Abstract
We assessed whether HIV status was associated with white matter hyperintensities (WMH), a neuroimaging correlate of cerebral small vessel disease (CSVD), in men aged ≥50 years. A cross-sectional substudy was nested within a larger cohort study. Virologically suppressed men living with HIV (MLWH) and demographically matched HIV-negative men aged ≥50 underwent magnetic resonance imaging (MRI) at 3 Tesla. Sequences included volumetric three-dimensional (3D) T1-weighted, fluid-attenuated inversion recovery and pseudocontinuous arterial spin labeling. Regional segmentation by automated image processing algorithms was used to extract WMH volume (WMHV) and resting cerebral blood flow (CBF). The association between HIV status and WMHV as a proportion of intracranial volume (ICV; log-transformed) was estimated using a multivariable linear regression model. Thirty-eight MLWH [median age 59 years (interquartile range, IQR 55–64)] and 37 HIV-negative [median 58 years (54–63)] men were analyzed. MLWH had median CD4+ count 570 (470–700) cells/μL and a median time since diagnosis of 20 (14–24) years. Framingham 10-year risk of cardiovascular disease was 6.5% in MLWH and 7.4% in controls. Two (5%) MLWH reported a history of stroke or transient ischemic attack and five (13%) reported coronary heart disease compared with none of the controls. The total WMHV in MLWH was 1,696 μL (IQR 1,229–3,268 μL) or 0.10% of ICV compared with 1,627 μL (IQR 1,032–3,077 μL), also 0.10% of ICV in the HIV-negative group (p = .43). In the multivariable model, WMHV/ICV was not associated with HIV status (p = .86). There was an age-dependent decline in cortical CBF [−3.9 mL/100 mL/min per decade of life (95% confidence interval 1.1–6.7 mL)] but no association between CBF and HIV status (p > .2 in all brain regions analyzed). In conclusion, we found no quantitative MRI evidence of an increased burden of CSVD in MLWH aged 50 years and older.
Keywords: HIV infection, cerebral small vessel disease, aging, magnetic resonance imaging, cerebral blood flow, leukoencephalopathy
Introduction
An increasing proportion of people living with HIV (PLWH) are older than 50, largely due to increased survival,1,2 and they are therefore at greater risk of comorbidities of older age such as cardiovascular disease (CVD), dementia, hypertension, and type 2 diabetes. It is possible that HIV infection in middle and older age is associated with an increased risk of cerebral small vessel disease (CSVD) in addition to that seen in the general population. CSVD is associated with circulating inflammatory mediators,3,4 and chronic immune activation and corresponding elevations of systemic inflammatory markers are seen in HIV even after virological suppression has been achieved with antiretroviral therapy (ART).5
An association between HIV infection and CSVD would be of major importance to those living with HIV because of the increased risk of stroke, dementia, and death in individuals with white matter hyperintensities (WMH) of presumed vascular origin,6 one of the key quantifiable neuroimaging features of CSVD.7 It is recognized that vascular pathology makes a substantial contribution to cognitive impairment and dementia in the general population.8 Cognitive impairment is thought to be highly prevalent in PLWH,9–11 and CSVD is one plausible mechanism for this.
While epidemiological evidence suggests higher rates of large vessel ischemic stroke in HIV infection,12–17 it cannot be assumed that a similar association exists for CSVD as it is likely to be a different disease process. Early studies of mainly untreated PLWH using older neuroimaging techniques found little evidence of an association between HIV and WMH.18,19 Furthermore, an autopsy study found no association between CSVD and HIV status.20 More recently, two other studies measuring the total volume of WMH found no overall difference between PLWH and HIV-negative participants.21,22 Other authors have suggested that there is an association between HIV and CSVD but did not compare neuroimaging and neuropathological measures to HIV-negative controls.23,24 However, a recent neuroimaging study of Dutch men living with HIV (MLWH), older than 45, and HIV-negative controls reported a greater burden of WMH of presumed vascular origin on brain magnetic resonance imaging (MRI).25
There is also some evidence to support an association between HIV infection and lower cerebral blood flow (CBF),26,27 although this is not consistent across all studies.28,29 A recent meta-analysis of studies of HIV-negative individuals showed that lower CBF is associated with higher numbers of WMH, at least in cross-sectional studies.30
In the present study, we aimed to assess whether HIV status was associated with an increased volume of WMH in men aged ≥50 years at two clinics in London, United Kingdom. We also sought to compare CBF between MLWH and HIV-negative men, and estimate the association between CBF and WMH burden.
Materials and Methods
Participant selection
We conducted a cross-sectional neuroimaging substudy within the Pharmacokinetic and Clinical Observations in PeoPle over fiftY (POPPY) study (ClinicalTrials.gov Identifier NCT01737047).31 Male participants enrolled in POPPY, ages 50 or older at two study sites in London, United Kingdom, were recruited between October 2013 and September 2015. Only those self-identifying with a white ethnic group were recruited. Participants provided written, informed consent and the study was approved by the National Research Ethics Service Committee London–Central, reference 13/LO/1088. Our target sample size was 40 in each group. We excluded participants with current active neurological, psychiatric, or substance use disorders.
