associated with the risk for developing T2DM. In a large longitudinal study on a euthyroid population without diabetes, Jun et al. (116) assessed the association between consecutive changes in serum TSH from the baseline values and the incidence of T2D during a 6-year follow-up. Cox proportional hazard models showed that the risk of incident T2D was significantly increased with each 1 mU/L increment of serum TSH; in particular, this risk was increased in subjects with the highest TSH change tertile compared with the lowest tertile (hazard ratio, 1.25; 95% CI, 1.05 to 1.48; P for trend = 0.011). Changes in serum TSH correlated with changes in gylcated hemoglobin (HbA1c), which represents the main indicator of the average glycemic control during 2 to 3 months (116). The same group of authors reported that individual changes in TSH and THs, even within the normal reference range, were an additional risk factor of incident T2DM during a 7-year longitudinal study on 6235 euthyroid subjects without T2D (117). A progressive increase in TSH with a decrease in T3 and FT4, suggesting the development of a more severe form of hypothyroidism, was independently associated with the risk of developing T2D regardless of sex and thyroid autoimmunity. An increase in TSH from baseline (range, –4.1 mU/L to +12.3 mU/L) was associated with a higher risk of T2D (HR, 1.27; 95% CI, 1.14 to 1.40 per SD). An increase in FT4 (range, –0.60 to +1.60 ng/dL) or T3 (range, –76.5 to +223 ng/dL) was associated with a lower risk of incidence of T2D. T3 directly increases islet β cell mass pathways (118) and controls insulin secretion (119) and intracellular glucose availability. These results suggest that subtle changes in the levels of serum TSH and THs, even within the physiological range, can induce insulin resistance or diabetes (120, 121).
The Rotterdam Study, a large prospective population-based cohort study, investigated the association of thyroid function with the incidence of T2D and the progression from prediabetes to diabetes (122). Higher TSH levels and lower FT4 levels were associated with an increased risk of diabetes and progression from prediabetes to diabetes. On the contrary, high and high-normal thyroid function was protective against the development of and/or progression to T2D (122).
A recent large cross-sectional study from China has found that decreased FT3, decreased FT3/FT4 ratios, and increased FT4 levels were independently related to a higher prevalence of T2D in both males and females (123). A higher prevalence of T2D had a negative correlation with FT3 and a positive correlation with FT4, even after the adjustment for confounding factors in both males and females. The decreased FT3/FT4 ratio could be considered the indicator of the inhibition of the peripheral deiodinase activity, which can lower the basal metabolic rate and explain the pathogenesis of T2D or could reflect the low T3 syndrome in inadequately controlled patients with diabetes (124, 125). In a further study, hypothyroidism increased the risk of developing diabetes (RR, 2.06; 95% CI, 1.42 to 2.99) (90), also showing that the risk of DM was also prominent in statin users with SHypo (90). The RR was 1.94 (95% CI, 1.13 to 3.34) and 1.20 (95% CI, 0.52 to 2.75) in statin users and nonusers, respectively (90). Mitochondrial dysfunction can represent the common mechanism related to both DM and TD that can be aggravated by statins. In fact, hypothyroidism and statins can both induce mitochondrial dysfunction (126–129). Interestingly, in this study, patients with hypothyroidism treated with replacement doses of L-T4 were not at increased risk for DM (90)
Risk of progression of TH deficiency in T2D
There is an increased risk of progression from subclinical to overt hypothyroidism in patients with T2D, especially in women (104). This progression may be deleterious in patients with T2D because the onset of overt TD may deteriorate glucose control in patients with diabetes. A progression rate of 5% per year from SHypo to overt Hypo was reported in patients with diabetes with positive TPO Abs (104). Both AITD and female sex are risk factors for progression of TD among patients with diabetes. Although the duration of diabetes can be a risk for AITD in children and adolescents with T1D (16), it is not the case in those suffering from T2D (84).
Central and Peripheral Effects of THs on the Regulation of β Cell Function, Glucose Tolerance, Hepatic Glucose Production, and Peripheral Glucose Utilization
TH exerts profound effects on the regulation of glucose homeostasis and lipid metabolism. These effects are mediated both throughout the central nervous system and the direct interaction of THs with peripheral target organs such as liver, white and brown adipose tissues (WAT and BAT, respectively), pancreatic β cells, and skeletal muscle (130) (Fig. 1).
Regulation of hepatic glucose and lipid metabolism
TH receptors (TRs) α1 and β1 are important for normal pancreatic islet development (131). Neonatal β cells have TH receptors, and their exposure to T3 determines the activation of the transcription factor MAFA, which stimulates β cell maturation and insulin secretion. Moreover, T3 promotes proliferation of pancreatic islet cells (132). It increases proinsulin mRNA expression (131) and also acts as a mitogenic prosurvival factor for pancreatic β cells through a mechanism that seems to involve MAPK/ERK activation (133). T3 is a physiological regulator of β cell function. It controls insulin secretion and glucose uptake, acting differently in the liver, skeletal muscle, and adipose tissue, which are the main targets of insulin action (130, 134). TH has insulin-antagonistic effects in the liver, whereas it acts synergically with insulin in the peripheral tissues. TH increases hepatic glucose output through increased hepatic expression of glucose transporter (GLUT)2 and stimulates the endogenous production of glucose through the increase in gluconeogenesis and glycogenolysis, which is responsible for the decrease of liver sensitivity to insulin (135). Treatment with TH increases alanine transport into hepatocytes and the conversion of alanine into glucose (136). An important effect of T3 is the increase of glucose 6-phosphate mRNA expression and the synthesis of PEPCK (137, 138). Hepatic PEPCK mRNA is stimulated 3.5-fold in thyrotoxic rats, and it is resistant to insulin suppression of hepatic glucose production compared with euthyroid rats (139). Other hepatic gluconeogenic enzymes are positively regulated by TH (140).
T3 can also centrally modulate hepatic glucose production and insulin sensitivity by acting on the sympathetic pathway, connecting the paraventricular hypothalamus to the liver (141). T3 administration in hypothalamic PVN increases hepatic glucose production independent of plasma T3, insulin, glucagon, and corticosterone. This effect is abolished by selective hepatic sympathectomy, supporting that T3-sensitive neurons in PVN mediate liver glucose production via sympathetic projections on the liver. TH facilitates the glycogenolytic and gluconeogenic effects of epinephrine and glucagon by inducing β2-adrenergic receptor mRNA and repression of inhibitory G protein RNA of the adenylate cyclase cascade (142). T3 further increases the dysregulation of liver glucose and lipid metabolism characteristic of insulin resistance by the induction of lipogenic enzymes (135). Both lipogenesis and lipolysis are stimulated by T3. TH may increase fatty acid uptake in the liver via the regulation of fatty acid transporter proteins and increases in hepatic lipogenesis (143–146). The activation of hepatic lipases and lipophagy has been implicated in the intrahepatic lipolysis induced by T3 (147). The conversion of glucose into fatty acids together with nonsuppressed gluconeogenesis perpetuates the hyperinsulinemic state. Hyperthyroidism and high-fat feeding result in significant impairment of islet function. In contrast, physiological T3 treatment prevents streptozocin-induced islet deterioration and maintains islet structure, size, and consistency (133).
Regulation of glucose metabolism in the skeletal muscle and adipose tissue
T3 upregulates the expression of genes involved in glucose transport and glycolisis in peripheral tissues (140). In the skeletal muscle, T3 modulates mRNA and protein expression of GLUT4, adenosine monophosphate–activated protein kinase, and acetyl coenzyme A carboxylase (148). Therefore, T3 increases basal and insulin-stimulated glucose transport in this tissue (149). The transcriptional regulation of the sarcoplasmic endoplasmic reticulum (SERCA1a, SERCA2a) and other important proteins can explain the TH-induced shift to faster contractile function in the muscle and the concomitant increase in both glycolytic and oxidative capacities (150–155). T3 can also act by a nongenomic mechanism because it is able to induce within 30 minutes an increase in insulin-dependent GLUT4-mediated glucose uptake, without interfering with other transporters such as GLUT1 and GLUT3 (156). Adipose tissue can modulate insulin sensitivity of skeletal muscle by the release of adipokines and, alternatively, the skeletal muscle can affect adipose tissue by the production of several myokines (157). Interestingly, both hypothyroidism and hyperthyroidism can interfere with the normal adipocyte–myocyte crosstalk, thus contributing to the insulin resistance (157). Another T3 target in the skeletal muscle is mitochondrial uncoupling protein (UCP)3; this effect explains the increased energy expenditure induced by TH excess (158–160).
Metabolic Changes and Glycemic Control in Patients With TD and DM
Metabolic changes in patients with hyperthyroidism and the effect of hyperthyroidism on glycemic control
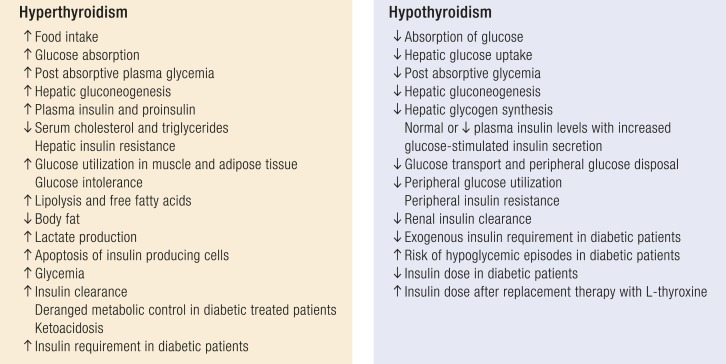
Glucose intolerance in patients with hyperthyroidism is prevalently due to hepatic insulin resistance because TH excess increases the endogenous glucose production and insulin requirement and reduces hepatic insulin sensitivity (Fig. 2) (134, 139). Fasting or postprandial insulin and proinsulin levels are elevated in hyperthyroidism, and free fatty acid concentrations are raised (161, 162). Glucose and insulin response is increased after an oral glucose tolerance test in patients with subclinical and overt hyperthyroidism (163). Moreover, hyperthyroidism has been associated with increased degradation of insulin (164). Gluconeogenesis is increased in both subclinical and overt hyperthyroidism when compared with euthyroidism. Moreover¸ TH excess increases β cell apoptosis, and this effect could be one of the major elements responsible for the deterioration of glucose tolerance in thyrotoxicosis leading to hyperglycemia. Peripheral glucose transport and tissue utilization are increased in hyperthyroidism with peripheral insulin resistance (162, 163). The insulin-stimulated glucose oxidation rate is increased in the muscle and adipose tissue of patients with hyperthyroidism. The considerable increase of glucose in peripheral tissues leads to an increased metabolism of glucose through the nonoxidative pathway. In fact, owing to the muscle insulin resistance, glucose is processed mainly by glycolysis that generates lactic acid; it is released into the circulation and returns to the liver, determining an increase of hepatic glucose production (130, 135, 164, 165). Peripheral insulin resistance can also be due to the secretion of hyperthyroidism-induced proinflammatory mediators (IL-6, TNFα, and several adipokines) by adipocytes (157).
Figure 2.
Metabolic changes and glycemic control in patients with TD.
Therefore, hyperthyroidism is associated with a hypermetabolic state with increased energy expenditure and weight loss despite increased appetite and food intake, reduced cholesterol levels, increased lipolysis, and gluconeogenesis. Insulin resistance, which is associated with hyperthyroidism, can be improved with the restoration of euthyroidism (162). Patients with hyperthyroidism can have an increased risk of severe hyperglycemia (166, 167), and preexisting DM is exacerbated by hyperthyroidism.
Metabolic changes in patients with hypothyroidism and the effect of hypothyroidism on glycemic control
Hypothyroidism is characterized by impaired glucose absorption from the gastrointestinal tract, delayed peripheral glucose assimilation, decreased or normal hepatic glucose output, and reduced liver and muscle gluconeogenesis and glycogenolysis (168) (Fig. 2). Insulin secretion has been reported to be normal, increased, or reduced, and the insulin half-life is prolonged (169).
Adipocytes and skeletal muscle of hypothyroid rats are less responsive to insulin (170–172) because overt and SHypo are associated with decreased glucose transport in myocytes (173). Glucose utilization is slowed in the peripheral tissues, and the rates of glucose oxidation and glycogen synthesis are decreased in hypothyroidism. The inability of insulin to sufficiently maintain glucose utilization by the muscles leads to insulin resistance in patients with subclinical and overt hypothyroidism (174, 175).
The Health ABC study revealed a positive correlation between subclinical and overt hypothyroidism and elevated fasting glucose levels at baseline (176). Several studies have demonstrated higher insulin levels in hypothyroidism with a lower insulin clearance (177). Insulin resistance, in both fasting and postglucose conditions, has been reported in patients with overt and SHypo, with a positive correlation between THs and the Matsuda index (173). The pathogenetic mechanism leading to insulin resistance in hypothyroidism may be related to the dysregulation of the leptin action at the hypothalamic level, the impaired GLUT4 translocation, and the increase in free fatty acids. Insulin-stimulated glucose transport has been found to be decreased in isolated monocytes from patients with overt and SHypo due to impaired translocation of GLUT4 glucose transporters on the plasma membrane, suggesting similar changes in peripheral tissues (173). Moreover, a community-based observational study in healthy subjects, the Fremantle diabetes study, showed that the combination of serum TSH and tissue insulin sensitivity has important effects on serum lipid parameters in T2D, supporting its important contribution to diabetic dyslipidemia (178). Hypothyroid thyroidectomized patients have insulin resistance even after acute L-T4 withdrawal (174).
Glucose-induced insulin secretion by the β cells is increased in hypothyroidism and is reduced after L-T4 therapy (179). The insulin requirement is decreased in insulin‐treated patients with diabetes developing hypothyroidism because of the impaired renal insulin clearance (180–182). Therefore, during treatment with insulin, patients with hypothyroidism with DM require decreased insulin doses and, when exogenous insulin is not decreased, symptomatic hypoglycemia can occur. Uncontrolled and untreated hypothyroidism (overt and SHyo) may induce recurrent hypoglycemic episodes in treated patients with T2D, and a reduction in the insulin dosage should be considered to prevent hypoglycemia. As a result, insulin doses should be modulated with the correction of hypothyroidism, and insulin requirements should be assessed in light of the increased risk of hypoglycemia (182).
Diagnosis of TD in Patients With DM
It is essential to recognize TD in patients with DM. However, this diagnosis can be difficult because DM can affect the evaluation of a concomitant TD (Table 4) (183–193). DM influences thyroid function by controlling TSH release at the level of hypothalamus and by affecting T4-to-T3 conversion in the peripheral tissues (183–186). Experimental-induced diabetes may cause alterations in the HPT axis by reducing the levels of plasma TRH and TSH, thereby affecting TH production (183, 184). Patients with diabetes may have an impaired TSH response to TRH stimulation with decreased T4-to-T3 conversion. A significant correlation between HbA1c concentration and TSH levels has been reported (187, 188).
Table 4.
Factors Limiting the Diagnosis of TD in Patients With DM
| • The clinical diagnosis may be delayed because symptoms are masked by DM |
| • Diabetes-induced alterations of HPT axis with reduced levels of TRH and TSH |
| • Decreased T4-to-T3 conversion, especially in poorly controlled diabetes |
| • Low T3 syndrome in diabetic ketoacidosis |
| • Potential interference of TH binding inhibitor on T3 production |
| • Effect of oral hypoglycemic agents on serum TSH levels |
The clinical diagnosis of DM may be delayed in patients with hyperthyroidism and, alternatively, the clinical features of decompensated diabetes may be masked by hyperthyroidism (189). The evaluation of serum TSH and FT3 can provide unreliable results for the coexistence of the low T3 syndrome (190, 191). Both T1D and T2D may induce a “low T3” condition with low serum total and FT3 and increased reverse T3 levels. An abnormal TH pattern associated with diabetes was attributed to the presence of TH binding inhibitor (THBI), an inhibitor of the extrathyroidal conversion enzyme (5′-deiodinase) of T4 to T3, and to the dysfunction of the HPT axis (192). These features were exacerbated by stress and poorly controlled diabetes. Additionally, some oral hypoglycemic agents can influence serum TSH levels (193).
Treatment of Thyroid Dyfunction in Patients With DM
Treatment of hyperthyroidism
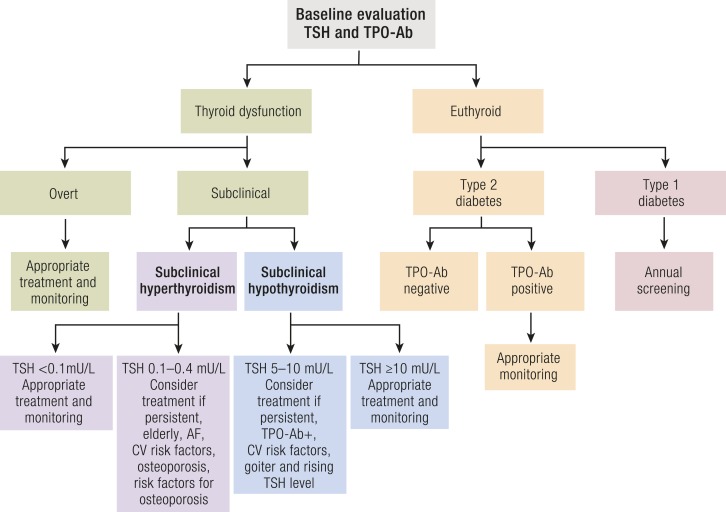
Hyperthyroidism is successfully treated with antithyroid drugs (ATDs), radioactive iodine (RAI), or surgery. Treatment of hyperthyroidism is recommended by the European Thyroid Association (ETA) and the American Thyroid Association (ATA) in patients with overt disease and in those with severe (grade 2) SHyper in both GD and toxic nodular thyroid disease (194, 195) for the increased risk of atrial fibrillation, heart failure, fractures, cognitive dysfunction, and all-cause and cardiovascular mortality (4, 5). Treatment of mild (grade 1) SHyper (TSH of 0.1 to 0.4 mU/L) can be considered when serum TSH is persistently low, especially in elderly patients and in those with a high cardiovascular risk (history of atrial fibrillation or stroke, heart failure, and coronary disease) or risk factors for osteoporosis (4, 5, 194–197). However, no prospective randomized controlled trial has been performed to evaluate whether treatment can improve the adverse outcomes associated with SHyper.
The choice of appropriate treatment should take into account the etiology and severity of hyperthyroidism, the age of the patients, associated comorbidities, the size of the goiter and patient’s preference. ATDs (e.g., methimazole and propylthiouracil) are appropriate in GD because a spontaneous remission of this autoimmune condition is possible after 12 to 18 months of therapy. Pretreatment with ATDs can be necessary in patients with toxic adenoma and multinodular goiter with severe hyperthyroidism or comorbidies, even though a definitive treatment with thyroid ablation therapy (e.g., RAI or surgery) would be preferred, especially in patients with persistent hyperthyroidism at high risk of adverse cardiac events or fractures (5, 194, 195) (Fig. 3). Surgery is usually reserved in the presence of large goiters, coexisting hyperparathyroidism, or suspicion of thyroid cancer.
Figure 3.
Algorithm for the diagnosis and treatment of TD in patients with T1D and T2D.
Treatment of hyperthyroidism with ATDs does not affect glycemic control, apart from possible iatrogenic hypothyroidism. Corticosteroids are occasionally used for treatment of Graves ophthalmopathy (GO) or to prevent its exacerbation after the administration of RAI. These drugs may worsen glycemic control or induce diabetes (166). Therefore, the negative effects of administered steroids on metabolic control should be considered in patients with hyperthyroidism and diabetes as well as the onset/exacerbation of GO.
The adjustment of insulin treatment should be considered in patients with diabetes after the occurrence of hyperthyroidism. The evaluation of thyroid function should be performed during ketoacidosis in patients with clinical symptoms and signs raising a suspicion of hyperthyroidism. However, the hormonal profile should be cautiously considered because of the frequent coexistence of a low T3 syndrome. Hyperglycemia should be reevaluated in subjects with hyperthyroidism after the control of TD.
Treatment of hypothyroidism
Treatment of hypothyroidism in adult patients with T2D is simple and available because hypothyroidism is successfully treated with oral L-T4 monotherapy. This treatment is recommended by both the ETA and ATA when serum TSH levels are >10.0 mU/L (198, 199) (Fig. 3). Uncontrolled diabetes may impair the effectiveness of L‐T4 treatment in hypothyroidism. Alternatively, L-T4 treatment may normalize fasting hyperinsulinemia and significantly improve insulin sensitivity in patients with hypothyroidism and T2D. This could suggest consideration of a potential benefit of L-T4 administration in treating even mild TH deficiency to improve the insulin resistance and dyslipidemia that is associated with SHypo (179). Prospective studies are warranted to address this issue.
Patients with hypothyroidism and DM have reduced insulin requirements, and therefore an increased insulin dose may be necessary when starting treatment with L-T4 (179). Excessive L‐T4 therapy inducing TSH suppression should be avoided because it may induce iatrogenic hyperthyroidism and cause a further impairment in glycemic metabolism. The lipid profile is usually partially normalized by L‐T4 replacement therapy (178); therefore, combination therapy with statins is frequently required to obtain greater improvement in lipid profile in patients with hypothyroidism. However, the risk of statin‐induced myopathy is greater in patients with hypothyroidism and DM (90, 200). Accordingly, when required, a lower statin dose should be administered in combination with other lipid‐lowering treatments. The use of statins should be considered only after the correction of possible hypothyroidism to prevent the risk of myopathy.
Effect of Antidiabetic Medications on Thyroid Function
Some antidiabetic drugs can affect thyroid function and impact the HPT axis. Therefore, the use of these drugs in patients with T2D diabetes can influence the evaluation of serum TSH and TH levels (Table 5). Specific recommendations should be considered during treatment with antidiabetic medications (Table 6).
Table 5.
