Abstract
The active form of the small GTPase RhoA is necessary and sufficient for formation of a cytokinetic furrow in animal cells. Despite the conceptual simplicity of the process, the molecular mechanisms that control it are intricate and involve redundancy at multiple levels. Here, we discuss our current knowledge of the mechanisms underlying spatiotemporal regulation of RhoA during cytokinesis by upstream activators. The direct upstream activator, the RhoGEF Ect2, requires activation due to autoinhibition. Ect2 is primarily activated by the centralspindlin complex, which contains numerous domains that regulate its subcellular localization, oligomeric state, and Ect2 activation. We review the functions of these domains and how centralspindlin is regulated to ensure correctly timed, equatorial RhoA activation. Highlighting recent evidence, we propose that although centralspindlin does not always prominently accumulate on the plasma membrane, it is the site where it promotes RhoA activation during cytokinesis.
Introduction
Cytokinesis, one of the most photogenic events in the life of a cell, requires precise positioning of the division machinery relative to the two segregated masses of DNA. Diverse strategies to accomplish this important cellular process have appeared during evolution. In plant cells, the division plane is determined by the converging plus ends of interpolar microtubules that direct the delivery and ultimate fusion of membranes containing cell wall materials to the cell center [1]. Many prokaryotes divide at the midcell. This site can be defined by the coordinated action of two inhibitory signals: an assembly inhibitor that oscillates between the two cell poles and a second inhibitory signal associated with the segregating masses of DNA [2]. The midcell is permissive for the assembly and treadmilling of prokaryotic tubulin, ftsZ, which directs the local synthesis of cell wall materials that mediate cell fission [3]. In animal cells, the position of the anaphase spindle directs the position of the cleavage furrow, as demonstrated by spindle manipulation experiments [4]. The spindle generates both positive and negative signals that create and pattern cortical contractility. At the peak of the positive signal and/or the minimum of the negative signal, the contractile ring, an actomyosin-based structure assembles, constricts, and eventually triggers abscission. Contractile ring assembly requires activation of the small GTPase RhoA and zones of active RhoA accumulate at sites of furrow formation [5–7]. Optogenetic induction of a membrane-associated zone of active RhoA reveals such zones are sufficient for furrow formation [8]. These findings suggest that a primary function of the spindle is to generate and pattern zones of RhoA activity.
RhoA directly activates formin-mediated f-actin assembly and indirectly promotes myosin II activation [9, 10]. The GTPase associates with the plasma membrane via C-terminal prenylation [11], a modification essential for its function. RhoA is activated by a conserved RhoGEF, Ect2 (for consistency, we will use the mammalian nomenclature throughout) (Figure 1 and 2) [12]. RhoA activation requires the interaction of RhoA•GDP with active Ect2 at the plasma membrane. This step is highly regulated, as Ect2 is autoinhibited (Figure 2) [13] and, in some cell types, not constitutively membrane bound [12]. Here we will review the current understanding of this process, with a focus on developments in the last ~5 years. The important steps that follow RhoA activation, contractile ring assembly, constriction and abscission have been recently reviewed [14, 15].
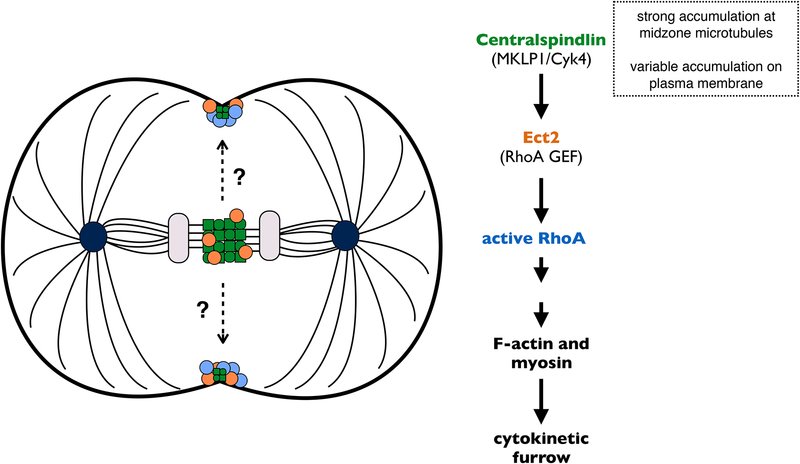
Figure 1. How is RhoA activated at the equatorial plasma membrane?
The centralspindlin complex (green) prominently accumulates at the spindle midzone and is responsible for recruiting and activating the RhoGEF Ect2 (orange) to the mid-plane during anaphase. Both Ect2 and active RhoA (blue) have been detected on the equatorial membrane, where they promote formation of an actomyosin-based contractile ring. There is also evidence for a pool of membrane-bound centralspindlin. Does this pool activate Ect2-RhoA at the plasma membrane, especially in cells where the spindle midzone does not lie adjacent to the membrane?
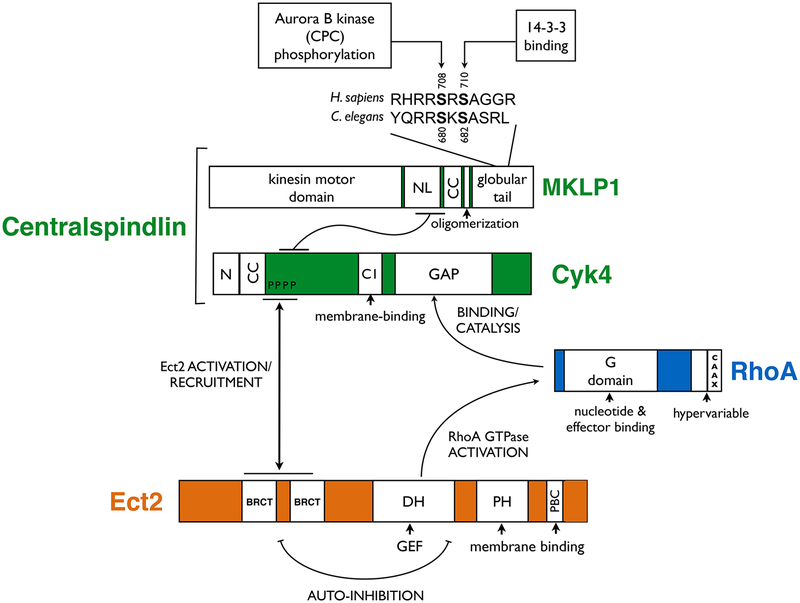
Figure 2. Domain organization of RhoA, Ect2 and centralspindlin.
Primary structure and predicted domains of the main proteins involved in RhoA activation during cytokinesis. The protein-protein interaction regions are indicated as are the main functional interactions. (N) N-terminal domain; (NL) neck linker region; (CC) coiled-coil domain; (PBC) poly-basic cluster.
Anaphase spindles are primarily composed of radial arrays of dynamic astral microtubules surrounding spindle poles and overlapping plus ends of microtubules at the middle of the spindle. Either one can spatially modulate furrow assembly, and a number of cell types enlist both strategies [16], presumably improving fidelity. Considerable variety is observed in the degree to which a given cell relies on one or the other. Anastral meiotic spindles rely exclusively on overlapping microtubules at the spindle midzone, representing one end of this spectrum. To a first approximation, overlapping microtubule plus ends are involved in promoting the local activation of RhoA at the equator and dynamic astral microtubules suppress it at poles. The centralspindlin complex, a major focus of this review, is a crucial component of the former. We begin by discussing the myriad domains of centralspindlin and how they contribute to RhoA activation. Subsequently we discuss how these reactions are spatially constrained to ensure RhoA activation at the equatorial plasma membrane - despite the lack of significant accumulation of centralspindlin at this site - and discuss our nascent understanding of the mechanism of inhibition of contractile activity by astral microtubules.
