Abstract
BACKGROUND
Metabolomics study may help identify novel mechanisms underlying arterial stiffening.
METHODS
We performed untargeted metabolomics profiling among 1,239 participants of the Bogalusa Heart Study. After quality control, 1,202 metabolites were evaluated for associations with augmentation index (AI) and pulse wave velocity (PWV), using multivariate linear regression adjusting for age, sex, race, education, smoking, drinking, body weight, body height, physical activity, and estimated glomerular filtration rate. Heart rate, blood pressure and antihypertensive medication usage, lipids, and fasting glucose were sequentially adjusted in the sensitivity analyses for significant metabolites. Weighted correlation network analysis was applied to build metabolite networks.
RESULTS
Six novel metabolites were negatively associated with AI, of which, 3-methyl-2-oxobutyrate had the lowest P value and the largest effect size (β = –6.67, P = 5.99 × 10–6). Heart rate contributed to a large proportion (25%–58%) of the association for each metabolite. Twenty-one novel metabolites were identified for PWV, of which, fructose (β = 0.61, P = 6.18 × 10–10) was most significant, and histidine had the largest effect size (β = –1.09, P = 2.51 × 10–7). Blood pressure played a major contribution (9%–54%) to the association for each metabolite. Furthermore, 16 metabolites were associated with arterial stiffness independent of traditional risk factors. Network analysis identified 2 modules associated with both AI and PWV (P < 8.00 × 10–4). One was composed of metabolites from the glycerolipids synthesis and recycling pathway, and the other was involved in valine, leucine, and isoleucine metabolism. One module related to sphingomyelin metabolism was associated with PWV only (P = 0.002).
CONCLUSIONS
This study has identified novel and important metabolites and metabolic networks associated with arterial stiffness.
Keywords: arterial stiffness, blood pressure, hypertension, metabolomics, metabolite networks
Arterial stiffness measured by aortic pulse wave velocity (PWV) and augmentation index (AI) is a marker of subclinical organ damage and an independent risk factor for cardiovascular disease events and mortality.1–5 Although many factors, including aging, diabetes, hypertension, dyslipidemia, and chronic kidney disease, contribute to arterial stiffening, the pathophysiological mechanisms of this process are still not fully understood.
Circulating low-weight metabolites represent the intermediate and end products of metabolic pathways and may reflect initial stages of arterial stiffness. Recent advances in metabolomics technology have allowed for global characterization of a large panel of metabolites from biological samples, which provides a unique opportunity for investigating arterial stiffness mechanisms. Previous metabolomics studies have identified important metabolites associated with arterial stiffness,6–8 revealing novel mechanisms of arterial stiffening.6–8 However, these studies either had small sample sizes or only assayed a limited number of metabolites, and omitted metabolite networks.
In this study, we performed untargeted metabolomics profiling using the most up-to-date metabolites panel in 1,239 participants of the Bogalusa Heart Study (BHS) to identify novel metabolites and metabolic pathways associated with arterial stiffness.
MATERIALS AND METHODS
Study participants
The BHS is a series of repeated surveys among a semirural biracial (35% black and 65% white) cohort of residents from Bogalusa, Louisiana. The study was established in 1973 by Dr Gerald Berenson to investigate the early natural history of cardiovascular disease. The current BHS sample includes 1,261 participants who were born between 1959 and 1979 and were screened at least twice during childhood and twice during adulthood. Blood samples and arterial stiffness measures of the 1,261 participants were collected during the 2013–2016 visit cycle. This study was approved by the institutional review boards at Tulane University.
Metabolites profiling and quality control
Among the 1,261 BHS participants, a random sample of 64 participants had blood samples collected twice to serve as blind duplicates. Therefore, metabolites were quantified from 1,325 fasting serum samples by Metabolon, Inc. (Durham, NC) using an untargeted, ultrahigh performance liquid chromatography-tandem mass spectroscopy-based metabolomics quantification protocol. Details of the profiling process and quality control measures are described in Supplementary materials. This untargeted approach identified 956 known biochemicals and 510 compounds of unknown identities. The unknown metabolites are tagged beginning with “X” and followed by numbers (e.g., X-12127).
We carried out further quality control to the metabolite data before analyses. For each metabolite, we calculated reliability coefficient (Spearman correlation coefficient) among the 64 blind duplicate samples. We removed 264 metabolites with either reliability coefficient ≤0.3 or missing rates or below-detection-limit rates ≥80%, and a total of 1,202 metabolites were included in the current analyses. A total of 167 metabolites had missing rates or below-detection-limit rates between 50% and 80%. We calculated median among values above the detection limits for each of the 167 metabolites and categorized these metabolites as 1 = missing or below-detection-limit, 2 = greater than detection-limit but less than median, and 3 = equal to or greater than median. For the remaining 1,035 metabolites with missing rates or below-detection-limit rates less than or equal to 50%, the normalized measures were used.
Arterial stiffness measures
Pulse wave velocity
Aortic PWV was measured using a Toshiba Ultrasound instrument (Xario, SSA-660A; Toshiba America Medical Systems, Tustin, CA) as described previously.9 A nondirectional transcutaneous Doppler flow probe (Toshiba PSK25AT, 2.5 mHz) was positioned at the suprasternal notch and another probe (Toshiba PCK703AT, 7.5 mHz) at the left femoral artery with participants in a supine position. The software of the instrument determined the time from the R-wave of the electrocardiogram to the foot of each waveform. Aortic PWV was then calculated by dividing the distance traveled by the time differential between the two waveforms. Aortic PWV was measured 3 times for each participant, and the average of the 3 measurements was used in all analyses.
