Abstract
Background
Stereotactic Body Radiotherapy (SBRT) has been adopted as the standard of care for inoperable early-stage non-small cell lung cancer (NSCLC), with local control rates consistently > 90%. However, data directly comparing the outcomes of SBRT with those of conventionally fractionated radiotherapy (CONV) is lacking.
Material and Methods
Between 1990 and 2013, 497 patients (525 lesions) with early-stage NSCLC (T1-T2N0M0) were treated with CONV (n=127) or SBRT (n=398). In this retrospective analysis, five endpoints were compared, with and without adjusting for clinical and dosimetric factors. Competing risks analysis was performed to estimate and compare the cumulative incidence of local failure (LF), nodal failure (NF), distant failure (DF) and disease progression. Overall survival (OS) was estimated by the Kaplan-Meier method and compared by the Cox regression model. Propensity score (PS) matched analysis was performed based on seven patient and clinical variables: age, gender, Karnofsky performance status (KPS), histology, T-stage, biologically equivalent dose (BED), and history of smoking.
Results
The median dose delivered for CONV was 75.6 Gy in 1.8 to 2.0 Gy fractions (range 60 to 90 Gy; median BED = 89.20 Gy) and for SBRT 48 Gy in 4 fractions (45 to 60 Gy in 3 to 5 fractions; median BED = 105.60 Gy). Median follow up was 24.4 months, and 3-year LF rates were 34.1% with CONV and 13.6% with SBRT (p<0.001). 3-year OS rates were 38.9% and 53.1%, respectively (p=0.018). PS matching showed a significant improvement of OS (p=0.0497) for SBRT. T-stage was the only variable correlating with all five endpoints.
Conclusion
SBRT compared to CONV is associated with improved LF rates and OS. Our data supports the continued use and expansion of SBRT as the standard of care treatment for inoperable early-stage NSCLC.
Keywords: stereotactic body radiation therapy (SBRT), conventional RT, non-small cell lung cancer
Introduction
Early-stage disease accounts for about 20% of new diagnoses of NSCLC. With new lung cancer screening guidelines for former and current smokers, we may see an increase in the proportion of early-stage cancers detected.[1] Traditionally, early-stage NSCLC was treated with surgical resection, resulting in high rates of local disease control of about 90 to 95% and 5-year survival ranging from 50 to 80%.[2,3] However, approximately 20% of stage I NSCLC patients do not undergo surgery due to medical or technical inoperability or because of patient refusal, according to a population study of the California Cancer Center registry.[2] Stage I NSCLC, left untreated, has a median survival of only 9 months.[2]
Definitive radiation therapy (RT) with curative intent has therefore been used as the standard non-invasive definitive treatment alternative to surgical resection. Historically, patients with inoperable early-stage NSCLC were treated with conventionally fractionated radiation therapy (CONV) to doses of 60 to 80 Gy in 1.8 to 2.0 Gy fractions. Retrospective analyses of CONV have shown a 5-year survival of 10 to 22% with 50 to 60% local control rates.[3–5]
Recent advances in imaging and radiation delivery techniques have allowed for the development of stereotactic body radiation therapy (SBRT). In the last ten years, SBRT has supplanted CONV for the treatment of early-stage NSCLC in inoperable patients at many institutions, including ours. This approach has been validated by several prospective single-arm studies, most notably the multi-center Radiation Therapy Oncology Group (RTOG) 0236 trial, which demonstrated significantly better local control compared to historical means, with rates of local control >90%.[6] However, there is limited data directly comparing outcomes and patterns of failure of CONV with SBRT.
At our institution, we have a longstanding experience with both CONV and SBRT. We spearheaded dose escalation using conventional fractionation prior to the development of SBRT, and over the past decade have treated a large patient population with a uniform approach to SBRT. Thus, we conducted a retrospective review of patients at our institution comparing patients treated for early-stage NSCLC with either CONV or SBRT. We hypothesized that SBRT would be associated with a significant improvement in local control and a possible benefit in overall survival.
Material and Methods
Study design and patients
We retrospectively identified all patients with early-stage NSCLC treated with RT and followed up at our institution from 1990 to 2013. Patient, treatment, and toxicity data was collected by review from the electronic medical records under a retrospective institutional review board waiver.