Effect of Antidiabetic Medications on Thyroid Function
| Metformin |
| • Lowering TSH effect in subjects with high-normal serum TSH levels (TSH levels >2.5–3 mU/L) and in patients with overt and SHypo |
| • Reversible effect after discontinuation of metformin |
| • The TSH lowering effect of metformin can be observed in diabetic patients with thyroid disorder also when treating with T4 |
| Sulfonylureas |
| • Goitrogenic activity of the first-generation sulfonylurea compounds |
| • Higher incidence of hypothyroidism in diabetic patients treated with the first-generation sulfonylureas compared with controls treated with diet alone or insulin |
| • No influence of second generation of sulfonylureas (glibenclamide and gliclazide) on TH metabolism |
| Thiazolidinediones |
| • Activation of PPAR-γ stimulates functional TSH receptor expression |
| • Increased recruitment and differentiation of orbital fibroblasts and stimulation of adipogenesis |
| • Increased risk of GO |
| Incretin mimetics |
| Animal models |
| • Activation of GLP-1 receptors on thyroid C cells |
| • Increased release of CT |
| • Increased C cell hyperplasia and adenomas |
| • Increased risk of medullary thyroid cancer at very high doses |
| Humans |
| • No effect on GLP-1 receptor activation |
| • No effects on serum CT levels |
| • No evidence for adverse effects |
| Insulin |
| • Enhanced levels of FT4 and reduced levels of T3 |
| • Modulation of TRH and TSH secretion |
Table 6.
Recommendations on the Treatment of TD in Patients With DM
| • TSH levels should be monitored after beginning metformin treatment in diabetic patients with overt and SHypo. |
| • There is insufficient evidence to recommend the assessment of serum TSH and FT4 levels and thyroid ultrasound in patients treated with sulphonylureas. |
| • Pioglitazone should not be administered to diabetic patients with GO. |
| • GLP-1 receptor agonists are not recommended in patients with a personal or family history of MTC or type 2 multiple endocrine neoplasia. |
| • The adjustment of insulin treatment should be considered in patients with diabetes after the occurrence of TD. |
| • The evaluation of thyroid function should be performed during ketoacidosis in patients with clinical symptoms and signs raising a suspicion of hyperthyroidism. |
| • Glycemic control should be reassessed in hyperthyroid subjects after the control of hyperthyroidism with ATDs. |
| • An increased insulin dose may be necessary when starting treatment with L-T4 in hypothyroid patients with DM. |
| • Excessive L‐T4 therapy inducing TSH suppression should be avoided because it may induce iatrogenic hyperthyroidism determining an impaired glycemic control. |
| • The use of statins should be considered only after the correction of hypothyroidism to prevent the risk of myopathy. |
Metformin
Metformin, an oral hypoglycemic biguanide, is the first drug of choice for treatment of patients with T2D because of its safety profile, efficacy in controlling glycemic levels, and reasonably good compliance and tolerance (201). The main side effect of metformin is gastrointestinal intolerance (diarrhea, nausea, dyspepsia, and abdominal pain), which may be observed in up to 28% of patients and lead to discontinuation of therapy in <2% of patients (201). The TSH-lowering effect of metformin in patients with T2D with primary hypothyroidism was first reported in 2006 (193). Subsequent studies have suggested that metformin may reduce serum TSH levels in patients with primary hypothyroidism; this effect was observed in both patients with hypothyroidism under LT4 replacement therapy and in untreated subjects (202, 203).
A meta-analysis assessed the changes in serum TSH levels in 206 patients before and after metformin treatment (204). It included seven studies, of which four studies were performed on 119 patients with overt hypothyroidism receiving L-T4 replacement therapy, two studies on 33 patients with SHypo not receiving L-T4, and one study on 54 euthyroid patients without any L-T4 therapy (204). Six data sets included subjects with diabetes and one study selected women receiving metformin for PCOS (204). The results showed that metformin reduces TSH levels in both overt and SHypo, whereas no change in TSH levels was observed in euthyroid patients (204). The results of this meta-analysis on the nonsignificant effect of metformin on serum TSH in euthyroid individuals were mainly based on one prospective study by Cappelli et al. (203) that examined 54 euthyroid patients with T2D. These data were subsequently confirmed in the cross-sectional study by Díez and Iglesias (205) on 828 patients with T2D and by Rezzónico et al. (206), which included only women with insulin resistance and thyroid nodules. In a second paper, Cappelli et al. (207) performed a large retrospective study on 393 patients with T2D and showed that metformin had a lowering TSH effect in subjects with high-normal basal serum TSH levels (TSH levels >2.5 mU/L) and in patients with hypothyroidism. A retrospective study in seven primary health care centers in Spain assessed 278 patients with T2D (110 females) and evaluated serum TSH levels before and 1 year after the onset of metformin treatment (208). Based on a mathematical model, a TSH cut-off point level of 2.98 mU/L was associated with a lowering effect of metformin (208). Therefore, a serum TSH cut-off of 2.5 to 3 mU/L can predict the effect of metformin on serum TSH.
The decline in serum TSH levels during treatment with metformin was not associated with alterations in plasma FT4 and FT3 concentrations in the studies previously discussed. Therefore, changes in serum TSH levels were independent from a potential effect of metformin on L-T4 absorption, as demonstrated by the lack of the effects on circulating TH (209, 210). Moreover, the effect of metformin was reversible after its discontinuation. Also, thyroid autoimmunity and obesity were not involved in the relationship between metformin and thyroid profile (209, 210).
Metformin is able to cross the blood–brain barrier and reach a high concentration in the pituitary (211). Therefore, the hypothetical mechanisms for the effect of metformin on serum TSH might be related to the potential effect on TRs or the modulation of the activity of type II deiodinase at the hypothalamic–pituitary level (212). Regarding the underlying mechanism of these effects, potential explanations include the possibilities that metformin might induce changes in the affinity of TRs, TH binding, bioavailability and metabolism of TH, or induce interference with the TSH assay. THs negatively regulate their production through the HPT axis. However, some conditions, such as reduced food availability, may downregulate the HPT axis, even in the presence of normal or lower TH levels (213, 214). Metformin mainly acts by suppressing hepatic gluconeogenesis via activation of AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1). These effects may probably counteract the hypothalamic T3 action on TSH secretion by inhibiting central AMPK (215). There is also evidence that metformin increases hypothalamic dopaminergic tone in association with improved insulin sensitivity and thereby could modulate the dopaminergic tone on TSH secretion (216). Moreover, metformin might affect deiodinase type 2 (D2) activity in glial cells, astrocytes, and tanycytes in the mediobasal hypothalamus, where D2 catalyzes the conversion of T4 to active T3 (217). Interestingly, D2 polymorphism generating less T3 has been associated with some degrees of insulin resistance, and metformin might enhance D2 activity providing more T3 at the pituitary level in patients with hypothyroidism (218).
In conclusion, available evidence supports the conclusion that metformin treatment is not associated with a significant modification of TSH values in subjects with an intact HPT axis (209). A TSH-lowering effect of metformin can be observed in patients with diabetes with thyroid disorder, independently of treatment with thyroxine. Although the effect of metformin is yet to be clearly established, literature results suggest that TSH levels should be monitored in patients with diabetes with overt hypothyroidism and SHypo during treatment with metformin. A possible adjunctive role of metformin during L-T4 therapy to obtain TSH suppression in thyroidectomized patients with differentiated thyroid cancer has been reported; it should be further investigated to reduce the adverse effects of exogenous SHyper on the heart and bone (219, 220).
Regarding the effects of metformin on thyroid morphology, one study showed that it significantly decreased nodule size by 30% to 50% of the initial volume in patients with insulin resistance (206). More importantly, it has also been reported that metformin exerts an antimitogenic and proapoptotic effect in thyroid carcinoma cell lines and increases the antiproliferative effect of chemotherapeutic agents, such as doxorubicin and cisplatin (221). It inhibits insulin-induced growth stimulation in differentiated and undifferentiated thyroid carcinoma and thyroid cancer stem cells (221).
Moreover, treatment with metformin could suppress the growth of metastatic medullary thyroid carcinoma (MTC) cells by downregulating the mTOR pathway (222). These observations suggest an important potential role of metformin as an adjuvant treatment in the management of thyroid cancer, especially in patients with diabetes.
Sulfonylureas
Antithyroid and goitrogenic activities of sulfonylurea have been reported. In animals receiving large doses of sulfonylureas, the weight of the thyroid gland was increased and iodine content and radioiodine uptake were reduced (223–226). A higher incidence of hypothyroidism was found in patients with diabetes treated with the first-generation sulfonylureas compared with controls treated with diet alone or insulin (227). Chlorpropamide and tolbutamide inhibit the binding of T3 and T4 to T4-binding globulin competitively in vitro and after IV administration. In one study they only had a minimal effect after oral administration (228). Studies on the second-generation sulfonylureas demonstrated that glibenclamide and gliclazide had no influence on TH metabolism (229–233). Therefore, there is insufficient evidence to recommend the assessment of serum TSH and FT4 levels and thyroid ultrasound in patients treated with sulfonylureas.
Thiazolidinediones
The thiazolidinediones (TZDs) are among one of several classes of oral hypoglycemic agents commonly used in T2D. They are potent agonists of the nuclear hormone receptor, peroxisome proliferator–activated receptor-γ (PPAR-γ), which is found predominantly in adipose tissue and plays a dominant role in adipocyte differentiation (234). The expression of PPAR-γ is greater in adipose and connective tissue from patients during the active stages of GO. The activation of PPAR-γ by its agonist, TZD, was shown to stimulate functional TSH receptor expression. It also induces the recruitment and differentiation of orbital fibroblasts into mature lipid-laden adipocytes, suggesting that the activation of PPAR-γ may play an important role in the stimulation of adipogenesis and the pathogenesis of GO induced by TZDs (235). Further studies have shown that patients with T2D can have increased eye protrusion during treatment with pioglitazone (234). Exacerbation of GO was described in patients with T2D following treatment with glitazones without apparent changes in TH levels. The withdrawal of pioglitazone treatment did not result in remission of GO (236–239). Thus, TZDs should be administered with caution in patients with diabetes with GD. It seems apparent that pioglitazone should not be administered to patients with diabetes with clinically active GO.
The results from one study suggest that rosiglitazone use in patients with T2D might reduce the risk of thyroid cancer (240).
Incretin mimetics
Incretins act by increasing the activity of human glucagon-like peptide-1 (GLP-1), an endogenous hormone released by the intestine in response to food; they are designed as additional treatment to metformin in patients with T2D to improve the control of blood glucose (241). These drugs are able to reduce HbA1c levels without inducing episodes of severe hyoglycaemia and can also induce weight loss in patients who are obese. Incretin mimetics (exenatide) or analogs (liraglutide) act as GLP-1 receptor agonists but are resistant to the degradation of dipeptidyl peptidase-4 (DPP-4). Sitagliptin and other similar drugs specifically inhibit DPP-4 and therefore increase the half-life of endogenous GLP-1. Treatment with incretins has been associated with an increased risk of thyroid cancer (242), although this risk is controversial and may differ between GLP-1 receptor agonists and DPP-4 inhibitors and among different DPP-4 inhibitors (242). There is weak evidence suggesting an association between sitagliptin and an increased risk of thyroid cancer in Taiwanese patients with T2D, especially during the first year of treatment (242). However, the lack of specific information on the pathology, grading, staging of thyroid cancer, and potential confounders (radiation, smoking, lifestyle, insulin resistance, inflammation, and genetic factors) does not permit examination of the potential mechanism of this association.
GLP-1 promotes β cell proliferation and inhibits apoptosis, stimulates insulin secretion, and reduces blood glucose in human subjects with T2D. GLP-1 controls glycemia via additional actions on glucose sensors, the inhibition of gastric emptying, food intake, and glucagon secretion (241).
Serum calcitonin (CT) is an important biomarker for C cell diseases such as MTC and hereditary C cell hyperplasia (243). Preclinical studies in mice and rats demonstrated that liraglutide activates GLP-1 receptors on the thyroid C cells, causing the release of CT with a dose-dependent effect on C cell pathology (244). Animal models have demonstrated an association between treatment with exenatide or liraglutide and the appearance of abnormalities of thyroid C cells, with progressive development of hyperplasia and adenomas (244–246). These lesions were preceded by an increase in plasma CT levels. Very high doses of liraglutide (45-fold human exposure) caused a small number of C cell carcinomas in female mice. In contrast, C cells within the monkey and human thyroid gland exhibited lower levels of GLP-1 receptor expression and did not respond to GLP-1 receptor agonists with an acute release of CT. Prolonged administration of liraglutide at very high doses did not produce C cell proliferation in monkeys and did not induce significant changes in CT levels in clinical studies in humans compared with controls (247, 248). A large population-based randomized study (the LEADER Trial) assessed the long-term effects of the GLP-1 receptor agonist liraglutide on serum CT concentrations during a 3.5- to 5-year period. Unstimulated serum CT concentrations were measured in >9340 patients with T2D receiving liraglutide or placebo (249). The results did not support any effect of GLP-1 receptor activation on serum CT levels, C cell hyperplasia, or C cell malignancy in humans, suggesting that the findings previously reported in rodents may not apply to humans (249). Therefore, there is no evidence for adverse effects of liraglutide in humans. The reported adverse events of this drug can be explained by the greater sensitivity and density of GLP‐1 receptors in rat C cells as well as the high dosage of the drug when employed in animal models. The Food and Drug Administration stated that patients taking a GLP-1 receptor agonist do not need to be monitored for the potential development of MTC with CT levels. Nevertheless, GLP-1 receptor agonists are not recommended in patients with a personal or family history of MTC or type 2 multiple endocrine neoplasia.
Insulin
Insulin enhances the levels of FT4 and suppresses the level of T3 by inhibiting the hepatic conversion of T4 to T3. Insulin modulates TRH and TSH levels (192). These effects can be worsened in poorly controlled patients with diabetes.
Polymorphism of the D2 Gene, Thr92Ala, and Increased Risk of T2D
Deiodinases are selenoenzymes that regulate the intracellular concentration of T3, thereby indirectly controlling the response of these tissues to THs. They control the activation and inactivation of T3 and T4 and therefore central and peripheral T3 levels (130, 250). Deiodinase type 1 (D1) is expressed predominantly in the liver, kidney, and thyroid in humans. D1 has an important role for the adaptation to iodine deficiency and for reducing the impact of elevated TH levels in hyperthyroidism. D2 is highly expressed in BAT, skeletal muscle, brain, and pituitary and has an essential role in preserving the availability of T3 levels in these tissues. D2 is the main source of circulating levels of T3 in euthyroid subjects (251, 252). The T3 generated intracellularly by D2 is transferred to the nucleus and regulates specific gene transcription. Polymorphisms in the D2 gene have been associated with interindividual variation in the TSH-free T4 “set point” (130). Deiodinase type 3 (D3) is predominantly expressed in the adult central nervous system, skin, vascular tissue, placenta, pregnant uterus, and fetal tissues. D3 protects the fetus from excessive maternal TH production. The deiodinase enzymes also differ in their subcellular localization, because D1 and D3 are expressed on the cell membrane, whereas D2 is expressed in the endoplasmic reticulum (130). T3 upregulates the expression of GLUT-4, the insulin-responsive glucose transporter in the skeletal muscle that mediates the rate-limiting step of glucose metabolism and increases glucose uptake. Thus, a lower intracellular D2-generated T3 decreases the transcription rate of GLUT-4 in the skeletal muscle and adipose tissue and is responsible for an impaired insulin-stimulated glucose disposal resulting in increased insulin resistance and contributing to weight gain (253). A single-nucleotide polymorphism (Thr92Ala) in the coding region of the human D2 gene was associated with increased insulin resistance in subjects with T2D and those who were nondiabetic (254). This polymorphism is extremely prevalent among various ethnic groups. It has been reported that the Ala allele frequencies vary among different populations: Indians (0.61), Han Chinese in Beijing (0.58), Mexican Americans (0.54), African Americans (0.51), British (0.34), and Finnish (0.24) (255). The Thr92Ala variants rs225011 and rs225015 were modestly associated with an early onset of T2D in Pima Indians, although none of these variants was significantly associated with either fasting insulin levels or rates of insulin-mediated glucose uptake (256). Moreover, this gene did not have a large impact on T2D at older ages and did not influence body mass index (BMI) in the Pima Indians. Associations with common D2 Thr92Ala polymorphism with insulin resistance were also found in Mexican Americans and Brazilian cohorts (254, 257). A Danish study reported a link between the D2 Ala/Ala genotype and glycemic traits of insulin resistance (258). In this study, an increased area under the serum insulin curve was observed during the oral glucose tolerance test, and an elevated fasting plasma glucose was found in the D2 Ala/Ala group, although no increased risk of T2D was reported (258). However, studies performed in other populations found an inconsistent association between the Thr92Ala D2 variant and insulin resistance and/or T2D (259, 260).
“GLP-1 receptor agonists are not recommended in patients with a personal or family history of MTC or type 2 multiple endocrine neoplasia.”
Environmental factors and lifestyle could influence the genetic predisposition factors for T2D and explain these conflicting results. Physical exercise might potentially overrule the effects on insulin resistance of the Thr92Ala D2 polymorphism by the exercise-induced increase in translocation of GLUT-4 to the cell membranes (261). Conversely, in sedentary individuals, low intracellular levels of T3 might reduce GLUT-4 transcription, leading to a decreased insulin-stimulated glucose disposal. A recent meta-analysis in 11,000 subjects confirmed the association of the D2 Ala/Ala genotype with increased insulin resistance (increased homeostatic model assessment index) (218). This study demonstrated that this genotype is also linked to a worse glycemic control (increased HbA1c levels) in a cohort of 1057 subjects with T2D (218). In a further meta-analysis, the homozygosis for the D2 Thr92Ala polymorphism was also associated with higher HbA1C levels in patients with T2D, suggesting that this polymorphism might be correlated with a worse glycemic control in patients with T2D (262).
In conclusion, all available studies underline the potential genetic contribution of D2 Thr92Ala polymorphism to the pathogenesis of T2D.
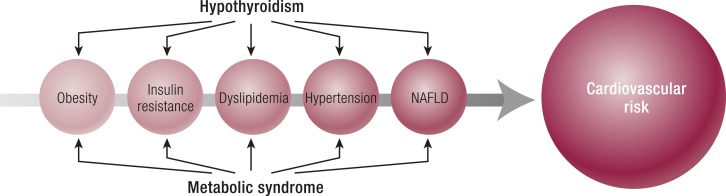
Underlying Mechanisms of the Association Between MetS in TD
MetS is characterized by a cluster of cardiovascular risk factors (81). Isulin resistance is the main clinical and pathogenic feature of this syndrome. The definition of MetS is based on central obesity plus any two of the following factors: (i) atherogenic dyslipidemia, with high triglycerides and low high-density lipoprotein cholesterol (HDL-C) or specific treatment of this lipid abnormality; (ii) high blood pressure or antihypertensive treatment; (iii) insulin resistance and elevated fasting plasma glucose concentration or previously diagnosed of T2D (263). A prothrombotic state and a proinflammatory state are usually associated with MetS, which is as an independent risk factor for CVDs and T2D (263, 264).
TH affects food intake, resting energy expenditure, and thermogenesis, and consequently metabolic alterations can develop in patients with TD. T3 influences both metabolism and thermogenesis by regulating (i) transcription factors involved in adipogenesis of WAT and BAT, (ii) appetite, and (iii) glucose and lipid metabolism and oxidation (108, 109, 265, 266). Alternatively, the combination of central nutritional state and hormonal signals, including leptin, dopamine, somatostatin, insulin, and adrenergic signaling, integrate information regarding overall nutritional status, circadian rhythms, and stress to modulate TH production (109, 130).
Effects of TH on thermogenesis
TH plays a significant role in energy expenditure through both central and peripheral actions. Obligatory thermogenesis is the generation of heat that accompanies metabolic processes. Facultative thermogenesis is important to maintain body temperature after cold exposure and increase energy expenditure after eating. The primary site of this adaptive thermogenesis in rodents is in BATs (130). The TH plays a major role in regulating both obligatory and adaptive thermogenesis by increasing the basal metabolic rate and by modulating the sympathetic nervous system activation of BAT (108, 130) (Fig. 1). TH stimulates basal metabolic rate by increasing ATP production for metabolic processes and by generating and maintaining ion gradients. D2 is expressed in the WAT, BAT, and skeletal muscle and is required for adaptive thermogenesis. There is an important central role for T3 in stimulating adrenergic-mediated thermogenesis due to direct actions on BAT. A central administration of T3 in rats leads to sympathetic nervous system activation and upregulation of BAT thermogenesis via the reduction in the hypothalamic AMP-activated protein kinase activity. The inhibition of this pathway resulted in increased lipogenesis with a net effect of increased thermogenesis and energy expenditure (213, 214).
Humans have both visceral and subcutaneous BAT. However, human BAT is considered more important in neonates than in the adults. There is more BAT in younger and leaner individuals and it is induced by cold temperature. Recent studies using positron emission tomography and CT imaging have shown a significant amount of BAT, especially in the subscapular and chest region (265). Although the importance of BAT for metabolic regulation in adults is controversial, functional imaging by positron emission tomography scan has demonstrated BAT activation after overnight cold exposure (266). Treatment with β-adrenergic blockers reduces BAT activity, suggesting the importance of catecholamine for the development and regulation of BAT. During cold exposure, D2 increases the generation of T3 in BAT, thus promoting heat production (130). The thermogenic effect of T3 in BAT is mediated by UCP1. The expression of UCP1 is required for BAT thermogenesis, and UCP1 is synergistically regulated by both noradreanaline and T3 (267). TRβ regulates UCP1 expression in BAT; TRα mediates sensitivity to adrenergic simulation. T3 and noradreanaline increase UCP1 expression by twofold separately. On the contrary, when both agents are combined they induce a 20-fold expression of UCP1 (268).
