Abstract
Approximately one third of stroke patients suffer visual field impairment as a result of their strokes. However, studies using the visual pathway as a paradigm for studying post-stroke recovery are limited. In this article, we propose that the visual pathway has many features that make it an excellent model system for studying post-stroke neuroplasticity and assessing the efficacy of therapeutic interventions. First, the functional anatomy of the visual pathway is well characterized, which makes it well suited for functional neuroimaging studies of post-stroke recovery. Second, there are multiple highly standardized and clinically available diagnostic tools and outcome measures that can be used to assess visual function in stroke patients. Finally, as a sensory modality, the assessment of vision is arguably less likely to be affected by confounding factors such as functional compensation and patient motivation. Given these advantages, and the general similarities between post-stroke visual field recovery and recovery in other functional domains, future neurorehabilitation studies should consider using the visual pathway to better understand the physiology of neurorecovery and test potential therapeutics.
Keywords: stroke, stroke rehabilitation, rehabilitation research, cortical blindness, vision disorders, hemianopia
Despite major advances in acute stroke care over the last two decades and a steady decline in stroke-related mortality in more recent years, stroke remains the leading cause of adult long-term disability in the United States. Nearly 800,000 Americans will have a stroke this year alone.1 Of this number, approximately 40% will be left with permanent disability.2 As the incidence of stroke increases in the young,3 the societal impact of stroke-related disability will only continue to rise. In response to these trends and the growing need for evidence-based interventions in neurorehabilitation, the American Heart Association and the National Institute of Neurological Disease and Stroke have identified neurorehabilitation as a high-priority area of research.4–6
Several recently published articles have highlighted the main challenges facing the field of post-stroke recovery and rehabilitation.7–11 A major issue is the inherent heterogeneity of the stroke population and the difficulty of achieving consistency in therapeutic interventions and reducing the variability of environmental inputs during the recovery period. Stroke survivors comprise a diverse group with a wide range of genetic backgrounds and social, medical, and psychiatric histories. Additionally, stroke lesions vary greatly from patient to patient in terms of size, location, and topology. Furthermore, individual exposure to post-stroke rehabilitation is hard to standardize due to differences in the availability of health care resources, variability among different regional and institutional cultures, and the range of patient effort and participation. Last but not least, a significant hurdle is posed by the paucity of good outcome measures that can detect and quantify meaningful differences in functional outcomes. Developing an ideal outcome measure is not a trivial proposition: it must be easy to administer, demonstrate strong inter-rater and intra-subject reliability, and have the ability to distinguish between compensation and true recovery as mediated, for instance, by neuroplasticity. Given these challenges, it is perhaps not surprising that the field of neurorehabilitation has struggled with several disappointingly negative studies, and that experts are calling for a critical reevaluation of clinical trial design that would address the unique needs of this area of inquiry.4,7
While certain aspects of patient heterogeneity will always be present, we propose in this article that studying post-stroke visual field recovery in patients suffering from homonymous hemianopia (loss of vision on one side of the visual field as a result of damage to the post-geniculate visual pathway, typically in the contralateral posterior hemisphere) can help us overcome many other challenges posed by stroke recovery research. The visual pathway has been relatively underutilized in neurorehabilitation studies, yet it has great potential to contribute to our understanding of the nature of spontaneous post-stroke neurological recovery and our ability to test neuroplasticity-enhancing therapeutic interventions. First, the functional anatomy of the early visual pathway is well characterized and has a highly retinotopic organization, making it very amenable to investigation by functional neuroimaging techniques during post-stroke recovery. Second, visual function can be measured readily and reliably using a range of widely available standardized ophthalmological methods. Third, relatively speaking, it is somewhat easier to distinguish compensation from true neurological recovery in the visual pathway. Fourth, visual input to a recovering brain is less likely to be affected by patient motivation or variability in environmental stimuli. Finally, there are important similarities between the visual pathway and other neurologic systems, such as the motor and language systems, in terms of the predictors and natural history of post-stroke recovery, suggesting that some findings from the visual pathway may generalize to other functional domains. Of note, while post-stroke visual impairments span a range of abnormalities, including impaired eye movement, attention, and higher visual processing, this paper will focus on visual field loss.
1. The functional anatomy of the visual system offers several advantages for studying structure-function relationships during post-stroke recovery.
While the computational details of how visual inputs are processed to generate perception are still under study, the basic functional anatomy of the visual pathway is well characterized. Starting over a hundred years ago, with studies linking cortical lesions to visual deficits,12 a large body of work has helped to elucidate structure-function relationships at each level of the visual pathway.13 The early visual cortex (V1) in particular is known to have a highly regular retinotopic organization.13 Moreover, there is evidence that retinotopic biases are carried forward into higher order visual areas.14 This high degree of resolution at a functional anatomical level is of great potential value for functional brain imaging studies seeking to shed light on the course of recovery following specific interventions.
