Abstract
Objectives: The aim of this study was to evaluate and compare the severity of acute kidney injury (AKI) induced by iodine contrast agent injection via the renal artery, ear vein, and femoral artery in a rabbit model.
Methods: Blood oxygenation level-dependent (BOLD) magnetic resonance (MR) scans were performed at 24 h prior to contrast injection and 1, 24, 48, and 72 h after injection. Iodixanol injection dose was 1.0, 1.5, 2.0, and 2.5 g iodine/kg, respectively. Hypoxia-inducible factor-1α (HIF-1α) expression was determined, and the BOLD-MRI parameter R2* was used to express tissue oxygenation. Increases in R2* levels reflect reductions in tissue oxygenation. Analyses including R2* value, dose response, histology, and HIF-1α were conducted.
Result: Injection of 1.0 g iodine/kg into the left renal artery resulted in significant increases in renal R2* values after 24 h. This was equivalent to the change of R2* after 2.0 g iodine/kg femoral artery injection. Renal injury scores and HIF-1α expression scores were significantly increased at 24 h. The R2* values exhibited a positive linear correlation with histological injury scores. The maximum effects occurred 24 h after iodixanol injection and returned to baseline levels within 72 h.
Conclusions: The renal injury induced by 1.0 g iodine/kg iodixanol through renal artery injection was more significant than that caused by the same dose of femoral artery and auricular vein injection, while similar to that caused by 2.0 g iodine/kg femoral artery injection.
Keywords: Magnetic resonance blood oxygenation level dependent imaging, injection route, contrast agent nephropathy, acute kidney injury
Introduction
Contrast-induced acute kidney injury (CIAKI) is a common iatrogenic clinical event resulted from the administration of iodine contrast agents during intra-arterial (IA) digital subtraction angiography (DSA) and intravenous (IV) CT angiography procedures [1]. Renal concentrations and time profiles of contrast may differ between IA and IV injection routes due to factors including in vivo blood circulation, capillary buffer, and dilutional effect of venous capacity as it interferes with the circulation of contrast agents. Therefore, this raises concerns, including whether CIAKI may be route-dependent (IA vs. IV injection), highlighting the need for comparison of CIAKI severity among different routes, and ultimately, how research results may guide clinical practitioners to make diagnoses and carry out appropriate treatment plans or, preferably, CIAKI preventive measures [2].
Of note, the majority of previous clinical studies have evaluated the risk of IA and IV injections using serum creatinine (Scr) levels after direct DSA and CT examination [2–5]. The confounding factors include inconsistent injection dosages, the use of different types, or molecular structures of contrast agent, and the physiological fluctuation of Scr values.
Renal hypoxia and direct nephrotoxicity of iodine contrast agent have been recognized as the main pathogenesis of CIAKI. BOLD MRI is currently the preferable methodology for measuring renal tissue deoxyhemoglobin levels for the study of renal oxygenation during AKI in humans and animals, as it is noninvasive and easy to measure. R2* is the parameter of BOLD, which reflects the tissue oxygenation bioavailability. An increase in R2* implies a high deoxyhemoglobin level and suggests poor oxygenation within the renal tissue [6]. BOLD-MR has been used to assess changes in tissue oxygenation in various renal diseases such as ureteral obstruction [7], donated and remaining kidneys [8], and atherosclerotic renal artery stenosis [9].
In addition, BOLD-MR can simultaneously measure the state of oxygenation of bilateral kidneys with different renal functions, respectively, which provides clearer imaging evidence of CIAKI. Hypoxia-inducible factor-1α (HIF-1α) is a transcriptional regulator that adapts to hypoxia [10]. Under hypoxic conditions, it accumulates and is upregulated in the nucleus [11], facilitating the accurate assessment of renal hypoxia in this study.
We hypothesized that CIAKI was injection route-dependent and dose-dependent, and hence, studied the relationship between route and dose. The nonionic dimer isotonic contrast medium iodixanol was injected through three different routes, the renal artery, ear vein, and femoral artery, in animal models. The CIAKI effect was longitudinally monitored by BOLD MRI within 72 h after injection. Histological changes and HIF-1α expression were studied to determine the MRI findings.
Materials and methods
Contrast
The contrast agent used was iodixanol (GE Healthcare, Shanghai, China).
Experiment 1: Comparison of the CIAKI-inducing effects of three different injection routes on the kidneys.
All the experimental procedures were approved by the Ethics Committee of First Hospital of China Medical University (AF-S0P-07–1.1–01) and complied with guidance for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996). A total of 126 male New Zealand white rabbits were obtained from the Department of Veterinary Medicine, China Medical University (aged 8–10 months, weighing 2.7–3.3 kg). The animals were randomly divided into the following groups: Group Renal Artery-Iodixanol (RA-Io) and Renal Artery-0.9% Saline (RA-S); Group Ear Vein-Iodixanol (EV-Io) and Ear Vein-0.9% Saline (EV-S); Group Femoral Artery-Iodixanol (FA-Io) and Femoral Artery-0.9% Saline (FA-S). Each group had 21 rabbits. Rabbits were anesthetized by auricular vein injection of pentobarbital, 30 mg/kg, followed by DSA puncture or BOLD-MRI scan.
Group RA-Io underwent femoral artery puncture with 5 F sheath insertion and DSA monitoring for guided catheter introduction into the left renal artery with a guide wire. Once through, the high-pressure syringe was connected and the iodixanol was injected at 1 mL/s with a dosage of 1.0 g iodine/kg dose. Groups EV-Io and FA-Io underwent 22 G venous indwelling needle puncture into the ear vein and the femoral artery, respectively, with the same injection rate/dosage as Group RA-Io. BOLD-MRI scans were performed at 24 h prior to contrast injection, and 1, 24, 48, and 72 h post-injection in each group to observe the changes in the R2* values of the renal cortex, outer medulla, and inner medulla. The experimental flow chart of group RA-Io, as an example, is outlined in Figure 1. The RA-S, EV-S, and FA-S groups were injected with the same volume of 0.9% saline according to different routes.
Figure 1.
Experimental flow chart of group RA-Io as an example. BOLD, blood oxygenation level-dependent.
