Abstract
Objectives. To assess the effect of 2-way short message service (SMS) with a nurse on postpartum contraceptive use among individual women and couples.
Methods. From 2016 to 2017, we conducted a randomized controlled trial at 2 public hospitals in western Kenya. We assigned eligible pregnant women to receive 2-way SMS with a nurse or no SMS, with the option to include male partners. We delivered automated family planning–focused SMS messages weekly until 6 months postpartum. Women and men receiving SMS could interact with nurses via SMS. In intention-to-treat analysis, we compared highly effective contraceptive (HEC) use at 6 months postpartum between groups using the χ2 test. We used Poisson regression in adjusted analysis.
Results. We randomized 260 women to 2-way SMS or control, and we enrolled 103 male partners. At 6 months postpartum, 69.9% women receiving SMS reported HEC use, compared with 57.4% in control (relative risk = 1.22; 95% confidence interval [CI] = 1.01, 1.47; P = .04). In analysis adjusted for baseline demographic differences, the adjusted relative risk for HEC use in the SMS group was 1.26 (95% CI = 1.04, 1.52; P = .02).
Conclusions. Two-way SMS with a nurse, including optional male participation, increased postpartum contraceptive use.
Trial Registration. ClinicalTrials.gov; identifier: NCT02781714.
Addressing high unmet need for contraception among postpartum women in low- and middle-income countries is a global public health priority.1,2 Using a prospective measure of unmet need, demographic and health survey data from 2006 to 2012 estimate that within 1 year after delivering, 65% of postpartum women in East and Southern Africa did not desire pregnancy for at least 2 years but were not using contraception.3 In Kenya, unmet need for limiting and spacing pregnancies within the first 2 years postpartum has been estimated at 57%.4 More recent data demonstrate that 33% of postpartum Kenyan women attending 6- and 9-month infant immunization visits report an unmet need for family planning (FP).5 Recognizing the missed opportunities for improving reproductive and child health outcomes,6 the FP2020 global initiative identified postpartum FP within 1 year of delivery as a critical component of global FP strategy.7
Postpartum women have specific needs that may not be well served by traditional FP programs. Pregnancy risk perception during lactation and prior to resuming menses influences FP use.8–10 Contraceptive side effect and safety concerns,9,10 low attendance of postpartum visits,11 and concern about partner disapproval12 are other barriers to meeting postpartum women’s needs. The majority of postpartum women in Kenya initiate short-acting methods, and method discontinuation is common.4,13 A recent study found that although 49% of women in a Nairobi urban slum cohort initiated a method by 6 months postpartum, almost half discontinued within 12 months.13
Male partners are known to play a critical role in reproductive decision-making,14,15 and there is great global interest in strategies to engage them in FP,16 but few programs have successfully incorporated men. Qualitative research from Kenya highlights men’s and women’s desire for men to be included in FP education, as well as widespread male resistance to FP.17,18 There is an urgent need for innovative strategies to meet women’s postpartum contraceptive needs and to understand optimal approaches to men’s engagement.
Interventions using mobile health (mHealth) technologies, including short message service (SMS), have shown promise in a variety of contexts in resource-limited settings, such as improvements in skilled delivery attendance19 and antiretroviral therapy adherence,20 although evidence is mixed.21 There is limited evidence of efficacy for mHealth approaches to improving outcomes in FP.22 Trials of mobile phone interventions in the United States and Cambodia demonstrated increased oral contraceptive continuation and postabortion contraceptive uptake,23,24 whereas a FP-focused SMS program in Kenya showed no effect on contraceptive use.25 Despite the paucity of data on the use of SMS for postpartum contraceptive education and counseling, SMS communication could be a valuable adjunct to facility-based care. In light of the specific barriers facing postpartum women’s contraceptive access, an interactive and tailored program allowing for SMS dialogue with a clinician (2-way SMS) and real-time support may promote uptake and continuation. Our intervention, the Mobile WACh XY trial, aimed to evaluate the effect of 2-way SMS on postpartum contraceptive use among women and couple dyads.
METHODS
Mobile WACh XY was an unblinded, randomized controlled trial implemented at 2 public hospitals in the Kisumu and Siaya counties of western Kenya. These facilities serve predominantly low-income, rural populations.
Participants
Female participants were recruited from antenatal clinics by study nurses and screened with a tablet-based questionnaire. Eligible women were 14 years of age or older; were pregnant, with an estimated gestational age of 28 weeks or more; were able to read and respond to SMS themselves or with assistance from a family member or friend in English, Kiswahili, or Dholuo; reported daily access to a mobile phone using the Safaricom network; planned to remain in the area; reported HIV negative status; and were not participating in another research study. We excluded HIV-infected women because of their distinct peripartum health needs, although a related trial specific to women living with HIV was ongoing at the same sites.26 The written informed consent process emphasized that SMS content included overt FP information, and that privacy concerns should be considered prior to enrollment. In Kenya, women aged 14 to 17 years who become pregnant or have children are legally considered adults.27
Staff asked enrolled women about their partnership status. If partnered, they had the opportunity to refer their male partners for recruitment into the trial; the partners would receive the same allocation if enrolled. Male partner referral was optional. Each referred partner was contacted by phone by male study staff, and study visits occurred in the community setting.
