Abstract
Sex differences in behavior—both sex-specific and shared behaviors—are fundamental to nearly all animal species. One often overlooked mechanism by which these behavioral differences can be generated is through sex-specific modulation of shared circuitry (i.e., circuits present in both sexes). In vertebrates this modulation is likely regulated by hormone-dependent mechanisms as well as by somatic sex itself; invertebrate models have particular promise for understanding the latter of these. Here we review molecular and behavioral evidence of sexual modulation of shared circuitry in the nematode C. elegans. Multiple behaviors in this species, both copulatory and not, are modulated by the genetic sex of shared neurons and circuit. These studies are close to uncovering the molecular mechanisms by which somatic sex modulates neural function in the worm, mechanisms which may be well conserved in more complex organisms. Improving our understanding of the modulation of neural circuit development and function by somatic sex may lend important insight into sex differences in the mammalian nervous system which, in turn, may have important implications for sex biases in disease.
1. Introduction
Sexual behaviors—and, by extension, sex-specific behaviors—are essential for survival across the animal kingdom. Through both natural and sexual selection, males and females of nearly all species have evolved distinct features in morphology and behavior [1]. In general, these sex differences can be thought of as either sexually dimorphic or sexually modulated. While a clear distinction between these classes is not always apparent, they provide a useful framework for thinking about the influence of sex on development and physiology.
Sexually dimorphic morphology, where each sex possesses structures that are absent in the other, is obvious in animal gonads and genitalia. In many species, other body parts also feature prominent sexual dimorphism. Similarly, sexually dimorphic behaviors are best known in courtship and copulation. In some cases, however, this assignment can be arbitrary or even misleading: for example, the mounting behavior exhibited by many male mammals is often considered a sex-specific behavior, though it can also be observed in females as a display of aggression or dominance. Regardless, the neural substrates for sexually dimorphic behavior are an area of intense current interest, and the relative contributions of shared vs. sex-specific neuroanatomy to these behaviors remains unclear.
In contrast to sexually dimorphic phenomena, sexually modulated features are morphological features and behavioral programs that appear in both sexes but that display differences in one or more aspects. For example, in many bird species, adults of both sexes produce song, but the characteristics of these vocalizations differ by sex. Similarly, the morphological or physiological features of structures present in both sexes may differ between males and females, illustrated in body size or pigmentation. Though just as pervasive as sexual dimorphism, sexual modulation has received relatively less attention. Understanding these processes holds great promise for revealing the regulatory mechanisms that link sex determination to sexual differentiation; moreover, it also has the potential to reveal key nodes in biological networks that can be “tuned” to give rise to adaptive variation in morphology and behavior.
The nematode C. elegans provides an ideal model in which to address the neural basis for sex differences in behavior, particularly with regard to the role of the modulation of non-sex-specific (“shared”) neural circuits. This species produces two sexes, male and hermaphrodite, the latter of which are somatic females that produce a limited number of self-sperm late in larval development. Thus, C. elegans hermaphrodites can produce both self-progeny and, upon fertilization by a male, cross-progeny. As such, selection is likely to favor distinct behavioral repertoires in hermaphrodites (which should favor the production of self-progeny) and in males (which need to locate and copulate with a mate in order to reproduce). Consistent with this, C. elegans adults exhibit numerous sex differences in behavior that are related both directly and indirectly to reproduction. Here, we review recent work to understand the means by which genetic sex (sometimes called “sex chromosome content”) modulates the development and function of shared neurons in service of these behaviors.
2. Sex differences in the C. elegans nervous system
A key advantage of C. elegans as an experimental model is the exceptional resolution with which its neuroanatomy is known. Like other sex differences, those in the C. elegans nervous system can be grouped into two types [2] (Fig. 1). The more obvious of these is in numbers and types of cells: each sex possesses its own set of sex-specific neurons. Hermaphrodites have eight sex-specific neurons, two HSNs and six VCs, that enable egg-laying behavior; these are absent in males. In contrast, males possess 87 sex-specific neurons, most of which are located in the tail [3]. These cells provide the bulk of the circuitry that subserves copulation, a multistep, male-specific behavior that is discussed elsewhere in this issue.
Figure 1. Sex differences in the C. elegans nervous system.
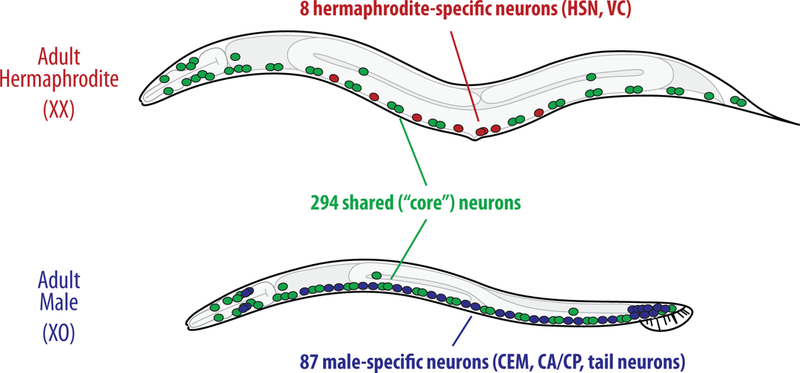
The C. elegans nervous system contains both shared and sex-specific neurons. The shared component comprises 294 neurons (represented in green) that are present in both sexes; these control behaviors that both sexes exhibit, including locomotion, chemosensation, and feeding. However, these neurons can be sexually modulated to produce subtle differences in these behaviors. In addition, each sex contains sex-specific neurons, which are present only in the hermaphrodite (red) or male (blue). Sex-specific neurons are important for sex-specific behaviors like egg laying and mating.