Indeed, functional magnetic resonance imaging (fMRI) is sufficiently sensitive to study normal visual function and retinotopy in healthy subjects15 and to measure changes that occur in the retinotopic map as a result of injury and during recovery.16–18 Retinotopic mapping with fMRI is typically accomplished by presenting flickering or moving checkerboard wedges, rings, or vertical and horizontal bars, while the subject maintains fixation on a central target in the scanner (Figure 1). The data are analyzed to determine the visual field location that showed the strongest and most consistent modulation of the blood oxygen level-dependent signal for each voxel independently. A priori knowledge about the retinotopic organization of visual cortex then allows researchers to delineate the borders between different visual areas from V1 to V4 based on the progression of the polar angle and eccentricity preferences across visual cortical areas.15 Further advances in fMRI retinotopic mapping now estimate the population receptive field size of each voxel in addition to its preferred stimulus location.19 Several groups have capitalized on this robust technique to study changes in retinotopy after ischemic damage to the visual cortex or its afferent inputs in stroke patients.16–18
Figure 1:
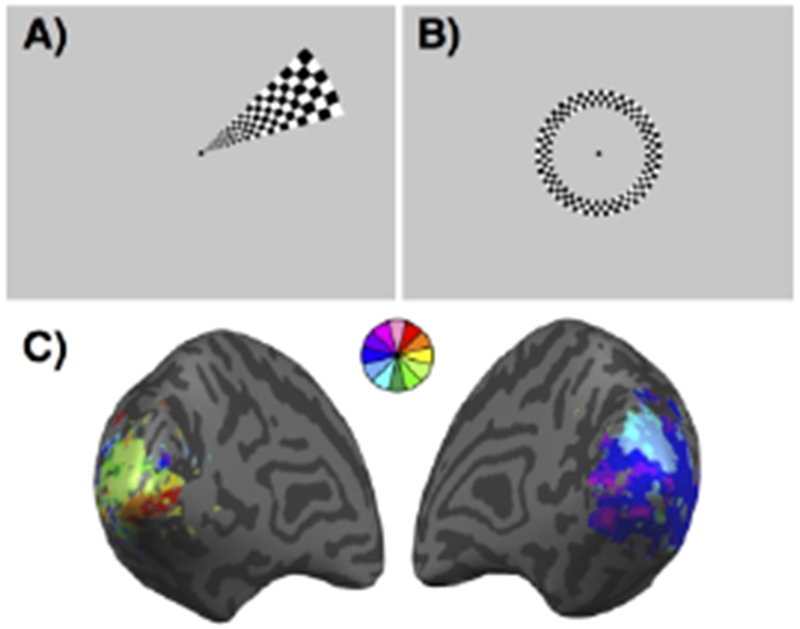
Retinotopic mapping. (A) Example of a wedge stimulus used to map polar angle visual preferences. Subjects fixate on the central dot while the flickering checkerboard wedge is presented in each of 12 non-overlapping polar angles multiple times. (B) Example of a ring stimulus used to map eccentricity visual preferences. Subjects fixate on the central dot while the flickering checkerboard ring is presented in each of 6 non-overlapping eccentricities multiple times. (C) Example of a retinotopic map from a stroke patient with a visual field cut. The map is pseudo-colored based on each voxel’s preferred wedge location.
Functional neuroimaging has also proved to be a powerful tool for studying post-stroke motor and language recovery. In fact, previous studies have detected similar types of plastic changes in all three systems: (1) increased perilesional activation,7,16,20 (2) perilesional cortical reorganization,18,21–23 (3) strengthening of existing cortico-cortical connections,24–28 and (4) recruitment of distant brain regions.16,29–33 Given these similarities, a greater understanding of post-stroke plastic changes in the visual pathway may shed light on recovery in the motor and language domains. Furthermore, studying post-stroke recovery in the visual pathway offers a number of additional advantages, including tolerance for a wide range of lesion sizes, elimination of task confounds, and standardization of testing conditions.
Tolerance for lesion size heterogeneity
Performing tasks while minimizing head movement inside the scanner can be difficult for some patients, limiting the type of stroke patients suitable for functional neuroimaging research. While strokes in the distribution of the middle cerebral artery (MCA) are far more common than strokes in other vascular territories, most functional neuroimaging studies of post-stroke motor and language recovery exclude patients with large strokes in the MCA territory because the extensive cortical and subcortical damage often associated with such lesions may result in severe aphasia or profound neglect, affecting the ability of many of these patients to understand and complete the required tasks.34 On the other hand, with the notable exception of those with significant thalamic or hippocampal involvement, patients with extensive ischemic injury in the distribution of the posterior cerebral artery (PCA) typically retain the ability to understand and follow directions. Given that up to 75% of patients with primary visual cortex damage retain foveal vision in the central 1-10 degrees,35 even patients with large PCA territory strokes, resulting in a dense homonymous hemianopia, are typically able to maintain central fixation during peripheral vision tasks. As a result, while functional neuroimaging studies of patients with strokes in the distribution of the MCA may be limited by lesion size, this variable poses less of a limitation in the PCA territory.
Elimination of task confounds
The use of functional neuroimaging to investigate mechanisms of post-stroke recovery can be confounded by neural activation events that are not causally related to recovery. For example, in a patient with hemiparesis, if the paretic arm is stronger at the time of a follow-up scan, it is not always possible to establish with certainty whether any observed increase in neural activity is the cause or consequence of improved limb strength. Similarly, if language production is more fluent at follow-up, it is difficult to attribute changes in brain activity to recovery alone. Other confounds, including handedness, learning effects, and effort,36 also affect our ability to directly relate functional recovery to changes in cortical activation. Studying post-stroke recovery in a sensory system, using perceptual rather than motor or language tasks, largely avoids these confounds by removing the variability in brain activity inherent in a patient’s ability to produce a behavior. This means that any changes in visual cortical activation, organization, or connectivity can be directly compared to changes in visual ability, allowing researcher to determine what cortical activation patterns are associated with good versus poor recovery. Given that vision is the major sensory input to the brain, and that over 30% of all strokes are associated with a visual field defect,37–39 the visual system is well positioned to offer insights that may generalize to mechanisms of post-stroke recovery in other domains.