Data collection
The POPPY cohort database was used to obtain clinical information relating to previous CVD, cardiovascular risk factors, current and previous ART, lymphocyte subset counts, anthropometric data, serum lipids, syphilis, hepatitis C virus (HCV) serology, and recreational drug use. Framingham 10-year CVD risk was calculated using the Framingham STATA module.32 Clinical assessments included frailty measurements, namely timed 5-m walk and grip strength.
Magnetic resonance image processing
Participants underwent a 1-h imaging protocol, including volumetric T1- and T2-weighted sequences, three-dimensional (3D) fluid-attenuated inversion recovery (FLAIR), pseudocontinuous arterial spin labeling (pCASL), and a 3D multiple echo recombined gradient echo sequence of the carotid arteries. Images were obtained on the same Phillips 3 Tesla system for all participants. Individuals with metallic implants such as coronary stents were only imaged if the safety of undergoing MRI could be confirmed.
WMH volume (WMHV) was extracted from volumetric 3D T1 and FLAIR images using automated image processing algorithms.33
CBF was calculated from pCASL sequence data after partial volume correction and adjustment for hematocrit.34 CBF maps were coregistered to structural sequences and CBF was measured in the following regions using automated segmentation algorithms: supratentorial gray matter, deep gray matter (basal ganglia and thalamus), frontal cortex, occipital cortex, parietal cortex, cingulate cortex, and total white matter.
Statistical analysis
MLWH who did not have a suppressed HIV-1 viral load (<50 copies/mL) on ART were excluded from the analysis. The main outcome variable of WMHV was expressed as a proportion of intracranial volume (ICV) and log transformed. This was compared between the MLWH and HIV-negative groups using the Mann–Whitney test. Other variables were compared between groups using the Mann–Whitney test and analyzed for their association with WMHV using linear regression; these included linear continuous variables [body mass index, waist circumference, diastolic and systolic blood pressure, and concentrations of total, high-density lipoprotein, and low-density lipoprotein (LDL) cholesterol] and binary variables [smoking status, self-reported history of ischemic heart disease, stroke or transient ischemic attack (TIA), injection drug use, psychoactive recreational drug use in the past 6 months, hepatitis C antibody status, and previous syphilis]. HIV status, age, and any other factors found to have at least a modest association (p < .2) with WMHV in bivariate models were analyzed in multivariable linear regression models.
Additional linear regression analyses, including the subgroup of MLWH, only explored associations between WMHV and HIV-related factors (linear continuous variables of CD4+ and CD8+ lymphocyte counts and CD4:CD8 ratio, time since HIV diagnosis, time between diagnosis and first initiation of ART, and total duration of ART, and binary categorization of current use of all antiretroviral drug classes as well as abacavir and tenofovir), adjusted for age. Finally, linear regression was used to assess the relationship between CBF in each region of interest and HIV status, and between CBF in each region of interest and WMHV.
Results
Participant characteristics
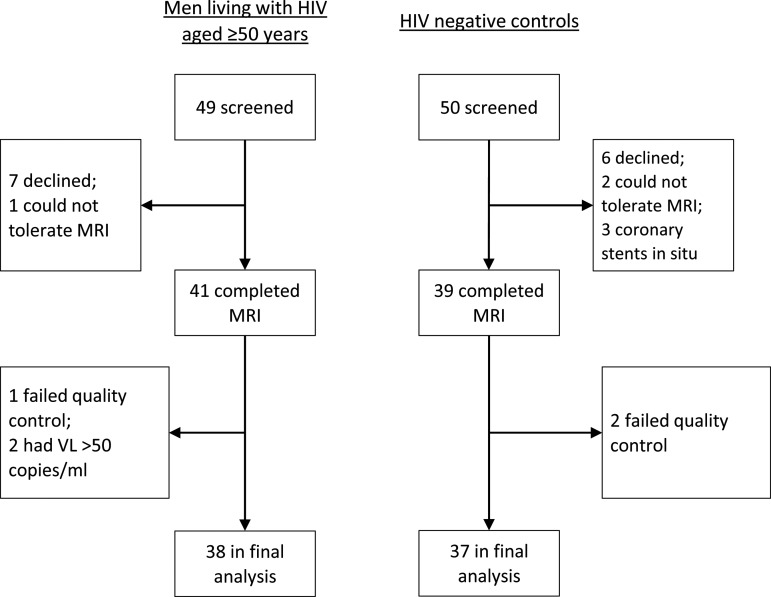
There were 38 MLWH and 37 HIV-negative controls in the final analysis (Fig. 1); their characteristics are shown in Table 1. The two groups were similar in cardiovascular risk factors, although HIV-negative participants had higher LDL concentration. MLWH had more previous syphilis (47% vs. 22%; p = .02), but the prevalence of hepatitis C was low in both groups (5% vs. 0%; p = .49). Two (5%) MLWH reported a history of stroke or TIA and five (13%) reported coronary heart disease (CHD) compared with none of the controls (p = .49 for stroke/TIA; p = .05 for CHD). MLWH had a markedly slower 5-m walk than controls [median 6.3 s (interquartile range, IQR 5.7–8.3) vs. 4.5 s (IQR 3.0–5.6), p < .001] although there was no difference in grip strength (p = .61). The most frequently reported recreational drugs were ketamine (16%), methamphetamine (“crystal meth,” 15%), amphetamine (15%), and cannabis (13%), with no differences between groups. MLWH had a median duration of infection of 20 years (IQR 14–24 years), a median duration of treatment of 15 years (IQR 12–20 years), and 17 (45%) had a history of AIDS. ART regimens included nucleoside reverse transcriptase inhibitor(s) in 34 (89%), non-nucleoside reverse transcriptase inhibitors in 19 (50%), protease inhibitors in 19 (50%), and an integrase inhibitor in 1 man (3%).