The second type of sex difference found in the C. elegans nervous system is more subtle, and has only recently been appreciated: neurons and circuits that are present in both sexes can differ functionally. This shared (or “core”) component of the nervous system comprises 294 neurons that are lineally identical between the sexes [2]. However, behavioral and gene-expression studies from multiple laboratories have demonstrated that shared neurons and circuits can have sex-specific properties, suggesting that biological sex plays a much broader role in the nervous system than had been previously thought.
3. Sex determination and sexual differentiation in C. elegans
Ultimately, all sex differences in biology arise from a primary sex-determining cue. In C. elegans, this is chromosomal status: hermaphrodite state is specified by two X chromosomes (XX), while males arise when only one is present (XO) [4,5]. This primary cue is “read” by autosomal and X-chromosome signal element genes that converge on xol-1, a regulator of both sex determination and dosage compensation [6,7]. Through a cryptic hedgehog-like pathway comprising her-1, tra-2 and the fem genes, xol-1 activity determines the state of the master regulator of C. elegans somatic sexual differentiation, tra-1 (Fig. 2). This gene’s active product, the transcription factor TRA-1A [8], is stabilized in XX animals, allowing it to promote hermaphrodite development and repress male development [9,10]. In XO animals, TRA-1A is degraded, allowing male development to occur. Importantly, tra-1 activity is both necessary and sufficient for all somatic sex differences. tra-1 XX loss-of-function mutants develop as fertile “pseudomales” that have a completely masculinized soma and a nearly normal male gonad [9]. In contrast, tra-1 XO gain-of-function mutants develop as somatic females [9]. Notably, the TRA-1A protein acts cell-autonomously to control sex differences in development, except in cases where it regulates sex-dependent signaling processes like vulval induction [9,11]. Although the genetic functions of tra-1 as a master sexual regulator are well-established, its expression pattern in the soma during development and in adulthood has not been carefully described, an important area for future work.
Figure 2. The somatic sex determination pathway.
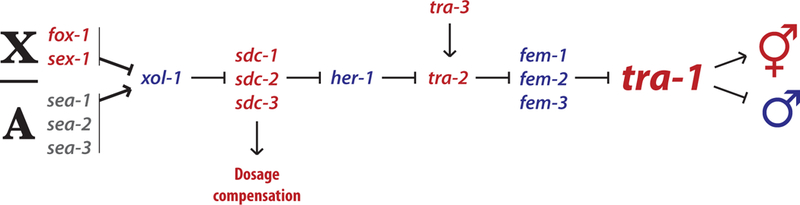
tra-1 is the master regulator of sexual state in C. elegans: high tra-1 activity confers hermaphrodite state while low activity confers male state. tra-1 activity is controlled through a multistep genetic hierarchy that is initiated by the ratio of X chromosomes to autosomes. By altering tra-1 activity in a tissue- or cell-type-specific manner, animals with mosaic sexual states can be generated. Genetic feminization can be achieved by increasing tra-1 activity in males by expressing constitutively active forms of tra-2 or tra-1. Conversely, masculinization can be brought about by expression of fem-3, which inhibits tra-1 function.
Downstream of tra-1 lie the developmental and physiological differences that characterize the male and hermaphrodite soma. Outside the nervous system, tra-1 directly regulates several transcription factors, particularly the doublesex-like genes mab-3 and dmd-3, to bring about sex differences in the intestine, tail tip, and elsewhere [12,13]. In the nervous system, one mechanism by which tra-1 establishes sexual dimorphism is through transcriptional regulation of cell death. Two direct tra-1 targets, egl-1 and ceh-30, control the sex-specific survival of the hermaphrodite-specific HSN neurons and the male-specific CEM neurons, respectively. egl-1 encodes a BH3-domain activator of the cell death pathway. In hermaphrodites, tra-1 acts through a cell-type-specific enhancer to repress egl-1 in the HSNs, allowing their survival; in males, tra-1 inactivity permits egl-1 expression, leading to their death [14]. This genetic logic is reversed in the case of the CEMs, where tra-1 represses ceh-30, a pro-survival homeodomain transcription factor, to cause cell death in hermaphrodites and survival in males [15,16]. In the case of other sex-specific neurons, tra-1 acts in more complex means to regulate the proliferation of neural precursors and specification of cell fate [17,18]. In these cases, the specific mechanisms by which tra-1 brings about sex differences in neuroanatomy remain unclear.