Randomization
Pregnant women were randomly assigned (1:1) to either 2-way SMS or control (no SMS) groups using random block randomization. Staff distributed sequentially numbered, sealed, opaque envelopes containing allocation assignments to each site. Participants and staff could not be masked to group allocation, because of the nature of the intervention. Staff enrolled male partners within 3 weeks of randomization and allocated them to the same group as their female partners.
Procedures
All women received standard antenatal care. Women allocated to SMS registered their mobile phone numbers and received an intervention orientation at enrollment. The Mobile WACh platform, described in detail elsewhere,26 is a human–computer hybrid communication system designed at the University of Washington that enables automated SMS and SMS dialogue between participants and clinicians (Figure A, available as a supplement to the online version of this article at http://www.ajph.org). The online system, hosted on a secure server, allows study nurses to view and answer messages and to manage visit dates and participant-specific details. Participants indicate their language of choice (English, Kiswahili, or Dholuo), preferred name, and preferred day and time to receive automated messages. Nurses demonstrated that sending SMS was free of charge, explained how to discontinue SMS, and explained that the SMS system should not be used for urgent medical needs.
We used the theory of planned behavior28 to guide message development; we refined FP message content and adapted the SMS platform from the findings of formative focus group discussions.29 Automated SMS contained a health education message, and each message ended with actionable advice or a question designed to promote SMS dialogue. Automated message content centered around FP (approximately two thirds of all messages), and included information about available methods and their effectiveness, postpartum pregnancy risk, contraceptive safety during lactation, anticipatory guidance about side effects, community misperceptions, and dual protection. The remaining third of messages were focused on general perinatal topics, such as healthy pregnancy and exclusive breastfeeding. The SMS platform sent automated system messages once weekly from enrollment to 6 months postpartum, with message content corresponding to participants’ gestational age or week postpartum. When postpartum women or couples indicated initiation of a method, they switched into method-specific automated messaging, which was designed to support continuation and side effect management related to the chosen method.
Women whose male partners were referred for the trial received messages in the couple’s specific language. If the male partner was not enrolled within the 3-week period, the SMS platform sent automated messages to the woman only. The nurse could reply to 1 or both members of the couple, as appropriate, and track conversations of both partners together on the online interface.
Study nurses administered an enrollment questionnaire to all female participants, and women were asked to notify the study team when they delivered. Study nurses called each participant 2 weeks after the estimated or actual delivery date. The nurses scheduled study visits at the facility at 6 and 14 weeks and 6 months postpartum, corresponding with the infant immunization schedule. At each visit, a nurse administered a questionnaire. FP use and date of initiation were ascertained by self-report. If a participant stated an intention to start a contraceptive method but had not yet initiated the method, she was not considered a contraceptive user. Participants received a phone call 2 weeks after a missed 6-week or 6-month visit to reschedule. For participants who could not be reached by phone, staff completed final study visits at home visits. We trained study staff to avoid clinical counseling during non-SMS interaction with participants. Male partners completed questionnaires with male study staff at enrollment and at 6 months postpartum in the community setting.
Outcomes
The primary outcome of the trial was current self-reported use of a highly effective contraceptive (HEC) method at 6 months postpartum. We defined highly effective methods as modern methods available in Kenya with a typical use failure rate of less than 10%,30 as follows: permanent contraception (male or female), contraceptive implant, copper intrauterine device (IUD), injectable contraception (depot medroxyprogesterone acetate), and oral contraception. Any contraceptive use included use of a HEC, barrier, calendar, or lactational amenorrhea method (at visits prior to 6 months postpartum). Secondary outcomes included HEC use at 6 and 14 weeks, any contraceptive use, exclusive breastfeeding, FP satisfaction, contraceptive discontinuation by 6 months postpartum (discontinuation of a HEC or barrier method without starting another method), and time to first initiation of any method. Dual contraceptive use was a prespecified secondary outcome, but it is not reported here because of inadequate capture of condom use with another method in data collection.
Statistical Analysis
We calculated that a sample size of 200 would be required to detect a 50% increase in HEC use at 6 months postpartum in the intervention versus control arm with 80% power and α = 0.05, assuming 40% estimated HEC use in the control population.5,31 To account for potential attrition, we increased our sample size to 260 women.
All analyses were intention to treat; we completed them in Stata 14.2 (StataCorp LP, College Station, TX). We compared categorical primary and secondary outcomes using the χ2 test. We calculated relative risk, with a value greater than 1 indicating higher contraceptive use in the intervention group. We used Poisson regression with robust standard errors to adjust for variables that were unbalanced between groups at baseline. In secondary analysis of initiation of any contraceptive method, we assessed the relationship between the intervention and time to initiation of any contraceptive method using Cox proportional hazards regression. We estimated probabilities of contraceptive initiation by 6 and 14 weeks and 6 months postpartum using the Kaplan–Meier method and we compared them using the Wald test. A test of proportional hazards demonstrated no violation of the assumption. We compared prespecified subgroup analyses of the primary outcome by male partner referred or enrolled status using the χ2 test. We registered the trial at ClinicalTrials.gov; identifier: NCT02781714.