FIG. 1.
Participant flowchart. MRI, magnetic resonance imaging; VL, viral load.
Table 1.
Characteristics of Enrolled Participants and Comparison Between the Two Groups
| Variable | MLWH (n = 38) | HIV negative (n = 37) | p-Value for group-wise comparisona |
|---|---|---|---|
| Age, median years (IQR) | 59 (55–64) | 58 (54–63) | .61 |
| Blood pressure, median mm Hg (IQR) | |||
| Systolic | 125 (117–131) | 126 (116–138) | .45 |
| Diastolic | 79 (73–84) | 76 (72–82) | .37 |
| Smoking, n (%) | |||
| Current | 9 (24) | 7 (19) | .82 |
| Previous | 15 (39) | 14 (38) | |
| Alcohol/week, median units (IQR) | 6 (2–15) | 14 (4–21) | .12 |
| Psychoactive drug use in past 6 months, n (%) | 11 (29) | 9 (24) | .65 |
| Medical history, n (%) | |||
| Coronary heart disease | 5 (13.2) | 0 | .05 |
| Stroke or TIA | 2 (5.3) | 0 | .49 |
| Syphilis | 18 (47.4) | 8 (21.6) | .02 |
| Hepatitis C | 2 (5.3) | 0 | .49 |
| Framingham 10-year CVD risk, median % (IQR)b | 6.5 (5.0,–9.4) | 7.4 (6.0–10.4) | .41 |
| Framingham 10-year CVD risk above 10%, n/N (%)b | 9/37 (24.3) | 8/29 (27.6) | .78 |
| Body mass index, median kg/m2 (IQR) | 25.4 (23.5–28.2) | 25.0 (23.3–28.0) | .66 |
| Waist circumference, median cm (IQR) | 93 (86–100) | 90 (86–100) | .56 |
| Lipids, median mmol/L (IQR)c | |||
| Total cholesterol | 4.8 (3.9–5.4) | 5.1 (4.2–5.8) | .14 |
| HDL | 1.3 (1.0–1.5) | 1.3 (1.1–1.6) | .55 |
| LDL | 2.4 (1.8–3.3) | 3.1 (2.6–3.5) | .06 |
| Cholesterol:HDL ratio | 3.4 (2.8–5.0) | 4.0 (3.2–4.6) | .74 |
| Years since tested HIV positive, median (IQR) | 20.4 (14.2–24.4) | n/a | — |
| Years since first started ART, median (IQR) | 15.1 (11.7–20.2) | n/a | — |
| Lymphocytes, median cells/mm3 (IQR) | |||
| Absolute CD4+ | 570 (470–700) | n/a | — |
| Percentage CD4+ | 34 (23–39) | ||
| CD8+ | 1,010 (690–1,320) | ||
| CD4+/CD8+ ratio | 0.61 (0.39–0.89) | ||
Groups were compared using the Mann–Whitney test for continuous variables and the chi-squared test for categorical variables.
Framingham risk could not be calculated in nine participants, due to missing lipid results.
Lipid concentrations are presented in SI units (mmol/L). Values can be converted to mg/dL by multiplying by 38.67. A total cholesterol concentration of 4.8 mmol/L equates to around 186 mg/dL.
ART, antiretroviral therapy; CVD, cardiovascular disease; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; MLWH, men living with HIV; TIA, transient ischemic attack.
