Abstract
Context: Oxidative stress plays an important role in development of diabetes mellitus. Satureja bachtiarica Bunge (Lamiaceae) is a rich source of bioactive compounds with antioxidant properties.
Objective: This study investigates the antidiabetic effect of hydroalcoholic extract of aerial parts of S. bachtiarica.
Methods and materials: Male Wistar rats were randomly divided into six groups (n = 8) including control (normal saline), diabetic [Streptozotocin (STZ)], intervention (STZ plus hydroalcoholic extract of S. bachtiarica at doses of 75, 150 and 250 mg/kg/d) and positive control (STZ plus captopril 50 mg/kg/d) groups. A single intraperitoneal (IP) injection of STZ (60 mg/kg) was used to induce diabetes and IP therapy with drugs was performed for four weeks.
Results: In diabetic rats, serum total antioxidant capacity (TAC) decreased significantly, but glucose, alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT) and malondialdehyde (MDA) increased significantly as compared to the control (p < 0.05). Treatment with extract (250 mg/kg) caused a significant decline in serum glucose, GGT, ALT, AST and MDA as well as a significant increase in serum TAC (p < 0.05). During the intervention period, diabetic rats showed significant weight loss, but extract (250 mg/kg) treated rats did not show any weight loss. Extract (250 mg/kg) up-regulated GLUT2 expression and down-regulated GLUT4 expression in the liver (p < 0.05). S. bachtiarica extract at all dosage levels prevented STZ-induced histological damage of liver, kidney and pancreas.
Discussion and conclusions:S. bachtiarica extract exhibits antidiabetic effects through modulation of oxidative stress and expression of GLUT2 and GLUT4.
Keywords: Satureja bachtiarica, diabetes, streptozotocine, oxidative stress, glucose transporter
Introduction
Diabetes is one of the most common metabolic disorders of the current era. The global prevalence of diabetes was estimated to be 8.8% in 2015 and predicted to rise to 10.4% in 2040 (Ogurtsova et al. 2017). In diabetes, the body loses its ability to produce insulin hormone or becomes resistant to the action of insulin, and, therefore, the produced insulin cannot function properly. As a result, the body’s function to use and metabolize glucose is demonstrably reduced and blood glucose level increases, which known as hyperglycaemia (American Diabetes Association [ADA] 2017). Chronic hyperglycaemia can lead to the damage of small blood vessels and subsequent failure of various organs, such as kidneys, eyes and nerves (Abouzed et al. 2018). Diabetes is also directly correlated with the increased risk of developing cardiovascular disease (Mahmoud et al. 2017).
Diabetes has two main types, in type 1 diabetes which comprises 5–10% of all diagnosed diabetes cases, the autoimmune destruction of pancreatic β-cells leads to the defective insulin secretion. In type 2 diabetes which accounts for 90–95% of cases of diabetes, the body’s cells are becoming progressively resistant to the action of insulin, which may ultimately lead to the destruction of pancreatic β-cells and complete impairment of insulin production (Oyenihi et al. 2017). Genetic factors, obesity and lack of physical activity are known to play an important role in the development of type 2 diabetes (Mahmoud et al. 2017).
Impairment of insulin signalling in skeletal muscle, liver and adipose tissues due to the progressive insulin resistance is the main cause of type 2 diabetes. Certain biochemical factors, such as inflammatory markers and adipokines, which are usually secreted from the liver and adipose tissues, are also involved in the development of type 2 diabetes (Oyenihi et al. 2017).
Hyperglycaemia and oxidative stress play an important role in the aetiology and pathology of diabetic complications. Chronic hyperglycaemia induces oxidative stress and increases the production of reactive oxygen species (ROS) and free radicals (Oyenihi et al. 2017). Several hypotheses have been proposed to explain the mechanisms of increased ROS generation during diabetes, including glucose autoxidation, non-enzymatic and progressive protein glycosylation, increased glycine end-product and polyol pathway (Sadek and Shaheen 2014). ROS, such as hydroxyl and superoxide radicals attack important cellular macromolecules, such as carbohydrates, nucleic acids, lipids and protein leading to cell damage and death (Sadek et al. 2017).
Type 2 diabetes is also associated with a variety of lipid disorders, and evidence suggests that hyperlipidaemia, especially hypercholesterolemia, plays a key role in the development of cardiovascular diseases and atherosclerosis in diabetic patients (Xu et al. 2019). The severity of lipid disorders in diabetic patients depends on the level of insulin secretion, insulin resistance, obesity, diet and the presence of underlying or secondary causes of hyperlipidaemia. Hyperlipidaemia exacerbates hyperglycaemia-induced oxidative stress and related tissue damage through increasing serum and tissues free fatty acids (FFA) (Xu et al. 2019).
The facilitated transport of glucose from the plasma membrane of the mammalian tissues is catalysed by a family of glucose transporters (GLUTs). GLUT2 isoform is found abundantly in the liver and pancreatic β-cells, and GLUT4 isoform is found in the skeletal muscle cells and adipocytes (Jung et al. 2006). Certain therapeutic interventions affecting these transporters have been shown to improve glucose and insulin metabolism and ultimately prevent and manage diabetic disorders (Zygmunt et al. 2010).
Regarding the role of oxidative stress and inflammation in the pathogenesis of type 2 diabetes, the researchers are now focused on the use of natural compounds with antioxidant, anti-inflammatory and hepatoprotective effects to manage diabetic complications (Coskun et al. 2005). Satureja bachtiarica Bunge (Lamiaceae) is widely distributed in Iran and has been reported to grow in western, central and southwestern provinces of the country (Soodi et al. 2016). In Iranian traditional medicine, S. bachtiarica is used as a carminative, appetizer and sexual enhancer. S. bachtiarica is also recommended for the treatment of cough, dyspnoea, diarrhoea and stomach cramps (Khadivi-Khub et al. 2015). Pharmacological studies have demonstrated antioxidant (Soodi et al. 2016), anticancer (Behdarvand Shoushtar and Ghasemi Pirbaloti 2017), neuroprotective (Soodi et al. 2012), anti-inflammatory and analgesic (Saghaei and Motamedi 2017) properties of S. bachtiarica extract. Therefore, this study investigates the efficacy of S. bachtiarica extract on STZ-induced hyperglycaemia, oxidative stress, tissue damage and expression of GLUT2 and GLUT4 receptors in diabetic rats.
Materials and methods
Chemicals
2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4″-disulphonic acid sodium salt (Ferrozine) were purchased from Sigma-Aldrich Chemical Co., St Louis, MO. Other chemicals were laboratory reagent grade and obtained from Merck Co. (Darmstadt, Germany).
