Abstract
Accurate evaluation of morphological and physiological traits is critical for selection of wheat (Triticum aestivum L.) cultivars exhibiting high yield, which is stable over different growing conditions. In order to use selection index based on high yield, high grain quality and drought tolerance in wheat, a set of 145 CIMMYT Wheat Physiological Germplasm Screening Nursery lines and seven local spring wheat varieties were phenotyped and evaluated for physiological and yield traits under two irrigation regimes during the 2011 and 2012 growing seasons in Xinjiang, China. The results showed that drought-stress significantly increased canopy temperature but reduced grain yield, grain weight per spike, normalized difference vegetation index at the flowering and grain filling stages, chlorophyll content at the grain filling stage, grain plumpness, grain number per spike, thousand-grain weight, and plant height. Grain weight per spike, plant height and grain plumpness explained 61.8% of the total phenotypic variation in grain yield under no-stress conditions, where they were the three principal factors most closely related to grain yield. Under drought-stress conditions, canopy temperature at the grain filling stage, plant height and grain plumpness were the three principal factors affecting grain yield, and contributed 44.8% of the total phenotypic variation in grain yield. Finally, ten genotypes, including three local varieties, ‘Xinchun 11’, ‘Xinchun 23’ and ‘Xinchun 29’, with appropriate plant height and high and stable yield under both no-stress and drought-stress conditions over the two years of trials, were identified and can be recommended as core parents for spring wheat drought tolerance breeding in Xinjiang, China.
Keywords: wheat, phenotype, evaluation, drought-stress
Introduction
Drought is one of the most severe abiotic factors limiting agricultural production in arid and semi-arid regions (Delmer 2005). Drought can be prolonged or seasonal, since rainfall in such areas is very seasonal, and periodic drought occurs regularly. About 230 million ha of farm land is used for wheat cultivation world-wide, and half of this area is regularly affected by drought (Trethowan et al. 2007). Xinjiang has a temperate continental climate, with low rainfall and low humidity, and is typical of an arid or semi-arid desert region. The data on wheat drought tolerance research in Xinjiang is valuable for wheat breeders, especially for those working in drought-prone regions of the world.
Drought-stress results in average wheat yield losses of 17%–70% (Nouriganbalani et al. 2009). Breeding crop cultivars for the ability to withstand drought is an important strategy, and is necessary to withstand both mild and severe stress conditions. However, better characterization of the ranges of drought-stress responses among available germplasm, and a comprehensive understanding of the physiological mechanisms associated with drought-stress response is crucial to ensure acceptable yield when drought occurs (Rizza et al. 2004). Three physiological and morphological mechanisms, namely drought escape, drought avoidance and drought tolerance, are known to be exhibited in response to drought (Levitt 1980), and crop plants may use combinations of different stress-withstanding mechanisms to cope with drought-stress at any one time (Cheng et al. 2016, Zhang et al. 2012).
Breeding crop cultivars with the ability to withstand drought should use a combination of different relevant traits as selection criteria, rather than a single trait. The traits for selection (or screening) for drought escape, avoidance or tolerance and the drought-stress breeding framework depend on the level and timing of stresses in the targeted area (Araus et al. 2002). Some physiological traits are related to drought coping, such as rapid ground cover or early vigor (Richards and Lukacs 2002), “stay-green” (Nawaz et al. 2013), canopy temperature and canopy temperature depression (Pinto et al. 2015, Rebetzke et al. 2013), stable carbon isotope discrimination (Cabrera-Bosquet et al. 2017, Rebetzke et al. 2008), the normalized difference vegetation index (NDVI) (Christopher et al. 2014, Lopes and Reynolds 2012) and chlorophyll content (measured as SPAD) (Barakat et al. 2015, Hamblin et al. 2014). Physiological and morphological traits under drought-stress conditions, such as grain yield per plant (Chen et al. 2012, Mwadzingeni et al. 2017), leaf rolling (Bogale et al. 2011, Kadioglu et al. 2012), and plant height (Jatoi et al. 2011), are also associated with drought coping, and can be used to screen for high-yield germplasm accessions under drought-stress conditions. Grain plumpness is significantly positively correlated with grain yield under stress conditions (Waldron 1933). The number of grains per spike and grain weight per spike have a positive association with grain yield. However, grain number per spike, thousand-grain weight and especially grain yield are more sensitive to drought-stress than are plant height and the number of spikelets per spike (Denčić et al. 2000, Rebetzke et al. 2016). Therefore, breeders usually place greater emphasis on yield performance under drought-stress conditions.
Drought indices (DIs), measures of drought based on the reduction of grain yield under drought-stress conditions, compared with that under normal irrigated conditions, have been used for drought tolerance evaluation. The stress susceptibility index (SSI) has also been proposed for measurement of yield stability, based on the differences between potential and actual yields under stress environments (Willick et al. 2018). Meanwhile, the drought resistance coefficient (Chinoy 1961) and the drought resistance index (DI) were used to identify genotypes achieving high yield under both stress and no-stress conditions (Lan 1998, Lan et al. 1990). Drought yield index (DYI), water yield index (WYI) and yield high water-use efficiency index (YH-WUEI) were suggested to be used together for evaluating drought tolerant genotypes, and also for simultaneous screening of genotypes for high water-use efficiency (Wu et al. 2005). Genotypes identified on the basis of high values of DYI, WYI and YH-WUEI have greater adaptability and stability, and achieve higher yields under both drought-stress and no-stress conditions. Higher DYI, WYI and YH-WUEI values (>1.05) were reported for accessions with high water-use efficiency and lower values (<0.95) were observed for accessions with low water-use efficiency, whereas values between 0.95 to 1.05 were shown to be associated with accessions exhibiting moderate water-use efficiency (Wu et al. 2005).
Wheat is one of the most important food crops in China and is the principal food crop in Xinjiang, China, a typical arid or semi-arid region. Drought-stress is the major yield-limiting abiotic factor for wheat production in Xinjiang, which has an average annual rainfall of approximately 200 mm, with less than 100 mm rainfall occurring during the wheat-growing season (Li et al. 2015). Without irrigation, there is no yield from crops grown in most regions of Xinjiang, as evaporation losses in the plains area are very high. However, irrigation water in Xinjiang is routinely in short supply during the wheat grain filling stage because of limited water availability. The main breeding objectives for wheat in Xinjiang are high and stable yield, with good adaptability to uncertain drought-stress conditions. In order to screen for the selection index of high yield and high grain quality under drought-stress in wheat, a set of 145 CIMMYT WPHYSGP nursery lines and seven local spring wheat varieties were phenotyped and evaluated for physiological and yield traits under two irrigation regimes during the 2011 and 2012 growing seasons in Xinjiang, China. In this paper, we report the evaluation and phenotyping of these wheat genotypes in response to drought, and the identification of the best-adapted genotypes, exhibiting high yields under both stress and no-stress conditions, as potential parents in wheat breeding programs in Xinjiang and areas with similar drought-prone climates.
Materials and Methods
Wheat genotypes
A total of 152 wheat genotypes were used in the trials, including seven Xinjiang locally bred spring wheat varieties, well adapted to conditions in the north of Xinjiang, and 145 lines from the CIMMYT Wheat Physiological Germplasm Screening (WPHYSGP) nursery in Mexico, where screening had been carried out for improved drought response. Of the seven local varieties, Xinchun 6, Xinchun 11, Xinchun 17 and Xinchun 29 are leading varieties in different regions of Xinjiang, while ‘Xinchun 6’ is also the local check variety in regional trials in Xinjiang.
Experimental conditions
Trials were conducted in two growing seasons (2011 and 2012) at the Junhu wheat experimental station of Changji, Xinjiang Academy of Agricultural Sciences, China: 43°96′N, 87°01′E, altitude 717.2 m, gray desert soil, soil depth 70 cm, soil pH~7.8, and 200 mm average annual precipitation.
Sowing was done by hand in plots with four rows, 2 m length, and 0.2 m inter-row spacing and 624 seeds m−2 sowing density. Seeds were planted on April 11, 2011 and March 31, 2012. Before planting, 15 g and 6.8 g of N and P, respectively, were applied per plot in the seedbed. At the seedling (two to three leaves) and jointing stages, 13.8 and 14.5 g N per plot, respectively, were applied. The herbicide 2-methy1-4-chlorophenoxy acetic acid was applied once at the rate of 0.15 g m−2 during the jointing stage.
In each of the two growing seasons, the experimental layout was randomized complete blocks design with three replicates under each of the irrigation regimes, namely no-stress (non-limited irrigation) and drought-stress (limited irrigation) conditions. Both of the no-stress and drought-stress treatments used drip irrigation which was independently controlled according to the irrigation regime, and the areas under the different irrigation regimes were separated by 4 m isolation zone. No-stress plots (T1) were watered seven times between April 28, 2011 and June 28, 2011, and eight times between April 18, 2012 and June 28, 2012, with irrigation intervals of 10 d. Drought-stress plots (T2) were irrigated twice in 2011 at the jointing and heading stages, respectively, and three times in 2012 at the jointing, heading and early grain filling stages. During the 2011 growing season, all plots of T1 and T2 received 105.3 mm rainfall, and plots T1 and T2 received an additional 420 mm and 120 mm irrigation water, respectively. During the 2012 growing season, all plots of T1 and T2 received 95 mm rainfall, and plots T1 and T2 received an additional 480 mm and 180 mm irrigation water, respectively.
