Abstract
Excessive accumulation of misfolded proteins was recently demonstrated in preeclampsia (PE). We examined levels and activity of circulatory proteasome and immunoproteasome (inflammatory subtype) in PE and HELLP syndrome. We analyzed samples from women with hypertensive pregnancy disorders (n=115), including PE with severe features and HELLP syndrome, and normotensive controls (n=45). Plasma proteasome and immunoproteasome immunoreactivity were determined by quantifying the α-subunit of the 20S core and β5i (LMP7), respectively. Plasma proteasome activity was analyzed with fluorogenic substrates. MG132, lactacystin, and ONX0914 were used to inhibit the circulating proteasome and immunoproteasome, respectively. Plasma cytokine profiles were evaluated by multiplex immunoassay. Placental expression of β5 (constitutive proteasome) and β5i (immunoproteasome) was interrogated by immunohistochemistry. Women with PE with severe features (sPE) had increased plasma 20S levels (P<0.001) and elevated lytic activities (chymotrypsin-like 7-fold; caspase-like 4.2-fold; trypsin-like 2.2-fold, P<0.001 for all) compared to pregnant controls. Women with features of HELLP displayed the highest plasma proteasome levels and activity, which correlated with decreased IFN-γ, and increased IL-8 and IL-10. In sPE and HELLP, chymotrypsin-like activity was suppressed by proteasome inhibitors including ONX0914. Compared to gestational age-matched controls, sPE placentas harbored increased β5 and β5i immunostaining in trophoblasts. β5i signal was elevated in HELLP with predominant staining in villous core, extravillous trophoblasts in placental islands, and extracellular vesicles in intervillous spaces. Pregnancy represents a state of increased proteostatic stress. sPE and HELLP were characterized by significant upregulation in circulating levels and lytic activity of the proteasome that was partially explained by placental immunoproteasome upregulation.
Keywords: proteasome, immunoproteasome, preeclampsia, HELLP, inflammation, cytokines
INTRODUCTION
Recently, our group demonstrated that PE shares pathophysiologic features with protein misfolding disorders, such as Alzheimer’s, Parkinson’s, and prion diseases.1 This novel concept stemmed from the discovery that urine of PE women exhibits congophilia due to increased excretion of misfolded proteins with affinity for the azo-dye Congo Red (CR) that binds amyloid or amyloid-like aggregates.2 We observed that the increased burden of misfolded proteins in PE associated with amyloid-like deposits in the placenta that resembled the plaques observed in brains of Alzheimer’s patients.1,3 Collectively, these observations led us to conclude that PE is a state of disturbed proteostasis that results from imbalance between production and/or clearance of misfolded proteins in pregnancy.
Misfolded proteins and their intermediates are toxic to cells and must be degraded before they perturb their metabolism, signaling, transport, and structural integrity.4 The ubiquitin-proteasome system (UPS) represents a highly ordered assembly of enzymatic activities that collectively degrades misassembled proteins and peptides.5 As represented schematically in Figure S1 in the online-only Data Supplement, the 26S proteasome is composed of two distinct sub-complexes: a barrel shaped catalytic core particle (also known as the 20S core) flanked by one or two terminal 19S cap regulatory particles.6 The 19S regulatory particle serves as proteasome activator, recognizing and translocating into the 20S core proteins flagged for degradation by addition of a polyubiquitin chain.7 The 20S core is comprised of axial stacking of two outer α-rings and two inner β-rings. The β-rings each contain seven β-type subunits of which three (β1, β2, β5) are responsible for proteasome’s ability to cleave peptide bonds after acidic (caspase-like: CAS-L), basic (trypsin-like: TRY-L), or hydrophobic (chymotrypsin-like: CHE-L) amnio-acids, respectively. The 20S proteasome progressively degrades proteins into peptides ranging in length from 3 to 15 amnio-acid residues, which are subsequently hydrolyzed into amnio-acids by other oligo- and amnio-carboxyl peptidases. These proteasomes have been termed constitutive as they function to maintain basic physiologic proteostasis.
The immunoproteasome is a specialized form of proteasome that has enhanced ability to degrade damaged proteins that are inefficiently degraded by the constitutive 26S particles.8 The switch to immunoproteasome is regulated by immunomodulatory cytokines [in particular interferon (IFN)-γ and TNF-α] and environmental stressors (oxidative stress, heat shock response) which induce expression of β1i, β2i, and β5i (immunosubunits) whose higher affinity for the proteasome assembly displaces the respective β-subunit of the constitutive proteasome.9 The immunoproteasome generates a repertoire enriched in antigenic peptides that are better suited to bind to MHC class I molecules.10 In addition to immune cells which express immunoproteasome subunits constitutively, the immunoproteasome plays critical roles in clearance of misfolded proteins from immune privileged sites such as the retina and brain even in the absence of inflammation.11,12 Despite placenta’s unequivocal immune privilege, there is very limited knowledge on expression and function of placental immunoproteasome.13
Proteasome and immunoproteasome-like assemblies have been recently found to circulate in blood and extracellular fluids of patients with cancer, trauma, sepsis, neurodegenerative, and autoimmune diseases.14 Their origin, biological role, and prognostic significance is intensely debated.15 So far, there is consensus that circulating proteasomes are limited to the 20S core and are released via deliberate cellular export.16 Additionally, extracellular proteasome are able to degrade non-ubiquitinated proteins provided that they are misfolded or oxidized; their activity is ATP-independent, albeit slightly less efficiently than that of fully assembled intracellular counterpart.14 Hypothetically, if in PE pregnancies there is an increased burden of misfolded proteins, clearance mechanisms must be upregulated as the maternal organism attempts to regain proteostasis. We tested our hypothesis by comparing levels and activity of circulating proteasome and immunoproteasome between non-pregnant women, healthy pregnant women, and women with hypertensive disorders of pregnancy. Our results demonstrate increased circulating proteasome enzymatic activities in early-onset preeclampsia with severe clinical features (sPE) and in hemolysis, elevated liver enzymes, and thrombocytopenia (HELLP) syndrome and suggest that protein processing by placental proteasome and immunoproteasome may play important roles in these clinical conditions.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design, Patients and Biological Samples
Using a case control study design we tested plasma samples retrieved from 160 women (Figure S2 in the online-only Data Supplement) of whom 115 had hypertensive pregnancy disorders: 1) chronic hypertension (crHTN, n=25, gestational age [GA] median [interquartile range]: 31 [28–36] weeks), 2) gestational hypertension (gHTN, n=25, GA: 30 [25–33] weeks), 3) preeclampsia without severe features (mPE, n=10, GA: 30 [23–32] weeks), 4) sPE (n=39, GA: 30 [24–34] weeks), and 5) HELLP syndrome (n=16, GA: 29 [25–32] weeks). The remaining 45 women were normotensive and served as controls: 6) non-pregnant women of reproductive age (NP-CRL, n=10), 7) healthy pregnant women [P-CRL, n=14, 29 [25–30] weeks), and 8) women with idiopathic spontaneous preterm birth (sPTB, n=21, GA: 25 [22–29] weeks). Non-pregnant and pregnant women with singletons were enrolled in the antepartum clinics and Labor and Delivery wards at Yale-New Haven Hospital (New Haven, CT) and The Ohio University Wexner Medical Center (Columbus, OH) from March 2004 to January 2016. Exclusion criteria were multiple gestations, presence of viral hepatitis infection, human immunodeficiency virus, multiple gestations, anhydramnios, abnormal karyotype, or congenital anomalies. All women provided written informed consent under research protocols approved by the Institutional Review Boards at both institutions.