Preparation of hydroalcoholic extract
Aerial parts of S. bachtiarica were collected from Khuzestan Province during summer 2017, and after identification by Dr. Rafieian-Kopaei, botanist, a specimen voucher (no: 987,689) was deposited at the Herbarium of Islamic Azad University of Izeh, Khuzestan. S. bachtiarica aerial parts were shade dried at room temperature and then pulverized with an electric mill. The extraction was carried out by using the maceration process. The plant powder was mixed with water and ethanol (70:30 v/v) and stored at room temperature in the dark for 48 h. Then, the resulting mixture was filtered using a filter paper, concentrated using a rotary evaporator, and then incubated at 37 °C to until complete dryness (Sowndhararajan and Kang 2013).
Evaluation of in vitro antioxidant effects
DPPH radical scavenging activity
Different concentrations of extract were prepared in distilled water, and 1 mL of resulting solutions were mixed with 1 mL of 0.1 mM DPPH solution (prepared in 95% ethanol) and allowed to stand for 15 min at room temperature and then, the absorbance of the samples were recorded at 517 nm using a spectrophotometer. The control was prepared using 1 mL of distilled water instead of the sample. The percentage of DPPH radicals scavenging activity was determined using the following formula: %DPPH radical scavenging activity = [(Acontrol – Asample)/Acontrol] × 100. IC50 value obtained by plotting a graph of concentration (X-axis) versus the percentage of inhibition (Y-axis) (Sowndhararajan and Kang 2013).
Fe2+ chelating activity
First, extract solution (1 mL) at different concentrations was mixed with 3.7 mL of distilled water. Then, 0.1 mL of 2 mM FeCl2 and 0.2 mL of 5 mM ferrozine were added to the mixture. After approximately 20 min, the optical absorbance was read at 562 nm. For the control sample, distilled water was used instead of the sample. Fe2+ chelating activity was calculated using the following formula. Fe2+ chelating activity (%) = [(Acontrol – Asample)/Acontrol] × 100. IC50 value was calculated from the plot of the chelating activity against the sample concentrations (Sowndhararajan and Kang 2013).
ABTS radical scavenging activity
ABTS working solution was prepared by reacting ABTS (7.4 mM, 10 mL) with potassium persulfate (2.6 mM, 10 mL) for 12 h at room temperature in the dark. Before the experiment, freshly prepared ABTS solution was diluted with methanol to reach an absorbance of 1.1 ± 0.02 at 734 nm. Then, 150 μL of the extract at different concentrations was added to 2850 μL of ABTS solution, and after incubation at ambient temperature for 12 h, the optical absorbance was recorded. The control sample was prepared using 150 μL of distilled water instead of the sample. ABTS scavenging activity was determined using the following formula. ABTS scavenging activity (%) = [(Acontrol – Asample)/AControl] × 100. IC50 value was determined from the plot of the scavenging activity against the sample concentrations (Sowndhararajan and Kang 2013).
Hydroxyl radical scavenging activity
First, 1, 10-phenanthroline solution (1.865 mM, 1 mL) and the extract at different concentration (2 mL) were added into a test tube and mixed well. Then, mL of the FeSO4 solution (1.865 mM) was added to the mixture. The reaction was started by adding 1 mL of H2O2 (3% v/v) and after 60 min of incubation in a 37 °C water bath, the absorbance was recorded at 536 nm. The solution containing extract without hydrogen peroxide was considered as the blank and solution without extract was considered as the negative control. Hydroxyl radical scavenging activity was determined using the following formula. Hydroxyl radical scavenging activity (%) = [(As-An)/(Ab-An)] × 100; where As is the absorbance of the sample, An is the absorbance of the negative control and Ab is the absorbance of the blank. IC50 value was determined from the plot of the scavenging activity against the sample concentration (Sowndhararajan and Kang 2013).
Laboratory animals
Male Wistar rats weighing 250–300 g were used in this study. Animals were kept in the Animal House of the Islamic Azad University of Izeh, Khuzestan at the appropriate temperature (21 ± 2 °C) and 12 h light/dark cycle with free access to water and food. All animal procedure was based on Guidelines for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research 1985).
Diabetes induction and animal grouping
The animals with a fasting blood glucose level of less than 130 mg/mL were used in this study. Intraperitoneal (IP) injection of a single dose of streptozotocin (STZ) (60 mg/kg) was used to induce diabetes in rats. After observing the symptoms of diabetes within 72 h (weight loss, increase in the blood glucose level (over 250 mg/mL) and polydipsia), treatment using the extract or captopril was performed for four weeks. Rats were divided randomly into six groups (n = 8) including a group of healthy rats (treated with normal saline, IP), a group of diabetic rats (treated with normal saline, IP), 3 groups of diabetic rats treated with S. bachtiarica extract at doses of 75, 150 and 250 mg/kg using IP injection and a groups of diabetic rats treated with captopril at a dose of 50 mg/kg using IP injection. After four weeks of treatment, rats were fasted for 12 h and then anesthetized with chloroform. Blood samples were collected from the heart of animals and centrifuged to separate the sera. Sera were immediately stored at −70 °C until biochemical analysis. Animals were then autopsied, and the liver, pancreas and kidney were removed and fixed in 10% formalin for pathophysiological analysis. The animal weight was measured by using a digital scale before and after treatment (Dewanjee et al. 2009).
Serum biochemical factors
The serum levels of lipids [total triglyceride (TG), total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C)], liver enzymes [aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT) and γ-glutamyltransferase (GGT)], as well as urea, creatinine and albumin were measured by commercial kits (Pars Azmoon Co., Tehran, Iran) using an autoanalyzer (Biotecnica BT-3000, Tokyo, Japan) (Dewanjee et al. 2009).
Serum malondialdehyde (MDA) assay
Briefly, 1.5 mL of 20% acetic acid, 1.5 mL of 0.8% thiobarbituric acid (TBA), 200 μL of 1.8% sodium dodecyl sulphate (SDS) and 700 μL of distilled water were added to the test tubes containing 100 μL of serum samples. The tubes were then immersed in a boiling water bath for 60 min, then 1 mL of distilled water and 5 mL of butanol/pyridine were added to the samples, and the samples were centrifuged and the optical absorbance of the supernatant was recorded at 532 nm (Dewanjee et al. 2009).
Total antioxidant capacity (TAC) measurement
Ferric reducing power (FRAP) assay was used to measure the serum TAC. The FRAP solution was prepared by adding 10 mL of acetate buffer (0.25 M, pH = 3.6), 5 mL of TPTZ (10 mM) [prepared in HCl (40 mM)], and 2.5 mL of 6H2OFeCl3 (20 mM). 25 μL of the serum was mixed with 1.5 mL of the FRAP solution, and the resulting mixture was left at 37 °C for 10 min and then the optical absorbance at 593 nm was recorded (Dewanjee et al. 2009).