Augmentation index
AI was measured using the HEM 9000 AI, Non-Invasive Blood Pressure Monitor with AI (Omron Healthcare Co, Ltd, Kyoto, Japan) as described previously.10 This instrument provides blood pressure and heart rate measures and estimates central aortic systolic blood pressure, radial AI, and an AI corrected to a heart rate of 75 beats/minutes. A total of 4 readings, 2 from the left radial artery and the other 2 from the right arm, were taken and averaged for analyses.
Covariates
Age, sex, race, education levels, and smoking and drinking status were based on self-report. Education was categorized into high school graduate or less vs. more than high school. Smoking and drinking status were defined as never, former, or current users. Anthropometric measures were collected by trained staff with participants in light clothing without shoes. Body weight and height were measured in duplicate to the nearest 0.1 kg and 0.1 cm, respectively. The means of weight and height were used to calculate body mass index in kilogram per square meter. Physical activity was measured using the International Physical Activity Questionnaire,11 and the total metabolic equivalent of task was calculated based on the International Physical Activity Questionnaire guideline for data processing and analysis.11 Heart rate and blood pressure were measured using the HEM 9000 AI, Non-Invasive Blood Pressure Monitor with AI (Omron Healthcare Co, Ltd). Estimated glomerular filtration rate (eGFR), blood lipids, and fasting glucose were measured using standard methods.
Statistical analyses
Characteristics of the study participants were presented as percentages for categorical variables and means and SDs or medians and interquartile ranges for continuous variables by race. All continuous variables were checked for normality and log-transformed as needed.
Single metabolite-based analyses
Multivariate linear regression models were applied to test the associations of each metabolite with PWV and AI, respectively, while controlling for age, gender, education, smoking, drinking, physical activity, body height, body weight, and eGFR in each race group. Such analyses were also conducted in the overall BHS participants with race added in the model. Bonferroni corrected P value of 4.16 × 10–5 (0.05/1202, correcting for 1,202 metabolites) was used for statistical significance. Metabolites meeting all the following 3 criteria were considered significant: (i) P < 4.16 × 10–5 in any of the analyses among African Americans, whites, or overall participants; (ii) nominally significant (P < 0.05) in both African Americans and whites; and (iii) effect directions were consistent in African Americans and whites. To test for robustness of the significant metabolites, we additionally built 3 sets of models among the overall participants as shown in the following equation:
where SBP is the systolic blood pressure, DBP is the diastolic blood pressure, LDLC is the low-density lipoprotein cholesterol, and HDLC is the high-density lipoprotein cholesterol. To estimate the impact of these factors on metabolite-arterial stiffness associations, we calculated changes in effect sizes as , where is the regression coefficient of a metabolite in the ith model, and i ranges from 1 to 3.
As metabolite profile may largely change after menopause, we performed sensitivity analyses for the significant metabolites excluding postmenopausal women. All single metabolite-based analyses were performed using SAS software (version 9.4).
Network-based analyses.
To identify metabolites networks that were associated with arterial stiffness, we constructed signed metabolite networks, in which all metabolites were positively correlated,12 using the weighted correlation network analyses (WGCNA) method implemented in R software.13 Minimum module size was 20 metabolites, and power beta was 5, so that models’ fitting index R-squared was larger than 0.8. Each network was noted by a unique color, and eigenmetabolite of each module, the first principal component of the module explaining the largest proportion of variance,12 was linked to arterial stiffness measures using multivariate linear regression models adjusting for age, sex, race, education, smoking, drinking, body weight, body height, physical activity, and eGFR.
RESULTS