NP-CRL reproductive age women were recruited following their annual exam. P-CRL women attended the antenatal clinic for routine prenatal care and had a normal pregnancy outcome (delivery of a healthy baby >37 weeks in the absence of complications of pregnancy). Clinical management of patients with sPTB and hypertensive disorders was left to the discretion of medical providers. For sPE women, delivery was recommended in the setting of worsening maternal or fetal status, which included persistent cerebral or visual symptoms, epigastric or right upper-quadrant pain, pulmonary edema, oliguria, placental abruption, worsening laboratory parameters such as evidence of impaired liver function, increased maternal serum creatinine levels (>1.1 mg/dL), HELLP syndrome, and abnormal fetal testing.17,18
GA was determined based on the last menstrual period confirmed by an ultrasound examination prior to 20 weeks.19 mPE was defined as blood pressure elevation of systolic ≥140 or diastolic ≥90 mmHg in addition to new onset proteinuria >300 mg in 24 hours or >0.3 urinary protein/creatinine ratio on two occasions 4–6 hours apart, in the absence of signs or symptoms consistent with a diagnosis of sPE.20 sPE was defined as systolic blood pressure >160 mmHg or diastolic >110 mmHg on at least two occasions 4 hours apart plus either of the following clinical severity features: neurologic or visual symptoms, renal insufficiency (serum creatinine >1.1 mg/dL), pulmonary edema, right upper quadrant pain, impaired liver function tests (elevated blood liver transaminases to twice the normal concentration) or thrombocytopenia (platelet count <100,000/µL).20 HELLP syndrome was defined as presence of the following clinical features: hemolysis (total bilirubin >1.2 mg/dL), impaired liver function tests, and low platelet count irrespective of blood pressures or proteinuria. gHTN was defined as elevated blood pressures presenting de novo after 20 weeks without proteinuria or clinical features of sPE or HELLP syndrome. crHTN was defined as a sustained elevated blood pressure prior to pregnancy or before 20 weeks gestation. sPTB was defined as spontaneous delivery of a neonate <34 weeks in the absence of intra-amniotic infection or histological chorioamnionitis (ruled out by histological examination of the placenta by a perinatal pathologist).
Blood samples were collected at the time of clinically indicated blood draws independent of our research protocol. Whole blood was retrieved by venipuncture onto Vacutainer tubes with sodium citrate as anticoagulant. The tubes were spun at 3,000g at 4°C for 20 minutes and the supernatant (plasma) aliquoted and immediately stored at −80°C until performance of the assays by investigators unaware of clinical classification.
Proteasome and Immunoproteasome Immunoassays
Plasma concentration of 20S proteasome was measured by enzyme-linked immunoassay (Proteasome ELISA kit, BML-PW0575, Enzo Life Sciences, Farmingdale, NY). Following dilution (2–50 fold), samples were assayed in duplicate in a 96-well plate pre-coated with capture monoclonal antibody directed against α6 subunit (clone MCP20, Enzo Life Sciences).21 The 20S proteasome levels were determined by comparison to a calibration curve produced in parallel from increasing concentrations of purified 20S proteasome. Plasma immunoproteasome concentration was measured in select clinical groups using an immunoassay targeted against the β5i subunit (LMP7/PSMB8, Cat#: MBS2509694, MyBioSource, San Diego, CA). Incubation and washing protocols were performed as instructed by the manufacturer, followed by reading at 450 nm with wavelength correction. Data were reported and plotted with the Softmax software Pro 5.4.1. The inter- and intra-assay coefficients of variation were <10%.
Proteasome Activity Assays
We carried out proteasome lytic activity assay using fluorogenic substrates that target CAS-L, CHE-L, and TRY-L activities of the proteasome. Plasma samples were prepared with 10% SDS in a 9:1 ratio and then incubated at room temperature for 15 minutes. The treated plasma samples were then plated with activity buffer and one of six different substrates that target CAS-L [substrate – (sub)-1: Ac-LLE-AMC; C35H44N4O9 and sub-2: Ac-GPLD-AMC; C29H37N5O9], CHE-L [sub-3: N-Succinyl-LLVY-AMC; C40H53N5O10 and sub-4: Ac-LLL-AMC; C36H48N4O7], and TRY-L [sub-5: Ac-RLR-AMC; C30H46N10O6 and sub-6: Boc-LRR-AMC] (Enzo Life Sciences). Substrates were diluted 1:80 in Proteasome Assay Buffer (Enzo Life Sciences) for a final concentration of 100µM. A 7-point standard curve generated by progressive dilution of 30μM 7-Amino-4-methylcoumarin (AMC) stock was run on each plate. Fluorescence measurements were obtained in a spectrofluorometer (Clariostar, BMG Labtech, Cary, NC) set at excitation 380 nm and emission 450 nm. Fluorescence readings were collected every 5 minutes over 5 hours of incubation at 37ºC. The slope of the fluorescence curve was calculated from a linear segment between 15 to 200 minutes with ≥20 readings. Values for blank wells were subtracted from values with substrates at each time point. Results were reported in fmol AMC/sec and reflected the rate of fluorogenic substrate hydrolysis by the proteasome.
To provide additional assurance that the measured lytic activity in plasma was accounted by the proteasome, activity assays of a subset of samples with elevated circulating proteasome activity (sPE, n=16 and HELLP, n=9 with initial activity >2-fold median of P-CRL group) were repeated in the presence of known pharmacologic proteasome inhibitors. Proteasome inhibitors fall into three chemical categories: peptide aldehydes, peptide boronates, and nonpeptide inhibitors.22 MG132 (peptide aldehyde) is a reversible specific inhibitor of ubiquitin-proteasome pathway and the least selective with respect to differentiating among proteasomal activities.23 Lactacystin is a natural microbial product that binds to proteasome subunits and irreversibly inhibits TRY-L and CHE-L proteolytic activities.24 ONX0914 selectively targets immunoproteasome activity by irreversibly binding to the β5i subunit thus primarily inhibiting CHE-L activity.24 MG132 and ONX0914 (both from Cayman Chemical Company, Ann Arbor, MI) were diluted in DMSO to make a 2mM working stock. Lactacystin (Enzo Life Sciences) was diluted in DMSO to make a 5mM working stock. Each sample was treated with either plain buffer or the above mentioned inhibitors, incubated at room temperature for 30 minutes, and then activity measured in the Clariostar Fluorimeter using the settings described above. The lytic activity in the presence of the inhibitor was divided by activity without inhibitor assessed within the same assay and interpreted as % remaining activity.
Plasma Pro-and Anti-inflammatory Cytokines and Other Biochemical Measures
Plasma cytokines were profiled using the V-PLEX Plus Proinflammatory Panel I (human) kit (Meso Scale Discovery, Rockville, MD) that assays ten markers of inflammation: IL-10, IL-13, IL-1β, IL-2, IL-4, IL-6, IL-8, TNF-α, IFN-γ, and IL-12p70. This multi-array 96-well plate format utilizes electrochemiluminescent labels conjugated to detection antibodies. This assay was performed on a MesoScale Quick Plex SQ120 instrument with data analysis performed by Discovery Workbench 4.0.12 software.
LDH activity (Stanbio Laboratory, Boerne, TX) level was evaluated to measure cell damage that could potentially contribute to release of intracellular proteasome in circulation. Hemoglobin levels were assessed using the leukomalachite green assay (Sigma Aldrich, St. Louis, MO) as a marker of hemolysis.
Immunohistochemistry for Proteasome and Immunoproteasome Subunits in Placenta of Women with Preeclampsia and HELLP Syndrome
Presence and location of subunits attributable to the constitutive proteasome and immunoproteasome was interrogated using 5μm paraffin sections of placental villous tissue from women enrolled in the following groups: sPTB (n=4), sPE (n=6), and HELLP (n=5). Placental tissue biopsies were retrieved immediately after delivery from the central portion of the placental disk, fixed in formalin, and embedded in paraffin. For immunohistochemistry, tissues were deparaffinized in xylene and rehydrated with graded ethanol to potassium-phosphate-buffered saline solution, pH 7.2. After antigen retrieval with citrate buffer, the sections were pretreated with 1% hydrogen peroxide for 15 minutes followed by blocking and overnight incubation (at 4°C) with polyclonal anti-β5 and anti-β5i antibodies (both 1:200 dilution). Specific β5 and β5i staining in the syncytiotrophoblast, cytotrophoblast, villous stroma and villous perivascular tissues were evaluated semi-quantitatively in a blinded fashion from 3 random fields per slide. Staining intensity was scored on a scale from 0 (absent) to 5 (intense).