Histological studies
The pancreas, liver and kidney tissues were fixed in 10% buffered formalin solution, processed and embedded in paraffin and 3–5 μm sections were prepared and placed on the glass slides. The slides were then stained with haematoxylin-eosin (H & E) and used for histological evaluation under a light microscope (Dewanjee et al. 2009).
Measurement of GLUT2 and GLUT4 gene expression
Samples of rat liver were frozen in an adequate volume of acid guanidinium thiocyanate solution and kept at −80 °C until RNA extraction. Total cellular RNA was extracted by the method of acid guanidinium thiocyanate phenol/chloroform extraction. The total tissue RNA concentration was measured by spectrophotometric absorbance (260 nm), and the quality of isolated RNA was verified by agarose gel electrophoresis with ethidium bromide staining. Purified total RNA (1 µg) was used as a substrate for reverse transcription. The reaction was performed by incubation of RNA with 1 µM oligo (dT) and 200 units of MMLV reverse transcriptase from a Clontech (Mountain View, CA) first strand cDNA synthesis kit. An aliquot (5 µL of a 1/10 dilution) of the cDNA of each sample was used for RT-PCR. The PCR primers used are shown in Table 1. DNA amplification was carried out in 1 × Taq polymerase buffer, 1.5 mM MgCI2 supplemented with 50 µM dNTP, 0.25 µM of 5′ and 3′-specific primers, 1 µCi of [α-32p] and 2 units of Taq polymerase (Promega C) in a final volume of 50 µL. The mixture was overlaid with mineral oil and amplified for 30 cycles (each consisting of denaturation for 1 min at 94 °C, annealing for 1 min at 60 °C, extension for 1 min at 72 °C) then extension for 7 min at 72 °C and storage at 4 °C in a Triothermoblock. cDNA products (10 µL) were size-separated by electrophoresis on a 10% acryl/bisacrylamide gel and stained with ethidium bromide (15 µg/mL). Each band was excised from the gel, and the quantity of 32p incorporated was measured in a scintillation counter (Weinstein et al. 1994).
Table 1.
Primers for GLUT2, GLUT4 and GAPDH gene.
| Genes | Forward | Reverse |
|---|---|---|
| GAPDH (control gen) | GTA TTG GGC GCC TGG TCA CC | CGC TCC TGG AAG ATG GTG ATG G |
| GLUT4 | ACATACCTGACAGGGCAAGG | CGCCCTTAGTTGGTCAGAAG |
| GLUT2 | GGCTAATTTCAGGACTGGTT | TTTCTTTGCCCTGACTTCCT |
Data analysis
Data were analysed using SPSS version 20 (SPSS Inc., Chicago, IL). Analysis of variance (ANOVA) followed by Duncan’s test used to identify statistical differences between means. All data were presented as mean ± SD and p value less than 0.05 was considered statistically significant.
Results
Results of in vitro antioxidant assays
In this study, the antioxidant activity of S. bachtiarica extract was evaluated in vitro by using five antioxidant tests. The results of in vitro assays showed that S. bachtiarica extract had strong DPPH radical scavenging activity (IC50 = 67.02 µg/mL), moderate ABTS and hydroxyl radicals scavenging activity (IC50 = 468.52 and 303.56 µg/mL, respectively) and relatively good ferrous iron chelating activity (IC50 = 1639.38 µg/mL), and strong ferric reducing activity (optical absorbance at 700 nm = 2.7).
Results of laboratory animals
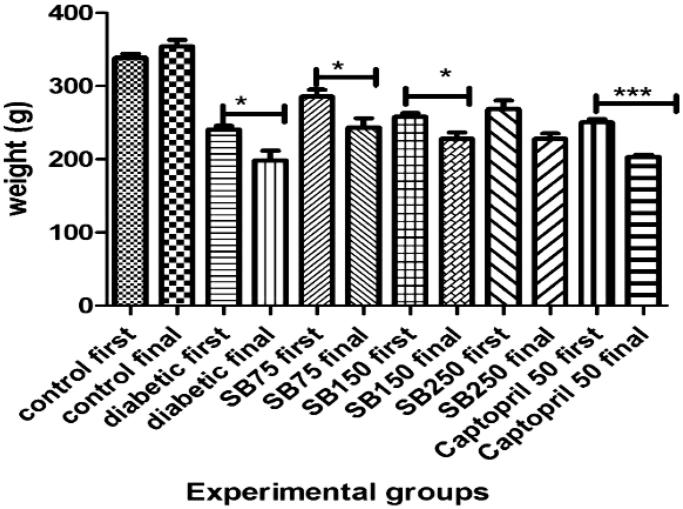
According to the results, STZ-treated rats exhibited significant weight loss over the 4 week period (p < 0.05), while there was no significant change in the weight of the control group. The weight of diabetic rats treated with S. bachtiarica extract at doses of 75 and 150 mg/kg also showed a significant reduction (p < 0.05), but, rats treated with extract at a dose of 250 mg/kg did not show any significant weight loss (Figure 1).
Figure 1.
Comparison of body weight change during the study period between the studied groups (SB: Satureja bachtiarica); *Significant difference compared to values before treatment (***p < 0.001 and *< 0.05).
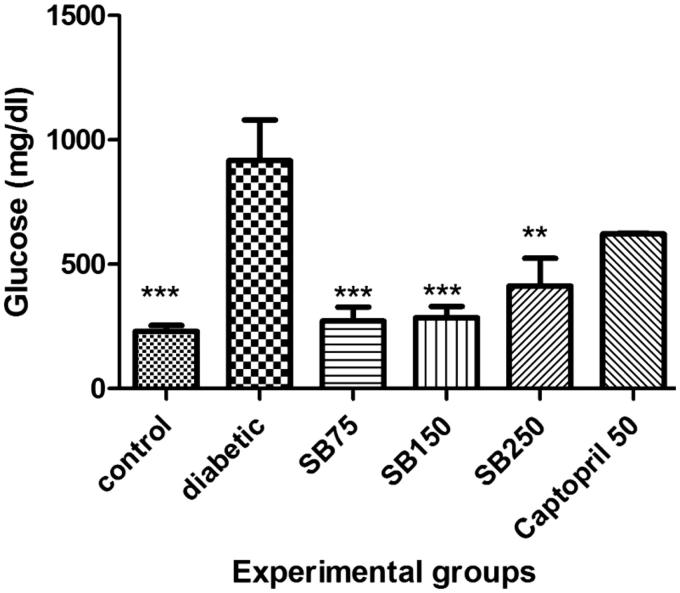
The blood glucose level in the diabetic group was significantly higher than that in the control group (p < 0.001). Administration of S. bachtiarica extract at doses of 75, 150, and 250 mg/kg to diabetic rats produced a significant decrease in the blood glucose level compared to the diabetic group (p < 0.001 and < 0.001). Captopril treatment also reduced blood glucose level compared to the diabetic rats, but the difference was not statistically significant (Figure 2).