The BHS participants were on average obese with a mean body mass index of 33.4 (SD = 8.9) among African Americans and 30.4 (SD = 6.9) among whites (Table 1). A total of 17.9% of white and 23.6% of African American participants were current smokers, and more than half of the participants were current drinkers. White participants were more likely to be male and have higher education and lower physical activity levels.
Table 1.
Characteristics of Bogalusa Heart Study participants
| Black (n = 428) | White (n = 811) | |
|---|---|---|
| Age, years, mean (SD) | 47.5 (5.6) | 48.5 (5.0) |
| Male, % | 37.4 | 43.2 |
| Menopause, % | 11.7 | 8.1 |
| Education, ≤high school, % | 64.7 | 43.7 |
| Smoking status, % | ||
| Current smoker | 23.6 | 17.9 |
| Former smoker | 27.6 | 30.1 |
| Never smoker | 48.8 | 52.0 |
| Drinking status, % | ||
| Current drinker | 51.2 | 57.8 |
| Former drinker | 30.9 | 33.0 |
| Never drinker | 17.9 | 9.2 |
| Body mass index, kg/m2, mean (SD) | 33.4 (8.9) | 30.4 (6.9) |
| Systolic blood pressure, mm Hg, mean (SD) | 128.0 (19.3) | 120.9 (14.8) |
| Diastolic blood pressure, mm Hg, mean (SD) | 81.5 (12.6) | 76.9 (10.3) |
| Antihypertensive medication, % | 47.1 | 28.4 |
| Pulse wave velocity, mean (SD) | 8.3 (1.5) | 7.8 (1.3) |
| Augmentation Index, mean (SD) | 25.7 (11.5) | 25.2 (12.0) |
| Physical activity, metabolic equivalent of task, mean (SD) | 8,251.5 (7,816.0) | 6,675.4 (6,869.0) |
Single metabolite-based analysis results
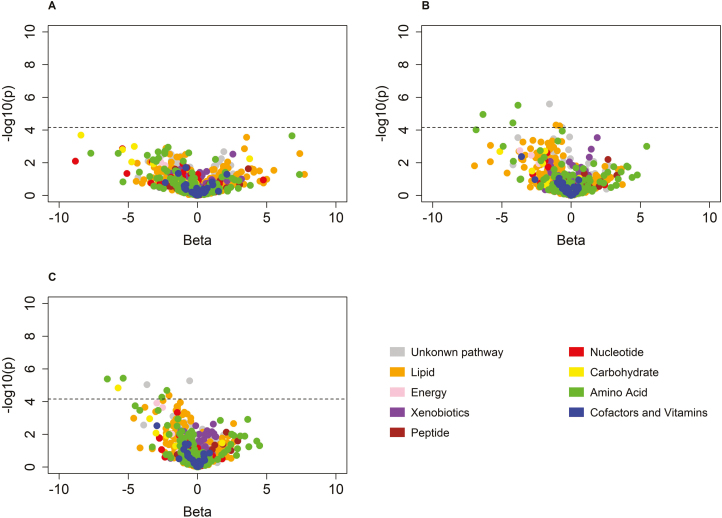
Single metabolite-based analyses identified 6 novel metabolites for AI (Figure 1, Table 2), including 2 metabolites in the amino acid super-pathway, 1 metabolite in the lipid super-pathway, and 2 metabolites of unknown identities. The 6 metabolites were all negatively associated with AI, with effect sizes ranging from –0.59 for X-12127 to –6.67 for 3-methyl-2-oxobutyrate among the overall participants. Four of those metabolites were also positively associated with PWV (Supplementary Table 1). Heart rate contributed to a large proportion of the association for each metabolite. After further controlling for heart rate, the effect sizes for the 6 metabolites changed by 25%–58% (Table 2). When blood pressure and antihypertensive medications were adjusted in the model, effect sizes for the 6 metabolites only slightly changed, ranging from 1% to 10%. However, when blood lipids and fasting glucose were adjusted in the full model, lactate and X-16135 became nonsignificant. Detailed information and race-specific associations for the 6 metabolites are presented in Supplementary Table 1.
Figure 1.
Volcano plots for the associations between 1,202 metabolites and augmentation index among African Americans (a), whites (b), and the overall Bogalusa Heart Study sample (c).
Table 2.
Metabolites significantly associated with augmentation index in single metabolite-based analyses
| Model 1 | Model 2 | Changes in β | Model 3 | Changes in β | Model 4 | Changes in β | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites by super-pathways | β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | |||
| Amino acid | |||||||||||
| 3-Methyl-2- oxobutyrate | –6.67 (1.47) | 5.99E–06 | –3.02 (1.42) | 3.36E–02 | 55% | –3.14 (1.42) | 2.74E–02 | –4% | –3.27 (1.57) | 3.78E–02 | –4% |
| 3-Methoxytyramine sulfate | –2.56 (0.56) | 6.21E–06 | –1.45 (0.54) | 7.27E–03 | 43% | –1.44 (0.54) | 7.59E–03 | 1% | –1.44 (0.56) | 9.60E–03 | 0% |
| Lipid | |||||||||||
| 1-Palmitoyl-2- arachidonoyl-GPE (16:0/20:4)* | –2.13 (0.51) | 3.03E–05 | –1.24 (0.49) | 1.09E–02 | 42% | –1.21 (0.49) | 1.35E–02 | 2% | –1.38 (0.65) | 3.50E–02 | –14% |
| Carbohydrate | |||||||||||
| Lactate | –6.11 (1.34) | 6.04E–06 | –2.57 (1.31) | 4.94E–02 | 58% | –2.83 (1.31) | 3.12E–02 | –10% | –2.39 (1.43) | 9.58E–02 | 16% |
| Unknown | |||||||||||
| X-12127 | –0.59 (0.13) | 3.37E–06 | –0.44 (0.12) | 2.55E–04 | 25% | –0.43 (0.12) | 3.59E–04 | 2% | –0.44 (0.12) | 3.70E–04 | –2% |
| X-16135 | –3.58 (0.83) | 1.99E–05 | –1.82 (0.80) | 2.31E–02 | 49% | –1.75 (0.80) | 2.83E–02 | 4% | –1.61 (0.82) | 5.10E–02 | 8% |
Bold P values are not significant. Abbreviation: PWV = pulse wave velocity; changes in Beta was calculated as (Betai–Betai+1)/Betai, and i =1–3.
Model 1: age, sex, race, education, smoking, drinking, body height, body weight, physical activity, and estimated glomerular filtration rate.
Model 2: all variables in model 1 plus heart rate.
Model 3: all variables in model 2 plus systolic blood pressure, diastolic blood pressure, and blood pressure lowering medication.
Model 4: all variables in model 3 plus low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, and fasting glucose.