Standardization of testing conditions
Finally, using the visual pathway to study post-stroke recovery allows remarkable standardization of testing conditions during functional neuroimaging experiments. Since neurons in the visual cortex are primarily driven by visual input from the environment,40 and since vision tasks are inherently perceptual rather than motor in nature, the input to the brain can be tightly controlled by the investigator in these studies. Furthermore the output, in the form of stimulus-dependent cortical activity as measured by fMRI, for instance, is less affected by behavioral variables, including patient comprehension, reaction time, and motivation, which can sometimes confound motor and language production tasks. Indeed, low-complexity, high-contrast visual stimuli for retinotopic mapping have long been adopted by the field41 and provide a reliable method for measuring the neural correlates of post-stroke visual field recovery. Eye tracking in the scanner enables researchers to control for compensatory eye movements, further enhancing the validity of the data. Last but not least, because vision is not a lateralized or dominant function of one cerebral hemisphere, the unaffected region of the visual field in a hemianopic patient can serve as a valuable within-subject control in functional neuroimaging studies.
2. Multiple standardized and automated tools exist for the evaluation of visual function.
Critical to any observational or interventional study of post-stroke recovery are reliable, validated, and commonly available methods to measure neurologic outcomes, functional status, and quality of life. The assessment of the visual pathway has the advantage of being amenable to highly standardized and automated evaluation methods that are already routinely used in patient care and research. There are many ways to evaluate the visual pathway, ranging from objective and quantitative assessments to more subjective patient-centered outcome measures.
One of the most commonly used measures of the visual pathway in the eye clinic is perimetry, which systematically maps a patient’s visual field by presenting stimuli in different locations and measuring the patient’s ability to detect them. Depending on the type of perimeter, the stimuli may be white or colored, static or moving, and may vary in size or light intensity. The concept of perimetry for visual field testing has existed for over 150 years, and now automated perimetry systems, with improved methods of test administration, standardization, and statistical analysis, are the norm.42 Widely available examples of automated perimeters include the Humphrey visual field analyzer (Carl Zeiss Meditec, Inc., Dublin, CA) (Figure 2) and the Octopus 900 perimeter (Haag Streit International, Koeniz, Switzerland). During visual field assessment, the patient is instructed to focus on a fixation point while a computer presents white stimuli of incremental brightness against a white background. The patient is asked to click a button whenever a stimulus is seen. The results are then compared with normative data from healthy age-matched controls to create a quantitative representation of the patient’s visual field. An important advantage of this test is that it is widely available, takes little time to administer, and has been extensively used in vision research, including natural history studies of post-stroke visual field recovery.43,44 Disadvantages include its reliance on patient understanding and concentration, and the need to maintain proper visual fixation. These issues can often be addressed by repeat testing in the same locations and by excluding patients with frequent visual fixation loss. Recent technological solutions addressing these issues will be discussed at greater length in the next section.
Figure 2:
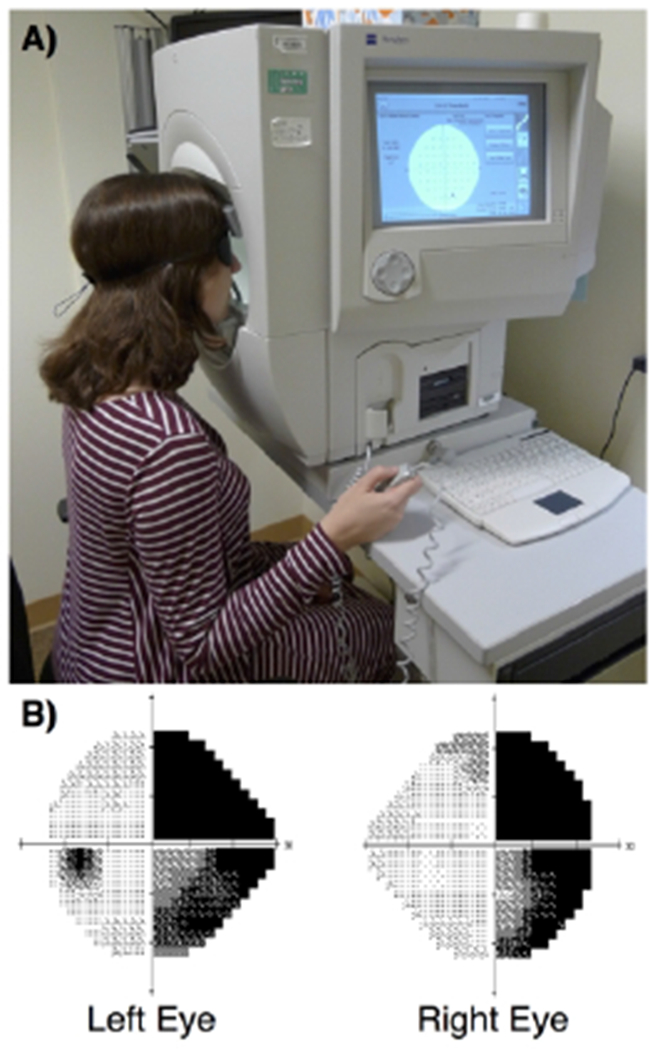
Humphrey Visual Field Analyzer. (A) The patient is positioned against the chin and forehead rest, instructed to maintain fixation on a visual target, presented with a series of bright white lights with varying intensity in different locations of the visual field, and asked to press a handheld button each time a stimulus is seen. This information is used to create visual field maps for each eye, where darker tones represent loss of vision at that location in the visual field. (B) Example of a Humphrey visual field map from a patient with right homonymous hemianopia. The focal area of darkness within the left visual field of the left eye corresponds to the anatomical blind spot, or optic disk.