All tumors were biopsy-proven and histology verified. No patients received chemotherapy, surgery or radiofrequency ablation (RFA) as a part of their treatment for this primary NSCLC. Patients with metastatic lung lesions or recurrent NSCLC were excluded. We excluded treatments prior to 1990, as three-dimensional treatment planning was not routinely used. Patients who received sub-therapeutic doses, defined as ≤60 Gy in ≥30 fractions of CONV or ≤ 45 Gy in 5 fractions for SBRT, were excluded from this study.
Baseline patient variables included age at diagnosis, sex, Karnofsky performance status (KPS) at diagnosis, and smoking history. Tumor and treatment characteristics included T-stage at diagnosis, use of 18F-flourodeoxyglucose Positron Emission Tomography (18F-FDG PET) or invasive mediastinal staging, and histology.
Treatment characteristics
Our CONV technique has been previously described.[7,8] Briefly, patients were immobilized in a supine position in a customized mold with their arms raised above the head. All patients underwent CT simulation. PTV included the gross tumor volume (GTV) plus a 15–20mm standard margin. The RT dose was prescribed to the isodose line surrounding the PTV, and the doses were corrected for heterogeneity. Typically, at least 95% of the PTV received prescription dose and the PTV dose in these small tumors was quite uniform with less than 10–12% variation. IMRT use (using sliding window dynamic multileaf collimation) began in 2004. Thereafter, patients could be assigned to either IMRT or the previously described 3-dimensional conformal radiation therapy (3DCRT); about 40% received IMRT. Megavoltage linear accelerator x-rays of 6 MV or higher energy were used for treatment delivery. Beginning in 1992 lung complication probabilities (NTCP) were calculated using Lyman’s model, with the model parameters of Burman et al. and the NTCP was limited to ≤25%.[9–11] If NTCP exceeded 25%, the plan had to be altered to reduce it or the prescription dose would be reduced. PET for simulation and target delineation was used since 2006.
Our SBRT method has been previously described as well. [12,13] SBRT was first used in our institution in early 2006. Briefly, patients were immobilized the same way as for conventional RT and - since 2008 - underwent a 4D-CT simulation to determine the internal target volume (ITV). The ITV was expanded with a 2 to 3 mm margin for microscopic disease extension to create a clinical target volume (CTV), which was then expanded 5 mm to the planning target volume (PTV). Dose was prescribed to the 100% isodose line; plans were designed and normalized so this isodose surrounded the PTV and at least 95% of the PTV received prescription. Our SBRT dose distributions were designed to be quite uniform with less than 10–12% dose variation, similarly to the CONV distributions. V20 was constrained to ≤12% for the total lung and ≤25% for the ipsilateral lung. Treatment setup was verified using a cone-beam CT scan, and SBRT was delivered with coplanar IMRT beams (dynamic multileaf collimation). Tumors were typically treated in a risk-adapted approach with 9 to 10 Gy × 5 fractions (n=94) for tumors within 2 cm from the proximal bronchial tree, 12 Gy × 4 fractions (n=123) for tumors within 1 cm from the chest wall, and 18 to 20 Gy × 3 fractions (n=135) for all other peripherally located tumors, delivered every other day.
Follow up
Patients were typically followed with a clinic visit and chest CT every 3 months for the first two years. From year two to four, patients were seen every six months, and annually thereafter. Progression in the form of LF, NF, and DF was determined by reviewing all imaging studies and based on clinical exams subsequent to treatment of the primary. Suspicious CT findings were typically further evaluated with a PET scan. Patients with suspicious PET results were set up for a biopsy whenever clinically feasible.
Statistical methods and design
Patient characteristics were compared between the treatment groups using the chi-squared test for categorical variables and the Wilcoxon rank sum test for continuous variables. Competing risks analysis was performed for incidence of LF, NF, DF, and progression (whichever of LF, NF, or DF occurred first). The risk of each event was estimated using a cumulative incidence function that accounted for death without the event of interest. Patients who were still alive and did not experience the event of interest were censored at the date of last scan or evaluation. The Fine and Gray method was used for univariate and multivariate analyses. Overall survival (OS) was analyzed by the Kaplan-Meier method and Cox regression models for univariate and multivariate analyses, with patients who were still alive censored at the date of last documented survival. All endpoints were determined from the date of the last fraction of radiation therapy. Patient factors with p<0.10 in univariate analysis (UVA) were incorporated in a multivariate analysis (MVA) for each endpoint.