Multiple centralspindlin domains contribute to RhoA activation
To activate RhoA and induce a cleavage furrow, the RhoGEF Ect2 itself requires activation. This role is performed by centralspindlin, a protein complex conserved in all metazoans. A centralspindlin heterotetramer contains a dimer of a kinesin-6 motor protein MKLP1 (aka Kif23 in mammals; ZEN-4 in C. elegans; and Pavarotti in Drosophila) and a dimer of a RhoGAP protein, Cyk4 (aka MgcRacGAP in mammals; CYK-4 in C. elegans; and RacGAP50C/Tumbleweed in Drosophila). Each subunit of the complex contains multiple domains required for cytokinesis (Figure 2). While both centralspindlin and Ect2 are well conserved, as is the requirement that they interact, certain aspects of their localization patterns are not consistent across cell types. In most somatic cells during interphase, centralspindlin subunits and Ect2 are primarily nuclear localized [17, 18], but a fraction can also be detected in the cytoplasm and/or the membrane [19]. In mammalian cells, membrane recruitment of Ect2 is cell cycle regulated [20] and accompanied by prominent recruitment to the spindle midzone, whereas in C. elegans blastomeres it is constitutively membrane-bound and only weakly detected at the spindle midzone (K. Longhini and MG, unpublished results). Prominent centralspindlin localization to the spindle midzone is a conserved feature of anaphase cells, but its cortical recruitment is more variable. In Drosophila it is readily detected at the cortex [21, 22], in C. elegans it can be detected during mid-cytokinesis [23, 24], but it is rarely detected at the cortex in cultured mammalian cells, prior to midbody assembly when it is highly concentrated on the membrane [25].
MKLP1 has five functional domains. It has an N-terminal motor domain, an extended neck linker region, a coiled-coil dimerization motif, an oligomerization motif, and a C-terminal globular region. Cyk4 has a short N-terminal unstructured region, a coiled-coil dimerization motif, an extended region that interacts with Ect2, a lipid binding C1 domain, and a C-terminal RhoGAP domain. The N-terminal region of Cyk4 and the extended neck linker region of MKLP1 mediate complex assembly which also requires that each protein dimerize [26].
While this review focuses on the role of centralspindlin in RhoA activation for furrow induction, centralspindlin also is required early in cytokinesis to organize the central spindle and during the final stage of cell abscission [27]. In addition to its cytokinetic functions, distinct interphase functions for this complex are now being defined. For example, centralspindlin recruits Ect2, and thereby activates RhoA, to regulate epithelial junctional integrity [19]. The ability of MKLP1 to bundle microtubules is also required in post-mitotic germline cell development [28] and neuronal axon extension [29].
Structure and function of MKLP1
Kinesin motor domain
One of the major functions of MKLP1 is to bundle microtubules in the spindle midzone during anaphase via its motor domain [17]. Although MKLP1 can associate with microtubules as a dimer, these interactions are transient; processive motility requires MKLP1 oligomerization [30]. However, MKLP1 oligomers are insufficient to bundle midzone microtubules and concentrate centralspindlin at this site. Cyk4/MKLP1 complex formation is required for bundling in vivo [25] and establishes a strong preference for antiparallel microtubule bundling in vitro [31]. Cyk4 binding conformationally restricts the two motor domains of a MKLP1 dimer [31, 32].
MKLP1 oligomerization motif and its regulation
A small, functionally conserved, 16-residue domain in MKLP1 makes an important contribution to centralspindlin function during cytokinesis by inducing oligomerization [24, 30, 33]. Oligomerization potentiates the weak processivity of MKLP1 on microtubules and consequently the signature accumulation of centralspindlin on plus ends of antiparallel microtubules [30]. In human cells, the ability of centralspindlin to oligomerize is inhibited by 14–3-3 proteins that directly bind phosphorylated S710 on MKLP1 prior to anaphase [33]. 14–3-3 proteins are highly helical, dimeric, multi-functional proteins that bind to specific interactors via phosphoserine motifs [34, 35]. The 14–3-3 binding site on MKLP1 is conserved; it is generated through phosphorylation by Ndr kinases [36]. However, during anaphase, the Aurora B kinase subunit of the Chromosome Passenger Complex (CPC), phosphorylates MKLP1 at S708 [37–39], which abolishes 14–3-3 binding [33]. Thus, the CPC promotes central spindle assembly by inducing oligomerization of centralspindlin.
An additional, vital role for centralspindlin oligomers emerged from analysis of PAR-5, a 14–3-3 protein in C. elegans [24]. Most species have several 14–3-3 isoforms, some of which are functionally redundant, making it challenging to characterize their cellular roles [35, 40]. PAR-5 is the only 14–3-3 isoform expressed in the early C. elegans embryo [41, 42]. Depleting this protein or, importantly, mutating its conserved binding site (S682) on ZEN-4/MKLP1 results in embryos with a global increase in active RhoA, and ectopic cleavage furrows associated with a striking localization of centralspindlin on the membrane. These results reveal a role for centralspindlin oligomers in RhoA activation at the plasma membrane. This function of centralspindlin is promoted by the CPC by antagonizing PAR-5 activity. As the CPC is dispensable for furrow formation in PAR-5-deficient embryos [24], centralspindlin oligomerization appears to be the primary function of the CPC in furrow formation (Figure 3). Thus, the property of centralspindlin to oligomerize promotes at least two functions of the complex, one based on microtubule binding and the other on membrane binding. Oligomerization likely increases the avidity of a weak microtubule binding motor domain in MKLP1 and a combination of weak membrane tethers in Cyk4.
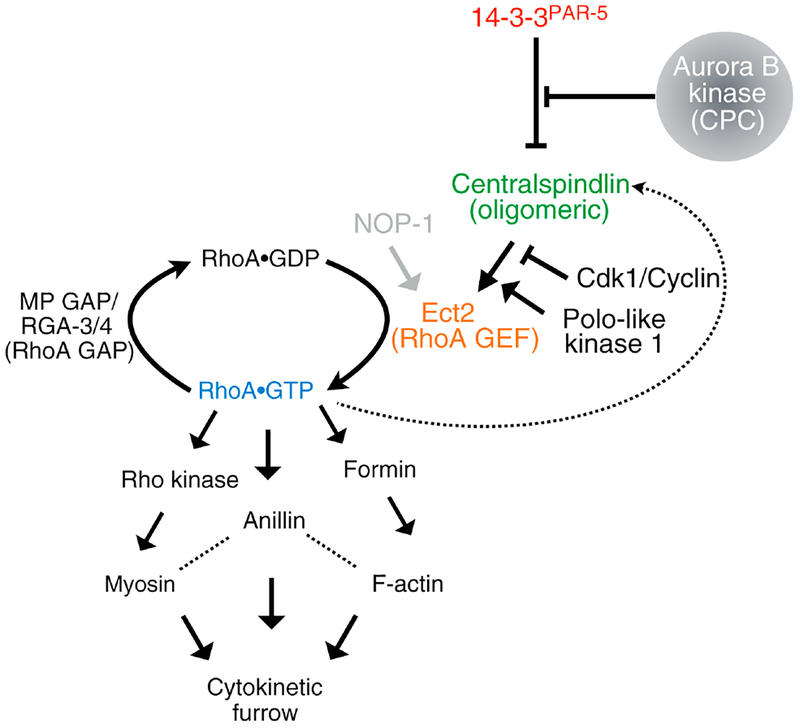
Figure 3. Molecular pathway leading to RhoA activation during cytokinesis.
GTP-bound RhoA activates formin-mediated actin assembly, myosin motors and binds a scaffold protein anillin, to generate a functional contractile ring. The RhoA GEF Ect2 is spatiotemporally regulated by centralspindlin. Polo-like kinase 1 phosphorylates Cyk4 permitting its interaction with Ect2, once Cdk1 activity falls in anaphase. 14–3-3 proteins bind MKLP1 preventing centralspindlin oligomerization. Aurora B kinase phosphorylation of MKLP1 prevents 14–3-3 binding, thus activating a functional, oligomeric form of centralspindlin that can localize at the central spindle and the plasma membrane. PAR-5 is the only active 14–3-3 protein in the early C. elegans embryo. NOP-1 is a C. elegans-specific Ect2 activator.
Structure and function of Cyk4
N-terminal MKLP1 and Ect2 docking sites
The N-terminus of Cyk4 is intimately involved in assembly of the centralspindlin complex [25]. C-terminal to this region are important residues that mediate interaction with the RhoGEF Ect2 [43, 44]. Although their domain structures suggest that the RhoGEF Ect2 and the RhoGAP Cyk4 function antagonistically, there is strong evidence that they cooperate to promote RhoA activation. In most cell types, depletion of either Ect2 or Cyk4 results in a failure to form an ingressing cleavage furrow. Ect2 and Cyk4 are required for the accumulation of RhoA and its effectors at the equatorial plasma membrane [44–47]. Depletion of MKLP1 has a weaker affect on contractile ring assembly [44], perhaps due to variable extents of depletion.
Ect2 exists in an inactive conformation owing to an intramolecular interaction between N terminal BRCT domains and C terminal DH-PH domains [13]. How is this GEF activated to promote RhoA function? The N-terminal BRCT domains of Ect2 are phosphopeptide binding modules [48] that also bind phospho-Cyk4 [43, 44] relieving autoinhibition.