Statistical Analysis
Statistical analyses were performed with Sigma Stat, version 2.03 (SPSS Inc., Chicago, IL) statistical software. Normality testing was performed using the Shapiro-Wilk test. Data were compared with Mann-Whitney Rank Sum test, 1-way ANOVA followed by Holm-Sidak tests (parametric) or Kruskal-Wallis ANOVA on ranks followed by Dunn’s tests (non-parametric). The effect of proteasome inhibitors was analyzed using 2-way ANOVA or 2-way repeated measures ANOVA with factors represented by the different substrates, enzymatic activities, or disease state (sPE or HELLP). Statistical analysis of immunoassay data was performed after logarithmic transformation. Multivariable regression was used to correct for possible influences of maternal age, GA, and other disease and non-disease related factors such as degree of hemolysis and time from sample collection to analysis. Comparisons between proportions were done with χ2 tests. Principal component analysis (PCA) for the plasma proteasome profile of each group was accomplished using the ‘prcomp’ function in R version 3.0.3. A P value of <0.05 was considered significant throughout the analysis.
RESULTS
Characteristics of the Clinical Groups
Demographic and outcome characteristics of the women who contributed plasma samples are presented in Table S1 in the online-only Data Supplement. Women with crHTN were of significant higher gravidity and parity. A higher proportion of African-American women contributed to crHTN, mPE, and sPE groups. Women who comprised the hypertensive groups had significantly elevated blood pressure and proteinuria levels. At delivery, women with sPTB, sPE, and HELLP were of significantly lower GAs and delivered babies with lower birthweights. Women with sPE and HELLP were more often delivered by Cesarean section.
Circulating 20S Proteasome Levels and Activity in Pregnant versus Non-Pregnant State
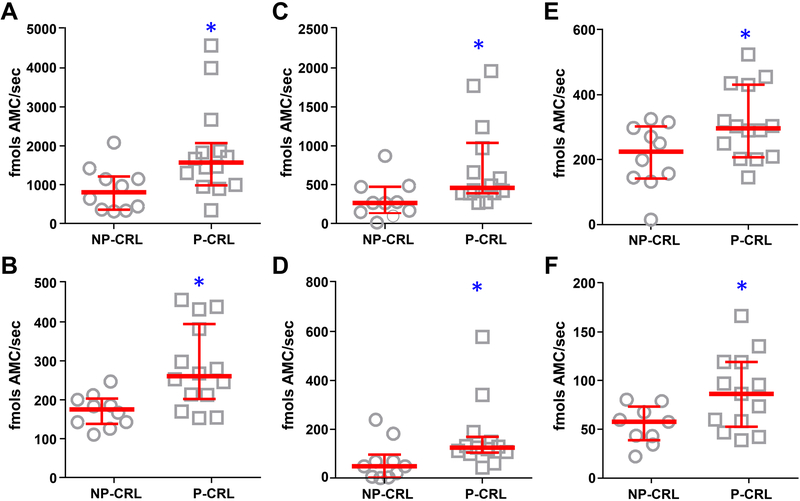
Pregnancy did not affect the circulating levels of 20S proteasome (Figure S3A in the online-only Data Supplement, P=0.208), but significantly up-regulated proteolytic activity (Figure 1). Compared to NP-CRL, pregnant women had increased plasmatic activity of each of the three proteasome enzymatic activities: CAS-L (Figure 1A–1B, sub-1, P=0.021; sub-2, P=0.004), CHE-L (Figure 1C–1D, sub-3, P=0.011; sub-4, P=0.020), and TRY-L (Figure 1E–1F, sub-5, P=0.033; sub-6, P=0.045).
Figure 1. Maternal plasma enzymatic activities of the circulating proteasome in pregnant (P-CRL, n=14) compared to non-pregnant (NP-CRL, n=10) controls.
Plasma of P-CRL women in second (n=5) and third (n=9) trimester displays significantly higher caspase-like (A, Z-LLE-AMC; B, Ac-GPLD-AMC), chymotrypsin-like (C, Suc-LLVY-AMC; D, Z-LLL-AMC), and trypsin-like (E, Ac-RLR-AMC; F, Ac-LRR-AMC) enzymatic activities than non-pregnant plasma. Data are presented as median (horizontal line) and interquartile range (vertical bars). Mann-Whitney Rank Sum Tests, * P<0.05 vs NP-CRL.
Circulating 20S Proteasome Levels and Activity in Preeclampsia and HELLP Syndrome
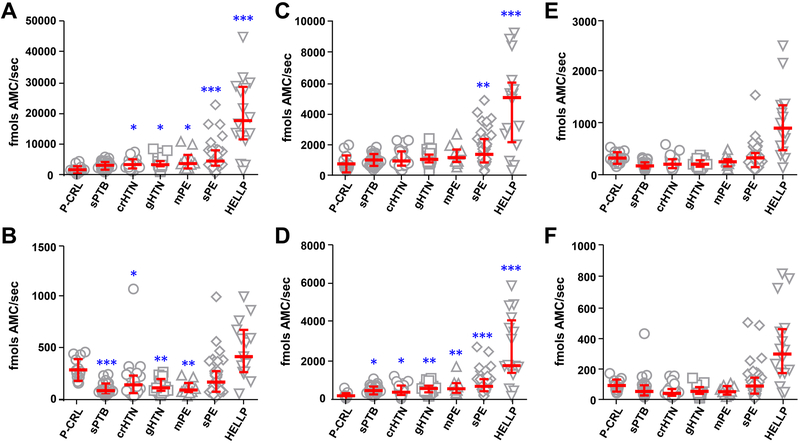
Compared to P-CRL, women with sPE (P=0.009) and HELLP (P<0.001) had significantly increased circulating levels of 20S proteasome that was not observed in the other clinical groups (Figure S3B in the online-only Data Supplement). Analysis of proteasome enzymatic activity demonstrated significant differences among groups for CAS-L (Figure 2A–2B), CHE-L (Figure 2C–2D), and TRY-L (Figure 2E–2F) substrates (Kruskal Wallis ANOVA P<0.001 for all). Among the clinical groups studied, plasma from women with sPE and especially HELLP displayed the highest median lytic activity against all substrates. CHE-L activity was the most consistently elevated as the increase was noted for both tested substrates. CAS-L activity of sPE and HELLP plasma was significantly elevated compared to P-CRL only for one of the two substrates (sub-1, P<0.001). Among lytic activities, TRY-L was overall the least prominent and did not reach significance for either sPE or HELLP group.
Figure 2. Maternal plasma enzymatic activities of the circulating proteasome in healthy pregnant controls compared to women with spontaneous preterm birth and hypertensive conditions of pregnancy.
Circulating proteasome activity in pregnant controls (P-CRL, n=14) and women with idiopathic spontaneous preterm birth (sPTB, n=21), chronic hypertension (crHTN, n=25), gestational hypertension (gHTN, n=25), preeclampsia without severe clinical feature (mPE, n=10), preeclampsia with severe clinical features (sPE, n=39), and sPE and clinical features of HELLP syndrome (HELLP, n=16) display differential caspase-like (A, Z-LLE-AMC; B, Ac-GPLD-AMC), chymotrypsin-like (C, Suc-LLVY-AMC; D, Z-LLL-AMC), and trypsin-like (E, Ac-RLR-AMC; F, Ac-LRR-AMC) enzymatic activities. Data are presented as median (horizontal line) and interquartile range (vertical bars). Kruskal-Wallis analysis of variance followed by multiple comparisons with Dunn’s test. * P<0.05, ** P<0.01, *** P<0.001 vs P-CRL group.
