Abstract
We hereby present a case of a 6-year-old boy with exposed brain matter following traumatic injury from a road traffic accident, in a third-world country with poor healthcare resources. The patient was taken immediately to operating theater where two general surgeons performed an emergent craniotomy and debridement. The patient survived the injury and surgery without neurological deficits or other surgical complications. He was discharged home in good condition.
INTRODUCTION
In the current report, we detail our global health experience providing trauma surgical care in a third-world country with low resources and poor healthcare options. Our case describes a 6-year-old boy who presented with exposed brain matter following traumatic injury from a road traffic accident. This accident occurred in a third-world country with poor healthcare resources. Despite limited tools and diagnostic modalities, the patient survived the injury and emergent craniotomy without neurological deficits or other surgical complications.
CASE REPORT
Patient presentation
The patient is a 6-year-old African male who presented at our tertiary surgical referral center in Central Africa after motor vehicle accident. His past medical, social, and family history were not obtained given that the patient spoke a Northern African dialect, whereas the staff at the local hospital spoke English and a Southern dialect. Furthermore, he presented without any family member or guardians, and one sheet of poorly documented medical history.
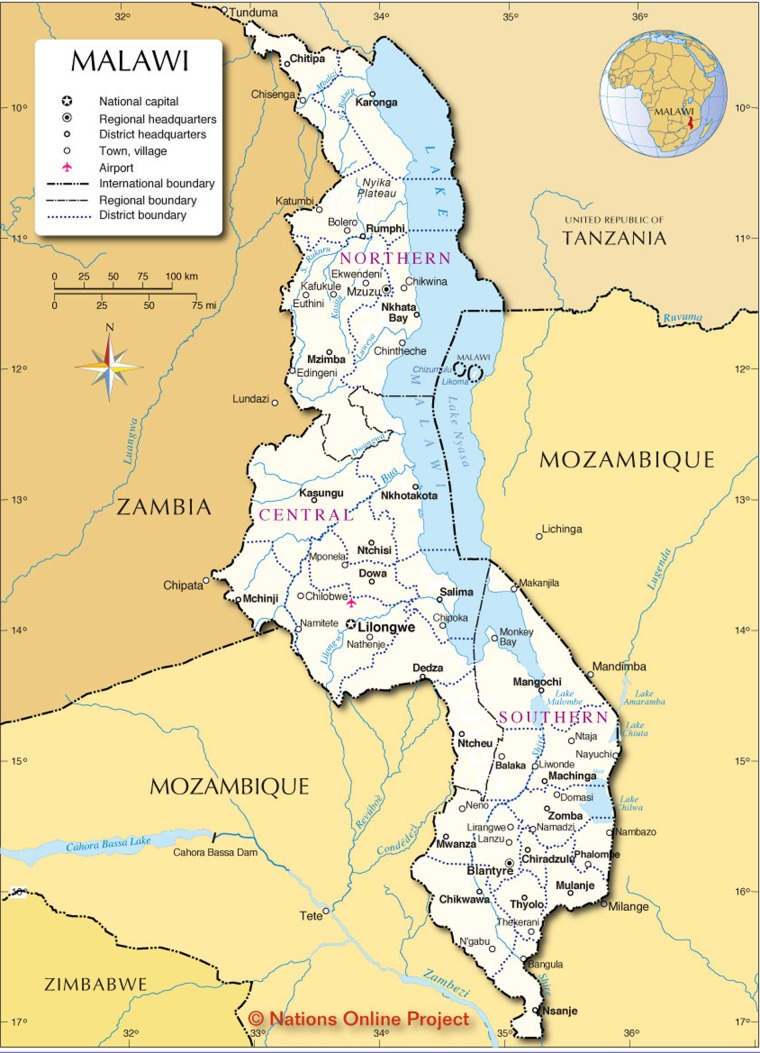
According to this record, he was struck by a motorcycle the preceding evening, approximately 12 hours prior to presentation. He was struck by a passing motorcyclist at an unknown speed, then launched and landed head-on into a large rock. This occurred at the border of Tanzania and Malawi which is approximately 150 miles from the tertiary hospital (Fig. 1). He initially presented to the closest medical facility, where he was evaluated by an orthopedic clinical technician (the only available in-house staff). His presumptive diagnosis from the technician was a depressed skull fracture with exposed brain parenchyma. No other physical examination findings or vital signs were documented. Head and lateral cervical spine X-rays were performed prior to transfer. As the tertiary hospital was the closest hospital with surgical capabilities, he was transferred for further management by police car. The patient suffered a witnessed seizure during transfer. He arrived on the morning of 26 May 2018 to the intensive care unit (ICU) at tertiary hospital.