Chemical inhibitors and analog sensitive mutants of Plk1 reveal that it plays multiple roles in cytokinesis. Plk1 kinase activity is required for its spindle midzone localization, yet it antagonizes midzone recruitment of PRC1 and centralspindlin during metaphase [49]. Plk1 inhibition leads to loss of Ect2 from the midzone; RhoA and downstream effectors fail to accumulate [50, 51] and Ect2 does not detectably bind Cyk4 [50, 52]. In vitro, Cyk4 phosphorylation promotes, though is not strictly required for, binding with Ect2 [44, 52]. The N-terminus of Cyk4 has seven Plk1 phosphorylation sites, four of which are evolutionarily conserved (S149, S153, S164 and S170). Mutating these sites to alanine attenuates assembly of a stable complex between centralspindlin and Ect2, failure to activate RhoA, and loss of Ect2 from the midzone [52, 53]. In sum, these results point to a model where Cyk4 binding contributes to Ect2 activation.
Although the structure of the Cyk4/Ect2 complex is not known, homology modeling of Ect2 BRCT domains allowed prediction of residues required for this interaction. Mutational analysis revealed that residues T153 and K195 are crucial for Cyk4 binding [52]. Recently, T153A and K195M (TK) mutations were generated in full-length Ect2 and expressed in cells. While one might expect that the TK mutation of Ect2 BRCT domains would phenocopy the non-phosphorylatable mutant of Cyk4, surprisingly, the TK mutant rescues cytokinesis defects in the absence of endogenous Ect2. This mutant is strongly impaired for its interaction with Cyk4 and accumulates extremely weakly at the spindle midzone and equatorial membrane. These results were interpreted to indicate that the Ect2/Cyk4 interaction is dispensable during wild-type cytokinesis [54]. However, an alternative interpretation bears consideration. As the BRCT domains are known to be involved in autoinhibition [13], T153 and K195 may participate in binding to the C-terminus of Ect2 to mediate Ect2 autoinhibition, perhaps via a C-terminal phosphorylation site or an acidic amino acid. If so, the TK mutant might be partially activated due to relief of autoinhibition. Importantly, Cyk4 is still required for cytokinesis in these mutants [54]. This suggests that a low level of interaction of the TK mutant with Cyk4, undetected via co-immunoprecipitation, might suffice for RhoA activation. Indeed, the same study shows that although cytokinesis requires membrane recruitment of Ect2, the required levels of Ect2 at the membrane can be very low, below the limit of detection.
Plk1 phosphorylation of Cyk4 is antagonized by PP2A activity. PP2A binds Cyk4 through these phosphorylations and an additional interaction with a nearby LxxIxE motif. Mutation of this motif results in hyperphosphorylation of Cyk4 and cytokinesis failure. However, cells are able to assemble intact central spindles and cleavage furrows that ingress extensively before regressing. Dephosphorylation of Cyk4 by PP2A is thus likely important for the late stages of cytokinesis and not for early RhoA activation [55–57].
Membrane binding via the C1 domain
Though conventionally and historically considered a microtubule bundling complex, centralspindlin has been reported to localize to the cleavage furrow in some cells [21, 58, 59]. However the mechanism by which it localizes, and the role of this pool of centralspindlin in cytokinesis has only been recently revealed. The Cyk4 component of centralspindlin contains a weak membrane-binding C1 domain. In human cells, this domain plays a role late in cytokinesis, linking the plasma membrane to the spindle microtubules as the midbody forms [60]. Because deletion of the HsCyk4 C1 domain does not impact cleavage furrow formation in these cells [60], it suggests that the membrane-binding property of the C1 domain is not required earlier for RhoA activation. However, the C1 domain is essential for centralspindlin-directed RhoA activation in C. elegans [24, 61]. This difference is likely due to redundant, microtubule-based mechanisms for generating membrane-associated centralspindlin [62], as disrupting spindle organization in human cells uncovers a role for the C1 domain in RhoA activation [24]. Ectopic localization of the Drosophila homolog of Cyk4 on the plasma membrane in S2 cells leads to hypercontractility [63], supporting the model that membrane-bound centralspindlin promotes RhoA activation.
RhoGAP domain
Although the GAP domain of Cyk4 is capable of serving as a canonical Rho family GAP in vitro, with a significant preference for Rac and Cdc42 over RhoA [27, 64, 65], genetic analyses indicate that the domain is primarily involved in RhoA activation during cytokinesis. Activation of RhoA is a curious function for a RhoGAP protein like Cyk4, which would ordinarily be predicted to turn a GTPase “off”. Another RhoGAP, MP-GAP (ARHGAP11A), has a conserved role in inactivating RhoA [66–68] (Figure 3).
The Cyk4 RhoGAP domain has been analyzed most extensively in the early C. elegans embryo. Two main models have been proposed for its function. One posits that Cyk4 GAP domain is primarily required to activate RhoA during cytokinesis. Another model proposes that its GAP activity is required during cytokinesis to turn off the GTPase Rac1. We recently elaborated on the merits of these models in a separate review [69]. A major concern with the latter model is that the experiments to support it have been performed in the presence of a parallel RhoA activation pathway peculiar to C. elegans (see NOP-1 section). In the absence of this second, non-essential, pathway, Cyk4 GAP mutants do not support furrow induction, thus favoring the model where this domain participates in RhoA activation. It is notable that, in this context, the active site of the GAP domain of Cyk4 is required for furrowing even when Rac1 is mutated [61].
The exact role performed by the Cyk4 GAP domain is likely to be context dependent. In Xenopus embryos, inactivating GAP function by abolishing the catalytic arginine finger increases the intensity and broadens the zone of RhoA activation during cytokinesis [70]. This is consistent with a conventional view of GAP function and favors a model where continuous GTPase flux is required for proper cytokinesis. However, deletion of the Cyk4 GAP domain in these embryos causes dramatic instability of the contractile ring [70], suggesting that the GAP domain performs an anchoring function in cytokinesis. Consistent with a role for the GAP domain in membrane recruitment, it is required for its recruitment to the bridges separating individual nuclei in the syncytial germline in C. elegans [71]. In other cell types, the Cyk4 catalytic arginine is either dispensable for furrow induction [72], required to limit cell-substrate adhesion via Rac effectors [65], or contrary to expectations for a conventional GAP, strictly required for furrow formation [73].
The very C-terminus of Cyk4 mediates a direct interaction with the microtubule bundling protein PRC1. While not strictly required for centralspindlin localization to the midzone, this interaction promotes stable accumulation of the complex and central spindle assembly, in the presence of spindle pulling forces [74].
Summary: Activation of Ect2 by Centralspindlin
Centralspindlin-mediated RhoA activation via Ect2 is complex and multifactorial. Genetic analysis indicates it requires physical interactions between all four proteins (Cyk4, MKLP1, Ect2, and RhoA) at the plasma membrane. Formation of this complex involves Plk1-mediated phosphorylation of Cyk4 and subsequent binding of this site by the auto-inhibitory N-terminus of Ect2 [52, 53]. Ect2 membrane recruitment is mediated by its PH domain and basic regions in the C-terminus [20]. Membrane recruitment of Cyk4 involves both its C1 and GAP domains and its ability to bind to MKLP1 [61]. The involvement of the GAP domain suggests that active RhoA could contribute to centralspindlin recruitment to the membrane and thus Ect2 activation, perhaps enabling positive feedback [61]. The Cyk4 GAP domain also appears to allosterically activate Ect2; this activation involves RhoA binding, potentially inducing a second means of positive feedback. These reactions also depend on centralspindlin oligomerization which requires antagonism of 14–3-3 by the CPC [24]. The oligomeric state of the complex may permit a division of labor - some GAP domains in the complex may directly engage Ect2, whereas others may contribute to membrane localization.
While individual interactions between domains have been established in vitro, activation of nucleotide exchange activity of Ect2 by centralspindlin has not yet been demonstrated. Because low levels of membrane-bound Ect2 are sufficient for furrow formation in vivo, and due to the relatively modest activity of Ect2 in vitro, it is likely that the stimulation by centralspindlin will be significant. A major challenge for the future is biochemical reconstitution of Ect2 activation. It is important to emphasize that not every interaction mentioned above is necessarily required during cytokinesis of every cell.