Experiment 2. Comparison of the CIAKI-inducing effects of different injection doses on the kidneys. A total of 126 male New Zealand white rabbits underwent femoral artery puncture and were equally divided into six groups, according to injection dose of iodixanol and same volume of 0.9% saline: Group FA-Io-1.5 g iodine/kg and FA-S-1.5; Group FA-Io-2.0 g iodine/kg and FA-S-2.0; Group FA-Io-2.5 g iodine/kg and FA-S-2.5. BOLD-MRI scans were performed 24 h prior to contrast injection, and 1, 24, 48, and 72 h post-injection. The changes in the R2* values of the renal cortex, outer medulla, and inner medulla were compared with those in Group RA-Io, and the association between the dose of iodine contrast, degree of renal injury, and different injection routes was evaluated.
MRI scan
A 3.0 T Twin Speed whole-body MR scanner (GE Medical Systems, Milwaukee, WI) was used, along with animal coils, a supine position scan, and advanced BOLD scanning using a multiple-echo spoiled gradient recalled echo protocol: Repetition time, 101.5 ms; echo time, 6.3–32 ms; flip angle, 30°; bandwidth, 31.25 kHz; matrix, 160 × 160; field of view, 18 cm × 18 cm; section thickness, 4 mm; and section number, 5.
Workstation software (GE Medical Systems, Chicago, IL) was used. On the central coronal slice, the region of interest (ROI) included an extensive section of the renal cortex, outer medulla, and inner medulla, as shown in Figure 2, while avoiding the majority of the sinuses, blood vessels, and adipose tissue.
Figure 2.
Renal R2* maps in a representative rabbit from the injection of contrast via the left renal artery. BOLD MRI was performed 24 h before contrast injection (baseline) and at 1, 24, 48, and 72 h after injection. All maps of the same row are displayed with the same window, level and color scale settings. Higher intensities on the R2* map indicate lower oxygenation of the underlying tissue.
Histological analysis
Following each MR scan, three rabbits from each group were sacrificed by auricular vein injection of 100 mg/kg pentobarbital. Their kidneys with the capsules were surgically removed, cut in half longitudinally, and fixed in 4% paraformaldehyde, followed by conventional dehydration and paraffin embedding. Serial cutting of 5-μm-thick slices was performed for conventional optical microscopy and staining with hematoxylin and eosin. Sections were semi-quantitatively analyzed by pathologists with at least 5 years of clinical experience and blinded to group assignment, to assess vacuolar vacuolation of tubular epithelial cells, intraluminal detritus accumulation, and lumen expansion. Scores of 0–4 were assigned to each histopathological change according to previously published criteria [12]: 0 points, normal kidney; 1 point, mild injury (0–5%); 2 points, moderate injury (5–25%); 3 points, severe injury (25–75%); and 4 points, severe damage (75–100%). A total of five non-overlapping areas of the cortex, outer medulla, and inner medulla were observed, and a mean score was calculated across those five areas to obtain a final severity score for each section.
HIF-1α immunohistochemistry analysis
HIF-1α was immunostained on 5-μm paraffin-embedded tissue sections using the streptavidin-peroxidase technique. Briefly, paraffin sections were deparaffinized, hydrated, and subjected to antigen retrieval and blocking of endogenous peroxidase activity. Slides were incubated with the primary antibody monoclonal anti-HIF-1α (H1alpha67; 1:200; Novus Biologicals, Littleton, CO) overnight at 4 °C, followed by incubation with non-biotinkit (EliVision™ superKIT-9922, Maixin, Fuzhou, China) reagents A and B for 15 min each to allow the horseradish peroxidase polymer to bind to the primary antibody. Diaminobenzidine was used for staining, followed by a hematoxylin counterstain.
The expression of HIF-1α in the renal tubular epithelial cells was observed by optical microscopy with a ×400 magnification visual field and blinded scored as 0–4 points: 0 points, weakly expressed or without nuclear staining; 1 point, positive rate of nuclear staining <25%; 2 points, positive rate of nuclear staining of 25–50%; 3 points, positive rate of nuclear staining of 50–75%; and 4 points, positive rate of nuclear staining >75%.
Statistical analysis
R2* values were compared between groups using analysis of variance (ANOVA) and the Tukey post hoc test. R2* values within each group were compared using repeated measures ANOVA and the Bonferroni post hoc test. Histology scores and HIF-1α expression scores were compared using the Kruskal–Wallis test and pairwise comparisons. Spearman’s correlation analysis was used to assess the correlation between renal tissue injury, HIF-1α expression scores, and MRI parameters. SPSS version 24.0 software (IBM Corp., Armonk, NY) was used for statistical analysis. p<.05 was considered to indicate a statistically significant difference.
Results
Characteristics and baseline values
The characteristics and baseline characteristics of all rabbits in all groups, including number, age, sex, body weight, Scr, and the R2* values of the cortex, outer medulla, and inner medulla are presented in Table 1, with no significant difference among the groups.
Table 1.
Baseline characteristics of iodixanol-injected and saline-injected rabbit groups.