Factors associated with volume of WMH
There were no differences in WMHV between MLWH and HIV-negative participants, whether analyzed by absolute volume or the proportion of total ICV (Table 2). Age was associated with WMHV (unadjusted; p = .02). Ten-year Framingham cardiovascular risk was also associated (p = .049), most likely due to the contribution of age to the risk calculation. Weaker associations (0.2 > p ≥ .05) were present for body mass index (BMI; p = .13), waist circumference (p = .09), and LDL cholesterol concentration (p = .10). Adjusting for age, BMI, and LDL (Fig. 2), WMHV was not associated with HIV status (p = .86), BMI (p = .20), or LDL (p = .16), but there was an association with increasing age [1.4-fold increase in WMHV/ICV per decade, 95% confidence interval (CI) 1.0–2.0, p = .038].
Table 2.
White Matter Hyperintensity Volume in Study Participants, Expressed as Median (Interquartile Range)
| MLWH (n = 38) | HIV negative (n = 37) | pa | |
|---|---|---|---|
| WMHV, μL | 1,696 (1,229–3,268) | 1,627 (1,032–3,077) | .43 |
| Total ICV, mL | 1,639 (1,577–1,711) | 1,682 (1,574–1,764) | .33 |
| WMHV, % of ICV | 0.10 (0.08–0.20) | 0.10 (0.06–0.19) | .36 |
Groups were compared by Mann–Whitney's test. When compared using Student's t-test, p was equal to .69, .22, and .64, respectively.
ICV, intracranial volume; WMHV, white matter hyperintensity volume.
FIG. 2.
Association between WMHV, HIV status, and other factors. Broken lines denote unadjusted (bivariate) analyses. Solid lines denote adjusted (multivariable) analyses. Factors were included in the multivariable model if they showed p < .2 in bivariate models. HIV status was also included. Deflections to the right denote a positive association (higher value of exposure variable correlated with larger volume of white matter hyperintensities). Lipid concentrations were analyzed in SI units (mmol/L). One mmol/L of LDL is equal to 38.67 mg/dL. BMI, body mass index; CI, confidence interval; LDL, low-density lipoprotein; WMHV, white matter hyperintensity volume (as a proportion of total intracranial volume).
Among the subgroup of MLWH, there was no association between WMHV and any of the exposure variables analyzed (absolute and percentage CD4 count, absolute CD8 count, CD4/CD8 ratio, time since HIV diagnosis, time since starting ART, history of AIDS, HCV status, and ART drug class).
Regional distribution of WMH
The anatomical distribution of WMH was compared between men living with HIV (MLWH) and controls. To do this, white matter and subcortical structures were subdivided by region (left and right frontal, parietal, occipital, and temporal lobes, and a combined basal ganglia/inferotemporal region) and by distance from the ventricular surface (in four concentric layers, numbered from one at the periventricular surface to four at the juxtacortical margin). The rationale and procedures for this subdivision method have been recently developed and the results can be displayed graphically as “bullseye” plots.35
Comparison of the median WMHV as a proportion of segmental brain volume between groups was done independently for each of the 36 subregions, using the Mann–Whitney test. After applying Bonferroni's correction for multiple comparisons, one subregion reached statistical significance, namely layer 4 of the combined basal ganglia/inferotemporal region [WMHV median 0.07% in MLWH (IQR 0.02%–0.10%) compared with median 0.009% in controls (IQR 0%–0.04%), p = .0009, critical p-value by Bonferroni's correction .0010]. Three other subregions reached conventional statistical significance (p < .05) but were deemed not statistically significant after Bonferroni's correction. These were layer 4 of the left occipital white matter (p = .02), layer 3 of the basal ganglia/inferotemporal region (p = .04), and layer 3 of the left occipital white matter (p = .04), and in all three subregions there was a greater proportion of WMH in brain tissue in MWLH than in controls.
CBF in HIV-positive and HIV-negative participants
Mean resting CBF, measured using pCASL, was compared between MLWH and HIV-negative men in the gray matter of the frontal, occipital, parietal and cingulate cortices, in subcortical gray matter, and in white matter. There were 34 participants in each group with pCASL images of sufficient quality. There was no difference between groups in any of these regions (Table 3). The same null findings were seen after adjustment for age. Age itself was the only significant factor associated with CBF, with a 3.9 mL/100 mL/min decrease in cortical CBF per decade of life (95% CI 1.1–6.7 mL) and a 2.8 mL/100 mL/min decrease in subcortical CBF per decade of life (95% CI 0.1–5.5 mL). Similar associations were seen between age and CBF in all cortical regions. There was no association between CBF and the total volume of WMH in cortical gray matter (p = .52), subcortical gray matter (p = .37), or white matter (p = .48).
Table 3.