RESULTS
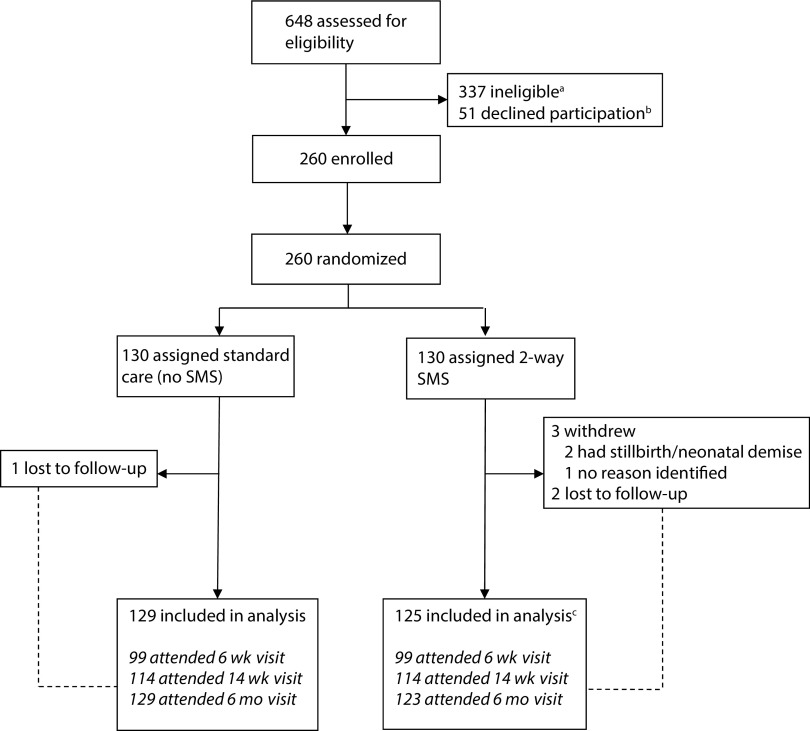
Between July 19 and December 6, 2016, staff assessed 648 women for eligibility; 377 (58%) were ineligible, 51(8%) declined participation or preferred to return for enrollment at a different time, and 260 (40%) were enrolled (Figure 1). The most common reasons for ineligibility (nonexclusive) were gestational age less than 28 weeks (46%), no daily access to a mobile phone (28%), lack of a Safaricom subscriber identification module (SIM) card (12%), and receipt of antenatal care elsewhere (18%). Three participants withdrew and 3 were lost to follow-up prior to the first study visit; a total of 254 participants were included in the analysis. An additional 2 intervention participants were lost to follow-up after attending the 14-week visit. Two intervention women requested discontinuation of the intervention (included in the analysis). Primary outcome data were available for 252 women (97% overall retention).
FIGURE 1—
CONSORT Flow Diagram for the Mobile WACh XY Randomized Controlled Trial: Kenya, 2016–2017
Note. SMS = short message service.
aReasons for ineligibility (participants could choose more than 1 reason, so percentages do not add up to 100%): gestational age less than 28 weeks: 155 (46%); no daily access to mobile phone: 95 (28%); no Safaricom SIM: 40 (12%); does not know phone number: 15 (4%); unwilling to receive SMS: 4 (1%); receiving antenatal care elsewhere: 60 (18%); other research study: 31(9%); HIV positive: 4 (1%); younger than 14 years: 2 (0.5%).
bThirty-one of 51 wanted to consult a spouse or return for enrolment at another time; some of these participants may have been rescreened and enrolled in the study.
cTwo participants who were included in the analysis stopped the SMS intervention upon their request.
Characteristics at Randomization
Baseline characteristics of the women are shown in Table 1 (for men’s baseline characteristics, see online Table A). Median age was 23 years (interquartile range [IQR] = 20–26.5), and median gestational age at enrollment was 32 weeks (IQR = 30–36). Most women were partnered (76.2%) and currently married (70.4%). Of 198 partnered women, 158 (79.8%) referred their male partners for inclusion in the trial and 103 (52.0%) were enrolled. The majority had an education of primary school or less (54.2%), were parous (56.5%), and had ever used a contraceptive method (58.5%). Half of enrolled women (50.0%) considered their current pregnancies to be unintended (i.e., they had not wanted another pregnancy) or mistimed. Forty-six (17.7%) shared their phones and 97 (37.3%) reported ever having used the Internet. The groups were balanced at baseline, with the exception of education category, parity, and desire for another child in the future.