Effects of hypothalamic pituitary thyroid axis on appetite regulation
The hypothalamic area of the brain is critical in the regulation of food intake. It contains the ARC, PVN, ventromedial nucleus (VMN), dorsomedial nucleus, and lateral hypothalamic area/perifornical area. The ARC, which is adjacent to the floor of the third ventricle, is the most important hypothalamic region in controlling appetite and is known as ‘‘the center of food intake.” This area is not fully protected from the circulation by the blood–brain barrier and receives information regarding energy status from peripheral circulating factors produced by peripheral organs such as leptin by adipose tissue, insulin and pancreatic polypeptide by the pancreas, gut hormones (ghrelin, obestatin, GLP-1, oxyntomodulin, peptide YY), and T3 by the thyroid gland (213, 269). The ARC is able to integrate these peripheral signals and regulate food intake through expression of orexigenic factors (appetite stimulation) such as neuropeptide Y (NPY) and agouti-related protein (AgRP), as well as the expression of anorexigenic factors (appetite inhibition) such as proopiomelanocortin (POMC), which encodes for α-melanocyte–stimulating hormone (α-MSH) and cocaine- and amphetamine-regulated transcript (CART) (270–273) (Fig. 1). Both NPY and AgRP increase food intake when administered intracerebroventricularly (ICV) to rats, whereas CART and α-MSH inhibit food intake when administered ICV. The NPY/AgRP and POMC/CART neurons from the ARC are extensively connected to other hypothalamic areas, including the lateral hypothalamic area and perifornical area. This last area is the site of production of other orexigenic neuropeptides, melanin-concentrating hormone, and the orexins. The ICV administration of orexins increases food intake.
The brain-derived neurotrophic factor (BDNF) is highly expressed in the VMN. The central infusion of BDNF reduces food intake and induces weight loss in rats (274). The VMN receives NPY/AgRP, and POMC neuronal projections from the ARC and POMC neurons from the ARC play a role in activating BDNF neurons in the VMN to decrease the food intake (275). The TRH, secreted from the hypothalamus, has direct anorectic effects and can regulate food intake independent of effects on the HPT axis. In rodents, central and peripheral administration of TRH reduces food intake (276). TSH has also been shown to reduce food intake when injected centrally into rats and could be involved with the seasonal alterations in food intake and body weight that occur in some species (277, 278). T3 in the brain is crucial for the feedback regulation of TSH secretion. T3 directly stimulates food intake at the level of the hypothalamus, independently of changes in energy expenditure. In rodent models, peripheral and central hypothalamic administration of T3 increases food intake (279). In fact, T3 can directly cross the blood–brain barrier, and the central administration of T3 activates the mTOR signaling pathway and is associated with increased expression of AgRP and NPY, as well as decreased POMC expression in hypothalamic ARC (280, 281). Direct administration of T3 into the VMN increases food intake in rats (282). The effects of T3 in the VMN may be mediated by glutamatergic neurons that modulate ARC POMC neurons and/or BDNF neurons (282).
D2 activity is particularly high in the ARC and median eminence, where it is expressed within astrocytes and tanycytes (130) in direct contact with NPY/AgRP neurons. Despite the reductions in peripheral and liver T3 levels during fasting, hypothalamic D2 activity is increased with fasting, resulting in an increase in local hypothalamic T3 production and a consequent mitochondrial proliferation in NPY/AgRP neurons, leading to increased appetite. Hypothalamic D2 mRNA shows a diurnal variation, peaking in the nocturnal feeding phase in rats. This suggests that local production of T3 in the hypothalamus may be involved in appetite regulation and supports the hypothesis that the mechanism to stimulate arcuate NPY/AgRP neurons may have an important physiological role in the regulation of food intake (283, 284).
“The administration of leptin to normal weight rodents produces a dramatic reduction in food intake and body weight….”
Leptin is an adipokine and its serum concentration is proportional to body fat content. Leptin acts on the hypothalamus to influence food intake (Fig. 1). The administration of leptin to normal weight rodents produces a dramatic reduction in food intake and body weight because leptin directly activates anorectic POMC neurons and inhibits orexigenic AgRP/NPY neurons (285). Both leptin and TH regulate signaling in the ACN (286–288). Leptin levels are reduced in hyperthyroidism, correlating with BMI.
Insulin has anorectic effects in the central nervous system and its ICV administration reduces food intake and body weight (289) (Fig. 1). Its anorectic effect involves insulin receptors on ARC neurons (289). Insulin binds to the insulin receptor and activates the insulin receptor substrate (IRS) proteins. These in turn allow IRS proteins to activate the phosphatidylinositol-3-OH kinase pathway. It is interesting that this IRS/phosphatidylinositol-3-OH kinase pathway is also activated by leptin, and therefore the similar effects of leptin and insulin on food intake may be mediated via this common pathway in the hypothalamus (290). Hypoglycemia potently stimulates food intake; however, this effect is due to low glucose, not elevated insulin. Arcuate NPY/AgRP neurons do not respond directly to hypoglycemia, but their activation during glucoprivation depends on catecholamine projections from the hindbrain (291, 292).
The hyperthyroidism-induced increase in food intake is associated with the dysregulation of the hypothalamic neuropeptide system, including increased NPY and AgRP expression, and decreased POMC expression in ARC. T3-treated rats have significantly lower plasma leptin levels compared with control rats; hypothalamic expression of NPY mRNA is increased fourfold, and hypothalamic POMC and CART mRNA are reduced by ∼80%, compared with control rats. The hyperthyroidism-induced increase in food intake is reversed by central treatment with the specific mTOR inhibitor rapamycin, resulting in weight loss (280, 281, 293). Despite increased appetite, hyperthyroidism is usually associated with a variable decrease in body weight owing to the increase in total energy expenditure (108). Oppositely, the correction of hyperthyroidism may be responsible for excessive weight gain, independent of the treatment modality of thyrotoxicosis, that is, surgery, RAI, or ATDs (108).
Regarding hypothyroidism, weight excess was reported in 54% patients with overt hypothyroidism, reflecting both the accumulation of body fat, due to decreased resting energy expenditure, and the increased water content of the body, consequent to a reduced capacity of excreting free water (108). In spite of adequate replacement therapy with L-T4 and the increase in resting energy expenditure, hypothyroid patients usually have only a modest and/or transient weight loss prevalently due to the excretion of excess body water, rather than reduction in fat mass (108).
Impact of obesity, insulin resistance, and other risk factors for MetS in patients with clinical and SHypo
Obesity
Obesity can induce insulin resistance and diabetes because the adipose tissue is an active endocrine tissue; it releases free fatty acids, leptin, adiponectin, resistin, and other products that play an important major role in glucose and lipid metabolism (294). An early marker for the risk of diabetes is the increased accumulation of lipid within nonadipose tissue, including the skeletal muscle and liver. Inflammation within the adipose tissue, particularly related to obesity and high fat consumption, may contribute to systemic insulin resistance (295).
TD is associated with changes in body weight and composition, body temperature, and total and resting energy expenditure independently of physical activity (108, 109, 296). Both subclinical and overt hypothyroidism are frequently associated with weight gain, decreased thermogenesis, and metabolic rate (297, 298). Moreover, slight variations in thyroid function, even within the laboratory reference range, can be associated with the development of regional obesity and the tendency to gain weight (299, 300). The NHANES 2007–2008 analysis of 3114 euthyroidhealthy men and women showed that TSH levels and FT3 were correlated with BMI and waist circumference (301). Moreover, raising TSH levels has been linked to a progressive increase in weight over time (296). The correlation between TSH and BMI could be mediated by leptin produced by adipose tissue because a positive correlation has been identified between serum leptin and serum TSH levels in individuals who are obese. TSH in turn can stimulate leptin secretion by human adipose tissue via TSH receptors that are expressed on adipocytes (287, 302, 303).
In vivo, the administration of recombinant human TSH at supraphysiological doses can induce the release of small, but significant amounts of leptin, which are proportional to the adipose mass (304). Leptin can also regulate D2, activating the conversion of T4 to T3 (296).
Isolated hyperthyrotropinemia is frequently observed in patients who are obese. It is a condition spontaneously reversible by losing weight without the necessity of specific treatment; in fact, it is usually associated with FT3 levels that are at the upper limit of the normal range (296). Alternatively, individuals who are morbidly obese show a high prevalence of overt and subclinical autoimmune hypothyroidism, accounting for 19.5% of hypothyroidsm (305, 306). A link has been reported between obesity, TSH increase, leptin increase, thyroid autoimmunity, alterations and development of subclinical and overt hypothyroidism, and deranged lipid profile, thereby bringing the thyroid/obesity association to a full circle (296, 306).
Insulin resistance
Indices of insulin resistance are closely linked to TSH and TH levels, even within the normal range in euthyroid eumetabolic subjects (106, 307–311). In a Korean study on 6241 nondiabetic euthyroid subjects, those with the lowest FT4 quartile had twice the risk of insulin resistance when compared with those in the highest quartile after adjusting for age, sex, metabolic, and lifestyle factors (312). In healthy euthyroid middle-aged subjects, FT3 and FT4 levels and the FT3/FT4 ratio were associated with various markers of unfavorable metabolic profile and cardiovascular risk (313). Furthermore, it has been shown that in both euthyroid adults with and without diabetes, small variations in TSH at different levels of insulin sensitivity might exert a marked effect on lipid levels (314). Regarding the effects of insulin on the thyroid gland, high circulating levels of insulin may induce increased thyroid proliferation with the development of larger thyroid volume and the formation of nodules. This goitrogenic action of insulin may explain why the thyroid gland was considered another victim of the insulin resistance syndrome (315).
Lipids, blood pressure, and nonalcoholic fatty liver disease
The lipid status is affected by TD (316, 317). THs modulate cholesterol synthesis, mobilization, and breakdown. In particular, they increase cholesterol synthesis via the rate-limiting enzyme 3-OH-methylglutaryl coenzyme A reductase and, in turn, stimulate its removal by enhancing the expression of low-density lipoprotein (LDL) receptors in the liver (318). An increase of total cholesterol and its subfractions is a common finding in hypothyroidism, and a direct relationship between serum TSH and total cholesterol levels has been described (318). Reduced expression of the hepatic LDL receptor gene, responsible for an impaired cholesterol clearance, may contribute to hypercholesterolemia in patients with hypothyroidism (318). The elevation of triglycerides in hypothyroidism is due to a decrease in the activity of hepatic triglyceride lipase (318).
Overt hypothyroidism is associated with hyperlipidemia, and even SHypo has been related to alterations in plasma lipids, although with conflicting results (319). Data from the EPIC-Norfolk study showed a higher total and LDL cholesterol (LDL-C) in women with SHypo and reported that, even in the euthyroid population, there were associations between TSH levels and lipid profiles (320). Other studies confirmed that the association between TSH and total cholesterol and LDL-C remained significant after the adjustment for age and sex (299, 321, 322). In the HUNT Study, among 30,656 euthyroid individuals, serum TSH levels within the euthyroid range were positively and significantly associated with total cholesterol, LDL-C, and triglycerides, with an inverse relationship between serum TSH and HDL-C (323). Higher TSH levels were also associated with higher triglycerides and lower HDL; furthermore, this relationship was stronger among individuals who were overweight (BMI >25 kg/m2) (323). Moreover, serum TSH was positively associated with total cholesterol and triglycerides among a cohort of 2771 euthyroid Hispanic individuals, and serum-free T4 was positively correlated with serum HDL-C (311).
Two meta-analyses found that systolic (324, 325) and diastolic blood pressure (325) were higher in the group with SHypo than in euthyroid controls.
One of the mechanisms that links the MetS with hepatic steatosis is the insulin stimulation of lipogenesis, which can lead to fatty liver and worsen insulin resistance (326). Longitudinal studies support the association of nonalcoholic fatty liver disease with either T2D or MetS. Moreover, SHypo and high serum TSH levels were associated with nonalcoholic fatty liver disease (327–329).
All of these studies suggest that atherogenic dyslipidemia, insulin resistance, and hypertension can be linked with overt and subclinical hypothyroid states, and consequently TH deficiency is significantly associated with some components of MetS.
Association between MetS and TH deficiency
The prevalence of MetS was reported to be 44% in patients with hypothyroidism and 35% in patients with SHypo compared with 33% in the control group (P = 0.016) (330). A significant increase in the odds of having MetS was found in participants with marked SHypo (TSH 10 to 20 mU/L). Two meta-analysis of cross-sectional studies concluded that SHypo might be associated with an increased risk of MetS (331, 332). However, heterogeneity was observed among the included studies because the authors did not apply a uniform definition of MetS for their study design and data synthesis. Another meta-analysis assessed the association between MetS and SHypo by employing the Adult Treatment Panel III criteria for MetS (333). Although this study did not report a significant difference in MetS prevalence between individuals with SHypo and euthyroid individuals (OR, 1.13; 95% CI, 0.95 to 1.34), the prevalence of central obesity (OR, 1.43; 95% CI, 1.04 to 1.96) was significantly higher in the group with SHypo, and hypertriglyceridemia was prevalent in the female group with SHypo (333).
“…all subjects with T1D should undergo annual functional screening by serum TSH measurement to detect asymptomatic TD….”
There are reports on higher TSH levels in patients with MetS than in healthy ones, as well as a high prevalence of MetS in subjects with TSH levels higher than normal when compared with those with normal TSH levels (176, 334). Even high-normal serum TSH levels and low-normal free T4 levels have been significantly associated with increased prevalence of MetS (335).
A large population-based study of older adults reported that increasing TSH levels were associated with greater odds of having MetS and found a significant association between continuous serum TSH levels and prevalent MetS among euthyroid participants (176). A study on a euthyroid German cohort (mean age 52 years) found that TSH in the upper normal range (2.5 to 4.5 mU/L) was associated with a 1.7-fold increased risk of MetS compared with a low-normal TSH (0.3 to 2.5 mU/L) (336). Euthyroid subjects with TSH values in the upper normal range (2.5 to 4.5 mU/L) were more obese (BMI >30 kg/m2), had higher triglyceride levels, and had an increased likelihood of having MetS (336). Another study on 1000 euthyroid postmenopausal women in Korea (mean age 59 years) reported an OR for MetS of 1.9 in participants with the highest vs lowest quartiles of TSH (337). Interestingly, the prevalence of MetS decreased significantly from the higher to lower FT4 tertiles within the euthyroid range (30.1% in the lowest FT4 tertile to 22.4% in the highest FT4 tertile) (P < 0.001) (107). Higher FT4 values were associated with lower odds of MetS (OR, 0.96; 95% CI, 0.92 to 0.99; P = 0.01) (107). Moreover, an association between FT4 and lipids in MetS was demonstrated (106).
All of these data suggest that there is a higher prevalence of metabolic abnormalities with increasing serum TSH levels.
Both MetS and hypothyroidism are independent risk factors for CVDs (338). The MetS has been linked to CVD and mortality in the general population (263, 338, 339). This high cardiovascular risk could be further increased when metabolic syndrome is associated with TH deficiency (340). SHypo was considered a risk factor for MetS (341). Consequently, a considerable overlap may occur in the pathogenetic mechanisms of atherosclerotic disease correlated with MetS and hypothyroidism (Fig. 4).
Figure 4.
The pathogenetic mechanism for atherosclerotic disease correlates with hypothyroidism and MetS.
Prognosis of Patients With T2D and Associated TD: Long-Term Morbidity and Mortality
DM is associated with increased morbidity and mortality. Patients with DM have an increased risk of heart disease, stroke, heart failure, kidney disease, blindness, amputations, and neuropathy (342–344). DM caused 1.5 million deaths in 2012, with an excess relative risk of mortality, ranging from 1.15 to 3.15, mainly attributable to cardiovascular causes (345–349).
Epidemiological studies have shown an increased cardiovascular risk in patients with subclinical and overt hypothyroidism (350). Insulin resistance, diastolic hypertension, increased systemic vascular resistance, increased arterial stiffness, endothelial dysfunction, and altered coagulability represent the main risk factors responsible for the increased cardiovascular mortality associated with TH deficiency (4). Individual participant data meta-analyses reported that SHypo was associated with an increased risk for coronary heart disease (CHD) events and mortality (350) and heart failure (351), especially in patients with serum TSH >10 mU/L.
The onset of TD can further increase the long-term morbidity and mortality associated with diabetes. The coexistence of both diabetes and thyroid disorders may worsen the risk of diabetic retinopathy (DR) and diabetic nephropathy (DN) in patients with diabetes (352–354). DR, a common microvascular complication of diabetes, can be completely asymptomatic until a significant visual impairment occurs, and patients often miss the opportunity for appropriate treatment. Risk factors for the development of DR include duration of diabetes, poor glycemic control, elevated blood pressure, and dyslipidemia (355). SHypo is linked to DR (353, 354). A meta-analysis including eight epidemiological studies confirmed this association showing that the onset of SHypo increased the risk of DR by 2.13-fold (95% CI, 1.41 to 3.23; P < 0.001) compared with euthyroid individuals (356).
A growing body of evidence suggests that hypothyroidism is a risk factor for incident chronic kidney disease, progression of chronic kidney disease , and higher death risk in kidney disease patients (352, 357). SHypo was associated with albuminuria in patients with T2D, and an increased serum TSH was an independent risk factor for the presence of albuminuria (357). A greater prevalence of DN was reported in a cross-sectional analysis on 588 patients with T2D and SHypo compared with euthyroid individuals (352). SHypo was associated with an OR of 3.15 (95% CI, 1.48 to 6.69) for the development of DN and was also responsible for an increased risk of cardiovascular events mediated by DN (352).
There are conflicting results on cardiovascular mortality in patients with diabetes and TH deficiency (358, 359). Patients with diabetes have up to a sixfold higher risk of future CHD events compared with nondiabetic individuals (360–362). The coexistence of both T2D and thyroid diseases could further increase the risk of CHD (363).
A meta-analysis of 36 articles assessed the prevalence of complications in patients with T2D with SHypo (103). The risks of diabetic microangiopathy and macroangiopathy were evaluated by assessing the risk of DN and DR, peripheral arterial disease (PAD), CHD, and diabetic peripheral neuropathy (DPN). The OR for DN was 1.74 (95% CI, 1.34 to 2.28) when pooling data from 10 studies, which included 869 individuals with T2D with SHypo compared with 3892 euthyroid individuals with T2D. The OR for DR was 1.42 (95% CI, 1.21 to 1.67) when pooling the results from 10 studies that enrolled 835 individuals with T2D with SHypo and 3737 euthyroid individuals with T2D. The OR for CHD was 1.59 (95% CI, 0.92 to 2.76) when pooling data from 10 studies that included 579 individuals with T2D with SHypo and 1317 euthyroid individuals with T2D. The pooled OR for PAD was 1.85 (95% CI, 1.35 to 2.54) when pooling data from four studies that included 312 cases and 489 controls. The pooled OR for DPN was 1.87 (95% CI, 1.06 to 3.28) when pooling data from three studies that included of 420 individuals with T2D with SHypo and 1290 euthyroid individuals with T2D. Therefore, the authors concluded that SHypo is prevalent in patients with T2D and may be responsible for a greater risk of diabetic complications such as DN, DR, PAD, and DPN, but not CHD (103).
Thyroid Dyfunction and Gestational Diabetes
Incidence and adverse effects of TD during pregnancy in patients with diabetes
DM and TD are among the most common endocrinopathies during pregnancy. Hypothyroidism can be responsible for a variety of adverse effects in pregnancy outcomes and offspring- and pregnancy-related complications (364, 365). The prevalence of hypothyroidism in women during the reproductive age is 2% to 3%, and chronic autoimmune thyroiditis is the main cause of hypothyroidism during pregnancy. Pregnancy has a profound impact on thyroid gland morphology and function. TH and daily iodine requirements increase during pregnancy because of important changes in the thyroid function and renal iodine excretion (364–366). Serum FT4 decreases whereas TSH gradually rises within normal limits during pregnancy owing to the influence of increasing concentrations of T4-binding globulin and the transplacental transfer of FT4. Moreover, the turnover of FT4 is increased for the enhanced activity of placental type III deiodinase. This trend is further increased in women with HT, leading to an increased risk of TH deficiency during pregnancy (364–366). Severe maternal hypothyroidism is associated with an increased risk of adverse pregnancy complications (fetal death, premature birth, low birth weight, pregnancy loss, and gestational hypertension) as well as detrimental effects on fetal neurocognitive development, leading to a lower intelligence quotient in the offspring (364–366). An increased risk of pregnancy complications is also associated with SHypo, especially in TPO Ab-positive women (364–366). Recent guidelines have recommended treating overt and SHypo on the basis of these adverse effects, especially in TPO Ab-positive women with a TSH greater than the pregnancy-specific reference range (364, 365). Moreover, the ATA guidelines have recommended L-T4 therapy for TPO Ab-negative women with a TSH >10.0 mIU/per liter (365), whereas the ETA guidelines recommend L-T4 replacement in women with SHypo who are TPO Ab-negative (364).
Gestational DM (GDM) is defined by the World Health Organization as “any degree of glucose intolerance with the onset or first recognition during pregnancy” (367, 368). The prevalence of GDM in pregnant women ranges from 1.7% to 20%, with a wide difference according to country, screening strategy, and diagnostic criteria (367, 369–371). The incidence of T2D in pregnant women is increased with both maternal age and increasing prepregnancy BMI (367–371). Moreover, pregnant women with a family history of diabetes have a much higher incidence of GDM than do those without a family history of diabetes (367–371). Patients with poorly controlled DM may have up to a 50% increased risk of pregnancy loss (372). Women diagnosed with GDM should receive lifelong screening for prediabetes and T2D because they have an increased risk for the development of T2D after delivery (368, 373).
