Abstract
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by selective and progressive loss of dopaminergic neurons. Genetic and environmental risk factors are associated with this disease. The genetic factors are composed of approximately 20 genes, such as SNCA, parkin, PTEN-induced kinase1 (pink1), leucine-rich repeat kinase 2 (LRRK2), ATP13A2, MAPT, VPS35, and DJ-1, whereas the environmental factors consist of oxidative stress-induced toxins such as 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP), rotenone, and paraquat. The analyses of their functions and mechanisms have provided important insights into the disease process, which has demonstrated that these factors cause oxidative damage and mitochondrial dysfunction. The most invaluable studies have been performed using disease model organisms, such as mice, fruit flies, and worms. Among them, Drosophila melanogaster has emerged as an excellent model organism to study both environmental and genetic factors and provide insights to the pathways relevant for PD pathogenesis, facilitating development of therapeutic strategies. In this review, we have focused on the fly model organism to summarize recent progress, including pathogenesis, neuro-protective compounds, and newer approaches.
Keywords: Environmental toxins, Genetic factors, Mitochondrial dysfunction, Oxidative stress, Parkinson’s disease
INTRODUCTION
Parkinson disease (PD) is the second-most common human neurodegenerative (ND) disorder after Alzheimer’s disease. The pathological features involve slow degeneration of the dopaminergic neurons in the substantia nigra (SN) and formation of intracytoplasmic Lewy body (LB) inclusion structures. Moreover, PD is characterized by neuronal inclusions composed of abnormal α-synuclein, which is generally referred to as the Lewy-related pathology (1). It leads to cellular toxicity and, eventually, PD pathogenesis. Most PD cases are idiopathic, which appears to be involved in multiple processes such as neuroinflammation, excitotoxicity, oxidative stress, environmental toxins, and accumulation of misfolded proteins from proteasome impairment (2).
Over the past 15 years, several gene mutations have been definitively shown to mediate familial PD. For instance, SNCA mutations (encoding α-synuclein to PARK1 (3) and PARK4 (4), LRRK2 (PARK8) (5), VPS35 (PARK17) (6), HtrA2 (PARK13) (7), and EIG4G1 (PARK18) (8) cause autosomal dominant forms of PD. Moreover, mutations in parkin (PARK2) (9), DJ-1 (PARK7) (10), pink1 (PARK6) (11), DNAJC6 (PARK19) (12), SYANJ1 (PARK20) (13), and ATP13A2 (PARK9) (14) are associated with autosomal-recessive forms of PD.
Mitochondrial dysfunction and oxidative stress are the symptoms of PD pathogenesis (15). Recent demonstrations that pink1, parkin, and DJ-1 play crucial roles in mitochondrial function and resistance to oxidative stress, reinforcing the central importance of these themes in PD pathogenesis. Moreover, it allows us to understand PD processes at the molecular and cellular levels.
Drosophila melanogaster, commonly known as the fruit fly, is a powerful organism for modeling human ND diseases. Nearly 75% of all human disease genes have Drosophila homologues (16). Drosophila models have successfully provided valuable insights into the elucidation of pathomechanisms and development of therapies for neurodegenerative diseases. The causal relationship among PD abnormalities, such as dopaminergic cell degeneration, inclusion body formation, and locomotion dysfunction, have been elucidated with the expression of α-synuclein in Drosophila models (17). Most recently, SPG7 mutants showed a short life span, progressive locomotion defects, and sensitivity to chemical and environmental stressors (18). Here, we reviewed in detail how these genetic and environmental factors are involved in PD with model organisms, especially D. melanogaster.
DOPAMINERGIC (DA) NEURONS IN PARKINSON DISEASE
PD is characterized by the death of DA neurons in the substantia nigra (SN) region of the brain. Oxidative stress plays a key role in the DA neurons’ degeneration. The susceptibility of the brain, especially the SN to oxidative stress, is augmented by various factors such as high oxygen demands, higher rates of oxidative metabolism, lower levels of protective antioxidant system, and an abundant neuronal network (19). These pathways produce abundant quantities of ROS species. Moreover, mitochondrial dysfunction and the impaired protein degradation pathway align to the degeneration of dopaminergic neurons which further influence PD-related protein expressions, such as LRRK2, α-Syn, PINK1, UCH-L1, and DJ-1 (20–22). The misexpression or overexpression of the above parameters in D. melanogaster was examined to unscramble the root cause and mechanisms of DA neuronal loss. Therefore, studies of molecular and cellular mechanisms between mitochondrial dysfunction and different genes are essential for establishing therapeutic treatment for PD.