Centralspindlin-independent RhoA activation
Although in most cells, Ect2 activation during cytokinesis requires centralspindlin, in C. elegans embryos, centralspindlin is partially redundant with a protein called NOP-1. NOP-1 is a nematode-specific protein that functions as a global activator of Ect2 during polarization and early embryonic development [75]. C. elegans embryos in which centralspindlin function is disrupted can form cytokinetic furrows that ultimately regress. However simultaneous loss of NOP-1 and centralspindlin abolishes furrow induction and active RhoA does not accumulate on the membrane (Figure 3) [75]. The mechanism by which NOP-1 activates Ect2 is currently unknown; the protein contains low complexity regions but lacks other recognizable domains.
However, elimination of this pathway provides a straightforward means to study the role of centralspindlin in furrow formation in this system. Thus far, compelling evidence for parallel activators of Ect2 during cytokinesis in other organisms is lacking.
Spatial Control of RhoA activation
Spindle midzone
In light of our current understanding of the mechanism by which centralspindlin promotes RhoA activation (Figure 3) how is this activity spatially regulated by the spindle? Overlapping plus ends of microtubules in the spindle midzone are bundled by, among other factors, the coordinated activity of centralspindlin oligomers, the microtubule associated protein (MAP) PRC1, and Kif4, a kinesin motor protein that functions in association with PRC1 [76, 77]. The bundling reaction results in stabilization of these midzone microtubules and stable accumulation of centralspindlin, and in some cell types, centralspindlin recruits Ect2 to the midzone [44].
Centralspindlin accumulation at the spindle midzone positions it in the presumptive plane of cell division. However, prominent accumulation at this site is not essential for initiation of a furrow, as cells can furrow in a centralspindlin-dependent manner without significant centralspindlin concentrating on the spindle midzone, as in PRC1-depleted cells [23, 78, 79]. Furthermore, centralspindlin accumulation at the midzone is not always sufficient for furrowing as observed in C. elegans embryos expressing a Cyk4 ΔC1 variant [24] (Figure 4). While the spindle-bound pool of centralspindlin plays a crucial role in midbody stabilization during the terminal stages of cytokinesis [60], the membrane-bound pool of centralspindlin is likely the most relevant for RhoA activation and furrow initiation, as the plasma membrane is the site of RhoA activation. Microtubule bundles with associated centralspindlin may facilitate its association with Ect2 at the plasma membrane if they are positioned sufficiently close to the cell cortex, as is seen in human cells expressing the Cyk4 ΔC1 variant [60] and in Drosophila spermatocytes [62]. The stark difference in phenotype in human cells and C. elegans embryos caused by the same Cyk4 ΔC1 allele may be a function of the distance between the spindle and the plasma membrane (Figure 4) [24]. Membrane and microtubule-associated pools of centralspindlin may compete with each other, however this has not been experimentally shown. Once cytokinesis is underway, this is unlikely to be a particularly dynamic competition as microtubule-associated centralspindlin exchanges slowly [30].
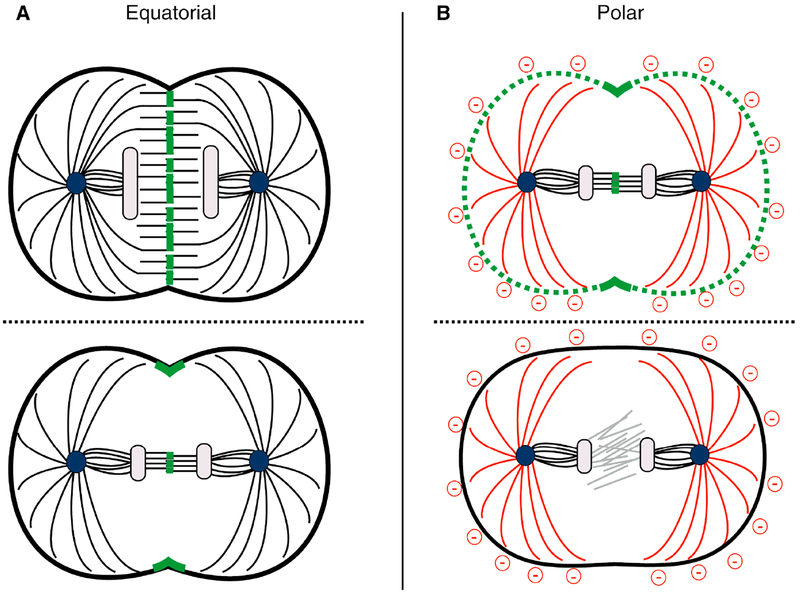
Figure 4. Spatial control of RhoA activity by the mitotic spindle.
Furrow induction involves centralspindlin-dependent RhoA activation at the equatorial plasma membrane and astral inhibition at the poles (red). Centralspindlin (green) also strongly accumulates at midzone microtubules, but this location can be too distant from the membrane to directly induce RhoA activation. A. In small cells, in which the spindle extends towards the membrane (top), centralspindlin can concentrate near the plasma membrane; in such cells the Cyk4 C1 domain is not required to generate a furrow. In cell types where the central spindle is far away from the plasma membrane (bottom), the membrane-binding C1 domain of Cyk4 is critical in localizing centralspindlin and thereby activating RhoA at the equator to generate a furrow. Equatorial accumulation of membrane-bound centralspindlin is likely induced by a combination of spindle midzone accumulation, local CPC activity, and directed microtubule-based transport.
B. Astral microtubules negatively regulate RhoA activity at the poles. In cells where the centralspindlin complex is globally activated (in the absence of 14–3-3 proteins), asters can still restrict furrow formation to the equatorial region (top). In the absence of RhoA activators (such as a cyk-4;nop-1 double mutant C. elegans embryo), cytokinetic furrows do not form (bottom).
Chromatin modulates CPC accumulation
Centralspindlin binds to the ends of distinct sets of microtubules, depending on the organization of the spindle. In monopolar cells, centralspindlin accumulates robustly at a clustered subset of microtubule plus ends in the cell periphery [80]. However, these sites do not become populated when a bipolar spindle is present, presumably due to the preferential recruitment of centralspindlin to antiparallel bundles at the spindle midzone (Figure 5). Indeed, in assays with artificial asters, centralspindlin accumulates between adjacent asters [81].
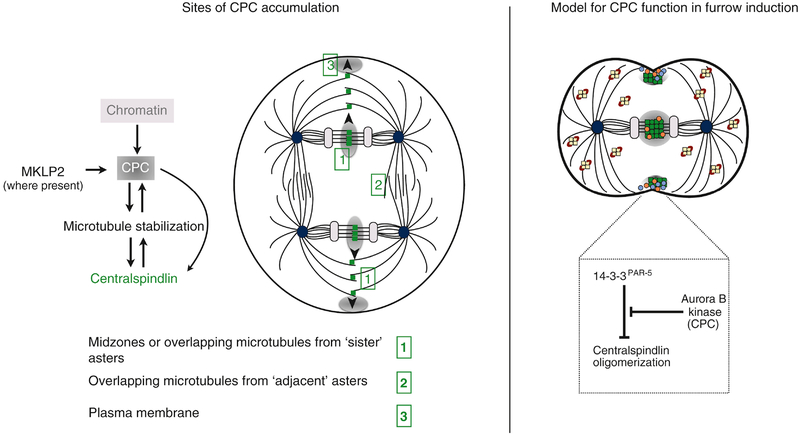
Figure 5. Spatial control of RhoA activity by the CPC.
A. Schematic of a binucleate cell undergoing mitosis. During anaphase, the CPC localizes at (1) the spindle midzone near separating chromosomes and subsequently propagates to nearby overlapping microtubules. CPC can also accumulate on (2) microtubules from overlapping adjacent asters but less efficiently than at sister asters. It also can accumulate at (3) the plasma membrane. Known requirements for its association at each site are listed. The complex may not occupy all these sites in every cell type and the regulators of its localization are not fully conserved, and therefore require further investigation.
B. The CPC (gray) is known to accumulate at the equatorial region of dividing veterbrate cells, specifically at the central spindle and the plasma membrane during anaphase. This could result in zones of centralspindlin activity where centralspindlin (green) locally forms clusters and accumulates with high avidity, allowing Ect2 (orange) and RhoA (blue) activation at the equatorial membrane and subsequent recruitment of actomyosin to generate the contractile ring. Centralspindlin is inactive (pale yellow) in other regions of the cell because of 14–3-3/PAR-5 (red) inhibition.