Figure 1:
Map of Central Africa. Patients home location is approximately 150 miles from our tertiary hospital, the closest hospital with surgical capabilities. Copyright Nations Online Project.
On primary trauma survey, he was noted to have an intact airway, breathing spontaneously, and had normal vital signs. His heart rate was 126 beats per minute, blood pressure was 128 mmHg systolic over 85 mmHg diastolic, temperature was 36.0 degrees Celsius, and he was breathing 33 times per minute. His initial Glasgow coma scale score was 11. He was given one point for eye opening as his eyelids were swollen shut; five points for best verbal response since he was able to converse appropriately; and five points for best motor response with localization of pain.
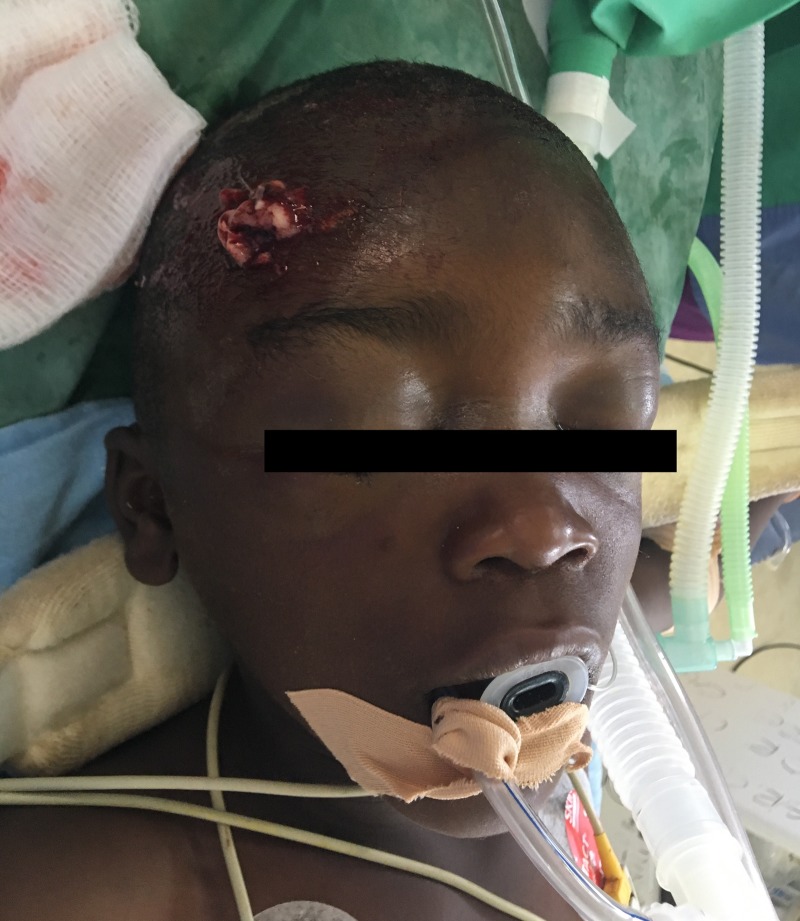
His secondary survey was notable for a small, 1 cm laceration in his right frontal cranium with blood and possible brain matter leaking from the defect (Fig. 2). A makeshift cervical spine collar was applied loosely, with the patient’s head rotated ninety-degrees to the left. He had no cervical spine tenderness and his collar was reapplied. Pupillary exam was impaired by severe bilateral eyelid edema, and the patient had no lateralizing neurologic deficits otherwise. There were no physical examination findings suggestive of basilar skull fracture. The remainder of his examination revealed superficial abrasions to his bilateral lower extremities but no other traumatic injuries.
Figure 2:
Six-year-old boy presenting with exposed brain matter after sustaining traumatic head injury following road traffic accident.