MITOCHONDRIAL DYSFUNCTION IN PD
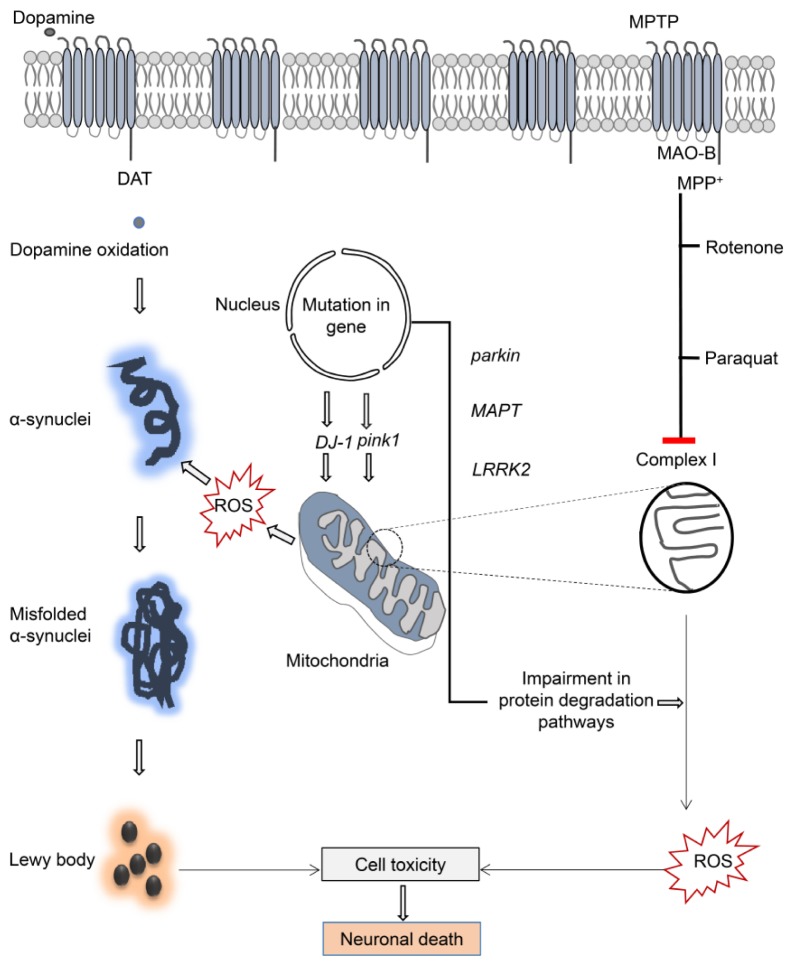
Most mitochondrial dysfunction results from damage to complex I or nicotinamide adenine dinucleotide phosphate (NADH): ubiquinone oxidoreductase—which forms a part of the oxidative phosphorylation system (23). PD pathogenesis results from impairment to complex I and complex I-mediated dopaminergic cell death resulting from Bax transcription activation (24). Furthermore, a clear correlation exists between ND diseases and impaired electron transport chain function. Iron containing cytochromes-associated movement plays a particularly prominent role in the mitochondrial membrane (25). As a result of this dysfunction, increased free radicals have been recorded, which is harmful to the proper functioning of cells. Oxidants, including hydrogen peroxide and superoxide radicals, are produced as byproducts of oxidative phosphorylation, making the mitochondria the main site of ROS generation within a cell. However, in pathological situations where mitochondrial respiratory defects occur, the amount of ROS produced by the electron transport chain increases dramatically, swamping the antioxidant protection mechanisms. PD has been shown to produce these conditions (Fig. 1). Evidence that oxidative stressors, such as ROS, are the culprits in these mitochondrial dysfunctions has recently emerged. The generation of oxidizing agents, such as hydrogen peroxide or superoxide, recapitulates the mitochondrial dysfunction (26).
Fig. 1.
Toxins and genetic factors responsible for PD. Schematic illustrations for related genes of PD and toxins in the mitochondria.
Excess free radicals are scavenged by enzymes such as glutathione peroxidase, catalase, and superoxide dismutase in normal mitochondria. However, when ROS build up, they interact with the membrane lipids and proteins, altering their conformations and, ultimately, disrupting their functioning. Furthermore, complex I inhibitors, like MPTP or rotenone, demonstrate preferential cytotoxicity to the DA neurons (27). The MPP+ (oxidized form of MPTP that is toxic) accumulates in the mitochondria, where it inhibits complex I in the mitochondrial electron transport chain complex (METC), thereby disrupting the flow of electrons along the METC (Fig. 1). This event results in decreased ATP production and increased ROS generation (28). Similar to MPTP, rotenone is another mitochondrial complex I inhibitor. Interestingly, rotenone toxicity is involved in oxidative damage to proteins and Lewy body-like inclusions (29). Other evidence for mitochondrial dysfunction related to oxidative stress and DA cell damage comes from findings that mutations in protein genes like α-synuclein, parkin, DJ-1, or pink1 are linked to the familial forms of PD (Fig. 1). Indeed, the latest study provides evidence that elevated mitochondrial Ca2+ is responsible for mitochondrial damage and neuronal death, which is controlled by a mitochondrial trafficking protein, Miro (30). The intercorrelated role of these proteins on mitochondrial dynamics reveals a common function in the mitochondrial stress response, which may provide a significant physiological basis for PD pathology (31).
MOLECULAR MODELS FOR PARKINSON DISEASE (Table 1)
Table 1.