| Group | RA-Io | EV-Io | FA-Io | FA-Io-1.5 | FA-Io-2.0 | FA-Io-2.5 |
|---|---|---|---|---|---|---|
| Number | 21 | 21 | 21 | 21 | 21 | 21 |
| Age (month) | 8.81 ± 0.87 | 8.76 ± 0.76 | 8.62 ± 0.67 | 8.48 ± 0.51 | 8.29 ± 0.46 | 8.71 ± 0.78 |
| Gender | Male | Male | Male | Male | Male | Male |
| Weight (kg) | 2.99 ± 0.10 | 2.96 ± 0.08 | 2.97 ± 0.10 | 2.93 ± 0.09 | 2.93 ± 0.08 | 2.95 ± 0.11 |
| SCr (μmol/L) | 34.19 ± 2.50 | 33.57 ± 2.50 | 33.76 ± 2.28 | 33.24 ± 2.21 | 34.00 ± 2.12 | 33.95 ± 2.58 |
| Cortex R2* (1/s) | 19.39 ± 1.01 | 19.21 ± 1.09 | 19.26 ± 1.26 | 19.31 ± 0.92 | 19.37 ± 1.06 | 19.32 ± 1.05 |
| OM R2* (1/s) | 23.86 ± 1.01 | 24.10 ± 0.94 | 24.12 ± 1.00 | 24.04 ± 1.09 | 24.01 ± 0.88 | 24.18 ± 1.09 |
| IM R2* (1/s) | 20.71 ± 1.00 | 20.53 ± 1.06 | 20.31 ± 0.98 | 20.41 ± 0.79 | 20.30 ± 0.98 | 20.62 ± 1.05 |
| Group |
RA-S |
EV-S |
FA-S |
FA-S-1.5 |
FA-S-2.0 |
FA-S-2.5 |
| Number | 21 | 21 | 21 | 21 | 21 | 21 |
| Age (month) | 8.90 ± 0.83 | 8.81 ± 0.68 | 8.76 ± 0.70 | 8.71 ± 0.78 | 8.90 ± 0.70 | 8.61 ± 0.80 |
| Gender | Male | Male | Male | Male | Male | Male |
| Weight (kg) | 2.99 ± 0.10 | 2.98 ± 0.09 | 3.01 ± 0.09 | 2.98 ± 0.08 | 3.01 ± 0.69 | 2.99 ± 0.05 |
| SCr (μmol/L) | 34.33 ± 2.13 | 33.52 ± 2.14 | 33.81 ± 2.16 | 34.19 ± 1.99 | 33.86 ± 2.06 | 33.81 ± 1.40 |
| Cortex R2* (1/s) | 19.22 ± 0.97 | 19.57 ± 0.95 | 19.11 ± 0.90 | 18.86 ± 0.89 | 18.82 ± 0.86 | 19.10 ± 0.73 |
| OM R2* (1/s) | 24.26 ± 0.98 | 23.97 ± 0.80 | 24.26 ± 0.86 | 24.13 ± 0.82 | 24.15 ± 0.88 | 23.98 ± 0.88 |
| IM R2* (1/s) | 20.23 ± 0.88 | 20.37 ± 0.87 | 20.30 ± 0.98 | 20.51 ± 0.77 | 20.38 ± 0.67 | 20.54 ± 0.66 |
There was no significant statistical difference among all groups with respect to Number, Age, Gender, Weight, or serum creatinine in all renal tissues. SCr: serum creatinine; OM: outer medulla; IM: inner medulla; RA-Io: Renal Artery-Iodixanol; RA-S: Renal Artery-0.9% Saline; EV-Io: Ear Vein-Iodixanol; EV-S: Ear Vein-0.9% Saline; FA-Io: Femoral Artery-Iodixanol; FA-S: Femoral Artery-0.9% Saline; FA-Io-1.5: Femoral Artery-Iodixanol-1.5 g iodine/kg; FA-S-1.5: Femoral Artery-0.9% Saline; FA-Io-2.0: Femoral Artery-Iodixanol-2.0 g iodine/kg; FA-S-2.0: Femoral Artery-0.9% Saline; FA-Io-2.5: Femoral Artery-Iodixanol-2.5 g iodine/kg; FA-S-2.5: Femoral Artery-0.9% Saline.
Experiment 1. Scr was significantly higher in the RA-Io group than that in the remaining groups at 24 h (Table 2). Figure 2 illustrates renal R2* maps in a representative rabbit at baseline and following the injection of contrast agent via the left renal artery. Clear images of the renal cortex, outer medulla, and inner medulla were obtained. R2* staining intensity may be used to extrapolate the relative oxygenation of renal tissues. At 24 h after the injection, the high-intensity region increased, signifying decreased tissue oxygenation. The changes of R2*, renal injury scores and HIF-1α expression in left kidney were significantly higher than that in right kidney at 24 h after injection of iodixanol into left renal artery (Tables 3–5), respectively. Figure 3(A) demonstrates the alterations in R2* values in Groups RA-Io, EV-Io, and FA-Io. The cortex, outer medulla, and inner medulla R2* levels increased 1 h after the injection and reached their peak at 24 h. Group RA-Io had the greatest alteration in the R2* value compared with groups EV-Io and FA-Io in the cortex, outer medulla, and inner medulla (RA-Io vs. EV-Io: all 24 h p<.001; RA-Io vs. FA-Io: all 24 h p<.001). Subsequently, the R2* levels of the cortex and inner medulla decreased to baseline levels within 48 h after the injection and the R2* level of the outer medulla decreased to baseline level within 72 h after the injection. The above results indicated maximum renal effects within 24 h after injection via the renal artery and no significant difference in renal effects between injections via the auricular vein and femoral artery (EV-Io vs. FA-Io: p = 1.000). There were no statistical differences between the groups with saline injection via the three different routes (Table 6).
Table 2.
The mean value of serum creatinine (mmol/L) at different time points in each group in experiment 1.
| Baseline | 1 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|
| RA-Io | 34.33 ± 2.52 | 42.00 ± 2.65 | 59.33 ± 3.51* | 41.67 ± 3.06 | 38.00 ± 4.36 |
| EV-Io | 32.67 ± 2.89 | 39.33 ± 2.52 | 46.33 ± 4.51*a | 38.67 ± 3.06 | 36.67 ± 2.52 |
| FA-Io | 33.67 ± 1.53 | 38.67 ± 2.08 | 46.33 ± 3.21*a | 38.33 ± 2.31 | 35.33 ± 2.08 |
| RA-S | 34.33 ± 1.53 | 35.33 ± 3.51 | 34.67 ± 1.53abc | 33.67 ± 3.06 | 33.00 ± 2.00 |
| EV-S | 35.00 ± 2.00 | 35.00 ± 2.00a | 32.33 ± 2.08abc | 35.00 ± 4.58 | 33.33 ± 1.53 |
| FA-S | 32.67 ± 3.79 | 32.00 ± 1.73ab | 34.00 ± 2.00abc | 33.33 ± 2.31 | 33.67 ± 2.52 |
One-way ANOVA Tukey p values,
a p<.05 vs. RA-Io,
b p<.05 vs. EV-Io,
c p<.05 vs. FA-Io,
*p<.05 vs. Baseline.
Table 3.