TABLE 1—
Characteristics of Female Trial Participants at Baseline: Kenya, 2016–2017
| Variable | 2-Way SMS (n = 130), No. (%) or Median (IQR) | Control (n = 130), No. (%) or Median (IQR) |
| Site | ||
| Ahero Sub-County Hospital, Kisumu County | 62 (47.7) | 62 (47.7) |
| Bondo County Hospital, Siaya County | 68 (52.3) | 68 (52.3) |
| Age, y | 22.5 (22–26) | 23 (19–28) |
| Education, y | 12 (10–16) | 12 (8–15) |
| Education* | ||
| Less than primary | 8 (6.2) | 10 (7.7) |
| Primary completed | 50 (38.5) | 73 (56.2) |
| Secondary completed | 38 (29.2) | 33 (25.4) |
| Postsecondary | 34 (26.2) | 14 (10.8) |
| Married | 90 (69.2) | 93 (71.5) |
| Polygamous marriage | 12 (9.2) | 13 (10.0) |
| Partnered at enrollment | 96 (73.9) | 102 (78.5) |
| Referred partnera | 80 (83.3) | 78 (76.4) |
| Partner enrolleda | 53 (55.2) | 50 (49.0) |
| Length of relationship, ya | 3 (1–6) | 3.5 (2–8) |
| Individual income, monthly, in Kenyan shillingsb | 0 (0–5000) | 400 (0–5000) |
| Gestational age at enrollment, wk | 33 (31–36) | 32 (30–35) |
| Parity* | ||
| 0 (nulliparous) | 62 (47.7) | 51 (39.2) |
| 1 | 39 (30.0) | 30 (23.1) |
| 2 | 15 (11.5) | 25 (19.2) |
| 3 | 7 (5.4) | 10 (7.7) |
| ≥ 4 | 7 (5.4) | 14 (10.8) |
| Current pregnancy unintended or mistimedc | 62 (48.1) | 68 (52.7) |
| Ever use FP | 73 (56.2) | 79 (60.8) |
| Ever use highly effective contraception | 62 (47.7) | 66 (50.8) |
| Desires no more childrend,* | 30 (23.3) | 44 (34.4) |
| Ever discussed FP with partnera | 74 (77.1) | 81 (79.4) |
| Believes male partner approves of FPa | 81 (84.4) | 79 (77.5) |
| Shares mobile phone | 22 (16.9) | 24 (18.5) |
| Using mobile phone for < 1 y | 17 (13.6) | 16 (12.4) |
| Has ever used the Internet | 53 (40.8) | 44 (33.9) |
Note. FP = family planning; IQR = interquartile range.
Percentages calculated on the basis of number partnered (n = 198) as denominator.
n = 112 for 2-way SMS; n = 109 for control.
n = 129 for 2-way SMS; n = 129 for control.
n = 129 for 2-way SMS; n = 128 for control.
Statistically significant (P < .05) differences between the groups (χ2 test).
Outcomes
The primary outcome of HEC use at 6 months postpartum was significantly higher among women in the SMS group (69.9%) than in the control group (57.4%) (relative risk [RR] = 1.22; 95% confidence interval [CI] = 1.01, 1.47; P = .04). In the adjusted analysis, 6-month HEC use remained associated with treatment assignment (adjusted RR [aRR] = 1.26; 95% CI = 1.04, 1.41; P = .02).
Overall contraceptive use was similar in the SMS and control groups at each of the 3 time points in the unadjusted analysis (at 6 weeks: RR = 1.00; 95% CI = 0.60, 1.66; P = .99; at 14 weeks: RR = 1.12; 95% CI = 0.89, 1.41; P = .34; at 6 months: RR = 1.18; 95% CI = 1.00, 1.38; P = .05; Table 2). When adjusted for education, parity, and desire for future children, the intervention group was more likely to use contraception at 6 months (aRR = 1.19; 95% CI = 1.01, 1.41; P = .04). Contraceptive users in both groups reported similarly high levels of satisfaction with their methods (Table 2). HEC use at 6 and 14 weeks postpartum did not differ between groups.
TABLE 2—
Effect of 2-Way Short Message Service (SMS) on Contraceptive Method Use and Method Satisfaction: Kenya, 2016–2017
| Time Point | 2-Way SMS, No. (%) | Control, No. (%) | RR (95% CI) | ARR (95% CI)a |
| 6 wk | ||||
| Any method use | 22 (22.7) | 23 (22.8) | 1.00 (0.60, 1.66) | 1.04 (0.61, 1.76) |
| HEC useb | 14 (14.4) | 19 (18.8) | 0.77 (0.41, 1.44) | 0.86 (0.45, 1.65) |
| LARC/PC use | 6 (6.2) | 10 (9.9) | 0.62 (0.24, 1.65) | 0.66 (0.22, 1.95) |
| 14 wk | ||||
| Any method use | 67 (59.3) | 61 (53.0) | 1.12 (0.89, 1.41) | 1.09 (0.86, 1.38) |
| HEC use | 58 (51.3) | 54 (47.0) | 1.09 (0.84, 1.42) | 1.07 (0.81, 1.41) |
| LARC/PC use | 26 (23.0) | 24 (20.9) | 1.10 (0.68, 1.80) | 1.04 (0.63, 1.71) |
| Satisfied with methodc,d | 62 (91.2) | 52 (86.7) | 1.05 (0.93, 1.19) | 1.07 (0.95, 1.20) |
| 6 mo | ||||
| Any method use | 93 (75.6) | 83 (64.3) | 1.18 (1.00, 1.38) | 1.19 (1.01, 1.41) |
| HEC usee | 86 (69.9) | 74 (57.4) | 1.22 (1.01, 1.47) | 1.26 (1.04, 1.52) |
| LARC/PC use | 34 (27.6) | 36 (27.9) | 1.00 (0.67, 1.48) | 0.92 (0.62, 1.38) |
| Satisfied with methodc,f | 86 (94.5) | 83 (97.7) | 0.97 (0.91, 1.03) | 0.96 (0.91, 1.02) |
Note. ARR = adjusted relative risk; CI = confidence interval; HEC = highly effective contraceptive; LARC = long-acting reversible contraception (implant, intrauterine device); PC = permanent contraception (bilateral tubal ligation); RR = relative risk.