Parkinson’s disease and their phenotypic expressions in animal models
| PD gene/locus | Mammalian/mouse | Drosophila melanogaster |
|---|---|---|
| SNCA/PARK1 | Expression of Human α-Syn (A53T): ↑ Accumulation of α-synuclein, ND and leading to cell death (75). Expression of Human α-Syn (A30P): Progressive motor disorder accompanied by accumulation of α synuclein in the soma and neurite (76). |
Expression of Human α-Syn (A30P and A53T) in pan-neuron: Dopaminergic cell degeneration, LB inclusion formation and locomotor dysfunction (17). |
| parkin/PARK2 | Expression of C-terminally truncated parkin in DA neuron: Motor deficit, nigrostriatal degeneration, α-synuclein accumulation (77). | KO mutants: ↓ Lifespan and locomotion, and male sterility (40). Loss of proper morphology of DA neurons and deficit in motor function (42). |
| PARK3 | ND in SN of brain and LB formation, presence of neurofibrillary tangles and Alzheimer plaques (78). | - |
| SNCA/PARK4 | Nigral degeneration with LB, vacuoles in neurons of the hippocampus and other brain parts (78). | - |
| UCH-L1/PARK5 | Rotenone induced mouse models: S-Nitrosylation of UCH-L1, ↑ α-synuclein aggregation (79). | KD mutants: ↓ Dopamine in the brain results in locomotor dysfunction (80). |
| pink1/PARK6 | KO mouse: Impairment in hindlimb and forelimb steps (81). | KO mutants: Mitophagy of flight muscle cells and dopaminergic neuron with aging (82). |
| DJ-1/PARK7 | KO mouse: Loss of DA neurons in SN of brain (83). |
DJ-1β mutant: ↓ Climbing activity (41). Exhibit taste impairment and memory defect (59). |
| LRRK2/PARK8 | Overexpression of LRRK2R1441G: Progressive motor deficit with immobility by 10-12 months (84). | Expression of RNA interference of JNKK or dominant-negative form of JNK increases fly survival time, locomotor activity, and decrease DA neuronal degeneration in LRRK2G2019S overexpression in DA neurons (63). |
| ATP13A2/PARK9 | KD mouse: Impairment in lysosomal degradation, α-synuclein accumulation and neurotoxicity (85). | - |
| Unknown/PARK10 | - | - |
| GIGYF2/PARK11 | Heterozygous Gigyf2+/− mouse: Exhibits motor dysfunction by 12-15 months (86). | KO mutants: Locomotor defects and early mortality (87). |
| Unknown/PARK12 | - | - |
| HtrA2/PARK13 | KO mouse: ↓ Climbing ability, movement disorders, and tremor (88). | KO mutants: Mitochondrial defects, loss of flight and climbing ability, male infertility, and increase of sensitivity to oxidative stress (89). |
| PLA2G6/PARK14 | KO mouse: Loss of DA neurons in SN and rescue by feeding L-DOPA in motor dysfunction (90). | KO mutants: Mitochondrial dysfunction and oxidative stress (91). |
| FBOX7/PARK15 | KO mouse: ↓ Proteasome activities and early-onset motor deficit (92). | Expression of FBXO7 rescues parkin mutant phenotypes, including locomotors dysfunction, DA neuron losses and muscle degeneration (93). |
| RAB7L1 (one of the candidate gene)/PARK16 | KD rodent: DA neuron degeneration as LRRK2 mutant phenotype. Overexpression of RAB7L1 reduces LRRK2 mutant induced DA neurodegeneration (94). |
KD Mutants: DA neuron degeneration as LRRK2 mutant phenotype. Overexpression of RAB7L1 in DA neurons rescues DA neurodegeneration (94). |
| VPS35/PARK17 | VPS35+/− mouse: Mitochondrial fusion and cellular respiration function impairments and neuronal loss (95). | KD of VPS35: Locomotor impairments, mild compound eye disorganization, and interommatidial bristleloss (37). |
| EIG4G1/PARK18 | Mutation in EIG4G1 (A502V, R1205H): Impairment in oxidative stress resistance (8). | - |
| DNAJC6/PARK19 | KO mouse: Early postnatal mortalities, and weight loss of surviving pups (96). | KD mutants: Loss of climbing abilities, decrease of lifespan, and DA neuron death (97). |
| SYNJ1/PARK20 | SYNJ1+/− mice: Progressive PD-like behavioral alterations and DA neurodegeneration (98). | KD mutants: ↓ Endogenous synaptic transmission at the neuromuscular junction, and 80% reduction of evoked transmission (99). |
PD genes and their phenotypic expressions in animal models, especially Drosophila melanogaster.
PD: Parkinson’s disease, UCH-L1: ubiquitin carboxyl-terminal esterase L1, PINK1: PTEN-induced putative kinase 1, LRRK2: leucine-rich repeat kinase 2, HtrA2: High temperature requirement protein A2, FBOX7: F-box protein 7, LOF: Loss of function, KD: Knockdown, KO: Knockout, DA: dopamine, ↓: Decreased/Reduced, ↑: Increased/Enhanced, LB: Lewy body.
SNCA (α-synuclein: αS)
SNCA encodes a small protein called α-synuclein. α-Synuclein is abundant in the brain; small amounts are detected in the heart, muscles, and other tissues. PD correlates with the formation of insoluble fibrillar aggregates in the central nervous system that contain α-synuclein (3) and misfolding of α-synuclein resulting from point mutations in SNCA (Fig. 2A). Aggregated monomeric α-synuclein generates β sheet-rich oligomers, inducing selective oxidation of the ATP synthase β subunit and mitochondrial lipid peroxidation. These oxidation events increase the probability of permeability transition pore opening, triggering mitochondrial swelling and, ultimately, cell death (32). A30P, A53T, and E46K (33) are three PD-related αS mutations. Among them, A30P and A53T are the most well-studied mutations. A53T transgenic mice displayed abnormally large accumulations of α-synuclein, causing rapid neurodegeneration and leading to cell death. A30P α-synuclein transgenic animals exhibit similar physiological and phenotypic characteristics to those found in humans, including the slow degeneration of DA neurons, formation of LB-like inclusions, and loss of locomotor functions (17). Similarly, a Drosophila-expressing A30P mutant causes a more rapid loss of climbing ability (34). Cathespin D, glucocerobrosidase, and proteinase K actively participate in accumulation of α-synuclein in the brain, resulting in DA neuronal loss along with decreased locomotor activity (35–38). N-terminal 32 amino acids of human α-synuclein contains mitochondrial targeting signal that plays a role in the association of these proteins with the inner mitochondrial membrane. Aggregated α-synuclein in the mitochondrial membrane of DA neurons results in complex I impairment, increased ROS production, and decreased mitochondrial transmembrane potential (39).