We also performed propensity score-matched analyses to reduce the selection bias related to nonrandomized treatment SBRT vs CONV. Propensity scores (PS) were computed as the conditional probability of SBRT vs CONV using a logistic regression model which included seven patient and clinical variables: age, gender, KPS, histology, T-stage, biologically equivalent dose (BED), and history of smoking. PS matched pairs were identified without replacement using a 1:1 nearest neighbor matching algorithm with caliper width equal to 0.15 and 0.10 based on lesion and patient data, respectively. These caliper widths were determined by decreasing the computed optimal caliper widths 0.67 and 0.67 (equals 0.2 of the standard deviation of the logit of the PS, recommended by Austin [14]) until the criteria of balance, that is absolute standardized mean difference (ASMD) between the two treatment groups <0.1, was satisfied for each of the variables included in the PS matching. After the matching procedure, the relationship between SBRT vs CONV group and LF, nodal failure, DF, disease progression was performed using Zhou, Fine, Labopin’s competing risks regression model for clustered data, and OS was performed using Cox proportional hazards regression model that stratified on the matched pairs.
Statistical significance for all analyses was 2-sided and used a 5% significance level (p<0.05). Statistical analyses were performed using R, version 3.0.1 (The R Project for Statistical Computing, Vienna, Austria) with the ‘survival’, ‘cmprsk’, ‘MatchIt’, and ‘crrSC’ packages.
Results
Patient characteristics
We identified 127 patients treated with conventionally fractionated radiation (CONV) and 370 patients (398 lesions) treated with SBRT that met our inclusion criteria. Baseline patient characteristics of both treatment groups are shown in Table 1. The median age was 76 years (range: 46 to 95), and the median KPS was 80% (range 40 to 100%). Most were former (79%) or current smokers (12%). The T-stage at diagnosis was T1 in 74% and T2 in 26%. Sixty-nine percent were adenocarcinoma, 25% squamous cell carcinoma, and 6% NSCLC, not otherwise specified (NOS). The SBRT group contained more T1 tumors (p<0.001), more former than current smokers (p=0.005), and more adenocarcinomas (p<0.001) than CONV. With regards to staging, the SBRT group was more likely to have undergone staging by FDG-PET, with 97% of patients staged by PET/CT in the SBRT group and 76% of patients staged by PET in the CONV group. However, the CONV group was more likely to be invasively staged by bronchoscopy or mediastinoscopy (46% vs 9% for SBRT). No significant difference in gender, KPS or age was noted between the groups. The median CONV dose delivered was 75.6 Gy in 1.80 Gy fractions (range 60 to 90 Gy; median BED = 89.20 Gy [α/β = 10]). The median SBRT dose delivered was 48 Gy in 4 fractions (range 45 to 60 Gy in 3 to 5 fractions; median BED = 105.60 Gy). The SBRT group, as expected, had a significantly higher mean BED (p<0.001) compared to CONV.
Table 1.
Patient characteristics
| Factor | Conventional, n=127 n (%) |
SBRT, n=398 n (%) |
p-value |
|---|---|---|---|
| Median Age (range) | 74 (46–93) | 77 (50–95) | 0.060 |
| Gender | 0.11 | ||
| Male | 69 (54) | 182 (46) | |
| Female | 58 (46) | 216 (54) | |
| KPS | 0.058 | ||
| < 80 | 47 (37) | 110 (28) | |
| ≥ 80 | 80 (63) | 288 (72) | |
| History of Smoking | 0.005 | ||
| Never | 10 (8) | 34 (9) | |
| Former | 91 (72) | 326 (82) | |
| Current | 26 (20) | 38 (10) | |
| T-Stage | < 0.001 | ||
| T1 | 66 (52) | 321 (81) | |
| T2 | 61 (48) | 77 (19) | |
| Histology | < 0.001 | ||
| Adenocarcinoma | 77 (61) | 283 (71) | |
| Squamous cell | 32 (25) | 101 (25) | |
| NSCLC NOS | 18 (14) | 14 (4) | |
| PET staging | < 0.0001 | ||
| Yes | 96 (76) | 388 (97) | |
| No | 31 (24) | 10 (3) | |
| Median Radiation dose (Gy, range) | 75.6 (60–90) | 48 (45–60) | |
| Median BED (Gy, range) | 89.2 (72.0–108.0) | 105.6 (85.5–180.0) | < 0.001 |
Outcomes
Median follow up for survivors was 101.1 months (range 6.9 to 148.5 months) and 23.3 months (range 2.2 to 75.2 months) for the CONV and SBRT group, respectively. 24 of 110 recurrences (21.8%) in the CONV group and 59 of 163 recurrences (36.2%) in the SBRT group were confirmed with a biopsy.