Bilateral renal R2* (1/s) values following left renal artery administration of iodixanol and 0.9% saline.
| Baseline | 1 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|
| Cortex | |||||
| RA-Io | 19.14 ± 1.09 | 24.62 ± 1.18 | 29.87 ± 1.17 | 22.85 ± 1.96 | 20.46 ± 1.07 |
| RA-Io-R | 19.52 ± 0.93 | 23.77 ± 1.68 | 25.10 ± 1.43a | 22.06 ± 2.64 | 19.47 ± 1.10 |
| RA-S | 18.33 ± 1.16 | 19.06 ± 1.24ab | 18.59 ± 1.11ab | 19.22 ± 1.30a | 19.23 ± 1.06 |
| RA-S-R | 18.54 ± 0.87 | 18.37 ± 0.95ab | 18.70 ± 0.88ab | 18.51 ± 1.17ab | 18.68 ± 1.25 |
| Tukey p Value |
.196 | .000 | .000 | .001 | .078 |
| Outer medulla | |||||
| RA-Io | 24.02 ± 0.95 | 29.10 ± 1.18 | 33.85 ± 1.05 | 29.12 ± 0.85 | 25.12 ± 2.14 |
| RA-Io-R | 23.89 ± 1.07 | 26.27 ± 1.22a | 28.27 ± 0.78a | 26.19 ± 1.00a | 23.99 ± 1.58 |
| RA-S | 23.21 ± 0.91 | 24.02 ± 0.87ab | 24.35 ± 0.85ab | 24.18 ± 0.85ab | 23.86 ± 1.15 |
| RA-S-R | 23.66 ± 1.19 | 23.86 ± 1.08ab | 24.33 ± 0.85ab | 23.94 ± 1.03ab | 23.88 ± 1.32 |
| Tukey p Value |
.563 | .000 | .000 | .000 | .470 |
| Inner medulla | |||||
| RA-Io | 20.62 ± 1.25 | 26.52 ± 0.99 | 29.40 ± 0.82 | 22.10 ± 1.61 | 21.32 ± 1.32 |
| RA-Io-R | 20.13 ± 1.12 | 23.46 ± 1.24a | 25.25 ± 0.88a | 21.38 ± 1.17 | 20.52 ± 1.38 |
| RA-S | 20.10 ± 1.02 | 19.93 ± 1.22ab | 19.86 ± 0.79ab | 19.66 ± 0.80a | 19.82 ± 0.87 |
| RA-S-R | 20.70 ± 0.90 | 19.38 ± 0.58ab | 20.04 ± 1.17ab | 19.43 ± 0.99ab | 19.85 ± 1.64 |
| Tukey p Value |
.679 | .000 | .000 | .002 | .202 |
One-way ANOVA Tukey p values.
a p<.05 vs. RA-Io,
b p<.05 vs. RA-Io-R.
RA-Io: the left renal, Left Renal Artery-Iodixanol; RA-Io-R: the right renal, Left Renal Artery-Iodixanol; RA-S: the left renal, Left Renal Artery-0.9% saline; RA-S-R: the right renal, Left Renal Artery-0.9% saline.
Table 4.
Bilateral renal injury scores following left renal artery administration of iodixanol and 0.9% saline.
| Baseline | 1 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|
| Cortex | |||||
| RA-Io | 0.00 ± 0.00 | 5.07 ± 0.46 | 6.93 ± 0.46* | 2.80 ± 0.40 | 1.20 ± 0.40 |
| RA-Io-R | 0.00 ± 0.00 | 4.53 ± 0.46 | 6.13 ± 0.46* | 2.40 ± 0.40 | 0.93 ± 0.46 |
| RA-S | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| RA-S-R | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| K-W p Value |
1.000 | .017 | .016 | .019 | .021 |
| Outer medulla | |||||
| RA-Io | 0.00 ± 0.00 | 4.27 ± 0.46 | 7.33 ± 0.46* | 5.07 ± 0.46 | 2.00 ± 0.40 |
| RA-Io-R | 0.00 ± 0.00 | 3.73 ± 0.46 | 6.53 ± 0.46* | 4.53 ± 0.46 | 1.60 ± 0.40 |
| RA-S | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| RA-S-R | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| K-W p Value |
1.000 | .017 | .016 | .017 | .019 |
| Inner medulla | |||||
| RA-Io | 0.00 ± 0.00 | 5.20 ± 0.40 | 6.53 ± 0.46* | 2.00 ± 0.40 | 1.20 ± 0.40 |
| RA-Io-R | 0.00 ± 0.00 | 4.73 ± 0.50 | 5.77 ± 0.40* | 1.60 ± 0.40 | 0.67 ± 0.46 |
| RA-S | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| RA-S-R | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| K-W p Value |
1.000 | .019 | .016 | .019 | .018 |
Kruskal–Wallis test and pairwise comparisons. *p<.05 vs. Baseline.
RA-Io: the left renal, Left Renal Artery-Iodixanol; RA-Io-R: the right renal, Left Renal Artery-Iodixanol; RA-S: the left renal, Left Renal Artery-0.9% saline; RA-S-R: the right renal, Left Renal Artery-0.9% saline.
Table 5.
Bilateral renal HIF-1α expression scores following left renal artery administration of iodixanol and 0.9% saline.
| Baseline | 1 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|
| Cortex | |||||
| RA-Io | 0.00 ± 0.00 | 2.00 ± 1.73 | 3.60 ± 3.47* | 2.40 ± 2.20 | 1.60 ± 1.40 |
| RA-Io-R | 0.00 ± 0.00 | 1.60 ± 1.47 | 3.20 ± 3.07* | 2.20 ± 2.00 | 1.40 ± 1.27 |
| RA-S | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| RA-S-R | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| K-W p Value |
1.000 | .017 | .016 | .019 | .021 |
| Outer medulla | |||||
| RA-Io | 0.00 ± 0.00 | 1.93 ± 0.23 | 3.47 ± 0.23* | 2.33 ± 0.23 | 1.4 ± 0.2 |
| RA-Io-R | 0.00 ± 0.00 | 1.67 ± 0.23 | 3.07 ± 0.23* | 2.07 ± 0.23 | 1.2 ± 0.2 |
| RA-S | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| RA-S-R | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| K-W p Value |
1.000 | .017 | .016 | .017 | .019 |
| Inner medulla | |||||
| RA-Io | 0.00 ± 0.00 | 2.20 ± 0.20 | 3.27 ± 0.23* | 1.47 ± 0.12 | 0.93 ± 0.23 |
| RA-Io-R | 0.00 ± 0.00 | 2.13 ± 0.12 | 2.87 ± 0.23* | 1.40 ± 0.20 | 0.93 ± 0.23 |
| RA-S | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| RA-S-R | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| K-W p Value |
1.000 | .021 | .016 | .021 | .019 |
Kruskal–Wallis test and pairwise comparisons. *p<.05 vs. Baseline.