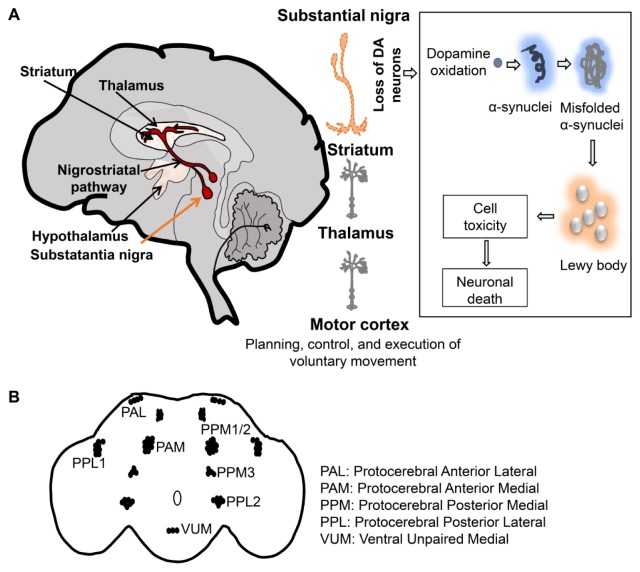
Fig. 2.
Clinical presentation of pathogenesis in PD and fly dopaminergic neuronal clusters. (A) Dopaminergic neurons in the substantia nigra and PD pathology related with Lewy body. (B) Dopaminergic neuronal clusters in a fly brain.
parkin
parkin mutation leads to an early onset form of PD, and its product encodes an E3 ligase, including functional domains such as the ubiquitin-like domain and RING finger domains. The first in vivo indication that parkin regulates mitochondrial integrity arose from studies on Drosophila parkin mutants. parkin fly mutants exhibit locomotor defects, reduced longevity, male sterility, DA neurodegeneration, and mitochondrial defects in several energy-intensive tissues such as muscles and brain (40, 41). D. parkin null mutants display degeneration of DA neurons in the PPL1 cluster and reduced TH- staining in the PPM1/2 cluster (Fig. 2B), resulting in reduced DA content in the brain. D. parkin loss-of-function mutants exhibit shrinkage of DA neurons with a decrease of tyrosine hydroxylase (TH) level and locomotor defects (42).
pink1
This gene encodes a putative serine/threonine kinase with a mitochondrial targeting sequence (11). pink1 mutants possess fragmentation in mitochondrial cristae and are very susceptible to oxidative stress. pink1 mutants are characterized by reduced lifespan, locomotor defects, degenerated flight muscle, and loss of DA neurons (43). D. pink1 mutants also have a defective thorax phenotype in three-day-old flies as well as age-dependent loss of DA neurons in the PPL1 cluster at the age of 30 days (44). Furthermore, pink1 loss of function in mice models resulted in locomotor defects and degenerated DA neurons (45). These studies provide cellular and behavioral phenotypes of pink1 mutant reproducing PD phenotypes.
The pink1 mutant flies share marked phenotypic similarities with parkin mutants. A pink1 mutant phenotype was rescued by parkin overexpression, whereas pink1 overexpression had no effect on parkin mutant phenotypes (46, 47). These observations suggest that Parkin acts downstream of Pink1 in the same pathway, which is conserved between flies and mammals. Genetic epistasis analyses revealed that proteins function in the same pathway to maintain mitochondrial fidelity, although they are localized differently; pink1 localizes to the mitochondria, and parkin resides in the cytosol (40, 47, 48). Cell studies have revealed parkin is recruited from the cytosol to depolarized mitochondria to mediate selective autophagic removal of the damaged organelle (mitophagy) (49). Furthermore, in Drosophila, pink1 directly phosphorylates parkin to control its translocation to the mitochondria (50). The above finding suggests that pink1 and parkin act in a common pathway.
DJ-1
DJ-1 binds to the subunits of mitochondrial complex I and regulates its activity (51). It is present in the mitochondrial matrix and intermembranal space (52). Its translocation into the mitochondria is enhanced by oxidative stress. DJ-1 KO mice elicit nigrostriatal DA neuron loss and accumulate defective mitochondria, which can be reversed by adenovirus-mediated DJ-1 overexpression; this phenomenon demonstrates DJ-1’s specific role in mitochondrial function (53).
DJ-1 encodes a highly conserved protein belonging to the ThiJ/PfPI superfamily of molecular chaperones (54). Two DJ-1 orthologs exist in Drosophila: DJ-1α and DJ-1β. DJ-1α is predominantly expressed in the male testis and, at a lower level, in the brain than DJ-1β. DJ-1α exhibits a role in oxidative stress, generating DA neurodegeneration, although the DJ-1β mutant contributes more to DA neuronal degeneration (55). DJ-1β decreases climbing ability (41) and increases sensitivity to environmental toxins such as H2O2, paraquat, and rotenone (56). DJ-1β loss of function results in accumulated ROS in adult brains, elevated levels of lipid peroxidation, and an increased catalase enzymatic activity (57). In Drosophila, both the aging process and oxidation challenge promote DJ-1β overoxidation at cysteine 104 (which is analogous to cysteine 106 in human DJ-1) which, in turn, irreversibly inactivates the protein DJ-1 (58). Aged flies demonstrate further vulnerability to oxidative stress, which suggests that DJ-1’s protective function against oxidative stress could be progressively lost through aging, increasing the risk of DA neuron loss. Recently, our group reported that the DJ-1β mutant has low sugar sensitivity and reduced taste-associative memory (59), which are relevant phenotypes because > 30% of PD patients have dementia. Our group also showed recovery from reduced memory defect by feeding health supplements such as omija. The fly model organism can be used for drug discovery in behavioral as well as cellular studies.
