Abstract
Foxp3+ regulatory CD4+ T (Treg) cells play an essential role in preventing overt immune responses against self and innocuous foreign antigens. Selective expansion of endogenous Treg cells in response to the administration of interleukin (IL)-2/antibody complex, such as the IL-2/JES6-1 complex (IL-2C) in mice, is considered an attractive therapeutic approach to various immune disorders. Here, we investigated the therapeutic potential of IL-2C in allergic airway inflammation models. IL-2C treatment ameliorated Th17-mediated airway inflammation; however, unexpectedly, IL-2C treatment exacerbated Th2-mediated allergic airway inflammation by inducing the selective expansion of Th2 cells and type-2 innate lymphoid cells. We also found that IL-2 signaling is required for the expansion of Th2 cells in lymphoproliferative disease caused by Treg cell depletion. Our data suggest that IL-2C is selectively applicable to the treatment of allergic airway diseases depending on the characteristics of airway inflammation.
Keywords: Airway inflammation, Foxp3+ regulatory T cell, IL-2/antibody complex, Th17, Th2
INTRODUCTION
Foxp3+ regulatory CD4+ T (Treg) cells play an essential role in preventing overt pro-inflammatory responses against self and innocuous environmental antigens (1). Multiple mechanisms are involved in the suppressive functions of Treg cells; these include the production of immunosuppressive cytokines, CTLA-4-mediated inhibition of antigen-presenting cell functions, and deprivation of cytokines such as interleukin (IL)-2 via the expression of high levels of CD25, an IL-2 receptor α-subunit required for the assembly of a high-affinity IL-2 receptor complex (1, 2).
Allergic airway disease is mediated by overt proinflammatory responses against inhaled innocuous antigens. This pulmonary disorder is a multifaceted disease, and various types of immune cells participate in the development and progression of allergic airway diseases (3). Reduced levels of Treg cells are associated with the pathogenesis of allergic airway disease (4). Hence, adoptive transfer of Treg cells can prevent the development or progression of airway disease (5). Considering the heterogeneity and complexity of allergic airway disease, the application of Treg cells with multi-targeting properties represents an attractive therapeutic approach for treating this condition (2, 3).
IL-2 is a trophic cytokine required for the expansion of effector cells as well as Treg cells (6). The IL-2/anti-IL-2 antibody complex has been shown to induce vigorous T cell proliferation in vivo (7). Depending on the clone of the anti-IL-2 monoclonal antibody, the IL-2/antibody complex exerts differential effects on the proliferation of T cell subsets. In mice, IL-2 complexed with the S4B6 clone of the anti-IL-2 antibody preferentially induces the expansion of CD8+ T cells. The IL-2/JES6-1 complex induces the preferential expansion of Treg cells by blocking the interaction of IL-2 with CD122 (IL-2Rβ) and CD132 (common γ-chain or IL-2Rγ) and promoting interaction with CD25 (8). In this regard, the potential therapeutic utility of the IL-2/JES6-1 complex in treating multiple sclerosis, organ transplantation, food allergy, and airway allergic diseases has been investigated (9–11).
Here, we evaluated the potential therapeutic utility of the IL-2/JES6-1 complex in two different endotypes of allergic airway inflammation. Unexpectedly, we found that the IL-2/JES6-1 complex did not ameliorate, but rather exacerbated, Th2-mediated airway inflammation. In contrast, this complex alleviated Th17-mediated airway inflammation. Exacerbation of airway inflammation by IL-2/JES6-1 complex was mediated by the selective expansion of Th2 cells and type-2 innate lymphoid cells. We additionally demonstrated that IL-2 signaling is required for GATA3 expression in CD4+ T cells, and IL-2 signaling is critical for the expansion of Th2 cells in a lymphoproliferative disease induced by Treg cell depletion.