RA-Io: the left renal, Left Renal Artery-Iodixanol; RA-Io-R: the right renal, Left Renal Artery-Iodixanol; RA-S: the left renal, Left Renal Artery-0.9% saline; RA-S-R: the right renal, Left Renal Artery-0.9% saline.
Figure 3.
These were the changes in R2* after contrast injection. Temporal alterations in R2* levels of the renal cortex, outer medulla, and inner medulla in every group. The R2* levels increased 1 h post-injection, and reached their peak after 24 h. Subsequently, the R2* levels of the cortex and inner medulla decreased to baseline levels within 48 h after injection, and the R2* level of the outer medulla decreased to baseline level within 72 h after injection (AB). Hypoxia was clearly dose responsive and that half as much contrast was need in the renal artery compared to the femoral artery to cause the same degree of hypoxia at 24 h (C).
Table 6.
Renal R2* (1/s) values following the administration of saline.
| 1 h | 24 h | 48 h | 72 h | Bonferroni p Value | |
|---|---|---|---|---|---|
| Cortex | |||||
| RA-S | 19.19 ± 1.07 | 18.85 ± 1.07 | 18.60 ± 1.27 | 18.73 ± 1.10 | .92 |
| EV-S | 19.02 ± 1.21 | 18.60 ± 1.11 | 18.88 ± 1.13 | 18.57 ± 1.13 | .93 |
| FA-S | 18.75 ± 1.11 | 18.51 ± 1.21 | 18.55 ± 1.22 | 18.65 ± 1.10 | .98 |
| FA-S-1.5 | 19.10 ± 0.88 | 19.09 ± 1.09 | 18.48 ± 1.09 | 18.84 ± 0.75 | .77 |
| FA-S-2.0 | 18.78 ± 1.14 | 18.80 ± 1.16 | 18.74 ± 0.83 | 18.92 ± 1.05 | .99 |
| FA-S-2.5 | 18.96 ± 0.90 | 19.13 ± 1.28 | 19.00 ± 1.18 | 18.89 ± 0.97 | .99 |
| Tukey p Value |
.97 | .92 | .96 | .99 | |
| Outer medulla | |||||
| RA-S | 24.25 ± 0.91 | 23.73 ± 0.98 | 23.95 ± 1.13 | 24.05 ± 1.20 | .93 |
| EV-S | 23.97 ± 0.95 | 23.65 ± 1.25 | 23.72 ± 1.05 | 23.41 ± 0.77 | .93 |
| FA-S | 24.07 ± 1.01 | 23.91 ± 1.07 | 23.93 ± 1.18 | 23.89 ± 1.09 | .99 |
| FA-S-1.5 | 23.99 ± 0.96 | 23.93 ± 1.01 | 23.97 ± 1.00 | 23.48 ± 1.05 | .86 |
| FA-S-2.0 | 23.75 ± 0.83 | 23.50 ± 1.23 | 23.93 ± 1.00 | 24.03 ± 0.97 | .50 |
| FA-S-2.5 | 23.83 ± 0.87 | 23.74 ± 1.00 | 23.79 ± 0.94 | 24.06 ± 1.01 | .96 |
| Tukey p Value |
.95 | .98 | .99 | .77 | |
| Inner medulla | |||||
| RA-S | 20.28 ± 1.46 | 19.76 ± 1.11 | 20.00 ± 0.97 | 19.80 ± 0.86 | .90 |
| EV-S | 19.76 ± 1.02 | 19.99 ± 1.19 | 19.58 ± 0.96 | 19.79 ± 0.96 | .88 |
| FA-S | 19.85 ± 0.99 | 19.98 ± 1.13 | 19.85 ± 1.02 | 19.86 ± 1.05 | .61 |
| FA-S-1.5 | 20.06 ± 1.26 | 19.83 ± 1.08 | 19.99 ± 1.04 | 19.84 ± 1.21 | .86 |
| FA-S-2.0 | 19.44 ± 1.07 | 19.83 ± 0.86 | 19.79 ± 1.07 | 19.95 ± 1.05 | .37 |
| FA-S-2.5 | 19.67 ± 1.03 | 19.99 ± 1.02 | 19.88 ± 1.08 | 19.63 ± 1.25 | .86 |
| Tukey p Value |
.85 | .99 | .98 | .99 |
Repeated measures ANOVA Bonferroni p values and one-way ANOVA Tukey p values, no statistical difference.
This table combines experiment 1 and 2. No changes in R2* when 0.9% saline is injected.
Experiment 2. Figure 3(B) illustrates the alterations in R2* values in Groups FA-Io-1.5, FA-Io-2.0, and FA-Io-2.5. The cortex, outer medulla, and inner medulla R2* levels increased 1 h after the injection, and reached their peak after 24 h. Group FA-Io-2.5 had the most notable change in R2* value compared with groups FA-Io-1.5 and FA-Io-2.0 in the cortex, outer medulla, and inner medulla (FA-Io-2.5 vs. FA-Io-1.5: all 24 h p<.001; FA-Io-2.5 vs. FA-Io-2.0: all 24 h p<.001). Subsequently, the R2* levels of the cortex and inner medulla in Groups FA-Io-1.5, FA-Io-2.0, and FA-Io-2.5 decreased to baseline levels within 48 h after the injection and the R2* level of the outer medulla decreased to baseline level within 72 h after the injection.
In Figure 3(C), when compared with Group RA-Io, the 24 h R2* level of the cortex, outer medulla, and inner medulla in Group FA-Io-2.0 were not significantly different (p = 1.000). The above results indicated that as the dose of injection was increased via the femoral artery, the renal effect increased. There was no significant difference between 2.0 g iodine/kg injection via the femoral artery and 1.0 g iodine/kg via the renal artery. There were no statistical differences between the groups with saline injection by different injection volumes (Table 6).