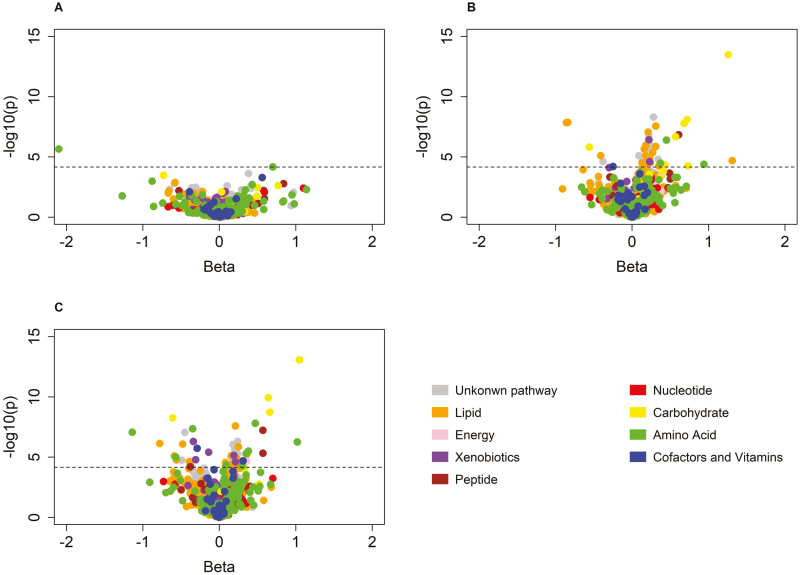
Twenty-one novel metabolites were associated with PWV (Figure 2 and Table 3). Fructose had the lowest P value (β = 0.61, P = 6.18 × 10–10), and histidine had the largest effect size (β = –1.09, P = 2.51 × 10–7). Nine of the metabolites were also associated with AI (Supplementary Table 2). As shown in Table 3, after controlling for heart rate, effect sizes for the metabolites only moderately changed, ranging from 3% to 21%. When further controlling for blood pressure and antihypertensive medication usage, associations of all metabolites became much weaker and less significant, decreasing by 9%–47%. After further adjusting for blood lipids and fasting glucose, 9 of the novel metabolites became nonsignificant. Detailed information and race-specific associations for the 21 metabolites are presented in Supplementary Table 2.
Figure 2.
Volcano plots for the associations between 1,202 metabolites and pulse wave velocity among African Americans (a), whites (b), and the overall Bogalusa Heart Study sample (c).
Table 3.
Metabolites significantly associated with pulse wave velocity in single metabolite-based analyses
| Metabolites by super-pathways | Model 1 | Model 2 | Changes in β | Model 3 | Changes in β | Model 4 | Changes in β | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | ||||
| Amino acid | |||||||||||
| Cysteine-glutathione disulfide | –0.30 (0.07) | 5.13E–06 | –0.25 (0.07) | 1.43E–04 | 17% | –0.15 (0.06) | 1.18E–02 | 40% | –0.09 (0.06) | 1.55E–01 | 40% |
| Histidine | –1.09 (0.21) | 2.51E–07 | –0.97 (0.21) | 3.41E–06 | 11% | –0.74 (0.19) | 9.30E–05 | 24% | –0.63 (0.19) | 7.64E–04 | 15% |
| Isoleucine | 1.03 (0.20) | 3.17E–07 | 1.00 (0.20) | 5.10E–07 | 3% | 0.73 (0.18) | 4.69E–05 | 27% | 0.37 (0.19) | 5.41E–02 | 49% |
| 3-Methyl-2-oxovalerate | 0.59 (0.14) | 3.47E–05 | 0.48 (0.14) | 8.94E–04 | 19% | 0.32 (0.13) | 1.25E–02 | 33% | 0.17 (0.14) | 2.26E–01 | 47% |
| Carbohydrate | |||||||||||
| Fructose | 0.61 (0.10) | 6.18E–10 | 0.56 (0.10) | 5.90E–09 | 8% | 0.38 (0.09) | 1.66E–05 | 32% | 0.27 (0.10) | 8.47E–03 | 29% |
| Mannose | 0.64 (0.11) | 2.39E–09 | 0.57 (0.11) | 1.06E–07 | 11% | 0.38 (0.10) | 1.08E–04 | 33% | 0.25 (0.15) | 8.52E–02 | 34% |
| Cofactors and vitamins | |||||||||||
| Oxalate (ethanedioate) | –0.28 (0.06) | 3.69E–06 | –0.26 (0.06) | 2.14E–05 | 7% | –0.12 (0.05) | 2.45E–02 | 54% | –0.11 (0.05) | 4.83E–02 | 8% |
| γ-Tocopherol/β-tocopherol | 0.30 (0.07) | 3.30E–05 | 0.29 (0.07) | 4.59E–05 | 3% | 0.17 (0.06) | 7.45E–03 | 41% | 0.16 (0.07) | 1.35E–02 | 6% |
| Lipid | |||||||||||
| Linoleoyl-linoleoyl-glycerol (18:2/18:2) [1]* | 0.20 (0.04) | 6.24E–06 | 0.19 (0.04) | 2.26E–05 | 5% | 0.09 (0.04) | 1.71E–02 | 53% | –0.02 (0.05) | 6.62E–01 | 122% |
| N-linoleoylserine* | –0.29 (0.06) | 6.94E–06 | –0.25 (0.06) | 1.11E–04 | 14% | –0.21 (0.06) | 2.85E–04 | 16% | –0.15 (0.06) | 1.09E–02 | 29% |
| Phosphoethanolamine | –0.38 (0.09) | 3.13E–05 | –0.36 (0.09) | 7.32E–05 | 5% | –0.19 (0.08) | 2.11E–02 | 47% | –0.13 (0.08) | 1.06E–01 | 32% |
| 1-(1-Enyl-palmitoyl)-2-linoleoyl-GPC (P-16:0/18:2)* | –0.76 (0.13) | 7.35E–09 | –0.67 (0.13) | 4.59E–07 | 12% | –0.61 (0.12) | 3.22E–07 | 9% | –0.44 (0.14) | 1.52E–03 | 28% |
| N-stearoyl-sphinganine (d18:0/18:0)* | 0.14 (0.03) | 1.50E–05 | 0.11 (0.03) | 1.10E–03 | 21% | 0.08 (0.03) | 8.30E–03 | 27% | 0.04 (0.03) | 2.27E–01 | 50% |
| Peptide | |||||||||||
| γ-Glutamylisoleucine* | 0.64 (0.11) | 4.30E–09 | 0.60 (0.11) | 3.64E–08 | 6% | 0.41 (0.10) | 2.63E–05 | 32% | 0.24 (0.10) | 2.14E–02 | 41% |
| γ-Glutamylvaline | 0.64 (0.13) | 6.58E–07 | 0.59 (0.13) | 3.46E–06 | 8% | 0.40 (0.11) | 4.30E–04 | 32% | 0.28 (0.12) | 1.77E–02 | 30% |
| γ-Glutamylglycine | –0.42 (0.10) | 1.87E–05 | –0.36 (0.10) | 1.87E–04 | 14% | –0.25 (0.09) | 3.94E–03 | 31% | –0.19 (0.09) | 2.99E–02 | 24% |