4. Sexual modulation of shared neurons and circuits
Multiple lines of evidence indicate that genetic sex has deep influences on the properties of nominally “shared” components of the nervous system. Several mechanisms can be imagined through which this could come about. First, sex differences in synaptic connectivity between shared neurons could alter the flow and processing of information between hermaphrodites and males. Indeed, recent analysis of neural connectivity in the male tail has identified several examples of this [3], pointing to interesting but unknown interactions between genetic sex and synaptic specification or stability. In contrast, though the detailed connectivity of the male head has not yet been reported, preliminary evidence suggests that synaptic sex differences in this region are relatively rare (S. Emmons and S. Cook, pers. comm.). One interpretation of this is that shared neurons may play important roles in copulatory behaviors by being incorporated into male-specific tail circuits, while sexually dimorphic connectivity may not have a major role in generating sex differences in head circuitry.
Second, sexually dimorphic morphology could be important for establishing sex differences in neural function. Ultrastructural studies have revealed several examples of this, including the tail PHC sensory neurons, which harbor striated rootlets in the male but not in the hermaphrodite [19], and the interneuron PDB, whose process exhibits elaborate male-specific branching [3]. The developmental underpinnings and functional significance of these morphological dimorphisms is not yet understood.
Finally, sex-specific neuromodulation of shared neurons and circuits could have important roles in regulating behavior. As they do in other systems, neuromodulators regulate behavioral states in C. elegans by altering neural excitability and modifying circuit composition. This effectively selects specific circuits to be activated while forcing others to remain latent [20]. Therefore, sex differences in neuromodulation could activate or inhibit shared circuitry to produce sexually dimorphic behaviors. Sex differences in neuromodulation could arise from cell-autonomous regulation by tra-1 of, for example, intracellular signaling components, sensory receptors, or receptors for neuromodulators themselves. Alternatively, tra-1 could regulate the production of neuromodulators, thereby having non-cell-autonomous effects on neurons that receive these signals. Indeed, neuromodulators are known to have important roles in sex-specific behaviors—e.g., serotonin in hermaphrodite egg-laying and PDF neuropeptide signaling in male mate-searching [21,22]. Moreover, many sex-specific neurons produce modulatory cues, including monoamines and neuropeptides [23,24]. However, the role of sexually dimorphic neuromodulation in regulating shared circuits, particularly in the head, remains unclear.
4.1. Sexual modulation of neural circuitry: molecular evidence
Although focused, systematic studies have yet to be undertaken, several examples of sexually dimorphic gene expression in shared C. elegans neurons have been identified. In most of these cases, the functional significance of these modifications is unknown; however, the observation that they emerge only upon adulthood suggests that they may have important roles in adult-specific sexual behavior.
By examining the expression of reporter genes, at least four examples of sexually dimorphic gene expression in shared neurons have been identified (Table 1A). The doublesex-family transcription factor gene mab-3 exhibits male-specific expression in the sensory neuron ADF [12], as does the candidate chemoreceptor srd-1 [25]. A second candidate chemoreceptor gene, srj-54, is male-specifically expressed in the AIM interneurons [26]; however, as these cells are not exposed to the environment, the product of srj-54 may function not as a classical chemoreceptor but as a receptor for a neuromodulatory signal. A third chemoreceptor, the diacetyl receptor odr-10, shows sexually modulated expression in the AWA olfactory neurons, where it modulates food detection (see section 4.2.2.) (Ryan et al., submitted). Interestingly, mab-3 is a direct target of tra-1 in the intestine, where it has an important role in repressing yolk expression [12]. Thus, its male-specific expression in ADF might be important for maintaining a male-like gene expression profile in this neuron; however, mab-3 does not appear to regulate srd-1 in this cell [12].
Table 1.
Genetic and functional sex differences in non-sex-specific C. elegans neurons
| A. Sexually dimorphic gene expression in shared neurons | ||
|---|---|---|
| Gene | Site of sexually dimorphic expression |
Description |
| odr-10 | AWA | High expression in adult hermaphrodites; low in adult males * |
| srj-54 | AIM | Male-specific expression in adults [26] |
| mab-3 | ADF | Male-specific expression [12] |
| srd-1 | ADF | Male-specific expression [25] |
| B. Behaviors controlled by sex-specific modulation of shared neurons/circuits | ||
|---|---|---|
| Sexually modulated behavior |
Site of sexual modulation | Evidence |
| Diacetyl attraction/Food-seeking | AWA | Genetic sex-reversal of AWA * |
| Ascaroside attraction | ASK, ADL and others | Laser ablation [41,44] and genetic sex-reversal ** |
| Non-ascaroside attraction | AWA, AWC and many additional candidates | Laser ablation and genetic sex reversal [46] |
| Locomotion | Sensory neurons | Genetic sex-reversal [34] |
| Sex-specific behavior | ||
| Male Mating | Sensory neurons | Genetic sex-reversal *** |
| Male Food-leaving | Sensory neurons | Genetic sex-reversal *** |
| Hermaphrodite Egg Laying | Sensory neurons | Genetic sex-reversal *** |
Ryan et al., submitted
K.A.F. and D.S.P., unpublished data
Renee M. Miller and D.S.P., unpublished data
In addition to these gene-by-gene approaches, microarray and deep RNA sequencing studies have also been undertaken to characterize sex differences in gene expression [27–30](K.A.F. and D.S.P., unpublished). Using total worm RNA, these studies have identified numerous genes with differential expression between males and hermaphrodites. However, many or even most of the genes identified using these approaches may be expressed in sex-specific somatic (or in some cases, germline) tissues. More focused studies—in particular, those using methods to specifically isolate RNA from the nervous system or even from specific neuron types—will be necessary to detect sex differences in genes that are only expressed in a handful of neurons.