LRRK2
The most common form of sporadic PD occurs due to mutations in the gene encoding LRRK2, which comprises a large domain GTPase and kinase activity. LRRK2 has been associated with a diverse set of cellular functions and signaling pathways, including mitochondrial function, vesicle trafficking, together with endocytosis, retromer complex modulation, and autophagy (60). The study in mice showed that the degeneration of dopamine neurons is enhanced due to combined effects of LRRK2G2019S mutation with environmental toxins such as MPTP (61). The overexpression of LRRK2 or LRRK2-G2019S lead to retinal degeneration, selective loss of DA neurons, decreased climbing activity, and early mortality in flies (62). LRRK2-induced neuronal degeneration is mediated by hemipterous (hep or JNKK). The expression of RNA interference of JNKK or dominant-negative form of JNK, a downstream kinase of JNKK, increases fly survival, locomotor activity, and decreases DA neuronal degeneration in LRRK2-G2019S mutant (63).
ENVIRONMENTAL RISK FACTORS FOR PD
MPTP
MPTP is the most commonly used toxin to generate a PD model. It is one of the first models to link the inhibition of mitochondria complex I to PD (64). Several animal species, such as sheep, cats, mice, rats, and monkeys have been treated with MPTP to recapitulate the phenotype of a PD model. Both monkeys and mice treated with MPTP have shown selectively progressive loss of DA neurons, but no LBs (65). Loss of DA neurons leads to reduced motor abilities, although there are no LBs. MPTP induces a high level of NO in flies. Resveratrol decreases MPTP-mediated oxidative stress in flies and increases their life span. Therefore, resveratrol can be used as a therapeutic agent against PD (66), which indicates that a MPTP toxin-induced model in D. melanogaster is a useful tool for PD pathophysiology.
Rotenone and paraquat
Several studies have looked at rotenone and paraquat (PQ) (a proposed mitochondrial complex I inhibitor) in Drosophila to investigate the susceptibility of PD genetic models and their role in neuronal cell death. Not only do these models induce DA neuron loss, but also show evidence of behavioral and histological changes, completing the pathological picture of PD (67). Paraquat causes oxidative stress in cells through the ROS generation. Rotenone blocks the mitochondrial electron transport chain through inhibition of complex I, as seen in MPTP. Rotenone also blocks mitosis and inhibits cell proliferation, which is caused by the perturbation of microtubule assembly and decreases the GTP hydrolysis rate (68). Chronic systemic exposure to rotenone in rats led to the development of several features of PD, including nigrostriatal DA degeneration. This model has been shown to reproduce almost all PD features, including the formation of intracellular inclusions that resemble LB (69).
THERAPEUTICS APPROACH IN PARKINSON DISEASE
Vitamin K2 acts as an electron carrier and enhances ATP production in the mitochondria. Defective mitochondria are also found in Parkinson’s patients with a pink1 or parkin mutation. Vitamin K2 may offer hope for a new PD treatment (70). Vitamin K2 is essential to electron transfer in Drosophila mitochondria. Heix mutants show severe mitochondrial defects that are rescued by vitamin K2, which serves as a mitochondrial electron carrier, helping to maintain normal ATP production. A major breakthrough in PD drug development was L-dopa, which protects the brain from oxidative stress and free radicals (71). Most pharmacological approaches to PD treatment are symptomatic and target the nigrostriatal dopaminergic pathway. The gold-standard drug is L-dopa—a precursor of dopamine—which crosses the blood–brain barrier and is converted to dopamine. Other drugs are used as monotherapy or combined with L-dopa to enhance its efficacy, including dopamine receptor agonists, catechol-O-methytransferase (COMT) inhibitors, and monoamine oxidase (MAO) inhibitors (72). Zinc is an essential trace metal and a component of several enzymes and transcriptional regulators. Unlike copper and iron, zinc is not redox-active and, under most conditions, it serves as an antioxidant. The condition of parkin mutants raised on zinc-supplemented food is greatly improved. Parkin mutants perform best at high zinc concentrations, where controls begin to show adverse effects as a result of the metal supplement. This is manifested in a higher frequency of reaching adulthood, extended lifespan, and improved motor abilities (73).
CONCLUSION AND FUTURE PERSPECTIVE
Drosophila mutants and transgenic models have been used to study the genetics and environmental factors responsible for PD. More than 20 genes are associated with PD, which shows interaction between genetics and environmental factors. The common endpoint of gene and toxins are believed to initiate mitochondrial dysfunction, which results in lower ATP and oxidative stress. Various antioxidants, such as zinc and vitamin K2, have shown good medicinal value in PD. Similarly, omija feeding has also helped resolve taste memory problems and learning defects. Until now, most studies have focused on mitochondrial dysfunction and correlated genes. In addition to mitochondrial dysfunction and oxidative stress, endoplasmic reticulum (ER) stress is another demanding model to study PD pathogenesis in D. melanogaster. ER stress can be reduced with piperine, which increases mesencephalic astrocyte-derived neurotrophic factor expression that ameliorates spinocerebrellar ataxia 17 (SCA17)-associated neuropathology in the TBP-105Q knock-in mouse model (74). The study of piperine’s involvement in controlling neurodegeneration would be a fascinating approach for effective prophylaxis. More powerful clinical treatments than L-dopa (a precursor of dopamine) are needed for PD patients, especially in an aging society.