Grain yield and DYI, WYI, and YH-WUEI
In both the 2011 and 2012 growing seasons, all the plants (except for those harvested earlier for individual agronomic traits) in the T1 and T2 plots were harvested by hand, threshed by machine, cleaned by hand, and the yield per plot weighed by hand. Weights were expressed at a moisture content of 13%. Performance of the genotypes in each year was evaluated by calculation of the drought yield index (DYI), the water yield index (WYI) and the yield high water use efficiency index (YH-WUEI):
where YS and YP are the grain yields of each genotype under drought-stress (T2) and no-stress conditions (T1), respectively, and ȲS and ȲP are the mean grain yields of all the genotypes under drought-stress and no-stress conditions, respectively.
Agronomic traits
In both the 2011 and 2012 growing seasons, days to heading, plant height, grain plumpness, thousand-grain weight, spike number per plant, spikelet number per spike, spike length, grain number per spike, grain weight per spike, and grain weight per plant were determined. Days to heading was measured as the number of days from planting until 50% of the main culm spikes emerged from the boot in each plot. Plant height was measured as the distance from the ground to the top of spike (excluding the awns) at maturity. Physiological maturity was recorded when the green color was about to disappear from the upper portion of the main culm peduncle and there was complete loss of green color from the flag leaves. Days to physiological maturity was counted as the number of days from planting to physiological maturity. Grain plumpness was classified on a nine-class scale, from 1 (very shriveled) to 9 (well rounded). Ten whole-plant samples from each T1 and T2 plot were taken before harvesting and used for collecting spike number per plant, spikelet number per spike, spike length, grain number per spike, grain weight per spike, and grain weight per plant. Weights were expressed at a moisture content of 13%.
Physiological measurements
Previous studies had shown that reduction in canopy temperature at solar noon correlated better with yield than it did in the morning or late afternoon (Reynolds et al. 1994). CT was measured in both 2011 and 2012 at the mid-grain filling stage (18 d after flowering), using a hand-held infrared thermometer Optris LS LT (Optris Infrared Sensing, Portsmouth, NH, USA) between 13:00 and 15:00 h during the day, selecting days with clear skies and low wind, keeping the sun behind the operator and measuring the temperature of the canopy exposed to the sun (Reynolds et al. 1994).
Normalized difference vegetation index (NDVI) was determined with the Green Seeker 505 (NTech Industries Inc., Sunnyvale, CA, USA) on the central rows of all plots. In both the 2011 and 2012 growing seasons, NDVI was measured at the jointing stage, flowering stage and mid-grain filling stage. NDVI reflected the stay-green traits of plants. It mainly reflected the growth potential of plants during the vegetative growth period, and reflected the transformation ability of photosynthesis products of plants after flowering. Some studies have shown that stay-green traits have great potential in selecting for water-stress adaptation (Christopher et al. 2014, 2016).
The SPAD Minolta 502 Plus (Konica Minolta, Tokyo, Japan) was used to non-destructively determine chlorophyll content, measuring 10 main culm flag leaves from each replicate plot, and recording the mean data. In both the 2011 and 2012 growing seasons, SPAD was measured at the flowering stage and the mid-grain filling stage.
Statistical analysis
Restricted maximum likelihood (REML) variance components analysis was conducted on the data in 2011 and 2012 (genotype, irrigation and year were the fixed model and row and column were the random model). Pearson’s correlation coefficients were determined between grain yield and other evaluated traits within the drought-stress and no-stress conditions. Parametric linear regression analyses and multiple linear regression analysis were conducted among evaluated traits within each of the two irrigation regimes. Significant differences among genotypes and irrigation regimes were determined using Fisher’s protected Least-Significant Difference at α0.05. Data analysis was performed using Genstat v.17.1 (VSN International Ltd., Hemel Hempstead, UK) and SPSS Statistics v.17.0.1 (IBM Corporation, Armonk, NY, USA).
Results
Differences in agronomic traits between different genotypes and irrigation regimes
REML variance components analysis of the 152 genotypes showed significant differences in grain yield (p < 0.001), thousand-grain weight (p < 0.001), grain plumpness (p < 0.001), CT (p < 0.01), NDVI (p < 0.001), SPAD (p < 0.001), grain number per spike (p < 0.001), grain weight per spike (p < 0.001), days to heading (p < 0.001) and plant height (p < 0.001) within and between the two irrigation treatments in both the 2011 and 2012 growing seasons (Table 1). The seasonal effect was not significant for grain yield, grain plumpness, CT, NDVI at flowering and grain filling stages, grain number per spike, grain weight per spike and days to heading. Genotype × Irrigation treatment interaction was also significant (p < 0.05) with regard to grain yield, thousand-grain weight, grain plumpness, CT, NDVI at the flowering and grain filling stages, days to heading and plant height in both the 2011 and 2012 growing seasons.
Table 1.
REML variance components analysis for two treatments in 2011 and 2012 growing seasons
| Trait | Source of variation | df | Wald statistic | F value |
|---|---|---|---|---|
| GY, kg/ha | Genotype (G) | 151 | 1810142.85 | 2.0*** |
| Irrigation treatment (I) | 1 | 3085829921.37 | 3431.8*** | |
| Year (Y) | 1 | 899176.21 | 1.0NS | |
| G × I | 151 | 41996942.06 | 46.7*** | |
| G × Y | 151 | 1020276.18 | 1.1NS | |
| I × Y | 1 | 216462509.56 | 240.7*** | |
| G × I × Y | 151 | 894905.09 | 1.0NS | |
|
| ||||
| TGW, g | Genotype (G) | 151 | 101.10 | 10.4*** |
| Irrigation treatment (I) | 1 | 15938.45 | 1644.9*** | |
| Year (Y) | 1 | 12.37 | 1.3* | |
| G × I | 151 | 22.19 | 2.3*** | |
| G × Y | 151 | 26.70 | 2.8*** | |
| I × Y | 1 | 1203.53 | 124.2*** | |
| G × I × Y | 151 | 12.24 | 1.3* | |
|
| ||||
| Grain plumpness | Genotype (G) | 151 | 2.65 | 4.4*** |
| Irrigation treatment (I) | 1 | 324.14 | 532.6*** | |
| Year (Y) | 1 | 0.83 | 1.2NS | |
| G × I | 151 | 48.25 | 79.3*** | |
| G × Y | 151 | 1.11 | 1.8*** | |
| I × Y | 1 | 67.14 | 110.3*** | |
| G × I × Y | 151 | 0.69 | 1.1NS | |
|
| ||||
| CT mid-grain filling | Genotype (G) | 151 | 60.88 | 21.0*** |
| Irrigation treatment (I) | 1 | 2433.76 | 840.1*** | |
| Year (Y) | 1 | 2.72 | 0.9NS | |
| G × I | 151 | 85.75 | 29.6*** | |
| G × Y | 151 | 2.88 | 1.0NS | |
| I × Y | 1 | 2.13 | 0.7NS | |
| G × I × Y | 151 | 2.11 | 0.7NS | |
|
| ||||
| NDVI jointing | Genotype (G) | 151 | 0.03 | 6.5*** |
| Irrigation treatment (I) | 1 | 0.32 | 71.7*** | |
| Year (Y) | 1 | 3.61 | 801.4*** | |
| G × I | 151 | 0.00 | 1.1NS | |
| G × Y | 151 | 0.01 | 2.6*** | |
| I × Y | 1 | 0.50 | 110.1*** | |
| G × I × Y | 151 | 0.00 | 0.8NS | |
|
| ||||
| NDVI flowering | Genotype (G) | 151 | 0.02 | 2.5*** |
| Irrigation treatment (I) | 1 | 20.54 | 2548.9*** | |
| Year (Y) | 1 | 0.01 | 0.9NS | |
| G × I | 151 | 8.86 | 1099.7*** | |
| G × Y | 151 | 0.01 | 1.3* | |
| I × Y | 1 | 1.47 | 182.5*** | |
| G × I × Y | 151 | 0.01 | 0.9NS | |
|
| ||||
| NDVI mid-grain filling | Genotype (G) | 151 | 0.02 | 2.0*** |
| Irrigation treatment (I) | 1 | 28.12 | 3034.3*** | |
| Year (Y) | 1 | 0.01 | 0.9NS | |
| G × I | 151 | 0.27 | 29.1*** | |
| G × Y | 151 | 0.01 | 1.4* | |
| I × Y | 1 | 0.54 | 58.4*** | |