| γ-Glutamylglutamate | 0.29 (0.07) | 2.01E–05 | 0.26 (0.07) | 1.27E–04 | 10% | 0.23 (0.06) | 1.89E–04 | 12% | 0.16 (0.06) | 1.24E–02 | 30% |
| γ-Glutamylleucine | 0.58 (0.14) | 2.90E–05 | 0.54 (0.14) | 7.11E–05 | 7% | 0.37 (0.12) | 2.32E–03 | 31% | 0.19 (0.13) | 1.34E–01 | 49% |
| Xenobiotics | |||||||||||
| Tartronate (hydroxymalonate) | –0.32 (0.07) | 6.10E–06 | –0.30 (0.07) | 2.79E–05 | 6% | –0.15 (0.06) | 2.40E–02 | 47% | –0.13 (0.06) | 4.20E–02 | 19% |
| Unknown | |||||||||||
| X-11315 | –0.44 (0.08) | 1.42E–07 | –0.38 (0.08) | 5.46E–06 | 14% | –0.23 (0.08) | 2.33E–03 | 39% | –0.14 (0.08) | 8.25E–02 | 39% |
| X-24334 | 0.25 (0.05) | 2.91E–07 | 0.22 (0.05) | 5.58E–06 | 12% | 0.16 (0.04) | 3.31E–04 | 27% | 0.12 (0.05) | 1.11E–02 | 25% |
Bold P values are not significant. Abbreviation: AI = augmentation index; changes in Beta was calculated as (Betai–Betai+1)/Betai, and i =1–3.
Model 1: age, sex, race, education, smoking, drinking, body height, body weight, physical activity, and estimated glomerular filtration rate.
Model 2: all variables in model 1 plus heart rate.
Model 3: all variables in model 2 plus systolic blood pressure, diastolic blood pressure, and blood pressure lowering medication.
Model 4: all variables in model 3 plus low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and fasting glucose.
Sensitivity analysis excluding women with menopause yields similar results as the analysis among the overall participants (Supplementary Tables 1 and 2). Finally, as shown in Supplementary Table 3, we successfully replicated 12 of the 21 metabolite–PWV associations reported in previous studies.6,14–17
Network-based analysis results
WGCNA generated 10 signed modules in which all metabolites were positively correlated (Table 4). Module sizes ranged from 20 to 477 metabolites. Modules “magenta,” “pink,” “purple,” and “yellow” had very good measures in parameters of density, centralization, and heterogeneity.
Table 4.
Network statistics for modules identified in the weighted correlation network analyses and their correlations with arterial stiffness measures.
| Augmentation Index* | Pulse wave velocity* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Module | Size | Density | Centralization | Heterogeneity | Correlation | P | Correlation | P | |
| Black | 34 | 0.18 | 0.12 | 0.31 | –0.017 | 0.50 | –0.069 | 0.01 | |
| Blue | 91 | 0.07 | 0.06 | 0.35 | 0.034 | 0.20 | –0.0038 | 0.90 | |
| Brown | 84 | 0.18 | 0.13 | 0.33 | –0.057 | 0.04 | 0.039 | 0.20 | |
| Green | 43 | 0.18 | 0.11 | 0.26 | –0.038 | 0.20 | 0.064 | 0.02 | |
| Magenta | 22 | 0.25 | 0.10 | 0.20 | 0.048 | 0.09 | –0.089 | 0.002 | |
| Pink | 33 | 0.22 | 0.10 | 0.21 | 0.009 | 0.80 | –0.008 | 0.80 | |
| Purple | 20 | 0.21 | 0.11 | 0.28 | –0.10 | 3.00E–4 | 0.13 | 2.00E–6 | |
| Red | 39 | 0.12 | 0.08 | 0.27 | –0.058 | 0.04 | 0.021 | 0.40 | |
| Turquoise | 477 | 0.08 | 0.12 | 0.46 | –0.027 | 0.30 | 0.076 | 0.007 | |
| Yellow | 61 | 0.19 | 0.10 | 0.23 | –0.094 | 8.00E–4 | 0.11 | 7.00E–5 | |
*Age, sex, race, education, smoking, drinking, body mass index, and physical activity adjusted.
Bolded are significant correlations after Bonferroni correction for 10 tests in each module.
Eigenmetabolites for the “purple” and “yellow” modules were significantly associated with both AI (P = 8.00 × 10–4 and 3.00 × 10–4, respectively) and PWV (P = 7.00 × 10–5 and 2.00 × 10–6, respectively). In addition, module “magenta” was significantly associated with PWV only (P = 0.002). Detailed information on metabolites included in these significant modules are listed in Supplementary Table 4. Module “magenta” included 20 metabolites related to sphingomyelin metabolism and 2 unknown metabolites, “X-24106” and “X-24870”. Module “purple” contained 20 metabolites in a pathway of the valine, leucine, and isoleucine metabolism and the alanine–glucose cycle. The “yellow” module had 61 metabolites involved in membrane glycerol lipids synthesis and recycling.