RESULTS
IL-2/antibody complex exacerbates allergic airway inflammation
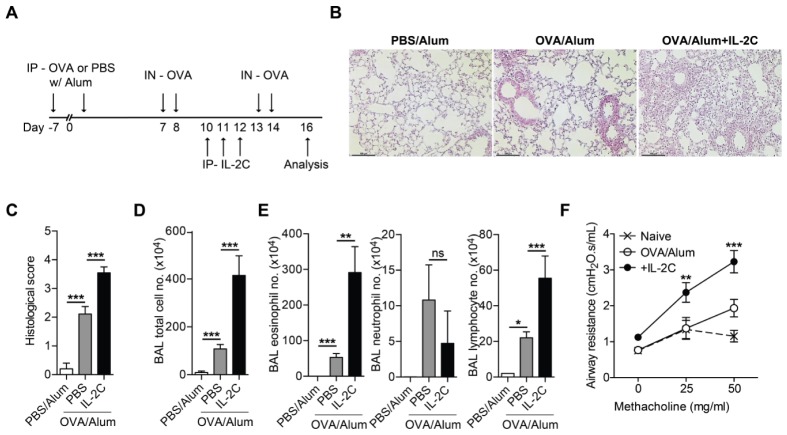
We tested whether the expansion of endogenous Treg cells following the administration of IL-2/JES6-1 complex (IL-2C) ameliorated allergic airway inflammation. C57BL/6 mice were intraperitoneally (i.p.) sensitized with ovalbumin (OVA) emulsified with aluminum hydroxide (Alum), and then intranasally (i.n.)-challenged with OVA. IL-2C was i.p. -injected during intranasal OVA challenge to examine the potential therapeutic effect of IL-2C treatment (Fig. 1A).
Fig. 1.
IL-2/antibody complex (IL-2C) exacerbates allergic airway inflammation. C57BL/6 mice were i.p.-sensitized with ovalbumin (OVA) or PBS plus Alum twice at 1-week interval and i.n.-challenged with OVA four times. IL-2/JES6-1 antibody complex (IL-2C) or PBS was i.p. injected once daily for three consecutive days during OVA-challenge (A) Experimental scheme. (B) Representative lung section stained with hematoxylin and eosin (scale bar, 100 μm). (C) Histological scores. (D) Total cell number in BAL fluids. (E) Number of cell infiltrates in BAL fluids: eosinophils (left), neutrophils (middle) and lymphocytes (right). (F) Airway hyper-responsiveness was determined at day 2 after final OVA-challenge (n = 4). P-value indicates statistical significance between OVA/Alum and OVA/Alum + IL-2C. Data in C-E are pooled from two independent experiments (n = 6–8). Error bars denote mean ± S.E.M; *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant.
In contrast with the previous findings indicating that IL-2C ameliorates airway inflammation (11), IL-2C treatment did not ameliorate, but rather markedly increased, allergic airway inflammation compared with that in OVA-sensitized/challenged mice that did not receive IL-2C treatment. IL-2C treatment increased lung inflammation, as determined by H&E staining of lung tissue sections (Fig. 1B, C). IL-2C treatment led to a prominent increase in total cell number in bronchoalveolar lavage (BAL) fluids (Fig. 1D), with marked infiltration of eosinophil but not neutrophil (Fig. 1E). IL-2C treatment also resulted in the increase of airway hypersensitiveness as measured by airway resistance to inhaled methacholine (Fig. 1F).
This unexpected effect of IL-2C on airway inflammation is not mouse strain-specific. IL-2C treatment increased airway inflammation in OVA-sensitized/challenged BALB/c mice (Fig. S1A, B). Interestingly, IL-2C treatment before intranasal OVA challenge did not exacerbate airway inflammation (Fig. S1B, C). Collectively, our data indicated that IL-2C treatment during the onset of disease exacerbates allergic airway inflammation.