Figure 2.
Comparison of blood glucose levels between the studied groups (SB: Satureja Bacchitaria); *Show significant difference compared to the diabetic group (***p < 0.001 and **< 0.01).
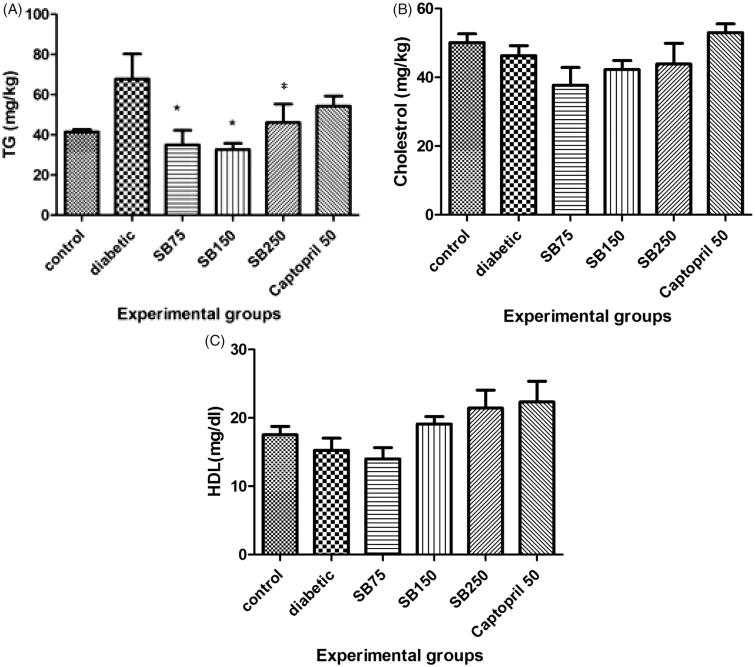
As shown in Figure 3, the serum TG level increased in diabetic rats as compared to the control group, but the increase was not statistically significant. Administration of S. bachtiarica extracts (75, 150 and 250 mg/kg) to diabetic rats significantly decreased blood TG level when compared to the diabetic group (p < 0.05, Figure 3). There were no significant differences in serum TC and HDL-C levels between the studied groups (Figure 3(B,C)).
Figure 3.
Comparison of serum triglyceride (TG) (A), total cholesterol (TC) (B), and high-density lipoprotein-cholesterol (HDL-C) (C) levels between the studied groups (SB: Satureja bachtiarica). In Figure A: *Show significant difference compared to the diabetic group (*p < 0.05).
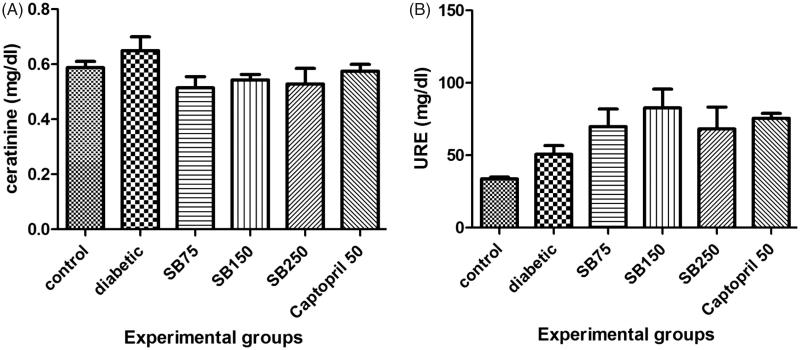
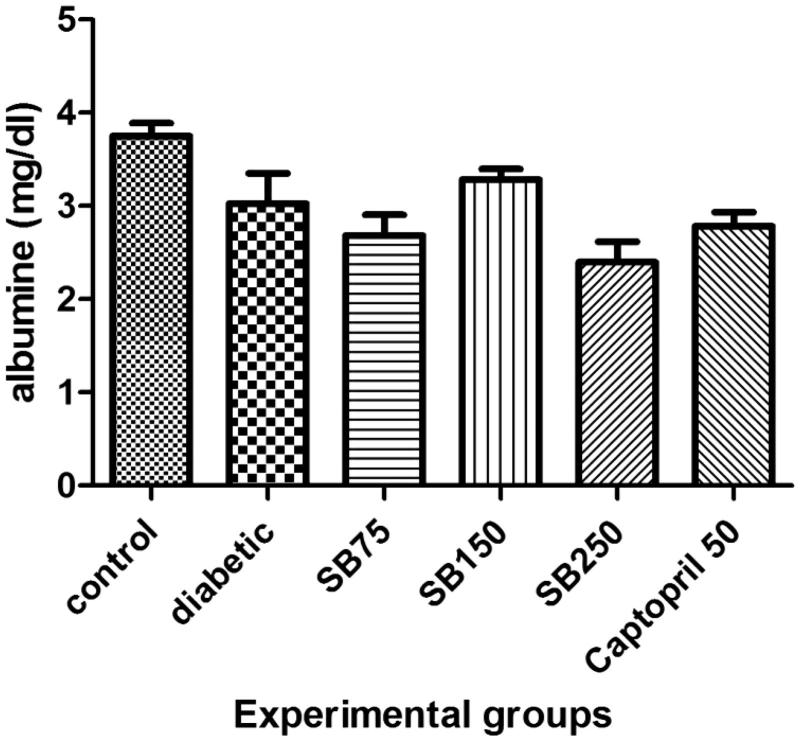
Serum urea and creatinine levels in healthy, diabetic and diabetic rats treated with extract or captopril showed no statistically significant differences (Figure 4(A,B)). Serum albumin level also showed no significant difference between the groups (Figure 5).
Figure 4.
Comparison of serum creatinine (A) and urea (B) levels between the studied groups (SB: Satureja bachtiarica).
Figure 5.
Comparison of serum albumin level between the studied groups (SB: Satureja bachtiarica).