Although centralspindlin binds to overlapping antiparallel microtubule ends, some overlapping plus ends recruit centralspindlin more efficiently than others. When cells contain multiple spindles, centralspindlin accumulation is favored at the overlapping plus ends between asters which had segregated chromosomes (“sister asters”) as compared to neighboring asters that did not participate in chromosome segregation [81]. The preference for sister asters is probably imposed by their strong accumulation of the CPC, apparently as a consequence of the dissociation of CPC from chromosomes and its subsequent binding on the nearest antiparallel microtubules (Figure 5) [82]. CPC accumulation creates gradients in Aurora B kinase activity [83] that appear to propagate CPC accumulation from an initial site at the center of an overlap zone to adjacent regions [81]. This bias in CPC accumulation is then amplified through a positive feedback loop in which Aurora B kinase activity promotes microtubule stabilization [84], and stabilized microtubules preferentially recruit CPC. This model is supported by experiments in which hyperactivation of the CPC or artificial stabilization of microtubules reduces the disparity in accumulation of CPC on sister asters relative to adjacent asters [81]. The CPC, in turn, regulates centralspindlin recruitment to microtubules, at least in part, by regulating centralspindlin oligomerization.
Chromosome Passenger Complex and MKLP2
The CPC also regulates centralspindlin recruitment to the membrane (Basant and Glotzer, unpublished). Suggestively, in human cells, CPC localizes to the equatorial cell cortex prior to furrow invagination [85]. The mechanism of CPC accumulation on the equatorial membrane is not well understood. INCENP, the scaffolding subunit of the CPC, contains a long, flexible, charged, single alpha helical (SAH) domain that has microtubule binding activity required for its localization to the spindle midzone, but it is dispensable for its accumulation at the cell cortex [86, 87]. Curiously, this helical region also associates with f-actin [84].
Factors that regulate CPC recruitment may regulate local RhoA activation (Figure 5). In mammalian cells, one such regulator is the kinesin-6 motor protein MKLP2. CPC and MKLP2 interact and prominently co-accumulate on the spindle midzone, as well as at the equatorial cortex [88]. MKLP2 is structurally related to MKLP1, however, MKLP2 does not interact with Cyk4. Nevertheless, these kinesins are regulated by similar mechanisms. Microtubule binding of both motors is inhibited by direct Cdk1 phosphorylation and activated by dephosphorylation upon mitotic exit; they also both oligomerize [30, 89, 90]. During anaphase, MKLP2 promotes dissociation of the CPC from chromosomes. This suggests a hierarchy of localization dependence: MKLP2 > CPC > centralspindlin.
Given this hierarchy, what are the determinants of MKLP2 localization? MKLP2 contains a myosin binding domain and its localization depends on myosin accumulation. Myosin has been proposed to promote MKLP2 localization [91]. However, given that myosin II accumulation is RhoA dependent and RhoA activation requires centralspindlin, and CPC and MKLP2 promote membrane localization of centralspindlin, this model appears circular, in that it suggests that a downstream effector of MKLP2 promotes its recruitment. Perhaps low levels of active RhoA can be generated at the equator prior to MKLP2/CPC accumulation.
Indeed, although MKLP2 localization is important, in cultured human cells with bipolar spindles, furrow formation is not strictly dependent on either MKLP2 or CPC, though cytokinesis fails to complete in their absence. The simplest interpretation is that a weak, albeit functional, MKLP2/CPC-independent mechanism promotes and regulates cortical accumulation of centralspindlin that creates an initial concentration of equatorial myosin that is then amplified by MKLP2 and CPC-induced recruitment of centralspindlin.
An additional mystery surrounds the role of MKLP2. While the function of Aurora B and centralspindlin are largely similar in C. elegans and vertebrate cells, C. elegans lacks an MKLP2 ortholog, raising the question of how CPC dissociates from chromosomes during anaphase in such cells. One possibility is that MKLP1 fulfills the function of both MKLP1 and MKLP2 in these organisms; however, Aurora B does not remain chromosome bound in C. elegans embryos depleted of MKLP1 [92]. Alternatively, another factor may contribute to CPC localization. A third possibility is that CPC may not have as high an affinity for chromatin in these organisms as it does in vertebrate cells, in which case specific factors would not be required for its dissociation during anaphase. Curiously, although the MKLP2 ortholog in Drosophila regulates CPC localization, it is not essential for cytokinesis in most cells [93].
Astral microtubules
Experiments in a variety of systems indicate the existence of a second pathway for spatial control of RhoA activation that works in parallel to the local accumulation of centralspindlin at the cell equator (Figure 4). This mechanism might be responsible for the initial, CPC-independent, equatorial activation of RhoA discussed above. Although the underlying molecular mechanism is not known, this pathway appears to involve inhibition of cortical contractility by dynamic astral microtubules, a model proposed many decades ago [94, 95].
Many insights into astral inhibition have come from studies of the early C. elegans embryo. When microtubules are attenuated, spindle assembly occurs in the embryo posterior, aligned with the short axis of the elliptical cell. Under these conditions, one furrow will form in the embryo anterior, distal to the astral microtubules and second furrow will form between the two asters [96]. Likewise, furrow formation still occurs when the spindle midzone in C. elegans embryos is disrupted genetically or by laser ablation and the position of the resulting furrow can be predicted from the position of the spindle asters in anaphase [97]. When the midzone is disrupted by inactivation of centralspindlin, these furrows are dependent on the presence of the aforementioned RhoA activator NOP-1, which given the lack of conservation of NOP-1, raises the possibility that these observations might not represent features of astral inhibition in all cell types. However, the well-conserved centralspindlin-directed furrowing pathway, when hyper-activated in C. elegans, is also restricted by astral inhibition. Depletion of 14–3-3/PAR-5 or mutation of MKLP1 such that it is not subject to regulation by 14–3-3/PAR-5 and the CPC, results in embryos that still furrow at the equator, albeit with less precision than in wild-type. This is surprising, as RhoA is initially globally activated under these conditions. When such embryos are treated with nocodazole to misposition the spindle to the posterior, ectopic oligomeric centralspindlin induces furrows in the anterior of the embryo, away from the asters, both in the presence or absence of NOP-1 [24]. These results suggest that astral microtubules can regulate contractility that results from either NOP-1- or centralspindlin-directed RhoA activation in the embryo. The simplest explanation of these results is that dynamic microtubules do not regulate NOP-1 or centralspindlin, but rather a common downstream factor.
Astral microtubules also regulate contractility in other contexts. For example, dynamic microtubules locally inhibit RhoA activation in both cultured mammalian cells and excitable cortices observed in activated echinoderm and Xenopus blastomeres [98, 99]. Microtubule disruption results in hypercontractility in C. elegans embryos [100].
The molecular basis for astral inhibition is not fully understood. Optogenetic experiments in cultured human cells indicate that dynamic astral microtubules do not impact the contractility resulting from artificial accumulation of a minimal RhoGEF domain [8]. If the astral pathway is active in these cells, then it must act upstream of the activators of RhoA, as it does not inhibit ectopically activated RhoA or the downstream effectors. In C. elegans embryos, Aurora A and its activator TPXL-1 are required for microtubules to inhibit accumulation of f-actin and the RhoA effector anillin from the anterior cortex [101]. Interestingly, Aurora A is largely dispensable for clearance of these downstream components from the posterior cortex, suggesting the existence of a separate regulatory mechanism.
A subset of astral microtubules may also play a positive role in delivering activators of contractility. For example, equatorially-directed astral microtubules can contribute to equatorial CPC localization [102] and centralspindlin localization (Figure 4, 5) [45]. In Xenopus, an SxIP motif in centralspindlin enables it to bind microtubule plus ends via EB1, but this motif is not well-conserved [103]. In echinoderm zygotes, but not in subsequent divisions, equatorially-directed microtubules appear to be preferentially stabilized [104, 105]. It is not clear whether this is a common role that microtubules play, nor is it known what distinguishes this subset of microtubules from the larger, radial array of microtubules that surrounds the spindle poles.
Concluding remarks
Activation of RhoA with precise spatiotemporal control during cytokinesis involves intricate, often redundant, mechanisms to regulate the centralspindlin-Ect2 complex. The importance of this complex in furrow induction and several aspects of regulation were well-established. Formerly, much attention focused on the role of this complex at the spindle midzone, particularly since the centralspindlin/Ect2 complex accumulates prominently at this site. However, protein abundance does not necessarily correlate with function. Additionally, given that RhoGEFs are potently activated by tethering to the plasma membrane [8, 106] and the demonstration that centralspindlin-mediated RhoA activation during cytokinesis in C. elegans embryos requires membrane targeting domains in centralspindlin and correlates with the degree of membrane recruitment [24, 61], we propose that the plasma membrane is the primary site where centralspindlin/Ect2 complex induces RhoA activation during cytokinesis. Recent observations support the conjecture that biologically relevant levels of this complex do not require high levels of protein accumulation [54].