Local failure (LF)
The 3-year cumulative incidence of LF was 34.1% in the CONV group and 13.6% in the SBRT group (p<0.001). PET scan showing FDG-avidity at the treated site was obtained in 27 of 49 (CONV) and 37 of 41 (SBRT) local recurrences. A confirmation biopsy was obtained for nine of 49 (CONV) and 20 of 41 (SBRT) lesions. On univariate analysis, CONV, male gender, squamous histology, T2 stage, and BED were correlated with increased risk of LF. [Figure 1] On multivariate analysis, the significant local control benefit with SBRT persisted, as did the increased risk of LF with male gender, squamous cell histology, and T2 stage. [Table 2]
Figure 1.
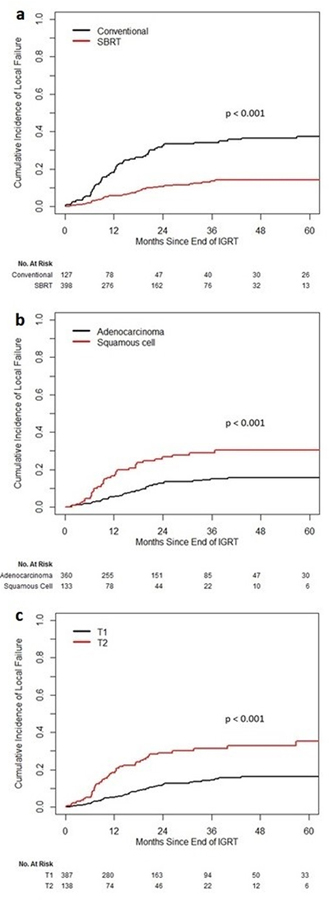
Cumulative Incidence of Local Failure a CONV vs. SBRT b adenocarcinoma vs. squamous cell c T1 vs. T2
Table 2.
UVA/MVA for Local Failure
| Factor | UVA | MVA | ||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | p-value | Hazard Ratio (95% Confidence Interval) | p-value | |
| SBRT vs CONV | 0.29 (0.19, 0.43) | < 0.001 | 0.42 (0.25, 0.70) | <0.001 |
| Gender (F vs M) | 0.59 (0.39, 0.90) | 0.014 | 0.63 (0.40, 0.997) | 0.049 |
| Histology (squamous vs. adeno, n=493) | 2.17 (1.40, 3.35) | < 0.001 | 2.24 (1.42, 3.55) | <0.001 |
| T-stage (T2 vs T1) | 2.58 (1.72, 3.88) | < 0.001 | 1.63 (1.02, 2.62) | 0.04 |
| BED (Gy, continuous) | 0.98 (0.97, 0.99) | < 0.001 | 0.993 (0.984, 1.002) | 0.14 |
| Age (continuous) | 1.01 (0.98, 1.03) | 0.63 | ||
| KPS (≥ 80 vs. < 80) | 1.22 (0.77, 1.93) | 0.39 | ||
| Smoking (former vs. never) | 0.66 (0.36, 1.24) | 0.20 | 0.63 (0.31, 1.27) | 0.20 |
| Smoking (current vs. never) | 0.44 (0.18, 1.09) | 0.076 | 0.45 (0.17, 1.19) | 0.11 |
Nodal failure (NF)
There was no significant difference between SBRT and CONV for nodal failure (3-year cumulative incidence: 17.8% and 14.4%, respectively; p=0.20 on multivariate analysis). Male gender (p=0.009) and T2-stage (p=0.012) were established as significant variables on multivariate analysis for increased nodal failure.