| G × I × Y | 151 | 0.01 | 1.2NS | |
|
| ||||
| SPAD flowering | Genotype (G) | 151 | 37.79 | 3.8*** |
| Irrigation treatment (I) | 1 | 593.15 | 59.9*** | |
| Year (Y) | 1 | 444.73 | 44.9*** | |
| G × I | 151 | 8.60 | 0.9NS | |
| G × Y | 151 | 10.61 | 1.1NS | |
| I × Y | 1 | 2.04 | 0.2NS | |
| G × I × Y | 151 | 7.54 | 0.8NS | |
|
| ||||
| SPAD mid-grain filling | Genotype (G) | 151 | 139.75 | 2.0*** |
| Irrigation treatment (I) | 1 | 41963.19 | 596.2*** | |
| Year (Y) | 1 | 53581.70 | 761.3*** | |
| G × I | 151 | 116.49 | 1.7*** | |
| G × Y | 151 | 128.74 | 1.8*** | |
| I × Y | 1 | 47971.88 | 681.6*** | |
| G × I × Y | 151 | 106.55 | 1.5*** | |
|
| ||||
| Spike length | Genotype (G) | 151 | 24.81 | 1.5*** |
| Irrigation treatment (I) | 1 | 52.10 | 3.3NS | |
| Year (Y) | 1 | 1215.27 | 75.9*** | |
| G × I | 151 | 14.68 | 0.9NS | |
| G × Y | 151 | 16.24 | 1.0NS | |
| I × Y | 1 | 34.90 | 2.2NS | |
| G × I × Y | 151 | 15.47 | 1.0NS | |
|
| ||||
| SPS | Genotype (G) | 151 | 16.74 | 16.2*** |
| Irrigation treatment (I) | 1 | 1.3 | 1.1NS | |
| Year (Y) | 1 | 7644.53 | 7386.7*** | |
| G × I | 151 | 0.86 | 0.8NS | |
| G × Y | 151 | 2.40 | 2.3*** | |
| I × Y | 1 | 14.42 | 13.9*** | |
| G × I × Y | 151 | 0.77 | 0.7NS | |
|
| ||||
| GNPS | Genotype (G) | 151 | 129.42 | 4.3*** |
| Irrigation treatment (I) | 1 | 17242.87 | 575.5*** | |
| Year (Y) | 1 | 35.47 | 1.2NS | |
| G × I | 151 | 11016.32 | 367.7*** | |
| G × Y | 151 | 39.59 | 1.3* | |
| I × Y | 1 | 4026.35 | 134.4*** | |
| G × I × Y | 151 | 26.80 | 0.9NS | |
|
| ||||
| GWPS | Genotype (G) | 151 | 0.20 | 3.0*** |
| Irrigation treatment (I) | 1 | 107.78 | 1581.8*** | |
| Year (Y) | 1 | 0.10 | 1.1NS | |
| G × I | 151 | 0.07 | 1.0NS | |
| G × Y | 151 | 0.07 | 1.0NS | |
| I × Y | 1 | 12.10 | 177.6*** | |
| G × I × Y | 151 | 0.06 | 0.9NS | |
|
| ||||
| Days to heading | Genotype (G) | 151 | 46.07 | 33.0*** |
| Irrigation treatment (I) | 1 | 1.35 | 1.0NS | |
| Year (Y) | 1 | 5.02 | 3.6NS | |
| G × I | 151 | 2.26 | 1.6*** | |
| G × Y | 151 | 5.27 | 3.8*** | |
| I × Y | 1 | 107.09 | 76.8*** | |
| G × I × Y | 151 | 1.57 | 1.1NS | |
|
| ||||
| Plant height | Genotype (G) | 151 | 387.63 | 11.9*** |
| Irrigation treatment (I) | 1 | 80000.46 | 2457.1*** | |
| Year (Y) | 1 | 25697.52 | 789.3*** | |
| G × I | 151 | 77.61 | 2.4*** | |
| G × Y | 151 | 40.74 | 1.3* | |
| I × Y | 1 | 18939.96 | 581.7*** | |
| G × I × Y | 151 | 37.04 | 1.1NS | |
Significant at the 0.05 probability level,
Significant at the 0.01 probability level,
Significant at the 0.001 probability level,
NS, non-significant at the 0.05 probability level.
Assessment of a set of CIMMYT WPHYSGP nursery lines and local varieties
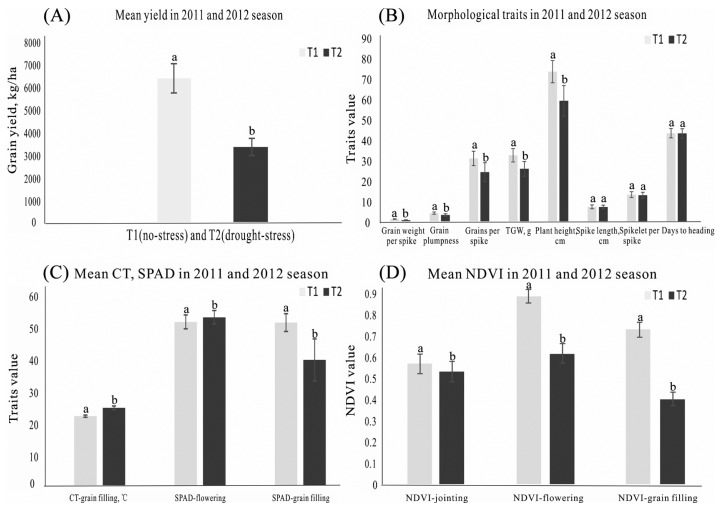
Comparison of the mean data of the 152 genotypes under trial showed that drought-stress significantly reduced grain yield, grain weight per spike, NDVI at the flowering and grain filling stages, SPAD at the grain filling stage, grain plumpness, grain number per spike, thousand-grain weight, plant height and CT under each of the two irrigation regimes (Fig. 1). The mean grain yields of all the genotypes under no-stress and stress conditions averaged over the two seasons were 6440.8 kg ha−1 and 3360.8 kg ha−1, respectively (Table 2), representing a significant decrease of 47.8% under drought-stress conditions. Grain weight per spike, NDVI at the flowering and grain filling stages, SPAD at the grain filling stage, grain plumpness, grain number per spike, thousand-grain weight, and plant height decreased by 50%, 45.2%, 30.7%, 22.9%, 22.7%, 21.8%, 20.5%, and 19.5%, respectively, under drought-stress, while CT increased by 12.2% under drought-stress conditions (Fig. 1B–1D). Spike length, spikelet number per spike and heading date values were very similar under both irrigation regimes (Fig. 1B). NDVI continued to rise from the seedling stage onwards, reaching its peak at the flowering stage then decreasing at the grain filling stage (Fig. 1D). NDVI at the jointing stage under the no-stress and stress conditions were similar because drought-stress occurred mainly after the flowering stage. NDVI under drought-stress was lower than that under no-stress conditions at the flowering and grain filling stages. Chlorophyll content at the flowering stage was higher under drought-stress conditions, compared to the no-stress conditions, but decreased sharply at the grain filling stage under the stress conditions (Fig. 1C).
Fig. 1.
(A) The mean ± SD grain yield of 152 spring wheat genotypes under two irrigation regimes, T1 (no drought stress) and T2 (drought stress conditons) in 2011 and 2012. (B) The mean ± SD grain weight per spike, grain plumpness, grains per spike, TKW, plant height, spike length, spikelet per spike, days to heading, of 152 spring wheat genotypes under two irrigation regimes, T1 (no drought stress) and T2 (drought stress conditons) in 2011 and 2012. (C) The mean ± SD CT at grain filling stage, SPAD at flowering stage, SPAD at grain filling stage of 152 spring wheat genotypes under two irrigation regimes, T1 (no drought stress) and T2 (drought stress conditons) in 2011 and 2012. (D) The mean ± SD grain NDVI of 152 spring wheat genotypes under two irrigation regimes, T1 (no drought stress) and T2 (drought stress conditons) in 2011 and 2012. The different letters showed significant at the 0.05 probability level according to the ANOVA.
Table 2.
Characteristics of a set of CIMMYT WPHYSGP nursery and local varieties in two regimes in 2011 and 2012 growing season at Changji, Xinjiang
| Trait | No stress condition | Stress condition | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Av | Min | Max | Av | Min | Max | |
| GY, kg/ha | 6440.8 | 3870.0 | 7995.0 | 3360.6 | 2066.0 | 4492.0 |
| TGW, g | 35.6 | 27.0 | 48.0 | 28.3 | 21.0 | 37.7 |
| Grain plumpness | 4.4 | 2.5 | 6.2 | 3.4 | 1.8 | 5.0 |
| CT mid-grain filling, °C | 22.2 | 21.3 | 23.4 | 24.9 | 23.0 | 27.2 |
| NDVI jointing | 0.5153 | 0.3726 | 0.6439 | 0.4806 | 0.3259 | 0.6327 |
| NDVI flowering | 0.8027 | 0.6433 | 0.9064 | 0.5565 | 0.3668 | 0.7068 |
| NDVI mid-grain filling | 0.6619 | 0.5515 | 0.7808 | 0.3625 | 0.2646 | 0.4948 |
| SPAD flowering | 52.5 | 44.7 | 61.3 | 54.0 | 49.0 | 61.8 |
| SPAD mid-grain filling | 52.3 | 45.0 | 60.6 | 40.3 | 25.0 | 63.0 |
| Spike length, cm | 7.8 | 4.8 | 9.8 | 7.7 | 5.1 | 9.8 |
| Spikelet per spike | 14.4 | 9.4 | 17.6 | 14.1 | 10.3 | 17.4 |
| Grains per spike | 33.9 | 15.4 | 48.3 | 26.5 | 16.1 | 37.2 |
| Grain weight per spike | 1.2 | 0.5 | 1.7 | 0.6 | 0.3 | 1.1 |
| Days to heading | 47.5 | 41.8 | 52.7 | 47.4 | 42.3 | 52.2 |
| Plant height, cm | 80.7 | 57.0 | 101.3 | 65.0 | 45.5 | 82.0 |
Evaluation of grain yield of test genotypes under two irrigation regimes
Under no-stress conditions, 22 genotypes showed at least 10% higher grain yield and water yield index (WYI) than the mean value of the 152 genotypes. These genotypes were 9645, ‘Xinchun 29’, 9611, 9650, 9637, 9653, 9657, ‘Xinchun 6’, 9692, 9638, 9655, 9606, ‘Xinchun 23’, 9639, 9744, 9608, 9727, 9630, 9649, ‘Xinchun 11’ and 9729. Among these genotypes, ‘Xinchun 29’, ‘Xinchun 6’, ‘Xinchun 23’ and ‘Xinchun 11’ were local varieties, while the others were CIMMYT lines (Table 3). Of the 22 genotypes, five genotypes (9645, ‘Xinchun 29’, 9611, 9650 and 9637) achieved 3% higher yield than the local check variety ‘Xinchun 6’. CIMMYT genotypes 9672, 9703, 9678, 9701, 9711, 9659, 9661, 9706, 9677, 9702, 9663, 9605, 9662, 9732, 9704, 9684, 9675, 9665, 9670, 9660, 9679, 9740 and 9664 showed 10% lower grain yield than the mean grain yield of the 152 genotypes and 21% lower grain yield than the check variety ‘Xinchun 6’, and also had lower water yield index values (WYI) (Table 3).