Investigations
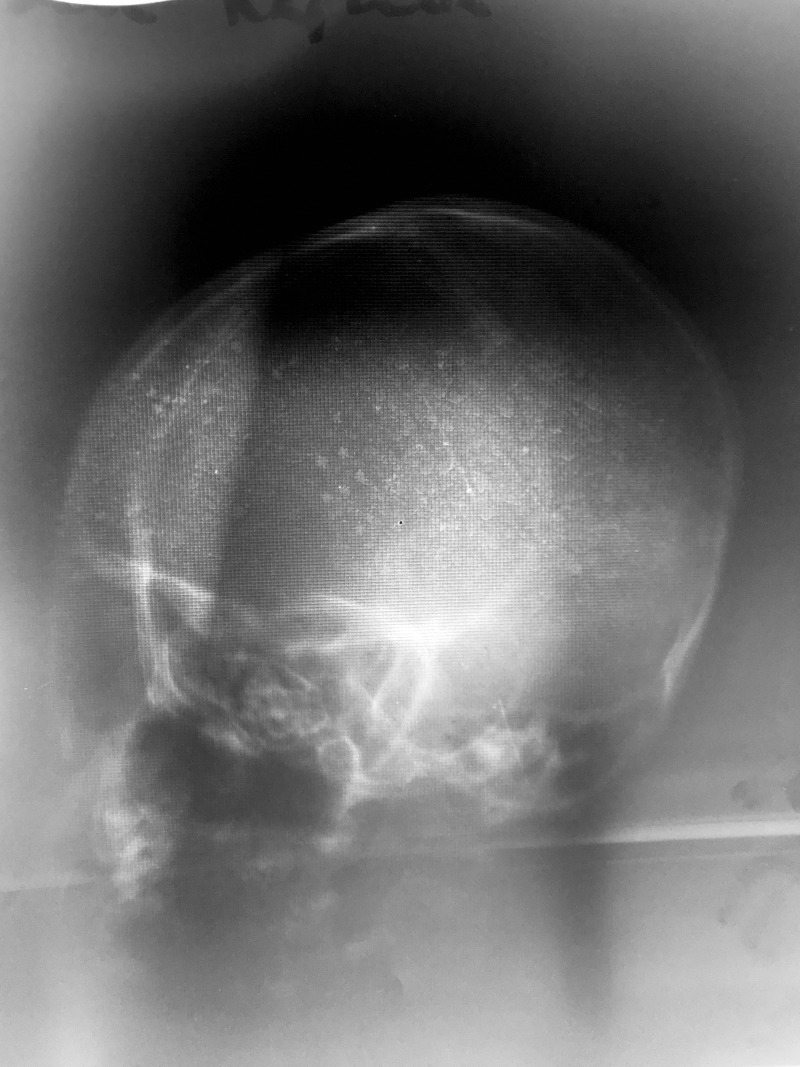
The patient’s initial work-up included a full blood count, type and cross-match for transfusion, and X-ray imaging of his head and lateral cervical spine. The X-rays were both performed at the primary medical facility. Head X-ray was of poor quality, but did not reveal any obvious injuries (Fig. 3). Cervical spine X-ray imaging was lost on transfer, and repeating the cervical spine X-ray at the tertiary hospital would have delayed operative treatment. Cross-sectional imaging was not performed, as CT scanning is not available at the tertiary hospital. His full blood count was notable for a hemoglobin of 11.3 g/dl and platelet count of 202 (x106 u/ml).
Figure 3:
Lateral head X-ray. No other imaging modalities were available or functional at time of patient presentation.
He was placed on supplemental oxygen per nasal cannula. Due to his open and contaminated head laceration, intravenous ceftriaxone was administered for prophylaxis. He was also given phenytoin for seizure prophylaxis [1]. Again, phenytoin was the only available antiepileptic available. One pint of cross-matched whole blood was ordered on call to operating theater. Blood product fractionation is not practiced at the tertiary hospital due to logistical blood-banking constraints.
Treatment
The patient arrived in the operating theater at 8 am on 26 May 2018. He was orotracheally intubated and general anesthesia was induced. The patient was prepped and draped in sterile fashion. Due to unforeseen circumstances, only the minor set of instruments were available for operation. Our suture options were limited to polyglactin (Vicryl), silk, and chromic gut.