These concerns aside, genome-wide approaches have identified members of three neuropeptide families—insulin-like peptides, FMRFamide-related peptides, and the neuropeptide-like proteins—as having sexually dimorphic expression. Some of these genes are known to be expressed in sex-specific neuron types [23], but recent evidence suggests that some insulin-like peptides may show sex differences in expression in shared circuitry [31]. Further studies of the expression patterns and functions of these modulators could reveal important roles for peptidergic signaling in generating sex differences in behavior.
4.2. Sexual modulation of neural circuitry: behavior
In contrast to the “bottom-up” approach of characterizing sex-dependent gene-expression differences in shared neurons, “top-down” studies have focused on the role of shared circuits in generating sex differences in behavior. These have identified sex differences in a variety of behaviors, including chemosensory and locomotor behaviors (Table 1B). They have also provided evidence that shared neurons and circuits have key roles in sex-specific reproductive behaviors.
An approach that has proven particularly powerful in these studies takes advantage of the ability to manipulate tra-1 function in a cell-type-specific manner. By expressing the tra-1 inhibitor fem-3 under the control of cell-type-specific promoters, particular cell types can be genetically “masculinized” [26,32]. Conversely, cell-type-specific expression of a tra-1(gf) allele is able to genetically “feminize” cells [33]; expression of a constitutively active form of tra-2 called tra-2(IC) has similar effects [34,35] (Fig. 2). By driving the expression of these sex-reversal transgenes with promoters that are active only postmitotically, it is possible to flip the sexual “state” of a cell without altering the cell lineages that give rise to sex-specific neurons.
4.2.1. Odortaxis
Chemosensation plays a critical role in the life of a worm. C. elegans relies on chemosensory cues to identify food, avoid toxins and predators, sense developmental cues, and mate [36]. Much of the worm’s chemosensory apparatus is in the head and, with the exception of the male-specific CEM (cephalic companion) sensory neurons, is sexually isomorphic. Nevertheless, studies have revealed that these shared structures differ functionally by sex. Earlier work from our group identified sex differences in olfactory function, such that hermaphrodites are more attracted to some canonical odorants (particularly diacetyl and benzaldehyde), while males may be more attracted to others (e.g., pyrazine) [26]. Using cell-type-specific genetic masculinization, we found that the genetic sex of sensory neurons themselves, rather than of downstream circuit components, was critical for these sex differences [26].
Subsequent work has demonstrated that a single olfactory neuron, AWA, is an important focus of sexual modulation. Specific genetic sex-reversal of this cell is sufficient to “flip” sex-typical patterns of diacetyl-pyrazine preference in both sexes. Moreover, these studies have found that the sex difference in diacetyl attraction results from sex-specific regulation of the diacetyl receptor odr-10: hermaphrodites express high levels of this receptor, while expression is low or even absent in males (Ryan et al., submitted). Though this regulation depends on the cell-autonomous action of tra-1, the mechanism(s) whereby tra-1 modulates odr-10 expression remains unknown.
4.2.2. Exploration and food-seeking
Sex differences also exist in exploratory and food-seeking behavior. When individual animals are placed on a small patch of food, hermaphrodites will remain on the patch to feed while males will leave to explore their surroundings. The observation that the presence of a mate will efficiently inhibit male exploration indicates that it reflects a sexually motivated mate-searching drive [37]. Interestingly, males will not explore if they are food-deprived, indicating the existence of active mechanisms that prioritize these behaviors by sex and feeding state. Male exploration is promoted by male-specific ray neurons and PDF neuropeptide signaling, as discussed elsewhere in this issue [21,38].
In addition to these inputs from male-specific neurons, recent work has indicated that the sexual state of the shared chemosensory neuron AWA is important for setting the relative priority of exploratory and feeding behaviors (Ryan et al., submitted). Interestingly, these studies have indicated that odr-10, whose expression is significantly lower in males than in hermaphrodites, is important for efficient food detection. When odr-10 levels are low, males are less attracted to food and instead tend to explore their environment. Transient food-deprivation upregulates odr-10 expression in AWA to increase food detection, accounting for the temporary suppression of exploratory behavior upon starvation. This dynamic regulation of odr-10, and the ability to switch between feeding-oriented and mating-oriented states, is critical for male reproductive fitness: well-fed males that are unable to repress odr-10 are less efficient at finding mates, reducing their reproductive success (Ryan et al., submitted).