Table 3.
Mean grain yield of 152 genotypes under no-stress conditions (T1) and drought-stress conditions (T2) averaged over the two growing seasons
| Genotype | Original | GY in T1, kg/ha | GY in T2, kg/ha | Genotype | Original | GY in T1, kg/ha | GY in T2, kg/ha | Genotype | Original | GY in T1, kg/ha | GY in T2, kg/ha |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 9645 | CIMMYT | 7995 (1) | 3881 (11) | 9696 | CIMMYT | 6707 (51) | 3077 (116) | 9699 | CIMMYT | 6234 (99) | 3841 (16) |
| Xinchun29 | Local | 7635 (2) | 3793 (21) | 9629 | CIMMYT | 6703 (52) | 3386 (71) | 9615 | CIMMYT | 6218 (100) | 3297 (86) |
| 9611 | CIMMYT | 7622 (3) | 3815 (18) | 9632 | CIMMYT | 6698 (53) | 3091 (115) | 9603 | CIMMYT | 6200 (101) | 3413 (65) |
| 9650 | CIMMYT | 7574 (4) | 3873 (13) | 9621 | CIMMYT | 6697 (54) | 3288 (87) | 9728 | CIMMYT | 6196 (102) | 3035 (122) |
| 9637 | CIMMYT | 7573 (5) | 3798 (20) | 9633 | CIMMYT | 6688 (55) | 3249 (94) | 9676 | CIMMYT | 6189 (103) | 2946 (130) |
| 9653 | CIMMYT | 7474 (6) | 3474 (56) | 9720 | CIMMYT | 6678 (56) | 3868 (14) | 9695 | CIMMYT | 6166 (104) | 3331 (79) |
| 9657 | CIMMYT | 7362 (7) | 3418 (64) | 9656 | CIMMYT | 6677 (57) | 3539 (43) | 9686 | CIMMYT | 6160 (105) | 3556 (40) |
| Xinchun6 | Local | 7356 (8) | 3341 (77) | 9736 | CIMMYT | 6662 (58) | 3608 (33) | 9723 | CIMMYT | 6141 (106) | 3032 (123) |
| 9692 | CIMMYT | 7322 (9) | 4124 (7) | 9658 | CIMMYT | 6662 (58) | 3526 (47) | 9714 | CIMMYT | 6136 (107) | 3333 (78) |
| 9638 | CIMMYT | 7315 (10) | 3894 (10) | 9626 | CIMMYT | 6660 (59) | 3531 (45) | 9721 | CIMMYT | 6096 (108) | 3466 (58) |
| 9655 | CIMMYT | 7300 (11) | 3704 (24) | 9745 | CIMMYT | 6656 (60) | 3069 (117) | 9609 | CIMMYT | 6069 (109) | 3271 (93) |
| 9635 | CIMMYT | 7291 (12) | 3203 (99) | 9654 | CIMMYT | 6652 (61) | 3599 (35) | 9718 | CIMMYT | 6050 (110) | 3299 (85) |
| 9606 | CIMMYT | 7267 (13) | 3875 (12) | 9680 | CIMMYT | 6651 (62) | 3534 (44) | 9613 | CIMMYT | 6032 (111) | 3329 (80) |
| Xinchun23 | Local | 7264 (14) | 3621 (32) | 9624 | CIMMYT | 6609 (63) | 3412 (66) | 9712 | CIMMYT | 6032 (111) | 3092 (114) |
| 9639 | CIMMYT | 7259 (15) | 3605 (34) | 9726 | CIMMYT | 6599 (64) | 3698 (26) | 9617 | CIMMYT | 6018 (112) | 2513 (144) |
| 9744 | CIMMYT | 7241 (16) | 3507 (50) | 9628 | CIMMYT | 6599 (64) | 3480 (55) | 9708 | CIMMYT | 6012 (113) | 3309 (83) |
| 9608 | CIMMYT | 7163 (17) | 3199 (100) | 9634 | CIMMYT | 6595 (65) | 2934 (131) | 9669 | CIMMYT | 6002 (114) | 3544 (42) |
| 9727 | CIMMYT | 7161 (18) | 3356 (74) | 9691 | CIMMYT | 6588 (66) | 2973 (128) | 9674 | CIMMYT | 5978 (115) | 2933 (132) |
| 9630 | CIMMYT | 7156 (19) | 3132 (110) | 9652 | CIMMYT | 6573 (67) | 3048 (120) | 9667 | CIMMYT | 5965 (116) | 3320 (81) |
| 9649 | CIMMYT | 7151 (20) | 3468 (57) | Xinchun33 | Local | 6558 (68) | 3701 (25) | 9673 | CIMMYT | 5938 (117) | 2755 (139) |
| Xinchun11 | Local | 7149 (21) | 3591 (36) | 9685 | CIMMYT | 6557 (69) | 3525 (48) | 9724 | CIMMYT | 5891 (118) | 3508 (49) |
| 9729 | CIMMYT | 7103 (22) | 4052 (8) | 9709 | CIMMYT | 6544 (70) | 3458 (60) | 9707 | CIMMYT | 5888 (119) | 3404 (68) |
| Xinchun17 | Local | 7093 (23) | 3460 (59) | 9610 | CIMMYT | 6523 (71) | 3486 (54) | 9713 | CIMMYT | 5871 (120) | 3059 (119) |
| 9746 | CIMMYT | 7085 (24) | 3577 (37) | 9648 | CIMMYT | 6521 (72) | 3365 (73) | 9666 | CIMMYT | 5848 (121) | 3280 (90) |
| 9622 | CIMMYT | 7071 (25) | 3629 (31) | 9717 | CIMMYT | 6503 (73) | 3319 (82) | 9620 | CIMMYT | 5798 (122) | 2910 (133) |
| 9730 | CIMMYT | 7054 (26) | 3808 (19) | 9737 | CIMMYT | 6499 (74) | 3219 (98) | 9716 | CIMMYT | 5791 (123) | 2987 (127) |
| 9741 | CIMMYT | 7051 (27) | 3066 (118) | 9668 | CIMMYT | 6486 (75) | 2856 (135) | 9672 | CIMMYT | 5784 (124) | 3037 (121) |
| 9642 | CIMMYT | 7050 (28) | 4169 (5) | 9742 | CIMMYT | 6485 (76) | 3487 (53) | 9703 | CIMMYT | 5687 (125) | 3351 (75) |
| 9688 | CIMMYT | 7038 (29) | 3553 (41) | 9647 | CIMMYT | 6449 (77) | 3561 (39) | 9678 | CIMMYT | 5686 (126) | 2902 (134) |
| 9738 | CIMMYT | 7037 (30) | 3400 (69) | 9683 | CIMMYT | 6429 (78) | 3500 (52) | 9701 | CIMMYT | 5683 (127) | 3281 (89) |
| 9646 | CIMMYT | 7000 (31) | 4262 (2) | 9690 | CIMMYT | 6427 (79) | 3348 (76) | 9711 | CIMMYT | 5657 (128) | 3095 (113) |
| 9734 | CIMMYT | 6996 (32) | 3237 (96) | 9682 | CIMMYT | 6426 (80) | 3393 (70) | 9659 | CIMMYT | 5611 (129) | 3133 (109) |
| Xinchun10 | Local | 6990 (33) | 3234 (97) | 9643 | CIMMYT | 6425 (81) | 4492 (1) | 9661 | CIMMYT | 5596 (130) | 2952 (129) |
| 9604 | CIMMYT | 6978 (34) | 3368 (72) | 9612 | CIMMYT | 6405 (82) | 4251 (3) | 9706 | CIMMYT | 5571 (131) | 3275 (92) |
| 9671 | CIMMYT | 6958 (35) | 3127 (111) | 9743 | CIMMYT | 6394 (83) | 3561 (39) | 9677 | CIMMYT | 5550 (132) | 2714 (140) |
| 9698 | CIMMYT | 6942 (36) | 3901 (9) | 9687 | CIMMYT | 6391 (84) | 3113 (112) | 9702 | CIMMYT | 5540 (133) | 3672 (29) |
| 9631 | CIMMYT | 6931 (37) | 3246 (95) | 9614 | CIMMYT | 6383 (85) | 3189 (102) | 9663 | CIMMYT | 5522 (134) | 3135 (108) |
| 9627 | CIMMYT | 6926 (38) | 3853 (15) | 9693 | CIMMYT | 6376 (86) | 3154 (105) | 9605 | CIMMYT | 5520 (135) | 3503 (51) |
| 9625 | CIMMYT | 6925 (39) | 3147 (107) | 9733 | CIMMYT | 6369 (87) | 2828 (136) | 9705 | CIMMYT | 5506 (136) | 2066 (147) |
| 9618 | CIMMYT | 6902 (40) | 3234 (97) | 9735 | CIMMYT | 6367 (88) | 3833 (17) | 9662 | CIMMYT | 5504 (137) | 2998 (126) |
| 9651 | CIMMYT | 6874 (41) | 3404 (68) | 9700 | CIMMYT | 6353 (89) | 3682 (28) | 9732 | CIMMYT | 5452 (138) | 2705 (141) |
| 9636 | CIMMYT | 6822 (42) | 3574 (38) | 9694 | CIMMYT | 6329 (90) | 3198 (101) | 9704 | CIMMYT | 5391 (139) | 3420 (63) |