Resting Cerebral Blood Flow in Men Living with HIV and HIV-Negative Men Aged 50 and Older
| Brain region | MLWH (n = 34)a | HIV negative (n = 34)a | pb |
|---|---|---|---|
| Frontal cortex | 50.1 (43.5–56.4) | 48.6 (44.4–55.5) | .90 |
| Occipital cortex | 47.4 (40.2–51.4) | 45.0 (39.8–52.9) | .69 |
| Parietal cortex | 47.9 (40.4–51.9) | 46.6 (41.2–52.5) | .92 |
| Cingulate cortex | 49.3 (45.4–56.0) | 49.7 (45.5–53.9) | .84 |
| Subcortical gray matter | 41.7 (37.4–46.8) | 40.4 (37.9–44.8) | .49 |
| White matter | 26.6 (25.0–29.2) | 26.6 (23.2–29.0) | .59 |
Cerebral blood flow is expressed in units of mL of blood per 100 mL of brain tissue per minute, and displayed as median (IQR).
Groups were compared by Mann–Whitney's test.
Discussion
We found no difference in the burden of CSVD, estimated by the volume of WMH, between MLWH ages 50 and older and demographically matched HIV-negative controls.
Previous evidence in the literature offers a mixture of results. In the Netherlands, Su et al. used similar techniques to ours to investigate 103 MLWH and 70 HIV-negative men ages 45 and older, and found a higher volume of WMH in MLWH (1.0 mL of white matter hyperintensity compared with 0.7 mL in controls, p = .008).25 Their HIV-positive participants had more tobacco consumption (expressed as pack-years) and a higher waist/hip ratio than controls. Cole et al. also found a higher burden of WMH in PLWH than in controls (1,126 μL compared with 824 μL, p = .015).29 Their cohort, based in the United Kingdom and the Netherlands, was more than 90% male and there may have been some overlap in the participant pool with the preceding Dutch study. However, they observed that there was no difference between groups in the rate of change over a 2-year period. In France, a study using the Fazekas and Schmidt radiological rating scale reported a prevalence of CSVD of 52% in 456 PLWH compared with 36% in 154 HIV-negative controls (odds ratio 2.3, 95% CI 1.5–3.6).36 The PLWH in their sample were 85% male compared with 77% in the control group (p = .03) and the positive group had statistically significantly higher rates of hypertension, hyperlipidemia, and previous CVD.
Null findings come from two other MRI studies, both conducted in the United States and again using measures of WMHV similar to ours. In one, there was no difference between the PLWH (n = 88) and the control group (n = 49) (p = .49), but neither the average WMHV, the gender, nor the cardiovascular risk profile of the participants was reported.21 Watson et al. also found no difference in WMHV between 65 PLWH and 29 HIV-negative controls (2.78 mL of WMH compared with 3.18 mL, p = .69).22 All participants in the latter study were older than 60, with the control group being on average 2 years older, and there were higher rates of smoking and hypercholesterolemia in the PLWH, but no difference in the prevalence of hypertension. Some studies measuring other biomarkers of CSVD such as retinal vessel calibers37,38 and cerebrovascular histology20 have similarly found no association with HIV.
We also found no evidence of an association between HIV status and resting CBF, supporting the findings of several other groups as well as our own unpublished arterial spin labeling data.28,29,39 However in a study of cerebral vasoreactivity, a dynamic measure of cerebrovascular endothelial function, an attenuated response to hypercapnia was found in HIV-positive participants.40 We observed decreasing gray matter perfusion with age, which is expected in the normal aging process.41
Our study focused on a specific demographic group recruited from two locations in the same city, which may limit the generalizability of our findings to other settings. We chose to restrict our sample by ethnic group and gender to eliminate the well-described and powerful confounding effects of these variables on vascular disease markers; the study would likely have been insufficiently powered to perform gender and ethnicity subgroup analyses. We chose an older male cohort because such individuals are at greater risk of vascular disease, and we targeted the predominant demographic and transmission risk group (white men who have sex with men) attending our local clinics and living with HIV in the United Kingdom and many high-income settings. It is a strength of our study that the HIV-negative controls had similar measured characteristics to the MLWH, and their attendance for sexual health and HIV testing at the same centers as the MLWH should have reduced the effect of unmeasured confounders. There was an incidental finding of a slower timed walk in the HIV-positive group, possibly indicating occult brain insult from another etiology unrelated to the primary outcome measure.
The study's power to detect associations between HIV and CSVD was limited by its small sample size. Age was the only variable found to have a strong association with the outcome measures. Most participants were in middle age and therefore at lower risk of cerebrovascular disease than an older age group. However, the difference observed between the two groups was very small compared with the overall distribution of values, and despite the small sample, the evidence for there being no true difference in WMHV on the basis of HIV status is strong.
The results of this study contribute to the accumulated evidence so far in this field. In summary, this evidence does not consistently find HIV to be a risk factor for CSVD. The inconsistencies between published studies may, in part, be due to differences in the method for measuring WMH and the lack of a gold standard.7 However, our findings do not diminish the need for assessment and optimization of individual patient's cardiovascular risks. Of the MLWH, 24% had a 10-year risk of CVD (Framingham model) of above 10% (the threshold above which the UK National Institute for Health and Care Excellence recommends statin therapy for primary prevention42). Signs, symptoms, and risk factors associated with CSVD should be addressed in PLWH in the same manner as in HIV-negative individuals.