IL-2/antibody complex increases the number of Th2 cells and type-2 innate lymphoid cells despite the increase in Foxp3+ regulatory T cells
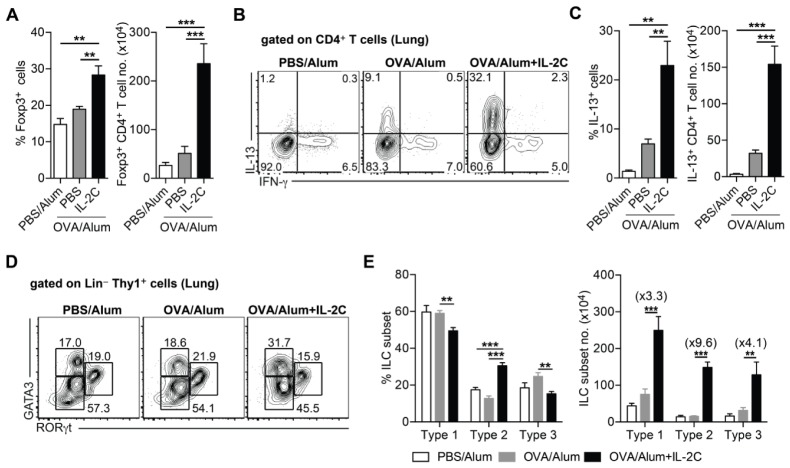
In order to elucidate the mechanism through which IL-2C exacerbates airway inflammation, we first examined CD4+ T cell responses in the lungs of OVA-sensitized/challenged mice with or without IL-2C treatment. As shown previously (11), IL-2C treatment increased the frequency and number of Treg cells at local sites (Fig. 2A). IL-2C prominently increased IL-13-producing CD4+ T cells in the lung (Fig. 2B, C); however, IFN-γ-producing CD4+ T cells was not influenced by the IL-2C treatment (data not shown).
Fig. 2.
IL-2/antibody complex increases the number of Th2 cells and type-2 innate lymphoid cells despite the increase in the number of Foxp3+ regulatory T cells. Allergic airway inflammation was induced as described in Fig. 1. (A) Frequencies of Foxp3+ cells gated on CD4+ T cells (left) and numbers of Foxp3+ CD4+ T cells (right) in the lung from indicated mice. (B) Representative FACS plots of IFN-γ and IL-13. (C) Frequencies of IL-13+ cells gated on CD4+ T cells (left) and number of IL-13+ CD4+ T cells (right) in the lung. (D) Representative FACS plots of GATA3 and RORγt gated on Lin− Thy1+ cells. (E) Frequencies of innate lymphoid cell (ILC) subsets; ILC1, type-1 (GATA3−, RORγt−); ILC2, type-2 (GATA3+, RORγt−); ILC3 type-3 (GATA3−, RORγt+) (left) and numbers of innate lymphoid cell subsets (right). Numbers in parentheses are fold-difference between OVA/Alum and OVA/Alum+IL-2C. Data are pooled from two independent experiments (n = 6–8). Error bars denote mean ± S.E.M; **P < 0.01, ***P < 0.001.
Furthermore, as previously reported (12, 13), IL-2C treatment selectively increased type-2 innate lymphoid cells (ILCs) expressing GATA3 (Fig. 2D, E). Despite the increase in the total number of lung-resident ILCs upon IL-2C treatment, the number of type-2 ILCs was more prominently increased in response to IL-2C treatment than that of Tbet+ type-1 or RORγt+ type-3 ILC subsets (Fig. 2D, E). Given that both Th2 and type-2 ILCs are key mediators in Th2-mediated airway inflammation (13), our data suggest that IL-2C exacerbates airway inflammation through the combined action of Th2 cells and type-2 ILCs.
IL-2/antibody complex ameliorates Th17-mediated airway inflammation
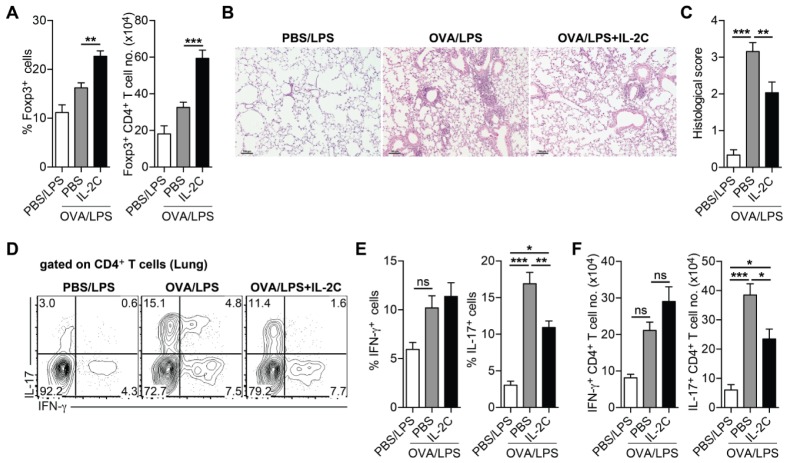
Allergic airway inflammation is heterogeneous and manifests as various characteristic endotypes (14). Experimentally, distinct from Th2-mediated airway inflammation induced by OVA/Alum sensitization, the use of lipopolysaccharide (LPS) as an adjuvant induces Th17-mediated airway inflammation and airway infiltration of neutrophils, but not that of eosinophils (15). To determine whether the exacerbating effect of IL-2C treatment is restricted only to Th2-mediated airway inflammation or whether IL-2C can also exacerbate the pathogenesis of Th17-mediated airway inflammation, mice were sensitized by intranasal treatment with OVA plus LPS and then i.n.-challenged with OVA. During OVA challenge, mice were treated with IL-2C (Fig. S2A).