Pathological analysis
The most commonly held theory about the pathomechanism of CI-AKI is tubular cell injury. Following the contrast injection, visible renal tubular lumen dilatation with luminal visible debris accumulation, and vacuolization of proximal convoluted tubule and distal tubule epithelial cells were observed and compared with normal kidney histology (Figure 4). These changes began at 1 h, progressing in number and size up to 24 h, followed by a gradual decline. The histology of the kidneys following saline injection was indistinguishable from that of normal kidneys.
Figure 4.
Histological effects after contrast injection via the left renal artery (HE stain, ×400). Vacuolation was primarily observed in the proximal and distal convoluted tubule epithelial cells (green arrow). Renal tubular lumen dilation with visible luminal debris accumulation (blue arrow) was observed. These alterations began at 1 h, progressing in number and size over 24 h, followed by a gradual decline.
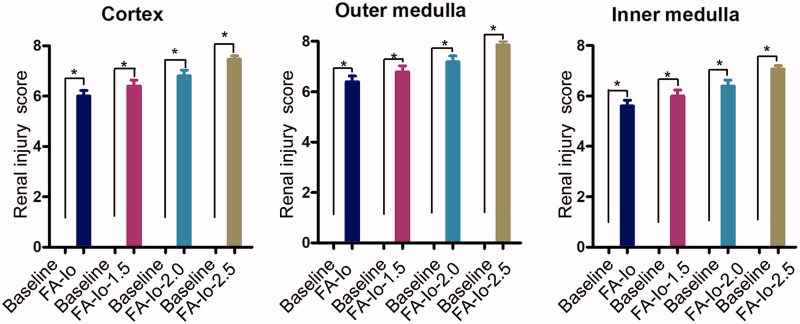
At 24 h post-injection, the severity scores of renal injury to the cortex, outer medulla, and inner medulla were significantly increased compared with the baseline values (all groups, p<.05) (Figure 5). There were higher injury scores as the contrast dose increased. Group FA-Io had the lowest score and Group FA-Io-2.5 had the highest score. There were no statistical differences between the groups.
Figure 5.
Groups FA-Io-1.0, 1.5, 2.0, and 2.5 renal injury scores in the cortex, outer medulla, and inner medulla at 24 h. There were higher injury scores as the contrast dose is increased. There was statistical difference compared to baseline. Results are presented as the mean ± standard error of the mean. *p<.05 vs. baseline.
Immunohistochemistry analysis
HIF-1α primarily accumulated in the cell nucleus and was used to determine the degree of tissue hypoxia. After contrast injection, HIF-1α expression increased precipitously at 24 h and subsequently declined toward baseline level (Figure 6).
Figure 6.
HIF-1α expression following contrast injection via the left renal artery (×400). HIF-1α expression increased precipitously at 24 h in the cortex, outer medulla, and inner medulla, and subsequently declined (black arrow).
At 24 h post-injection, the expression scores of HIF-1α in the cortex, outer medulla, and inner medulla were significantly increased compared with the baseline values (all groups, p<.05) (Figure 7). There were greater HIF-1α expression scores as contrast dose increased. Group FA-Io had the lowest score, while Group FA-Io-2.5 had the highest score. There was no statistical difference among the groups.
Figure 7.
Groups FA-Io-1.0, 1.5, 2.0, and 2.5 HIF-1α expression scores in the cortex, outer medulla, and inner medulla at 24 h. There were greater HIF-1α expression scores as contrast dose increased. There was statistical difference compared to baseline. Results are presented as the mean ± standard error of the mean. *p<.05 vs. baseline.
Correlation of MRI parameters with histology and immunohistochemistry
The R2* levels of the renal cortex, outer medulla, and inner medulla had a significant positive correlation with the tissue severity scores (p<.001, r = 0.887; p<.001, r = 0.865; p<.001, r = 0.815, respectively), and HIF-1α expression (p<.001, r = 0.786; p<.001, r = 0.857; p<.001, r = 0.808, respectively) (Figure 8).
Figure 8.
Positive correlations of R2* values to renal injury severity scores (red circles) and to HIF-1α expression (green triangles).
Discussion
This study focused on contrast agent administration via three different routes, including the renal artery, ear vein, femoral artery on renal injury and renal oxygen content. Contrast agent induced peak renal damage after 24 h. At the same dose, injection via the ear vein and femoral artery led to a similar degree of injury, which was less severe compared with injection via the renal artery. In this study, it was likely that the concentration of contrast agent was higher in the renal artery compared with that following indirect injection. Injection via the renal artery induced as much as twice the renal injury compared with injection via the femoral artery at the same dose. Renal histology and HIF-1α immune expression were evaluated to confirm the MRI findings.
In previous studies of CIAKI-induced kidney injury via arteriovenous pathways [2,13–16], confounding factors have included the following: (1) Injection of contrast agents with variable osmotic pressures (hypertonic, 1000–1800 mOsm/kg; hypotonic, 500–700 mOsm/kg; isotonic, 290 mOsm/kg). Hypertonic contrast agents may themselves cause vascular endothelial damage and red blood cell shrinkage and may even affect microcirculation [17]. (2) The total dose of iodine contrast agents administered has varied and arterial DSA diagnosis and treatment approaches have also varied according to the complexity of the lesion. In extreme scenarios, the maximum amount of contrast agent administered may be more than 10 times the minimum amount injected [18]. (3) The fluctuation of Scr levels may also interfere with the accuracy of diagnosis of AKI, as certain patients’ Scr levels fluctuate even without exposure to contrast agents, thus obscuring the diagnostic criteria for CIAKI [19]. Our results of this study underlined that contrast product can damage the kidneys, despite recent publications stating the opposite [20]. (4) Patients with IA procedures usually have comorbidities such as diabetes mellitus, chronic kidney disease, a history of AKI, and congestive heart failure. These comorbidities are high-risk factors for CIAKI which may lead to the conclusion that IA contrast agent administration would cause more damage compared with IV administration [3]. (5) IA injection routes do not distinguish between renal and non-renal arteries.