The incidence of TD during pregnancy in women with T1D is threefold higher than in the general population, particularly in the first trimester of pregnancy and in the first year postpartum (374). TD was observed in 22.5% of women with T1D in the first trimester and 18.4% in the third trimester; the prevalent form of TD was subclinical or overt hypothyroidism (374). Alternatively, pregnant women with hypothyroidism or isolated hypothyroxinemia can have adverse metabolic characteristics (e.g., raised blood glucose, HbA1c, insulin resistance, and gestational diabetes) (375–377). A meta-analysis of six cohort studies showed that the incidence of GDM in patients with SHypo was 1.35-fold higher than the incidence in the control group (378). The risk for gestational diabetes is increased even in women with high TSH levels within the normal reference range (378). A large meta-analysis reported that the presence of thyroid autoantibodies did not increase the risk of GDM in euthyroid pregnant women, and their positivity in the first trimester did not have a predictive value for the risk of GDM (379). Conversely, the combination of high serum TSH and thyroid autoimmunity in early pregnancy was associated with a fourfold increased risk for GMD (RR, 4.3; 95% CI, 2.1 to 8.9) and a threefold increased risk for low birth weight neonates (RR, 3.1; 95% CI, 1.2 to 8.0) after adjustment for several confounders (380).
A prospective study of 6031 Chinese pregnant women showed that low FT4 levels during pregnancy were a risk factor for GDM and preeclampsia (381). Conversely, the incidence of GDM decreased in women with higher FT4 levels, showing a protective effect for the development of GDM (382). GDM can induce adverse outcomes such as miscarriage, excessive fetal growth, birth trauma, and neonatal metabolic abnormalities. These complications may be further increased when hypothyroidism develops in patients with GMD (365). Additionally, women with GDM are at a high risk of developing chronic hypertension and vascular complications (383, 384). An association has been reported between overt hypothyroidism during pregnancy and subsequent diabetes morbidity later in life (385).
Risk of postpartum thyroiditis in patients with diabetes
Postpartum thyroiditis (PPTD) is caused by a destructive autoimmune thyroiditis in the postpartum period, mediated by a rebound of both cellular and humoral immunity. The clinical presentation can vary from hyperthyroidism to hypothyroidism or both and is associated with a significant increased incidence of depression (386). PPTD developed in up to 25% to 38% of women with T1D (387, 388). The incidence of PPTD is threefold to fourfold higher in women with T1D compared with unselected populations (388, 389).
Screening of TD in pregnant patients with DM
The screening of TD and PPTD is recommended in pregnant women with T1D or other autoimmune disorders by the ATA/American Association of Clinical Endocrinologists (365), the Endocrine Society (390), the British Thyroid Association (BTA) (391), and the American Diabetes Association (ADA) (392–394). The ATA/ACE and the Endocrine Society also recommend the screening of TD in women with morbid obesity (BMI ≥40 kg/m2), TPO positivity, family or personal history of TD, and age >30 years, before and after pregnancy (365, 390). TSH and TPO Abs should be evaluated in women with T1D planning pregnancy or at the beginning of pregnancy. When thyroid function is normal, the screening should be repeated in the first trimester. In case of positive TPO Abs and normal serum TSH during pregnancy, it is essential to recheck thyroid function at 3, 6, and 12 months after delivery. Screening for postpartum thyroiditis is recommended in patients with T1D at 3 and 6 months postpartum (390).
Necessity for a Screening Program to Assess TD in Patients With Diabetes: Differences Among Guidelines
Screening of TD in patients with T1D
TD is a frequent condition in patients with T1D with a rising prevalence with age. The onset of TD is responsible for poorly controlled T1D because overt or SHypo can be associated with increased risk of symptomatic hypoglycemia whereas hyperthyroidism alters glucose metabolism, potentially resulting in the deterioration of metabolic control.
The assessment of TD in patients with T1D meets the criteria for a screening program because of the following points: (i) TD and type 1 DM are frequently linked; (ii) clinical and laboratory diagnoses can be easily performed; (iii) the delay or lack of diagnosis of TD may worsen the prognosis of T1D; and (iv) an effective therapy for TD and diabetes is available (Table 7). Therefore, the cost-benefit ratio could be favorable for TD screening in T1D.
Table 7.
Major Criteria Supporting the Screening of TD in Patients With Type 1 DM
| • TD and T1D are frequently associated. |
| • The clinical and laboratory diagnosis is easily performed. |
| • The delay or lack of diagnosis of TD can worsen the prognosis of T1D. |
| • Early treatment of these disorders can improve their prognosis. |
| • The cost-benefit ratio is favorable for a screening of TD. |
Consistent with these considerations, screening for TD is currently recommended in children, adolescents, and adults with T1D by the ATA (199), BTA (391), ADA (392–394), American Association of Clinical Endocrinologists (395), and the International Society for Pediatric and Adolescent Diabetes (396).
The BTA (391), ADA (392–394), and the International Society for Pediatric and Adolescent Diabetes (396) recommend that thyroid Ab tests and thyroid function should be considered close to the time of diagnosis of T1D and repeated when clinical symptoms suggest the possibility of thyroid disease. Thyroid tests should be rechecked every year. Therefore, all subjects with T1D should undergo annual functional screening by serum TSH measurement to detect asymptomatic TD, particularly those with positive TPO Abs. L-T4 replacement therapy should be started in diabetic patients with overt, subclinical, and mild hypothyroidism to reduce the risk of hyperlipidemia and atherosclerotic heart disease (4, 198, 199). The evaluation and treatment of hyperthyroidism should be started in patients with suppressed serum TSH and elevated TH levels (194, 195). According to the ADA, the screening of TD should include TSH, TPO Abs, and thyroglobulin Abs (392–394). The ADA specifies that TSH should be measured after obtaining satisfactory glycemic control. Long-term follow-up should include a periodic assessment of thyroid function (with TSH determination). Thus, tests should be run at 1- to 2-year intervals when TPO Abs are initially negative and more frequent (up to every 6 months) when TPO Abs are positive or when there are symptoms of TD such as goiter, abnormal growth rate in pediatric age, or unexplained glycemic variation. The US Preventive Services Task Force did not have specific recommendations about T1D (397). However, in general, it suggested that the screening of TD should be performed by means of thyroid autoantibodies and serum TSH because they are the most sensitive ways of identifying patients with thyroid autoimmunity and TD (397). Multiple tests during 3 to 6 months should be performed to confirm or rule out any abnormal findings. Follow-up testing of serum T4 levels in persons with persistently abnormal TSH levels can differentiate between subclinical (normal T4) and overt (abnormal T4) TD (397). All of these recommendations confirm the importance of screening TD in patients with T1D and vice versa to improve symptoms, quality of life, and prognosis (see Table 8 for a summary of guidleines) (391–398).
Table 8.
Summary of Guidelines on a Screening Program for TD in Patients With T1D and T2D, in the General Population, and in Pregnant Patients
| Guidelines (Ref.) | T1D | T2D | In the General Population | In Pregnant Patients |
|---|---|---|---|---|
| BTA (391) | TSH and TPO Ab at diagnosis and every year | Thyroid function tests at baseline. Routine annual thyroid function testing is not recommended | Not applicable | TSH and Abs are recommended in diabetic patients in pregnancy and postpartum |
| ADA (392–394) | TSH, TPO Ab, and Tg Ab evaluation at diagnosis Measure TSH soon after the diagnosis of T1D and after glucose control. Annual screening when TPO Abs are initially negative and more frequent (up to every 6 mo) when TPO Abs are positive or when there are symptoms of TD such as goiter, abnormal growth rate in pediatric age, or unexplained glycemic variation A1c, islet cell Abs, and glutamic acid decarboxylase Abs at the time of diagnosis of AITD | Thyroid palpation in all diabetic patients TSH in adults >50 y of age or in patients with dyslipidemia | TSH screening at the age of 45 y and in younger adults who are overweight with one or more of the following risk factors: (i) positive family history of diabetes (ii) coming from high-risk minority ethnic/racial groups (iii) presence of hyperlipidemia (iv) being physically inactive (v) experiencing signs/symptoms of insulin resistance and/or (vi) presence of hypertension or treatment of hypertension (vii) women with polycystic ovary syndrome or other clinical conditions associated with insulin resistance (e.g. severe obesity, acanthosis nigricans) | Women who were diagnosed with GDM should have lifelong testing at least every 3 y |
| AACE (395) | Thyroid palpation and TSH at diagnosis and at regular intervals in patients with goiter and AITD | Thyroid palpation and TSH at diagnosis and at regular intervals in patients with goiter and AITD Screening of TD in older patients, especially women | Annual screening for patients with two or more risk factors Individuals at risk for DM whose glucose values are in the normal range should be screened every 3 y | Pregnant females with DM risk factors should be screened at the first prenatal visit for undiagnosed T2D using standard criteria. At 24- to 28-wk gestation, all pregnant subjects should be screened for gestational diabetes Assessment of thyroid function in pregnant patients with diabetes recommended |
| ISPAD (396) | Thyroid Ab tests and thyroid function at close diagnosis and repeated when clinical symptoms suggest the possibility of thyroid disease Annual screening in asymptomatic patients when thyroid function is normal | No specific recommendations | Screening in all children and adolescents at risk for glucose intolerance or diabetes. The risks include: (i) a combination of BMI greater than the 85th percentile for height, (ii) ethnicity, (iii) family history of CVD in first- or second-degree members, and (iv) signs of insulin resistance (as defined by the ADA). Screening should begin at age 10 y or at the onset of puberty (when puberty occurs at an earlier age) using the fasting plasma glucose test, and rescreening should be carried out every 2 y | Not applicable |
| USPTF (397) | No specific recommendations | No specific recommendations | Insufficient evidence to recommend a screening program in nonpregnant asymptomatic adults | Not applicable |
| ATA (398) | More frequent TSH evaluation in patients with T1D | More frequent TSH evaluation in patients with T2D | Evaluation of serum TSH in asymptomatic adults 35 y or age every 5 y and in individuals with symptoms and signs potentially attributable to TD and those with risk factors for its development. More frequent testing in high-risk patients | Not applicable |
Abbreviations: AACE, American Association of Clinical Endocrinologists; ISPAD, International Society for Pediatric and Adolescent Diabetes; Ref., reference; USPTF, US Preventive Task Force.
The possible coexistence or potential onset of T1D in AITD patients, especially in the case of younger subjects and/or a family history of T1D, can be evaluated by measuring fasting glucose, HbA1c, islet cell Abs, and glutamic acid decarboxylase Abs at the time of diagnosis of AITD. In case of a T1D–AITD association, a genetic study is advised along with a serological screening of first-degree relatives in the context of probable APS (21). Owing to the well-known increased clustering of both endocrine and nonendocrine autoimmune diseases in patients with coexistent T1D and AITD (APS3) and their relatives, serological screening with the measurement of the glandular autoantibodies is recommended in all APS3 patients and their first-degree relatives (19–22). When serological screening is positive, functional testing follows. Also recommended is the serological screening for the prevalent autoimmune nonendocrine disorders in APS, that is, celiac disease, autoimmune gastritis, pernicious anemia, and Sjögren syndrome. Typing of the MHC HLA class II DR/DQ antigens and eventually testing the PTPN22 and CTLA-4 SNPs help differentiate between patients with polyglandular vs monoglandular autoimmunity (32, 33, 399).
A longitudinal evaluation of thyroid function should be subsequently monitored every 1 to 2 years or obtained at any time growth rate is abnormal.
Screening of TD in patients with T2D
T2D accounts for ∼14% of the health expenditure (US$245 billion last year) in the United States to treat complications such as myocardial infarction, stroke, retinopathy, end-stage renal disease, and care for ulcerative lesions (400). Regarding the screening of T2D, the ADA suggests the screening of this disorder at the age of 45 years as well as screening younger adults who are overweight with one or more of the following risk factors: (i) a positive family history of diabetes; (ii) coming from high-risk minority ethnic/racial groups; (iii) the presence of hyperlipidemia; (iv) being physically inactive; (v) experiencing signs/symptoms of insulin resistance (i.e., acanthosis nigricans, polycystic ovarian syndrome, history of GMD, previous prediabetes diagnosis); and/or (vi) the presence of hypertension or treatment of hypertension (390) (Table 9). The ADA recommends rescreening patients with these risk factors only every 3 years based on the rationale that someone who tests negative would be highly unlikely to develop the disease complications within the 3-year time period (392–394).
Table 9.
Benefits and Limits of a Screening Program in Patients With T2DM
| • Asymptomatic TD is frequently observed in patients with T2D. |
| • Undetected thyroid disorders can compromise the metabolic control and amplify the risk of cardiovascular events. |
| • The screening of TD could prevent the high cardiovascular complications of T2D. |
| • The diagnosis of TD in patients with T2D is difficult because some oral antidiabetic drugs can interfere with the evaluation of serum TSH levels. |
| • Mild subclinical thyroid disease can progress at a low rate, suggesting a long-term screening program. |
| • The use of age-specific TSH range should be considered to avoid the misdiagnosis of hypothyroidism in elderly patients with T2D. |
| • There are insufficient data to assess the specific tests for screening (TSH alone, TSH and TPO, or TSH and FT4), the exact interval of periodic screening, and the cost-benefit ratio. |
Regarding the screening of TD, the ATA recommends that adults should be screened by the measurement of serum TSH concentration, beginning at age 35 years and every 5 years thereafter (398). This screening is justified because it is cost-effective within the context of the periodic health examination. Moreover, the ATA suggests that individuals with symptoms and signs potentially attributable to TD and those with risk factors for its development may require more frequent TSH testing (398). Conversely, despite the potential association between TD and CVD, the US Preventive Services Task Force concluded that there is no clear evidence for the necessity of a screening program of TD in asymptomatic subjects (397).
The necessity of a screening program for TD in patients with T2D is a controversial issue because current evidence is insufficient to assess the specific tests for screening (TSH alone, TSH and TPO Abs, or TSH and FT4), the exact interval of periodic screening, and the cost-benefit ratio. The ADA recommends thyroid palpation in all diabetic patients and the evaluation of serum TSH in adults >50 years of age and in patients with dyslipidemia (392–394). The ATA recommends testing TSH at the diagnosis of diabetes and every 5 years thereafter (398). According to the BTA, thyroid function tests are recommended in T2D only when there is a suspicion of an AITD (391). They suggest that patients with T2D should have their thyroid function checked at diagnosis, but routine annual thyroid function testing is not recommended.
However, most of the available guidelines are too general and not detailed regarding routine screening and monitoring of T2D and TD. Even current guidelines from the ETA (194, 198) and ATA (195, 199, 401) do not specifically address screening of thyroid function or treatment of TD in individuals with T2D.
The following considerations could support the benefit of an early detection of TD in patients with T2D: (i) TD, especially hypothyroidsm, is frequently observed in patients with T2D; (ii) many patients with T2D may be asymptomatic even in the presence of overt hypothyroidism; (iii) clinical symptoms of TD may be masked by a poor metabolic control, which is more frequent when TD develops; (iv) undetected thyroid disorders may compromise the metabolic control and amplify the risk of cardiovascular events in patients with T2D; and (v) the screening of TD may allow early treatment, preventing high cardiovascular and metabolic complications (Table 9).
Conversely, the subsequent considerations may limit the necessity of a screening program for TD in patients with T2D: (i) there is evidence that the mild form of STDs can progress at a low rate (<1% during 5 years), suggesting a long-term screening program; (ii) it is difficult to diagnose TD in T2D patients for the frequent association of comorbidity and drug interferences; (iii) some oral antidiabetic drugs interfere with the evaluation of serum TSH levels; and (iv) the use of age-specific TSH range should be considered to avoid misdiagnosis of hypothyroidism in elderly patients with T2D (3, 4) (Table 9).
All of these considerations support that the evaluation of serum TSH alone could be inappropriate for screening or monitoring TD in patients with T2D, even in those with an acute presentation of DM such as ketoacidosis, hyperosmolar conditions, and hypoglycemic episodes. The assessment of both FT4 and TPO could be necessary in a screening program, but this assessment further increases the cost of screening.
Therefore, there is insufficient evidence to evaluate whether a specific screening of TD policy is necessary in T2D. These uncertainties do not allow us to adopt specific recommendations from the guidelines published by the major endocrine and diabetes societies (see Table 8 for a summary of guidelines) (391–398).
Unanswered Questions and Future Needs
More studies are needed to better understand the possible role of TD in the development and progression of DM and vice versa. In the future, the use of more sensitive peripheral markers of thyroid function may help clinicians to identify and personalize the treatment of TD in patients with diabetes, particularly in those in which these disorders are difficult to manage.
Large prospective studies are necessary to clarify the adverse effects of TD and DM when associated. Randomized controlled trials could verify whether the treatment of thyroid disorders can counteract the expected risks. Futures studies assessing TD in the early stage and performing an adequate treatment of TD in patients with DM could help understand the prognosis of these associated disorders when appropriately treated.
The discovery of TH analogs to improve metabolic control may help clinicians improve the cardiovascular risk factors associated with DM, MetS, and TH deficiency.
Future studies could clarify the potential role of metformin to improve TSH suppression therapy with L-T4 in patients with differentiated thyroid cancer and the prognosis of medullary thyroid cancer.
Finally, specific guidelines are necessary to recommend a systematic approach for the correct diagnosis and treatment of TD in patients with T1D and T2D.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant RO-1 38325-35 (to P.R.R.).
Disclosure Summary The authors have nothing to disclose.