ACKNOWLEDGEMENTS
We thank Jeeyoung Lee for valuable comments. B.A. was supported by the Global Scholarship Program for Foreign Graduate Students at Kookmin University in Korea. This work is supported by grants to Y.L. from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B 03931273 and NRF-2018R1A2B6004202).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Whitton P. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 4.Singleton A, Farrer M, Johnson J, et al. α-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841–841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 5.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Zimprich A, Benet-Pagès A, Struhal W, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakabayashi K, Takahashi H. Pathology of familial Parkinson’s disease. Brain Nerve. 2007;59:851–864. [PubMed] [Google Scholar]
- 8.Chartier-Harlin M-C, Dachsel JC, Vilariño-Güell C, et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet. 2011;89:398–406. doi: 10.1016/j.ajhg.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 10.Bonifati V, Rizzu P, Squitieri F, et al. DJ-1 (PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol Sci. 2003;24:159–160. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- 11.Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 12.Köroğlu Ç, Baysal L, Cetinkaya M, Karasoy H, Tolun A. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Parkinsonism Relat Disord. 2013;19:320–324. doi: 10.1016/j.parkreldis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Quadri M, Fang M, Picillo M, et al. Mutation in the SYNJ1 Gene Associated with Autosomal Recessive, Early-Onset P arkinsonism. Hum Mutat. 2013;34:1208–1215. doi: 10.1002/humu.22373. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez A, Heimbach A, Gründemann J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1190. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 15.Greenamyre JT, Hastings TG. Parkinson’s--divergent causes, convergent mechanisms. Science. 2004;304:1120–1122. doi: 10.1126/science.1098966. [DOI] [PubMed] [Google Scholar]
- 16.Bilen J, Bonini NM. Drosophila as a model for human neurodegenerative disease. Annu Rev Genet. 2005;39:153–171. doi: 10.1146/annurev.genet.39.110304.095804. [DOI] [PubMed] [Google Scholar]
- 17.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 18.Pareek G, Thomas RE, Pallanck LJ. Loss of the Drosophila m-AAA mitochondrial protease paraplegin results in mitochondrial dysfunction, shortened lifespan, and neuronal and muscular degeneration. Cell Death Dis. 2018;9:304. doi: 10.1038/s41419-018-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 20.Kann O, Kovács R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 21.Mosharov EV, Larsen KE, Kanter E, et al. Interplay between cytosolic dopamine, calcium, and α-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehay B, Bourdenx M, Gorry P, et al. Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol. 2015;14:855–866. doi: 10.1016/S1474-4422(15)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blum D, Torch S, Lambeng N, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/S0301-0082(01)00003-X. [DOI] [PubMed] [Google Scholar]
- 24.Perier C, Bové J, Wu D-C, et al. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:8161–8166. doi: 10.1073/pnas.0609874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 26.Trushina E, McMurray C. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 27.Blesa J, Przedborski S. Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front Neuroanat. 2014;8:155. doi: 10.3389/fnana.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizuno Y, Sone N, Saitoh T. Effects of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J Neurochem. 1987;48:1787–1793. doi: 10.1111/j.1471-4159.1987.tb05737.x. [DOI] [PubMed] [Google Scholar]
- 29.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 30.Lee K-S, Huh S, Lee S, et al. Altered ER–mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc Natl Acad Sci U S A. 2018;115:E8844–E8853. doi: 10.1073/pnas.1721136115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris KL, Hao R, Chen L-F, et al. Convergence of parkin, PINK1 and α-synuclein on stress-induced mitochondrial morphological remodelling. J Neurochem. 2015;290:13862–13874. doi: 10.1074/jbc.M114.634063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludtmann MH, Angelova PR, Horrocks MH, et al. α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun. 2018;9:2293. doi: 10.1038/s41467-018-04422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blandini F, Armentero MT. Animal models of Parkinson’s disease. FEBS J. 2012;279:1156–1166. doi: 10.1111/j.1742-4658.2012.08491.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen AY, Xia S, Wilburn P, Tully T. Olfactory deficits in an alpha-synuclein fly model of Parkinson’s disease. PLoS One. 2014;9:e97758. doi: 10.1371/journal.pone.0097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khair A, Salema B, Dhanushkodi NR, et al. Silencing of Glucocerebrosidase Gene in Drosophila Enhances the Aggregation of Parkinson’s Disease Associated α-Synuclein Mutant A53T and Affects Locomotor Activity. Front Neurosci. 2018;12:81. doi: 10.3389/fnins.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis MY, Trinh K, Thomas RE, et al. Glucocerebrosidase deficiency in Drosophila results in α-synuclein-independent protein aggregation and neurodegeneration. PLoS Genet. 2016;12:e1005944. doi: 10.1371/journal.pgen.1005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura E, Hasegawa T, Konno M, et al. VPS35 dysfunction impairs lysosomal degradation of α-synuclein and exacerbates neurotoxicity in a Drosophila model of Parkinson’s disease. Neurobiol Dis. 2014;71:1–13. doi: 10.1016/j.nbd.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki M, Fujikake N, Takeuchi T, et al. Glucocerebrosidase deficiency accelerates the accumulation of proteinase K-resistant α-synuclein and aggravates neurodegeneration in a Drosophila model of Parkinson’s disease. Hum Mol Genet. 2015;24:6675–6686. doi: 10.1093/hmg/ddv372. [DOI] [PubMed] [Google Scholar]