Acknowledgments
The authors acknowledge all participants in this study. MRI was carried out at the Imaging Department at University College Hospital, London, with the invaluable support of Lorna Smith, Nicky Stevens, and David Atkinson. Additional support at the Institute of Neurology, UCL, was provided by Fiona Kennedy, Mark White, and Gary Zhang. The study was funded by Clinical Research and Development Committee Research Funding from the UCL Medical School General Charitable Trust. The POPPY study (of which this is a substudy) is supported by investigator-initiated grants from Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Merck, and ViiV Healthcare, and from a National Institute for Health Research (NIHR) Senior Investigator Award (NF-SI-0514-10075). The research is supported by the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The clinical sites are grateful for NIHR Clinical Research Network support.
The POPPY study includes the following individuals: POPPY Management Team (Marta Boffito, Paddy Mallon, Frank Post, Caroline Sabin, Memory Sachikonye, Alan Winston); POPPY Scientific Steering Committee (Jane Anderson, David Asboe, Marta Boffito, Lucy Garvey, Paddy Mallon, Frank Post, Anton Pozniak, Caroline Sabin, Memory Sachikonye, Jaime Vera, Ian Williams, Alan Winston); Caldecot Centre, King's College Hospital (Frank Post, Lucy Campbell, Selin Yurdakul, Sara Okumu, Louise Pollard); Research Department of Infection and Population Health, University College London (Ian Williams, Damilola Otiko, Laura Phillips, Rosanna Laverick, Michelle Beynon, Anna-Lena Salz); Elton John Centre, Brighton and Sussex University Hospital (Martin Fisher, Amanda Clarke, Jaime Vera, Andrew Bexley, Celia Richardson); HIV Molecular Research Group, School of Medicine, University College Dublin (Paddy Mallon, Alan Macken, Bijan Ghavani-Kia, Joanne Maher, Maria Byrne, Ailbhe Flaherty, Sumesh Babu); Homerton Sexual Health Services, Homerton University Hospital (Jane Anderson, Sifiso Mguni, Rebecca Clark, Rhiannon Nevin-Dolan, Sambasivarao Pelluri); Ian Charleson Day Centre, Royal Free Hospital (Margaret Johnson, Nnenna Ngwu, Nargis Hemat, Martin Jones, Anne Carroll); Imperial Clinical Trials Unit, Imperial College London (Andrew Whitehouse, Laura Burgess, Daphne Babalis); St. Mary's Hospital London, Imperial College Healthcare NHS Trust (Alan Winston, Lucy Garvey, Jonathan Underwood, Matthew Stott, Linda McDonald); St Stephen's Centre, Chelsea and Westminster Hospital (Marta Boffito, David Asboe, Anton Pozniak, Chris Higgs, Elisha Seah, Stephen Fletcher, Michelle Anthonipillai, Ashley Moyes, Katie Deats, Irtiza Syed, Clive Matthews, Peter Fernando); Methodology, Statistics and Analysis Group (Caroline Sabin, Davide De Francesco, Emmanouil Bagkeris).
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Data were presented as a poster at the 9th IAS Conference on HIV Science, Paris, France, July 23–26, 2017.
Contributor Information
Collaborators: on behalf of the Pharmacokinetics and clinical Observations in PeoPle over fiftY (POPPY) Study Group, Marta Boffito, Paddy Mallon, Frank Post, Caroline Sabin, Memory Sachikonye, Alan Winston, Jane Anderson, David Asboe, Marta Boffito, Lucy Garvey, Paddy Mallon, Frank Post, Anton Pozniak, Caroline Sabin, Memory Sachikonye, Jaime Vera, Ian Williams, Alan Winston, Frank Post, Lucy Campbell, Selin Yurdakul, Sara Okumu, Louise Pollard, Ian Williams, Damilola Otiko, Laura Phillips, Rosanna Laverick, Michelle Beynon, Anna-Lena Salz, Martin Fisher, Amanda Clarke, Jaime Vera, Andrew Bexley, Celia Richardson, Paddy Mallon, Alan Macken, Bijan Ghavani-Kia, Joanne Maher, Maria Byrne, Ailbhe Flaherty, Sumesh Babu, Jane Anderson, Sifiso Mguni, Rebecca Clark, Rhiannon Nevin-Dolan, Sambasivarao Pelluri, Margaret Johnson, Nnenna Ngwu, Nargis Hemat, Martin Jones, Anne Carroll, Andrew Whitehouse, Laura Burgess, Daphne Babalis, Alan Winston, Lucy Garvey, Jonathan Underwood, Matthew Stott, Linda McDonald, Marta Boffito, David Asboe, Anton Pozniak, Chris Higgs, Elisha Seah, Stephen Fletcher, Michelle Anthonipillai, Ashley Moyes, Katie Deats, Irtiza Syed, Clive Matthews, Peter Fernando, Caroline Sabin, Davide De Francesco, and Emmanouil Bagkeris
Authors' Contributions
L.J.H. conceived and led the study. C.H.S., M.S., S.O., and J.C. extracted and processed the MRI data. All authors contributed to the analysis and interpretation of data. L.J.H. conducted the main analyses and wrote the article. All authors read and approved the final version.