Optical coherence tomography (OCT) is another widely available diagnostic test that can provide objective information about the visual system (Figure 3). This technique uses near-infrared light in an interferometer to measure retinal nerve fiber layer thickness. Previous studies using OCT have shown a progressive thinning of the retinal nerve fiber layer following strokes affecting the occipital lobes and optic radiations,45–49 likely due to trans-synaptic retrograde degeneration of retinal ganglion cells.50 In clinical trials of post-stroke visual field recovery, retinal nerve fiber layer thickness could therefore potentially serve as a biomarker of a patient’s physiologic response to the intervention under investigation.49
Figure 3:
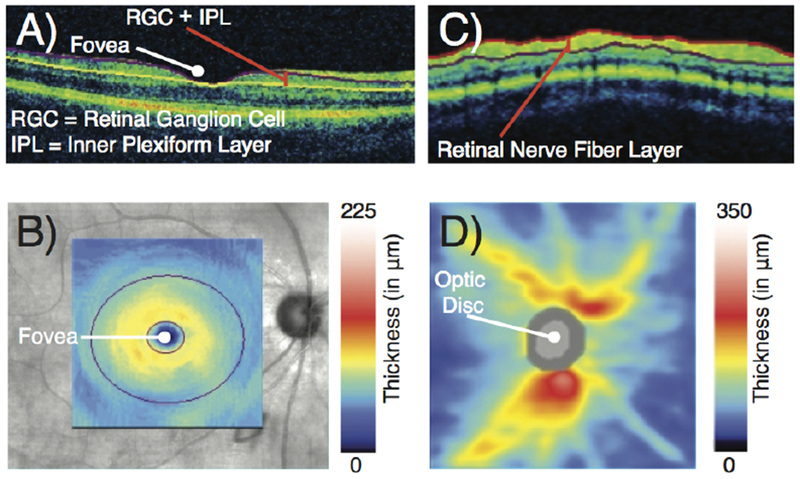
Optical coherence tomography. (A) Tomogram through the fovea shows the ganglion cell complex layer, which is comprised of the retinal ganglion cell layer and the inner plexiform layer. (B) Thickness of the ganglion cell complex layer. (C) Circular tomogram around the optic disc shows the retinal nerve fiber layer. (D) Thickness of the retinal nerve fiber layer.
Finally, in recent years, there has been an appropriate growth of interest in outcome measures that assess a patient’s health-related quality of life. The 25-Item National Eye Institute Visual Function Questionnaire is a survey specifically designed to provide a vision-targeted assessment of health-related quality of life by measuring the influence of visual disability on daily visual and social functioning and emotional wellbeing.51 This questionnaire has previously been used to determine how stroke-related vision loss impacts quality of life.52,53 It has also been used to track clinically significant visual improvement after visual retraining therapy.54
3. Compensatory strategies can be monitored and distinguished from true recovery in the visual system.
Patients with visual field defects often develop, or are trained55 to develop, compensatory strategies that rely on conscious or unconscious eye movements. Indeed these learned compensatory eye movements hampered early attempts at developing interventions to promote visual field recovery.56 The issue of compensation is non-trivial. When designing neurorehabilitation trials, it is critical to differentiate compensation from neurological recovery to be able to determine if the intervention has an effect on neuroplasticity. Compensation refers to approximating a lost function by developing a novel strategy for attaining the same behavioral goal. For example, a stroke patient may perform a motor task effectively, without recovery of function in the muscles affected by the stroke, if other muscle groups can be engaged to produce movements that result in a sufficiently similar action.57 This distinction is not purely academic. While regaining function via compensatory movements is undoubtedly beneficial to the patient, ideal rehabilitation interventions would target functional recovery mediated by neuroplasticity (not compensatory strategies) early in the recovery process, when a critical window for neural reorganization may still be open.58,59 Furthermore, compensatory strategies can sometimes lead to maladaptive muscle activation patterns that may contribute to post-stroke arthralgias and limit functional recovery.60 To properly assess recovery and differentiate restoration of native function from compensatory behaviors, measures are needed that can detect not only if, but also how, a given task is accomplished.57 In the field of motor recovery, future studies will likely use methods such as electromyography and kinematics to capture and quantify recovery.61 However, these techniques are not yet readily available in most clinical settings and can be labor-intensive in terms of data collection and analysis.