Our experiment utilized a high-pressure syringe that was able to inject contrast agent into the rabbits at an equal concentration (320 mg iodine/mL), equal amount (1.0 g iodine/kg), and equal injection rate (1.0 mL/s), thus minimizing the above confounding factors.
Each contrast injection route has unique characteristics while being excreted through systemic circulation, meaning that the agent will eventually reach the kidney at a unique peak level or ratio compared with other routes. In the renal artery injection group, mechanical catheterization of the renal artery and exposure to relatively concentrated contrast agent would likely exacerbate the renal injury [4,21]. Theoretically, the contrast agent injected via ear vein will undergo buffering by the pulmonary capillary bed. The contrast agent injected via the femoral artery undergoes buffering twice by the capillary bed of the lower extremity and the capillary bed of the pulmonary circulation, reducing the contrast concentration and inducing comparatively less renal damage [22]. However, only a small fraction of the contrast medium passes through the aorta directly to the kidneys, ∼20% of the cardiac output [13], which may explain the similar results in the ear vein and femoral artery groups. However, injection via the femoral artery led to dose-dependent renal injury characteristics [23].
The R2* value increased rapidly 1 h after the contrast injection and peaked at 24 h. This may be attributed to the following: (1) Catheter stimulation and a high iodine-stimulated angiotensin II-induced vasoconstrictor effect resulting in decreased blood flow and reduced renal oxygen supply [24]. (2) The viscosity of the contrast agent, iodixanol, increases exponentially as the concentration increases [25]. The concentration-dependent exponential increase in the viscosity of iodixanol may result in a slowing down of renal perfusion and an inhibited oxygen supply [26]. (3) The glomerular filtration rate (GFR) decreases due to contrast media-induced renal dysfunction, resulting in increased tubular resistance and tubular dilation, compression of adjacent blood vessels and impaired renal oxygen delivery [27,28].
In this study, renal artery injection caused a significant increase in the R2* values, suggesting an exacerbation of hypoxia in the kidney. Ear vein and femoral artery injection had weaker effects on the kidneys. The R2* value peaked at 24 h after the contrast injection and subsequently decreased back to baseline, a trend consistent with contrast agent excretion. A reported 99% of iodixanol is eliminated in the urine within 24 h of injection [29]. The significant correlation between R2* values and HIF-1α expression indicates that BOLD MRI may be an important imaging technique that reflects the status of renal oxygenation.
It is not only hypoxia, but also direct cell toxicity that lead to contrast agent-induced kidney damage. In this study, kidney injury was observed following the injection of iodixanol, including cytoplasmic vacuolation in tubular epithelial cells, intraluminal detritus accumulation, and luminal dilation. Reduced renal oxygenation and direct renal tubular cytotoxicity are considered the key etiology of CIAKI. Reduced kidney oxygenation is associated with hypoxic injury and the formation of reactive oxygen species (ROS) [30]. Direct tubular cytotoxicity leads to oxidative stress, and impaired nitric oxide and tubular feedback, and aggravates renal insufficiency in vivo [31]. Injection via the renal artery and bolus injection of iodixanol increased the severity of renal hypoxia, prolonged the exposure of tubular cells to the contrast agent and increased nephrotoxicity.
These data suggested that iodine contrast agent injection route and injection dose have a certain effect on the kidney. Direct renal artery injection should be avoided in clinical use of contrast agent. In addition, injection led to dose-dependent renal injury characteristics; clinicians should be advised to choose the method that uses the least contrast agent.
This study has certain limitations: (1) SCr was measured. CIAKI is commonly defined by a clinical change in SCr [32], but SCr corresponds to the renal function of the bilateral kidneys and would not accurately reflect alterations in left renal function following left renal artery bypass. When the renal function of a patient differs between the two kidneys, the change in Scr alone may be insufficient to correctly infer the side on which the contrast agent had greater impact. BOLD and histological examinations can make up for this deficiency. (2) The R2* values were manually sketched. In measuring the R2* values of the renal cortex, outer medulla, and inner medulla, a larger ROI is used to maximize the sensitivity and objectivity of the R2* values by maximizing the measured tissue envelope to reduce deviations [33]. (3) BOLD-MR may detect kidney tissue hypoxia, but it cannot distinguish between a reduced oxygen supply or increased oxygen consumption. Although BOLD technology is only used in research, this study may provide a basis for its future applications in the clinic.
In conclusion, renal damage was most pronounced at 24 h after contrast injection. Ear vein and femoral artery injection led to less renal damage compared with injection via the renal artery, which caused as much as twice the amount of damage.
Funding Statement
This study was supported by National Natural Science Foundation of China (8157070408), Jilin Provincial Health and Family Planning Commission 2017 Science and Technology Ability Promotion Project (2017Q038), and Jilin City Science and Technology Plan Project (201537038).
Acknowledgments
The authors thank Li-zhi Xie, Chun-rong Han for expert technical assistance with MRI, pathology.
Ethics approval
All the experimental procedures were approved by the Ethics Committee of First Hospital of China Medical University (AF-S0P-07–1.1–01) and complied with guidance for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996).
Informed consents
Informed consents have been obtained from all participants before enrollment in this study.
Disclosure statement
All authors declare that they have no conflict of interest.