Distant failure (DF)
When comparing cumulative incidences there was no significant difference between CONV and SBRT in terms of risk for DF (p=0.20). T2 stage was the only variable significantly correlated with increased DF on univariate (p=0.002) and multivariate analysis (p=0.003). [Supplemental Material Figure S1] It should be noted that the failure pattern with SBRT shifted from LF to DF compared with CONV.
Disease progression
The 3-year cumulative incidence of disease progression was 48.1% in the CONV group compared to 33.8% in the SBRT group (UVA: p<0.001, MVA: p=0.021). [Supplemental Material Figure S2] On multivariate analysis, other factors significant for lower incidence of progression were current smokers vs. never smokers (p=0.05), adenocarcinoma histology (p=0.046) and T1-stage (p=0.002).
Overall survival (OS)
The 3-year OS rate was 38.9% in the CONV group and 53.1% in the SBRT group (p=0.018). Other factors significantly associated with longer OS included younger age, higher KPS, T1-stage, adenocarcinoma subtype, and BED. [Figure 2] On MVA, treatment with SBRT did not retain statistical significance as a predictor of longer OS (p=0.10). Younger age, higher KPS, adenocarcinoma subtype, and T1-stage remained significant for longer OS on MVA. [Table 3]
Figure 2.
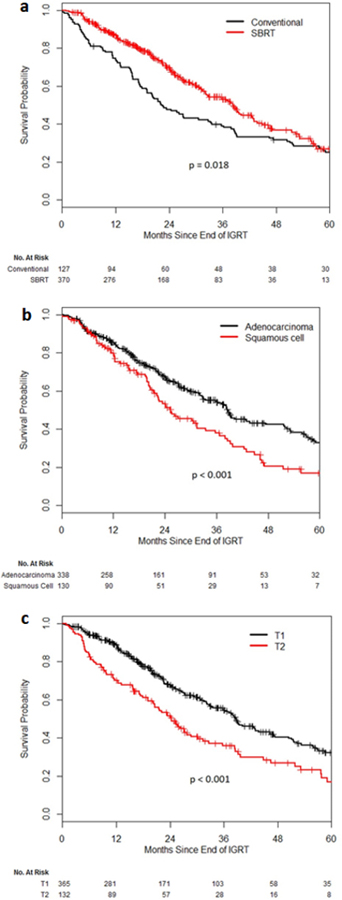
Overall Survival Probability a CONV vs. SBRT b adenocarcinoma vs. squamous cell c T1 vs. T2
Table 3.
UVA/MVA for Overall Survival
| Factor | UVA | MVA | ||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | p-value | Hazard Ratio (95% Confidence Interval) | p-value | |
| SBRT vs CONV | 0.73 (0.57, 0.95) | 0.018 | 0.77 (0.55, 1.06) | 0.10 |
| Age (continuous) | 1.03 (1.01, 1.04) | < 0.001 | 1.03 (1.02, 1.05) | <0.001 |
| KPS (≥ 80 vs. < 80) | 0.62 (0.49, 0.80) | < 0.001 | 0.66 (0.51, 0.85) | 0.002 |
| Histology (squamous vs. adeno, n=493) | 1.63 (1.25, 2.12) | < 0.001 | 1.58 (1.20, 2.07) | 0.001 |
| T-stage (T2 vs T1) | 1.80 (1.40, 2.31) | < 0.001 | 1.38 (1.03, 1.85) | 0.03 |
| BED (Gy, continuous) | 0.9959 (0.9922, 0.9996) | 0.028 | 0.999 (0.995, 1.004) | 0.70 |
| Smoking (former vs. never) | 1.12 (0.73, 1.71) | 0.61 | ||
| Smoking (current vs. never) | 0.89 (0.53, 1.49) | 0.65 | ||
| Gender (F vs M) | 1.06 (0.83, 1.35) | 0.63 | ||
Propensity score matching
PS matching was based on 493 lesions, excluding tumors whose histology was categorized as ‘NSCLC not otherwise specified’. For LF, NF, DF and progression, 94 matched pairs were identified. However, the criteria of balance could not be satisfied for sex (ASMD=0.127) and T stage (ASMD=0.108). No significant difference was shown for SBRT vs CONV for LF (hazard ratio (HR) 0.62, 95% confidence interval (CI) 0.36–1.08; p=0.09), NF (HR 1.59, 95% CI 0.75–3.4; p=0.23), DF (HR 0.88, 95% CI 0.44–1.76; p=0.73) or disease progression (HR 0.87, 95% CI 0.54–1.4; p=0.57). PS matching for OS resulted in 86 matched pairs and all ASMDs were less than 0.10, indicating a balance between both groups. The comparison showed a statistically significant improvement of OS with SBRT (HR 0.58, 95% CI 0.34–0.999; p=0.0487).