In no way does this proposal imply that association of centralspindlin with the plasma membrane is independent of microtubules. In fact, we suggest that microtubules both promote and inhibit the membrane association of centralspindlin and hence its ability to complex with Ect2 (Figure 5). First, by promoting equatorial accumulation of CPC, microtubules indirectly induce direct binding of centralspindlin to the plasma membrane. Second, through the ability of antiparallel microtubule bundles to recruit centralspindlin directly and indirectly via CPC, such bundles at the periphery of the spindle midzone may bring centralspindlin/Ect2 in the immediate vicinity of the plasma membrane. Third, plus-end directed trafficking by centralspindlin could promote its delivery to the plasma membrane [30, 45]. In addition to these mechanisms that promote membrane associated centralspindlin/Ect2, the spindle midzone may functionally sequester centralspindlin/Ect2. Finally, through mechanisms that are yet to be fully defined, astral microtubules are proposed to inhibit the accumulation of centralspindlin and/or Ect2 at polar regions of the cell. We must also consider that there are examples of polarity-derived cues directing the site of cleavage furrow formation, independent of the spindle [22]. Presumably such cells have the capacity to locally induce RhoA activation, much like the pseudocleavage furrow of the early C. elegans embryo. As emphasized throughout this review, different cells are known to utilize different combinations of these mechanisms to promote equatorial activation of RhoA. Indeed, it is likely that the aforementioned redundancy has impeded our ability to understand the mechanism of cytokinetic furrowing.
The coming years promise to reveal important features of the above mechanisms. We need to better understand how asters clear RhoA activators from the poles, how CPC localization on the membrane is controlled, and the molecular details by which centralspindlin enhances Ect2 activity towards RhoA and whether active RhoA has a direct role in this process thereby generating positive feedback. Finally, while these core mechanisms are largely conserved among metazoans, a number of additional context-dependent factors such as polarity proteins, inter-cellular tension [107, 108], cell adhesion [8, 109], cell size, and relative abundance of cytokinetic proteins can impact the position of the division plane; these mechanisms also remain to be fully understood.
Acknowledgements
MG gratefully acknowledges support from NIGMS R01GM085087
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Angika Basant, The Francis Crick Institute, London, UK.
Michael Glotzer, The University of Chicago, Chicago, IL USA.
References:
- 1.Austin JR, Seguí-Simarro JM, and Staehelin LA (2005). Quantitative analysis of changes in spatial distribution and plus-end geometry of microtubules involved in plant-cell cytokinesis. J Cell Sci 118, 3895–3903. [DOI] [PubMed] [Google Scholar]
- 2.Tsang M-J, and Bernhardt TG (2015). Guiding divisome assembly and controlling its activity. Curr Opin Microbiol 24, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisson-Filho AW, Hsu Y-P, Squyres GR, Kuru E, Wu F, Jukes C, Sun Y, Dekker C, Holden S, VanNieuwenhze MS, et al. (2017). Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science 355, 739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rappaport R (1986). Establishment of the Mechanism of Cytokinesis in Animal Cells. 105, 245–281. [DOI] [PubMed] [Google Scholar]
- 5.Kishi K, Sasaki T, Kuroda S, Itoh T, and Takai Y (1993). Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI). J Cell Biol 120, 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yonemura S, Hirao-Minakuchi K, and Nishimura Y (2004). Rho localization in cells and tissues. 295, 300–314. [DOI] [PubMed] [Google Scholar]
- 7.Bement WM, Benink HA, and Dassow, von G (2005). A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol 170, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner E, and Glotzer M (2016). Local RhoA activation induces cytokinetic furrows independent of spindle position and cell cycle stage. J Cell Biol 213, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otomo T, Otomo C, Tomchick DR, Machius M, and Rosen MK (2005). Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell 18, 273–281. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura F (2005). Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol 15, 371–377. [DOI] [PubMed] [Google Scholar]
- 11.Allal C, Favre G, Couderc B, Salicio S, Sixou S, Hamilton AD, Sebti SM, Lajoie-Mazenc I, and Pradines A (2000). RhoA prenylation is required for promotion of cell growth and transformation and cytoskeleton organization but not for induction of serum response element transcription. J Biol Chem 275, 31001–31008. [DOI] [PubMed] [Google Scholar]
- 12.Tatsumoto T, Xie X, Blumenthal R, Okamoto I, and Miki T (1999). Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol 147, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J-E, Billadeau DD, and Chen J (2005). The tandem BRCT domains of Ect2 are required for both negative and positive regulation of Ect2 in cytokinesis. J Biol Chem 280, 5733–5739. [DOI] [PubMed] [Google Scholar]
- 14.Mierzwa B, and Gerlich DW (2014). Cytokinetic abscission: molecular mechanisms and temporal control. Dev Cell 31, 525–538. [DOI] [PubMed] [Google Scholar]
- 15.Green RA, Paluch E, and Oegema K (2012). Cytokinesis in animal cells. Annu Rev Cell Dev Biol 28, 29–58. [DOI] [PubMed] [Google Scholar]
- 16.Dassow, von G (2009). Concurrent cues for cytokinetic furrow induction in animal cells. Trends Cell Biol 19, 165–173. [DOI] [PubMed] [Google Scholar]
- 17.Matuliene J, Matuliene J, and Kuriyama R (2004). Role of the midbody matrix in cytokinesis: RNAi and genetic rescue analysis of the mammalian motor protein CHO1. Mol Biol Cell 15, 3083–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatsumoto T, Xie X, Blumenthal R, Okamoto I, and Miki T (1999). Human Ect2 Is an Exchange Factor for Rho Gtpases, Phosphorylated in G2/M Phases, and Involved in Cytokinesis. J Cell Biol 147, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, and Yap AS (2012). Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su K-C, Takaki T, and Petronczki M (2011). Targeting of the RhoGEF Ect2 to the equatorial membrane controls cleavage furrow formation during cytokinesis. Dev Cell 21, 1104–1115. [DOI] [PubMed] [Google Scholar]
- 21.Adams RR, Tavares AA, Salzberg A, Bellen HJ, and Glover DM (1998). pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev 12, 1483–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabernard C, Prehoda KE, and Doe CQ (2010). A spindle-independent cleavage furrow positioning pathway. Nature 467, 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbrugghe KJC, and White JG (2004). SPD-1 is required for the formation of the spindle midzone but is not essential for the completion of cytokinesis in C. elegans embryos. Curr Biol 14, 1755–1760. [DOI] [PubMed] [Google Scholar]
- 24.Basant A, Lekomtsev S, Tse YC, Zhang D, Longhini KM, Petronczki M, and Glotzer M (2015). Aurora B kinase promotes cytokinesis by inducing centralspindlin oligomers that associate with the plasma membrane. Dev Cell 33, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishima M, Kaitna S, and Glotzer M (2002). Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell 2, 41–54. [DOI] [PubMed] [Google Scholar]
- 26.Pavicic-Kaltenbrunner V, Mishima M, and Glotzer M (2007). Cooperative assembly of CYK-4/MgcRacGAP and ZEN-4/MKLP1 to form the centralspindlin complex. Mol Biol Cell 18, 4992–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jantsch-Plunger V, Gönczy P, Romano A, Schnabel H, Hamill D, Schnabel R, Hyman AA, and Glotzer M (2000). CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol 149, 1391–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou K, Rolls MM, and Hanna-Rose W (2013). A postmitotic function and distinct localization mechanism for centralspindlin at a stable intercellular bridge. Dev Biol 376, 13–22. [DOI] [PubMed] [Google Scholar]