On the other hand, standard visual perimetry methods, which are widely available in the clinical setting, can provide longitudinal measurements of visual field deficits enabling assessment of whether there has been interval improvement. The reliability indices enable monitoring of whether the patient breaks fixation, which could happen if the patient is using a compensatory strategy such as moving their head or initiating a compensatory saccade into the blind hemifield. In standard Humphrey perimetry, the head position is fixed, and eye fixation loss is monitored by recording pupil movements with a gaze tracker and observing the patient’s response to visual stimuli presented in the region of the physiologic blind spot. In newer systems, eye tracking data are used in real time to pause testing automatically when the patient loses central fixation, as in the Octopus 900 perimeter, or to present stimuli in a gaze-contingent manner, as in the MAIA Microperimeter (CenterVue, Padova, Italy). Therefore visual field recovery studies, in comparison to motor recovery studies, can use readily available clinical tools to monitor visual field changes with relatively limited confounding by compensatory head or eye movements.
4. The frequency, intensity, and complexity of visual inputs experienced by different patients are less likely to be affected by patient motivation or environmental variability.
A major challenge in rehabilitation research is the variability in patient environments and exposure to rehabilitation treatments.7,61 Not only do health insurance policies and health care centers differ in the rehabilitation resources they offer to patients, but individual patients may also move through several health care environments with differing therapeutic priorities during the course of their recovery, making it difficult to study treatment effects across patients, institutions, and geographic regions. Additionally, studies on motor recovery suggest that patient effort and the number of exercise repetitions vary considerably during a given rehabilitation session and may further contribute to variations in outcome.62 In contrast, barring any premorbid optic neuropathy, retinopathy, or visual field defects, stroke patients are likely exposed to a less variable amount of background visual stimuli, not only because indoor environments can have high amounts of clutter, organization, local contrast, texture, and variety of colors much like complex outdoor scenery63, but also because they receive continuous visual stimuli throughout the day.
While new experience-dependent strategies show promise in enhancing visual field recovery,64,65 the current standard of care for stroke patients with visual field defects is focused on teaching compensatory or substitutive strategies.66 However, this does not alter the fact that the visual system is constantly being exposed to potentially neuroplasticity-enhancing visual stimuli. Patient resources and access to medical care are unlikely to affect the amount of visual stimulation received substantially. Furthermore, post-stroke visual field recovery is probably less susceptible to the effects of patient motivation and engagement than other domains, such as motor and language function.
In light of these considerations, observational studies of post-stroke visual field recovery, as well as interventional studies testing the effectiveness of pharmacologic interventions for stroke-related vision loss, are probably less likely to suffer from high therapeutic and environmental variability. In the near future, we may be able to investigate the effect of differences in background visual experience on post-stroke visual field recovery by using new light-weight video technologies and automated tools to quantify contrast levels and scene complexity encountered in each patient’s visual experience in order to compare exposure to visual stimuli among different patients in different environments.67
5. Post-stroke visual field recovery is similar in important ways to post-stroke neurological recovery in other functional domains.
Meta-analyses seeking to draw general conclusions from studies of post-stroke motor, language, and visual field recovery have been frustrated by polymethodology and inconsistent results.68 Be that as it may, a number of underlying similarities have emerged across different functional domains, suggesting that stroke patients may recover from seemingly disparate neurologic deficits in similar ways, and findings in one domain have the potential to generate testable hypotheses concerning recovery in other functional domains.
First, greater initial symptom severity and larger lesion size are both strongly associated with poor recovery across multiple functional modalities,69 including vision.39,43 Furthermore, treatment with systemic thrombolytic therapy improves the likelihood of post-stroke visual field recovery37 in the same way that it improves the chances of a good functional recovery from other stroke-related neurologic deficits.
Second, post-stroke functional recovery follows a similar time course and occurs in comparable proportions of affected patients across a range of stroke-related neurologic deficits.69 Stroke patients with visual field defects experience the most improvement in the first few months after their stroke,37,39,44 as do those with stroke-related impairments in motor or language function. 70,71 Reports of post-stroke visual field recovery vary with respect to the time course and degree of improvement. Some amount of spontaneous recovery may occur in as many as 72% of stroke survivors with visual field defects, particularly those with partial versus complete hemianopia,72 but full recovery is less common, especially in patients with complete homonymous hemianopia in the acute stage.38,73
Third, multiple different mechanisms of neuroplasticity are known to play a role during post-stroke recovery across several functional domains (see Section 1). All of these signatures of neuroplasticity have been observed in the recovering visual pathway after a stroke.
Fourth, the available evidence indicates that similar therapeutic principles can be applied to improve recovery across different functional domains. For example, motor and visual field recovery both appear to be enhanced by repetitive, task-specific exercises in a dose-dependent way.29,55,68,74
Finally, several pharmacological agents have shown promise as potential mediators of enhanced recovery in recent years. Amphetamine and other stimulants may be beneficial in this regard,75 and selective serotonin reuptake inhibitors such as fluoxetine, administered in the first few months after a stroke, may improve motor outcomes.76 Whether or not post-stroke visual field recovery can also be enhanced by fluoxetine is an area of active investigation by our group (NCT02737930).