OVA/LPS-sensitized mice displayed increased airway infiltration of neutrophils, but not eosinophils, upon i.n.-OVA challenge (Fig. S2B–D). In contrast to the effect of IL-2C in Th2-mediated airway inflammation, the total cell number in BAL fluids was significantly reduced in response to IL-2C treatment with the profound reduction in neutrophil number (Fig. S2C, D). As expected, IL-2C treatment significantly increased lung-resident Treg cells (Fig. 3A). IL-2C treatment significantly reduced lung inflammation as evidenced by H&E staining of lung tissue sections (Fig. 3B, C), and also reduced the frequency and number of lung Th17 cells (Fig. 3D–F). Consequently, Airway hypersensitiveness was reduced by IL-2C treatment relative to OVA/LPS-sensitized and OVA-challenged mice (Fig. S2E). These results suggest that IL-2C treatment alleviates Th17-mediated airway inflammation and selectively exacerbates Th2-mediated airway inflammation.
Fig. 3.
IL-2/antibody complex alleviates Th17-mediated airway inflammation. C57BL/6 mice were i.n.-sensitized with OVA or PBS plus LPS and then i.n.-challenged with OVA. During OVA-challenge, OVA-sensitized mice were i.p.-treated with PBS or IL-2C once daily for three consecutive days. (A) Frequencies of Foxp3+ cells gated on CD4+ T cells (left) and numbers of Foxp3+ CD4+ T cells (right) in the lung from indicated mice. (B) Representative lung section stained with hematoxylin and eosin (scale bar, 100 μm). (C) Histological scores. (D) Representative FACS plots of IFN-γ and IL-17 gated on CD4+ T cells in the lung. (E) Frequencies of IFN-γ+ (left) and IL-17+ cells (right) gated on CD4+ T cells in the lung. (F) Numbers of IFN-γ+ (left) and IL-17+ CD4+ T cells (right). Data are pooled from two independent experiments (n = 6–8). Error bars denote mean ± S.E.M; *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant.
IL-2-mediated signaling is required for the expansion of Th2 cells
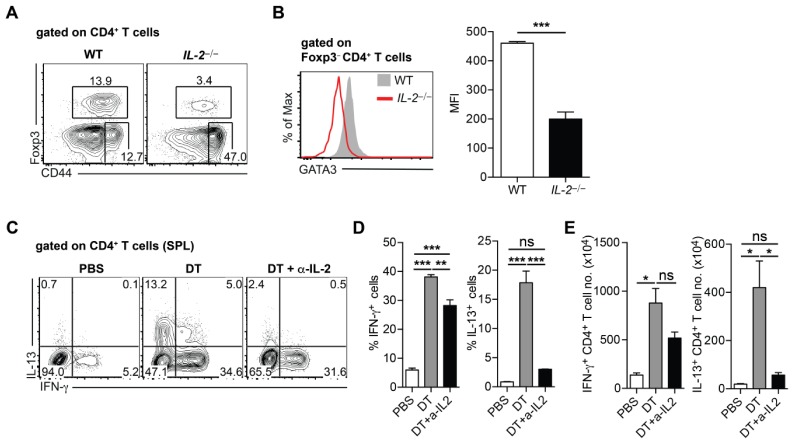
Next, we sought to elucidate the mechanisms underlying the selective expansion of Th2 cells in airway inflammation in response to IL-2C treatment. We first examined GATA3 expression of CD4+ T cells in IL-2−/− mice to examine the role of IL-2 signaling in the generation of Th2 cells. IL-2−/− mice displayed the reduced level of Treg cells and concomitant increase of CD44hi effector T cells, supporting the role of IL-2 signaling in Treg cell proliferation and resistance to apoptosis in Treg cells (16). Interestingly, the level of GATA3 expression was severely decreased in CD4+ T cells relative to that in WT mice (Fig. 4A, B). These results suggest that IL-2 signaling is required for GATA3 expression in CD4+ T cells under steady-state conditions.