Glossary
Abbreviations
- Ab
antibody
- ADA
American Diabetes Association
- AgRP
agouti-related protein
- AITD
autoimmune thyroid disease
- APC
antigen-presenting cell
- APS3
autoimmune polyglandular syndrome type 3 variant
- ARC
arcuate nucleus
- ATA
American Thyroid Association
- ATD
antithyroid drug
- BAT
brown adipose tissue
- BDNF
brain-derived neurotrophic factor
- BMI
body mass index
- BTA
British Thyroid Association
- CART
cocaine- and amphetamine-regulated transcript
- CHD
coronary heart disease
- CLEC16A
C-type lectin domain family 16 member A
- CT
calcitonin
- CTLA
cytotoxic T lymphocyte antigen
- CVD
cardiovascular disease
- D1
deiodinase type 1
- D2
deiodinase type 2
- D3
deiodinase type 3
- DM
diabetes mellitus
- DN
diabetic nephropathy
- DPN
diabetic peripheral neuropathy
- DPP-4
dipeptidyl peptidase-4
- DR
diabetic retinopathy
- ETA
European Thyroid Association
- FOXP3
forkhead box P3
- GD
Graves disease
- GDM
gestational DM
- GLP-1
glucagon-like peptide-1
- GLUT
glucose transporter
- GO
Graves ophthalmology
- HDL
high-density lipoprotein
- HDL-C
HDL cholesterol
- HLA
human leukocyte antigen
- HNF
hepatocyte nuclear factor
- HPT
hypothalamic–pituitary–thyroid
- HT
Hashimoto thyroiditis
- ICV
intracerebroventricular(ly)
- IL-2Rα
IL-2 receptor α
- IRS
insulin receptor substrate
- LDL
low-density lipoprotein
- LDL-C
LCL cholesterol
- LYP
lymphoid tyrosine phosphatase
- MetS
metabolic syndrome
- MHC
major histocompatibility complex
- MODY
maturity-onset diabetes of the young
- MTC
medullary thyroid carcinoma
- NHANES
National Health and Nutrition Examination Survey
- NPY
neuropeptide Y
- PAD
peripheral arterial disease
- PEPCK
phosphoenolpyruvate carboxykinase
- POMC
proopiomelanocortin
- PPAR-γ
peroxisome proliferator–activated receptor-γ
- PPTD
postpartum thyroiditis
- PTPN22
protein tyrosine phosphatase nonreceptor type 22
- PVN
paraventricular nucleus
- RAI
radioactive iodine
- RR
relative risk
- SHyper
subclinical hyperthyroidism
- SHypo
subclinical hypothyroidism
- SNP
single nucleotide polymorphism
- STD
subclinical thyroid disorder
- TD
thyroid dysfunction
- TH
thyroid hormone
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TPO
thyroperoxidase
- TR
TH receptor
- TZD
thiazolidinedione
- UCP
uncoupling protein
- VMN
ventromedial nucleus
- WAT
white adipose tissue
References
- 1. Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith PA. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf). 1977;7(6):481–493. [DOI] [PubMed] [Google Scholar]
- 2. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. [DOI] [PubMed] [Google Scholar]
- 3. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 4. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–1154. [DOI] [PubMed] [Google Scholar]
- 5. Biondi B, Cooper DS. Subclinical hyperthyroidism. N Engl J Med. 2018;378(25):2411–2419. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. World Health Statistics 2016: Monitoring health for the sustainable development goals. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 7. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 9. Neufeld M, Maclaren N, Blizzard R. Autoimmune polyglandular syndromes. Pediatr Ann. 1980;9(4):154–162. [PubMed] [Google Scholar]
- 10. Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med. 2004;350(20):2068–2079. [DOI] [PubMed] [Google Scholar]
- 11. Shun CB, Donaghue KC, Phelan H, Twigg SM, Craig ME. Thyroid autoimmunity in type 1 diabetes: systematic review and meta-analysis. Diabet Med. 2014;31(2):126–135. [DOI] [PubMed] [Google Scholar]
- 12. Jin P, Huang G, Lin J, Yang L, Xiang B, Zhou W, Zhou Z. High titre of antiglutamic acid decarboxylase autoantibody is a strong predictor of the development of thyroid autoimmunity in patients with type 1 diabetes and latent autoimmune diabetes in adults. Clin Endocrinol (Oxf). 2011;74(5):587–592. [DOI] [PubMed] [Google Scholar]
- 13. Gambelunghe G, Forini F, Laureti S, Murdolo G, Toraldo G, Santeusanio F, Brunetti P, Sanjeevi CB, Falorni A. Increased risk for endocrine autoimmunity in Italian type 2 diabetic patients with GAD65 autoantibodies. Clin Endocrinol (Oxf). 2000;52(5):565–573. [DOI] [PubMed] [Google Scholar]
- 14. Libman IM, Sun K, Foley TP, Becker DJ. Thyroid autoimmunity in children with features of both type 1 and type 2 diabetes. Pediatr Diabetes. 2008;9(4 Pt 1):266–271. [DOI] [PubMed] [Google Scholar]
- 15. Fatourechi A, Ardakani HM, Sayarifard F, Sheikh M. Hypothyroidism among pediatric patients with type 1 diabetes mellitus, from patients’ characteristics to disease severity. Clin Pediatr Endocrinol. 2017;26(2):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleiner HF, Bjøro T, Midthjell K, Grill V, Åsvold BO. Prevalence of thyroid dysfunction in autoimmune and type 2 diabetes: the population-based HUNT study in Norway. J Clin Endocrinol Metab. 2016;101(2):669–677. [DOI] [PubMed] [Google Scholar]
- 17. Umpierrez GE, Latif KA, Murphy MB, Lambeth HC, Stentz F, Bush A, Kitabchi AE. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003;26(4);1181–1185. [Google Scholar]
- 18. Kordonouri O, Klinghammer A, Lang EB, Grüters-Kieslich A, Grabert M, Holl RW. Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care. 2002;25(8):1346–1350. [DOI] [PubMed] [Google Scholar]
- 19. Kahaly GJ. Polyglandular autoimmune syndromes. Eur J Endocrinol. 2009;161(1):11–20. [DOI] [PubMed] [Google Scholar]
- 20. Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: immunogenetics and long-term follow-up. J Clin Endocrinol Metab. 2003;88(7):2983–2992. [DOI] [PubMed] [Google Scholar]
- 21. Kahaly GJ, Frommer L. Polyglandular autoimmune syndromes. J Endocrinol Invest. 2018;41(1):91–98. [DOI] [PubMed] [Google Scholar]
- 22. Dittmar M, Kahaly GJ. Genetics of the autoimmune polyglandular syndrome type 3 variant. Thyroid. 2010;20(7):737–743. [DOI] [PubMed] [Google Scholar]
- 23. Tomer Y, Menconi F. Type 1 diabetes and autoimmune thyroiditis: the genetic connection. Thyroid. 2009;19(2):99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang W, Connor E, Rosa TD, Muir A, Schatz D, Silverstein J, Crockett S, She JX, Maclaren NK. Although DR3-DQB1*0201 may be associated with multiple component diseases of the autoimmune polyglandular syndromes, the human leukocyte antigen DR4-DQB1*0302 haplotype is implicated only in beta-cell autoimmunity. J Clin Endocrinol Metab. 1996;81(7):2559–2563. [DOI] [PubMed] [Google Scholar]
- 25. Chikuba N, Akazawa S, Yamaguchi Y, Kawasaki E, Takino H, Yoshimoto M, Ohe N, Yamashita K, Yano A, Nagataki S. Immunogenetic heterogeneity in type 1 (insulin-dependent) diabetes among Japanese—class II antigen and autoimmune thyroid disease. Diabetes Res Clin Pract. 1995;27(1):31–37. [DOI] [PubMed] [Google Scholar]
- 26. Chuang LM, Wu HP, Chang CC, Tsai WY, Chang HM, Tai TY, Lin BJ. HLA DRB1/DQA1/DQB1 haplotype determines thyroid autoimmunity in patients with insulin-dependent diabetes mellitus. Clin Endocrinol (Oxf). 1996;45(5):631–636. [DOI] [PubMed] [Google Scholar]
- 27. Kim EY, Shin CH, Yang SW. Polymorphisms of HLA class II predispose children and adolescents with type 1 diabetes mellitus to autoimmune thyroid disease. Autoimmunity. 2003;36(3):177–181. [DOI] [PubMed] [Google Scholar]
- 28. Levin L, Ban Y, Concepcion E, Davies TF, Greenberg DA, Tomer Y. Analysis of HLA genes in families with autoimmune diabetes and thyroiditis. Hum Immunol. 2004;65(6):640–647. [DOI] [PubMed] [Google Scholar]
- 29. Golden B, Levin L, Ban Y, Concepcion E, Greenberg DA, Tomer Y. Genetic analysis of families with autoimmune diabetes and thyroiditis: evidence for common and unique genes. J Clin Endocrinol Metab. 2005;90(8):4904–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hashimoto K, Maruyama H, Nishiyama M, Asaba K, Ikeda Y, Takao T, Iwasaki Y, Kumon Y, Suehiro T, Tanimoto N, Mizobuchi M, Nakamura T. Susceptibility alleles and haplotypes of human leukocyte antigen DRB1, DQA1, and DQB1 in autoimmune polyglandular syndrome type III in Japanese population. Horm Res. 2005;64(5):253–260. [DOI] [PubMed] [Google Scholar]
- 31. Weinstock C, Matheis N, Barkia S, Haager MC, Janson A, Marković A, Bux J, Kahaly GJ. Autoimmune polyglandular syndrome type 2 shows the same HLA class II pattern as type 1 diabetes. Tissue Antigens. 2011;77(4):317–324. [DOI] [PubMed] [Google Scholar]
- 32. Flesch BK, Matheis N, Alt T, Weinstock C, Bux J, Kahaly GJ. HLA class II haplotypes differentiate between the adult autoimmune polyglandular syndrome types II and III. J Clin Endocrinol Metab. 2014;99(1):E177–E182. [DOI] [PubMed] [Google Scholar]
- 33. Barkia Beradhi S, Flesch BK, Hansen MP, Matheis N, Kahaly GJ. HLA class II differentiates between thyroid and polyglandular autoimmunity. Horm Metab Res. 2016;48(4):232–237. [DOI] [PubMed] [Google Scholar]
- 34. Faas S, Trucco M. The genes influencing the susceptibility to IDDM in humans. J Endocrinol Invest. 1994;17(7):477–495. [DOI] [PubMed] [Google Scholar]
- 35. Holl RW, Bohm B, Loos U, Grabert M, Heinze E, Homoki J. Thyroid autoimmunity in children and adolescents with type 1 diabetes mellitus. Effect of age, gender and HLA type. Horm Res. 1999;52(3):113–118. [DOI] [PubMed] [Google Scholar]
- 36. Dittmar M, Ide M, Wurm M, Kahaly GJ. Early onset of polyglandular failure is associated with HLA-DRB1*03. Eur J Endocrinol. 2008;159(1):55–60. [DOI] [PubMed] [Google Scholar]
- 37. Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, Davies TF, Ban Y, Jacobson EM, Concepcion ES, Li CW, Tomer Y. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci USA. 2008;105(37):14034–14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huber A, Menconi F, Corathers S, Jacobson EM, Tomer Y. Joint genetic susceptibility to type 1 diabetes and autoimmune thyroiditis: from epidemiology to mechanisms. Endocr Rev. 2008;29(6):697–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deng L, Mariuzza RA. Recognition of self-peptide–MHC complexes by autoimmune T-cell receptors. Trends Biochem Sci. 2007;32(11):500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Villano MJ, Huber AK, Greenberg DA, Golden BK, Concepcion E, Tomer Y. Autoimmune thyroiditis and diabetes: dissecting the joint genetic susceptibility in a large cohort of multiplex families. J Clin Endocrinol Metab. 2009;94(4):1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen S, Dadi H, Shaoul E, Sharfe N, Roifman CM. Cloning and characterization of a lymphoid-specific, inducible human protein tyrosine phosphatase, Lyp. Blood. 1999;93(6):2013–2024. [PubMed] [Google Scholar]
- 42. Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999;189(1):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vang T, Miletic AV, Bottini N, Mustelin T. Protein tyrosine phosphatase PTPN22 in human autoimmunity. Autoimmunity. 2007;40(6):453–461. [DOI] [PubMed] [Google Scholar]
- 44. Heward JM, Brand OJ, Barrett JC, Carr-Smith JD, Franklyn JA, Gough SC. Association of PTPN22 haplotypes with Graves’ disease. J Clin Endocrinol Metab. 2007;92(2):685–690. [DOI] [PubMed] [Google Scholar]
- 45. Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: back to the future. J Autoimmun. 2007;28(2–3):85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bottini N, Vang T, Cucca F, Mustelin T. Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Semin Immunol. 2006;18(4):207–213. [DOI] [PubMed] [Google Scholar]
- 47. Kahles H, Ramos-Lopez E, Lange B, Zwermann O, Reincke M, Badenhoop K. Sex-specific association of PTPN22 1858T with type 1 diabetes but not with Hashimoto’s thyroiditis or Addison’s disease in the German population. Eur J Endocrinol. 2005;153(6):895–899. [DOI] [PubMed] [Google Scholar]
- 48. Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, Ball SG, James RA, Quinton R, Perros P, Pearce SH. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves’ disease. J Clin Endocrinol Metab. 2004;89(11):5862–5865. [DOI] [PubMed] [Google Scholar]
- 49. Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, Eisenbarth GS, Comings D, Mustelin T. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36(4):337–338. [DOI] [PubMed] [Google Scholar]
- 50. Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, Vella A, Nutland S, Rance HE, Maier L, Barratt BJ, Guja C, Ionescu-Tîrgoviste C, Savage DA, Dunger DB, Widmer B, Strachan DP, Ring SM, Walker N, Clayton DG, Twells RC, Gough SC, Todd JA. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53(11):3020–3023. [DOI] [PubMed] [Google Scholar]
- 51. Kawasaki E, Awata T, Ikegami H, Kobayashi T, Maruyama T, Nakanishi K, Shimada A, Uga M, Kurihara S, Kawabata Y, Tanaka S, Kanazawa Y, Lee I, Eguchi K. Systematic search for single nucleotide polymorphisms in a lymphoid tyrosine phosphatase gene (PTPN22): association between a promoter polymorphism and type 1 diabetes in Asian populations [published correction appears in Am J Med Genet A. 2007;143(15):18212–18213]. Am J Med Genet A. 2006;140(6):586–593. [DOI] [PubMed] [Google Scholar]
- 52. Dittmar M, Kahaly GJ. Immunoregulatory and susceptibility genes in thyroid and polyglandular autoimmunity. Thyroid. 2005;15(3):239–250. [DOI] [PubMed] [Google Scholar]
- 53. Dultz G, Matheis N, Dittmar M, Röhrig B, Bender K, Kahaly GJ. The protein tyrosine phosphatase non-receptor type 22 C1858T polymorphism is a joint susceptibility locus for immunthyroiditis and autoimmune diabetes. Thyroid. 2009;19(2):143–148. [DOI] [PubMed] [Google Scholar]
- 54. Tomer Y, Dolan LM, Kahaly G, Divers J, D’Agostino RB Jr, Imperatore G, Dabelea D, Marcovina S, Black MH, Pihoker C, Hasham A, Hammerstad SS, Greenberg DA, Lotay V, Zhang W, Monti MC, Matheis N; SEARCH for Diabetes in Youth Study . Genome wide identification of new genes and pathways in patients with both autoimmune thyroiditis and type 1 diabetes. J Autoimmun. 2015;60:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24(1):65–97. [DOI] [PubMed] [Google Scholar]
- 56. Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162(10):5813–5820. [PubMed] [Google Scholar]
- 57. Donner H, Rau H, Walfish PG, Braun J, Siegmund T, Finke R, Herwig J, Usadel KH, Badenhoop K. CTLA4 alanine-17 confers genetic susceptibility to Graves’ disease and to type 1 diabetes mellitus. J Clin Endocrinol Metab. 1997;82(1):143–146. [DOI] [PubMed] [Google Scholar]
- 58. Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Rønningen KS, Guja C, Ionescu-Tîrgovişte C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. [DOI] [PubMed] [Google Scholar]
- 59. Kavvoura FK, Akamizu T, Awata T, Ban Y, Chistiakov DA, Frydecka I, Ghaderi A, Gough SC, Hiromatsu Y, Ploski R, Wang PW, Ban Y, Bednarczuk T, Chistiakova EI, Chojm M, Heward JM, Hiratani H, Juo SH, Karabon L, Katayama S, Kurihara S, Liu RT, Miyake I, Omrani GH, Pawlak E, Taniyama M, Tozaki T, Ioannidis JP. Cytotoxic T-lymphocyte associated antigen 4 gene polymorphisms and autoimmune thyroid disease: a meta-analysis. J Clin Endocrinol Metab. 2007;92(8):3162–3170. [DOI] [PubMed] [Google Scholar]
- 60. Ban Y, Taniyama M, Tozaki T, Yanagawa T, Yamada S, Maruyama T, Kasuga A, Tomita M, Ban Y. No association of type 1 diabetes with a microsatellite marker for CTLA-4 in a Japanese population. Autoimmunity. 2001;34(1):39–43. [DOI] [PubMed] [Google Scholar]
- 61. Cinek O, Drevínek P, Sumník Z, Bendlová B, Kolousková S, Snajderová M, Vavrinec J. The CTLA4 +49 A/G dimorphism is not associated with type 1 diabetes in Czech children. Eur J Immunogenet. 2002;29(3):219–222. [DOI] [PubMed] [Google Scholar]
- 62. Takara M, Kouki T, DeGroot LJ. CTLA-4 AT-repeat polymorphism reduces the inhibitory function of CTLA-4 in Graves’ disease. Thyroid. 2003;13(12):1083–1089. [DOI] [PubMed] [Google Scholar]
- 63. Wang XB, Kakoulidou M, Giscombe R, Qiu Q, Huang D, Pirskanen R, Lefvert AK. Abnormal expression of CTLA-4 by T cells from patients with myasthenia gravis: effect of an AT-rich gene sequence. J Neuroimmunol. 2002;130(1–2):224–232. [DOI] [PubMed] [Google Scholar]
- 64. Anjos S, Nguyen A, Ounissi-Benkalha H, Tessier MC, Polychronakos C. A common autoimmunity predisposing signal peptide variant of the cytotoxic T-lymphocyte antigen 4 results in inefficient glycosylation of the susceptibility allele. J Biol Chem. 2002;277(48):46478–46486. [DOI] [PubMed] [Google Scholar]
- 65. Zhang L, Zhao Y. The regulation of Foxp3 expression in regulatory CD4+CD25+T cells: multiple pathways on the road. J Cell Physiol. 2007;211(3):590–597. [DOI] [PubMed] [Google Scholar]
- 66. Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J Exp Med. 2005;201(5):769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brand OJ, Lowe CE, Heward JM, Franklyn JA, Cooper JD, Todd JA, Gough SC. Association of the interleukin-2 receptor alpha (IL-2Rα)/CD25 gene region with Graves’ disease using a multilocus test and tag SNPs. Clin Endocrinol (Oxf). 2007;66(4):508–512. [DOI] [PubMed] [Google Scholar]
- 68. Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7(12):1258–1265. [DOI] [PubMed] [Google Scholar]
- 69. Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor α promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94(7):3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Louis E, Franchimont D, Piron A, Gevaert Y, Schaaf-Lafontaine N, Roland S, Mahieu P, Malaise M, De Groote D, Louis R, Belaiche J. Tumour necrosis factor (TNF) gene polymorphism influences TNF-α production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans. Clin Exp Immunol. 1998;113(3):401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dittmar M, Kaczmarczyk A, Bischofs C, Kahaly GJ. The proinflammatory cytokine TNF-α -308 AA genotype is associated with polyglandular autoimmunity. Immunol Invest. 2009;38(3–4):255–267. [DOI] [PubMed] [Google Scholar]
- 72. Chow J, Rahman J, Achermann JC, Dattani MT, Rahman S. Mitochondrial disease and endocrine dysfunction. Nat Rev Endocrinol. 2017;13(2):92–104. [DOI] [PubMed] [Google Scholar]
- 73. Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345(13):971–980. [DOI] [PubMed] [Google Scholar]
- 74. Xu J, Capezzone M, Xu X, Hershman JM. Activation of nicotinamide N-methyltransferase gene promoter by hepatocyte nuclear factor-1β in human papillary thyroid cancer cells. Mol Endocrinol. 2005;19(2):527–539. [DOI] [PubMed] [Google Scholar]
- 75. Xu J, Filetti S, Hershman JM. Expression of hepatocyte nuclear factor-1α mRNA in human anaplastic thyroid cancer cell lines and tumors. Thyroid. 2008;18(5):533–539. [DOI] [PubMed] [Google Scholar]
- 76. American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. [DOI] [PubMed] [Google Scholar]
- 77. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. [DOI] [PubMed] [Google Scholar]
- 78. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J; China National Diabetes and Metabolic Disorders Study Group . Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–1101. [DOI] [PubMed] [Google Scholar]
- 79. Esteghamati A, Gouya MM, Abbasi M, Delavari A, Alikhani S, Alaedini F, Safaie A, Forouzanfar M, Gregg EW. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care. 2008;31(1):96–98. [DOI] [PubMed] [Google Scholar]
- 80. Akbar DH, Ahmed MM, Al-Mughales J. Thyroid dysfunction and thyroid autoimmunity in Saudi type 2 diabetics. Acta Diabetol. 2006;43(1):14–18. [DOI] [PubMed] [Google Scholar]
- 81. Robertson RP. Antagonist: diabetes and insulin resistance—philosophy, science, and the multiplier hypothesis. J Lab Clin Med. 1995;125(5):560–564, discussion 565. [PubMed] [Google Scholar]
- 82. Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61(4):778–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22(1):141–146. [DOI] [PubMed] [Google Scholar]
- 85. Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Bonadonna RC, Muggeo M; Bruneck Study . Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck Study. Diabetes Care. 2003;26(4):1251–1257. [DOI] [PubMed] [Google Scholar]
- 86. Díez JJ, Sánchez P, Iglesias P. Prevalence of thyroid dysfunction in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119(4):201–207. [DOI] [PubMed] [Google Scholar]
- 87. Friedrich N, Schwarz S, Thonack J, John U, Wallaschofski H, Völzke H. Association between parity and autoimmune thyroiditis in a general female population. Autoimmunity. 2008;41(2):174–180. [DOI] [PubMed] [Google Scholar]
- 88. Papazafiropoulou A, Sotiropoulos A, Kokolaki A, Kardara M, Stamataki P, Pappas S. Prevalence of thyroid dysfunction among greek type 2 diabetic patients attending an outpatient clinic. J Clin Med Res. 2010;2(2):75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brandt F, Thvilum M, Almind D, Christensen K, Green A, Hegedüs L, Brix TH. Morbidity before and after the diagnosis of hyperthyroidism: a nationwide register-based study. PLoS One. 2013;8(6):e66711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gronich N, Deftereos SN, Lavi I, Persidis AS, Abernethy DR, Rennert G. Hypothyroidism is a risk factor for new-onset diabetes mellitus: a cohort study. Diabetes Care. 2015;38(9):1657–1664. [DOI] [PubMed] [Google Scholar]
- 91. Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedüs L. Type and extent of somatic morbidity before and after the diagnosis of hypothyroidism. A nationwide register study. PLoS One. 2013;8(9):e75789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Perros P, McCrimmon RJ, Shaw G, Frier BM. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med. 1995;12(7):622–627. [DOI] [PubMed] [Google Scholar]