| 9641 | CIMMYT | 6822 (42) | 3528 (46) | 9697 | CIMMYT | 6310 (91) | 3161 (104) | 9684 | CIMMYT | 5383 (140) | 3302 (84) |
| 9616 | CIMMYT | 6819 (43) | 3183 (103) | 9602 | CIMMYT | 6301 (92) | 2668 (142) | 9675 | CIMMYT | 5304 (141) | 3150 (106) |
| 9644 | CIMMYT | 6816 (44) | 3791 (22) | 9710 | CIMMYT | 6296 (93) | 3691 (27) | 9665 | CIMMYT | 5165 (142) | 3032 (123) |
| 9725 | CIMMYT | 6767 (45) | 3758 (23) | 9715 | CIMMYT | 6296 (93) | 3278 (91) | 9670 | CIMMYT | 5146 (143) | 2796 (138) |
| 9719 | CIMMYT | 6764 (46) | 3411 (67) | 9689 | CIMMYT | 6292 (94) | 4191 (4) | 9660 | CIMMYT | 5140 (144) | 3022 (124) |
| 9623 | CIMMYT | 6748 (47) | 3351 (75) | 9619 | CIMMYT | 6278 (95) | 2287 (146) | 9679 | CIMMYT | 5051 (145) | 3012 (125) |
| 9722 | CIMMYT | 6729 (48) | 3422 (62) | 9731 | CIMMYT | 6274 (96) | 2819 (137) | 9740 | CIMMYT | 4303 (146) | 2480 (145) |
| 9607 | CIMMYT | 6719 (49) | 3451 (61) | 9739 | CIMMYT | 6269 (97) | 3665 (30) | 9664 | CIMMYT | 3870 (147) | 2525 (143) |
| 9681 | CIMMYT | 6711 (50) | 3285 (88) | 9640 | CIMMYT | 6235 (98) | 4143 (6) |
Ranking order of the mean grain yield of genotypes in no-stress (T1) conditions and drought-stress conditions (T2) is shown in brackets.
Under stress conditions, 26 genotypes achieved grain yields 10% higher than that of ‘Xinchun 6’ as well as high DYI. These genotypes were 9643, 9646, 9612, 9689, 9642, 9640, 9692, 9729, 9698, 9638 9645, 9606, 9650, 9720, 9627, 9699, 9735, 9611, 9730, 9637, ‘Xinchun 29’, 9644, 9725, 9655, ‘Xinchun 33’ and 9726. Of these superior genotypes, only ‘Xinchun 29’ and ‘Xinchun 33’ were local varieties, while the others were from CIMMYT. CIMMYT genotypes 9660, 9679, 9662, 9716, 9691, 9661, 9676, 9634, 9674, 9620, 9678, 9668, 9733, 9731, 9670, 9673, 9677, 9732, 9602, 9664, 9617, 9740, 9619, and 9705 had lower yields, with values 10% and 9.5% less than the mean grain yield of the 152 genotypes and the check variety, ‘Xinchun 6’, respectively.
Higher DYI, WYI and YH-WUEI values >1.05 were observed in 24 genotypes, consisting of twenty-one CIMMYTY nursery lines and three local varieties (Table 4). These genotypes showed high grain yield potential and good yield stability under both no-stress and drought-stress conditions. Acceptable plant height was considered to be between 70 and 90 cm, which was adapted to and acceptable for local irrigated wheat production. Ten genotypes, ‘Xinchun 29’, 9692, 9638, 9606, ‘Xinchun 23’, 9639, ‘Xinchun 11’, 9729, 9746 and 9730 exhibited acceptable plant heights and had high and stable yield under both no-stress and stress conditions. Twenty-four CIMMYT genotypes were classified as exhibiting low water-use efficiency (DYI < 0.95, WYI < 0.95, YH-WUEI < 0.95) and they were 9675, 9663, 9659, 9711, 9712, 9713, 9672, 9665, 9660, 9679, 9662, 9716, 9661, 9674, 9620, 9678, 9670, 9673, 9677, 9732, 9664, 9617, 9740 and 9705. The other 106 genotypes were classified as exhibiting moderate water-use efficiency. Among them, CIMMYT genotypes 9630, 9741, 9671, 9625 and 9616 showed high grain yield under no-stress conditions (WYI > 1.05), but low grain yield under drought-stress conditions (DYI < 0.95), indicating that they were very sensitive to drought-stress. CIMMYT genotypes 9702 and 9669 showed high grain yield and drought yield index (DYI > 1.05) under drought-stress conditions. However, low grain yield and WYI (<0.95) were observed for these two genotypes under no-stress conditions.
Table 4.
Screening of 24 genotypes with the highest drought yield index (DYI), water yield index (WYI) and yield high water use efficiency index (YH-WUEI) over 1.05 averaged over the two growing seasons
| Genotype | Origin | Plant height, cm in T1 | Plant height, cm in T2 | GY in T1, kg/ha | GY in T2, kg/ha | DYI | WYI | YHWUEI |
|---|---|---|---|---|---|---|---|---|
| 9645 | CIMMYT | 91.74 | 71.64 | 7995 | 3881 | 1.15 | 1.24 | 1.20 |
| Xinchun29 | Local | 84.67 | 68.62 | 7635 | 3793 | 1.13 | 1.19 | 1.16 |
| 9611 | CIMMYT | 95.98 | 67.76 | 7622 | 3815 | 1.14 | 1.18 | 1.16 |
| 9650 | CIMMYT | 95.15 | 71.07 | 7574 | 3873 | 1.15 | 1.18 | 1.16 |
| 9637 | CIMMYT | 91.25 | 72.82 | 7573 | 3798 | 1.13 | 1.18 | 1.15 |
| 9692 | CIMMYT | 85.18 | 64.79 | 7322 | 4124 | 1.23 | 1.14 | 1.18 |
| 9638 | CIMMYT | 87.09 | 64.86 | 7315 | 3894 | 1.16 | 1.14 | 1.15 |
| 9655 | CIMMYT | 91.69 | 68.89 | 7300 | 3704 | 1.10 | 1.13 | 1.12 |
| 9606 | CIMMYT | 85.38 | 71.68 | 7267 | 3875 | 1.15 | 1.13 | 1.14 |
| Xinchun23 | Local | 89.61 | 69.56 | 7264 | 3621 | 1.08 | 1.13 | 1.10 |
| 9639 | CIMMYT | 85.82 | 67.04 | 7259 | 3605 | 1.07 | 1.13 | 1.10 |
| Xinchun11 | Local | 74.61 | 58.11 | 7149 | 3591 | 1.07 | 1.11 | 1.09 |
| 9729 | CIMMYT | 90.01 | 75.29 | 7103 | 4052 | 1.21 | 1.10 | 1.15 |
| 9746 | CIMMYT | 83.90 | 68.52 | 7085 | 3577 | 1.06 | 1.10 | 1.08 |
| 9622 | CIMMYT | 100.75 | 81.98 | 7071 | 3629 | 1.08 | 1.10 | 1.09 |
| 9730 | CIMMYT | 88.87 | 70.08 | 7054 | 3808 | 1.13 | 1.10 | 1.11 |
| 9642 | CIMMYT | 77.20 | 64.32 | 7050 | 4169 | 1.24 | 1.09 | 1.17 |
| 9688 | CIMMYT | 88.26 | 69.58 | 7038 | 3553 | 1.06 | 1.09 | 1.07 |
| 9646 | CIMMYT | 85.87 | 73.90 | 7000 | 4262 | 1.27 | 1.09 | 1.18 |
| 9698 | CIMMYT | 88.63 | 67.16 | 6942 | 3901 | 1.16 | 1.08 | 1.12 |
| 9627 | CIMMYT | 90.66 | 65.97 | 6926 | 3853 | 1.15 | 1.08 | 1.11 |
| 9636 | CIMMYT | 86.39 | 68.24 | 6822 | 3574 | 1.06 | 1.06 | 1.06 |
| 9644 | CIMMYT | 90.90 | 74.93 | 6816 | 3791 | 1.13 | 1.06 | 1.09 |
| 9725 | CIMMYT | 80.22 | 69.65 | 6767 | 3758 | 1.12 | 1.05 | 1.08 |
Genotypes are listed according to their ranking for mean grain yield under no-stress conditions.