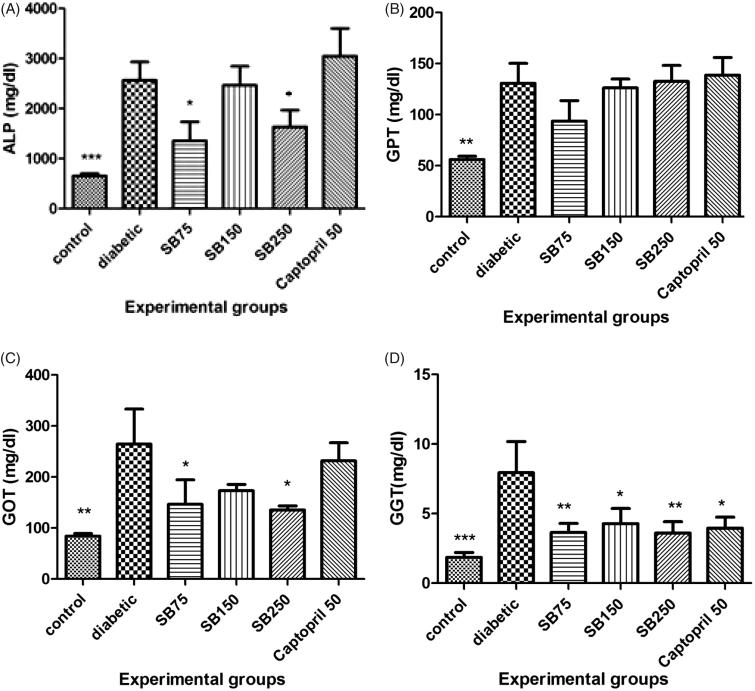
STZ-treated rats showed a significant elevation in the serum levels of ALP, ALT, AST and GGT as compared to the control group (p < 0.001 and p < 0.01). Administration of S. bachtiarica extract at 75 and 250 mg/kg significantly decreased serum ALP (p < 0.05) and AST (p < 0.05) levels and at 75, 150 and 250 mg/kg, significantly decreased serum GGT level (p < 0.05 and 0.01, Figure 6(A–D)).
Figure 6.
Comparison of alkaline phosphatase (A), alanine aminotransferase (B), aspartate aminotransferase (C) and gamma glutamyltransferase (D) between the studied groups (SB: Satureja bachtiarica). *Show significant difference compared to the diabetic group (***p < 0.001, **< 0.01 and *< 0.05).
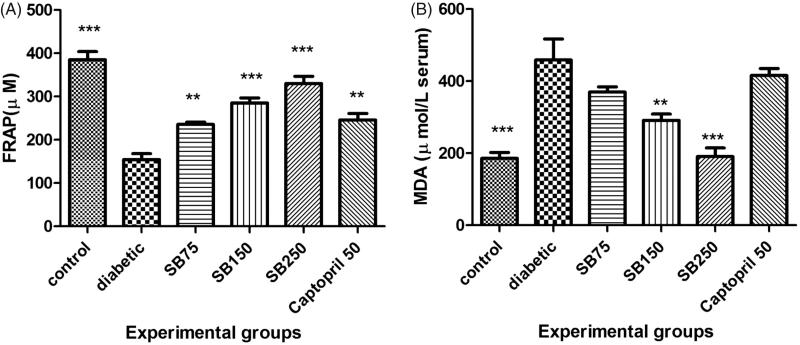
STZ-induced diabetic rats also showed a significant reduction of serum TAC and a significant increase of serum MDA when compared to the healthy rats (p < 0.001, Figure 6(A,B)). Treatment of diabetic rats with S. bachtiarica extract at doses of 75, 150 and 250 mg/kg considerably increased serum TAC (p < 0.01 and < 0.001), and at doses of 150 and 250 mg/kg, significantly decreased serum MDA level (p < 0.01 and < 0.001, Figure 7(A,B)).
Figure 7.
Comparison of serum total antioxidant capacity (A) and malondialdehyde level (B) among the studied groups (SB: Satureja bachtiarica). *Show significant difference compared to diabetic group (***p < 0.001 and *< 0.05).
Treatment of diabetic rats with S. bachtiarica extract at doses of 150 and 250 mg/kg caused a significant decrease and at a dose of 75 mg/kg caused a significant increase in the expression of GLUT2. Also, S. bachtiarica extract at 250 and 75 mg/kg increased and at 150 mg/kg, decreased the expression of GLUT4 (Table 2).
Table 2.
Comparison of GLUT2 and GLUT4 gene expression between studied groups.
| Groups | GLUT4 | GLUT2 |
|---|---|---|
| Diabetic | 1.00 ± 0.22 | 1.00 ± 0.27 |
| SB75 | 4.85 ± 1.28* | 1.96 ± 0.48 |
| SB150 | 0.44 ± 0.03* | 0.06 ± 0.02* |
| SB250 | 1.79 ± 0.34* | 0.10 ± 0.08* |
| Captopril | 0.10 ± 0.02 | 0.03 ± 0.00 |
*Significant difference compared to diabetic group (*< 0.05).
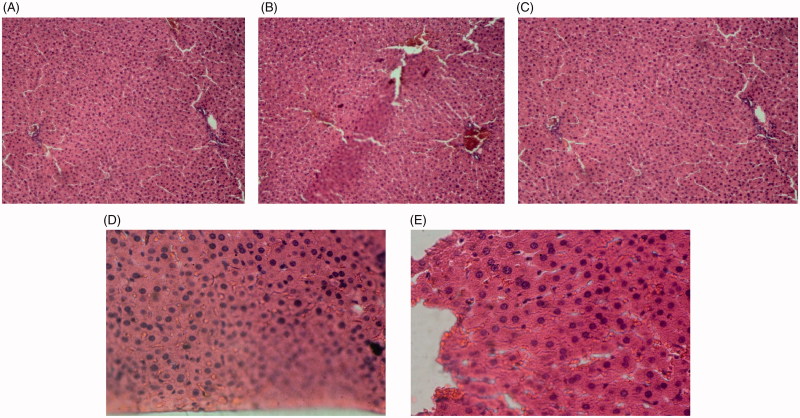
As histological examination shows, the liver tissue of the control group had a completely normal histological structure. In diabetic rats, severe hyperaemia and destruction of hepatic lobule, hepatocytes and sinusoids were observed. In diabetic rats treated with different doses of extract, the sinusoids, hepatocytes, hepatic lobule, central veins and portal triads were completely normal (Image 1).
Image 1.
Liver tissue sections of control rats (A), diabetic rats (B), diabetic rats treated with 75 mg/kg of S. bachtiarica extract (C), diabetic rats treated with 150 mg/kg of S. bachtiarica extract (D) and diabetic rats treated with 250 mg/kg of S. bachtiarica extract (E); haematoxylin-eosin staining; magnification x40.
