Abstract
A 3-year-old female was treated with neoadjuvant chemotherapy (NACT) for a PRETEXT IV hepatoblastoma. POST-TEXT IV findings merited a liver transplant (LT), but multiple limitations precluded it. The initial future liver remnant (FLR) was small (21.3%). Monosegment 6 ALPPS was a rational approach given that the inferior right hepatic vein (IRHV) provided adequate outflow. Therefore, the procedure was performed after parental informed consent. On PO15 of the first stage, FLR had reached 32.6% and then the second-stage was carried out. The patient was discharged on POD 31, and she is about to reach the 5-year disease-free survival milestone.
INTRODUCTION
When major hepatic resections are considered, the FLR is a predictor of post-hepatectomy liver failure (PHLF), the most severe complication in major liver surgery [1]. The ALPPS technique consists of portal vein ligation and transection of the liver parenchyma in a first stage. FLR is customarily evaluated after 7–21 days, and if it is considered adequate, then the hepatectomy is performed. ALPPS has made monosegmental liver remnant growth possible in a relative short time [2] but reports of pediatric cases are rather scarce. In adults, monosegmental ALPPS has shown promising results—in terms of resectability—but severe morbidity is an important problem that has to be considered during patient selection [2, 3].
Patients with unresectable pediatric hepatoblastoma after NACT are recommended to receive liver transplant [4] but donor shortage is a significant problem in many institutions. This paper illustrates, to the best of our knowledge, the first case of a successful pediatric monosegmental ALPPS procedure for a PRETEXT IV hepatoblastoma, with a near 5-year disease-free survival.
CASE REPORT
Patient selection
A 3-year-old female was admitted to a pediatric hospital with nausea, vomiting and hepatomegaly. A percutaneous tumor biopsy established the diagnosis of hepatoblastoma. Shortly after the biopsy, the patient underwent emergency laparotomy and liver packing due to hemoperitoneum, once she was hemodynamically stable, she was referred to our center and admitted to the ICU in respiratory distress that required mechanic ventilation.
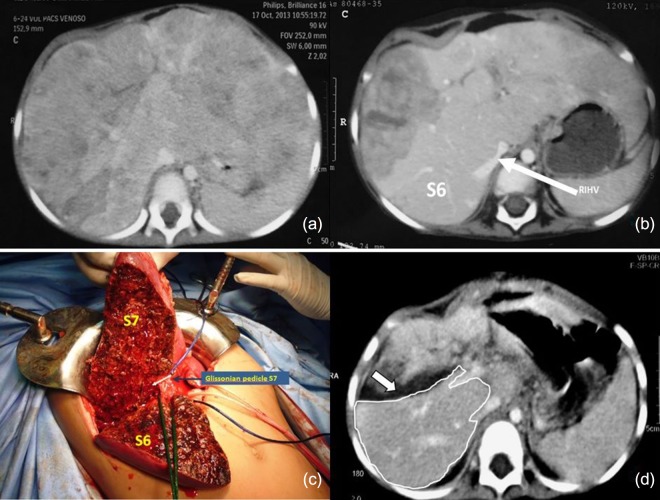
Tumor staging revealed a PRETEXT IV hepatoblastoma (Fig. 1a) with Alpha-fetoprotein (AFP) levels of 1 059,240 ng/ml. After 15 days in the ICU, when the patient’s condition had improved, we initiated NACT (3 courses of Doxorubicin and Carboplatin). At its completion, the AFP levels had decreased to 1 573 ng/ml and a follow-up CT scan showed tumor involvement of segments 1-7 except for s6 (POST-TEXT IV). The latter segment was vascularized through the IRHV but it was deemed insufficient (FLR 21.34%) (Fig. 1b). The absence of a liver donor within reasonable time prompted a multidisciplinary committee meeting that approved an ALPPS procedure after securing informed consent from the parents.
Figure 1:
(a) Tumor staging CT (PRETEXT IV), shows all liver sections being invaded by tumor (b) The CT scan post NACT indicates partial tumor response, with persistence of tumor compromising the four sections (POST-TEXT IV). The IRHV is identified (white arrow). No tumor is visible in s6 (c) First stage of ALPPS, the posterior pedicle and its branches were tagged and s7 pedicle clipped (d) FLR on PO15 is outlined in white. The white arrow shows the transection plane.
OPERATIVE TECHNIQUE
The patient underwent the first stage on April 9, 2014. An initial exploratory laparotomy confirmed absence of extrahepatic disease, and we also found firm adhesions as result of the hemoperitoneum caused by the biopsy that required the laparotomy with liver packing. All segments were found to have macroscopic disease except for segment 6 (Fig. 1c). A careful hilar and pedicle dissection was executed, with particular attention being paid to the posterior division and inferior hepatic vein. The left portal vein was ligated, and the other elements of the liver pedicle (including the anterior branch of the right portal vein) were tagged for the second stage. Transection was carried out with a Cavitron Ultrasonic Surgical Aspirator (CUSA) with the intention of identifying and preserving the segment six pedicle. The s7 pedicle was then clipped with the expectation of segment necrosis, which was confirmed in a postoperative CT scan. An estimated blood loss of 1 600cc prompted a transfusion of 750cc of red blood cells. Total operative time was 6 hours. The patient returned to the ICU and had an uneventful postoperative course after the first stage. Liver volumetry on POD 15 showed an FLR (segment 6) of 120cc (32.57%) (Fig. 1d).