Relationships between grain yield and agronomic traits
Significant negative correlations were obtained between grain yield and CT at the mid-grain filling stage, with coefficients of −0.440 and −0.494 (p < 0.001) for no-stress and drought-stress conditions, respectively (Table 5). At the mid-grain filling stage, CT also correlated significantly negatively with plant height, days to heading, spikelets per spike, spike length, grain number per spike and grain weight per spike under either irrigation regimes, and with thousand-grain weight but only under drought-stress conditions.
Table 5.
Pearson’ correlation coefficients under no-stress conditions (A) and drought-stress conditions (B) averaged over 2011 and 2012
| (A) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| GY | TGW | SPS | SL | SPAD-GF | SPAD-F | GP | PH | NDVI-GF | NDVI-F | NDVI-E | HD | GWPS | GNPS | |
| TGW | 0.450*** | |||||||||||||
| SPS | 0.512*** | NS | ||||||||||||
| SL | 0.602*** | 0.229** | 0.824*** | |||||||||||
| SPAD-GF | NS | 0.284*** | NS | NS | ||||||||||
| SPAD-F | NS | 0.301*** | NS | NS | 0.469*** | |||||||||
| GP | 0.413*** | 0.546*** | NS | NS | NS | NS | ||||||||
| PH | 0.740*** | 0.389*** | 0.651*** | 0.703*** | NS | NS | 0.354*** | |||||||
| NDVI-GF | 0.249** | NS | NS | NS | NS | NS | NS | NS | ||||||
| NDVI-F | 0.543*** | NS | 0.656*** | 0.692*** | NS | NS | NS | 0.645*** | 0.449*** | |||||
| NDVI-E | 0.551*** | 0.282*** | 0.551*** | 0.576*** | NS | NS | NS | 0.604*** | NS | 0.637*** | ||||
| HD | 0.541*** | NS | 0.709*** | 0.734*** | NS | NS | NS | 0.728*** | 0.339*** | 0.770*** | 0.639*** | |||
| GWPS | 0.567*** | 0.397*** | 0.642*** | 0.734*** | 0.162* | 0.171* | NS | 0.512*** | NS | 0.471*** | 0.382*** | 0.491*** | ||
| GNPS | 0.316*** | −0.198* | 0.693*** | 0.683*** | NS | NS | −0.1791* | 0.342*** | NS | 0.409*** | 0.238** | 0.509*** | 0.716*** | |
| CT-GF | −0.440*** | NS | −0.424*** | −0.477*** | −0.209** | NS | NS | −0.532*** | −0.275*** | −0.535*** | −0.439*** | −0.512*** | −0.291*** | −0.222** |
| (B) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| GY | TGW | SPS | SL | SPAD-GF | SPAD-F | GP | PH | NDVI-GF | NDVI-F | NDVI-E | HD | GWPS | GNPS | |
| TGW | 0.379*** | |||||||||||||
| SPS | 0.301*** | 0.369*** | ||||||||||||
| SL | 0.300*** | 0.462*** | 0.863*** | |||||||||||
| SPAD-GF | 0.173* | 0.519*** | 0.396*** | 0.270*** | ||||||||||
| SPAD-F | NS | NS | −0.242** | −0.251** | NS | |||||||||
| GP | 0.489*** | 0.698*** | 0.219** | 0.2448** | 0.388*** | NS | ||||||||
| PH | 0.494*** | 0.334*** | 0.440*** | 0.447*** | NS | −0.241** | 0.386*** | |||||||
| NDVI-GF | 0.222** | 0.348*** | NS | NS | 0.468*** | NS | 0.387*** | NS | ||||||
| NDVI-F | 0.442*** | 0.492*** | 0.545*** | 0.539*** | 0.369*** | −0.287*** | 0.417*** | 0.426*** | 0.474*** | |||||
| NDVI-E | 0.255** | 0.484*** | 0.524*** | 0.583*** | 0.264** | NS | 0.300*** | 0.436*** | NS | 0.506*** | ||||
| HD | 0.321*** | 0.562*** | 0.736*** | 0.687*** | 0.503*** | −0.275*** | 0.436*** | 0.335*** | 0.378*** | 0.631*** | 0.560*** | |||
| GWPS | 0.482*** | 0.463*** | 0.590*** | 0.634*** | 0.385*** | NS | 0.411*** | 0.252** | 0.249** | 0.424*** | 0.252** | 0.435*** | ||
| GNPS | 0.268*** | −0.189* | 0.422*** | 0.377*** | NS | NS | NS | NS | NS | NS | NS | NS | 0.650*** | |
| CT-GF | −0.494*** | −0.290*** | −0.492*** | −0.393*** | −0.265*** | NS | −0.228** | −0.274*** | −0.276*** | −0.476*** | −0.309*** | −0.471*** | −0.393*** | −0.294*** |
Significant at the 0.05 probability level.
Significant at the 0.01 probability level.
Significant at the 0.001 probability level.
NS, non-significant at the 0.05 probability level.
Grain yield was positively correlated with NDVI at the jointing (correlation coefficients of 0.551 [p < 0.001]), flowering (r = 0.543; p < 0.001) and mid-grain filling stages (r = 0.249; p < 0.001) in each of the two irrigation regimes. The correlation coefficients were more significant at the jointing and flowering stages than at the mid-grain filling stage under no-stress conditions. However, under drought-stress conditions, the correlation between NDVI and grain yield at the flowering stage was more significant than that at the jointing and mid-grain filling stages. At the jointing stage, NDVI was significantly correlated with plant height, days to heading, thousand-grain weight, spike length, spikelet number per spike, grain number per spike and grain weight per spike under either irrigation regimes, and with grain plumpness under only drought-stress conditions. At the flowering stage, NDVI was significantly correlated with plant height, days to heading, spike length, spikelet number per spike and grain weight per spike under either irrigation regimes, and with thousand-grain weight and grain plumpness under drought-stress conditions. At the mid-grain filling stage, NDVI was significantly correlated with thousand-grain weight, grain plumpness, grain weight per spike and days to heading under drought-stress conditions. However, at the mid-grain filling stage, NDVI was correlated with days to heading only under no-stress conditions.
At the mid-grain filling stage, chlorophyll content (SPAD) was positively correlated with grain yield under drought-stress conditions. However, no correlation was observed between SPAD and grain yield at the flowering stage under drought-stress nor at the flowering or mid-grain filling stages under no-stress conditions. At the mid-grain filling stage, SPAD was also positively correlated with thousand-grain weight, grain weight per spike and days to heading. Significant positive correlations were obtained between grain yield and the traits plant height, days to heading, spike length, spikelet number per spike, grain number per spike, grain weight per spike, grain plumpness and thousand-grain weight.
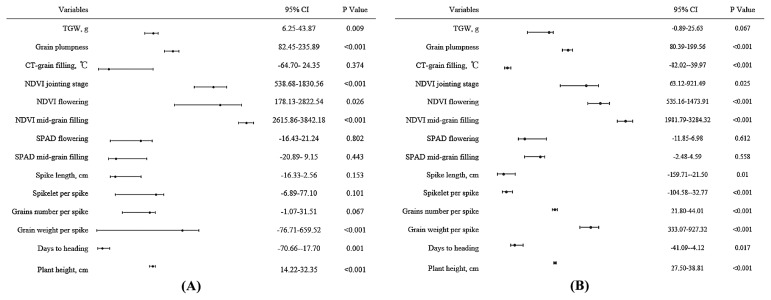
Multiple linear regression analysis indicated that grain yield was significantly (p < 0.001) related to grain weight per spike, plant height and grain plumpness under the no-stress conditions (Fig. 2A). The three principal factors, grain weight per spike (r = 0.318; p < 0.001), plant height (r = 0.549; p < 0.001) and grain plumpness (r = 0.172; p < 0.001) explained 61.8% of the total phenotypic variation in grain yield under no-stress conditions in 2011 and 2012. Multiple linear regression analysis also found significantly (p < 0.001) relationships between grain yield and CT, plant height and grain plumpness under drought-stress conditions (Fig. 2B). The three principal factors, CT at the grain filling stage (r = 0.244; p < 0.001), plant height (r = 0.244; p < 0.001) and grain plumpness (r = 0.239; p < 0.001) together explained 44.8% of the total phenotypic variation in grain yield under drought-stress conditions in 2011 and 2012.
Fig. 2.
Regression analysis between grain yield and other traits under no-stress (A) and drought-stress (B) in 2011 and 2012.