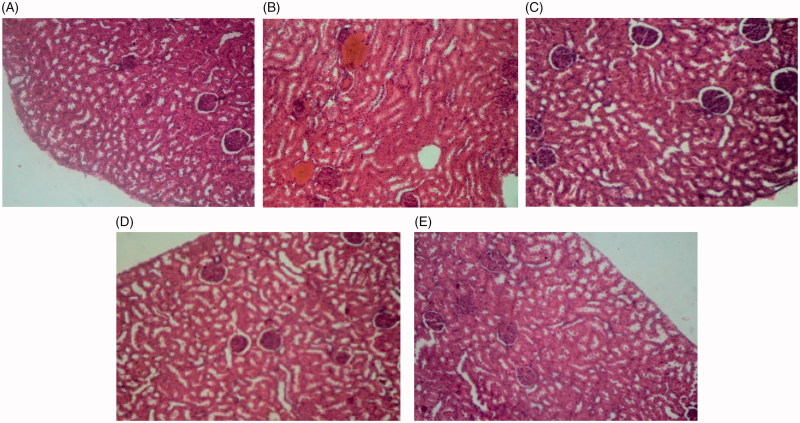
In control rats, nephron, as well as proximal and distal tubules, had normal structure. In the diabetic group, destruction of the renal tissue in the cortex and complete degeneration of the nephrons were observed. In diabetic rats treated with S. bachtiarica extract, tubules of the nephron, renal corpuscle, Bowman’s capsule were healthy and normal (Image 2).
Image 2.
Renal tissue sections of control rats (A), diabetic rats (B), diabetic rats treated with 75 mg/kg of S. bachtiarica extract (C), diabetic rats treated with 150 mg/kg of S. bachtiarica extract (D) and diabetic rats treated with 250 mg/kg of S. bachtiarica extract (E); haematoxylin-eosin staining; magnification x40.
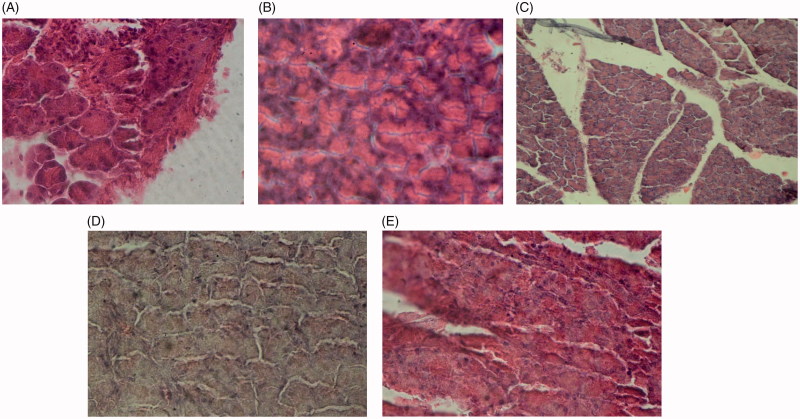
As Image 3 shows, in non-diabetic healthy rats, the pancreatic acinar cells and islets of Langerhans were healthy and had a normal appearance. In the diabetic group, hyperaemia and the acidophilia of the acinar cells were observed. In diabetic rats treated with S. bachtiarica extract, the acinar cells and islets of Langerhans were normal and healthy.
Image 3.
Pancreas tissue sections of control rats (A), diabetic rats (B), diabetic rats treated with 75 mg/kg of S. bachtiarica extract (C), diabetic rats treated with 150 mg/kg of S. bachtiarica extract (D) and diabetic rats treated with 250 mg/kg of S. bachtiarica extract (E); haematoxylin-eosin staining; magnification x40.
Discussion
This study investigated the protective effects of S. bachtiarica extract on STZ-induced diabetes in rats. It was observed that the induction of diabetes in rat causes a significant increase in blood glucose and MDA and a significant decrease in serum TAC.
STZ, an antibiotic produced by Streptomyces achromogenes, is typically used to induce diabetes in experimental models (Abouzed et al. 2018). STZ is taken up by the pancreatic β-cells through the GLUT2 glucose transporter and causes degenerative changes in the cells which leads to the decreased insulin secretion and ultimately hyperglycaemia (Eleazu et al. 2013). The mechanism of STZ action on pancreatic β-cells has not yet been fully understood; however, it is believed that oxidative stress plays an important role in the development of the STZ-induced complications (Sadek et al. 2017). STZ is an unstable molecule that accumulates in the pancreatic β-cells and degrades to carbonium radicals. Carbonium radicals are highly reactive and induce direct toxic effects on pancreatic islet cell; they also show indirect toxic effects by increasing the generation of ROS (Ghanema and Sadek 2012; Sadek et al. 2017). STZ was reported to cause β-cell damage through the induction of DNA fragmentation and methylation (Cardinal et al. 2001). It has also been shown that STZ causes inflammatory response through enhanced infiltration of mononuclear cells and excessive production of inflammatory cytokines in the islets of Langerhans that eventually leads to the β-cells injury and death (Abouzed et al. 2018). Also, studies have shown that increased nitric oxide (NO) production in the pancreas of STZ-treated rats plays an important role in the degeneration of β-cells (Pacher et al. 2005). In STZ-treated rats, serum NO level was reported to be elevated (Coskun et al. 2005), and inhibitors of nitric oxide synthases (NOS) such as LNG-NMDA and aminoguanidine resulted in remarkably reduced hyperglycaemia and β-cells damage (Lukic et al. 1991; Corbett and McDaniel 1996). In our study, hyperglycaemia, lipid peroxidation and decreased serum TAC in STZ-diabetic rats confirmed these mechanisms.
In diabetic rats, treatment with S. bachtiarica extract (150 and 250 mg/kg) improved hyperglycaemia, decreased lipid peroxidation and increased serum TAC. In previous studies, the antioxidant effects of S. bachtiarica have been observed both in vitro (Hashemi et al. 2011) and in vivo (Soodi et al. 2016) which are in harmony with our study. In this study, S. bachtiarica extract significantly ameliorated histopathological damage of pancreas in STZ-treated rats, which confirms its protective effects on pancreatic β cells.
The weight of diabetic rats significantly decreased after 4 weeks of treatment, while there was no significant change in weight of control rats and diabetic rats treated with 250 mg/kg of S. bachtiarica extract. Loss of body weight in diabetic rats may be due to the proteolytic degradation of structural proteins into amino acids that are eventually oxidized and used as a source of energy because the body’s cells are unable to absorb blood glucose as a source of metabolic energy. Amino acids may also be used as gluconeogenic precursors in the liver (Sathish Sekar et al. 2005), Also, glycogenolysis and lipolysis play a role in the weight loss of diabetic rats (Dewanjee et al. 2009). The ability of S. bachtiarica extract (250 mg/kg) to prevent the weight loss of diabetic rats can be due to its beneficial effects in reducing blood glucose.
