Abstract
Objective: The purpose of this study was to evaluate the efficacy and safety of a multicomponent nutraceutical (MCN) on facial skin. Methods: A randomized, placebo-controlled, single-blind trial was conducted involving two groups of female subjects affected by facial skin photoaging. For two months, volunteers took a daily dose of MCN containing 200mg of hyaluronic acid, 500mg of L-carnosine, and 400mg of methylsulfonylmethane, or a placebo. At Day 0 (T0) and Day 60 (T60), face skin hydration, elasticity, and sebometry were measured with an instrumental skin tester, and digital images of facial wrinkles were scored. A subject-based quali-/ quantitative assessment evaluating satisfaction/ quality of life was performed at T60. Results: The MCN and placebo groups each included 25 volunteers (mean ages: 49.3 and 47.8 years, respectively). After 60 days of MCN intake, glabella skin hydration and elasticity improved by 15.2 percent and 22.6 percent, respectively (p=0.03; p=0.004), glabella sebaceous secretion decreased by 24.2 percent (p=0.01), skin hydration and elasticity of the periocular area increased by 12.6 percent and 15.9 percent, respectively, and skin hydration and elasticity of the oral commissural area increased by 17.6 percent and 16 percent, respectively (p<0.001). No significant variation occurred in the placebo group. Wrinkle depth improved slightly in the MCN group (p=0.043 in the periocular area) but not in the placebo group. A slight improvement in joint pain and mucosae/ hair appearance was reported in the questionnaire in the MCN group only. Conclusions: Our results suggest that MCN is safe and effective for facial skin aesthetics and well-being.
Keywords: Carnosine, face, hyaluronic acid, MSM, Nutraceutical, skin hydration, skin elasticity
Skin aging is related to several interacting factors, including the progressive reduction and alteration of hyaluronic acid and other glycosaminoglicanes; oxidative stress, hyperproduction of free radicals, and impairment of radical scavenging mechanisms, which characterize all aging processes; glycation of different basic elements, namely proteins such as elastin and collagen, with advanced glycation end-product (AGE) formation; photoaging ultraviolet (UV) light exposure; endocrinosenescence, which tends to alter skin metabolism especially in the postmenopausal period in women; and postural/gravity interaction with skin sagging.1,2 Any conservative intervention that promotes an improvement in skin hydration, elasticity, aesthetics, and wellness in general should target most of these pathophysiologic processes. Literature on the oral supplementation of different moleculae in the form of food supplements shows interesting outcomes on facial skin health and aesthetics.3–7 While more invasive treatments can restore texture and hydration to the facial skin, nutrition and nutraceuticals might also play a relevant role in skin rejuvenation, as different metabolic processes form the basis of any skin pathophysiological deterioration.1
The present clinical/instrumental randomized controlled trial (RCT) (active vs. placebo) evaluated the safety and efficacy of a multicomponent nutraceutical (MCN) for targeting most of the skin aging processes, as described. The MCN contains three skin/ mucosae-oriented ingredients. The first, hyaluronic acid, is a polysaccharide that constitutes skin and mucosae tissues, as well as joint cartilage.3,8,9,11 The second, l-carnosine, is a dipeptide (β alanin+ l-histidine) shown to be one of the most effective antiglycation agents available,11 reducing AGE negative effects and the related cross-linking with skin proteins and lipids, which might ultimately reduce the “caramelization” of the tissues.2 Of note, carnosine has shown antioxidant power and a pH alkalinizing feature, which influences connective tissue.12,14 Lastly, methylsulfonylmethane (MSM) is a sulfur-based component that contributes to stabilizing and synergizing protein-to-protein linkages and reduces inflammatory mediators.15,16 Sulfur is also a basic principle for the formation of glutathione, the most important natural antioxidant in human beings.13
The secondary objective of the present RCT was to evaluate any possible beneficial effect on mucosae, hair, and joints.
METHODS
A randomized, placebo-controlled, single-center, single-blind clinical/instrumental trial was performed. The trial included 50 female subjects between the ages of 40 and 65 years with skin chronoaging and/or photoaging. Exclusion criteria were allergy to one or more of the ingredients of the MCN; being younger or older than 40 to 65 years of age; ongoing therapy with drugs that could positively or negatively impact skin or mucosae tissue (e.g., corticoids, antibiotics, anti-inflammatory drugs); active cancer; and active treatment of facial skin.
Through clinical and instrumental investigations, different parameters concerning the facial skin were assessed and scored. Patient-reported outcomes (PROs) and physician judgements were included in the protocol to assess the final results. The 50 subjects were enrolled after a dermatologist’s clinical examination and then randomized into two groups by way of an online randomizer (https://www.random.org/sequences/). In Group A, 25 subjects took two tablets of the active MCN per day for two months, whereas 25 subjects in Group B took two tablets of the placebo daily for two months. Both groups of subjects were unaware of the nature of their tablets. The MCN (Hyaluseon; Proeon SURL, San Benedetto del Tronto, Italy) composition was as follows: 200mg/day of hyaluronic acid (molecular weight: 1200–1,500kDa) of vegetable origin, 500mg of L-carnosine, and 400mg of MSM. The placebo tablets were made of microcrystalline cellulose and transparent gelatin and did not contain any active substances.