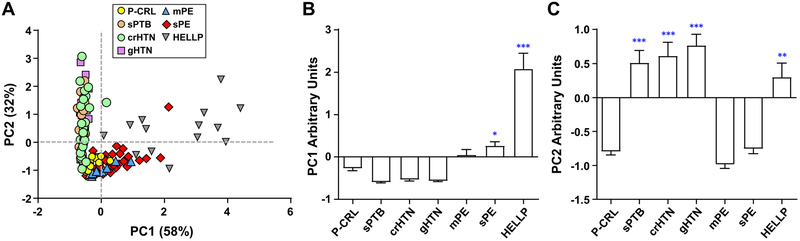
To further understand how the combination of lytic activities relate to clinical phenotypes, we performed PCA using the lytic activities against the six substrates. Two components (PC1 and PC2) were extracted that contributed 58% and 32% of total variance, respectively (Figure 3A). mPE and sPE cases distributed primarily along PC1 in a separate trajectory from crHTN, gHTN, and sPTB, which aligned along PC2 commonly characterized by lytic activity against sub-2 (CAS-L) and sub-6 (TRY-L). HELLP cases had the widest distribution area in the PCA space with contribution both from PC1 and PC2. PC1 had the strongest correlation with CHE-L activity (sub-4: r=0.965; sub-3: r=0.943, P<0.001 both) followed by CAS-L revealed by sub-1 (r=0.941, P=0.001) and TRY-L as revealed by sub-5 (r=0.844, P<0.001). PC2 had the strongest correlation with lytic activity against sub-6 (TRY-L, r=0.935, P<0.001) and sub-2 (CAS-L, r=0.935, P<0.001). The comparison among groups illustrates significant differences from P-CRL in PC1 for sPE and HELLP groups (Figure 3B) and in PC2 for sPTB, crHTN, gHTN, and HELLP (Figure 3C). Together these results support the notion that the lytic profile of the proteasome in women with HELLP syndrome differs from that of women with sPE alone.
Figure 3. Maternal plasma circulating proteasome profile analyzed by principal component analysis.
(A) Scatterplot of the first two principal components (PC1 and PC2) that explain 90% of the variance in the data. This analysis revealed that women with preeclampsia without severe clinical feature (mPE, n=10), preeclampsia with severe clinical features (sPE, n=39), and women with hemolysis, elevated liver enzymes, and thrombocytopenia (HELLP syndrome, n=16) co-cluster along a different trajectory from idiopathic spontaneous preterm birth (sPTB, n=21), chronic hypertension (crHTN, n=25), and gestational hypertension (gHTN, n=25). Pregnant controls (P-CRL, n=14) are closely grouped near the graph’s origin. Bar graphs (mean and standard error) of (B) PC1 and (C) PC2 for each clinical group with statistical significance. 1-way analysis of variance followed by multiple comparisons with Holm-Sidak method. * P<0.05, ** P<0.01, *** P<0.001 vs P-CRL group.
Effect of Pharmacological Inhibitors on Proteasome Activity of Women with Preeclampsia and HELLP Syndrome
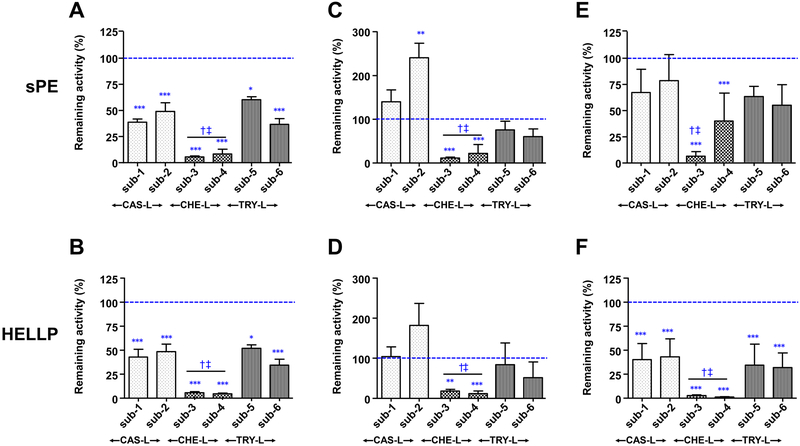
MG132, lactacystinm, and ONX0914 repressed lytic activity against most of the six substrates although differences were observed (Figure 4). The average remaining activity in the presence of MG132 for substrates measuring CAS-L, CHE-L and TRY-L activity was 44±3%, 6±1%, and 46±3%, respectively (Figure 4, 2-way RM ANOVA P<0.001 both for difference from baseline and among enzymatic activities). MG132 was equally effective in inhibiting circulating proteasome activities of sPE and HELLP plasma (Figure 4A and 4B, respectively; 2-way ANOVA, P=0.517). Different from MG132, lactacystin was only effective in repressing CHE-L activity with similar level of potency for sPE and HELLP plasma (Figure 4C and 4D, respectively; residual CHE-L activity in sPE: 24±10% and HELLP: 16±5%; P<0.001 for differences from baseline and P=0.694 among substrates). Interestingly, lactacystin did not inhibit TRY-L activity in either sPE or HELLP and a paradoxal stimulatory effect on CAS-L activity was observed in some samples that reached significance only for sub-2 (Figure 4C).
Figure 4. Effect of pharmacologic inhibition of circulating proteasome and immunoproteasome in women with preeclampsia with severe features (sPE) or HELLP syndrome.
The effect of (A, B) MG132, (C, D) lactacystin, and (E, F) ONX0914 on caspase-like (CAS-L), chymotrypsin-like (CHE-L), and trypsin-like (TRY-L) enzymatic activities, respectively. Each enzymatic activity was tested for two substrates (sub) as indicated. Data are presented as mean and standard error (vertical bars). Statistical analysis: 2-way repeated measures analysis of variance followed by multiple comparisons with Holm-Sidak tests (on log-transformed data). * P<0.05, ** P<0.01, *** P<0.001 for activity remaining after treatment with inhibitor versus baseline for each sample (blue interrupted line). † P<0.05 CHE-L vs CAS-L activities; ‡ P<0.05 CHE-L vs TRY-L activities.
When plasma samples from women with sPE were treated with ONX0914 (immunoproteasome inhibitor), there was no significant effect on CAS-L or TRY-L activity, but a significant reduction in CHE-L was noted (Figure 4E, residual CHE-L activity in sPE: 22±18%, P<0.001 for difference from baseline). In HELLP, ONX0914 had a profound inhibitory effect reducing almost entirely CHE-L activity (Figure 4F, remaining activity 1±1%, P<0.001 vs baseline). CAS-L and TRY-L activities were also inhibited, albeit to a lesser extent (42±12% and 33±12%, respectively, remaining activities; P<0.001 vs CHE-L for both). When analyzed comparatively, ONX0914 was more effective at inhibiting lytic activities in HELLP compared to sPE plasma (2-way ANOVA, P=0.009). This result supports a higher contribution of the immunoproteasome to the lytic profile of plasma from HELLP patients.
Maternal Cytokine Profile and Proteasome Activity
Because the replacement of immunoproteasome catalytic subunits including β5i is known to be induced by an inflammatory environment, we evaluated the maternal plasma cytokine profile of a subset of P-CRL (n=9), sPE (n=13), and HELLP (n=7) women. We observed significant differences in concentrations of IL-8, IL-10, and IFN-γ (Figure S4A-S4C in the online-only Data Supplement). Women with HELLP had significantly higher levels of circulatory IL-8 (P<0.001) and IL-10 (P<0.001), but lower levels of IFN-γ (P=0.040) compared to P-CRL. Although levels of TNF-α were not statistically different among groups (Figure S4D), women with HELLP had significantly higher IL-10:IFN-γ (Figure S4E) and IL-10:TNF-α (Figure S4F) ratios in peripheral blood. Both ratios correlated directly with proteasome immunoreactivity and with individual enzymatic activities (P<0.001 for all).