Author Disclosure Statement
L.J.H. has received speakers' fees and support for conference attendance from Gilead Sciences and Janssen Pharmaceuticals, honoraria for advisory boards from Gilead Sciences, and grants from Janssen-Cilag, the UK National Institute of Health Research, the Rosetrees Trust, and ViiV Healthcare. I.G.W. has received grants from the UK National Institute of Health Research—Health Technology Assessment program, Janssen-Cilag, and Merck Sharp Dohme. X.G. is CEO of Gold Standard Phantoms. A.W. reports research grants to Imperial College on his behalf, and personal advisory board fees and speaker fees from Janssen, Gilead, ViiV Healthcare, Merck-Sharpe-Dohme and Bristol-Meyers Squibb. C.A.S. has received honoraria for participation in data safety and monitoring boards, advisory boards, speaker panels, and for preparation of educational materials from Gilead Sciences, ViiV Healthcare, and Janssen-Cilag. All other authors have nothing to disclose.
References
- 1. Kirwan PD, Chau C, Brown AE, Gill ON, Delpech VC: HIV in the UK—2016 Report. Public Health England, London, UK, 2016 [Google Scholar]
- 2. Centers for Disease Control and Prevention: Diagnosis of HIV infection among adults aged 50 years and older in the United States and dependent areas, 2010–2014. HIV Surveillance Supplemental Report, Atlanta, GA, 2016 [Google Scholar]
- 3. Rouhl RP, Damoiseaux JG, Lodder J, et al. : Vascular inflammation in cerebral small vessel disease. Neurobiol Aging 2012;33:1800–1806 [DOI] [PubMed] [Google Scholar]
- 4. Shoamanesh A, Preis SR, Beiser AS, et al. : Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology 2015;84:825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Appay V, Kelleher AD: Immune activation and immune aging in HIV infection. Curr Opin HIV AIDS 2016;11:242–249 [DOI] [PubMed] [Google Scholar]
- 6. Debette S, Markus HS: The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wardlaw JM, Smith EE, Biessels GJ, et al. : Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy MP, Corriveau RA, Wilcock DM: Vascular contributions to cognitive impairment and dementia. Biochim Biophys Acta 2016;1862:857–859 [DOI] [PubMed] [Google Scholar]
- 9. Heaton RK, Clifford DB, Franklin DRJ, et al. : HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simioni S, Cavassini M, Annoni JM, et al. : Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010;24:1243–1250 [DOI] [PubMed] [Google Scholar]
- 11. Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes OA: Higher frequency of dementia in older HIV-1 individuals: The Hawaii Aging with HIV-1 Cohort. Neurology 2004;63:822–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benjamin LA, Corbett EL, Connor MD, et al. : HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: A case-control study. Neurology 2016;86:324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ovbiagele B, Nath A: Increasing incidence of ischemic stroke in patients with HIV infection. Neurology 2011;76:444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Worm SW, Kamara DA, Reiss P, et al. : Evaluation of HIV protease inhibitor use and the risk of sudden death or nonhemorrhagic stroke. J Infect Dis 2012;205:535–539 [DOI] [PubMed] [Google Scholar]
- 15. Yen Y-F, Chen M, Jen I, et al. : Association of HIV and opportunistic infections with incident stroke: A nationwide population-based cohort study in Taiwan. J Acquir Immune Defic Syndr 2017;74:117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rasmussen LD, Engsig FN, Christensen H, et al. : Risk of cerebrovascular events in persons with and without HIV: A Danish nationwide population-based cohort study. AIDS 2011;25:1637–1646 [DOI] [PubMed] [Google Scholar]
- 17. Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ: Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the Southeastern United States. AIDS Res Hum Retroviruses 2013;29:1068–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manji H, Connolly S, McAllister R, et al. : Serial MRI of the brain in asymptomatic patients infected with HIV: Results from the UCMSM/Medical Research Council neurology cohort. J Neurol Neurosurg Psychiatry 1994;57:144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McArthur JC, Kumar AJ, Johnson DW, et al. : Incidental white matter hyperintensities on magnetic resonance imaging in HIV-1 infection. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 1990;3:252–259 [PubMed] [Google Scholar]