DISCUSSION
Through global profiling of serum metabolites in a biracial cohort, we robustly identified 27 novel metabolites associated with AI or PWV and confirmed 12 of 21 metabolite–PWV associations reported in previous studies. Network-based WGCNA identified 3 biologically intriguing modules for AI and/or PWV. These findings provide important clues to delineate the mechanisms of arterial stiffening.
Six novel metabolites were negatively associated with AI, and heart rate contributed to a large proportion of the associations. Heart rate is a strong determinant for AI. The 6 novel metabolites might influence AI through heart rate. For example, lactate was strongly associated with heart rate in previous studies on physical training.18,19 Further studies on associations of those metabolites with heart rate are warranted.
Twenty-one novel metabolites were associated with PWV, and the associations were largely driven by blood pressure and antihypertensive medication usage. Elevated blood pressure is an important risk factor for arterial stiffness. These metabolites may be associated with PWV through influencing blood pressure. For example, cysteine-glutathione disulfide prevents hypertension through attenuating arterial alterations.20 In this study, this metabolite was negatively associated with PWV. Similarly, histidine has antihypertensive effects through activating the central histamine H3 receptors and decreasing nitric oxide content.21 Histidine was also negatively associated with PWV in this study. On contrary, isoleucine can significantly elevate blood pressure.22 In this study, it is positively associated with PWV. It is possible that some of the metabolites were downstream products in response to antihypertensive medication usage. Further studies on how those metabolites mediating the effect of antihypertensive medication on blood pressure may delineate the role of those metabolites in arterial stiffness. Nine of the novel metabolites became nonsignificant after further controlling for blood lipids and fasting glucose, suggesting that these metabolites may influence PWV through blood lipids and/or glucose. Meanwhile, these 9 metabolites belong to amino acid, carbohydrate, or lipids super-pathways. These metabolites may be upstream or downstream metabolites for blood lipids and/or glucose metabolisms. Future studies on the role of those metabolites in lipids and glucose metabolisms may help to delineate their impact on arterial stiffness.
Sixteen novel metabolites (4 for AI and 12 for PWV) were significantly associated with arterial stiffness independent of traditional risk factors, including hypertension, diabetes, and dyslipidemia, suggesting that those metabolites may have influenced arterial stiffness through unknown physiological mechanisms. Of those 16 metabolites, fructose, γ-tocopherol/β-tocopherol, and 3 γ-glutamyl peptides (γ-glutamylisoleusine, γ-glutamylvaline, γ-glutamylglutamate) were positively associated with PWV. Fructose is used as a sweetener in many food items. Recent studies indicated that fructose might be a key factor for metabolic syndrome23 and caused deeper vascular alteration in female rats.24 In addition, a small clinical trial in human demonstrated that fructose also increased serum c-reactive protein levels.25 γ-Tocopherol and β-tocopherol are 2 isomers of vitamin E that are most prevalent in plant seeds and widely used as dietary supplements. High level of vitamin E has prooxidant effect, and high dosage vitamin E supplementation may increase all-cause mortality.26 γ-Glutamylvaline and γ-glutamylisoleucine are peptides that have been used to enhance the mouthfulness flavor and induce long-lasting savory taste of chicken broth. Previous studies suggested that the 2 peptides were both associated with diabetes,27 an important risk factor for arterial stiffness. In addition, γ-glutamylvaline can activate the extracellular calcium-sensing receptor in the gastrointestinal tract and inhibit tumor necrosis factor-α signaling and reduce inflammation.28 γ-Glutamylglutamate is a dipeptide composed of γ-glutamate and glutamic acid. Glutamic acid a fast-excitatory neurotransmitter and a key molecule in cellular metabolism.29 Excessive accumulation of glutamic acid outside cells can lead to neuronal damage and eventual cell death, a phenomenon seen in stroke.29
Of the 16 novel metabolites associated arterial stiffness independent of traditional risk factors, 3 amino acids (3-methyl-2-oxobutyrate, 3-methoxytyramine sulfate, and histidine), oxalate, 3 lipids [1-palmitoyl-2-arachidonoyl-GPE (16:0/20:4)*, N-linoleoylserine*, and 1-(1-enyl-palmitoyl)-2-linoleoyl-GPC (P-16:0/18:2)*], peptide γ-glutamylglycine, and xenobiotics tartronate were negatively associated with arterial stiffness measures. The 3-methyl-2-oxobutyrate is a degradation product of valine and a precursor to leucine. Both valine and leucine can reduce serum levels of superoxide dismutase and glutathione peroxidase and improve the endothelial dysfunctions.30 The 3-methoxytyramine sulfate is an end product of dopamine and is involved in tyrosine metabolism. In human, dopamine D2 receptor agonist can cause adverse cardiovascular morbidity.31–33 Histidine is an essential amino acid. In a study among 1,898 female participants, although not significant, higher dietary intake of histidine resulted in lower PWV.16 The 3 lipid-related metabolites are involved in phosphatidylethanolamine, fatty acid amide, and plasmalogen metabolisms. Phosphatidylethanolamine can regulate autophagy and modulate aging process.34 Fatty acid amides are important signaling molecules in the nervous system and are involved in sleep and angiogenesis.35 Plasmalogens are important lipids that prevent membrane lipids from oxidation. Decreasing plasmalogen levels have been observed among people with metabolic syndrome, type 2 diabetes, and cardiovascular disease.36 γ-Glutamylglycine is an excitatory amino acid receptor antagonist.37 Tartonate is an inhibitor of the malic enzyme that converts pyruvate into phosphoenolpyruvate and plays a major role in the metabolism of lactate.38
WGCNA identified three metabolite pathways associated with arterial stiffness, including a pathway of sphingolipid metabolism; a pathway of valine, leucine, and isoleucine metabolism along with the alanine-glucose cycle; and a pathway of membrane glycerol lipids synthesis and recycling. Sphingolipids are involved in the development of diabetes and its complications.39 Previous studies also suggested that valine, leucine, and isoleucine were correlated with arterial stiffness.14,16 Our study provided evidence that the metabolism pathway of these amino acids was also involved in arterial stiffness. Studies of the glycerol lipids synthesis and recycle in arterial stiffness are lacking. Our study provided novel evidence that this pathway is also involved in arterial stiffening.