ARR computed by Poisson regression with robust standard errors, adjusted for education, category, parity, and desire for future children.
Includes hormonal, intrauterine, and permanent contraception.
Satisfied or highly satisfied with method on 5-point Likert scale.
n = 128.
Primary outcome.
n = 178.
Injectable contraception was the most popular method in both groups, accounting for 23.2% of participants at 14 weeks. At 6 months, 31.7% of all attendees were using injection (online Table B). Long-acting reversible contraception use, specifically the contraceptive implant, was also high in both groups. Implant users made up 19.7% and 25.4% of participants at 14 weeks and 6 months, respectively. No participants reported lactational amenorrhea as their method of contraception at the 14-week or 6-month visits. Method mix was similar between the groups at 14 weeks, but by 6 months postpartum more women in the intervention group were using HEC (Table 2). Contraceptive discontinuation at 6 months was comparable in the SMS and control groups at 1.6% (P = .96).
Most women (31.8% at 6 weeks, 57.9% at 14 weeks, and 67.7% at 6 months) reported having resumed sexual intercourse by 6 months. These proportions did not differ by group at any time point (data not shown). Fertility intentions were similar between groups at the 6-month visit; 66 (26.2%) reported a desire to stop childbearing, and among 184 who wanted to become pregnant again, 163 (88.6%) preferred to delay the next pregnancy by at least 3 years (data not shown). Only 1 repeat pregnancy (of an SMS-group participant who experienced neonatal death) was reported during study follow-up. Exclusive breastfeeding was similar between groups at all the time points (data not shown).
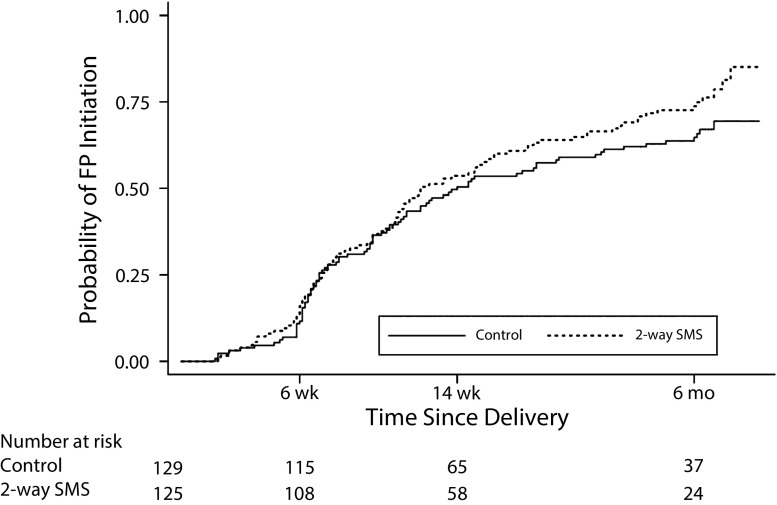
Time to first initiation of any contraception, adjusted for education, parity, and desire for future children, did not differ by group (hazard ratio = 1.31; 95% CI = 0.96, 1.78; P = .09; Figure 2). By 6 months postpartum, the probability of FP method initiation was higher in the intervention group than in the control group, but the difference was not statistically significant (0.74 vs 0.65; P = .12).
FIGURE 2—
Probability of Family Planning (FP) Initiation Over Time Since Delivery: Kenya, 2016–2017
Note. SMS = short message service.
We performed stratified analyses of HEC use at 6 months postpartum by both male partner referral status (determined prerandomization) and male partner enrollment status (i.e., partners enrolled after referral; online Table B). When we stratified data by partner referral status, there was no statistically significant difference in HEC use at 6 months postpartum between the groups in any stratum. HEC use at 6 months postpartum was higher in the intervention than in the control group among women whose partners were not enrolled (n = 92; 72.5% vs 51.9%; P = .045), but not among those with enrolled partners (n = 100; 80.4% vs 77.6%; P = .73) or unpartnered women (n = 60; 50.0% vs 32.1%; P = .16).
SMS Platform Use
Over the study period, the SMS platform delivered over 6800 messages to female and male intervention group participants. The overall response rate (sent at least 1 SMS) was 94.6% among women and 80.7% among men. Female participants sent 3188 messages (median = 20 messages per woman; IQR = 9–38), the majority in response to system messages. Male participants sent 387 messages (median = 7 messages per man; IQR = 3–13). Study nurses sent 1950 messages in response to participant questions. The largest proportions (41% among women and 34% among men) of incoming messages from participants were related to FP, followed by antenatal and delivery concerns.