Conclusion
Stroke is a heterogeneous disease and post-stroke recovery is a highly complicated process that cannot easily be encapsulated by a single sensory modality like vision. Nonetheless, the visual pathway has a number of characteristics that make it suitable for generating and testing hypotheses about therapeutic interventions that may promote post-stroke functional recovery in other domains. While studies of post-stroke visual field recovery will never supplant similar work in the motor and language domains, it is tempting to speculate that a better understanding of visual field recovery may have broader implications for the larger field of neurorehabilitation. The underlying similarity of the visual system to other functional modalities in terms of the predictors and natural history of post-stroke recovery further suggests that findings in the visual domain may be more generally applicable. We already have at our disposal a battery of widely available diagnostic tools and validated outcome measures that can be used to study visual function at the anatomical, physiological, psychophysical, and psychosocial levels. Many of these tools are standard of care in the clinical realm. Yet as widely available as they are, the concept of using the visual pathway as a model system to study post-stroke recovery is currently underutilized in the research context. The visual pathway can be used to test new neuroplasticity-enhancing drugs, for instance, or answer longitudinal questions about the mechanisms of post-stroke recovery in the context of a clinical trial using fMRI. However, to date there have been no large multicenter studies using vision to study recovery in clinical trial networks like NeuroNext and StrokeNet. We hope that this article will encourage others to consider using observational and interventional studies of post-stroke visual field recovery to help us address some of the vital challenges facing the field of neurorehabilitation today.
Acknowledgements/Sources of Funding:
Preparation of this manuscript was supported in part by NIH National Research Service Award Institutional Research Training Grant 2T32NS007338-16 to the University of Rochester providing support for AB, NIH grant R01 NS089609 to BZM, NIH grant F30 EY027988 to CLS, and a grant to BS from the Schmitt Program on Integrative Brain Research at the University of Rochester.
Footnotes
Conflict of Interest Statement:
The Authors declare that there is no conflict of interest.
References:
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics ‘2017 Update: A Report from the American Heart Association. Vol 135; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young J, Forster A. Review of stroke rehabilitation. BMJ. 2007;334(7584):86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Béjot Y, Delpont B, Giroud M. Rising Stroke Incidence in Young Adults: More Epidemiological Evidence, More Questions to Be Answered. J Am Heart Assoc. 2016;5(5):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winstein CJ, Stein J, Arena R, et al. AHA / ASA Guideline Guidelines for Adult Stroke Rehabilitation and Recovery.; 2016. [Google Scholar]
- 5.Winstein CJ, Stein J, Arena R, et al. Correction to: Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2017;48(2):e78–e78. [DOI] [PubMed] [Google Scholar]
- 6.Vickrey BG, Brott TG, Koroshetz WJ. Research priority setting: A summary of the 2012 NINDS stroke planning meeting report. Stroke. 2013;44(8):2338–2342. [DOI] [PubMed] [Google Scholar]
- 7.Cramer SC, Wolf SL, Adams HP, et al. Stroke Recovery and Rehabilitation Research Issues, Opportunities, and the National Institutes of Health StrokeNet. Stroke. 2017;(48):813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabil Neural Repair. 2017;31(9):793–799. [DOI] [PubMed] [Google Scholar]
- 9.Spong CY, Kennedy E, National S. National Institutes of Health research plan on rehabilitation. Rehabil Psychol. 2017;62(3):397–400. [DOI] [PubMed] [Google Scholar]
- 10.Corbett D, Carmichael ST, Murphy TH, et al. Enhancing the Alignment of the Preclinical and Clinical Stroke Recovery Research Pipeline: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable Translational Working Group *. Neurorehabil Neural Repair. 2017;31(8): 699–707. [DOI] [PubMed] [Google Scholar]
- 11.Campbell GB, Skidmore ER, Whyte EM, Matthews JT. Overcoming practical challenges to conducting clinical research in the inpatient stroke rehabilitation setting. Top Stroke Rehabil. 2015;22(5):386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishman RS. Gordon Holmes, the cortical retina, and the wounds of war. The seventh Charles B. Snyder Lecture. Doc Ophthalmol. 1997;93(March 1915):9–28. [DOI] [PubMed] [Google Scholar]
- 13.Wandell BA, Dumoulin SO, Brewer AA. Visual Field Maps in Human Cortex. Neuron. 2007;56(2):366–383. [DOI] [PubMed] [Google Scholar]
- 14.Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center–periphery organization of human object areas. NatNeurosci. 2001;4:533–539. [DOI] [PubMed] [Google Scholar]
- 15.Sereno MI, Dale AM, Reppas JB, et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. [DOI] [PubMed] [Google Scholar]
- 16.Vaina LM, Soloviev S, Calabro FJ, Buonanno F, Passingham R, Cowey A. Reorganization of Retinotopic Maps after Occipital Lobe Infarction. J Cogn Neurosci. 2014;26(6):1266–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho YC, Cheze A, Sitoh YY, et al. Residual neurovascular function and retinotopy in a case of hemianopia. Ann Acad Med Singapore. 2009;38(9):827–831. [PubMed] [Google Scholar]