Discussion
The development of SBRT has significantly altered the management of inoperable early-stage NSCLC in the past decade. SBRT has been increasingly adopted as a new standard of care for inoperable tumors. Current literature mainly includes prospective single-arm phase I or II [6,15,16] or retrospective studies [13,17–20] with several ongoing prospective trials comparing CONV and SBRT for the treatment of early-stage NSCLC. So far, there is only one phase II study with complete results published.[21] Here we provide, to our knowledge, the largest single-institution cohort of patients with inoperable early-stage NSCLC treated in a uniform manner with either high-dose conventional RT or SBRT.
We found that SBRT was associated with a significant improvement in local control and OS in UVA although this significance could not be shown for OS in MVA. When applying propensity score matching to eliminate imbalances in patient and clinical variables between the two groups, we did show a significant improvement of OS with SBRT but no significant difference in LF. A population-based, propensity-matched analysis of SBRT in inoperable early-stage lung cancer during the early years of SBRT implementation (2003–2006) showed a similar 3-year OS benefit of 48% (SBRT) vs. 40% (CONV) (p=0.001).[22] Comparable results have been published by Jeppesen et al.[20] In their retrospective study of 132 early-stage NSCLC patients treated with SBRT (n=100) or CONV (n=32) between 1998 and 2012, median OS of 36.1 months (SBRT) vs. 24.4 months (CONV) (p=0.02) was reported with survival rates of 82% vs. 75% at one and 34% vs. 10% at five years, respectively. No significant difference was found in LF rates in their analysis.
In our study, T1 when compared to T2 stage was a positive predictor of outcome for all endpoints. Adenocarcinoma histology was similarly associated with lower rates of LF, disease progression and improved OS compared to squamous cell. This is in line with previous studies of SBRT and conventional RT which have identified T-stage and histology as a predictor of local control and/or OS.[16,19,23–25] Although we utilized multivariate analysis and PS matching to account for the influence of different prognostic factors, the SBRT group showed several favorable characteristics which contribute in part to the improved survival and disease control. Patients in the SBRT cohort were significantly more likely to have a T1 vs. T2 stage and adenocarcinoma vs. squamous cell histology.
Several interesting prospective trials comparing SBRT and CONV in early-stage NSCLC are currently ongoing or have recently been published. In the SPACE study (NCT01920789), a Phase II multi-center randomized trial in Sweden, 102 early-stage NSCLC patients were randomized to either conventionally fractionated radiation (2 Gy x 35 fractions, n=53) or SBRT (22 cGy x 3 fractions, n=49).[21] The investigators found no significant difference in progression-free survival or OS. SBRT produced slightly superior outcomes in regard to toxicity and quality of life. Although the SPACE study presents the first randomized comparison of SBRT and CONV, some limitations remain. The two study arms diverged in T-stage, gender and ECOG performance status. In addition, around one-third of patients (36%) did not have a histo-pathological verification for their NSCLC.