- 29.del Castillo U, Lu W, Winding M, Lakonishok M, and Gelfand VI (2015). Pavarotti/MKLP1 regulates microtubule sliding and neurite outgrowth in Drosophila neurons. Curr Biol 25, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutterer A, Glotzer M, and Mishima M (2009). Clustering of centralspindlin is essential for its accumulation to the central spindle and the midbody. Curr Biol 19, 2043–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies T, Kodera N, Kaminski Schierle GS, Rees E, Erdelyi M, Kaminski CF, Ando T, and Mishima M (2015). CYK4 Promotes Antiparallel Microtubule Bundling by Optimizing MKLP1 Neck Conformation. PLoS Biol 13, e1002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White EA, Raghuraman H, Perozo E, and Glotzer M (2013). Binding of the CYK-4 subunit of the centralspindlin complex induces a large scale conformational change in the kinesin subunit. 288, 19785–19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douglas ME, Davies T, Joseph N, and Mishima M (2010). Aurora B and 14–3-3 coordinately regulate clustering of centralspindlin during cytokinesis. Curr Biol 20, 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, and Cantley LC (1997). The structural basis for 14–3-3:phosphopeptide binding specificity. Cell 91, 961–971. [DOI] [PubMed] [Google Scholar]
- 35.Aitken A (2006). 14–3-3 proteins: a historic overview. Semin. Cancer Biol 16, 162–172. [DOI] [PubMed] [Google Scholar]
- 36.Fesquet D, de Bettignies G, Bellis M, Espeut J, and Devault A (2015). Binding of Kif23-iso1/CHO1 to 14–3-3 is regulated by sequential phosphorylations at two LATS kinase consensus sites. PLoS ONE 10, e0117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guse A, Mishima M, and Glotzer M (2005). Phosphorylation of ZEN-4/MKLP1 by aurora B regulates completion of cytokinesis. Curr Biol 15, 778–786. [DOI] [PubMed] [Google Scholar]
- 38.Kaitna S, Mendoza M, Jantsch-Plunger V, and Glotzer M (2000). Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol 10, 1172–1181. [DOI] [PubMed] [Google Scholar]
- 39.Severson AF, Hamill DR, Carter JC, Schumacher J, and Bowerman B (2000). The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr Biol 10, 1162–1171. [DOI] [PubMed] [Google Scholar]
- 40.Fu H, Subramanian RR, and Masters SC (2000). 14–3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol 40, 617–647. [DOI] [PubMed] [Google Scholar]
- 41.Morton DG, Shakes DC, Nugent S, Dichoso D, Wang W, Golden A, and Kemphues KJ (2002). The Caenorhabditis elegans par-5 gene encodes a 14–3-3 protein required for cellular asymmetry in the early embryo. Dev Biol 241, 47–58. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, and Shakes DC (1997). Expression patterns and transcript processing of ftt-1 and ftt-2, two C. elegans 14–3-3 homologues. J Mol Biol 268, 619–630. [DOI] [PubMed] [Google Scholar]
- 43.Somers WG, and Saint R (2003). A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell 4, 29–39. [DOI] [PubMed] [Google Scholar]
- 44.Yüce O, Piekny A, and Glotzer M (2005). An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol 170, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimura Y, and Yonemura S (2006). Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci 119, 104–114. [DOI] [PubMed] [Google Scholar]
- 46.Zhao W-M, and Fang G (2005). MgcRacGAP controls the assembly of the contractile ring and the initiation of cytokinesis. Proc Natl Acad Sci U S A 102, 13158–13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, Lee J-S, and Miki T (2006). Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell 17, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manke IA, Lowery DM, Nguyen A, and Yaffe MB (2003). BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science 302, 636–639. [DOI] [PubMed] [Google Scholar]
- 49.Hu C-K, Ozlü N, Coughlin M, Steen JJ, and Mitchison TJ (2012). Plk1 Negatively Regulates PRC1 to Prevent Premature Midzone Formation before Cytokinesis. Mol Biol Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petronczki M, Glotzer M, Kraut N, and Peters J-M (2007). Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev Cell 12, 713–725. [DOI] [PubMed] [Google Scholar]
- 51.Burkard ME, Randall CL, Larochelle S, Zhang C, Shokat KM, Fisher RP, and Jallepalli PV (2007). Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc Natl Acad Sci U S A 104, 4383–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfe BA, Takaki T, Petronczki M, and Glotzer M (2009). Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol 7, e1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burkard ME, Maciejowski J, Rodriguez-Bravo V, Repka M, Lowery DM, Clauser KR, Zhang C, Shokat KM, Carr SA, Yaffe MB, et al. (2009). Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol 7, e1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kotýnková K, Su K-C, West SC, and Petronczki M (2016). Plasma Membrane Association but Not Midzone Recruitment of RhoGEF ECT2 Is Essential for Cytokinesis. Cell Rep 17, 2672–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bastos RN, Cundell MJ, and Barr FA (2014). KIF4A and PP2A-B56 form a spatially restricted feedback loop opposing Aurora B at the anaphase central spindle. J Cell Biol 207, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hertz EPT, Kruse T, Davey NE, López-Méndez B, Sigurðsson JO, Montoya G, Olsen JV, and Nilsson J (2016). A Conserved Motif Provides Binding Specificity to the PP2A-B56 Phosphatase. Mol Cell. [DOI] [PubMed] [Google Scholar]
- 57.Wu C-G, Chen H, Guo F, Yadav VK, Mcilwain SJ, Rowse M, Choudhary A, Lin Z, Li Y, Gu T, et al. (2017). PP2A-B’ holoenzyme substrate recognition, regulation and role in cytokinesis. Cell Discov 3, 17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verbrugghe KJC, and White JG (2007). Cortical centralspindlin and G alpha have parallel roles in furrow initiation in early C. elegans embryos. J Cell Sci 120, 1772–1778. [DOI] [PubMed] [Google Scholar]
- 59.Green RA, Mayers JR, Wang S, Lewellyn L, Desai A, Audhya A, and Oegema K (2013). The midbody ring scaffolds the abscission machinery in the absence of midbody microtubules. J Cell Biol 203, 505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lekomtsev S, Su K-C, Pye VE, Blight K, Sundaramoorthy S, Takaki T, Collinson LM, Cherepanov P, Divecha N, and Petronczki M (2012). Centralspindlin links the mitotic spindle to the plasma membrane during cytokinesis. Nature 492, 276–279. [DOI] [PubMed] [Google Scholar]
- 61.Zhang D, and Glotzer M (2015). The RhoGAP activity of CYK-4/MgcRacGAP functions non-canonically by promoting RhoA activation during cytokinesis. elife 4, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inoue YH, Savoian MS, Suzuki T, Máthé E, Yamamoto M-T, and Glover DM (2004). Mutations in orbit/mast reveal that the central spindle is comprised of two microtubule populations, those that initiate cleavage and those that propagate furrow ingression. J Cell Biol 166, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Avino PP, Savoian MS, Capalbo L, and Glover DM (2006). RacGAP50C is sufficient to signal cleavage furrow formation during cytokinesis. J Cell Sci 119, 4402–4408. [DOI] [PubMed] [Google Scholar]
- 64.Touré A, Dorseuil O, Morin L, Timmons P, Jégou B, Reibel L, and Gacon G (1998). MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem 273, 6019–6023. [DOI] [PubMed] [Google Scholar]
- 65.Bastos RN, Penate X, Bates M, Hammond D, and Barr FA (2012). CYK4 inhibits Rac1-dependent PAK1 and ARHGEF7 effector pathways during cytokinesis. J Cell Biol 198, 865–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schonegg S, Constantinescu AT, Hoege C, and Hyman AA (2007). The Rho GTPase-activating proteins RGA-3 and RGA-4 are required to set the initial size of PAR domains in Caenorhabditis elegans one-cell embryos. Proc Natl Acad Sci U S A 104, 14976–14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmutz C, Stevens J, and Spang A (2007). Functions of the novel RhoGAP proteins RGA-3 and RGA-4 in the germ line and in the early embryo of C. elegans. Development 134, 3495–3505. [DOI] [PubMed] [Google Scholar]
- 68.Zanin E, Desai A, Poser I, Toyoda Y, Andree C, Moebius C, Bickle M, Conradt B, Piekny A, and Oegema K (2013). A conserved RhoGAP limits M phase contractility and coordinates with microtubule asters to confine RhoA during cytokinesis. Dev Cell 26, 496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basant A, and Glotzer M (2017). A GAP that Divides. F1000Res 6, 1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller AL, Dassow, von G, and Bement WM (2008). Control of the cytokinetic apparatus by flux of the Rho GTPases. Biochem Soc Trans 36, 378–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee K-Y, Green RA, Gutierrez E, Gomez-Cavazos JS, Kolotuev I, Wang S, Desai A, Groisman A, and Oegema K (2017). CYK-4 functions independently of its centralspindlin partner ZEN-4 to cellularize oocytes in germline syncytia. bioRxiv, 196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamada T, Hikida M, and Kurosaki T (2006). Regulation of cytokinesis by mgcRacGAP in B lymphocytes is independent of GAP activity. 312, 3517–3525. [DOI] [PubMed] [Google Scholar]