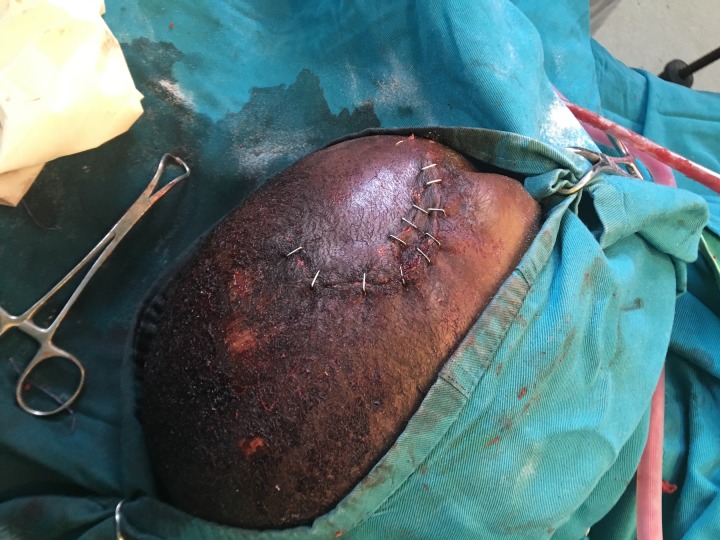
Our operative course began with an evaluation of the patient’s open skull fracture. We noted a 2 cm laceration with obvious brain matter extravasating from the wound. A 10 cm curvilinear incision was made around the defect. A scalp flap was created around the defect and hemostasis was achieved. On closer inspection, there was a stellate compound skull fracture with visible contamination and exposed, devitalized brain matter. The bone and brain matter were debrided leaving a skull defect measuring approximately 7 cm by 8 cm overlying the right frontal lobe. No bipolar electrocautery device was available, so monopolar electrocautery was used to achieve hemostasis. The galea aponeurosis layer was then reapproximated over the cranial defect using 2–0 polyglactin suture. The scalp layer was closed with skin staples, thus concluding the operation (Fig. 4).
Figure 4:
Stapled closure of craniotomy incision and traumatic scalp laceration.
Outcome and follow-up
Postoperatively, the patient was transferred back to the ICU. He was continued on ceftriaxone for seven days for meningitis prophylaxis. Sinus precautions were instituted with presumed sinus injury, as further imaging studies were unavailable. Given the severity of his traumatic brain injury (TBI), phenytoin was ordered to be continued for seven days, however the hospital supply of phenytoin was depleted after only two days. He did not suffer any post-traumatic seizures after surgery. He was able to follow commands on the fifth postoperative date after which his diet was advanced appropriately. He was transferred out of the ICU to the general pediatrics ward on the seventh postoperative date where he continued to improve.
The patient remained in the hospital into postoperative Day 14 for skin staple removal. He was then discharged home in good condition, tolerating a diet, following commands appropriately, and without any neurologic deficits. Most recently, he was seen in surgical clinic on 13 June 2018 and has continued to recover well.
DISCUSSION
In this report, we present a case of a 6-year-old boy with exposed brain matter following traumatic injury from a road traffic accident. Our aims are to detail our global health experience providing trauma surgical care in a third-world country with low resources and poor health care options. Moreover, we hope to shed light on healthcare conditions in Central Africa and working with limitations in surgical treatment options.
Central Africa is the poorest region in the world [2]. The healthcare system is severely limited by access to primary care, infrastructure, and an overall lack of physicians. Recent reports estimate a mere 18 physicians per million citizens in Central Africa, as compared to 2 500 per million in the United States [2]. Despite this discrepancy, the healthcare needs of these people are often greater than their first-world counterparts.
TBI is a leading cause of death and disability worldwide [3]. Outcomes after TBI are exceedingly poor in third-world countries, due to inadequate modalities for diagnosis, monitoring, and treatment. The global health burden of TBI is estimated at $400 billion USD annually due to these deficiencies in prevention and long-term care [4]. Further efforts to quantify the economic and healthcare impact of TBI in sub-Saharan Africa are limited due to several factors [5]. First, TBI is difficult to diagnose and capture with limited imaging modalities and ‘silent’ symptoms. Second, the incidence and prevalence of TBI are under-reported due to the absence of data surveillance and reporting systems. Third, TBI may be a leading cause of trauma-related mortality, however, mortality often goes unreported in many central and district hospitals in the region.