- 18.Papanikolaou A, Keliris GA, Papageorgiou TD, et al. Population receptive field analysis of the primary visual cortex complements perimetry in patients with homonymous visual field defects. Proc Natl Acad Sci. 2014;111(16):E1656–E1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumoulin SO, Wandell BA. Population Receptive Field Estimates in Human Visual Cortex. Neuroimage. 2008;39:647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leger A, Demonet JF, Ruff S, et al. Neural substrates of spoken language rehabilitation in an aphasic patient: an fMRI study. Neuroimage. 2002;17(1):174–183. [DOI] [PubMed] [Google Scholar]
- 21.Henriksson L, Raninen A, Nasanen R, Hyvarinen L, Vanni S. Training-Induced Cortical Representation of a Hemianopic Hemifield. J Neurol Neurosurg Psychiatry. 2007;78:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Functional MRI detects posterior shifts in primary sensorimotor cortex activation after stroke. Stroke. 2001;32:1134–1139. [DOI] [PubMed] [Google Scholar]
- 23.Calautti C, Leroy F, Guincestre JY, Baron JC. Displacement of primary sensorimotor cortex activation after subcortical stroke: a longitudinal PET study with clinical correlation. Neuroimage. 2003;19:1650–1654. [DOI] [PubMed] [Google Scholar]
- 24.Jang SH, Lee H Do. Recovery of Visual Field Defect via Corpus Callosum in a Patient with Cerebral Infarct. Neuro-Ophthalmology. 2015;39:88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park C, Chang WH, Ohn SH, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011; 42:STROKEAHA--110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruber T, Schlaug G, Lindenberg R. Compensatory role of the cortico-rubro-spinal tract in motor recovery after stroke. Neurology. 2012;79(6):515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breier JI, Juranek J, Papanicolaou AC. Changes in maps of language function and the integrity of the arcuate fasciculus after therapy for chronic aphasia. Neurocase. 2011;17(6):506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic broca’s aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci. 2009; 1169:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melnick M, Merriam E, Heeger D, Huxlin KR. Training-induced recovery of fMRI-based motion adaptation signals in VI damaged humans. J Vis. 2017;17(7). [Google Scholar]
- 30.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural Correlates of Outcome after Stroke: a Cross-Sectional fMRI Study. Brain. 2003;126:1430–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loubinoux I, Carel C, Pariente J, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20(4):2166–2180. [DOI] [PubMed] [Google Scholar]
- 32.Saur D, Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain. 2006; 129(6): 1371–1384. [DOI] [PubMed] [Google Scholar]
- 33.Fridriksson J, Moser D, Bonilha L, et al. Neural correlates of phonological and semantic-based anomia treatment in aphasia. Neuropsychologia. 2007;45(8): 1812–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calautti C, Baron J-C. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke, A J Cereb Circ. 2003;34(6): 1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- 35.Leff A A historical review of the representation of the visual field in primary visual cortex with special reference to the neural mechanisms underlying macular sparing. Brain Lang. 2004;88(3):268–278. [DOI] [PubMed] [Google Scholar]
- 36.Poldrack RA. Imaging Brain Plasticity: Conceptual and Methodological Issues — A Theoretical Review. Neuroimage. 2000;13:1–13. [DOI] [PubMed] [Google Scholar]
- 37.Ali M, Hazelton C, Lyden P, Pollock A, Brady M, Collaboration. V. Recovery from post-stroke visual impairment: evidence from a clinical trials resource. Neurorehabil Neural Repair. 2013;27(2): 133–141. doi: 10.1177/1545968312454683. [DOI] [PubMed] [Google Scholar]
- 38.Rowe FJ, Wright D, Brand D, et al. Research Article A Prospective Profile of Visual Field Loss following Stroke : Prevalence , Type , Rehabilitation , and Outcome. Biomed Res Int. 2013;2013:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray CS, French JM, Bates D, Cartridge NE, Venables GS, James OF. Recovery of Visual Fields in Acute Stroke: Homonymous Hemianopia Associated with Adverse Prognosis. Age Ageing. 1989; 18(6):419–421. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert CD, Li W. Top-down influences on visual processing. Nat Rev Neurosci. 2013. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wandell BA, Winawer J. Imaging retinotopic maps in the human brain. Vision Res. 2011. ;51(7):718–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson CA, Wall M, Thompson HS. A history of perimetry and visual field testing. Optom Vis Sci. 2011. doi: 10.1097/OPX.0b013e3182004c3b. [DOI] [PubMed] [Google Scholar]
- 43.Çelebisoy M, Çelebisoy N, Bayam E, Köse T. Recovery of Visual-Field Defects After Occipital Lobe Infarction: a Perimetric Study. J Neurol Neurosurg Psychiatry. 2011;82(6):695–702. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Homonymous hemianopia in stroke. J Neuroophthalmol. 2006;26(3):180–183. doi: 10.1016/j.ajo.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Jindahra P, Petrie A, Plant GT. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain. 2012;135(2):534–541. doi: 10.1093/brain/awr324. [DOI] [PubMed] [Google Scholar]
- 46.Park H-YL, Park YG, Cho A-H, Park CK. Transneuronal Retrograde Degeneration of the Retinal Ganglion Cells in Patients with Cerebral Infarction. Ophthalmology. 2013;120(6):1292–1299. doi: 10.1016/j.ophtha.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 47.Keller J, Sanchez-Dalmau BF, Villoslada P. Lesions in the Posterior Visual Pathway Promote Trans-Synaptic Degeneration of Retinal Ganglion Cells. PLoS One. 2014;9(5):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamashita T, Miki A. Reduced retinal ganglion cell complex thickness in patients with posterior cerebral artery infarction detected using spectral-domain optical coherence tomography. J Ophthalmol. 2012;56:502–510. [DOI] [PubMed] [Google Scholar]