Circulating β5i Immunoreactivity in Pregnancy, Preeclampsia, and HELLP syndrome
Because ONX0914 was more effective at inhibiting proteasome activity in HELLP women, we evaluated the level of plasma β5i (LMP7) for women with sPE and HELLP relative to P-CRL and NP-CRL. We found that pregnancy per se resulted in >20-fold increase in circulating β5i (LMP7) (NP-CRL vs P-CRL, Figure S5A in the online-only Data Supplement, P<0.001). There were no additional changes in β5i concentrations in peripheral blood of women with either sPE or HELLP (Figure S5B, P>0.05).
Immunolocalization of β5 and β5i Subunits in Placenta
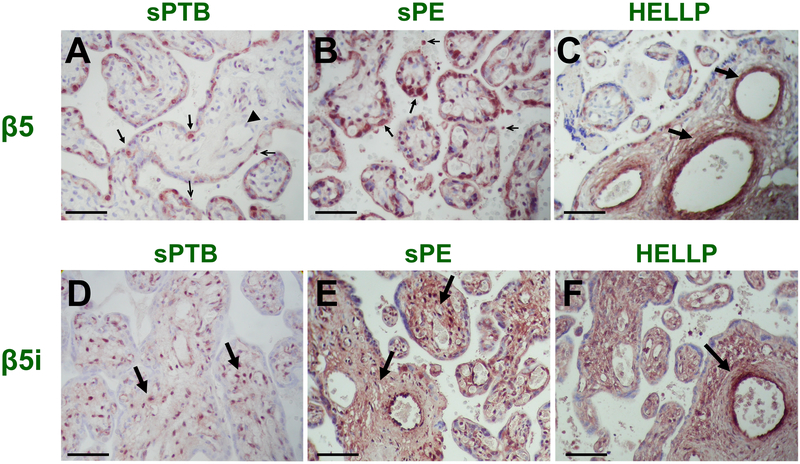
By using immunohistochemistry and GA-matched sPTB specimens as reference, we determined that human placenta expresses both β5 and β5i proteasome subunits constitutively, albeit in different cellular compartments of the placental villi (Figure 5). The β5 subunit of the constitutive proteasome in sPTB placenta localized predominantly to syncytiotrophoblast and underlining cytotrophoblasts (Figure 5A, closed black arrows). Cells in the villous stroma including those lining the fetal blood vessels stained only minimally (Figure 5A, black arrowhead). The intervillous space appeared overall clear, although a few vesicular acellular formations smaller in diameter than a red blood cell were noted (Figure 5A, open arrows). The staining intensity for the β5 subunit was more conspicuous in preeclamptic placentae for all the above locations with cytotrophoblasts harboring the strongest signal (Figure 5B, closed black arrows). Staining was also more intense in the villous stroma and on the outer edge of the syncytiotrophoblast. The vesicular agglomerations in the intervillous space were more numerous and of varying sizes (Figure 5B, open arrows). A similar distribution of β5 immunostaining was seen in HELLP syndrome placentas. The most distinctive feature in HELLP was intense perivascular β5 staining of intermediate villi (Figure 5C, black arrows). Results of semi-quantitative analysis of histological staining are presented in Figure S6A in the online-only Data Supplement.
Figure 5. Immunostaining for β5 and β5i subunits in placenta.
Representative immunostaining for β5 subunit of the constitutive proteasome in placentas of a women with (A) idiopathic spontaneous preterm birth (sPTB, 32 weeks), (B) preeclampsia with severe features (sPE, 32 weeks) or (C) HELLP syndrome (30 weeks). Representative immunostaining for β5i subunit of the immunoproteasome in the same cases (D) sPTB, (E) sPE, and (F) HELLP syndrome. Areas of interest are marked with black arrows or arrowheads as described in results. Scale bar: 50 nm.
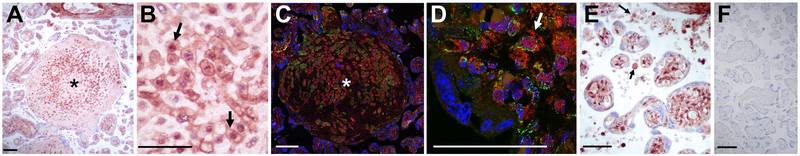
In contrast to the constitutive proteasome, staining for the β5i subunit of the immunoproteasome in sPTB placentas predominated in cells of the villous stroma (Figure 5D, black arrows) whereas cytotrophoblasts and syncytiotrophoblast had minimal staining. In placenta of both sPE (Figure 5E) and HELLP syndrome (Figure 5F), an intense, diffuse staining pattern was observed with β5i immunoreactivity occupying the entire stromal area of the terminal villi. Similar to β5 staining, a distinctive feature for HELLP placenta was intense β5i staining around larger caliber vessels (Figure 5F). Semi-quantitative analysis was suggestive of significant upregulation of β5i immunoreactivity in sPE with further increase in placentas of women with HELLP syndrome (Figure S6B in the online-only Data Supplement). Additional notable β5i staining patterns in HELLP placentas was the intense staining of extravillous trophoblasts (EVT) in placental cell islands (Figure 6A, asterisk; Figure 6B, black arrows) as confirmed by co-immunostaining with the EVT marker HLA-G (Figure 6C, asterisk; Figure 6D, white arrow). Lastly, numerous acellular round formations of varying sizes and β5i staining intensities were seen agglomerated in maternal intervillous space (Figure 6E, black arrows).
Figure 6. Additional β5i immunostaining patterns in context of HELLP syndrome.
(A) Remarkable staining of extravillous trophoblasts (EVT) within a placental cell island (asterisk). (B) Placental disk EVTs (arrows) shown at higher magnification. (C) Placental cell island (asterisk) imaged after double immunofluorescence for β5i (red signal) and human leukocyte antigen (HLA)-G (EVT marker, green signal), plus DAPI (nuclear counterstain, blue signal). (D) Cellular co-localization of β5i and HLA-G in EVTs (arrow) shown at higher magnification. (E) Micrograph illustrating acellular formations floating in the intervillous space and stained positive for β5i (arrows). (F) Negative control (non-immune IgG) of the same placenta. Scale bar: 50 nm.
DISCUSSION
A more comprehensive understanding of the functional changes associated with PE may lead to future therapeutic interventions designed based on molecular signatures of the disease. Because our prior studies indicated PE is characterized by increased protein misfolding, this study was focused on the levels and activity of constitutive proteasome and immunoproteasome, which are the major pathways for degradation of misfolded proteins in eukaryotes.
Pregnancy and Proteasome Activity
Previous studies reporting 20S proteasome subunits circulating freely in human blood effectively confirmed the existence of an extracellular proteasome.25,26 By using electron microscopy it was determined that the human circulating proteasome is comprised of intact 20S particles that display hydrolyzing activity that can be inhibited by lactacystin.16 It was proposed that cell proliferation and cytolysis of erythrocytes, immunocompetent cells, and vascular endothelial cells are the most probable sources of the circulating proteasome.15,16 The current study establishes the existence of a circulating proteasome in human gestation, and provides evidence that its levels are not significantly changed compared to the non-pregnant state at least as measured by its α subunit. Because cell proliferation is key for fetal growth and trophoblast proliferation, differentiation, and invasion, it is possible that the increased intra-vascular volume characteristic to human gestation can mask a potential outpouring of 20S proteasome in the maternal circulation through a dilutional effect.