A recent case series from Central Africa estimates the overall mortality rate after TBI at 30%, with 80% of survivors able to make a meaningful recovery [6]. Moreover, half of all trauma-related mortalities are secondary to central nervous system injury [6]. The tertiary hospital in particular exists as the sole referral center for all surgical emergencies in our specific region of Central Africa (Fig. 1). The surgical conditions referred to our hospital consist of abdominal emergencies, gynecologic and urologic conditions, as well as the full range of traumatic injuries. Unfortunately, the capabilities of this hospital are often outstripped by patient needs. The only available blood tests include a full blood count, liver function panel, and few electrolytes. Diagnostic imaging is limited to ultrasound and plain films, as the nearest CT scanner is 200 miles south in Lilongwe. Invasive hemodynamic monitoring does not exist, even in the ICU, nor does intracranial pressure monitoring. Furthermore, as in the case of our patient, cervical spine immobilization is unreliable.
In the authors’ experience, we encountered many TBI victims following road traffic accidents, the majority of whom were deceased or comatose on arrival. Due to inability to obtain head CT scans or perform invasive monitoring (e.g. intracranial pressure monitors, hyperventilation, vasopressors, etc.), our treatment modalities were limited to supportive care which included nasogastric enteral nutrition and tracheostomy insertion.
Approximately once a month, as in the present report, a patient presents with a depressed skull fracture. Per neurosurgical guidelines, all compound skull fractures with evidence of dural penetration require emergent neurosurgical intervention, if meaningful recovery is possible [7]. As neurosurgical staff are not available at our tertiary hospital, the Joint Trauma System Clinical Practice Guidelines recommend that trauma surgeons may perform cranial procedures with appropriate indications [8]. Open skull fractures call for debridement of devitalized brain matter and bone fragments, in addition to prophylactic antibiotics. Ceftriaxone was given to help prevent wound infection and meningitis. The use of prophylactic antibiotics has been shown to decrease infectious complications after open and basilar skull fractures (8.7% vs 0.9%, P < 0.05) [9]. Seizure prophylaxis is also recommended in the Neurosurgery guidelines following severe TBI [10]. These guidelines recommend a seven-day course of antiepileptic medications (phenytoin), if the benefits of therapy are thought to outweigh the potential complications. In our case, the patient had witnessed seizure-like activity prior to transfer to our tertiary hospital, thus prompting our treatment with phenytoin.
Our report is the first described surgical treatment of a compound skull fracture with exposed brain matter in sub-Saharan Africa. Despite the inherent limitation with access to healthcare resources, these traumatic injuries can be surgically managed with good outcomes. This case highlights the importance of recognition and prompt treatment of compound skull fractures.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest.
GRANTS AND FINANCIAL SUPPORT
Funding was from the University of Cincinnati Department of Surgery.
REFERENCES
- 1. Thompson K, Pohlmann-Eden B, Campbell LA, Abel H. Pharmacological treatments for preventing epilepsy following traumatic head injury. Cochrane Database Syst Rev 2015;8:CD009900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. General Global Surgery Rotation [Internet]. University of Cincinnati; 2016 [cited 2018 Jul 4]. Available from: https://med.uc.edu/surgery/residency-training/general-surgery/global-surgery-rotation.
- 3. Johnson WD, Griswold DP. Traumatic brain injury: a global challenge. Lancet Neurol 2017;16:949–50. [DOI] [PubMed] [Google Scholar]
- 4. Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. . Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017;16:987–1048. [DOI] [PubMed] [Google Scholar]
- 5. Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 2007;22:341–53. [PubMed] [Google Scholar]
- 6. Eaton J, Hanif AB, Grudziak J, Charles A. Epidemiology, management, and functional outcomes of traumatic brain injury in Sub-Saharan Africa. World Neurosurg 2017;108:650–5. [DOI] [PubMed] [Google Scholar]
- 7. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. . Surgical management of depressed cranial fractures. Neurosurgery 2006;58:S56–60. discussion Si-iv. [DOI] [PubMed] [Google Scholar]
- 8. Neurosurgery and Severe Head Injury [Internet]. Joint Trauma System: The Department of Defense Center of Excellence for Trauma. Joint Trauma System; 2017. [cited 2018Jul4]. Available from: http://jts.amedd.army.mil/index.cfm/PI_CPGs/cpgs.
- 9. Demetriades D, Charalambides D, Lakhoo M, Pantanowitz D. Role of prophylactic antibiotics in open and basilar fractures of the skull: a randomized study. Injury 1992;23:377–80. [DOI] [PubMed] [Google Scholar]
- 10. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. . Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017;80:6–15. [DOI] [PubMed] [Google Scholar]