DISCUSSION
In this randomized trial of a 2-way FP-focused SMS intervention with optional enrollment of male partners, HEC use at 6 months postpartum was significantly higher in the intervention group. Women randomized to SMS were 26% more likely to be using an HEC method at 6 months. We observed a high level of participant engagement in SMS dialogue with study nurses, which supports the conclusion that semiautomated but personalized SMS effectively facilitates FP initiation in the postpartum period.
To our knowledge, no other published studies have reported on messaging tailored for women and couples in the postpartum period. Our finding of increased HEC use in the intervention arm is consistent with some mHealth contraception studies in non-postpartum cohorts, including a daily 1-way SMS program in the United States that improved oral contraception continuation at 6 months23 and increased postabortion contraception use among women who received an interactive voice response intervention.24 However, a free SMS-based service (m4RH) providing FP information requested by consumers and made widely available in Kenya did not show an effect on contraceptive use, although participants did improve their scores on contraceptive knowledge tests.25 The authors concluded that SMS messaging alone is insufficient to change behavior. By contrast, our findings suggest that it may require a more personalized and interactive SMS approach to engage participants at the level needed to motivate and support contraceptive use. The mechanisms by which SMS interventions have an effect on contraceptive and other health outcomes probably differ on the basis of the content and delivery of the intervention and participant context, and they require additional research. Our findings are consistent with an earlier randomized controlled trial using the Mobile WACh SMS platform, which used a non-FP-focused messaging strategy to promote urban maternal child health service utilization.32 That trial demonstrated a high prevalence of contraceptive initiation in all arms by 6 months, as well as higher rates of exclusive breastfeeding among those receiving SMS.32 These findings suggest that message content—rather than simply receiving SMS—matters in motivating specific behavior change.
Contraceptive use was higher than anticipated in our study in both arms, likely because of sampling from a population already accessing health care services and steadily increasing contraceptive prevalence in Kenya.33 We based our power calculation on population-based data sources from Kenya suggesting a 40% to 50% contraceptive prevalence at 6 months postpartum,5,31 which is substantially lower than observed in our study: 64% of control participants were using a method by 6 months. It is possible that study participation, regardless of SMS exposure, encouraged care-seeking behavior. Although injectable contraception was the largest contributor to method mix at all time points, contraceptive implant use was unexpectedly high in both groups. Approximately 25% of participants were using implants at 6 months postpartum, in contrast to 12.4% in the Nyanza region in the 2014 demographic and health survey.33 High implant use may be related to counseling about the implant being a safe method during lactation. Alternatively, longer duration of use may have been attractive to women during the study period, which spanned 2 government health worker strikes, leading to unpredictable availability of FP services.
In stratified analysis, we observed a larger effect size of the SMS intervention among partnered women whose partners were not enrolled, compared with subgroups of couple dyads and unpartnered women. Among women with enrolled partners, contraceptive uptake was high in both trial arms and higher than among women without enrolled partners. This finding is consistent with our hypothesis that women whose male partners enrolled would be more likely to use contraception, perhaps because they were in more stable relationships and knew their partners to be supportive. Enrolled male partners engaged with the system, although at lower frequency, as we expected.34 However, the study was not powered to detect a difference within subgroups. This study demonstrates that the inclusion of men in a targeted FP mHealth intervention is feasible, despite recruitment challenges related to male availability and migration for work.35 Men engaged in the intervention, and optional male involvement did not compromise female trial participation. We developed and implemented a strategy for woman-initiated inclusion of male partners. Building on this experience, future community-based work should explore how to reach men who may be more resistant to FP for couples-based interventions.
This trial has several strengths, including high retention (97%). Most of the intervention is reproducible, as the platform sends preprogrammed, automated SMS, which enhances potential for scale-up. Two-way messaging was dynamic to participants’ needs and could not be completely standardized, although investigators regularly reviewed staff SMS responses for quality assurance and fidelity.
Our study also has limitations. We were unable to abstract data on contraceptive use from clinic records, as unique identifiers were unavailable. Self-report of contraceptive use could result in overreporting due to social desirability bias. Service delivery was interrupted by health worker strikes, which may have altered contraceptive initiation, choice, and continuation, although impacts would be expected to be distributed evenly between groups. Our results are not generalizable to women who do not have daily access to a mobile phone and may not represent women in the community not engaged in public facility-based care.
We did not observe an effect on HEC use at 6 or 14 weeks postpartum, perhaps because of lack of statistical power to detect a difference when fewer women had attended study visits. Alternatively, the intervention may have had an increasing effect over time as women interacted via SMS. Consistent with this hypothesis, the Kaplan–Meier curves comparing time to contraceptive initiation between groups diverged most at 6 months. We chose 6 months postpartum as the most clinically relevant time to measure the primary outcome, since most women will have ceased exclusive breastfeeding and resumed sex.3
Few reproductive health-focused mHealth programs have rigorously evaluated their effects on contraceptive outcomes. Our findings highlight the effectiveness of 2-way SMS to support contraceptive use, and future studies should assess optimal duration of SMS support. It is possible that SMS dialogue may have a larger effect on contraceptive continuation in the first 12 to 18 months postpartum than on initiation. Novel methods for remote data capture may enable more accurate assessment of contraceptive practices in real time. Finally, although enthusiasm for mHealth SMS programs is warranted, with increasing evidence of benefit and relatively low cost of the technology, interventions’ cost-effectiveness and potential for integration into health care delivery remain research and implementation priorities.