The CHISEL trial (NCT01014130), a phase III randomized trial by the Trans-Tasman Radiation Oncology Group (TROG) [26], has completed patient accrual, and first results have been presented in abstract form at the IASLC World Conference on Lung Cancer 2017. The target sample size was 100, and patients were randomized to arm 1: stereotactic ablative body radiotherapy (SABR, equivalent to SBRT) (54 Gy in 3 fractions or 48 Gy in 4 fractions) or arm 2: conventionally fractionated RT (66 Gy in 33 fractions or 50 Gy in 20 fractions) with the possibility of concurrent chemotherapy according to institutional practice at the treatment site. Eligibility criteria included histological or cytological disease confirmation, stage T1N0 or T2aN0 (PET), peripheral location of tumor (>2cm from bifurcation of lobar bronchi), and inoperability or refusal of surgery. SBRT resulted in longer freedom from LF (primary outcome), as well as higher OS compared to conventionally fractionated RT. SBRT did however result in a higher rate of toxicities with one patient reporting grade 4 dyspnea and nine patients reporting grade 3 toxicities versus two patients with grade 3 toxicities in the conventional RT arm. It will be interesting to see how the full results from the CHISEL trial compare to our study and the SPACE study. It will also be of interest how many patients will have received concurrent chemotherapy for node-negative NSCLC.
Another study, currently recruiting patients across 20 centers in Canada, is the Ontario Clinical Oncology Group’s (OCOG) LUSTRE trial (NCT01968941).[27,28] Estimated enrollment is 324 (2:1 ratio SBRT:CONV, start of enrollment 2014) and primary outcome is local control. Secondary outcomes include OS, disease-free survival, toxicity, quality of life and cost-utility.
Weaknesses of our analysis are consistent with the inherent limitations of retrospective studies, including imbalance of variables like T-stage and follow up time between the groups, as well as differences in margins, plan normalization, and image guidance. Additionally, considering that the SBRT cohort was treated more recently than most of the CONV patients, newer radiation and imaging techniques may have had an impact on the improved outcomes. Nevertheless, our large cohort of 497 patients (525 lesions) treated in uniform manner makes this study unique. Results from prospective studies should provide further insight into whether SBRT leads to a significant improvement of local control and OS, unbiased by medical and technological advances.
In summary, the results of our study support the use of SBRT as the definitive treatment of choice in stage I inoperable NSCLC. We confirmed the predictive value of T-stage and adenocarcinoma histology for increased survival and local control. Further results from randomized trials are expected to strengthen the evidence for definitive SBRT.
Supplementary Material
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
This work was presented as an oral presentation at the ASTRO 2014 Annual Meeting but has not been submitted for publication elsewhere.
Conflict of interest: None
References
- 1.The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raz DJ, Zell JA, Ou S-HI, et al. Natural History of Stage I Non-Small Cell Lung Cancer. Chest 2007;132:193–9. [DOI] [PubMed] [Google Scholar]
- 3.Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer 2005;103:2118–27. [DOI] [PubMed] [Google Scholar]
- 4.Dosoretz, Katin, Blitzer, et al. Medically Inoperable Lung Carcinoma: The Role of Radiation Therapy. Semin Radiat Oncol 1996;6:98–104. [DOI] [PubMed] [Google Scholar]
- 5.Kaskowitz L, Graham MV, Emami B, et al. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1993;27:517–23. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA 2010;303:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sim S, Rosenzweig KE, Schindelheim R, et al. Induction chemotherapy plus three-dimensional conformal radiation therapy in the definitive treatment of locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2001;51:660–5. [DOI] [PubMed] [Google Scholar]
- 8.Sura S, Yorke E, Jackson A, et al. High-Dose Radiotherapy for the Treatment of Inoperable Non-Small Cell Lung Cancer. The Cancer Journal 2007;13:238–42. [DOI] [PubMed] [Google Scholar]
- 9.Burman C, Kutcher GJ, Emami B, et al. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991;21:123–35. [DOI] [PubMed] [Google Scholar]