As previously shown, the circulating 20S proteasome units are proteolytically active.16 Our analysis detected increased serum CAS-L, CHE-L, and TRY-L enzymatic activities at the beginning of the third trimester, qualifying this observation as a newly described maternal physiologic adaptation to pregnancy. Specifically, we noted an increased efficiency with which proteins can be processed during gestation. The observed increased proteolytic activity is not surprising. First, there is an increased need for protein degradation because the maternal level of many proteins (e.g. α-fetoprotein, amylase, prolactin, hormone binding globulins) increases in gestation.27 Second, in addition to the protein “crowding” phenomenon characteristic to human pregnancy, hormonal activity specifically estrogens, stimulate proteasome enzymatic competence, as previously described.28 However, during gestation the functional capability of the proteasome could be organ, species, and pregnancy status dependent. Iorga et al. reported decreased cardiac proteasome activity during mouse pregnancy, implying that more research is needed in this area.29
Proteasome Levels and Activity in sPE and HELLP
The levels of circulating proteasome were significantly increased in sPE and HELLP. Women with hypertensive disorders including sPE and HELLP have a wide range of metabolic disturbances, vascular endothelial damage, and intra-vascular volume depletion, which may partially account for our results.30,31,32 However, upregulation of constitutive proteasome levels could be a compensatory mechanism needed to process the high load of circulating misfolded proteins (e.g. serpina-1) characteristic to women with sPE and HELLP.33 This assertion is supported by our recent work that demonstrated thioflavin T (ThT) fluorescence, a marker of amyloid-like aggregates, is increased in serum of women with sPE and HELLP.3 ThT fluorescence was independently associated with HELLP, inferring that an increased level of proteasome is needed to process the ThT-positive aggregates whose cytotoxicity may associate with hemolysis, elevated liver enzymes, and low platelet levels.3 Pereira et al. have demonstrated that magnesium markedly stimulates CAS-L catalytic activity of purified bovine pituitary proteasome, while inhibiting TRY-L and CHE-L activities.34 We do not believe that magnesium sulfate present in the plasma sample could have affected the results given that the proteasome activity buffer itself contains magnesium, which is added in the assay for optimal lytic activity under standard conditions. However, it remains to be investigated whether the modulation of proteasome activities by Mg2+ contributes to its therapeutic effect in women with PE.
Previously, the attention of most investigators has been focused on understanding the capacity of the proteasome to cleave cancer-related proteins.35,36,37 Our study expands the focus of proteasome research to human pregnancy, with specific focus on sPE and HELLP. Proteasome β5 subunits, along with β1 and β2 subunits are responsible for the ability to cleave and process peptide bonds at the C-terminal side of acidic, basic, and hydrophobic amino-acids residues, respectively.6 Herein, we observed that compared to P-CRLs and other hypertensive disorders, CAS-L and CHE-L, but not TRY-L enzymatic activity of the maternal constitutive proteasome was consistently elevated in both sPE and HELLP in a substrate dependent fashion. Among many functions, CAS-L cleavage can inactivate poly(ADP-ribose) polymerase.38 Because excessive stimulation of poly(ADP-ribose) polymerase contributes to endothelial dysfunction, the observed increase in CAS-L activity could be a compensatory mechanism aimed to limit the endothelial damage characteristic to PE.39 We confirmed that proteasome proteolytic capabilities can be modulated using specific proteasome and immunoproteasome inhibitors. Our experiments aimed to inhibit the enzymatic activity of the circulating proteasome were designed to demonstrate that this proteolytic machinery is functionally relevant and independent of other serum proteases. As a drug class, proteasome inhibitors have clinical applications as anticancer agents due to accumulation of misfolded proteins, which in cells with high turnover triggers apoptosis. For translational relevance, however, given the abundance of toxic circulating misfolded proteins in sPE or HELLP, clinician scientists must develop drugs that carry the ability to boost the proteolytic activity of the proteasome in a safe fashion for both mother and fetus. IFN-γ, ubiquitin-like modifiers, and tyrosine-kinase inhibitors carry such stimulatory properties, as reported in outside pregnancy studies.40,41 There has been emerging interest in screening small molecule libraries for compounds that boost proteasome activity. Among these, the naturally occurring oleuropein (a constituent of olive oil), and betulinic acid may find future application in prevention of PE.42
Another area that has received increased attention is the effect of environmental toxins that interfere with function of the proteasome. Among these, arsenic has been extensively linked to a variety of chronic diseases and to adverse pregnancy outcomes.43 Recent investigations have shown that arsenic compromises protein quality control and proteostasis through inhibition of the proteasome.44
PE, HELLP, and the Immunoproteasome
In PE, activated neutrophils, monocytes, and natural-killer cells initiate inflammation, suggesting that the innate immune system is actively engaged.45 Proteasomal CHE-L peptidase activity is required for essential adaptive immunity functions including differentiation, maturation, survival, and cytokine synthesis of dendritic cells.46 Increased serum CHE-L activity in sPE and HELLP may be involved in inducing the Th1/Th2 imbalance where dendritic cells could have a crucial role.47 In prior studies, analysis of the cytokine profile of mothers with PE showed that production of pro-inflammatory Th1 cytokines (e.g. TNF-α, IL-2, INF-γ) was dominant while production of regulatory Th2 cytokines (e.g. IL-10) was suppressed.48,49 In our study, the maternal serum cytokine profile demonstrated heterogeneity and significant differences between sPE and HELLP. For example, IFN-γ, a main immunoproteasome inducer, was significantly downregulated in HELLP. This could be the result of depressed T and B cell activity.50,51 Considering the above, one might expect that the level and enzymatic activity of the immunoproteasome to be significantly lower in HELLP compared to sPE. Yet, the results of our ONX0914 experiments demonstrated high level of immunoproteasome inhibition suggesting that this system is functionally active in HELLP. Interestingly, immunohistochemistry confirmed a significant upregulation of both constitutive proteasome and immunoproteasome in sPE and HELLP. Whereas our inhibition experiments suggested that at least a part of the elevated proteasome activity in sPE and HELLP is due to the immunoproteasome, this does not appear to result from additional incorporation of β5i, but rather from increased activity of a circulating immunoproteasome characteristic to the pregnant state.
Implications for the Localization of Placental Proteasome Subunits
Histological data demonstrated increased upregulation of proteasome and immunoproteasome subunits in the placental villus stroma and in the perivascular space of women with sPE and HELLP. This may not be surprising considering that placenta of women with PE display more often chronic villitis, which is characterized by inappropriate access of maternal T lymphocytes to the villous stroma, expansion of fetal antigen-presenting cells (Hofbauer cells), and vascular occlusive pathology.52,53 An important observation is that placenta expresses LMP7 constitutively, which concurs with its immuno-privileged status. Constitutive expression of the immunoproteasome in hematopoietic-derived cells may also explain the observed increased β5i immunostaining in the perivascular space.54 Clearly, the upregulation of stromal staining for both constitutive and immunoproteasome in PE suggests that the clearance provided by the trophoblasts is insufficient. Conspicuous staining of EVTs in placental cell islands of the chorionic disk suggests these cells may participate in clearing misfolded proteins and in generating antigenic peptides. Although the origin, proper denomination, and role of these cells has been intensely debated, they are known to increase in numbers in conditions associated with chronic placental hypoxia including PE.55
Study Limitations
While we attempted to measure proteasome enzymatic activities in placental lysates, the mixing between cellular and extracellular compartments and the need to add broad spectrum protease inhibitors to generate the lysates prevents us from measuring intracellular proteasome activity at the level of the placenta. A prior study has reported a decrease in placental proteasome activity and hypothesized that reduced protein clearance at the level of the placenta plays a role in PE pathogenesis.56 Our current data do not contradict this possibility as immunohistochemistry clearly showed an altered distribution of proteasome subunits in PE placentas.
Additional Considerations
The ability of the proteasome to generate not only products of linear cleavage of a parental protein, but spliced peptides through sequential cleavage and condensation is now emerging in the field of cancer biology as an important source of epitopes driving the anti-tumor immune response.57,58 More intriguing is that such spliced epitopes could originate from two distinct proteins (trans-splicing) which meet inside the barrel shaped proteasomes.58 The heightened activities of the proteasome in circulation and placenta of pregnant women and its additional elevation in sPE and HELLP syndrome may be an important source of peptide biomarkers with de novo activities that cannot be inferred from genomic or transcriptomic datasets, yet may contribute to the disturbed immune tolerance in preeclampsia and hence need to be further studied.