4.2.3. Response to sex pheromones
Aside from copulatory behavior perse, one of the most sexually dimorphic behaviors in C. elegans is its response to pheromones called ascarosides, glycosides of the dideoxysugar ascarylose. Both sexes secrete a number of ascaroside variants that vary by sex, developmental stage and environmental condition [39–43]. These molecules have been proposed to serve as a modular chemical “language” that animals use to exchange information about population density, nutritional conditions and the presence of toxins, pathogens and predators [40]. One important function of certain ascarosides—particularly ascr#2 (also called “C6”), ascr#3 (“C9”) and ascr#8—is that they are potent, synergistic, sex-specific attractors of males and are produced preferentially by hermaphrodites [39,41]. Hermaphrodites are weakly repelled by this same mixture; thus, these compounds are considered sex pheromones [41].
Recent work from several groups has begun to shed light on the mechanisms that regulate ascaroside attraction in C. elegans males. Interestingly, ascarosides are sensed by male-specific head sensory neurons (the CEMs) as well as non-sex-specific “shared” head sensory neurons [41,44]. Removal of the CEM neurons from males still leaves substantial pheromone attraction intact [41], indicating that the sexual state of shared circuitry is likely to be important for making pheromone response sexually dimorphic. Preliminary studies from our group using genetic sex-reversal approaches have confirmed this idea, highlighting roles for sex differences in sensory function (K.A.F. and D.S.P., unpublished). Ablation studies have shown that the sensory neurons ASK are important for pheromone attraction in males [41], and that the amphid neuron ADL, which may have a role in ascr#3 repulsion in hermaphrodites, is less active in males [44]. One interesting possibility is that sex differences in neuromodulatory signaling impinge on sensory or interneurons in the pheromone circuit to differentially regulate its activity. Along these lines, reducing the activity of the neuropeptide receptor NPR-1 can bring about some ascr#3 attraction in hermaphrodites [45]. However, male pheromone attraction does not result from sex differences in NPR-1 signaling [44], indicating that biological sex uses other unknown mechanisms to tune neural circuit function. Understanding these mechanisms is an important goal for further studies.
C. elegans hermaphrodites also secrete non-ascaroside compounds that attract males, the response to which also depends on the sexual state of shared circuitry [32,46]. The molecular identity of these non-ascaroside pheromones is unknown, and the circuitry that controls the responses that they elicit seems to overlap only partially with the ascaroside response circuitry. Genetic masculinization of the hermaphrodite nervous system is sufficient to confer attraction to these cues, indicating a central role for the sex-specific modulation of shared neurons [32]. Interestingly, recent work has indicated that TGFß signaling plays an important role in generating sex-specific attraction to these compounds [46].
Hermaphrodite-derived pheromones are also important for sex-specifically modulating olfactory plasticity [33]. Although salt is normally a moderate attractant to C. elegans, pairing salt exposure with starvation reverses the valence of this cue, making it a repellant [47]. This “salt chemotaxis learning” occurs in both males and hermaphrodites. However, if males are salt-conditioned under starvation conditions but in the presence of other hermaphrodites, males will remain attracted to salt. Males require both a hermaphrodite-specific contact cue as well as hermaphrodite-secreted pheromones to block the induction of salt aversion. Interestingly, genetic feminization of the nervous system is sufficient to restore salt avoidance in males, indicating an important role for the genetic sex of shared neurons [33]. However, masculinizing the hermaphrodite nervous system is not sufficient to prevent salt avoidance in the presence of other hermaphrodites, suggests that both male-specific and shared circuitry are important for male olfactory plasticity. While the specific focus of genetic sex in the salt chemotaxis learning has yet to be identified, this work demonstrates an important role for sexual modulation of neural function that allows males to use past mating experiences to tune their olfactory system to promote reproductive success.
4.2.4. Motor behavior
C. elegans adults also show sex differences in locomotion. Specifically, adult males move at a higher velocity and with a higher amplitude compared to adult hermaphrodites [37,48]. These differences are thought to contribute to increased exploratory activity, thereby promoting males’ ability to locate mates. Recent work on the mechanisms underlying this have shown that the higher body bend frequency of males and differences in the shape of male and hermaphrodite body waves contribute to the increased speed observed in males [34]. Furthermore, genetic sex-reversal experiments revealed an important role for the sexual state of the shared nervous system in generating the sex difference in body bend frequency. Surprisingly, sensory neurons, rather than command interneurons or motor neurons, appear to be an important site through which genetic sex acts to modulate locomotion [34]. Though the means through which this modulation occurs is unknown, there is precedent for sensory regulation of locomotor frequency through the mechanical sensing of food [49] and it is likely that this extends to other stimuli. Further work should help reveal the specific modulatory mechanisms that are targeted by sex to alter body-bend frequency.
In contrast to body-bend frequency, other aspects of locomotor behavior, particularly body wave geometry, are not primarily determined by the sexual state of the nervous system. Interestingly, sex-specific properties of muscle appear to have important roles in modulating wave propagation, indicating that distributed sexual modifications act to coordinate sex-typical locomotion [34].