- 93. Celani MF, Bonati ME, Stucci N. Prevalence of abnormal thyrotropin concentrations measured by a sensitive assay in patients with type 2 diabetes mellitus. Diabetes Res. 1994;27(1):15–25. [PubMed] [Google Scholar]
- 94. Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res. 2013;2013:390534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Díez JJ, Iglesias P. Subclinical hyperthyroidism in patients with type 2 diabetes. Endocrine. 2012;42(1):157–163. [DOI] [PubMed] [Google Scholar]
- 96. Gopinath B, Wang JJ, Kifley A, Wall JR, Leeder SR, Mitchell P. Type 2 diabetes does not predict incident thyroid dysfunction in the elderly. Diabetes Res Clin Pract. 2008;82(3):e11–e13. [DOI] [PubMed] [Google Scholar]
- 97. Chubb SA, Davis WA, Inman Z, Davis TM. Prevalence and progression of subclinical hypothyroidism in women with type 2 diabetes: the Fremantle Diabetes Study. Clin Endocrinol (Oxf). 2005;62(4):480–486. [DOI] [PubMed] [Google Scholar]
- 98. Gyawali P, Takanche JS, Shrestha RK, Bhattarai P, Khanal K, Risal P, Koju R. Pattern of thyroid dysfunction in patients with metabolic syndrome and its relationship with components of metabolic syndrome. Diabetes Metab J. 2015;39(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Al-Geffari M, Ahmad NA, Al-Sharqawi AH, Youssef AM, Alnaqeb D, Al-Rubeaan K. Risk factors for thyroid dysfunction among type 2 diabetic patients in a highly diabetes mellitus prevalent society. Int J Endocrinol. 2013;2013:417920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Song F, Bao C, Deng M, Xu H, Fan M, Paillard-Borg S, Xu W, Qi X. The prevalence and determinants of hypothyroidism in hospitalized patients with type 2 diabetes mellitus. Endocrine. 2017;55(1):179–185. [DOI] [PubMed] [Google Scholar]
- 101. Díez JJ, Iglesias P. An analysis of the relative risk for hypothyroidism in patients with type 2 diabetes. Diabet Med. 2012;29(12):1510–1514. [DOI] [PubMed] [Google Scholar]
- 102. Chen G, Wu J, Lin Y, Huang B, Yao J, Jiang Q, Wen J, Lin L. Associations between cardiovascular risk, insulin resistance, β-cell function and thyroid dysfunction: a cross-sectional study in She ethnic minority group of Fujian Province in China. Eur J Endocrinol. 2010;163(5):775–782. [DOI] [PubMed] [Google Scholar]
- 103. Han C, He X, Xia X, Li Y, Shi X, Shan Z, Teng W. Subclinical hypothyroidism and type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0135233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gray RS, Borsey DQ, Irvine WJ, Seth J, Clarke BF. Natural history of thyroid function in diabetics with impaired thyroid reserve: a four year controlled study. Clin Endocrinol (Oxf). 1983;19(4):445–451. [DOI] [PubMed] [Google Scholar]
- 105. Park HT, Cho GJ, Ahn KH, Shin JH, Hong SC, Kim T, Hur JY, Kim YT, Lee KW, Kim SH. Thyroid stimulating hormone is associated with metabolic syndrome in euthyroid postmenopausal women. Maturitas. 2009;62(3):301–305. [DOI] [PubMed] [Google Scholar]
- 106. Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92(2):491–496. [DOI] [PubMed] [Google Scholar]
- 107. Mehran L, Amouzegar A, Tohidi M, Moayedi M, Azizi F. Serum free thyroxine concentration is associated with metabolic syndrome in euthyroid subjects. Thyroid. 2014;24(11):1566–1574. [DOI] [PubMed] [Google Scholar]
- 108. Santini F, Marzullo P, Rotondi M, Ceccarini G, Pagano L, Ippolito S, Chiovato L, Biondi B. Mechanisms in endocrinology: the crosstalk between thyroid gland and adipose tissue: signal integration in health and disease. Eur J Endocrinol. 2014;171(4):R137–R152. [DOI] [PubMed] [Google Scholar]
- 109. Duntas LH, Biondi B. The interconnections between obesity, thyroid function, and autoimmunity: the multifold role of leptin. Thyroid. 2013;23(6):646–653. [DOI] [PubMed] [Google Scholar]
- 110. Menendez C, Baldelli R, Camiña JP, Escudero B, Peino R, Dieguez C, Casanueva FF. TSH stimulates leptin secretion by a direct effect on adipocytes. J Endocrinol. 2003;176(1):7–12. [DOI] [PubMed] [Google Scholar]
- 111. Denroche HC, Huynh FK, Kieffer TJ. The role of leptin in glucose homeostasis. J Diabetes Investig. 2012;3(2):115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tian L, Song Y, Xing M, Zhang W, Ning G, Li X, Yu C, Qin C, Liu J, Tian X, Sun X, Fu R, Zhang L, Zhang X, Lu Y, Zou J, Wang L, Guan Q, Gao L, Zhao J. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology. 2010;52(4):1401–1409. [DOI] [PubMed] [Google Scholar]
- 113. Wang T, Xu J, Bo T, Zhou X, Jiang X, Gao L, Zhao J. Decreased fasting blood glucose is associated with impaired hepatic glucose production in thyroid-stimulating hormone receptor knockout mice. Endocr J. 2013;60(8):941–950. [DOI] [PubMed] [Google Scholar]
- 114. Al-Hamodi Z, Al-Habori M, Al-Meeri A, Saif-Ali R. Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetol Metab Syndr. 2014;6(1):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ortiga-Carvalho TM, Oliveira KJ, Soares BA, Pazos-Moura CC. The role of leptin in the regulation of TSH secretion in the fed state: in vivo and in vitro studies. J Endocrinol. 2002;174(1):121–125. [DOI] [PubMed] [Google Scholar]
- 116. Jun JE, Jin SM, Jee JH, Bae JC, Hur KY, Lee MK, Kim SW, Kim JH. TSH increment and the risk of incident type 2 diabetes mellitus in euthyroid subjects. Endocrine. 2017;55(3):944–953. [DOI] [PubMed] [Google Scholar]
- 117. Jun JE, Jee JH, Bae JC, Jin SM, Hur KY, Lee MK, Kim TH, Kim SW, Kim JH. Association between changes in thyroid hormones and incident type 2 diabetes: a seven-year longitudinal study. Thyroid. 2017;27(1):29–38. [DOI] [PubMed] [Google Scholar]
- 118. Lin Y, Sun Z. Thyroid hormone potentiates insulin signaling and attenuates hyperglycemia and insulin resistance in a mouse model of type 2 diabetes. Br J Pharmacol. 2011;162(3):597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Blanchet E, Bertrand C, Annicotte JS, Schlernitzauer A, Pessemesse L, Levin J, Fouret G, Feillet-Coudray C, Bonafos B, Fajas L, Cabello G, Wrutniak-Cabello C, Casas F. Mitochondrial T3 receptor p43 regulates insulin secretion and glucose homeostasis. FASEB J. 2012;26(1):40–50. [DOI] [PubMed] [Google Scholar]
- 120. Kim SR, Tull ES, Talbott EO, Vogt MT, Kuller LH. A hypothesis of synergism: the interrelationship of T3 and insulin to disturbances in metabolic homeostasis. Med Hypotheses. 2002;59(6):660–666. [DOI] [PubMed] [Google Scholar]
- 121. Yavuz DG, Yüksel M, Deyneli O, Ozen Y, Aydin H, Akalin S. Association of serum paraoxonase activity with insulin sensitivity and oxidative stress in hyperthyroid and TSH-suppressed nodular goitre patients. Clin Endocrinol (Oxf). 2004;61(4):515–521. [DOI] [PubMed] [Google Scholar]
- 122. Chaker L, Ligthart S, Korevaar TI, Hofman A, Franco OH, Peeters RP, Dehghan A. Thyroid function and risk of type 2 diabetes: a population-based prospective cohort study. BMC Med. 2016;14(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gu Y, Li H, Bao X, Zhang Q, Liu L, Meng G, Wu H, Du H, Shi H, Xia Y, Su Q, Fang L, Yu F, Yang H, Yu B, Sun S, Wang X, Zhou M, Jia Q, Guo Q, Chang H, Wang G, Huang G, Song K, Niu K. The relationship between thyroid function and the prevalence of type 2 diabetes mellitus in euthyroid subjects. J Clin Endocrinol Metab. 2017;102(2):434–442. [DOI] [PubMed] [Google Scholar]
- 124. Köhrle J. Thyroid hormone transporters in health and disease: advances in thyroid hormone deiodination. Best Pract Res Clin Endocrinol Metab. 2007;21(2):173–191. [DOI] [PubMed] [Google Scholar]
- 125. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29(7):898–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kvetny J, Wilms L, Pedersen PL, Larsen J. Subclinical hypothyroidism affects mitochondrial function. Horm Metab Res. 2010;42(5):324–327. [DOI] [PubMed] [Google Scholar]
- 127. Venditti P, Bari A, Di Stefano L, Di Meo S. Role of mitochondria in exercise-induced oxidative stress in skeletal muscle from hyperthyroid rats. Arch Biochem Biophys. 2007;463(1):12–18. [DOI] [PubMed] [Google Scholar]
- 128. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. [DOI] [PubMed] [Google Scholar]
- 129. Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Bang LE, Bundgaard H, Nielsen LB, Helge JW, Dela F. Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J Am Coll Cardiol. 2013;61(1):44–53. [DOI] [PubMed] [Google Scholar]
- 130. Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Furuya F, Shimura H, Yamashita S, Endo T, Kobayashi T. Liganded thyroid hormone receptor-α enhances proliferation of pancreatic β-cells. J Biol Chem. 2010;285(32):24477–24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fukuchi M, Shimabukuro M, Shimajiri Y, Oshiro Y, Higa M, Akamine H, Komiya I, Takasu N. Evidence for a deficient pancreatic β-cell response in a rat model of hyperthyroidism. Life Sci. 2002;71(9):1059–1070. [DOI] [PubMed] [Google Scholar]
- 133. Verga Falzacappa C, Mangialardo C, Madaro L, Ranieri D, Lupoi L, Stigliano A, Torrisi MR, Bouchè M, Toscano V, Misiti S. Thyroid hormone T3 counteracts STZ induced diabetes in mouse. PLoS One. 2011;6(5):e19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Potenza M, Via MA, Yanagisawa RT. Excess thyroid hormone and carbohydrate metabolism. Endocr Pract. 2009;15(3):254–262. [DOI] [PubMed] [Google Scholar]
- 135. Duntas LH, Orgiazzi J, Brabant G. The interface between thyroid and diabetes mellitus. Clin Endocrinol (Oxf). 2011;75(1):1–9. [DOI] [PubMed] [Google Scholar]
- 136. Singh SP, Snyder AK. Effect of thyrotoxicosis on gluconeogenesis from alanine in the perfused rat liver. Endocrinology. 1978;102(1):182–187. [DOI] [PubMed] [Google Scholar]
- 137. Park EA, Jerden DC, Bahouth SW. Regulation of phosphoenolpyruvate carboxykinase gene transcription by thyroid hormone involves two distinct binding sites in the promoter. Biochem J. 1995;309(Pt 3):913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Park EA, Song S, Vinson C, Roesler WJ. Role of CCAAT enhancer-binding protein β in the thyroid hormone and cAMP induction of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem. 1999;274(1):211–217. [DOI] [PubMed] [Google Scholar]
- 139. Klieverik LP, Sauerwein HP, Ackermans MT, Boelen A, Kalsbeek A, Fliers E. Effects of thyrotoxicosis and selective hepatic autonomic denervation on hepatic glucose metabolism in rats. Am J Physiol Endocrinol Metab. 2008;294(3):E513–E520. [DOI] [PubMed] [Google Scholar]
- 140. Brenta G. Why can insulin resistance be a natural consequence of thyroid dysfunction? J Thyroid Res. 2011;2011:152850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Klieverik LP, Janssen SF, van Riel A, Foppen E, Bisschop PH, Serlie MJ, Boelen A, Ackermans MT, Sauerwein HP, Fliers E, Kalsbeek A. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci USA. 2009;106(14):5966–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Feng X, Jiang Y, Meltzer P, Yen PM. Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Mol Endocrinol. 2000;14(7):947–955. [DOI] [PubMed] [Google Scholar]
- 143. Ness GC. Thyroid hormone. Basis for its hypocholesterolemic effect. J Fla Med Assoc. 1991;78(6):383–385. [PubMed] [Google Scholar]
- 144. Lopez D, Abisambra Socarrás JF, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim Biophys Acta. 2007;1771(9):1216–1225. [DOI] [PubMed] [Google Scholar]
- 145. Shin DJ, Osborne TF. Thyroid hormone regulation and cholesterol metabolism are connected through sterol regulatory element-binding protein-2 (SREBP-2). J Biol Chem. 2003;278(36):34114–34118. [DOI] [PubMed] [Google Scholar]
- 146. Sinha RA, Singh BK, Yen PM. Thyroid hormone regulation of hepatic lipid and carbohydrate metabolism. Trends Endocrinol Metab. 2014;25(10):538–545. [DOI] [PubMed] [Google Scholar]
- 147. Sinha RA, You SH, Zhou J, Siddique MM, Bay BH, Zhu X, Privalsky ML, Cheng SY, Stevens RD, Summers SA, Newgard CB, Lazar MA, Yen PM. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J Clin Invest. 2012;122(7):2428–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Crunkhorn S, Patti ME. Links between thyroid hormone action, oxidative metabolism, and diabetes risk? Thyroid. 2008;18(2):227–237. [DOI] [PubMed] [Google Scholar]
- 149. Weinstein SP, O’Boyle E, Haber RS. Thyroid hormone increases basal and insulin-stimulated glucose transport in skeletal muscle. The role of GLUT4 glucose transporter expression. Diabetes. 1994;43(10):1185–1189. [DOI] [PubMed] [Google Scholar]
- 150. Simonides WS, Brent GA, Thelen MH, van der Linden CG, Larsen PR, van Hardeveld C. Characterization of the promoter of the rat sarcoplasmic endoplasmic reticulum Ca2+-ATPase 1 gene and analysis of thyroid hormone responsiveness. J Biol Chem. 1996;271(50):32048–32056. [DOI] [PubMed] [Google Scholar]
- 151. Hartong R, Wang N, Kurokawa R, Lazar MA, Glass CK, Apriletti JW, Dillmann WH. Delineation of three different thyroid hormone-response elements in promoter of rat sarcoplasmic reticulum Ca2+ATPase gene. Demonstration that retinoid X receptor binds 5′ to thyroid hormone receptor in response element 1. J Biol Chem. 1994;269(17):13021–13029. [PubMed] [Google Scholar]
- 152. Solanes G, Pedraza N, Calvo V, Vidal-Puig A, Lowell BB, Villarroya F. Thyroid hormones directly activate the expression of the human and mouse uncoupling protein-3 genes through a thyroid response element in the proximal promoter region. Biochem J. 2005;386(Pt 3):505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zorzano A, Palacín M, Gumà A. Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol Scand. 2005;183(1):43–58. [DOI] [PubMed] [Google Scholar]
- 154. Desvergne B, Petty KJ, Nikodem VM. Functional characterization and receptor binding studies of the malic enzyme thyroid hormone response element. J Biol Chem. 1991;266(2):1008–1013. [PubMed] [Google Scholar]
- 155. Dümmler K, Müller S, Seitz HJ. Regulation of adenine nucleotide translocase and glycerol 3-phosphate dehydrogenase expression by thyroid hormones in different rat tissues. Biochem J. 1996;317(Pt 3):913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Teixeira SS, Tamrakar AK, Goulart-Silva F, Serrano-Nascimento C, Klip A, Nunes MT. Triiodothyronine acutely stimulates glucose transport into L6 muscle cells without increasing surface GLUT4, GLUT1, or GLUT3. Thyroid. 2012;22(7):747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Havekes B, Sauerwein HP. Adipocyte-myocyte crosstalk in skeletal muscle insulin resistance; is there a role for thyroid hormone? Curr Opin Clin Nutr Metab Care. 2010;13(6):641–646. [DOI] [PubMed] [Google Scholar]
- 158. de Lange P, Feola A, Ragni M, Senese R, Moreno M, Lombardi A, Silvestri E, Amat R, Villarroya F, Goglia F, Lanni A. Differential 3,5,3′-triiodothyronine-mediated regulation of uncoupling protein 3 transcription: role of fatty acids. Endocrinology. 2007;148(8):4064–4072. [DOI] [PubMed] [Google Scholar]
- 159. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18(2):141–144. [DOI] [PubMed] [Google Scholar]
- 161. Dimitriadis GD, Raptis SA. Thyroid hormone excess and glucose intolerance. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S225–S239. [DOI] [PubMed] [Google Scholar]
- 162. Cavallo-Perin P, Bruno A, Boine L, Cassader M, Lenti G, Pagano G. Insulin resistance in Graves’ disease: a quantitative in-vivo evaluation. Eur J Clin Invest. 1988;18(6):607–613. [DOI] [PubMed] [Google Scholar]
- 163. Maratou E, Hadjidakis DJ, Peppa M, Alevizaki M, Tsegka K, Lambadiari V, Mitrou P, Boutati E, Kollias A, Economopoulos T, Raptis SA, Dimitriadis G. Studies of insulin resistance in patients with clinical and subclinical hyperthyroidism. Eur J Endocrinol. 2010;163(4):625–630. [DOI] [PubMed] [Google Scholar]
- 164. Randin JP, Tappy L, Scazziga B, Jequier E, Felber JP. Insulin sensitivity and exogenous insulin clearance in Graves’ disease. Measurement by the glucose clamp technique and continuous indirect calorimetry. Diabetes. 1986;35(2):178–181. [DOI] [PubMed] [Google Scholar]
- 165. Foss MC, Paccola GM, Saad MJ, Pimenta WP, Piccinato CE, Iazigi N. Peripheral glucose metabolism in human hyperthyroidism. J Clin Endocrinol Metab. 1990;70(4):1167–1172. [DOI] [PubMed] [Google Scholar]
- 166. Solá E, Morillas C, Garzón S, Gómez-Balaguer M, Hernández-Mijares A. Association between diabetic ketoacidosis and thyrotoxicosis. Acta Diabetol. 2002;39(4):235–237. [DOI] [PubMed] [Google Scholar]
- 167. Donckier JE. Endocrine diseases and diabetes. In: Pickup JC, Williams G, eds. Text Book of Diabetes Mellitus. Vol 27. Chichester, UK: Blackwell; 2003:21–27. [Google Scholar]
- 168. Okajima F, Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Glucose-turnover values and futile-cycle activities obtained with 14C- and 3H-labelled glucose. Biochem J. 1979;182(2):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lenzen S, Bailey CJ. Thyroid hormones, gonadal and adrenocortical steroids and the function of the islets of Langerhans. Endocr Rev. 1984;5(3):411–434. [DOI] [PubMed] [Google Scholar]
- 170. Czech MP, Malbon CC, Kerman K, Gitomer W, Pilch PF. Effect of thyroid status on insulin action in rat adipocytes and skeletal muscle. J Clin Invest. 1980;66(3):574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Dimitriadis G, Parry-Billings M, Bevan S, Leighton B, Krause U, Piva T, Tegos K, Challiss RA, Wegener G, Newsholme EA. The effects of insulin on transport and metabolism of glucose in skeletal muscle from hyperthyroid and hypothyroid rats. Eur J Clin Invest. 1997;27(6):475–483. [DOI] [PubMed] [Google Scholar]
- 172. Cettour-Rose P, Theander-Carrillo C, Asensio C, Klein M, Visser TJ, Burger AG, Meier CA, Rohner-Jeanrenaud F. Hypothyroidism in rats decreases peripheral glucose utilisation, a defect partially corrected by central leptin infusion. Diabetologia. 2005;48(4):624–633. [DOI] [PubMed] [Google Scholar]
- 173. Maratou E, Hadjidakis DJ, Kollias A, Tsegka K, Peppa M, Alevizaki M, Mitrou P, Lambadiari V, Boutati E, Nikzas D, Tountas N, Economopoulos T, Raptis SA, Dimitriadis G. Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur J Endocrinol. 2009;160(5):785–790. [DOI] [PubMed] [Google Scholar]
- 174. Brenta G, Celi FS, Pisarev M, Schnitman M, Sinay I, Arias P. Acute thyroid hormone withdrawal in athyreotic patients results in a state of insulin resistance. Thyroid. 2009;19(6):665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Dimitriadis G, Mitrou P, Lambadiari V, Boutati E, Maratou E, Panagiotakos DB, Koukkou E, Tzanela M, Thalassinos N, Raptis SA. Insulin action in adipose tissue and muscle in hypothyroidism. J Clin Endocrinol Metab. 2006;91(12):4930–4937. [DOI] [PubMed] [Google Scholar]
- 176. Waring AC, Rodondi N, Harrison S, Kanaya AM, Simonsick EM, Miljkovic I, Satterfield S, Newman AB, Bauer DC; Health, Ageing, and Body Composition (Health ABC) Study . Thyroid function and prevalent and incident metabolic syndrome in older adults: the Health, Ageing and Body Composition Study. Clin Endocrinol (Oxf). 2012;76(6):911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Stanická S, Vondra K, Pelikánová T, Vlcek P, Hill M, Zamrazil V. Insulin sensitivity and counter-regulatory hormones in hypothyroidism and during thyroid hormone replacement therapy. Clin Chem Lab Med. 2005;43(7):715–720. [DOI] [PubMed] [Google Scholar]
- 178. Chubb SA, Davis WA, Davis TM. Interactions among thyroid function, insulin sensitivity, and serum lipid concentrations: the Fremantle diabetes study. J Clin Endocrinol Metab. 2005;90(9):5317–5320. [DOI] [PubMed] [Google Scholar]
- 179. Handisurya A, Pacini G, Tura A, Gessl A, Kautzky-Willer A. Effects of T4 replacement therapy on glucose metabolism in subjects with subclinical (SH) and overt hypothyroidism (OH). Clin Endocrinol (Oxf). 2008;69(6):963–969. [DOI] [PubMed] [Google Scholar]
- 180. Mohn A, Di Michele S, Di Luzio R, Tumini S, Chiarelli F. The effect of subclinical hypothyroidism on metabolic control in children and adolescents with Type 1 diabetes mellitus. Diabet Med. 2002;19(1):70–73. [DOI] [PubMed] [Google Scholar]
- 181. Mouradian M, Abourizk N. Diabetes mellitus and thyroid disease. Diabetes Care. 1983;6(5):512–520. [DOI] [PubMed] [Google Scholar]
- 182. Johnson JL. Diabetes control in thyroid disease. Diabetes Spectr. 2006;19(3):148–153. [Google Scholar]
- 183. Schröder-van der Elst JP, van der Heide D. Effects of streptozocin-induced diabetes and food restriction on quantities and source of T4 and T3 in rat tissues. Diabetes. 1992;41(2):147–152. [DOI] [PubMed] [Google Scholar]
- 184. Moura EG, Pazos CC, Rosenthal D. Insulin deficiency impairs thyroid peroxidase activity. A study in experimental diabetes mellitus In: Medeiros-Neto G, Gaitan E, eds. Frontiers in Endocrinology. Boston, MA: Springer; 1986:627–630. [Google Scholar]
- 185. Chopra IJ, Wiersinga W, Frank H. Alterations in hepatic monodeiodination of iodothyronines in the diabetic rat. Life Sci. 1981;28(15–16):1765–1776. [DOI] [PubMed] [Google Scholar]
- 186. Ortiz-Caro J, Obregón MJ, Pascual A, Jolin T. Decreased T4 to T3 conversion in tissues of streptozotocin-diabetic rats. Acta Endocrinol (Copenh). 1984;106(1):86–91. [DOI] [PubMed] [Google Scholar]