Studies have reported that STZ-induced diabetes is associated with lipid disorders (increased TG, TC, LDL and VLDL, as well as decreased HDL) (Almeida et al. 2012). Several biochemical mechanisms have been proposed for lipid disorders due to diabetes. Following the induction of diabetes, the activity of hormone-sensitive lipases increases, which results in the breakdown of triglycerides stored in adipocytes into fatty acids and their release into the blood. It has also been reported that the activity of lipoprotein lipase (endothelium-bound glycoprotein), which plays an important role in the degradation of triacylglycerol, chylomicrons and VLDL, is reduced following induction of diabetes (Almeida et al. 2012). In our study, STZ-treated rats demonstrated an insignificant and minor increase in serum TG and also an insignificant decrease in serum HDL, treatment with of S. bachtiarica extract at all doses significantly reduced serum TG when compared to the diabetic rats.
In this study, significant increases in serum ALP, ALT, AST and GGT were observed in STZ-treated rats, but no significant changes were observed in the serum levels of creatinine, urea and albumin. STZ exhibits various biological activities including toxicity, carcinogenicity, teratogenesis and mutagenesis, and not only damages pancreatic beta cells, but also produces toxic effects in other tissues and organs of the body, such as the kidney, liver and gastrointestinal tract (Quine and Raghu 2005; El Rabey et al. 2017; Abouzed et al. 2018). In this study, the results of the histopathological examination showed that STZ injection causes tissue damage in not only pancreas but also liver and kidney. Increased blood levels of liver enzymes also confirmed histological damage of the liver. However, we did not observe any increase in the biochemical markers of renal injury. Studies have reported that STZ-induced damage to liver, kidney and gastrointestinal tract increase dramatically from the time of injection to six weeks later (Piyachaturawat et al. 1988; Zafar et al. 2009). So, the lack of a significant change in serum creatinine and urea in our study may be related to the shorter study period (4 weeks). Treatment of diabetic rats with S. bachtiarica extract significantly reduced serum GGT, ALT and AST levels, with comparatively greater activity at a dose of 250 mg/kg. The extract at all doses also ameliorated histological damage of pancreases, liver and kidney tissues. The observed protective effects of S. bachtiarica extract in diabetic rats are probably related to its antioxidant effects and reduction of STZ-induced oxidative stress as well as reduction of STZ-induced hyperglycaemia.
Treatment of diabetic rats with S. bachtiarica extract at a dose of 250 mg/kg significantly reduced the expression of GLUT2 and increased the expression of GLUT4 in the liver. GLUT2 is a trans-membrane transporter protein that transports glucose between the liver and blood and plays important role in the hepatic glucose uptake. Unlike GLUT4, GLUT2 does not require insulin for facilitation of glucose transport (Villanueva-Peñacarrillo et al. 2001).
In a previous study, a 2-fold increase in the levels of GLUT2 protein and mRNA has been reported in the liver tissue of STZ-treated diabetic rats, and antidiabetic medications have been shown to lower blood glucose by modulating their levels (Brichard et al. 1993). The GLUT4 isoform is the most important GLUT in muscle and adipose tissues (Kandror and Pilch 1996). Insulin stimulates GLUT4 translocation from the storage vesicles to the plasma membrane and ultimately absorption of glucose in muscle and fat tissues. Resistance to insulin’s stimulating effects on glucose uptake is an important feature of obesity, X syndrome and diabetes mellitus. Up-regulation of GLUT4 expression and an increase of its translocation to the cell surface considerably increase the permeability of the cell membrane to glucose and improves insulin action on the metabolism of glucose (Villanueva-Peñacarrillo et al. 2001). Some studies have shown that plant polyphenols play an important role in the preventing and managing diabetes by increasing the expression of GLUT4 in adipose (Jung et al. 2006) and muscle tissues (Zygmunt et al. 2010). As our results indicated, 250 mg/kg dose of S. bachtiarica extract showed an effective role in the improvement of diabetes-related disorders and also a 1.97-fold increase in GLUT4 expression and a decrease in GLUT2 expression in the liver.
Conclusions
S. bachtiarica extract has protective effects on hyperglycaemia and diabetes-induced hepatic and renal injury in rats. S. bachtiarica extract exerts its effects by decreasing the oxidative stress markers and modulating the expression of glucose transporter genes. It is recommended to identify the active chemical constituents of S. bachtiarica extract, evaluate their effects on animal models of diabetes, and then develop an appropriate clinical trial.
Acknowledgements
The authors would like to thank the staff of laboratory of Islamic Azad University Izeh, Iran for assisting in conducting this work. This article has been derived from the MSc thesis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abouzed TK, Contreras MDM, Sadek KM, Shukry M, H. Abdelhady D, Gouda WM, Abdo W, Nasr NE, Mekky RH, Segura-Carretero A, et al. . 2018. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in Wistar rats in relation to their metabolite fingerprint. Diabetes Res Clin Pract. 140:253–264. [DOI] [PubMed] [Google Scholar]
- Almeida D, Braga CP, Novelli ELB, Fernandes A. 2012. Evaluation of lipid profile and oxidative stress in STZ-induced rats treated with antioxidant vitamin. Braz Arch Biol Technol. 55:527–536. [Google Scholar]
- American Diabetes Association [ADA]. 2017. 2. Classification and diagnosis of diabetes. Diabetes Care. 40:11–24. [DOI] [PubMed] [Google Scholar]
- Behdarvand Shoushtar A, Ghasemi Pirbaloti A. 2017. Cytotoxic effect of hydroalcoholic extract from Satureja bachtiarica Bunge on Hela cancer cells line. J Herbal Drugs. 7:223–229. [Google Scholar]
- Brichard S, Desbuquois B, Girard J. 1993. Vanadate treatment of diabetic rats reverses the impaired expression of genes involved in hepatic glucose metabolism: effects on glycolytic and gluconeogenic enzymes, and on glucose transporter GLUT2. Mol Cell Endocrinol. 91:91–97. [DOI] [PubMed] [Google Scholar]