4.2.5. The role of shared circuitry in reproductive behaviors
A variety of studies have shown that the circuits for sex-specific reproductive behaviors comprise shared as well as sex-specific neurons. This appears to be especially true in males, as sex-specific circuitry is well connected to shared circuitry [3]. One way this recruitment occurs is through modulation of shared behavioral programs by sex-specific circuitry to increase mating efficiency. For example, male-specific circuitry in the tail can modulate general locomotion programs to generate reversals when required during mating [50]. This is achieved through male-specific cholinergic innervation of AVA, a shared command interneuron that plays a major role in switching from forward to backward locomotion. Conversely, shared circuitry can also modulate activity in sex-specific circuitry to ensure proper execution of reproductive behavior. For instance, shared touch receptor neurons release neuropeptides that likely act on downstream male-specific circuitry to prevent repetitive turning behavior during mating [51]. Furthermore, unpublished work from our group has found that genetic sex-reversal of shared circuitry compromises male mating, mate-searching, and hermaphrodite egg-laying behaviors (R.M. Miller and D.S.P., unpublished data). Together, these data suggest that the sexual properties of recruited shared circuitry are important for sex-specific reproductive behaviors.
5. Outlook
In most species, the extent to which neural circuits differ anatomically (i.e., the extent to which they are sexually dimorphic) is not known, though it is thought that these differences are generally subtle and highly specific. Similarly, the degree to which shared circuits are differentially modulated by sex is unclear. Studies of the sexual modulation of gene expression and behavior in C. elegans have highlighted an important, active role for shared neurons and circuits. One recurring theme of these studies is that sensory function itself is highly sexually modulated: the importance of this can be seen in odortaxis and food sensation (e.g., regulation of odr-10 in AWA), in pheromone response (e.g., sex differences in the pheromone-response properties of ADL), and in locomotion (the importance of the sexual state of sensory neurons in setting body-bend frequency). Interestingly, this parallels a number of recent studies from other systems in which modulated sensory function has been shown to be an important determinant of behavioral plasticity [52]. Understanding the mechanisms through which sensory function is sexually modulated, and its significance for generating adaptive sex differences in behavior, will be important areas for future work.
One aspect that has not yet been addressed concerns the developmental point at which tra-1 activity establishes sex differences in shared circuitry. Interestingly, while some sex differences (e.g. the survival of sex-specific neurons) appear in the embryo, others do not become apparent until adulthood (e.g. odr-10 and srj-54 expression, pheromone attraction and locomotion). Furthermore, it is largely unknown where tra-1 is expressed and whether its activity is constant throughout development and adulthood. One possibility is that tra-1 functions only during early development to establish sex differences that then later become activated. Another is that tra-1 is dynamically regulated in each cell depending on developmental stage. In both instances, convergence with other developmental signals could be important for activating sex differences in older animals or regulating tra-1. These issues warrant further investigation.
In more complex animals, sex differences in the nervous system have important roles in development and behavior; in humans, such differences are likely to be related to sex differences in susceptibility to a variety of neurological and neuropsychiatric disorders. While a model based on the organizational and activational functions of gonadal steroids has guided the field for many years [53], numerous recent studies have shown that the genetic sex of the nervous system itself is a key determinant of some sex differences in development and physiology. As a result, a more nuanced view has emerged, in which multiple cues from the gonad and nervous system itself interact to specify sex differences and also to compensate for their undesired effects [54,55]. Importantly, however, the molecular mechanisms through which somatic sex modulates neural development and function are almost completely unknown. Genetically tractable invertebrate models are likely to be able to make important contributions to this. Although upstream sex-determination mechanisms vary widely between species, downstream regulatory processes that connect the output of sex determination to the modulation of developmental and physiological processes may be conserved [56]. Because studies in C. elegans are now approaching complete descriptions of the mechanisms that link genetic sex to modulation of neural function and behavior, they may shed important light onto similar processes that operate in much more complex systems.
Acknowledgments
We thank members of the C. elegans research community and of the Portman laboratory for ongoing discussion and intellectual exchange, and are particularly grateful to S. Cook and S. Emmons (Albert Einstein College of Medicine) for sharing data on male head connectivity prior to publication. We thank Deborah A. Ryan and Renee R. Miller for their critical review of this manuscript. Current work in the Portman laboratory is supported by NIH grants F31 NS086283 (K.A.F.) and R21 NS082849 (D.S.P.).