ACKNOWLEDGMENTS
This study was funded by the Society of Family Planning, with additional support from National Institutes of Health–National Institute of Allergy and Infectious Diseases (grant K01 AI116298).
We thank all of the women and men who participated in the trial, and the team who made the study possible, including Peninah Kithao, Valarie Kemunto, Peter Oloo, Richard Sigar, Quinter Rege, Grace Obinge, and Lusi Osborn. Trevor Perrier adapted the SMS platform for Mobile WACh XY and provided essential technical support for the intervention. We appreciate Ken Tapia’s (University of Washington Center for AIDS Research) assistance with randomization and Barbra Richardson’s biostatistics consultation. Sarah Prager, Elizabeth Micks, and reviewers with the Fellowship in Family Planning provided valuable insights. We also thank the UW-Kenya staff for logistical support, and the University of Washington Kizazi Working Group/Global Center for the Integrated Health of Women, Adolescents, and Children (Global WACh) for providing comments on study design and data analysis. Finally, we appreciate Ministry of Health support for the study in Kisumu and Siaya counties.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
HUMAN PARTICIPANT PROTECTION
This study was approved by the institutional review boards at Kenyatta National Hospital/University of Nairobi (Ethics and Research Committee) and the University of Washington (Human Subjects Division).
Footnotes
See also Dulli, p. 836.
REFERENCES
- 1.World Health Organization. Statement for collective action for postpartum family planning. 2012. Available at: https://www.mchip.net/sites/default/files/PPFP%20Statement%20for%20Action.pdf. Accessed March 28, 2019.
- 2.Cleland J, Shah IH, Daniele M. Interventions to improve postpartum family planning in low- and middle-income countries: program implications and research priorities. Stud Fam Plann. 2015;46(4):423–441. doi: 10.1111/j.1728-4465.2015.00041.x. [DOI] [PubMed] [Google Scholar]
- 3.Rossier C, Bradley SEK, Ross J, Winfrey W. Reassessing unmet need for family planning in the postpartum period. Stud Fam Plann. 2015;46(4):355–367. doi: 10.1111/j.1728-4465.2015.00037.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore Z, Pfitzer A, Gubin R, Charurat E, Elliott L, Croft T. Missed opportunities for family planning: an analysis of pregnancy risk and contraceptive method use among postpartum women in 21 low-and middle-income countries. Contraception. 2015;92(1):31–39. doi: 10.1016/j.contraception.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Achwoka D, Pintye J, McGrath CJ et al. Uptake and correlates of contraception among postpartum women in Kenya: results from a national cross-sectional survey. Contraception. 2018;97(3):227–235. doi: 10.1016/j.contraception.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleland J, Conde-Agudelo A, Peterson H, Ross J, Tsui A. Contraception and health. Lancet. 2012;380(9837):149–156. doi: 10.1016/S0140-6736(12)60609-6. [DOI] [PubMed] [Google Scholar]
- 7. Family Planning 2020. Postpartum/postabortion family planning. 2017. Available at: http://www.familyplanning2020.org/microsite/ppfp. Accessed January 12, 2017.
- 8.Keogh SC, Urassa M, Kumogola Y, Kalongoji S, Kimaro D, Zaba B. Postpartum contraception in northern Tanzania: patterns of use, relationship to antenatal intentions, and impact of antenatal counseling. Stud Fam Plann. 2015;46(4):405–422. doi: 10.1111/j.1728-4465.2015.00040.x. [DOI] [PubMed] [Google Scholar]
- 9.Ndugwa RP, Cleland J, Madise NJ, Fotso JC, Zulu EM. Menstrual pattern, sexual behaviors, and contraceptive use among postpartum women in Nairobi urban slums. J Urban Health. 2011;88(suppl 2):S341–S355. doi: 10.1007/s11524-010-9452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naanyu V, Baliddawa J, Peca E, Karfakis J, Nyagoha N, Koech B. An examination of postpartum family planning in western Kenya: “I want to use contraception but I have not been told how to do so.”. Afr J Reprod Health. 2013;17(3):44–53. [PubMed] [Google Scholar]
- 11.Vernon R. Meeting the family planning needs of postpartum women. Stud Fam Plann. 2009;40(3):235–245. doi: 10.1111/j.1728-4465.2009.00206.x. [DOI] [PubMed] [Google Scholar]
- 12.Keesara S, Juma PA, Harper CC, Newmann SJ. Barriers to postpartum contraception: differences among women based on parity and future fertility desires. Cult Health Sex. 2018;20(3):247–261. doi: 10.1080/13691058.2017.1340669. [DOI] [PubMed] [Google Scholar]
- 13.Mumah JN, Machiyama K, Mutua M, Kabiru CW, Cleland J. Contraceptive adoption, discontinuation, and switching among postpartum women in Nairobi’s urban slums. Stud Fam Plann. 2015;46(4):369–386. doi: 10.1111/j.1728-4465.2015.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodoo FN. Men matter: additive and interactive gendered preferences and reproductive behavior in Kenya. Demography. 1998;35(2):229–242. [PubMed] [Google Scholar]