PERSPECTIVES
Pregnancy represents a state of increased proteostatic stress. The demonstrated up-regulation in proteasome level and activity in sPE and HELLP may result from a (mal)adaptive response of the maternal organism to counteract misfolded protein excess.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
This study demonstrates the existence of circulating plasma proteasome and immunoproteasome, which are upregulated during normal gestation in both levels and activity, consistent with physiologic adaptation to an increased need to process defective proteins.
Women with PE with severe clinical features and especially those with HELLP syndrome display additional upregulation in levels and enzymatic activity of the circulating proteasome and immunoproteasome that can be inhibited pharmacologically.
The constitutive proteasome and immunoproteasome subunit components are present in the human placenta with discernable differences among women with spontaneous idiopathic preterm birth, PE, and HELLP syndrome.
What is relevant?
The clinical manifestations of PE and HELLP syndrome could be the result of defective protein processing by the ubiquitin-proteasome system at the level of the placenta leading to buildup of misfolded proteins in the maternal circulation.
There may be therapeutic potential in targeting the activity of the proteasome to mitigate some clinical manifestations of sPE and HELLP syndrome.
Summary
The up-regulation in proteasome level and activity in sPE and HELLP could be a compensatory maternal mechanism to counteract the misfolded protein excess observed in these conditions.
ACKNOWLEDGEMENTS
We are indebted to the nurses, fellows, residents, and faculty at Yale-New Haven Hospital, The Ohio State University Wexner Medical Center, and to all women who participated in the study. IAB, KEB formulated the hypothesis, designed the study, performed the experiments, analyzed and interpreted the data, and drafted the manuscript. IAB had full access to all the data in the study and takes responsibility for its integrity and the data analysis. CSB participated with the study design drafting of the manuscript, analyzed the histological data and together with KEB collected biological specimens, and followed up the patients prospectively to the point of delivery. MA and ML assisted with ELISA assays and immunohistochemistry. All the coauthors participated with critical interpretation of the data, writing of the paper, and have reviewed and approved the final version.
SOURCE OF FUNDING
This work was supported by a grant from National Institutes of Health R01 HD 084628–01 (to IAB).
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- 1.Buhimschi IA, Nayeri UA, Zhao G, Shook LL, Pensalfini A, Funai EF, Bernstein IM, Glabe CG, Buhimschi CS. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci Transl Med. 2014;6(245):245ra92. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen MØ, Mikkelsen K, Behrens MA, Pedersen JS, Enghild JJ, Skrydstrup T, Malmendal A, Nielsen NC. NMR reveals two-step association of Congo Red to amyloid β in low-molecular-weight aggregates. J Phys Chem B. 2010;114:16003–16010. [DOI] [PubMed] [Google Scholar]
- 3.Millen KR, Buhimschi CS, Zhao G, Rood KM, Tabbah S, Buhimschi IA. Serum and urine thioflavin-T-enhanced fluorescence in severe preeclampsia. Hypertension. 2018; 71:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enam C, Geffen Y, Ravid T, Gardner RG. Protein Quality Control Degradation in the Nucleus. Annu Rev Biochem. 2018;87:725–749. [DOI] [PubMed] [Google Scholar]
- 5.Fredrickson EK, Gardner RG. Selective destruction of abnormal proteins by ubiquitin-mediated protein quality control degradation. Semin Cell Dev Biol. 2012; 23:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka K The proteasome: overview of structure and functions. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:12–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. [DOI] [PubMed] [Google Scholar]
- 8.Sijts EJ, Kloetzel PM. The role of the proteasome in the generation of MHC class I ligands and immune responses. Cell Mol Life Sci. 2011;68:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisamatsu H, Shimbara N, Saito Y, Kristensen P, Hendil KB, Fujiwara T, Takahashi E, Tanahashi N, Tamura T, Ichihara A, Tanaka K. Newly identified pair of proteasomal subunits regulated reciprocally by interferon gamma. J Exp Med. 1996;183:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unno M, Mizushima T, Morimoto Y, Tomisugi Y, Tanaka K, Yasuoka N, Tsukihara T. The structure of the mammalian 20S proteasome at 2.75 A resolution. Structure. 2002;10:609–618. [DOI] [PubMed] [Google Scholar]
- 11.Hussong SA, Roehrich H, Kapphahn RJ, Maldonado M, Pardue MT, Ferrington DA. A novel role for the immunoproteasome in retinal function. Invest Ophthalmol Vis Sci. 2011;52:714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccinini M, Mostert M, Croce S, Baldovino S, Papotti M, Rinaudo MT. Interferon-gamma-inducible subunits are incorporated in human brain 20S proteasome. J Neuroimmunol. 2003;135:135–140. [DOI] [PubMed] [Google Scholar]
- 13.Roby KF, Yang Y, Gershon D, Hunt JS. Cellular distribution of proteasome subunit Lmp7 mRNA and protein in human placentas. Immunology. 1995;86:469–474. [PMC free article] [PubMed] [Google Scholar]
- 14.Sixt SU, Dahlmann B. Extracellular, circulating proteasomes and ubiquitin - incidence and relevance. Biochim Biophys Acta. 2008;1782:817–823. [DOI] [PubMed] [Google Scholar]
- 15.Bochmann I, Ebstein F, Lehmann A, Wohlschlaeger J, Sixt SU, Kloetzel PM, Dahlmann B. T lymphocytes export proteasomes by way of microparticles: a possible mechanism for generation of extracellular proteasomes. J Cell Mol Med. 2014;18:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoeger A, Blau M, Egerer K, Feist E, Dahlmann B. Circulating proteasomes are functional and have a subtype pattern distinct from 20S proteasomes in major blood cells. Clin Chem. 2006;52:2079–2086. [DOI] [PubMed] [Google Scholar]
- 17.American Congress of Obstetrics and Gynecology Practice Bulletin Number 33. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167. [DOI] [PubMed] [Google Scholar]
- 18.Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169:1000–1006. [DOI] [PubMed] [Google Scholar]
- 19.American College of Obstetricians and Gynecologists. Committee opinion no 611: method for estimating due date. Obstet Gynecol. 2014;124:863–866. [DOI] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists.; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 21.Fukasawa H, Kaneko M, Niwa H, Matsuyama T, Yasuda H, Kumagai H, Furuya R. Circulating 20S proteasome is independently associated with abdominal muscle mass in hemodialysis patients. PLoS One. 2015;10:e0121352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisselev AF, van der Linden WA, Overkleeft HS. Proteasome inhibitors: an expanding army attacking a unique target. Chem Biol. 2012;19:99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan D, Hideshima T, Anderson KC. Proteasome inhibition in multiple myeloma: therapeutic implication. Annu Rev Pharmacol Toxicol. 2005;45:465–476. [DOI] [PubMed] [Google Scholar]
- 24.Bogyo M, Wang EW. Proteasome inhibitors: complex tools for a complex enzyme. Curr Top Microbiol Immunol. 2002;268:185–208. [DOI] [PubMed] [Google Scholar]
- 25.Wada M, Kosaka M, Saito S, Sano T, Tanaka K, Ichihara A. Serum concentration and localization in tumor cells of proteasomes in patients with hematologic malignancy and their pathophysiologic significance. J Lab Clin Med. 1993;121:215–223. [PubMed] [Google Scholar]