Fig. 4.
IL-2-mediated signaling is required for the induction of Th2 cells. (A) Representative FACS plots showing the frequencies of Foxp3+ cells and effector T cells (CD44hi Foxp3−) gated on CD4+ T cells in spleen (SPL) from wild-type (WT) and IL-2−/− mice. (B) Representative histogram showing GATA3 expression (left) and mean fluorescence intensity (MFI) of GATA3 expression gated on CD4+ T cells in SPL from WT and IL-2−/− mice. (C–E) Foxp3-DTR mice were treated with PBS or diphtheria toxin (DT). DT-treated mice were injected with PBS or anti-IL-2 depleting antibody. Cytokine production from CD4+ T cells in SPL was examined. (C) Representative FACS plots of IFN-γ and IL-13 gated on CD4+ T cells in the SPL from indicated mice. (D) Frequencies of IFN-γ+ (left) or IL-13+ (right) cells gated on CD4+ T cells. (E) Number of IFN-γ+ (left) and IL-13+ CD4+ T cells (right). Data represent one of two independent experiments (n = 3–4). Error bars denote mean ± S.E.M; *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant.
It was previously reported that the deficiency of Treg cells leads to a marked increase in both Th1 and Th2 cells, as seen in Foxp3null mice (17). To identify the role of IL-2 signaling in the generation of Th2 cells in this inflammatory setting, Foxp3-DTR mice, which specifically express diphtheria toxin (DT) receptor in their Foxp3+ cells, were treated with DT to deplete Foxp3+ cells in vivo. A cohort of Foxp3-DTR mice treated with DT was injected with anti-IL-2 monoclonal antibody to prevent IL-2 signaling on CD4+ T cells. In vivo depletion of Foxp3+ cells induced a prominent increase of IFN-γ- and IL-13-producing CD4+ T cells in the spleen (Fig. 4C–E). Interestingly, the blockade of IL-2 signaling effectively reduces the number of Th2 cells but not that of Th1 cells (Fig. 4C–E). Collectively, these results indicate that IL-2-mediated signaling is important for the generation of Th2 cells under inflammation.
DISCUSSION
The data presented herein demonstrate that IL-2C exacerbates Th2-mediated airway inflammation but alleviates Th17-mediated airway inflammation. Our data suggest that the effects of IL-2C treatment on allergic airway inflammation are contradictory depending on the characteristics of airway inflammation.
IL-2 signaling promotes Th2 differentiation but negatively regulates Th17 differentiation by activating STAT5 phosphorylation and inactivating STAT3 phosphorylation (18–20). Activation of IL-2/STAT5 and NF-κB pathway through T cell receptor stimulation is required for the robust increase of Th2 cytokine gene expression and enhanced Th2 cell differentiation (20–22). Considering that CD25 is up-regulated in effector CD4+ T cells at early activation stage (23), IL-2C treatment may influence the in vivo generation of effector CD4+ T cell subsets in a T cell-intrinsic manner. In this regard, in Th2-mediated airway inflammation, amplification of IL-2/STAT5 signaling by IL-2C treatment promotes the generation of pathogenic Th2 cells. In line with this, IL-2−/− mice displayed reduced GATA3 expression in CD4+ T cells relative to WT mice. Furthermore, in lymphoproliferative diseases caused by the depletion of Treg cells, IL-2 blockade selectively suppresses Th2 but not Th1 responses. However, in Th17-mediated airway inflammation, IL-2C treatment inhibits the generation of pathogenic Th17 cells, presumably in a T cell-intrinsic manner.