- 49.Schneider CL, Prentiss E, Busza A, Williams ZR, Sahin B, Mahon BZ. Do areas of retinal ganglion cell degeneration coincide with areas of decreased representation in V1 following stroke? J Vis. 2017;17(7). [Google Scholar]
- 50.Johnson H, Cowey A. Transneuronal retrograde degeneration of retinal ganglion cells following restricted lesions of striate cortex in the monkey. Exp Brain Res. 2000;132:269–275. [DOI] [PubMed] [Google Scholar]
- 51.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol (Chicago, Ill 1960). 2001;119(7):1050–1058. doi: 10.1097/00132578-200201000-00028. [DOI] [PubMed] [Google Scholar]
- 52.Gall C, Franke GH, Sabel BA. Vision-Related Quality of Life in First Stroke Patients with Homonymous Visual Field Defects. Health Qual Life Outcomes. 2010;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen CS, Lee AW, Clarke G, et al. Vision-Related Quality of Life in Patients with Complete Homonymous Hemianopia Post Stroke. Top Stroke Rehabil. 2009;16:445–453. [DOI] [PubMed] [Google Scholar]
- 54.Hayes A, Chen CS, Clarke G, Thompson A. Functional Improvements Following the Use of the NVT Vision Rehabilitation Program for Patients with Hemianopia Following Stroke. NeuroRehabilitation. 2012;31(1):19–30. [DOI] [PubMed] [Google Scholar]
- 55.Pollock A, Hazelton C, Henderson CA, et al. Interventions for visual field defects in patients with stroke. Cochrane Database Syst Rev. 2011;(10):1–61. doi:10.1002/14651858.CD008388.pub2 .10.1002/14651858.CD008388.pub2www.cochranelibrary.com. www.cochranelibrary.com [DOI] [PubMed] [Google Scholar]
- 56.Horton JC. Disappointing results from Nova Vision’s visual restoration therapy. Br J Ophthalmol. 2005;89(1):1–2. doi: 10.1136/bjo.2004.058214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levin MF, Kleim JA, Wolf SL. What Do Motor “Recovery” and “Compensation” Mean in Patients Following Stroke? 2009:313–319. [DOI] [PubMed] [Google Scholar]
- 58.Van Kordelaar J, Van Wegen E, Kwakkel G. Impact of time on quality of motor control of the paretic upper limb after stroke. Arch Phys Med Rehabil. 2014;95(2):338–344. doi: 10.1016/j.apmr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Zeiler SR, Krakauer JW. The interaction between training and plasticity in the post-stroke brain. Curr Opin Neurol. 2013;26(6):609–616. doi: 10.1097/WC0.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. 2000:940–953. [DOI] [PubMed] [Google Scholar]
- 61.Kwakkel G Standardised measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable (SRRR). Int J Stroke. 2017;in press(5):451–461. doi: 10.1177/1747493017711813. [DOI] [PubMed] [Google Scholar]
- 62.Lenze EJ, Munin MC, Quear T, et al. Significance of poor patient participation in physical and occupational therapy for functional outcome and length of stay. Arch Phys Med Rehabil. 2004;85(10):1599–1601. doi: 10.1016/j.apmr.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 63.Corchs SE, Ciocca G, Bricolo E, Gasparini F. Predicting complexity perception of real world images. PLoS One. 2016;11(6):1–22. doi: 10.1371/journal.pone.0157986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melnick MD, Tadin D, Huxlin KR. Relearning to See in Cortical Blindness. Neurosci. 2015;22(2):199–212. doi: 10.1177/1073858415621035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanna KL, Hepworth LR, Rowe FJ. The treatment methods for post-stroke visual impairment: A systematic review. Brain Behav. 2017;(November 2016):1–26. doi: 10.1002/brb3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rowe FJ, Wright D, Brand D, et al. A prospective profile of visual field loss following stroke: Prevalence, type, rehabilitation, and outcome. Biomed Res Int. 2013;2013. doi: 10.1155/2013/719096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forsythe A, Mulhern G, Sawey M. Confounds in pictorial sets: The role of complexity and familiarity in basic-level picture processing. Behav Res Methods. 2008;40(1): 116–129. doi: 10.3758/BRM.40.1.116. [DOI] [PubMed] [Google Scholar]
- 68.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 69.Ramsey LE, Siegel JS, Lang CE, Strube M, Shulman GL, Corbetta M. Behavioural clusters and predictors of performance during recovery from stroke. Nat Hum Behav. 2017;1:1–24. doi: 10.1016/j.parkreldis.2015.11.029.Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry. 1986;49(1): 11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hendricks HT, Van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: A systematic review of the literature. Arch Phys Med Rehabil. 2002;83(11):1629–1637. doi: 10.1053/apmr.2002.35473. [DOI] [PubMed] [Google Scholar]
- 72.Hepworth LR, Rowe FJ, Walker MF, et al. Post-stroke Visual Impairment: A Systematic Literature Review of Types and Recovery of Visual Conditions. 2016;5(September 2015): 1–43. doi: 10.9734/OR/2016/21767. [DOI] [Google Scholar]
- 73.Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Natural history of homonymous hemianopia. Neurology. 2006;66(6):901–905. doi: 10.1212/01.wnl.0000203338.54323.22. [DOI] [PubMed] [Google Scholar]
- 74.Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke. 2014;45(7):2053–2058. doi: 10.1161/STROKEAHA.114.004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker-batson D, Mehta J, Smith P, Johnson M. Progress in Neuro-Psychopharmacology & Biological Psychiatry Amphetamine and other pharmacological agents in human and animal studies of recovery from stroke. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:225–230. doi: 10.1016/j.pnpbp.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Chollet F, Tardy J, Albucher JF, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): A randomised placebo-controlled trial. Lancet Neurol. 2011; 10(2): 123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]