- Cardinal JW, Margison GP, Mynett KJ, Yates AP, Cameron DP, Elder RH. 2001. Increased susceptibility to streptozotocin-induced beta-cell apoptosis and delayed autoimmune diabetes in alkylpurine-DNA-N-glycosylase-deficient mice. Mol Cell Biol. 21:5605–5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JA, McDaniel ML. 1996. The use of aminoguanidine, a selective iNOS inhibitor, to evaluate the role of nitric oxide in the development of autoimmune diabetes. Methods. 10:21–30. [DOI] [PubMed] [Google Scholar]
- Coskun O, Kanter M, Korkmaz A, Oter S. 2005. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res. 51:117–123. [DOI] [PubMed] [Google Scholar]
- Dewanjee S, Das AK, Sahu R, Gangopadhyay M. 2009. Antidiabetic activity of Diospyros peregrina fruit: effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem Toxicol. 47:2679–2685. [DOI] [PubMed] [Google Scholar]
- El Rabey HA, Al-Seeni MN, Bakhashwain AS. 2017. The antidiabetic activity of Nigella sativa and propolis on streptozotocin-induced diabetes and diabetic nephropathy in male rats. J Evid Based Complementary Altern Med. 9:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleazu CO, Eleazu KC, Chukwuma S, Essien UN. 2013. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Dis. 12:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanema A II, Sadek KM. 2012. Olive leaves extract restored the antioxidant perturbations in red blood cells hemolysate in streptozotocin induced diabetic rats. World Acad Sci Eng Technol. 6:124–130. [Google Scholar]
- Hashemi MB, Niakousari M, Saharkhiz MJ. 2011. Antioxidant activity of Satureja bachtiarica Bunge essential oil in rapeseed oil irradiated with UV rays. Eur J Lipid Sci Technol. 113:1132–1137. [Google Scholar]
- Institute for Laboratory Animal Research 1985. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; p. 220. [Google Scholar]
- Jung UJ, Lee MK, Park YB, Kang MA, Choi MS. 2006. Effect of Citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol. 38:1134–1145. [DOI] [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. 1996. Compartmentalization of protein traffic in insulin-sensitive cells. Am J Physiol Endocrinol Metab. 271:1–14. [DOI] [PubMed] [Google Scholar]
- Khadivi-Khub A, Salehi-Arjmand H, Movahedi K, Hadian J. 2015. Molecular and morphological variability of Satureja bachtiarica in Iran. Plant Syst Evol. 301:77–93. [Google Scholar]
- Lukic M, Stosic-Grujicic S, Ostojic N, Chan W, Liew F. 1991. Inhibition of nitric oxide generation affects the induction of diabetes by streptozocin in mice. Biochem Biophys Res Commun. 178:913–920. [DOI] [PubMed] [Google Scholar]
- Mahmoud MF, El Ashry F, El Maraghy NN, Fahmy A. 2017. Studies on the antidiabetic activities of Momordica charantia fruit juice in streptozotocin-induced diabetic rats. Pharm Biol. 55:758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogurtsova K, da Rocha Fernandes J, Huang Y, Linnenkamp U, Guariguata L, Cho N, Cavan D, Shaw J, Makaroff L. 2017. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 128:40–50. [DOI] [PubMed] [Google Scholar]
- Oyenihi AB, Chegou NN, Oguntibeju OO, Masola B. 2017. Centella asiatica enhances hepatic antioxidant status and regulates hepatic inflammatory cytokines in type 2 diabetic rats. Pharm Biol. 55:1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Obrosova IG, Mabley JG, Szabó C. 2005. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 12:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piyachaturawat P, Poprasit J, Glinsukon T, Wanichanon C. 1988. Gastric mucosal lesions in streptozotocin-diabetic rats. Cell Biol Int Rep. 12:53–63. [DOI] [PubMed] [Google Scholar]
- Quine SD, Raghu PS. 2005. Effects of (-)-epicatechin, a flavonoid on lipid peroxidation and antioxidants in streptozotocin-induced diabetic liver, kidney and heart. Pharmacol Rep. 57:610–615. [PubMed] [Google Scholar]
- Sadek KM, Lebda MA, Nasr SM, Shoukry M. 2017. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed Pharmacother. 92:1085–1094. [DOI] [PubMed] [Google Scholar]
- Sadek KM, Shaheen H. 2014. Biochemical efficacy of vitamin D in ameliorating endocrine and metabolic disorders in diabetic rats. Pharm Biol. 52:591–596. [DOI] [PubMed] [Google Scholar]
- Saghaei F, Motamedi S. 2017. The antinociceptive and anti-inflammatory effects of hydroalcoholic extract of Satureja bachtiarica on mouse. J Herb Drugs. 8:179–184. [Google Scholar]
- Sathish Sekar D, Sivagnanam K, Subramanian S. 2005. Antidiabetic activity of Momordica charantia seeds on streptozotocin induced diabetic rats. Int J Pharm Sci. 60:383–387. [PubMed] [Google Scholar]
- Soodi M, Moradi S, Sharifzadeh M, Saeidnia S. 2012. Satureja bachtiarica methanolic extract ameliorate beta amyloid induced memory impairment. Res Pharm Sci. 7:802–823. [Google Scholar]
- Soodi M, Saeidnia S, Sharifzadeh M, Hajimehdipoor H, Dashti A, Sepand MR, Moradi S. 2016. Satureja bachtiarica ameliorate beta-amyloid induced memory impairment, oxidative stress and cholinergic deficit in animal model of Alzheimer’s disease. Metab Brain Dis. 31:395–404. [DOI] [PubMed] [Google Scholar]
- Sowndhararajan K, Kang SC. 2013. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J Biol Sci. 20:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva-Peñacarrillo ML, Puente J, Redondo A, Clemente F, Valverde I. 2001. Effect of GLP-1 treatment on GLUT2 and GLUT4 expression in type 1 and type 2 rat diabetic models. Endocrine. 15:241–248. [DOI] [PubMed] [Google Scholar]
- Weinstein SP, O’Boyle E, Fisher M, Haber RS. 1994. Regulation of GLUT2 glucose transporter expression in liver by thyroid hormone: evidence for hormonal regulation of the hepatic glucose transport system. Endocrinology. 135:649–654. [DOI] [PubMed] [Google Scholar]
- Xu L, Song P, Xu J, Zhang H, Yu C, Guan Q, Zhao M, Zhang X. 2019. Viscus fat area contributes to the Framingham 10-year general cardiovascular disease risk in patients with type 2 diabetes mellitus. Life Sci. 220:69–75. [DOI] [PubMed] [Google Scholar]
- Zafar M, Naqvi SUH, Ahmed M, Kaimkhani ZA. 2009. Altered liver morphology and enzymes in streptozotocin induced diabetic rats. Int J Morphol. 27:77–84. [Google Scholar]
- Zygmunt K, Faubert B, MacNeil J, Tsiani E. 2010. Naringenin, a Citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem Biophys Res Commun. 398:178–183. [DOI] [PubMed] [Google Scholar]