Abstract
The amniotic membrane (AM) has an increasing role as a scaffold for tissue repair due to its unique biological properties. The implantation of AM on pancreatic anastomosis after pancreaticoduodenectomy might improve the anastomotic healing and strengthen its structure, however has never been used in pancreatic surgery. We present the first application of AM after a pancreaticoduodenectomy was performed for a malignant tumor of the pancreatic head. After completing the pancreatic anastomosis, the AM was placed around the pancreatic anastomosis and fixed to it with single stiches. The AM, due to its physical characteristics, could be easily manipulated and adapted to the pancreatic anastomosis. This interesting and unique case shows that covering a pancreatic anastomosis with the AM is safe and technically feasible. The AM has no adverse effects while it may eventually provide a beneficial impact over the anastomotic healing.
INTRODUCTION
Post-operative pancreatic fistula (POPF) after pancreatic resection is a potentially life-threatening complication [1]. Even although over the past decades the outcome has improved thanks to advances in surgical techniques and centralization of pancreatic surgery [2], a POPF still occurs in up to 27% of patients undergoing a pancreatic resection in high volume centers [1]. Several strategies have been proposed like pancreatic duct stenting [3] or the use of biologic glues combined with collagen patches [4], but further improvements are needed. Amniotic membrane (AM) is a tissue of fetal origin composed of three major layers [5]. Many studies suggest that AM implantation is effective in tissue regeneration and prevention of fluid leakage at many surgical sites. AM has gained widespread attention for the reconstruction of the ocular surface, for the treatment of diabetic ulcers, as a patch graft for dural repair [6] and in the urology for the ureteral strictures and vescico-vaginal fistulas [7]. AM is a rich source of biologically active factors and exhibits anti-inflammatory and anti-microbial features [8]. However, to our knowledge, the implantation of AM has never been used in pancreatic surgery. The ability of the AM to promote tissues’s healing and strength, may also hold true to preserve the integrity of a pancreatic anastomosis. This is the first report of an AM implantation over a pancreatic anastomosis after pancreaticoduodenectomy (PD).
CASE REPORT
A 73-year-old man presented to our department with jaundice without pain, and elevated Ca 19.9 (250 U/ml). Abdominal ultrasound and computed tomography (CT) show a 3 cm solid mass of pancreatic head without distant metastasis. Histology confirmed the diagnosis of pancreatic adenocarcinoma. The patient was otherwise in good health and had no history of alcohol or drug abuses. Curative surgery was proposed.
AM preparation
The AM is provided by Fondazione Banca dei Tessuti di Treviso Onlus. The placenta is sourced from donors undergoing cesarean sections and processed shortly after retrieval, according to Italian requirements. The AM is detached from the chorion and rinsed with sterile saline solution. The membrane is flattened on a nitrocellulose membrane filter (Merck Millipore), with its stromal side in contact with the filter. The AM is immersed in a cocktail of antibiotics including vancomycin 100 μg/ml (Hospira), meropenem 200 μg/ml (Fresenius Kabi Italia), gentamicin 200 μg/ml (Fisiopharma) at +4 °C for 24 h in sterile conditions. The AM was then cut into patches and immersed in cryopreservant solution. Cryopreservation was achieved using a programmable cryogenic freezer (Planer KryoSave Integra, 750-30), which triggers a controlled cooling rate. The AM patches were stored in vapor-phase liquid nitrogen.
Surgical technique
The PD procedure was performed with open technique through a median abdominal incision. The pylorus-preserving variant was performed. Intraoperative frozen sections of the pancreatic and biliary resection margins were assessed, as routinely. Given the Fistula Risk Score [9] was 1, pancreato-jejunostomy (PJ) was performed in an end-to-side fashion with single layer non-absorbable suture with a with 3-0 braided polyester. A 10 × 15 cm2 AM patch was placed around the pancreatic anastomoses starting from the posterior surface (Fig. 1): the caudal and the cranial flap were overlapped on the anterior surface to wrap the anastomosis. The membrane was than fixed with 4-0 Monocryl sutures at the jejunal surface and at the peri-pancreatic tissue and an end-to-side bilio-enteric anastomosis was carried out. Reconstruction was completed by an end-to-side antecolic duodeno-jejunostomy and two drains were placed ventral and dorsal to the PJ, without friction with the AM (Fig. 2).
Figure 1:
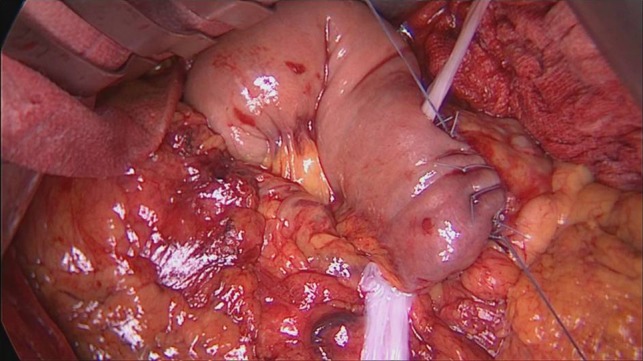
AM displacement begins from posterior face of pancreo-jejunal anastomosis.