- 39.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of α-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J, Kim SY, Cha G-H, Lee SB, Kim S, Chung J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 42.Cha G-H, Kim S, Park J, et al. Parkin negatively regulates JNK pathway in the dopaminergic neurons of Drosophila. Proc Natl Acad Sci U S A. 2005;102:10345–10350. doi: 10.1073/pnas.0500346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Gehrke S, Imai Y, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehmann S, Jardine J, Garrido-Maraver J, Loh SH, Martins LM. Folinic acid is neuroprotective in a fly model of Parkinson’s disease associated with pink1 mutations. Matters. 2017;3:e201702000009. [Google Scholar]
- 45.Moisoi N, Fedele V, Edwards J, Martins LM. Loss of PINK1 enhances neurodegeneration in a mouse model of Parkinson’s disease triggered by mitochondrial stress. Neuropharmacology. 2014;77:350–357. doi: 10.1016/j.neuropharm.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark IE, Dodson MW, Jiang C, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 47.Park J, Lee SB, Lee S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 48.Clark IE, Dodson MW, Jiang C, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 49.Narendra D, Tanaka A, Suen D-F, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Y, Park J, Kim S, et al. PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem Biophys Res Commun. 2008;377:975–980. doi: 10.1016/j.bbrc.2008.10.104. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi T, Ishimori C, Takahashi-Niki K, et al. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem Biophys Res Commun. 2009;390:667–672. doi: 10.1016/j.bbrc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Shimoji M, Thomas B, et al. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- 53.Heo JY, Park JH, Kim SJ, et al. DJ-1 null dopaminergic neuronal cells exhibit defects in mitochondrial function and structure: involvement of mitochondrial complex I assembly. PLoS One. 2012;7:e32629. doi: 10.1371/journal.pone.0032629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucas JI, Marín I. A new evolutionary paradigm for the Parkinson disease gene DJ-1. Mol Biol Evol. 2006;24:551–561. doi: 10.1093/molbev/msl186. [DOI] [PubMed] [Google Scholar]
- 55.Menzies FM, Yenisetti SC, Min K-T. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15:1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 56.Meulener M, Whitworth AJ, Armstrong-Gold CE, et al. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 57.Irrcher I, Aleyasin H, Seifert E, et al. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- 58.Meulener MC, Xu K, Thomson L, Ischiropoulos H, Bonini NM. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc Natl Acad Sci U S A. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poudel S, Lee Y. Impaired Taste Associative Memory and Memory Enhancement by Feeding Omija in Parkinson’s Disease Fly Model. Mol Cells. 2018;41:646–652. doi: 10.14348/molcells.2018.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallings R, Manzoni C, Bandopadhyay R. Cellular processes associated with LRRK2 function and dysfunction. FEBS J. 2015;282:2806–2826. doi: 10.1111/febs.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karuppagounder SS, Xiong Y, Lee Y, et al. LRRK2 G2019S transgenic mice display increased susceptibility to 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-mediated neurotoxicity. J Chem Neuroanat. 2016;76:90–97. doi: 10.1016/j.jchemneu.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Z, Wang X, Yu Y, et al. A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci U S A. 2008;105:2693–2698. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang D, Thomas JM, Li T, Lee Y, Liu Z, Smith W. Drosophila hep pathway mediates Lrrk2-induced neurodegeneration. Biochem Cell Biol. 2017;96:441–449. doi: 10.1139/bcb-2017-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissues Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- 65.Tieu K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb Perspect Med. 2011;1:a009316. doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abolaji AO, Adedara AO, Adie MA, Vicente-Crespo M, Farombi EO. Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced oxidative damage and behavioural deficits in Drosophila melanogaster. Biochem Biophys Res Commun. 2018;503:1042–1048. doi: 10.1016/j.bbrc.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 67.Trinh K, Andrews L, Krause J, et al. Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson’s disease through an NRF2-dependent mechanism. J Neurosci. 2010;30:5525–5532. doi: 10.1523/JNEUROSCI.4777-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivastava P, Panda D. Rotenone inhibits mammalian cell proliferation by inhibiting microtubule assembly through tubulin binding. FEBS J. 2007;274:4788–4801. doi: 10.1111/j.1742-4658.2007.06004.x. [DOI] [PubMed] [Google Scholar]
- 69.Sherer TB, Betarbet R, Testa CM, et al. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vos M, Esposito G, Edirisinghe JN, et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science. 2012;336:1306–1310. doi: 10.1126/science.1218632. [DOI] [PubMed] [Google Scholar]
- 71.Mena MA, Casarejos MJ, Solano RM, de Yebenes JG. Half a century of L-DOPA. Curr Top Med Chem. 2009;9:880–893. [PubMed] [Google Scholar]