- 187. Islam S, Yesmine S, Khan SA, Alam NH, Islam S. A comparative study of thyroid hormone levels in diabetic and non-diabetic patients. Southeast Asian J Trop Med Public Health. 2008;39(5):913–916. [PubMed] [Google Scholar]
- 188. Bazrafshan HR, Ramezani MA, Salehei A, Shirafkan AA, Mohammadian S, Frfajollahi M, Raiszadehe F, Azizi F. Thyroid dysfunction and its relation with diabetes mellitus (NIDDM) J Gorgan Uni Med Sci. 2000;2(1):5–11. [Google Scholar]
- 189. Dimitriadis G, Hatziagelaki E, Mitrou P, Lambadiari V, Maratou E, Raptis AE, Gerich JE, Raptis SA. Effect of hyperthyroidism on clearance and secretion of glucagon in man. Exp Clin Endocrinol Diabetes. 2011;119(4):214–217. [DOI] [PubMed] [Google Scholar]
- 190. Kunishige M, Sekimoto E, Komatsu M, Bando Y, Uehara H, Izumi K. Thyrotoxicosis masked by diabetic ketoacidosis: a fatal complication. Diabetes Care. 2001;24(1):171. [DOI] [PubMed] [Google Scholar]
- 191. Naeije R, Golstein J, Clumeck N, Meinhold H, Wenzel KW, Vanhaelst L. A low T3 syndrome in diabetic ketoacidosis. Clin Endocrinol (Oxf). 1978;8(6):467–472. [DOI] [PubMed] [Google Scholar]
- 192. Suzuki Y, Nanno M, Gemma R, Tanaka I, Taminato T, Yoshimi T. The mechanism of thyroid hormone abnormalities in patients with diabetes mellitus [in Japanese]. Nippon Naibunpi Gakkai Zasshi. 1994;70(4):465–470. [DOI] [PubMed] [Google Scholar]
- 193. Vigersky RA, Filmore-Nassar A, Glass AR. Thyrotropin suppression by metformin. J Clin Endocrinol Metab. 2006;91(1):225–227. [DOI] [PubMed] [Google Scholar]
- 194. Biondi B, Bartalena L, Cooper DS, Hegedüs L, Laurberg P, Kahaly GJ. The 2015 European Thyroid Association guidelines on diagnosis and treatment of endogenous subclinical hyperthyroidism. Eur Thyroid J. 2015;4(3):149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–1421. [DOI] [PubMed] [Google Scholar]
- 196. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131. [DOI] [PubMed] [Google Scholar]
- 197. Biondi B, Palmieri EA, Fazio S, Cosco C, Nocera M, Saccà L, Filetti S, Lombardi G, Perticone F. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. J Clin Endocrinol Metab. 2000;85(12):4701–4705. [DOI] [PubMed] [Google Scholar]
- 198. Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, Wemeau JL. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism In Adults . Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200–1235. [DOI] [PubMed] [Google Scholar]
- 200. Bitzur R, Cohen H, Kamari Y, Harats D. Intolerance to statins: mechanisms and management. Diabetes Care. 2013;36(Suppl 2):S325–S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GF. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA. 2016;316(3):313–324. [DOI] [PubMed] [Google Scholar]
- 202. Isidro ML, Penín MA, Nemiña R, Cordido F. Metformin reduces thyrotropin levels in obese, diabetic women with primary hypothyroidism on thyroxine replacement therapy. Endocrine. 2007;32(1):79–82. [DOI] [PubMed] [Google Scholar]
- 203. Cappelli C, Rotondi M, Pirola I, Agosti B, Gandossi E, Valentini U, De Martino E, Cimino A, Chiovato L, Agabiti-Rosei E, Castellano M. TSH-lowering effect of metformin in type 2 diabetic patients: differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients. Diabetes Care. 2009;32(9):1589–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lupoli R, Di Minno A, Tortora A, Ambrosino P, Lupoli GA, Di Minno MN. Effects of treatment with metformin on TSH levels: a meta-analysis of literature studies. J Clin Endocrinol Metab. 2014;99(1):E143–E148. [DOI] [PubMed] [Google Scholar]
- 205. Díez JJ, Iglesias P. Relationship between serum thyrotropin concentrations and metformin therapy in euthyroid patients with type 2 diabetes. Clin Endocrinol (Oxf). 2013;78(4):505–511. [DOI] [PubMed] [Google Scholar]
- 206. Rezzónico J, Rezzónico M, Pusiol E, Pitoia F, Niepomniszcze H. Metformin treatment for small benign thyroid nodules in patients with insulin resistance. Metab Syndr Relat Disord. 2011;9(1):69–75. [DOI] [PubMed] [Google Scholar]
- 207. Cappelli C, Rotondi M, Pirola I, Agosti B, Formenti A, Zarra E, Valentini U, Leporati P, Chiovato L, Castellano M. Thyreotropin levels in diabetic patients on metformin treatment. Eur J Endocrinol. 2012;167(2):261–265. [DOI] [PubMed] [Google Scholar]
- 208. Santos-Palacios S, Brugos-Larumbe A, Guillén-Grima F, Garmendia-Madariaga A, Galofré JC. Does metformin have a “buffer effect” on serum TSH levels in euthyroid diabetic patients? Hormones (Athens). 2015;14(2):280–285. [DOI] [PubMed] [Google Scholar]
- 209. Pappa T, Alevizaki M. Metformin and thyroid: an update. Eur Thyroid J. 2013;2(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Al-Alusi MA, Du L, Li N, Yeh MW, He X, Braverman LE, Leung AM. Metformin does not suppress serum thyrotropin by increasing levothyroxine absorption. Thyroid. 2015;25(10):1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Łabuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopień B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep. 2010;62(5):956–965. [DOI] [PubMed] [Google Scholar]
- 212. Krysiak R, Okopien B. Thyrotropin-lowering effect of metformin in a patient with resistance to thyroid hormone. Clin Endocrinol (Oxf). 2011;75(3):404–406. [DOI] [PubMed] [Google Scholar]
- 213. López M, Alvarez CV, Nogueiras R, Diéguez C. Energy balance regulation by thyroid hormones at central level. Trends Mol Med. 2013;19(7):418–427. [DOI] [PubMed] [Google Scholar]
- 214. López M, Varela L, Vázquez MJ, Rodríguez-Cuenca S, González CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, Martínez de Morentin PB, Tovar S, Nogueiras R, Carling D, Lelliott C, Gallego R, Oresic M, Chatterjee K, Saha AK, Rahmouni K, Diéguez C, Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16(9):1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Chau-Van C, Gamba M, Salvi R, Gaillard RC, Pralong FP. Metformin inhibits adenosine 5′-monophosphate-activated kinase activation and prevents increases in neuropeptide Y expression in cultured hypothalamic neurons. Endocrinology. 2007;148(2):507–511. [DOI] [PubMed] [Google Scholar]
- 216. Ortega-González C, Cardoza L, Coutiño B, Hidalgo R, Arteaga-Troncoso G, Parra A. Insulin sensitizing drugs increase the endogenous dopaminergic tone in obese insulin-resistant women with polycystic ovary syndrome. J Endocrinol. 2005;184(1):233–239. [DOI] [PubMed] [Google Scholar]
- 217. Mohácsik P, Zeöld A, Bianco AC, Gereben B. Thyroid hormone and the neuroglia: both source and target. J Thyroid Res. 2011;2011:215718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Dora JM, Machado WE, Rheinheimer J, Crispim D, Maia AL. Association of the type 2 deiodinase Thr92Ala polymorphism with type 2 diabetes: case-control study and meta-analysis. Eur J Endocrinol. 2010;163(3):427–434. [DOI] [PubMed] [Google Scholar]
- 219. Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid. 2010;20(2):135–146. [DOI] [PubMed] [Google Scholar]
- 220. Casteràs A, Zafon C, Ciudin A, Mesa J. Are levothyroxine requirements lower in thyroidectomized diabetic patients on metformin treatment? Thyroid. 2013;23(12):1510–1513. [DOI] [PubMed] [Google Scholar]
- 221. Chen G, Xu S, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab. 2012;97(4):E510–E520. [DOI] [PubMed] [Google Scholar]
- 222. Klubo-Gwiezdzinska J, Jensen K, Costello J, Patel A, Hoperia V, Bauer A, Burman KD, Wartofsky L, Vasko V. Metformin inhibits growth and decreases resistance to anoikis in medullary thyroid cancer cells. Endocr Relat Cancer. 2012;19(3):447–456. [DOI] [PubMed] [Google Scholar]
- 223. Brown J, Solomon DH. Mechanism of antithyroid effects of a sulfonylurea in the rat. Endocrinology. 1958;63(4):473–480. [DOI] [PubMed] [Google Scholar]
- 224. Nikkila EA, Jakobson T, Jokipii SG, Karlsson K. Thyroid function in diabetic patients under long-term sulfonylurea treatment. Acta Endocrinol (Copenh). 1960;33(IV):623–629. [DOI] [PubMed] [Google Scholar]
- 225. Feely J, McLaren S, Shepherd AM, Maclean D, Stevenson IH, Swift CG, Isles TE. Antithyroid effect of chlorpropamide? Hum Toxicol. 1983;2(1):149–153. [DOI] [PubMed] [Google Scholar]
- 226. Robuschi G, Emanuele R, Cavalli Sforza LT, Arsenio L, Strata A, Gnudi A, Roti E. Effect of iodine administration on thyroid function in diabetic patients. Acta Diabetol Lat. 1984;21(4):357–360. [DOI] [PubMed] [Google Scholar]
- 227. Tranquada RE, Solomon DH, Brown J, Greene R. The effect of oral hypoglycemic agents on thyroid function in the rat. Endocrinology. 1960;67(3):293–297. [DOI] [PubMed] [Google Scholar]
- 228. Hershman JM, Craane TJ, Colwell JA. Effect of sulfonylurea drugs on the binding of triiodothyronine and thyroxine to thyroxine-binding globulin. J Clin Endocrinol Metab. 1968;28(11):1605–1610. [DOI] [PubMed] [Google Scholar]
- 229. Kilo C, Dudley J, Kalb B. Evaluation of the efficacy and safety of Diamicron in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1991;14(Suppl 2):S79–S82. [DOI] [PubMed] [Google Scholar]
- 230. England ML, Hartnell JM, Hershman JM, Levin SR. Glyburide does not alter thyroid function. Diabetes Res. 1986;3(9):471–474. [PubMed] [Google Scholar]
- 231. Ikeda T, Ito Y, Murakami I, Mokuda O, Tokumori Y, Tominaga M, Mashiba H. Effect of glibenclamide on thyroid hormone metabolism in rats. Horm Metab Res. 1986;18(8):517–520. [DOI] [PubMed] [Google Scholar]
- 232. Shen SW, Bressler R. Clinical pharmacology of oral antidiabetic agents (second of two parts). N Engl J Med. 1977;296(14):787–793. [DOI] [PubMed] [Google Scholar]
- 233. Efe B, Entok E, Güney E, Erenoğlu E, Kebapçı M. Effects of second generation sulfonylureas on the thyroid. Turk J Endocrinol Metab. 1999;4:173–176. [Google Scholar]
- 234. Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351(11):1106–1118. [DOI] [PubMed] [Google Scholar]
- 235. Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-γ (PPARγ), and thyrotropin receptor by PPARγ agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2002;87(5):2352–2358. [DOI] [PubMed] [Google Scholar]
- 236. Pasquali D, Pierantoni GM, Fusco A, Staibano S, Colantuoni V, De Bellis A, Bellastella A, Sinisi AA. Fenofibrate increases the expression of high mobility group AT-hook 2 (HMGA2) gene and induces adipocyte differentiation of orbital fibroblasts from Graves’ ophthalmopathy. J Mol Endocrinol. 2004;33(1):133–143. [DOI] [PubMed] [Google Scholar]
- 237. Pistrosch F, Herbrig K, Oelschlaegel U, Richter S, Passauer J, Fischer S, Gross P. PPARγ-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 2005;183(1):163–167. [DOI] [PubMed] [Google Scholar]
- 238. Mimura LY, Villares SM, Monteiro ML, Guazzelli IC, Bloise W. Peroxisome proliferator-activated receptor-γ gene expression in orbital adipose/connective tissues is increased during the active stage of Graves’ ophthalmopathy. Thyroid. 2003;13(9):845–850. [DOI] [PubMed] [Google Scholar]
- 239. Dorkhan M, Lantz M, Frid A, Groop L, Hallengren B. Treatment with a thiazolidinedione increases eye protrusion in a subgroup of patients with type 2 diabetes. Clin Endocrinol (Oxf). 2006;65(1):35–39. [DOI] [PubMed] [Google Scholar]
- 240. Tseng CH. Rosiglitazone may reduce thyroid cancer risk in patients with type 2 diabetes. Ann Med. 2013;45(8):539–544. [DOI] [PubMed] [Google Scholar]
- 241. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. [DOI] [PubMed] [Google Scholar]
- 242. Tseng CH. Sitagliptin use and thyroid cancer risk in patients with type 2 diabetes. Oncotarget. 2016;7(17):24871–24879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Essig GF Jr, Porter K, Schneider D, Debora A, Lindsey SC, Busonero G, Fineberg D, Fruci B, Boelaert K, Smit JW, Meijer JA, Duntas L, Sharma N, Costante G, Filetti S, Sippel RS, Biondi B, Topliss DJ, Pacini F, Maciel RM, Walz PC, Kloos RT. Fine needle aspiration and medullary thyroid carcinoma: the risk of inadequate preoperative evaluation and initial surgery when relying upon FNAB cytology alone. Endocr Pract. 2013;19(6):920–927. [DOI] [PubMed] [Google Scholar]
- 244. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Mølck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation [published correction appears in Endocrinology. 2012;153(2):1000]. Endocrinology. 2010;151(4):1473–1486. [DOI] [PubMed] [Google Scholar]
- 245. Martín-Lacave I, Bernab R, Sampedro C, Conde E, Fernández-Santos JM, San Martín MV, Beato A, Galera-Davidson H. Correlation between gender and spontaneous C-cell tumors in the thyroid gland of the Wistar rat. Cell Tissue Res. 1999;297(3):451–457. [DOI] [PubMed] [Google Scholar]
- 246. Capen CC, Martin SL. The effects of xenobiotics on the structure and function of thyroid follicular and C-cells. Toxicol Pathol. 1989;17(2):266–293. [DOI] [PubMed] [Google Scholar]
- 247. Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab. 2011;96(7):2027–2031. [DOI] [PubMed] [Google Scholar]
- 248. Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab. 2011;96(3):853–860. [DOI] [PubMed] [Google Scholar]
- 249. Hegedüs L, Sherman SI, Tuttle RM, von Scholten BJ, Rasmussen S, Karsbøl JD, Daniels GH; LEADER Publication Committee on behalf of the LEADER Trial Investigators. No evidence of increase in calcitonin concentrations or development of C-Cell malignancy in response to liraglutide for up to 5 years in the LEADER Trial. Diabetes Care. 2018;41(3):620–622. [DOI] [PubMed] [Google Scholar]
- 250. Gereben B, McAninch EA, Ribeiro MO, Bianco AC. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol. 2015;11(11):642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Bianco AC, Larsen PR. Cellular and structural biology of the deiodinases. Thyroid. 2005;15(8):777–786. [DOI] [PubMed] [Google Scholar]
- 252. Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest. 2005;115(9):2524–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Chidakel A, Mentuccia D, Celi FS. Peripheral metabolism of thyroid hormone and glucose homeostasis. Thyroid. 2005;15(8):899–903. [DOI] [PubMed] [Google Scholar]
- 254. Mentuccia D, Proietti-Pannunzi L, Tanner K, Bacci V, Pollin TI, Poehlman ET, Shuldiner AR, Celi FS. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the β-3-adrenergic receptor. Diabetes. 2002;51(3):880–883. [DOI] [PubMed] [Google Scholar]
- 255.National Center for Biotechnology Information. 1000 Genomes browser. Available at: https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/. Accessed 30 April 2019.
- 256. Nair S, Muller YL, Ortega E, Kobes S, Bogardus C, Baier LJ. Association analyses of variants in the DIO2 gene with early-onset type 2 diabetes mellitus in Pima Indians. Thyroid. 2012;22(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Canani LH, Capp C, Dora JM, Meyer EL, Wagner MS, Harney JW, Larsen PR, Gross JL, Bianco AC, Maia AL. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005;90(6):3472–3478. [DOI] [PubMed] [Google Scholar]
- 258. Grarup N, Andersen MK, Andreasen CH, Albrechtsen A, Borch-Johnsen K, Jørgensen T, Auwerx J, Schmitz O, Hansen T, Pedersen O. Studies of the common DIO2 Thr92Ala polymorphism and metabolic phenotypes in 7342 Danish white subjects. J Clin Endocrinol Metab. 2007;92(1):363–366. [DOI] [PubMed] [Google Scholar]
- 259. Maia AL, Dupuis J, Manning A, Liu C, Meigs JB, Cupples LA, Larsen PR, Fox CS. The type 2 deiodinase (DIO2) A/G polymorphism is not associated with glycemic traits: the Framingham Heart Study. Thyroid. 2007;17(3):199–202. [DOI] [PubMed] [Google Scholar]
- 260. Mentuccia D, Thomas MJ, Coppotelli G, Reinhart LJ, Mitchell BD, Shuldiner AR, Celi FS. The Thr92Ala deiodinase type 2 (DIO2) variant is not associated with type 2 diabetes or indices of insulin resistance in the old order of Amish. Thyroid. 2005;15(11):1223–1227. [DOI] [PubMed] [Google Scholar]
- 261. Thorell A, Hirshman MF, Nygren J, Jorfeldt L, Wojtaszewski JF, Dufresne SD, Horton ES, Ljungqvist O, Goodyear LJ. Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am J Physiol. 1999;277(4):E733–E741. [DOI] [PubMed] [Google Scholar]
- 262. Zhang X, Sun J, Han W, Jiang Y, Peng S, Shan Z, Teng W. The type 2 deiodinase Thr92Ala polymorphism is associated with worse glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Diabetes Res. 2016;2016:5928726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. [DOI] [PubMed] [Google Scholar]
- 264. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. [DOI] [PubMed] [Google Scholar]
- 265. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–1508. [DOI] [PubMed] [Google Scholar]
- 267. Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, Brent GA. Thyroid hormone-sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform-specific. J Clin Invest. 2001;108(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Bianco AC, Sheng XY, Silva JE. Triiodothyronine amplifies norepinephrine stimulation of uncoupling protein gene transcription by a mechanism not requiring protein synthesis. J Biol Chem. 1988;263(34):18168–18175. [PubMed] [Google Scholar]
- 269. Dhillo WS. Appetite regulation: an overview. Thyroid. 2007;17(5):433–445. [DOI] [PubMed] [Google Scholar]
- 270. Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA. 1998;95(25):15043–15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21(6):1375–1385. [DOI] [PubMed] [Google Scholar]
- 272. Zarjevski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, Jeanrenaud B. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology. 1993;133(4):1753–1758. [DOI] [PubMed] [Google Scholar]
- 273. Abbott CR, Rossi M, Wren AM, Murphy KG, Kennedy AR, Stanley SA, Zollner AN, Morgan DG, Morgan I, Ghatei MA, Small CJ, Bloom SR. Evidence of an orexigenic role for cocaine- and amphetamine-regulated transcript after administration into discrete hypothalamic nuclei. Endocrinology. 2001;142(8):3457–3463. [DOI] [PubMed] [Google Scholar]
- 274. Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131(2):229–238. [DOI] [PubMed] [Google Scholar]
- 275. Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Choi YH, Hartzell D, Azain MJ, Baile CA. TRH decreases food intake and increases water intake and body temperature in rats. Physiol Behav. 2002;77(1):1–4. [DOI] [PubMed] [Google Scholar]
- 277. Lin MT, Chu PC, Leu SY. Effects of TSH, TRH, LH and LHRH on thermoregulation and food and water intake in the rat. Neuroendocrinology. 1983;37(3):206–211. [DOI] [PubMed] [Google Scholar]
- 278. Boelen A, Wiersinga WM, Fliers E. Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid. 2008;18(2):123–129. [DOI] [PubMed] [Google Scholar]
- 279. Kong WM, Martin NM, Smith KL, Gardiner JV, Connoley IP, Stephens DA, Dhillo WS, Ghatei MA, Small CJ, Bloom SR. Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology. 2004;145(11):5252–5258. [DOI] [PubMed] [Google Scholar]
- 280. Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, Diano S. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007;5(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Varela L, Martínez-Sánchez N, Gallego R, Vázquez MJ, Roa J, Gándara M, Schoenmakers E, Nogueiras R, Chatterjee K, Tena-Sempere M, Diéguez C, López M. Hypothalamic mTOR pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. J Pathol. 2012;227(2):209–222. [DOI] [PubMed] [Google Scholar]
- 282. Hrabovszky E, Kalló I, Turi GF, May K, Wittmann G, Fekete C, Liposits Z. Expression of vesicular glutamate transporter-2 in gonadotrope and thyrotrope cells of the rat pituitary. Regulation by estrogen and thyroid hormone status. Endocrinology. 2006;147(8):3818–3825. [DOI] [PubMed] [Google Scholar]
- 283. Hollenberg AN. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid. 2008;18(2):131–139. [DOI] [PubMed] [Google Scholar]
- 284. Ghamari-Langroudi M, Vella KR, Srisai D, Sugrue ML, Hollenberg AN, Cone RD. Regulation of thyrotropin-releasing hormone-expressing neurons in paraventricular nucleus of the hypothalamus by signals of adiposity. Mol Endocrinol. 2010;24(12):2366–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. [DOI] [PubMed] [Google Scholar]
- 286. Houseknecht KL, Mantzoros CS, Kuliawat R, Hadro E, Flier JS, Kahn BB. Evidence for leptin binding to proteins in serum of rodents and humans: modulation with obesity. Diabetes. 1996;45(11):1638–1643. [DOI] [PubMed] [Google Scholar]
- 287. Oge A, Bayraktar F, Saygili F, Guney E, Demir S. TSH influences serum leptin levels independent of thyroid hormones in hypothyroid and hyperthyroid patients. Endocr J. 2005;52(2):213–217. [DOI] [PubMed] [Google Scholar]
- 288. Seoane LM, Carro E, Tovar S, Casanueva FF, Dieguez C. Regulation of in vivo TSH secretion by leptin. Regul Pept. 2000;92(1-3):25–29. [DOI] [PubMed] [Google Scholar]
- 289. Woods SC, Lotter EC, McKay LD, Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282(5738):503–505. [DOI] [PubMed] [Google Scholar]
- 290. Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5(6):566–572. [DOI] [PubMed] [Google Scholar]
- 291. Grossman SP. The role of glucose, insulin and glucagon in the regulation of food intake and body weight. Neurosci Biobehav Rev. 1986;10(3):295–315. [DOI] [PubMed] [Google Scholar]
- 292. Mobbs CV, Isoda F, Makimura H, Mastaitis J, Mizuno T, Shu IW, Yen K, Yang XJ. Impaired glucose signaling as a cause of obesity and the metabolic syndrome: the glucoadipostatic hypothesis. Physiol Behav. 2005;85(1):3–23. [DOI] [PubMed] [Google Scholar]