- 73.Zavortink M, Contreras N, Addy T, Bejsovec A, and Saint R (2005). Tum/RacGAP50C provides a critical link between anaphase microtubules and the assembly of the contractile ring in Drosophila melanogaster. J Cell Sci 118, 5381–5392. [DOI] [PubMed] [Google Scholar]
- 74.Lee K-Y, Esmaeili B, Zealley B, and Mishima M (2015). Direct interaction between centralspindlin and PRC1 reinforces mechanical resilience of the central spindle. Nat Commun 6, 7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tse YC, Werner M, Longhini KM, Labbé J-C, Goldstein B, and Glotzer M (2012). RhoA activation during polarization and cytokinesis of the early Caenorhabditis elegans embryo is differentially dependent on NOP-1 and CYK-4. Mol Biol Cell 23, 4020–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurasawa Y, Earnshaw WC, Mochizuki Y, Dohmae N, and Todokoro K (2004). Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation. EMBO J 23, 3237–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bieling P, Telley IA, and Surrey T (2010). A minimal midzone protein module controls formation and length of antiparallel microtubule overlaps. Cell 142, 420–432. [DOI] [PubMed] [Google Scholar]
- 78.Vernì F, Somma MP, Gunsalus KC, Bonaccorsi S, Belloni G, Goldberg ML, and Gatti M (2004). Feo, the Drosophila homolog of PRC1, is required for central-spindle formation and cytokinesis. Curr Biol 14, 1569–1575. [DOI] [PubMed] [Google Scholar]
- 79.Mollinari C, Kleman J-P, Saoudi Y, Jablonski SA, Perard J, Yen TJ, and Margolis RL (2005). Ablation of PRC1 by small interfering RNA demonstrates that cytokinetic abscission requires a central spindle bundle in mammalian cells, whereas completion of furrowing does not. Mol Biol Cell 16, 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu C-K, Coughlin M, Field CM, and Mitchison TJ (2008). Cell polarization during monopolar cytokinesis. J Cell Biol 181, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Field CM, Groen AC, Nguyen PA, and Mitchison TJ (2015). Spindle-to-cortex communication in cleaving, polyspermic Xenopus eggs. Mol Biol Cell 26, 3628–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landino J, Norris SR, Li M, Ballister ER, Lampson MA, and Ohi R (2017). Two mechanisms coordinate the recruitment of the Chromosomal Passenger Complex to the plane of cell division. Mol Biol Cell, mbc.E17-06–0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fuller BG, Lampson MA, Foley EA, Rosasco-Nitcher S, Le KV, Tobelmann P, Brautigan DL, Stukenberg PT, and Kapoor TM (2008). Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453, 1132–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Landino J, and Ohi R (2016). The Timing of Midzone Stabilization during Cytokinesis Depends on Myosin II Activity and an Interaction between INCENP and Actin. Curr Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eckley DM, Ainsztein AM, Mackay AM, Goldberg IG, and Earnshaw WC (1997). Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells. J Cell Biol 136, 1169–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Horst A, Vromans MJM, Bouwman K, van der Waal MS, Hadders MA, and Lens SMA (2015). Inter-domain Cooperation in INCENP Promotes Aurora B Relocation from Centromeres to Microtubules. Cell Rep 12, 380–387. [DOI] [PubMed] [Google Scholar]
- 87.Samejima K, Platani M, Wolny M, Ogawa H, Vargiu G, Knight PJ, Peckham M, and Earnshaw WC (2015). The Inner Centromere Protein (INCENP) Coil Is a Single α-Helix (SAH) Domain That Binds Directly to Microtubules and Is Important for Chromosome Passenger Complex (CPC) Localization and Function in Mitosis. J Biol Chem 290, 21460–21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gruneberg U, Neef R, Honda R, Nigg EA, and Barr FA (2004). Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J Cell Biol 166, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mishima M, Pavicic V, Gruneberg U, Nigg EA, and Glotzer M (2004). Cell cycle regulation of central spindle assembly. Nature 430, 908–913. [DOI] [PubMed] [Google Scholar]
- 90.Kitagawa M, Fung SYS, Hameed UFS, Goto H, Inagaki M, and Lee SH (2014). Cdk1 Coordinates Timely Activation of MKlp2 Kinesin with Relocation of the Chromosome Passenger Complex for Cytokinesis. Cell Rep. [DOI] [PubMed] [Google Scholar]
- 91.Kitagawa M, Fung SYS, Onishi N, Saya H, and Lee SH (2013). Targeting Aurora B to the Equatorial Cortex by MKlp2 Is Required for Cytokinesis. PLoS ONE 8, e64826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Powers J, Bossinger O, Rose D, Strome S, and Saxton W (1998). A nematode kinesin required for cleavage furrow advancement. Curr Biol 8, 1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cesario JM, Jang JK, Redding B, Shah N, Rahman T, and McKim KS (2006). Kinesin 6 family member Subito participates in mitotic spindle assembly and interacts with mitotic regulators. J Cell Sci 119, 4770–4780. [DOI] [PubMed] [Google Scholar]
- 94.Wolpert L (1966). The mechanical properties of the membrane of the sea urchin egg during cleavage. 41, 385–396. [DOI] [PubMed] [Google Scholar]
- 95.White JG, and Borisy GG (1983). On the mechanisms of cytokinesis in animal cells. J Theor Biol 101, 289–316. [DOI] [PubMed] [Google Scholar]
- 96.Dechant R, and Glotzer M (2003). Centrosome separation and central spindle assembly act in redundant pathways that regulate microtubule density and trigger cleavage furrow formation. Dev Cell 4, 333–344. [DOI] [PubMed] [Google Scholar]
- 97.Bringmann H, and Hyman AA (2005). A cytokinesis furrow is positioned by two consecutive signals. Nature 436, 731–734. [DOI] [PubMed] [Google Scholar]
- 98.Murthy K, and Wadsworth P (2008). Dual role for microtubules in regulating cortical contractility during cytokinesis. J Cell Sci 121, 2350–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bement WM, Leda M, Moe AM, Kita AM, Larson ME, Golding AE, Pfeuti C, Su K-C, Miller AL, Goryachev AB, et al. (2015). Activator-inhibitor coupling between Rho signalling and actin assembly makes the cell cortex an excitable medium. Nat Cell Biol 17, 1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kurz T, Pintard L, Willis JH, Hamill DR, Gönczy P, Peter M, and Bowerman B (2002). Cytoskeletal regulation by the Nedd8 ubiquitin-like protein modification pathway. Science 295, 1294–1298. [DOI] [PubMed] [Google Scholar]
- 101.Mangal S, Sacher J, Kim T, Osório DS, Motegi F, Carvalho AX, Oegema K, and Zanin E (2018). TPXL-1 activates Aurora A to clear contractile ring components from the polar cortex during cytokinesis. J Cell Biol 90, jcb.201706021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murata-Hori M, and Wang Y-L (2002). Both midzone and astral microtubules are involved in the delivery of cytokinesis signals: insights from the mobility of aurora B. J Cell Biol 159, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Breznau EB, Murt M, Blasius TL, Verhey KJ, and Miller AL (2017). The MgcRacGAP SxIP motif tethers Centralspindlin to microtubule plus ends in Xenopus laevis. J Cell Sci 130, 1809–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Foe VE, and Dassow, von G (2008). Stable and dynamic microtubules coordinately shape the myosin activation zone during cytokinetic furrow formation. J Cell Biol 183, 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dassow, von G, Verbrugghe KJC, Miller AL, Sider JR, and Bement WM (2009). Action at a distance during cytokinesis. J Cell Biol 187, 831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inoue T, Heo WD, Grimley JS, Wandless TJ, and Meyer T (2005). An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat Methods 2, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guillot C, and Lecuit T (2013). Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues. Dev Cell 24, 227–241. [DOI] [PubMed] [Google Scholar]
- 108.Founounou N, Loyer N, and Le Borgne R (2013). Septins Regulate the Contractility of the Actomyosin Ring to Enable Adherens Junction Remodeling during Cytokinesis of Epithelial Cells. Dev Cell 24, 242–255. [DOI] [PubMed] [Google Scholar]
- 109.Campinho P, Behrndt M, Ranft J, Risler T, Minc N, and Heisenberg C-P (2013). Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nat Cell Biol 15, 1405–1414. [DOI] [PubMed] [Google Scholar]