Discussion
CIMMYT hexaploid spring wheat germplasm has played a global role in assisting wheat breeding with respect to high yield potential and quality improvement (Bhatta et al. 2018, Wang et al. 2009, Zhang et al. 2011). We evaluated the drought-stress response of a set of WPHYSGP lines, introduced from CIMMYT, under the two different irrigation regimes during the 2011 and 2012 growing seasons. Based on yield, DYI, WYI, and YH-WUEI, 24 elite genotypes with high and stable yield under drought-stress and no-stress conditions were identified. In view of the demand for varieties of appropriate suitable plant height, 10 of these genotypes can be recommended as core parents for breeding for improved drought response in Xinjiang, China. The DYI, WYI and YH-WUEI were shown to be very useful for evaluating drought tolerance and to be powerful in identifying genotypes with high yield potential and high water-use efficiency as well (Li et al. 2006, Wu et al. 2005). Interestingly, three out of seven (43%) locally bred varieties were included in the 10 selected superior lines, compared with seven out of 145 (4.8%) CIMMYT lines.
In the present study, drought-stress was associated with a noticeable decrease in grain yield, plant height, grain number per spike, grain weight per spike, thousand-grain weight, grain plumpness, NDVI at the flowering and grain filling stages and chlorophyll content at the grain filling stage, and to an increase in CT and chlorophyll content at the flowering stage. Plant height and grain weight per plant generally exhibit above-average heritability and have been shown to be very sensitive to drought-stress, so could be recommended as selection criteria for drought-response improvement (Chen et al. 2012, Christopher et al. 2016). However, another study also showed that the yield components number of kernels per spike and thousand-grain weight, and especially grain yield were even more sensitive to drought-stress than was plant height (Denčić et al. 2000, Mwadzingeni et al. 2016). Improvements in grain yield in recent years were often accompanied by increased thousand-grain weight (Feng et al. 2018, Singh et al. 2007, Stallmann et al. 2018). Our study showed that grain weight per spike, grain yield, NDVI at the flowering and grain filling stages, grain plumpness, grain number per spike, thousand-grain weight, plant height and CT were more sensitive to drought-stress than were NDVI at the jointing stage, spikelet number per spike, spike length and days to heading. Plants were watered at both jointing and heading stages of drought-stress, and spike development was completed before the heading stage, so drought-stress had little effect on NDVI at the jointing stage, spikelet number per spike, spike length and days to heading. The drought stress affected the growth of wheat after flowering stage, especially grain filling, thereby resulting in more change in grain weight per spike, grain yield, NDVI at the flowering and grain filling stages, grain plumpness, grain number per spike, thousand-grain weight, plant height and CT. Grain yield was negatively correlated with CT and positively correlated with plant height, grain number per spike, grain weight per spike, thousand-grain weight, grain plumpness, and NDVI. Low CT and high NDVI reflected better growth potential. The grain number per spike, grain weight per spike, thousand-grain weight and grain plumpness all reflected results of grain filling, so they were positively correlated with grain yield. We also found that grain weight per spike, plant height and grain plumpness were the three principal factors related to yield and could explain 61.8% of the total phenotypic variation of grain yield under no-stress conditions, so that grain weight per spike and grain plumpness could be used as selection indicator for high yield under stress conditions wheat breeding. Plant height was a key parameter affecting lodging and thus grain yield and grain quality, so the appropriate plant height was an important indicator for wheat breeding.
Many studies have reported that chlorophyll content (measured here as SPAD) was positively correlated with yield under drought-stress conditions (Hamblin et al. 2014, Yıldırım et al. 2010). In our study, chlorophyll content at the grain filling stage was positively associated with yield under drought-stress conditions but not under no-stress conditions. Furthermore, chlorophyll content at the flowering stage was not associated with grain yield under either irrigation regime. The reason for this may be the result of the SPAD measurement affected by leaf glaucousness.
Cooler canopies has been associated with increased stomatal conductance and increased grain yield under irrigated condition (Fischer et al. 1998) and with increased rooting depth and greater ability to extract moisture from deeper soil profiles under drought-stress conditions (Lopes and Reynolds 2010, Rutkoski et al. 2016). CT, dry weight stem−1, grains spike−1 and water-soluble carbohydrate concentration had high mean heritability and were recommended for use in selection for stress tolerance in plant breeding programs (Rattey et al. 2011, Stallmann et al. 2018). Lower CT was a trait that was proposed for breeding and early-stage selection, aimed at increasing genetic gain for grain yield in water-limited environments (Reynolds et al. 2009, Rutkoski et al. 2016). Our study confirmed that CT was negatively associated with grain yield under well-watered conditions as well as under drought-stress conditions. CT, especially at the grain filling stage, plant height and grain plumpness were the three principal factors associated with grain yield under drought-stress conditions.
NDVI has been used as a criterion to estimate relative biomass before heading (Reynolds et al. 2007) and the relative character of “stay-green” after flowering (Christopher et al. 2014). “Stay-green” traits may provide cumulative effects, together with other traits, which improve adaptation under stress conditions (Christopher et al. 2016, Lopes and Reynolds 2012). NDVI showed a positive relationship with grain yield under well-irrigated condition, with an even stronger association with grain yield under drought conditions (Christopher et al. 2016). In the present study, we found that NDVI in the jointing stage, the flowering stage and the mid-grain filling stage were all significantly positively correlated with yield under both no-stress and stress conditions.
Wheat drought tolerance is a complex quantitative trait and it is difficult to identify a single trait index which reflects both wheat yield potential and drought tolerance (Chen et al. 2012). Based on our study, plant height, grain number per spike, grain weight per spike, thousand-grain weight, grain plumpness, NDVI and CT could be recommended as indicators of drought tolerance improvement in spring wheat. Especially when breeding for improved varieties for cultivation in irrigated regions, more attention should be paid to screening for low values of CT and high values of grain weight per spike and grain plumpness, in combination with appropriate plant height in spring wheat breeding programs.
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China (Grant No. 2016YFD0300110), the GCP project (Grant No. G7010.02.01-5), the basic research funding of non-profit scientific research institutes in the Xinjiang Uygur Autonomous Region (Grant No. KY2017066), and a project of the Key Laboratory of Crop Ecophysiology and Farming System in Desert Oasis Region, Ministry of Agriculture (Grant No. 25107020-201605). We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Abbreviations
- CT
canopy temperature
- DI
drought resistance index
- DYI
drought yield index
- GNPS
grain number per spike
- GP
grain plumpness
- GWPS
grain weight per spike
- GY
grain yield
- HD
days to heading
- NDVI
normalized difference vegetation index
- PH
plant height
- SL
spike length
- SPAD
soil and plant analyzer development
- SPS
spikelet number per spike
- SSI
stress susceptibility index
- TGW
thousand-grain weight
- T1
no-stress conditions
- T2
drought-stress conditions
- WPHYSGP
Wheat Physiological Germplasm Screening Nursery
- WYI
water yield index
- YH-WUEI
yield high water-use efficiency index
Literature Cited
- Araus, J.L., Slafer, G.A., Reynolds, M.P. and Royo, C. (2002) Plant breeding and drought in C3 cereals: what should we breed for? Ann. Bot. 89: 925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat, M.N., Saleh, M., Aldoss, A.A., Moustafa, K.A., Elshafei, A.A. and Alqurainy, F.H. (2015) Identification of new SSR markers linked to leaf chlorophyll content, flag leaf senescence and cell membrane stability traits in wheat under water stressed condition. Acta Biol. Hung. 66: 93–102. [DOI] [PubMed] [Google Scholar]
- Bhatta, M., Morgounov, A., Belamkar, V., Poland, J. and Baenziger, P.S. (2018) Unlocking the novel genetic diversity and population structure of synthetic Hexaploid wheat. BMC Genomics 19: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogale, A., Tesfaye, K. and Geleto, T. (2011) Morphological and physiological attributes associated to drought tolerance of Ethiopian durum wheat genotypes under water deficit. J. Bindivers. Environ. Sci. 1: 22–36. [Google Scholar]
- Cabrera-Bosquet, L., Grieder, C., Alvarez Prado, S., Sánchez, C. and Araus, J.L. (2017) Kernel δ18O reflects changes in apical dominance and plant transpiration in tropical maize. J. Agron. Crop Sci. 203: 277–285. [Google Scholar]
- Chen, X., Min, D., Yasir, T.A. and Hu, Y.G. (2012) Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). Field Crops Res. 137: 195–201. [Google Scholar]