References
- 1. Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. [DOI] [PubMed] [Google Scholar]
- 2. Kooiman J, Le Haen PA, Gezgin G, et al. Contrast-induced acute kidney injury and clinical outcomes after intra-arterial and intravenous contrast administration: risk comparison adjusted for patient characteristics by design. Am Heart J. 2013;165:793–799, 799. e791. [DOI] [PubMed] [Google Scholar]
- 3. McDonald JS, Leake CB, McDonald RJ, et al. Acute kidney injury after intravenous versus intra-arterial contrast material administration in a paired cohort. Invest Radiol. 2016;51:804–809. [DOI] [PubMed] [Google Scholar]
- 4. Tong GE, Kumar S, Chong KC, et al. Risk of contrast-induced nephropathy for patients receiving intravenous vs. intra-arterial iodixanol administration. Abdom Radiol. 2016;41:91–99. [DOI] [PubMed] [Google Scholar]
- 5. Dong M, Jiao Z, Liu T, et al. Effect of administration route on the renal safety of contrast agents: a meta-analysis of randomized controlled trials. J Nephrol. 2012;25:290–301. [DOI] [PubMed] [Google Scholar]
- 6. Zhou HY, Chen TW, Zhang XM. Functional magnetic resonance imaging in acute kidney injury: present status. BioMed Res Int. 2016;2016:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pedersen M, Dissing TH, Merkenborg JAN, et al. Validation of quantitative BOLD MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int. 2005;67:2305–2312. [DOI] [PubMed] [Google Scholar]
- 8. Seif M, Eisenberger U, Binser T, et al. Renal blood oxygenation level-dependent imaging in longitudinal follow-up of donated and remaining kidneys. Radiology. 2016;279:795–804. [DOI] [PubMed] [Google Scholar]
- 9. Gloviczki M, Saad L, Textor SC. Blood oxygen level-dependent (BOLD) MRI analysis in atherosclerotic renal artery stenosis. Curr Opin Nephrol Hypertens. 2013;22:519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang W, Zhou X, Yao Q, et al. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am J Physiol Renal Physiol. 2017;313:F906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuan Q, Bleiziffer O, Boos AM, et al. PHDs inhibitor DMOG promotes the vascularization process in the AV loop by HIF-1a up-regulation and the preliminary discussion on its kinetics in rat. BMC Biotechnol. 2014;14:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ulusoy S, Ozkan G, Mungan S, et al. GSPE is superior to NAC in the prevention of contrast-induced nephropathy: might this superiority be related to caspase 1 and calpain 1? Life Sci. 2014;103:101–110. [DOI] [PubMed] [Google Scholar]
- 13. Nyman U, Almen T, Jacobsson B, et al. Are intravenous injections of contrast media really less nephrotoxic than intra-arterial injections? Eur Radiol. 2012;22:1366–1371. [DOI] [PubMed] [Google Scholar]
- 14. Stratta P, Izzo C, Canavese C, et al. Letter to the editor re: are intravenous injections of contrast media really less nephrotoxic than intra-arterial injections? Eur Radiol. 2013;23:1260–1263. [DOI] [PubMed] [Google Scholar]
- 15. Nyman U, Almen T, Jacobsson B, et al. Reply to letter to the editor re: are intravenous injections of contrast media really less nephrotoxic than intra-arterial injections? Eur Radiol. 2013;23:1264–1265. [DOI] [PubMed] [Google Scholar]
- 16. From AM, Bartholmai BJ, Williams AW, et al. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc. 2008;83:1095–1100. [DOI] [PubMed] [Google Scholar]
- 17. Hogstrom B, Ikei N. Physicochemical properties of radiographic contrast media, potential nephrotoxicity and prophylaxis. Clin Exp Pharmacol Physiol. 2015;42:1251–1257. [DOI] [PubMed] [Google Scholar]
- 18. Karlsberg RP, Dohad SY, Sheng R. Sheng R and iodixanol peripheral computed tomographic angiography study investigator P: contrast medium-induced acute kidney injury: comparison of intravenous and intraarterial administration of iodinated contrast medium. J Vasc Interv Radiol. 2011;22:1159–1165. [DOI] [PubMed] [Google Scholar]
- 19. Newhouse JH, Kho D, Rao QA, et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. Am J Roentgenol. 2008;191:376–382. [DOI] [PubMed] [Google Scholar]
- 20. Aycock RD, Westafer LM, Boxen JL. Acute kidney injury after computed tomography: a meta-analysis. Ann Emerg Med. 2017;71:30881–30888. [DOI] [PubMed] [Google Scholar]
- 21. Chou SH, Wang ZJ, Kuo J, et al. Persistent renal enhancement after intra-arterial versus intravenous iodixanol administration. Eur J Radiol. 2011;80:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol. 2011;21:2527–2541. [DOI] [PubMed] [Google Scholar]
- 23. Lauver DA, Carey EG, Bergin IL, et al. Sildenafil citrate for prophylaxis of nephropathy in an animal model of contrast-induced acute kidney injury. PLoS One. 2014;9:e113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sendeski M, Patzak A, Pallone TL, et al. Iodixanol, constriction of medullary descending vasa recta, and risk for contrast medium-induced nephropathy. Radiology. 2009;251:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jost G, Lenhard DC, Sieber MA, et al. Changes of renal water diffusion coefficient after application of iodinated contrast agents: effect of viscosity. Invest Radiol. 2011;46:796–800. [DOI] [PubMed] [Google Scholar]
- 26. Jung F, Rampling M. Role of blood viscosity in the microcirculation. Clin Hemorheol Microcirc. 2016;64:251–254. [DOI] [PubMed] [Google Scholar]
- 27. Wu CJ, Bao ML, Wang Q, et al. Acute kidney damage induced by low- and iso-osmolar contrast media in rats: comparison study with physiologic MRI and histologic-gene examination. J Magn Reson Imaging. 2017;45:291. [DOI] [PubMed] [Google Scholar]
- 28. Zhang YD, Wang J, Zhang J, et al. Effect of iodinated contrast media on renal function evaluated with dynamic three-dimensional MR renography. Radiology. 2014;270:409–415. [DOI] [PubMed] [Google Scholar]
- 29. Svaland MG, Haider T, Langseth-Manrique K, et al. Human pharmacokinetics of iodixanol. Invest Radiol. 1992;27:130. [DOI] [PubMed] [Google Scholar]
- 30. Quintavalle C, Brenca M, De Micco F, et al. In vivo and in vitro assessment of pathways involved in contrast media-induced renal cells apoptosis. Cell Death Dis. 2011;2:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu ZZ, Schmerbach K, Lu Y, et al. Iodinated contrast media cause direct tubular cell damage, leading to oxidative stress, low nitric oxide, and impairment of tubuloglomerular feedback. Am J Physiol Renal Physiol. 2014;306:F864–F872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chalikias G, Drosos I, Tziakas DN. Contrast-induced acute kidney injury: an update. Cardiovasc Drugs Ther. 2016;30:215–228. [DOI] [PubMed] [Google Scholar]
- 33. Thacker JM, Li LP, Li W, et al. Renal blood oxygenation level-dependent magnetic resonance imaging: a sensitive and objective analysis. Invest Radiol. 2015;50:821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]