This study has important strengthens. First, we used the most up-to-date profiling technology and databases to identify a comprehensive list of metabolites from serum samples. This allowed us to identify many novel metabolites associated with arterial stiffness. Second, stringent quality assurance and QC procedures were applied to examine metabolites, arterial stiffness, and important covariates. Third, the BHS is a biracial cohort, which allowed us to identify important metabolites common to both African Americans and whites. Our study also has limitations. First, the study was a cross-sectional analysis; therefore, the temporal relationship between the identified metabolites and arterial stiffness is still unclear. It is possible that participants with a high risk of arterial stiffness were advised to take nutrient supplements, which could cause reverse associations. Second, this study may have missed important race-specific metabolites. Future research in multiethnic groups is warranted to validate some of the metabolites that were only significant in one ethnic group. Finally, all participants were recruited from Bogalusa, Louisiana, and familial correlations may exist for some participants. However, data on familial relationship were not collected. A small proportion of the BHS participants had genome-wide genotype data, which can be used to infer a kinship matrix. However, not all individuals with metabolomics data have genotypes. To include more participants and increase statistical power of the current analyses, we did not include the kinship matrix in our model. Future multiomics studies incorporating the genomic and metabolomics data are warranted to delineate the mechanisms of arterial stiffness.
To conclude, this study identified 27 novel metabolites associated with arterial stiffness and confirmed 12 metabolite–PWV associations reported in previous studies. For the novel metabolites, a large proportion of the associations with AI can be attributed to heart rate, and the associations with PWV were mainly driven by blood pressure and/or antihypertensive medication usage. Finally, 16 metabolites were associated with AI and/or PWV independent of traditional risk factors for arterial stiffness. Network-based analyses revealed 2 modules associated with both AI and PWV and 1 module associated with PWV only.
Supplementary Material
ACKNOWLEDGMENT
The work was supported by multiple grants from the National Institutes on Health, including awards R21AG051914 and R01AG041200 from the National Institute on Aging and award 1P20GM109036-01A1 from the National Institute of General Medical Sciences. Shengxu Li was partly supported by award 13SDG14650068 from American Heart Association. Changwei Li was partly supported by a grant from the University of Georgia Research Foundation, Inc. We thank Mr. Kevin Spiegel for the editing assistance.
DISCLOSURE
The author(s) declared no conflict of interest.
REFERENCES
- 1. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 3. Janner JH, Godtfredsen NS, Ladelund S, Vestbo J, Prescott E. High aortic augmentation index predicts mortality and cardiovascular events in men from a general population, but not in women. Eur J Prev Cardiol 2013; 20:1005–1012. [DOI] [PubMed] [Google Scholar]
- 4. Janner JH, Godtfredsen NS, Ladelund S, Vestbo J, Prescott E. The association between aortic augmentation index and cardiovascular risk factors in a large unselected population. J Hum Hypertens 2012; 26:476–484. [DOI] [PubMed] [Google Scholar]
- 5. Rosenbaum D, Giral P, Chapman J, Rached FH, Kahn JF, Bruckert E, Girerd X. Radial augmentation index is a surrogate marker of atherosclerotic burden in a primary prevention cohort. Atherosclerosis 2013; 231:436–441. [DOI] [PubMed] [Google Scholar]
- 6. Menni C, Mangino M, Cecelja M, Psatha M, Brosnan MJ, Trimmer J, Mohney RP, Chowienczyk P, Padmanabhan S, Spector TD, Valdes AM. Metabolomic study of carotid-femoral pulse-wave velocity in women. J Hypertens 2015; 33:791–796; discussion 796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson SG, Sanders TA, Cruickshank JK. Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension 2009; 53:839–845. [DOI] [PubMed] [Google Scholar]
- 8. Jung S, Kim M, Lee YJ, Lee SH, Lee JH. Associations between metabolomic-identified changes of biomarkers and arterial stiffness in subjects progressing to impaired fasting glucose. Clin Endocrinol (Oxf) 2015; 83:196–204. [DOI] [PubMed] [Google Scholar]
- 9. Li S, Chen W, Srinivasan SR, Berenson GS. Childhood blood pressure as a predictor of arterial stiffness in young adults: the bogalusa heart study. Hypertension 2004; 43:541–546. [DOI] [PubMed] [Google Scholar]
- 10. Chester R, Sander G, Fernandez C, Chen W, Berenson G, Giles T. Women have significantly greater difference between central and peripheral arterial pressure compared with men: the Bogalusa Heart Study. J Am Soc Hypertens 2013; 7:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 12. Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol 2005; 4:Article17. [DOI] [PubMed] [Google Scholar]
- 13. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008; 9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zagura M, Kals J, Kilk K, Serg M, Kampus P, Eha J, Soomets U, Zilmer M. Metabolomic signature of arterial stiffness in male patients with peripheral arterial disease. Hypertens Res 2015; 38:840–846. [DOI] [PubMed] [Google Scholar]