References
- 1.Lande R (1980) Sexual Dimorphism, Sexual Selection, and Adaptation in Polygenic Characters. Evolution (N Y) 34: 292–305. doi: 10.2307/2407393. [DOI] [PubMed] [Google Scholar]
- 2.Portman DS (2007) Genetic control of sex differences in C. elegans neurobiology and behavior. Adv Genet 59: 1–37. doi: 10.1016/S0065-2660(07)59001-2. [DOI] [PubMed] [Google Scholar]
- 3.Jarrell TA, Wang Y, Bloniarz AE, Brittin CA, Xu M, et al. (2012) The connectome of a decision-making neural network. Science 337: 437–444. doi: 10.1126/science.1221762. [DOI] [PubMed] [Google Scholar]
- 4.Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nigon V, Dougherty EC (1949) Reproductive patterns and attempts at reciprocal crossing of Rhabditis elegans maupas, 1900, and Rhabditis briggsae Dougherty and nigon, 1949 (Nematoda: Rhabditidae). J Exp Zool 112: 485–503. doi: 10.1002/jez.1401120307. [DOI] [PubMed] [Google Scholar]
- 6.Meyer BJ (2005) X-Chromosome dosage compensation. WormBook: 1–14. doi: 10.1895/wormbook.1.8.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarkower D (2006) Somatic sex determination. WormBook: 1–12. doi: 10.1895/wormbook.1.84.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarkower D, Hodgkin J (1992) Molecular analysis of the C. elegans sex-determining gene tra-1: a gene encoding two zinc finger proteins. Cell 70: 237–249. [DOI] [PubMed] [Google Scholar]
- 9.Hodgkin J (1987) A genetic analysis of the sex-determining gene, tra-1, in the nematode Caenorhabditis elegans. Genes Dev 1: 731–745. doi: 10.1101/gad.1.7.731. [DOI] [PubMed] [Google Scholar]
- 10.Schvarzstein M, Spence AM (2006) The C. elegans sex-determining GLI protein TRA-1A is regulated by sex-specific proteolysis. Dev Cell 11: 733–740. doi: 10.1016/j.devcel.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Hunter C, Wood W (1990) The tra-1 gene determines sexual phenotype cell-autonomously in C. elegans. Cell 63: 1193–1204. [DOI] [PubMed] [Google Scholar]
- 12.Yi W, Ross JM, Zarkower D (2000) Mab-3 is a direct tra-1 target gene regulating diverse aspects of C. elegans male sexual development and behavior. Development 127: 4469–4480. [DOI] [PubMed] [Google Scholar]
- 13.Mason DA, Rabinowitz JS, Portman DS (2008) dmd-3, a doublesex-related gene regulated by tra-1, governs sex-specific morphogenesis in C. elegans. Development 135: 2373–2382. doi: 10.1242/dev.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conradt B, Horvitz HR (1999) The TRA-1A sex determination protein of C. elegans regulates sexually dimorphic cell deaths by repressing the egl-1 cell death activator gene. Cell 98: 317–327. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz H, Horvitz H (2007) The C. elegans protein CEH-30 protects male-specific neurons from apoptosis independently of the Bcl-2 homolog CED-9. Genes Dev: 3181–3194. doi: 10.1101/gad.1607007.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peden E, Kimberly E, Gengyo-Ando K, Mitani S, Xue D (2007) Control of sex-specific apoptosis in C. elegans by the BarH homeodomain protein CEH-30 and the transcriptional repressor UNC-37/Groucho. Genes Dev 21: 3195–3207. doi: 10.1101/gad.1607807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross JM, Kalis AK, Murphy MW, Zarkower D (2005) The DM domain protein MAB-3 promotes sex-specific neurogenesis in C. elegans by regulating bHLH proteins. Dev Cell 8: 881–892. doi: 10.1016/j.devcel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Kalis AK, Kissiov DU, Kolenbrander ES, Palchick Z, Raghavan S, et al. (2014) Patterning of sexually dimorphic neurogenesis in the caenorhabditis elegans ventral cord by Hox and TALE homeodomain transcription factors. Dev Dyn 243: 159–171. doi: 10.1002/dvdy.24064. [DOI] [PubMed] [Google Scholar]
- 19.Sulston JE, Albertson DG, Thomson JN (1980) The Caenorhabditis eiegans Male : Postembryonic Nongonadal Structures Development of. 576: 542–576. [DOI] [PubMed] [Google Scholar]
- 20.Bargmann CI (2012) Beyond the connectome: how neuromodulators shape neural circuits. Bioessays 34: 458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 21.Barrios A, Ghosh R, Fang C, Emmons SW, Barr MM (2012) PDF-1 neuropeptide signaling modulates a neural circuit for mate-searching behavior in C. elegans. Nat Neurosci 15. doi: 10.1038/nn.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD (1982) Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216: 1012–1014. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Kim K (2008) Neuropeptides. WormBook: 1–36. doi: 10.1895/wormbook.1.142.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sulston J, Dew M, Brenner S (1975) Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 163: 215–226. doi: 10.1002/cne.901630207. [DOI] [PubMed] [Google Scholar]
- 25.Troemel ER, Chou JH, Dwyer ND, Colbert H, Bargmann CI (1995) Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218. [DOI] [PubMed] [Google Scholar]