- 26.Roth GA, Moser B, Krenn C, Roth-Walter F, Hetz H, Richter S, Brunner M, Jensen-Jarolim E, Wolner E, Hoetzenecker K, Boltz-Nitulescu G, Ankersmit HJ. Heightened levels of circulating 20S proteasome in critically ill patients. Eur J Clin Invest. 2005;35:399–403. [DOI] [PubMed] [Google Scholar]
- 27.Joseph JC, Baker C, Sprang ML, Bermes EW. Changes in plasma proteins during pregnancy. Ann Clin Lab Sci. 1978;8:130–141. [PubMed] [Google Scholar]
- 28.Reed JL, Dimayuga FO, Davies LM, Keller JN, Bruce-Keller AJ. Estrogen increases proteasome activity in murine microglial cells. Neurosci Lett. 2004;367:60–65. [DOI] [PubMed] [Google Scholar]
- 29.Iorga A, Dewey S, Partow-Navid R, Gomes AV, Eghbali M. Pregnancy is associated with decreased cardiac proteasome activity and oxidative stress in mice. PLoS One. 2012;7:e48601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szpera-Goździewicz A, Majcherek M, Boruczkowski M, Goździewicz T, Dworacki G, Wicherek L, Bręborowicz GH. Circulating endothelial cells, circulating endothelial progenitor cells, and von Willebrand factor in pregnancies complicated by hypertensive disorders. Am J Reprod Immunol. 2017;77: doi: 10.1111/aji.12625. [DOI] [PubMed] [Google Scholar]
- 31.Possomato-Vieira JS, Khalil RA. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv Pharmacol. 2016;77:361–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell DM, Campbell AJ. Evans Blue disappearance rate in normal and pre-eclamptic pregnancy. Clin Exp Hypertens B. 1983;2:163–169. [DOI] [PubMed] [Google Scholar]
- 33.Buhimschi IA, Zhao G, Funai EF, Harris N, Sasson IE, Bernstein IM, Saade GR, Buhimschi CS. Proteomic profiling of urine identifies specific fragments of SERPINA1 and albumin as biomarkers of preeclampsia. Am J Obstet Gynecol. 2008;199:551.e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira ME, Yu B, Wilk S. Enzymatic changes of the bovine pituitary multicatalytic proteinase complex, induced by magnesium ions. Arch Biochem Biophys. 1992;294:1–8. [DOI] [PubMed] [Google Scholar]
- 35.Wei X, Zeng W, Xie K, Diao P, Tang P. Potential use of chymotrypsin-like proteasomal activity as a biomarker for prostate cancer. Oncol Lett. 2018;15:5149–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manasanch EE, de Larrea CF, Zingone A, Steinberg SM, Kwok M, Tageja N, Bhutani M, Kazandjian D, Roschewski M, Wu P, Carter G, Zuchlinski D, Mulquin M, Lamping L, Costello R, Burton D, Gil LA, Figg WD, Maric I, Calvo KR, Yuan C, Stetler-Stevenson M, Korde N, Landgren O. Enzymatic activities of circulating plasma proteasomes in newly diagnosed multiple myeloma patients treated with carfilzomib, lenalidomide and dexamethasone. Leuk Lymphoma. 2017;58:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonfili L, Cuccioloni M, Cecarini V, Mozzicafreddo M, Palermo FA, Cocci P, Angeletti M, Eleuteri AM. Ghrelin induces apoptosis in colon adenocarcinoma cells via proteasome inhibition and autophagy induction. Apoptosis. 2013;18:1188–1200. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. [DOI] [PubMed] [Google Scholar]
- 39.Crocker IP, Kenny LC, Thornton WA, Szabo C, Baker PN. Excessive stimulation of poly(ADP-ribosyl)ation contributes to endothelial dysfunction in pre-eclampsia. Br J Pharmacol. 2005;144:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoo L, Yoon AR, Yun CO, Chung KC. Covalent ISG15 conjugation to CHIP promotes its ubiquitin E3 ligase activity and inhibits lung cancer cell growth in response to type I interferon. Cell Death Dis. 2018;9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buhimschi AD, Armstrong HA, Toure M, Jaime-Figueroa S, Chen TL, Lehman AM, Woyach JA, Johnson AJ, Byrd JC, Crews CM. Targeting the C481S Ibrutinib-Resistance Mutation in Bruton’s Tyrosine Kinase Using PROTAC-Mediated Degradation. Biochemistry. 2018;57:3564–3575. [DOI] [PubMed] [Google Scholar]
- 42.Huang L, Chen CH. Proteasome regulators: activators and inhibitors. Curr Med Chem. 2009;16:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shih YH, Islam T, Hore SK, Sarwar G, Shahriar MH, Yunus M, Graziano JH, Harjes J, Baron JA, Parvez F, Ahsan H, Argos M. Associations between prenatal arsenic exposure with adverse pregnancy outcome and child mortality. Environ Res. 2017;158:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tillotson J, Zerio CJ, Harder B, Ambrose AJ, Jung KS, Kang M, Zhang DD, Chapman E. Arsenic Compromises Both p97 and Proteasome Functions. Chem Res Toxicol. 2017;30:1508–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redman CWG, Sacks GP, Sargent IL Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. [DOI] [PubMed] [Google Scholar]
- 46.Naujokat C, Berges C, Höh A, Wieczorek H, Fuchs D, Ovens J, Miltz M, Sadeghi M, Opelz G, Daniel V. Proteasomal chymotrypsin-like peptidase activity is required for essential functions of human monocyte-derived dendritic cells. Immunology. 2007;120:120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez-Sepulveda A, Torres MJ, Khoury M, Illanes SE. Innate immune system and preeclampsia. Front Immunol. 2014;5:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rein DT, Schondorf T, Gohring UJ, Kurbacher CM, Pinto I, Breidenbach M, Mallmann P, Kolhagen H, Engel H. Cytokine expression in peripheral blood lymphocytes indicates a switch to T(HELPER) cells in patients with preeclampsia. J Reprod Immunol. 2002;54:133–142. [DOI] [PubMed] [Google Scholar]
- 49.Azizieh F, Raghupathy R, Makhseed M. Maternal cytokine production patterns in women with pre-eclampsia. Am J Reprod Immunol. 2005;54:30–37. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Su X, Xu W, Zhou R. Interleukin-18 and interferon gamma levels in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol. 2014;72:504–514. [DOI] [PubMed] [Google Scholar]
- 51.Cunningham DS, Christie TL, Evans EE, McCaul JF. Effect of the HELLP syndrome on maternal immune function. J Reprod Med. 1993;38:459–464. [PubMed] [Google Scholar]
- 52.Benzon S, Zekić Tomaš S, Benzon Z, Vulić M, Kuzmić Prusac I. Involvement of T lymphocytes in the placentae with villitis of unknown etiology from pregnancies complicated with preeclampsia. J Matern Fetal Neonatal Med. 2016;29:1055–1060. [DOI] [PubMed] [Google Scholar]
- 53.Salafia CM, Pezzullo JC, López-Zeno JA, Simmens S, Minior VK, Vintzileos AM. Placental pathologic features of preterm preeclampsia. Am J Obstet Gynecol. 1995;173:1097–1105. [DOI] [PubMed] [Google Scholar]
- 54.Ebstein F, Kloetzel PM, Kruger E, Seifert U. Emerging roles of immunoproteasomes beyond MHC class I antigen processing. Cell Mol Life Sci. 2012;69:2543–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redline RW, Patterson P Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum Pathol. 1995;26:594–600. [DOI] [PubMed] [Google Scholar]
- 56.Rajakumar A, Michael HM, Daftary A, Jeyabalan A, Gilmour C, Conrad KP. Proteasomal activity in placentas from women with preeclampsia and intrauterine growth restriction: implications for expression of HIF-alpha proteins. Placenta. 2008;29:290–299. [DOI] [PubMed] [Google Scholar]
- 57.Berkers CR, de Jong A, Schuurman KG, Linnemann C, Meiring HD, Janssen L, Neefjes JJ, Schumacher TN, Rodenko B, Ovaa H. Definition of Proteasomal Peptide Splicing Rules for High-Efficiency Spliced Peptide Presentation by MHC Class I Molecules. J Immunol 2015;195:4085–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vigneron N, Ferrari V, Stroobant V, Abi Habib J, Van den Eynde BJ. Peptide splicing by the proteasome. J Biol Chem. 2017;292:21170–21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.