- 20. Morgello S, Murray JM, Van Der Elst S, Byrd DA: HCV, but not HIV, is a risk factor for cerebral small vessel disease. Neurol Neuroimmunol Neuroinflamm 2014;1:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seider TR, Gongvatana A, Woods AJ, et al. : Age exacerbates HIV-associated white matter abnormalities. J Neurovirol 2016;22:201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watson C, Busovaca E, Foley JM, et al. : White matter hyperintensities correlate to cognition and fiber tract integrity in older adults with HIV. J Neurovirol 2017;23:422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMurtray A, Nakamoto B, Shikuma C, Valcour V: Small-vessel vascular disease in human immunodeficiency virus infection: The Hawaii Aging with HIV cohort study. Cerebrovasc Dis 2007;24:236–241 [DOI] [PubMed] [Google Scholar]
- 24. Soontornniyomkij V, Umlauf A, Chung SA, et al. : HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS 2014;28:1297–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Su T, Wit FW, Caan MW, et al. : White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on combination antiretroviral therapy. AIDS 2016;30:2329–2339 [DOI] [PubMed] [Google Scholar]
- 26. Ances BM, Roc AC, Wang J, et al. : Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology 2006;66:862–866 [DOI] [PubMed] [Google Scholar]
- 27. Ances BM, Sisti D, Vaida F, et al. : Resting cerebral blood flow. A potential biomarker of the effects of HIV in the brain. Neurology 2009;73:702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Towgood KJ, Pitkanen M, Kulasegaram R, et al. : Regional cerebral blood flow and FDG uptake in asymptomatic HIV-1 men. Hum Brain Mapp 2013;34:2484–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cole JH, Caan MW, Underwood J, et al. : No evidence for accelerated ageing-related brain pathology in treated HIV: Longitudinal neuroimaging results from the Comorbidity in Relation to AIDS (COBRA) project. Clin Infect Dis 2018;66:1899–1909 [DOI] [PubMed] [Google Scholar]
- 30. Shi Y, Thrippleton MJ, Makin SD, et al. : Cerebral blood flow in small vessel disease: A systematic review and meta-analysis. J Cereb Blood Flow Metab 2016;36:1653–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bagkeris E, Burgess L, Mallon PW, et al. : Cohort profile: The Pharmacokinetic and clinical Observations in PeoPle over fiftY (POPPY) study. Int J Epidemiol 2018;47:1391e–1392e [DOI] [PubMed] [Google Scholar]
- 32. Linden A: Framingham: Stata module for calculating the Framingham 10-year Cardiovascular Disease Risk Prediction. Ann Arbor, MI, Boston College Department of Economics; 2015 [Google Scholar]
- 33. Sudre CH, Cardoso MJ, Bouvy WH, Biessels GJ, Barnes J, Ourselin S: Bayesian model selection for pathological neuroimaging data applied to white matter lesion segmentation. IEEE Trans Med Imaging 2015;34:2079–2102 [DOI] [PubMed] [Google Scholar]
- 34. Alsop DC, Detre JA, Golay X, et al. : Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73:102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sudre CH, Gomez Anson B, Davagnanam I, et al. : Bullseye's representation of cerebral white matter hyperintensities. J Neuroradiol 2018;45:114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moulignier A, Savatovsky J, Assoumou L, et al. : Silent cerebral small-vessel disease is twice as prevalent in middle-aged individuals with well-controlled, combination antiretroviral therapy-treated human immunodeficiency virus (HIV) than in HIV-uninfected individuals. Clin Infect Dis 2018;66:1762–1769 [DOI] [PubMed] [Google Scholar]
- 37. Haddow LJ, Laverick R, Leung I, et al. : Measurement of retinal vessels as a biomarker of cerebrovascular aging in older HIV-positive men compared with controls. J Acquir Immune Defic Syndr 2018;77:199–205 [DOI] [PubMed] [Google Scholar]
- 38. Pathai S, Weiss HA, Lawn SD, et al. : Retinal arterioles narrow with increasing duration of anti-retroviral therapy in HIV infection: A novel estimator of vascular risk in HIV? PLoS One 2012;7:e51405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haddow LJ, Sokolska M, Fallatah SM, Gilson RJC, Jäger HR, Golay X: A study of the effect of early, untreated HIV on cerebral perfusion and arterial transit time in the basal ganglia using arterial spin labelling. In: 15th European AIDS Conference, Barcelona, Spain, 2015 [Google Scholar]
- 40. Chow FC, Boscardin WJ, Mills C, et al. : Cerebral vasoreactivity is impaired in treated, virally suppressed HIV-infected individuals. AIDS 2016;30:45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soni N, Jain A, Kumar S, Pandey CM, Awasthi A: Arterial spin labeling magnetic resonance perfusion study to evaluate the effects of age and gender on normal cerebral blood flow. Neurol India 2016;64:32–38 [DOI] [PubMed] [Google Scholar]
- 42. Cardiovascular disease: Risk assessment and reduction, including lipid modification. National Institute for Health and Care Excellence, London, UK, 2014 [PubMed] [Google Scholar]