- 26.Lee K, Portman DS (2007) Neural sex modifies the function of a C. elegans sensory circuit. Curr Biol 17: 1858–1863. doi: 10.1016/j.cub.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Jiang M, Ryu J, Kiraly M, Duke K, Reinke V, et al. (2001) Genome-wide analysis of developmental and sex-regulated gene expression profiles in Caenorhabditis elegans. Proc Natl Acad Sci U S A 98: 218–223. doi: 10.1073/pnas.011520898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoemke K, Yi W, Ross JM, Kim S, Reinke V, et al. (2005) Genome-wide analysis of sex-enriched gene expression during C. elegans larval development. Dev Biol 284: 500–508. doi: 10.1016/j.ydbio.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Thomas CG, Li R, Smith HE, Woodruff GC, Oliver B, et al. (2012) Simplification and desexualization of gene expression in self-fertile nematodes. Curr Biol 22: 2167–2172. doi: 10.1016/j.cub.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinke V, Gil IS, Ward S, Kazmer K (2004) Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 131: 311–323. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- 31.Ritter AD, Shen Y, Bass JF, Jeyaraj S, Deplancke B, et al. (2013) Complex expression dynamics and robustness in C. elegans insulin networks: 1–12. doi: 10.1101/gr.150466.112.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White JQ, Nicholas TJ, Gritton J, Truong L, Davidson ER, et al. (2007) The sensory circuitry for sexual attraction in C. elegans males. Curr Biol 17: 1847–1857. doi: 10.1016/j.cub.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Sakai N, Iwata R, Yokoi S, Butcher RA, Clardy J, et al. (2013) A sexually conditioned switch of chemosensory behavior in C. elegans. PLoS One 8: e68676. doi: 10.1371/journal.pone.0068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mowrey WR, Bennett JR, Portman DS (2014) Distributed Effects of Biological Sex Define Sex-Typical Motor Behavior in Caenorhabditis elegans. J Neurosci 34: 1579–1591. doi: 10.1523/JNEUROSCI.4352-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lum D, Kuwabara PE, Zarkower D, Spence AM (2000) Direct protein–protein interaction between the intracellular domain of TRA-2 and the transcription factor TRA-1A modulates feminizing activity in C. elegans. Genes Dev 14: 3153–3165. [PMC free article] [PubMed] [Google Scholar]
- 36.Bargmann CI (2006) Chemosensation in C. elegans. WormBook: 1–29. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipton J, Kleemann G, Ghosh R, Lints R, Emmons SW (2004) Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. J Neurosci 24: 7427–7434. doi: 10.1523/JNEUROSCI.1746-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrios A, Nurrish S, Emmons SW (2008) Sensory regulation of C. elegans male mate-searching behavior. Curr Biol 18: 1865–1871. doi: 10.1016/j.cub.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izrayelit Y, Srinivasan J, Campbell SL, Jo Y, von Reuss SH, et al. (2012) Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem Biol 7: 1321–1325. doi: 10.1021/cb300169c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasan J, von Reuss SH, Bose N, Zaslaver A, Mahanti P, et al. (2012) A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol 10: e1001237. doi: 10.1371/journal.pbio.1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, et al. (2008) A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 454: 1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butcher RA, Fujita M, Schroeder FC, Clardy J (2007) Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol 3: 420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 43.Artyukhin AB, Yim JJ, Srinivasan J, Izrayelit Y, Bose N, et al. (2013) Succinylated octopamine ascarosides and a new pathway of biogenic amine metabolism in Caenorhabditis elegans. J Biol Chem 288: 18778–18783. doi: 10.1074/jbc.C113.477000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jang H, Kim K, Neal SJ, Macosko E, Kim D, et al. (2012) Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans. Neuron 75: 585–592. doi: 10.1016/j.neuron.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, et al. (2009) A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458: 1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White JQ, Jorgensen EM (2012) Sensation in a single neuron pair represses male behavior in hermaphrodites. Neuron 75: 593–600. doi: 10.1016/j.neuron.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saeki S, Yamamoto M, Iino Y (2001) Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol 204: 1757–1764. [DOI] [PubMed] [Google Scholar]
- 48.Hodgkin J (1974) Genetic and anatomical aspects of the Caenorhabditis elegans male PhD thesis, Univ Cambridge. [Google Scholar]
- 49.Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631. [DOI] [PubMed] [Google Scholar]
- 50.Sherlekar AL, Janssen A, Siehr MS, Koo PK, Caflisch L, et al. (2013) The C. elegans male exercises directional control during mating through cholinergic regulation of sex-shared command interneurons. PLoS One 8: e60597. doi: 10.1371/journal.pone.0060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu T, Kim K, Li C, Barr MM (2007) FMRFamide-like neuropeptides and mechanosensory touch receptor neurons regulate male sexual turning behavior in Caenorhabditis elegans. J Neurosci 27: 7174–7182. doi: 10.1523/JNEUROSCI.1405-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sengupta P (2012) The belly rules the nose: feeding state-dependent modulation of peripheral chemosensory responses. Curr Opin Neurobiol: 1–8. doi: 10.1016/j.conb.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breedlove SM, Cooke BM, Jordan CL (1999) The orthodox view of brain sexual differentiation. Brain Behav Evol 54: 8–14. doi:6607. [DOI] [PubMed] [Google Scholar]
- 54.McCarthy MM, Arnold AP (2011) Reframing sexual differentiation of the brain. Nat Neurosci 14: 677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ (2012) Sex differences in the brain: the not so inconvenient truth. J Neurosci 32: 2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkins AS (1995) Moving up the hierarchy: a hypothesis on the evolution of a genetic sex determination pathway. Bioessays 17: 71–77. doi: 10.1002/bies.950170113. [DOI] [PubMed] [Google Scholar]