- 293. Herwig A, Campbell G, Mayer CD, Boelen A, Anderson RA, Ross AW, Mercer JG, Barrett P. A thyroid hormone challenge in hypothyroid rats identifies T3 regulated genes in the hypothalamus and in models with altered energy balance and glucose homeostasis. Thyroid. 2014;24(11):1575–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354(24):2552–2563. [DOI] [PubMed] [Google Scholar]
- 295. Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307(5708):373–375. [DOI] [PubMed] [Google Scholar]
- 296. Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab. 2010;95(8):3614–3617. [DOI] [PubMed] [Google Scholar]
- 297. Hoogwerf BJ, Nuttall FQ. Long-term weight regulation in treated hyperthyroid and hypothyroid subjects. Am J Med. 1984;76(6):963–970. [DOI] [PubMed] [Google Scholar]
- 298. al-Adsani H, Hoffer LJ, Silva JE. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab. 1997;82(4):1118–1125. [DOI] [PubMed] [Google Scholar]
- 299. Knudsen N, Laurberg P, Rasmussen LB, Bülow I, Perrild H, Ovesen L, Jørgensen T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90(7):4019–4024. [DOI] [PubMed] [Google Scholar]
- 300. Kitahara CM, Platz EA, Ladenson PW, Mondul AM, Menke A, Berrington de González A. Body fatness and markers of thyroid function among U.S. men and women. PLoS One. 2012;7(4):e34979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Fox CS, Pencina MJ, D’Agostino RB, Murabito JM, Seely EW, Pearce EN, Vasan RS. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008;168(6):587–592. [DOI] [PubMed] [Google Scholar]
- 302. Feldt-Rasmussen U. Thyroid and leptin. Thyroid. 2007;17(5):413–419. [DOI] [PubMed] [Google Scholar]
- 303. Zimmermann-Belsing T, Brabant G, Holst JJ, Feldt-Rasmussen U. Circulating leptin and thyroid dysfunction. Eur J Endocrinol. 2003;149(4):257–271. [DOI] [PubMed] [Google Scholar]
- 304. Santini F, Galli G, Maffei M, Fierabracci P, Pelosini C, Marsili A, Giannetti M, Castagna MG, Checchi S, Molinaro E, Piaggi P, Pacini F, Elisei R, Vitti P, Pinchera A. Acute exogenous TSH administration stimulates leptin secretion in vivo. Eur J Endocrinol. 2010;163(1):63–67. [DOI] [PubMed] [Google Scholar]
- 305. Michalaki MA, Vagenakis AG, Leonardou AS, Argentou MN, Habeos IG, Makri MG, Psyrogiannis AI, Kalfarentzos FE, Kyriazopoulou VE. Thyroid function in humans with morbid obesity. Thyroid. 2006;16(1):73–78. [DOI] [PubMed] [Google Scholar]
- 306. Marzullo P, Minocci A, Tagliaferri MA, Guzzaloni G, Di Blasio A, De Medici C, Aimaretti G, Liuzzi A. Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. J Clin Endocrinol Metab. 2010;95(8):3965–3972. [DOI] [PubMed] [Google Scholar]
- 307. Kim BJ, Kim TY, Koh JM, Kim HK, Park JY, Lee KU, Shong YK, Kim WB. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin Endocrinol (Oxf). 2009;70(1):152–160. [DOI] [PubMed] [Google Scholar]
- 308. Iwen KA, Schröder E, Brabant G. Thyroid hormones and the metabolic syndrome. Eur Thyroid J. 2013;2(2):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Ortega E, Koska J, Pannacciulli N, Bunt JC, Krakoff J. Free triiodothyronine plasma concentrations are positively associated with insulin secretion in euthyroid individuals. Eur J Endocrinol. 2008;158(2):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Roef G, Lapauw B, Goemaere S, Zmierczak HG, Toye K, Kaufman JM, Taes Y. Body composition and metabolic parameters are associated with variation in thyroid hormone levels among euthyroid young men. Eur J Endocrinol. 2012;167(5):719–726. [DOI] [PubMed] [Google Scholar]
- 311. Garduño-Garcia JJ, Alvirde-Garcia U, López-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O, Choza R, Sauque L, Garay-Sevilla ME, Malacara JM, Gomez-Perez FJ, Aguilar-Salinas CA. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 2010;163(2):273–278. [DOI] [PubMed] [Google Scholar]
- 312. Shin JA, Mo EY, Kim ES, Moon SD, Han JH. Association between lower normal free thyroxine concentrations and obesity phenotype in healthy euthyroid subjects. Int J Endocrinol. 2014;2014:104318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Roef GL, Rietzschel ER, Van Daele CM, Taes YE, De Buyzere ML, Gillebert TC, Kaufman JM. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. 2014;24(2):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Bakker SJ, ter Maaten JC, Popp-Snijders C, Slaets JP, Heine RJ, Gans RO. The relationship between thyrotropin and low density lipoprotein cholesterol is modified by insulin sensitivity in healthy euthyroid subjects. J Clin Endocrinol Metab. 2001;86(3):1206–1211. [DOI] [PubMed] [Google Scholar]
- 315. Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid. 2008 r;18(4):461–464. [DOI] [PubMed] [Google Scholar]
- 316. Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab. 2012;97(2):326–333. [DOI] [PubMed] [Google Scholar]
- 317. Walsh JP, Bremner AP, Bulsara MK, O’leary P, Leedman PJ, Feddema P, Michelangeli V. Thyroid dysfunction and serum lipids: a community-based study. Clin Endocrinol (Oxf). 2005;63(6):670–675. [DOI] [PubMed] [Google Scholar]
- 318. Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12(4):287–293. [DOI] [PubMed] [Google Scholar]
- 319. Duntas LH, Wartofsky L. Cardiovascular risk and subclinical hypothyroidism: focus on lipids and new emerging risk factors. What is the evidence? Thyroid. 2007;17(11):1075–1084. [DOI] [PubMed] [Google Scholar]
- 320. Boekholdt SM, Titan SM, Wiersinga WM, Chatterjee K, Basart DC, Luben R, Wareham NJ, Khaw KT. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf). 2010;72(3):404–410. [DOI] [PubMed] [Google Scholar]
- 321. de Moura Souza A, Sichieri R. Association between serum TSH concentration within the normal range and adiposity. Eur J Endocrinol. 2011;165(1):11–15. [DOI] [PubMed] [Google Scholar]
- 322. Lee YK, Kim JE, Oh HJ, Park KS, Kim SK, Park SW, Kim MJ, Cho YW. Serum TSH level in healthy Koreans and the association of TSH with serum lipid concentration and metabolic syndrome. Korean J Intern Med (Korean Assoc Intern Med). 2011;26(4):432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Asvold BO, Vatten LJ, Nilsen TI, Bjøro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007;156(2):181–186. [DOI] [PubMed] [Google Scholar]
- 324. Ye Y, Xie H, Zeng Y, Zhao X, Tian Z, Zhang S. Association between subclinical hypothyroidism and blood pressure—a meta-analysis of observational studies. Endocr Pract. 2014;20(2):150–158. [DOI] [PubMed] [Google Scholar]
- 325. Cai Y, Ren Y, Shi J. Blood pressure levels in patients with subclinical thyroid dysfunction: a meta-analysis of cross-sectional data. Hypertens Res. 2011;34(10):1098–1105. [DOI] [PubMed] [Google Scholar]
- 326. Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18(2):239–253. [DOI] [PubMed] [Google Scholar]
- 327. Kim SH, Lee JW, Hwang HJ. Associations between combinations of body mass index plus non-alcoholic fatty liver disease and diabetes mellitus among Korean adults. Asia Pac J Clin Nutr. 2011;20(1):14–20. [PubMed] [Google Scholar]
- 328. Ortiz-Lopez C, Lomonaco R, Orsak B, Finch J, Chang Z, Kochunov VG, Hardies J, Cusi K. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care. 2012;35(4):873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Liu L, Yu Y, Zhao M, Zheng D, Zhang X, Guan Q, Xu C, Gao L, Zhao J, Zhang H. Benefits of levothyroxine replacement therapy on nonalcoholic fatty liver disease in subclinical hypothyroidism patients. Int J Endocrinol. 2017;2017:5753039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Erdogan M, Canataroglu A, Ganidagli S, Kulaksızoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest. 2011;34(7):488–492. [DOI] [PubMed] [Google Scholar]
- 331. Yi-Cong Ye, Hong-Zhi Xie, Xi-Liang Zhao, Shu-Yang Zhang. Subclinical hypothyroidism and the metabolic syndrome: a meta-analysis of cross-sectional studies. World J Metaanal. 2013;1(2):90–96. [Google Scholar]
- 332. Yang L, Lv X, Yue F, Wei D, Liu W, Zhang T. Subclinical hypothyroidism and the risk of metabolic syndrome: a meta-analysis of observational studies. Endocr Res. 2016;41(2):158–165. [DOI] [PubMed] [Google Scholar]
- 333. Eftekharzadeh A, Khamseh ME, Farshchi A, Malek M. The association between subclinical hypothyroidism and metabolic syndrome as defined by the ATP III criteria. Metab Syndr Relat Disord. 2016;14(3):137–144. [DOI] [PubMed] [Google Scholar]
- 334. Heima NE, Eekhoff EM, Oosterwerff MM, Lips PT, van Schoor NM, Simsek S. Thyroid function and the metabolic syndrome in older persons: a population-based study. Eur J Endocrinol. 2012;168(1):59–65. [DOI] [PubMed] [Google Scholar]
- 335. Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92(2):491–496. [DOI] [PubMed] [Google Scholar]
- 336. Ruhla S, Weickert MO, Arafat AM, Osterhoff M, Isken F, Spranger J, Schöfl C, Pfeiffer AF, Möhlig M. A high normal TSH is associated with the metabolic syndrome. Clin Endocrinol (Oxf). 2010;72(5):696–701. [DOI] [PubMed] [Google Scholar]
- 337. Park SB, Choi HC, Joo NS. The relation of thyroid function to components of the metabolic syndrome in Korean men and women. J Korean Med Sci. 2011;26(4):540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. [DOI] [PubMed] [Google Scholar]
- 339. Butler J, Rodondi N, Zhu Y, Figaro K, Fazio S, Vaughan DE, Satterfield S, Newman AB, Goodpaster B, Bauer DC, Holvoet P, Harris TB, de Rekeneire N, Rubin S, Ding J, Kritchevsky SB; Health ABC Study . Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47(8):1595–1602. [DOI] [PubMed] [Google Scholar]
- 340. Delitala AP, Fanciulli G, Pes GM, Maioli M, Delitala G. Thyroid hormones, metabolic syndrome and its components. Endocr Metab Immune Disord Drug Targets. 2017;17(1):56–62. [DOI] [PubMed] [Google Scholar]
- 341. Lai Y, Wang J, Jiang F, Wang B, Chen Y, Li M, Liu H, Li C, Xue H, Li N, Yu J, Shi L, Bai X, Hou X, Zhu L, Lu L, Wang S, Xing Q, Teng X, Teng W, Shan Z. The relationship between serum thyrotropin and components of metabolic syndrome. Endocr J. 2011;58(1):23–30. [DOI] [PubMed] [Google Scholar]
- 342. Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–1523. [DOI] [PubMed] [Google Scholar]
- 343. Vaur L, Gueret P, Lievre M, Chabaud S, Passa P; DIABHYCAR Study Group (type 2 DIABetes, Hypertension, CARdiovascular Events and Ramipril) study . Development of congestive heart failure in type 2 diabetic patients with microalbuminuria or proteinuria: observations from the DIABHYCAR (type 2 DIABetes, Hypertension, CArdiovascular Events and Ramipril) study. Diabetes Care. 2003;26(3):855–860. [DOI] [PubMed] [Google Scholar]
- 344. McMurray JJV, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2(10):843–851. [DOI] [PubMed] [Google Scholar]
- 345. Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM; South Tees Diabetes Mortality Study . Cause-specific mortality in a population with diabetes: South Tees Diabetes Mortality Study. Diabetes Care. 2002;25(1):43–48. [DOI] [PubMed] [Google Scholar]
- 346. Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, Cameron AJ, Dwyer T, Taylor HR, Tonkin AM, Wong TY, McNeil J, Shaw JE. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116(2):151–157. [DOI] [PubMed] [Google Scholar]
- 347. Lind M, Svensson AM, Kosiborod M, Gudbjörnsdottir S, Pivodic A, Wedel H, Dahlqvist S, Clements M, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972–1982. [DOI] [PubMed] [Google Scholar]
- 348. Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–1732. [DOI] [PubMed] [Google Scholar]
- 349. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J; Thyroid Studies Collaboration . Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, Nanchen D, den Elzen WP, Balmer P, Luben RN, Iacoviello M, Triggiani V, Cornuz J, Newman AB, Khaw KT, Jukema JW, Westendorp RG, Vittinghoff E, Aujesky D, Rodondi N; Thyroid Studies Collaboration . Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Chen HS, Wu TE, Jap TS, Lu RA, Wang ML, Chen RL, Lin HD. Subclinical hypothyroidism is a risk factor for nephropathy and cardiovascular diseases in Type 2 diabetic patients. Diabet Med. 2007;24(12):1336–1344. [DOI] [PubMed] [Google Scholar]
- 353. Yang JK, Liu W, Shi J, Li YB. An association between subclinical hypothyroidism and sight-threatening diabetic retinopathy in type 2 diabetic patients. Diabetes Care. 2010;33(5):1018–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Kim BY, Kim CH, Jung CH, Mok JO, Suh KI, Kang SK. Association between subclinical hypothyroidism and severe diabetic retinopathy in Korean patients with type 2 diabetes. Endocr J. 2011;58(12):1065–1070. [DOI] [PubMed] [Google Scholar]
- 355. Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–163. [DOI] [PubMed] [Google Scholar]
- 356. Wu J, Yue S, Geng J, Liu L, Teng W, Liu L, Chen L. Relationship between diabetic retinopathy and subclinical hypothyroidism: a meta-analysis. Sci Rep. 2015;5:12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Yasuda T, Kaneto H, Kuroda A, Yamamoto T, Takahara M, Naka T, Miyashita K, Fujisawa K, Sakamoto F, Katakami N, Matsuoka TA, Shimomura I. Subclinical hypothyroidism is independently associated with albuminuria in people with type 2 diabetes. Diabetes Res Clin Pract. 2011;94(3):e75–e77. [DOI] [PubMed] [Google Scholar]
- 358. Sathyapalan T, Manuchehri AM, Rigby AS, Atkin SL. Subclinical hypothyroidism is associated with reduced all-cause mortality in patients with type 2 diabetes. Diabetes Care. 2010;33(3):e37. [DOI] [PubMed] [Google Scholar]
- 359. Chubb SA, Davis WA, Davis TM. Subclinical hypothyroidism and mortality in women with type 2 diabetes. Clin Endocrinol (Oxf). 2006;64(4):476–477. [DOI] [PubMed] [Google Scholar]
- 360. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. [DOI] [PubMed] [Google Scholar]
- 361. Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care. 2005;28(12):2901–2907. [DOI] [PubMed] [Google Scholar]
- 362. Stamler J, Vaccaro O, Neaton JD, Wentworth D, and The Multiple Risk Factor Intervention Trial Research Group . Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434–444. [DOI] [PubMed] [Google Scholar]
- 363. Jia F, Tian J, Deng F, Yang G, Long M, Cheng W, Wang B, Wu J, Liu D. Subclinical hypothyroidism and the associations with macrovascular complications and chronic kidney disease in patients with Type 2 diabetes. Diabet Med. 2015;32(8):1097–1103. [DOI] [PubMed] [Google Scholar]
- 364. Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A. Negro, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3(2):76–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–389. [DOI] [PubMed] [Google Scholar]
- 366. Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocr Rev. 2014;35(3):433–512. [DOI] [PubMed] [Google Scholar]
- 367. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. [DOI] [PubMed] [Google Scholar]
- 368. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. [DOI] [PubMed] [Google Scholar]
- 369. World Health Organization Laboratory Diagnosis and Monitoring of Diabetes Mellitus. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 370. Schneider S, Bock C, Wetzel M, Maul H, Loerbroks A. The prevalence of gestational diabetes in advanced economies. J Perinat Med. 2012;40(5):511–520. [DOI] [PubMed] [Google Scholar]
- 371. Philips JC, Emonts P, Pintiaux A, Kirkpatrick C, Scheen AJ. Management of gestational diabetes [in French]. Rev Med Liege. 2013;68(9):489–496. [PubMed] [Google Scholar]
- 372. Dudley DJ. Diabetic-associated stillbirth: incidence, pathophysiology, and prevention. Clin Perinatol. 2007;34(4):611–626. [DOI] [PubMed] [Google Scholar]
- 373. Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, Fowler S, Kahn SE; Diabetes Prevention Program Research Group . Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93(12):4774–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Gallas PR, Stolk RP, Bakker K, Endert E, Wiersinga WM. Thyroid dysfunction during pregnancy and in the first postpartum year in women with diabetes mellitus type 1. Eur J Endocrinol. 2002;147(4):443–451. [DOI] [PubMed] [Google Scholar]
- 375. Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract. 2014;20(7):703–714. [DOI] [PubMed] [Google Scholar]
- 376. Bassols J, Prats-Puig A, Soriano-Rodríguez P, García-González MM, Reid J, Martínez-Pascual M, Mateos-Comerón F, de Zegher F, Ibáñez L, López-Bermejo A. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab. 2011;96(12):3717–3723. [DOI] [PubMed] [Google Scholar]
- 377. Knight BA, Shields BM, Hattersley AT, Vaidya B. Maternal hypothyroxinaemia in pregnancy is associated with obesity and adverse maternal metabolic parameters. Eur J Endocrinol. 2016;174(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol. 2012;119(5):983–988. [DOI] [PubMed] [Google Scholar]
- 379. Yang Y, Li Q, Wang Q, Ma X. Thyroid antibodies and gestational diabetes mellitus: a meta-analysis. Fertil Steril. 2015;104(3):665–671.e3. [DOI] [PubMed] [Google Scholar]
- 380. Karakosta P, Alegakis D, Georgiou V, Roumeliotaki T, Fthenou E, Vassilaki M, Boumpas D, Castanas E, Kogevinas M, Chatzi L. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab. 2012;97(12):4464–4472. [DOI] [PubMed] [Google Scholar]
- 381. Zhang Y, Dai X, Yang S, Zhang C, Han M, Huang HF, Fan J. Maternal low thyroxin levels are associated with adverse pregnancy outcomes in a Chinese population. PLoS One. 2017;12(5):e0178100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Yang S, Shi FT, Leung PC, Huang HF, Fan J. Low thyroid hormone in early pregnancy is associated with an increased risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2016;101(11):4237–4243. [DOI] [PubMed] [Google Scholar]
- 383. Riise HK, Sulo G, Tell GS, Igland J, Nygård O, Iversen AC, Daltveit AK. Association between gestational hypertension and risk of cardiovascular disease among 617 589 Norwegian women. J Am Heart Assoc. 2018;7(10):e008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Nerenberg K, Daskalopoulou SS, Dasgupta K. Gestational diabetes and hypertensive disorders of pregnancy as vascular risk signals: an overview and grading of the evidence. Can J Cardiol. 2014;30(7):765–773. [DOI] [PubMed] [Google Scholar]
- 385. Männistö T, Vääräsmäki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, Bloigu A, Järvelin MR, Suvanto E. Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J Clin Endocrinol Metab. 2010;95(3):1084–1094. [DOI] [PubMed] [Google Scholar]
- 386. Roti E, Uberti E. Post-partum thyroiditis—a clinical update. Eur J Endocrinol. 2002;146(3):275–279. [DOI] [PubMed] [Google Scholar]
- 387. Lazarus JH. Clinical manifestations of postpartum thyroid disease. Thyroid. 1999;9(7):685–689. [DOI] [PubMed] [Google Scholar]
- 388. Gerstein HC. Incidence of postpartum thyroid dysfunction in patients with type I diabetes mellitus. Ann Intern Med. 1993;118(6):419–423. [DOI] [PubMed] [Google Scholar]
- 389. Alvarez-Marfany M, Roman SH, Drexler AJ, Robertson C, Stagnaro-Green A. Long-term prospective study of postpartum thyroid dysfunction in women with insulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1994;79(1):10–16. [DOI] [PubMed] [Google Scholar]
- 390. De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(8):2543–2565. [DOI] [PubMed] [Google Scholar]
- 391. British Thyroid Association and Association of Clinical Biochemistry Guidelines 2016. www.british-thyroid-association.org/current-bta-guidelines. Accessed 30 April 2019.
- 392. American Diabetes Association. Standards of medical care in diabetes—2018. Diabetes Care. 2008;31(Suppl 1):S12–S54. [DOI] [PubMed] [Google Scholar]
- 393. American Diabetes Association Standards of medical care in diabetes–2009. Diabetes Care. 2009;32(Suppl 1):S13–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. American Diabetes Association 11. Children and adolescents. Diabetes Care. 2016;39(Suppl. 1):S86–S93. [DOI] [PubMed] [Google Scholar]
- 395. Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, Segal RL. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8(6):457–469. [PubMed] [Google Scholar]
- 396. Kordonouri O, Maguire AM, Knip M, Schober E, Lorini R, Holl RW, Donaghue KC; International Society for Pediatric and Adolescent Diabetes . ISPAD clinical practice consensus guidelines 2006–2007. Other complications and associated conditions. Pediatr Diabetes. 2007;8(3):171–176. [DOI] [PubMed] [Google Scholar]
- 397. Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(1):35–45. [DOI] [PubMed] [Google Scholar]
- 398. Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG, Smith SA, Daniels GH, Cohen HD. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160(11):1573–1575. [DOI] [PubMed] [Google Scholar]
- 399. Houcken J, Degenhart C, Bender K, König J, Frommer L, Kahaly GJ. PTPN22 and CTLA-4 polymorphisms are associated with polyglandular autoimmunity. J Clin Endocrinol Metab. 2018;103(5):1977–1984. [DOI] [PubMed] [Google Scholar]
- 400. Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. [DOI] [PubMed] [Google Scholar]
- 401. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM; American Thyroid Association Task Force on Thyroid Hormone Replacement . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24(12):1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]