The patient was then scheduled for the second stage on POD 16. Abdominal cavity exploration and adhesiolysis were performed without difficulty. Segment 7 showed necrosis and no purulent fluid was recovered. The left and right anterior branches of the portal vein, as well as the glissonean s7 pedicle were clipped, stitched and divided, including the hepatic veins. The outflow of s6 remained patent through the right inferior hepatic vein which was spared. A left trisectionectomy extended to s7 with s1 resection (Fig. 2a) was performed. 200cc of red blood cells were transfused. Operative time was 200 minutes.
Figure 2:
(a) Gross pathologic specimen after left trisectionectomy extended to s7. The tumor invades all segments resected, viable tumor was found in the microscopic study. (b) Last CT scan control is shown, with no evidence of recurrence in the liver remnant nor signs of portal hypertension. The white arrow shows the posterior portal branch and s6 pedicle. The black arrow points at the IRHV.
Post-operative course
The patient’s postoperative recovery showed no signs of liver failure. On POD 6 of the second stage she was medically treated for pneumonia that resolved without incident; she was discharged on POD 30. The pathologic examination of the surgical specimen revealed epithelial-type hepatoblastoma with 70% necrosis, and surgical margins free of disease (R0 resection).
Follow-up
The liver volume on December 2015 was 453cc (Fig. 2b). After three cycles of post-operative adjuvant chemotherapy, the AFP levels remained within normal range (<7 ng/ml). A follow-up control performed on November 17, 2018 continued to be without evidence of disease (AFP levels 1.56 ng/ml). Clinical and laboratory parameters showed normal liver function. The BMI-for-age was at the 85th percentile.
DISCUSSION
Future liver remnant is a crucial factor when considering a hepatectomy. Given its predictive importance, some patients are deemed ineligible for surgical resection and offered as alternatives, either a liver transplant or non-surgical therapies. Considering that the ALPPS procedure has a high morbidity rate when compared to conventional hepatectomy, it is critical to emphasize that a thorough patient selection is of paramount importance. The determining factor to consider this patient for ALPPS was the urgency to resect the tumor within an appropriate time after NACT. Given that liver transplant was not possible due to the unavailability of a donor within a reasonable period, a monosegmental ALPPS was approved in a multidisciplinary committee meeting, and then explained to the parents who agreed by signing an informed consent. The much improved medical condition of this patient after intensive multidisciplinary treatment played an important role in the decision-making process.
There are a few publications of ALPPS performed in pediatric populations [5, 6, 8] describing complications that range between no morbidity [5] pleural effusion [6], pneumonia [7], ileus and abnormal liver tests [8]. In the present case, the patient developed pneumonia after the second intervention that resolved uneventfully. This postoperative course leads us to infer that this procedure might be well tolerated in pediatric patients. Qazi et al. [7] reported a patient whose difficulty to undergo liver transplant resulted in ALPPS. Unfortunately, early recurrence most likely resulted in a fatal outcome. In the complete opposite of the spectrum, our patient has been able to reach long-term cancer free survival with good quality of life. This outcome leads us to believe that the evolution is less dependent of the surgical procedure per se and may be more related to the biology of the tumor.
Future studies are warranted to identify the population that will best benefit from this procedure and to determine the parameters that will allow a thorough evaluation of patients in order to avoid complications and achieve the best oncological outcome.
To the best of our knowledge, this is the first case of pediatric hepatoblastoma successfully treated with monosegmental ALPPS where the patient has not only reached long-term disease-free survival but also an optimal quality of life.
REFERENCES
- 1. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713–24. https://linkinghub.elsevier.com/retrieve/pii/S1365182X1530472X. [DOI] [PubMed] [Google Scholar]
- 2. Pineda-Solís K, Paskar D, Tun-Abraham M, Hernandez-Alejandro R. Expanding the limits of resectability: Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) using monosegment 6, facilitated by an inferior right hepatic vein. J Surg Oncol 2017;115:959–62. [DOI] [PubMed] [Google Scholar]
- 3. Schadde E, Malagó M, Hernandez-Alejandro R, Li J, Abdalla E, Ardiles V, et al. Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery 2015;157:676–89. http://linkinghub.elsevier.com/retrieve/pii/S0039606014007892. [DOI] [PubMed] [Google Scholar]
- 4. Trobaugh-Lotrario AD, Meyers RL, O’Neill AF, Feusner JH. Unresectable hepatoblastoma: current perspectives. Hepat Med 2017;9:1–6. http://www.ncbi.nlm.nih.gov/pubmed/28203111%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5293365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan A, Chung PH, Poon RT. Little girl who conquered the ‘ALPPS’. World J Gastroenterol 2014;20:10208–11. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4123352&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiederkehr JC, Avilla SG, Mattos E, Coelho IM, Ledesma JA, Conceição AF, et al. Associating liver partition with portal vein ligation and staged hepatectomy (ALPPS) for the treatment of liver tumors in children. J Pediatr Surg 2015;50:1227–31. 10.1016/j.jpedsurg.2014.10.019. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 7. Qazi AQ, Syed AA, Khan AW, Hanif F. Early multifocal recurrence of hepatoblastoma in the residual liver after R0 liver resection with ALPPS procedure: a case report. Ann Transl Med 2016;4:375–5. http://atm.amegroups.com/article/view/11942/12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong JC, Kim J, Browning M, Wagner A, Lerret S, Segura AD, et al. Modified Associating liver partition and portal vein ligation for staged hepatectomy for hepatoblastoma in a small infant. Ann Surg 2017;266:e16–7. http://insights.ovid.com/crossref?an=00000658-201708000−00030. [DOI] [PubMed] [Google Scholar]