- 72.Payami H, Factor SA. Promise of pharmacogenomics for drug discovery, treatment and prevention of Parkinson’s disease. A perspective. Neurotherapeutics. 2014;11:111–116. doi: 10.1007/s13311-013-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saini N, Schaffner W. Zinc supplement greatly improves the condition of parkin mutant Drosophila. Biol Chem. 2010;391:513–518. doi: 10.1515/bc.2010.052. [DOI] [PubMed] [Google Scholar]
- 74.Guo J, Cui Y, Liu Q, et al. Piperine ameliorates SCA17 neuropathology by reducing ER stress. Mol Neurodegener. 2018;13:4. doi: 10.1186/s13024-018-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee MK, Stirling W, Xu Y, et al. Human α-synuclein-harboring familial Parkinson’s disease-linked Ala-53→ Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dalfo E, Gomez-Isla T, Rosa J, et al. Abnormal α-synuclein interactions with Rab proteins in α-synuclein A30P transgenic mice. J Neuropathol Exp Neuron. 2004;63:302–313. doi: 10.1093/jnen/63.4.302. [DOI] [PubMed] [Google Scholar]
- 77.Lu X-H, Fleming SM, Meurers B, et al. Bacterial artificial chromosome transgenic mice expressing a truncated mutant Parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant α-synuclein. J Neurosci. 2009;29:1962–1976. doi: 10.1523/JNEUROSCI.5351-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gasser T. Genetics of Parkinson’s disease. J neurol. 2001;248:833–840. doi: 10.1007/s004150170066. [DOI] [PubMed] [Google Scholar]
- 79.Kumar R, Jangir DK, Verma G, et al. S-nitrosylation of UCHL1 induces its structural instability and promotes α-synuclein aggregation. Sci Rep. 2017;7:44558. doi: 10.1038/srep44558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tran HH, Dang SN, Nguyen TT, et al. Drosophila Ubiquitin C-Terminal Hydrolase Knockdown Model of Parkinson’s Disease. Sci Rep. 2018;8:4468. doi: 10.1038/s41598-018-22804-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelm-Nelson CA, Brauer AF, Barth KJ, et al. Characterization of early-onset motor deficits in the Pink1−/− mouse model of Parkinson disease. Brain Res. 2018;1680:1–12. doi: 10.1016/j.brainres.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cornelissen T, Vilain S, Vints K, Gounko N, Verstreken P, Vandenberghe W. Deficiency of parkin and PINK1 impairs age-dependent mitophagy in Drosophila. eLife. 2018;7:e35878. doi: 10.7554/eLife.35878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rousseaux MW, Marcogliese PC, Qu D, et al. Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease. Proc Natl Acad Sci U S A. 2012;109:15918–15923. doi: 10.1073/pnas.1205102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y, Liu W, Oo TF, et al. Mutant LRRK2 R1441G BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, α-synuclein accumulation, and neurotoxicity. J Neurosci. 2012;32:4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giovannone B, Tsiaras WG, de la Monte S, et al. GIGYF2 gene disruption in mice results in neurodegeneration and altered insulin-like growth factor signaling. Hum Mol Genet. 2009;18:4629–4639. doi: 10.1093/hmg/ddp430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim M, Semple I, Kim B, et al. Drosophila Gyf/GRB10 interacting GYF protein is an autophagy regulator that controls neuron and muscle homeostasis. Autophagy. 2015;11:1358–1372. doi: 10.1080/15548627.2015.1063766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martins LM, Morrison A, Klupsch K, et al. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tain LS, Chowdhury RB, Tao RN, et al. Drosophila HtrA2 is dispensable for apoptosis but acts downstream of PINK1 independently from Parkin. Cell Death Differ. 2009;16:1118–1125. doi: 10.1038/cdd.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Q, Yen A, Rymarczyk G, et al. Impairment of PARK14-dependent Ca 2+ signalling is a novel determinant of Parkinson’s disease. Nat Commun. 2016;7:10332. doi: 10.1038/ncomms10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiu C-C, Yeh T-H, Lu C-S, et al. PARK14 PLA2G6 mutants are defective in preventing rotenone-induced mitochondrial dysfunction, ROS generation and activation of mitochondrial apoptotic pathway. Oncotarget. 2017;8:79046–79060. doi: 10.18632/oncotarget.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vingill S, Brockelt D, Lancelin C, et al. Loss of FBXO7 (PARK15) results in reduced proteasome activity and models a parkinsonism-like phenotype in mice. EMBO J. 2016;35:2008–2025. doi: 10.15252/embj.201593585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burchell VS, Nelson DE, Sanchez-Martinez A, et al. The Parkinson’s disease–linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat Neurosci. 2013;16:1257–1265. doi: 10.1038/nn.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.MacLeod DA, Rhinn H, Kuwahara T, et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang F-L, Liu W, Hu J-X, et al. VPS35 deficiency or mutation causes dopaminergic neuronal loss by impairing mitochondrial fusion and function. Cell Rep. 2015;12:1631–1643. doi: 10.1016/j.celrep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yim Y-I, Sun T, Wu L-G, et al. Endocytosis and clathrin-uncoating defects at synapses of auxilin knockout mice. Proc Natl Acad Sci U S A. 2010;107:4412–4417. doi: 10.1073/pnas.1000738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song L, He Y, Ou J, et al. Auxilin underlies progressive locomotor deficits and dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Cell Rep. 2017;18:1132–1143. doi: 10.1016/j.celrep.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 98.Pan P-Y, Li X, Wang J, et al. Parkinson’s Disease-Associated LRRK2 Hyperactive Kinase mutant Disrupts Synaptic Vesicle Trafficking in Ventral midbrain Neurons. J Neurosci. 2017;47:11366–11376. doi: 10.1523/JNEUROSCI.0964-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-X. [DOI] [PubMed] [Google Scholar]