Figure 2:
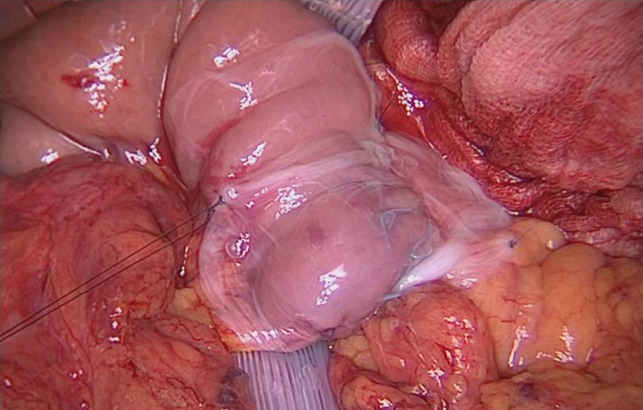
AM surrounds the entire PJ anastomosis and it is fixed to surrounding tissue by single stiches.
There was no postoperative pancreatic fistula [1]. All abdominal drains were removed on Day 3 after surgery. Delayed gastric emptying occurred in the postoperative period and the patient was treated with prokinetic drugs and total parenteral nutrition. Abdomen ultrasound and magnetic resonance showed, however, no peri-pancreatic collections, or intra-abdominal abscess. The upper gastrointestinal contrast series and the endoscopy highlighted an ulceration of the duodeno-jejunostomy which resulted in an anastomotic stricture. The patient underwent a successful endoscopic balloon dilatation and the anastomosis expanded well. The postoperative course was then uneventful and no clinical adverse reactions directly related to the AM were observed.
DISCUSSION
Although there have been significant advances in standardization of surgical procedures and outcome metrics, leakage of pancreatic anastomosis is still a significant cause of postoperative morbidity and mortality in patients undergoing pancreatic resection [2, 10]. Despite knowledge of the interference made by the pancreatic juice with the proper sealing and healing of the anastomosis, no widely used material or treatment breaks down the rate of pancreatic leakage [3, 4]. Human AM is one of the oldest biomaterials and has been used to treat very different pathologies since the beginning of the 20th century.
It has gained importance because of its ability to reduce inflammation, to enhance suture holding capacity, and to serve as a sealant. The AM showed a good tensile strength to the suture knots and was therefore feasible to stitch the membrane to the anastomosis. The AM may have a supportive mechanical role to preserve the integrity of the pancreatic anastomosis as a biologic bandage by sticking the anastomotic margins. Because the AM is easily adaptable to the desired shape, it has been possible to fit the PJ anastomosis and reach a possible watertight closure that may eventually lead to a decrease in the incidence of POPF.
Furthermore, the AM is reported to be also as a scaffold, upon which cells can migrate, proliferate and regenerate, inducing the rapid formation of granulation tissue. This capacity of the AM may potentially strengthen the pancreatic anastomosis thereby improving the anastomotic healing. Moreover, the AM had shown anti-microbial properties [8] and unlike to other synthetic materials used to seal the anastomotic site, we may speculate that it could be able to limit the infection of peri-anastomotic collections.
CONCLUSIONS
This case report has not the purpose to give conclusion about the outcome but it was necessary to assess applicability and tolerability of the AM over a pancreatic anastomosis. Although further studies are needed, this case shows no contraindications for this technique. After positive results of this first case, a prospective study is currently underway to evaluate the outcome on POPF of AM implantation after PD.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Pulvirenti A, Marchegiani G, Pea A, Allegrini V, Esposito A, Casetti L, et al. Clinical Implications of the 2016 International Study Group on Pancreatic Surgery Definition and Grading of Postoperative Pancreatic Fistula on 775 Consecutive Pancreatic Resections. Ann Surg 2018;268:1069–1075. [DOI] [PubMed] [Google Scholar]
- 2. Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011;364:2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel K, Teta A, Sukharamwala P, Thoens J, Szuchmacher M, DeVito P. External pancreatic duct stent reduces pancreatic fistula: a meta-analysis and systematic review. Int J Surg 2014;12:827–832. [DOI] [PubMed] [Google Scholar]
- 4. Mita K, Ito H, Fukumoto M, Murabayashi R, Koizumi K, Hayashi T, et al. Pancreaticojejunostomy using a fibrin adhesive sealant (TachoComb) for the prevention of pancreatic fistula after pancreaticoduodenectomy. Hepatogastroenterology 2011;58:187–191. [PubMed] [Google Scholar]
- 5. Bourne GL. The microscopic anatomy of the human amnion and chorion. Am J Obstet Gynecol 1960;79:1070–1073. [DOI] [PubMed] [Google Scholar]
- 6. Tomita T, Hayashi N, Okabe M, Yoshida T, Hamada H, Endo S, et al. New dried human amniotic membrane is useful as a substitute for dural repair after skull base surgery. J Neurol Surg B Skull Base 2012;73:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barski D, Gerullis H, Ecke T, Varga G, Boros M, Pintelon I, et al. Repair of a vesico-vaginal fistula with amniotic membrane—step 1 of the IDEAL recommendations of surgical innovation. Cent European J Urol 2015;68:459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stock SJ, Kelly RW, Riley SC, Calder AA. Natural antimicrobial production by the amnion. Am J Obstet Gynecol 2007;196:255.e1-6. [DOI] [PubMed] [Google Scholar]
- 9. Callery MP, Pratt WB, Kent TS, Chaikof EL, VOllmer CM. A prospectively validated clinical risk score acurately predicts pancreatic fistula after pancreaticoduodenectomy. J Am Coll Surg 2013;216:1–14. [DOI] [PubMed] [Google Scholar]
- 10. Bassi C, Andrianello S. Identifying key outcome metrics in pancreatic surgery, and how to optimally achieve them. HPB 2017;19:178–181. [DOI] [PubMed] [Google Scholar]