In both Th2- and Th17-mediated airway inflammation models, IL-2C treatment significantly increased the number of lung-resident Treg cells. Endogenous Treg cell expansion by IL-2C treatment may contribute to the reduction of pathogenic Th17 cells in Th17-mediated airway inflammation. However, IL-2C-mediated expansion of endogenous Treg cells did not result in the suppression of pathogenic Th2 cells in Th2-mediated airway inflammation. Given that Treg cells exert their suppressive functions through multiple mechanisms (2, 3), it is also possible that Treg cells suppress Th2 responses primarily through the deprivation of IL-2. Hence, exogenous administration of IL-2C interferes with the Treg cell-mediated suppression of Th2 responses despite the increase in the number of tissue-resident Treg cells.
Type-2 ILCs are known to promote Th2 responses by producing pro-Th2 cytokines such as IL-5, IL-13, and IL-4 (24), and also by presenting cognate antigens through the expression of MHC class II (25). Accordingly, the depletion of type-2 ILCs has been shown to impair protective Th2 responses during Nippostrongylus brasiliensis infection (25). Type-2 ILCs constitutively express CD25 and IL-2C treatment can expand type-2 ILCs (12). We also found that type-2 ILCs were preferentially increased by IL-2C treatment. In this context, IL-2C-mediated expansion of type-2 ILCs may play an important role in promoting the local expansion of Th2 cells. We also found that the preferential expansion of type-2 ILCs by IL-2C treatment in Th17-induced airway inflammation model (data not shown). However, during the onset of allergic airway diseases, the precise role of IL-2C-mediated alteration in lung-resident ILCs in the expansion of pathogenic effector CD4+ T cells remains to be further investigated.
Previously, it was reported that IL-2C administration alleviates airway inflammation induced by soluble egg antigen from Schistosoma mansoni through the expansion of functional IL-10-producing Treg cells in vivo (11). However, in the present work, we were unable to demonstrate the therapeutic benefit of IL-2C in Th2-mediated airway inflammation, presumably due to the difference in the experimental regimens. In addition, given that commensal microbiota influence the pathogenesis of allergic airway diseases (26, 27), the observed discrepancy in the effect of IL-2C on allergic airway disease may be attributable to the difference in the composition of the commensal microbiota. However, it still remains to be further investigated whether commensal microbiota influence IL-2C-mediated regulation of allergic airway inflammation.
In conclusion, our data demonstrate that the IL-2/antibody complex, which expands endogenous Treg cells in vivo, exacerbates Th2-mediated allergic airway inflammation. Therefore, the therapeutic application of IL-2/antibody complex needs to be carefully considered based on the characteristics of allergic airway diseases.
MATERIALS AND METHODS
Animals
Specific pathogen-free (SPF) C57BL/6J, BALB/c, IL-2−/− mice were purchased from The Jackson Laboratory. Foxp3-IRES-DTR-GFP (Foxp3-DTR) mice were kindly provided by Dr. Alexander Rudensky (Memorial Sloan Kettering Cancer Center, USA) via Dr. Dipayan Rudra (Institute for Basic Science, Korea). Mice were housed at the animal facility of POSTECH Biotech Center in accordance with the guidelines of the institutional animal care and use committee (IACUC #: POSTECH-2017-0053).
Reagents
For preparation of the IL-2/JES6-1 complex, 1 μg of recombinant murine IL-2 (ProSpec, Israel) was mixed with JES6-1 (5 μg/mouse, Bio X cell, USA) in sterile PBS with gentle agitation for overnight at 4°C. Freshly prepared IL-2/JES6-1 complex was i.p.-injected into mice once a day for three consecutive days. To in vivo deplete Foxp3+ T cells in Foxp3-DTR mice, mice were i.p.-injected with DT (1 μg/mouse, Sigma-Aldrich) every other day for a week. For in vivo blockade of IL-2 signaling, anti-IL-2 mAb (JES6-1) was produced from hybridoma (clone #JES6-1A12 obtained from ATCC) and anti-IL-2 mAb (200 μg/mouse) was i.p.-injected into mice every other day for a week.