- 10.Kutcher GJ, Burman C, Brewster L, et al. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys 1991;21:137–46. [DOI] [PubMed] [Google Scholar]
- 11.Yorke ED, Jackson A, Rosenzweig KE, et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 2002;54:329–39. [DOI] [PubMed] [Google Scholar]
- 12.Spratt DE, Wu AJ, Adeseye V, et al. Recurrence Patterns and Second Primary Lung Cancers After Stereotactic Body Radiation Therapy for Early-Stage Non-Small-Cell Lung Cancer: Implications for Surveillance. Clinical lung cancer 2015;17:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuaron JJ, Yorke ED, Foster A, et al. Stereotactic Body Radiation Therapy for Primary Lung Cancers >3 Centimeters. J Thorac Oncol 2013;8:1396–401. [DOI] [PubMed] [Google Scholar]
- 14.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: Results of a prospective trial. Lung cancer (Amsterdam, Netherlands) 2010;68:72–7. [DOI] [PubMed] [Google Scholar]
- 16.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic Body Radiation Therapy for Early-Stage Non–Small-Cell Lung Carcinoma: Four-Year Results of a Prospective Phase II Study. Int J Radiat Oncol Biol Phys 2009;75:677–82. [DOI] [PubMed] [Google Scholar]
- 17.Marwaha G, Stephans KL, Woody NM, et al. Lung Stereotactic Body Radiation Therapy: Regional Nodal Failure Is Not Predicted by Tumor Size. J Thorac Oncol 2014;9:1693–7. [DOI] [PubMed] [Google Scholar]
- 18.Onishi H, Shirato H, Nagata Y, et al. Stereotactic Body Radiotherapy (SBRT) for Operable Stage I Non–Small-Cell Lung Cancer: Can SBRT Be Comparable to Surgery? Int J Radiat Oncol Biol Phys 2011;81:1352–8. [DOI] [PubMed] [Google Scholar]
- 19.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated Stereotactic Radiotherapy (HypoFXSRT) for Stage I Non-small Cell Lung Cancer: Updated Results of 257 Patients in a Japanese Multi-institutional Study. J Thorac Oncol 2007;2:S94–S100. [DOI] [PubMed] [Google Scholar]
- 20.Jeppesen SS, Schytte T, Jensen HR, et al. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: An updated retrospective study on local failure and survival rates. Acta Oncologica 2013;52:1552–8. [DOI] [PubMed] [Google Scholar]
- 21.Nyman J, Hallqvist A, Lund J-Å, et al. SPACE – A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121:1–8. [DOI] [PubMed] [Google Scholar]
- 22.Koshy M, Malik R, Mahmood U, et al. Stereotactic body radiotherapy and treatment at a high volume facility is associated with improved survival in patients with inoperable stage I non-small cell lung cancer. Radiother Oncol 2015;114:148–54. [DOI] [PubMed] [Google Scholar]
- 23.Woody NM, Stephans KL, Andrews M, et al. A Histologic Basis for the Efficacy of SBRT to the lung. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2017;12:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa H, Nakayama Y, Kitamoto Y, et al. Effect of Histologic Type on Recurrence Pattern in Radiation Therapy for Medically Inoperable Patients with Stage I Non-Small-Cell Lung Cancer. Lung 2006;184:347–53. [DOI] [PubMed] [Google Scholar]
- 25.Hörner-Rieber J, Bernhardt D, Dern J, et al. Histology of non-small cell lung cancer predicts the response to stereotactic body radiotherapy. Radiotherapy and Oncology 2017;125:317–24. [DOI] [PubMed] [Google Scholar]
- 26.Trans-Tasman Radiation Oncology Group (TROG). Hypofractionated Radiotherapy (Stereotactic) Versus Conventional Radiotherapy for Inoperable Early Stage I Non-Small Cell Lung Cancer (NSCLC): NCT01014130: Bethesda (MD): National Library of Medicine (US); 2009. http://clinicaltrials.gov/show/NCT01014130 [accessed May 17, 2017].
- 27.Ontario Clinical Oncology Group (OCOG). Stereotactic Body Radiotherapy Versus Conventional Radiotherapy in Medically-Inoperable Non-Small Lung Cancer Patients (LUSTRE): NCT01968941: Bethesda (MD): National Library of Medicine (US); 2014. https://clinicaltrials.gov/show/NCT01968941 [accessed May 17, 2017].
- 28.Swaminath A, Wierzbicki M, Parpia S, et al. Canadian Phase III Randomized Trial of Stereotactic Body Radiotherapy Versus Conventionally Hypofractionated Radiotherapy for Stage I, Medically Inoperable Non–Small-Cell Lung Cancer – Rationale and Protocol Design for the Ontario Clinical Oncology Group (OCOG)-LUSTRE Trial. Clinical lung cancer 2017;18:250–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