- 15.Greene ME, Biddlecom AE. Absent and problematic men: demographic accounts of male reproductive roles. Popul Dev Rev. 2000;26(1):81–115. [Google Scholar]
- 16.Hardee K, Croce-Galis M, Gay J. Men as Contraceptive Users: Programs, Outcomes, and Recommendations. Washington, DC: USAID, Population Council, The Evidence Project; 2016. [Google Scholar]
- 17.Harrington EK, Dworkin S, Withers M, Onono M, Kwena Z, Newmann SJ. Gendered power dynamics and women’s negotiation of family planning in a high HIV prevalence setting: a qualitative study of couples in western Kenya. Cult Health Sex. 2016;18(4):453–469. doi: 10.1080/13691058.2015.1091507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Withers M, Dworkin SL, Zakaras JM et al. “Women now wear trousers”: men’s perceptions of family planning in the context of changing gender relations in western Kenya. Cult Health Sex. 2015;17(9):1132–1146. doi: 10.1080/13691058.2015.1043144. [DOI] [PubMed] [Google Scholar]
- 19.Lund S, Hemed M, Nielsen B et al. Mobile phones as a health communication tool to improve skilled attendance at delivery in Zanzibar: a cluster-randomised controlled trial. BJOG. 2012;119(10):1256–1264. doi: 10.1111/j.1471-0528.2012.03413.x. [DOI] [PubMed] [Google Scholar]
- 20.Lester RT, Ritvo P, Mills EJ et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 21.Linnemayr S, Huang H, Luoto J et al. Text messaging for improving antiretroviral therapy adherence: no effects after 1 year in a randomized controlled trial among adolescents and young adults. Am J Public Health. 2017;107(12):1944–1950. doi: 10.2105/AJPH.2017.304089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith C, Gold J, Ngo TD, Sumpter C, Free C. Mobile phone-based interventions for improving contraception use. Cochrane Database Syst Rev. 2015;6:CD011159. doi: 10.1002/14651858.CD011159.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castaño PM, Bynum JY, Andrés R, Lara M, Westhoff C. Effect of daily text messages on oral contraceptive continuation. Obstet Gynecol. 2012;119(1):14–20. doi: 10.1097/AOG.0b013e31823d4167. [DOI] [PubMed] [Google Scholar]
- 24.Smith C, Ngo TD, Gold J et al. Effect of a mobile phone-based intervention on post-abortion contraception: a randomized controlled trial in Cambodia. Bull World Health Organ. 2015;93(12):842A–850A. doi: 10.2471/BLT.15.160267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson D, Juras R, Riley P, Chatterji M, Sloane P. A randomized controlled trial of the impact of a family planning mHealth service on knowledge and use of contraception. Contraception. 2017;95(1):90–97. doi: 10.1016/j.contraception.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Drake AL, Unger JA, Ronen K et al. Evaluation of mHealth strategies to optimize adherence and efficacy of Option B+ prevention of mother-to-child HIV transmission: rationale, design and methods of a 3-armed randomized controlled trial. Contemp Clin Trials. 2017;57:44–50. doi: 10.1016/j.cct.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National AIDS and STI Control Programme (NASCOP) & Kenya Medical Research Institute (KEMRI) Guidelines for conducting adolescent HIV sexual and reproductive health research in Kenya. 2015. Available at: http://icop.or.ke/wp-content/uploads/2016/10/Adolescents-Guidance-on-HIV-SRH-Research.pdf. Accessed December 1, 2017.
- 28.Glanz K, Rimer BK, Viswanath K. Health Behavior and Health Education: Theory, Research, and Practice. 4th ed. San Francisco, CA: Jossey-Bass; 2008. [Google Scholar]
- 29.Harrington EK, McCoy EE, Drake AL et al. Engaging men in an mHealth approach to support postpartum family planning among couples in Kenya: a qualitative study. Reprod Health. 2019;16(1):17. doi: 10.1186/s12978-019-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winfrey W, Rakesh K. Use of Family Planning in the Postpartum Period. Rockville, MD: ICF International; 2014. DHS Comparative Report No. 36. [Google Scholar]
- 32.Unger J, Ronen K, Perrier T et al. Short message service communication improves exclusive breastfeeding and early postpartum contraception in a low- to middle-income country setting: a randomised trial. BJOG. 2018;125(12):1620–1629. doi: 10.1111/1471-0528.15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenya Demographic and Health Survey 2014. Nairobi, Kenya: Kenya National Bureau of Statistics, ICF Macro; 2015. [Google Scholar]
- 34. Perrier T, Harrington EK, Ronen K, et al. Male partner engagement in family planning SMS conversations at Kenyan health clinics. Paper presented at: Association for Computing Machinery Special Interest Group on Computers and Society Conference on Computing and Sustainable Societies; June 21–22, 2018; Menlo Park, CA.
- 35.Harrington EK, Drake AL, Matemo D et al. Experience including men in a novel short message service (SMS) approach to improve postpartum family planning education and counseling in Kenya. Contraception. 2017;96(4):301. [Google Scholar]