OVA-induced airway inflammation
For Th2-induced allergic airway inflammation, mice were sensitized by i.p.-injection with PBS or 100 μg of OVA in PBS (Sigma-Aldrich) emulsified in 2 mg of alum (Imject alum. Thermo Scientific). For i.n.-challenge, mice were anesthetized with Avertin (2,2,2-Tribromoethanol, Sigma-Aldrich) and administered 50 μg of OVA through the nasal route. Mice were sacrificed 48 h after a final challenge, and airway infiltrates and pro-inflammatory responses in the lung were examined. For Th17-induced allergic airway inflammation, mice were i.n.-sensitized with PBS or 100 μg of OVA plus 10 μg of lipopolysaccharide from E. coli strain O26:B6 (LPS, Sigma-Aldrich); then, mice i.n.-challenged with 50 μg of OVA.
Cell isolation
Spleens were collected from the mice and single-cell suspensions were generated by mechanical disruption. For isolating single-cell suspensions from the lung tissue, collected lung tissues were minced with a razor blade and further digested with DNase I and Collagenase D for 45 minutes at 37°C in RPMI medium containing FBS (3% vol/vol), HEPES (20 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), sodium pyruvate (1 mM), and non-essential amino acids (1 mM). For isolating bronchoalveolar lavage (BAL) cells, mice were anesthetized and trachea were cannulated and lavaged with sterile PBS. Fluids were centrifuged and cell pellets were recovered to enumerate BAL cells.
Lung histopathology
Lung tissues were fixed with 4% (w/v) paraformaldehyde. Fixed samples were paraffin-embedded, cut into 5-μm sections, and stained with hematoxylin and eosin by standard procedures. Images were acquired with a Nikon ECLIPSE TS100 microscope and processed using imaging software NIS-Elements F 4.00.00. Histology scores were measured as described previously (28).
Flow cytometry
Isolated cells were stained with Ghost viability dye (Tonbo) to exclude dead cells. Cells were surface-stained with the following fluorochrome-conjugated antibodies (eBioscience, Biolegend, Tonbo, and R&D Biosciences): anti-CD16/32 Fc Blocker (93), anti-CD4 (RM4-5), anti-TCRβ (H57-597), anti-CD44 (IM7), anti-Thy1.2 (53-2.1), anti-B220 (RA3-6B2), anti-I-A/I-E (M5/114/15.2), anti-CD11c (N418), anti-CD11b (M1/70), anti-Ly6G (RB6-8C5), and anti-Siglec F (E50-2440). For intracellular staining, surface-stained cells were fixed and permeabilized with a Foxp3 staining kit (eBioscience) according to manufacturer’s instructions, and stained with the following antibodies: anti-Foxp3 (FJK-16s), anti-GATA3 (TWAJ), anti-RORγt (B2D), anti-IFN-γ (XMG1.2), anti-IL17a (eBio17/B7), anti-IL13 (eBio13A), and anti-IL10 (JES5-16E3). For in vitro T cell stimulation, isolated cells were cultured for 3 h in RPMI-1640 medium containing 10% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml), and 55 μM β-mercaptoethanol in the presence of PMA/Ionomycin and protein transport inhibitors (eBioscience). Stained cells were acquired on a FACS Fortessa or FACS Canto-II flow cytometer with DIVA software (BD Biosciences), and FACS data were analyzed using FlowJo software (TreeStar).
Assessment of airway hyper-responsiveness (AHR)
At 2 day after the final OVA challenge, mice were anesthetized by i.p. injection of sodium pentobarbital (Hanlim Pharmaceutical, Korea, 120 mg/kg of body weight). Mice were tracheostomized and connected via the endotracheal cannula to a flexiVent system (SCIREQ Inc., Montreal, Canada). Airway resistance was determined in response to progressive concentrations of inhaled methacholine administration (0, 25, 50 mg/ml). Data were acquired by the flexiWare V8.0 software.
Statistical analyses
Mean and S.E.M. values were calculated using Prism 6 (GraphPad Software). Statistical significance between two variables was determined by unpaired two-tailed t-tests. Where appropriate, two-way or one-way ANOVA followed by Tukey’s multiple comparisons test were performed. P-values less than 0.05 were considered to indicate statistical significance.