- 15. Paapstel K, Kals J, Eha J, Tootsi K, Ottas A, Piir A, Zilmer M. Metabolomic profiles of lipid metabolism, arterial stiffness and hemodynamics in male coronary artery disease patients. IJC Metab Endocr 2016; 11:13–18. [Google Scholar]
- 16. Jennings A, MacGregor A, Welch A, Chowienczyk P, Spector T, Cassidy A. Amino acid intakes are inversely associated with arterial stiffness and central blood pressure in women. J Nutr 2015; 145:2130–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohnishi H, Saitoh S, Takagi S, Ohata J, Isobe T, Kikuchi Y, Takeuchi H, Shimamoto K. Pulse wave velocity as an indicator of atherosclerosis in impaired fasting glucose: the Tanno and Sobetsu study. Diabetes Care 2003; 26:437–440. [DOI] [PubMed] [Google Scholar]
- 18. Bouhel E, Jouini A, Gmada N, Nefzi A, Ben Abdallah K, Tabka Z. Heart rate and blood lactate responses during Taekwondo training and competition. Sci Sports 2006; 21:285–290. [Google Scholar]
- 19. Belcher CP, Pemberton CL. The use of the blood lactate curve to develop training intensity guidelines for the sports of track and field and cross-country. Int J Exerc Sci 2012; 5:148–159. [Google Scholar]
- 20. Vasdev S, Stuckless J. Antihypertensive effects of dietary protein and its mechanism. Int J Angiol 2010; 19:e7–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toba H, Nakamori A, Tanaka Y, Yukiya R, Tatsuoka K, Narutaki M, Tokitaka M, Hariu H, Kobara M, Nakata T. Oral L-histidine exerts antihypertensive effects via central histamine H3 receptors and decreases nitric oxide content in the rostral ventrolateral medulla in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 2010; 37:62–68. [DOI] [PubMed] [Google Scholar]
- 22. Cicero AF, Colletti A, Rosticci M, Cagnati M, Urso R, Giovannini M, Borghi C, D’Addato S. Effect of lactotripeptides (Isoleucine-Proline-Proline/Valine-Proline-Proline) on blood pressure and arterial stiffness changes in subjects with suboptimal blood pressure control and metabolic syndrome: a double-blind, randomized, crossover clinical trial. Metab Syndr Relat Disord 2016; 14:161–166. [DOI] [PubMed] [Google Scholar]
- 23. Khitan Z, Kim DH. Fructose: a key factor in the development of metabolic syndrome and hypertension. J Nutr Metab 2013; 2013:682673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sangüesa G, Shaligram S, Akther F, Roglans N, Laguna JC, Rahimian R, Alegret M. Type of supplemented simple sugar, not merely calorie intake, determines adverse effects on metabolism and aortic function in female rats. Am J Physiol Heart Circ Physiol 2017; 312:H289–H304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jameel F, Phang M, Wood LG, Garg ML. Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis 2014; 13:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller ER III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142:37–46. [DOI] [PubMed] [Google Scholar]
- 27. Suhre K, Meisinger C, Döring A, Altmaier E, Belcredi P, Gieger C, Chang D, Milburn MV, Gall WE, Weinberger KM, Mewes HW, Hrabé de Angelis M, Wichmann HE, Kronenberg F, Adamski J, Illig T. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One 2010; 5:e13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang H, Kovacs-Nolan J, Kodera T, Eto Y, Mine Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim Biophys Acta 2015; 1852:792–804. [DOI] [PubMed] [Google Scholar]
- 29. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 2000; 130:1007S–1015S. [DOI] [PubMed] [Google Scholar]
- 30. Cojocaru E, Filip N, Ungureanu C, Filip C, Danciu M. Effects of valine and leucine on some antioxidant enzymes in hypercholesterolemic rats. Health 2014; 6:2313–2321. [Google Scholar]
- 31. Abou Farha K, Baljé-Volkers C, Tamminga W, den Daas I, van Os S. Dopamine D2R agonist-induced cardiovascular effects in healthy male subjects: potential implications in clinical settings. ISRN Neurol 2014; 2014:956353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arbouw ME, Movig KL, Guchelaar HJ, Neef C, Egberts TC. Dopamine agonists and ischemic complications in Parkinson’s disease: a nested case-control study. Eur J Clin Pharmacol 2012; 68:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsieh PH, Hsiao FY. Risk of heart failure associated with dopamine agonists: a nested case-control study. Drugs Aging 2013; 30:739–745. [DOI] [PubMed] [Google Scholar]
- 34. Rockenfeller P, Koska M, Pietrocola F, Minois N, Knittelfelder O, Sica V, Franz J, Carmona-Gutierrez D, Kroemer G, Madeo F. Phosphatidylethanolamine positively regulates autophagy and longevity. Cell Death Differ 2015; 22:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farrell EK, Chen Y, Barazanji M, Jeffries KA, Cameroamortegui F, Merkler DJ. Primary fatty acid amide metabolism: conversion of fatty acids and an ethanolamine in N18TG2 and SCP cells. J Lipid Res 2012; 53:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 2012; 1822:1442–1452. [DOI] [PubMed] [Google Scholar]
- 37. Sawada S, Yamamoto C. Gamma-D-glutamylglycine and cis-2,3-piperidine dicarboxylate as antagonists of excitatory amino acids in the hippocampus. Exp Brain Res 1984; 55:351–358. [DOI] [PubMed] [Google Scholar]
- 38. Gutierrez G, Fernandez E, Hurtado FJ, Kiiski R, Chakravarthy S, Ronco JJ, Arbelaez G. Hydroxymalonate inhibits lactate uptake by the rabbit hindlimb. J Appl Physiol (1985) 1994; 76:2735–2741. [DOI] [PubMed] [Google Scholar]
- 39. Russo SB, Ross JS, Cowart LA. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handb Exp Pharmacol 2013; 216:373–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.