- Cheng, L.X., Wang, Y.P., He, Q., Li, H.J., Zhang, X.J. and Zhang, F. (2016) Comparative proteomics illustrates the complexity of drought resistance mechanisms in two wheat (Triticum aestivum L.) cultivars under dehydration and rehydration. BMC Plant Biol. 16: 188–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinoy, J.J. (1961) Physiology of drought resistance in wheat. II. Evaluation of drought resistance in eight varieties of wheat on the basis of growth analysis. J. Exp. Bot. 16: 131–139. [Google Scholar]
- Christopher, J.T., Veyradier, M., Borrell, A.K., Harvey, G., Fletcher, S. and Chenu, K. (2014) Phenotyping novel stay-green traits to capture genetic variation in senescence dynamics. Funct. Plant Biol. 41: 1035–1048. [DOI] [PubMed] [Google Scholar]
- Christopher, J.T., Christopher, M.J., Borrell, A.K., Fletcher, S. and Chenu, K. (2016) Stay-green traits to improve wheat adaptation in well-watered and water-limited environments. J. Exp. Bot. 67: 5159–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer, D.P. (2005) Agriculture in the developing world: Connecting innovations in plant research to downstream applications. Proc. Natl. Acad. Sci. USA 102: 15739–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denčić, S., Kastori, R., Kobiljski, B. and Duggan, B. (2000) Evaluation of grain yield and its components in wheat cultivars and landraces under near optimal and drought conditions. Euphytica 113: 43–52. [Google Scholar]
- Feng, F., Han, Y., Wang, S., Yin, S., Peng, Z., Zhou, M., Gao, W., Wen, X., Qin, X. and Siddique, K.H.M. (2018) The effect of grain position on genetic improvement of grain number and thousand grain weight in winter wheat in north China. Front Plant Sci 9: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, R.A., Rees, D., Sayre, K.D., Lu, Z.M., Condon, A.G. and Saavedra, A.L. (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 38: 1467–1475. [Google Scholar]
- Hamblin, J., Stefanova, K. and Angessa, T.T. (2014) Variation in chlorophyll content per unit leaf area in spring wheat and implications for selection in segregating material. PLoS ONE 9: e92529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatoi, W.A., Baloch, M.J., Kumbhar, M.B., Khan, N.U. and Kerio, M.I. (2011) Effect of water stress on physiological and yield parameters at anthesis stage in elite spring wheat cultivars. Sarhad J. Agric. 27: 59–65. [Google Scholar]
- Kadioglu, A., Terzi, R., Saruhan, N. and Saglam, A. (2012) Current advances in the investigation of leaf rolling caused by biotic and abiotic stress factors. Plant Sci. 182: 42–48. [DOI] [PubMed] [Google Scholar]
- Lan, J.S., Hu, F.S. and Zhang, J.R. (1990) The concept and statistical method of drought resistance index in crops. Acta Agriculturae Boreali-Sinica 5: 20–25. [Google Scholar]
- Lan, J.S. (1998) Comparison of evaluating methods for agronomic drought resistance in crops. Acta Agriculturae Boreali-occidentalis Sinica 7: 85–87. [Google Scholar]
- Levitt, J. (1980) Responses of plants to environmental stresses. Academic Press; pp. 3642–3645. [Google Scholar]
- Li, J.F., Lu, Y.H., Wu, Z.L., Cai, X.L., Fan, L., Fan, Z.R., Wang, Z.Y. and Zhang, Y.Q. (2006) The character on drought resistance and high water use efficiency of spring wheat in Xinjiang. Xinjiang Agricultural Sciences 43: 253–259. [Google Scholar]
- Li, R.X., Huang, C.L., Du, Z.Q. and Liu, Y.F. (2015) Trend and abrupt change of temperature and precipitation in Xinjiang over three decades. Chinese Agricultural Science Bulletin 31: 187–192. [Google Scholar]
- Lopes, M.S. and Reynolds, M.P. (2010) Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Funct. Plant Biol. 37: 147–156. [Google Scholar]
- Lopes, M.S. and Reynolds, M.P. (2012) Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J. Exp. Bot. 63: 3789–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwadzingeni, L., Shimelis, H., Tesfay, S. and Tsilo, T.J. (2016) Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front Plant Sci 7: 1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwadzingeni, L., Shimelis, H., Rees, D.J. and Tsilo, T.J. (2017) Genome-wide association analysis of agronomic traits in wheat under drought-stressed and non-stressed conditions. PLoS ONE 12: e0171692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz, A., Farooq, M., Cheema, S.A., Yasmeen, A. and Wahid, A. (2013) Stay green character at grain filling ensures resistance against terminal drought in wheat. Int. J. Agric. Biol. 15: 1560–8530. [Google Scholar]
- Nouriganbalani, A., Nouriganbalani, G. and Hassanpanah, D. (2009) Effects of drought stress condition on the yield and yield components of advanced wheat genotypes in Ardabil, Iran. J. Food Agric. Environ. 7: 228–234. [Google Scholar]
- Pinto, R.S., Reynolds, M.P., Pinto, R.S. and Reynolds, M.P. (2015) Common genetic basis for canopy temperature depression under heat and drought stress associated with optimized root distribution in bread wheat. Theor. Appl. Genet. 128: 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattey, A.R., Shorter, R. and Chapman, S.C. (2011) Evaluation of CIMMYT conventional and synthetic spring wheat germplasm in rainfed sub-tropical environments. II. Grain yield components and physiological traits. Field Crops Res. 124: 195–204. [Google Scholar]
- Rebetzke, G.J., Condon, A.G., Farquhar, G.D., Appels, R. and Richards, R.A. (2008) Quantitative trait loci for carbon isotope discrimination are repeatable across environments and wheat mapping populations. Theor. Appl. Genet. 118: 123–137. [DOI] [PubMed] [Google Scholar]
- Rebetzke, G.J., Rattey, A.R., Farquhar, G.D., Richards, R.A. and Condon, A.G. (2013) Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in wheat. Funct. Plant Biol. 40: 14–33. [DOI] [PubMed] [Google Scholar]
- Rebetzke, G.J., Bonnett, D.G. and Reynolds, M.P. (2016) Awns reduce grain number to increase grain size and harvestable yield in irrigated and rainfed spring wheat. J. Exp. Bot. 67: 2573–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, M., Dreccer, F. and Trethowan, R. (2007) Drought-adaptive traits derived from wheat wild relatives and landraces. J. Exp. Bot. 58: 177–186. [DOI] [PubMed] [Google Scholar]
- Reynolds, M., Manes, Y., Izanloo, A. and Langridge, P. (2009) Phenotyping approaches for physiological breeding and gene discovery in wheat. Ann. Appl. Biol. 155: 309–320. [Google Scholar]
- Reynolds, M.P., Balota, M., Delgado, M., Amani, I. and Fischer, R.A. (1994) Physiological and morphological traits associated with spring wheat yield under hot, irrigated conditions. Aust. J. Plant Physiol. 21: 717–730. [Google Scholar]
- Richards, R.A. and Lukacs, Z. (2002) Seedling vigour in wheat—sources of variation for genetic and agronomic improvement. Aust. J. Agric. Res. 53: 41–50. [Google Scholar]
- Rizza, F., Badeck, F.W., Cattivelli, L., Lidestri, O., Di Fonzo, N. and Stanca, A.M. (2004) Use of a water stress index to identify barley genotypes adapted to rainfed and irrigated conditions. Crop Sci. 44: 2127–2137. [Google Scholar]
- Rutkoski, J., Poland, J., Mondal, S., Autrique, E., Perez, L.G., Crossa, J., Reynolds, M. and Singh, R. (2016) Canopy temperature and vegetation indices from high-throughput phenotyping improve accuracy of pedigree and genomic selection for grain yield in wheat. G3 (Bethesda) 6: 2799–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R.P., Huerta-Espino, J., Sharma, R., Joshi, A.K. and Trethowan, R. (2007) High yielding spring bread wheat germplasm for global irrigated and rainfed production systems. Euphytica 157: 351–363. [Google Scholar]
- Stallmann, J., Schweiger, R. and Muller, C. (2018) Effects of continuous versus pulsed drought stress on physiology and growth of wheat. Plant Biol. (Stuttg) 20: 1005–1013. [DOI] [PubMed] [Google Scholar]
- Trethowan, R.M., Reynolds, M., Buck, H.T., Nisi, J.E. and Salomón, N. (2007) Drought resistance: genetic approaches for improving productivity under stress. pp. 895–901. [Google Scholar]
- Waldron, L.R. (1933) Yield and protein content of hard red spring wheat under condition of high temperature and low moisture. JAR 47: 129–146. [Google Scholar]
- Wang, Z.Y., Fan, Z.R., Wu, Z.L. and Chen, Y. (2009) Utilization of CIMMYT gemplasm in Xinjiang. China Seed Industry: 12–13. [Google Scholar]
- Willick, I.R., Lahlali, R., Vijayan, P., Muir, D., Karunakaran, C. and Tanino, K.K. (2018) Wheat flag leaf epicuticular wax morphology and composition in response to moderate drought stress are revealed by SEM, FTIR-ATR and synchrotron X-ray spectroscopy. Physiol. Plant. 162: 316–332. [DOI] [PubMed] [Google Scholar]
- Wu, Z.L., Huang, G.H., Fan, Z.R., Li, J.F. and Cai, X.L. (2005) The approach to the screening method of wheat germplasm with high water use efficiency. Journal of Triticeae Crops 25: 143–146. [Google Scholar]
- Yıldırım, M., Kılıç, H., Kendal, E. and Karahan, T. (2010) Applicability of chlorophyll meter readings as yield predictor in durum wheat. J. Plant Nutr. 34: 151–164. [Google Scholar]
- Zhang, Y., Li, S.-Z., Wu, Z.-L., Yang, W.-X., Yu, Y.-X., Xia, X.-C., He, Z.-H. and Zhao, D.-H. (2011) Contribution of CIMMYT wheat germplasm to genetic improvement of grain yield in spring wheat of Sichuan, Yunnan, Gansu, and Xinjiang provinces. Acta Agronomica Sinica 37: 1752–1762. [Google Scholar]
- Zhang, Z., Liu, X., Wang, X., Zhou, M., Zhou, X., Ye, X. and Wei, X. (2012) An R2R3 MYB transcription factor in wheat, TaPIMP1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense- and stress-related genes. New Phytol. 196: 1155–1170. [DOI] [PubMed] [Google Scholar]