SUPPLEMENTARY INFORMATION
ACKNOWLEDGEMENTS
This work was supported by Project IBS-R005-D1 from the Institute for Basic Science, National Research Foundation, Korean Ministry of Science, Information/Communication Technology and Future Planning and by the Global Ph.D. fellowship program (NRF-2015H1A2A1031937) through the National Research Foundation of Korea funded by the Ministry of Education (for E.O).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol. 2016;138:639–652. doi: 10.1016/j.jaci.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorburn AN, Hansbro PM. Harnessing regulatory T cells to suppress asthma: from potential to therapy. Am J Respir Cell Mol Biol. 2010;43:511–519. doi: 10.1165/rcmb.2009-0342TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamessier E, Nieves A, Lorec AM, et al. T-cell activation during exacerbations: a longitudinal study in refractory asthma. Allergy. 2008;63:1202–1210. doi: 10.1111/j.1398-9995.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- 5.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 8.Spangler JB, Tomala J, Luca VC, et al. Antibodies to Interleukin-2 Elicit Selective T Cell Subset Potentiation through Distinct Conformational Mechanisms. Immunity. 2015;42:815–825. doi: 10.1016/j.immuni.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster KE, Walters S, Kohler RE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smaldini PL, Trejo F, Cohen JL, Piaggio E, Docena GH. Systemic IL-2/anti-IL-2Ab complex combined with sublingual immunotherapy suppresses experimental food allergy in mice through induction of mucosal regulatory T cells. Allergy. 2018;73:885–895. doi: 10.1111/all.13402. [DOI] [PubMed] [Google Scholar]
- 11.Wilson MS, Pesce JT, Ramalingam TR, Thompson RW, Cheever A, Wynn TA. Suppression of murine allergic airway disease by IL-2:anti-IL-2 monoclonal antibody-induced regulatory T cells. J Immunol. 2008;181:6942–6954. doi: 10.4049/jimmunol.181.10.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roediger B, Kyle R, Yip KH, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roediger B, Kyle R, Tay SS, et al. IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. J Allergy Clin Immunol. 2015;136:1653–1663 e1657. doi: 10.1016/j.jaci.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Wesolowska-Andersen A, Seibold MA. Airway molecular endotypes of asthma: dissecting the heterogeneity. Curr Opin Allergy Clin Immunol. 2015;15:163–168. doi: 10.1097/ACI.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YS, Hong SW, Choi JP, et al. Vascular endothelial growth factor is a key mediator in the development of T cell priming and its polarization to type 1 and type 17 T helper cells in the airways. J Immunol. 2009;183:5113–5120. doi: 10.4049/jimmunol.0901566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitehouse G, Gray E, Mastoridis S, et al. IL-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc Natl Acad Sci U S A. 2017;114:7083–7088. doi: 10.1073/pnas.1620835114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 18.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Yang XP, Ghoreschi K, Steward-Tharp SM, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson MR, Ulrich BJ, Hummel SA, et al. Paracrine IL-2 Is Required for Optimal Type 2 Effector Cytokine Production. J Immunol. 2017;198:4352–4359. doi: 10.4049/jimmunol.1601792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagami S, Nakajima H, Suto A, et al. Stat5a regulates T helper cell differentiation by several distinct mechanisms. Blood. 2001;97:2358–2365. doi: 10.1182/blood.V97.8.2358. [DOI] [PubMed] [Google Scholar]
- 22.Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 23.Garcia S, DiSanto J, Stockinger B. Following the development of a CD4 T cell response in vivo: from activation to memory formation. Immunity. 1999;11:163–171. doi: 10.1016/S1074-7613(00)80091-6. [DOI] [PubMed] [Google Scholar]
- 24.Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliphant CJ, Hwang YY, Walker JA, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong SW, Kim KS, Surh CD. Beyond Hygiene: Commensal Microbiota and Allergic Diseases. Immune Netw. 2017;17:48–59. doi: 10.4110/in.2017.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd CM, Gonzalo JA, Nguyen T, et al. Resolution of bronchial hyperresponsiveness and pulmonary inflammation is associated with IL-3 and tissue leukocyte apoptosis. J Immunol. 2001;166:2033–2040. doi: 10.4049/jimmunol.166.3.2033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.