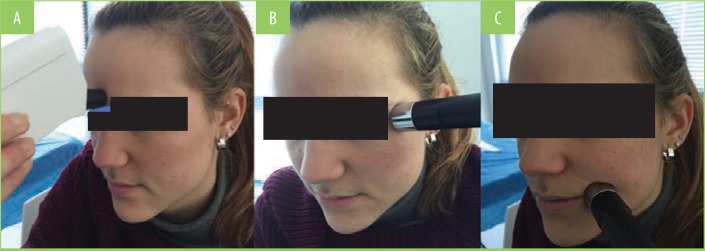
At Day 0 (T0) and Day 60 (T60), all patients underwent an instrumental examination using a validated diagnostic medical device suitable for testing multiple skin parameters (Multi Skin Test Center MC 750 system; Courage+Khazaka electronic GmbH, Cologne, Germany).17–19 Three different probes were used to measure three facial skin parameters at TO and T60 in three prefixed areas (the glabella and left periocular and left oral commissurals, Figure 1). The three parameters measured were skin hydration, assessed through capacitance measurement; sebum content, measured using a photometric method; and skin elasticity (biological skin age), determined through suction. Skin elasticity was calculated as a percentage of skin resistance to suction and expressed in comparison to the original state. The examinations were performed at the same time of the day for each participant, and all participants were instructed to avoid wearing facial makeup in the hours prior to the instrumental examination. These three parameters were evaluated using a using a dedicated software program provided by the medical device (Multi Skin Test Center MC 750 system). For each facial area, the measurement was repeated three times and the median value was included for the final statistical analysis. A second instrumental assessment of the three facial skin areas was based on digital photo imaging (DPI). At T0 and T60, digital photographic images were taken for each of the three areas and stored to be reviewed and compared by the same investigator (VQ). Specific attention was paid to reproduce the same camera settings, distance, and light conditions at T0 and T60.
FIGURE 1.
Face points evaluated using the Multi Skin Test Center MC 750 system—A) glabella, B) periocular, C) oral commissural
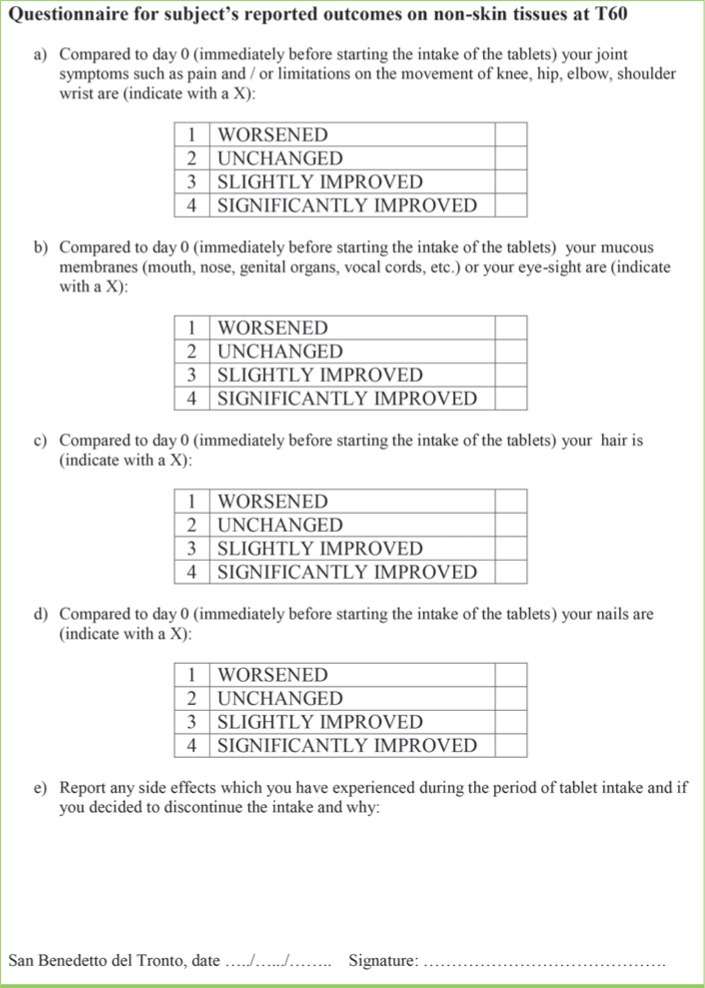
A wrinkle assessment scale20 was used to score each image with regard to the facial wrinkle appearance, with a reference scale ranging from 0.5 points (no visible wrinkles) to 3 points (evident wrinkles deeper than 3mm). In order to qualitatively and quantitatively assess evaluation of subjects on the possible efficacy of the MCN and placebo, a questionnaire was completed by all the subjects at T60 (Figure 2). Visual analog scale (VAS) assessment (1=very unsatisfied, 2=unsatisfied, 3=satisfied, and 4=fully satisfied) was performed to assess self-reported patient judgment of wrinkle improvement, quality of life, and degree of satisfaction at T60. Lastly, all subjects were asked to report any effects on mucosae (i.e., mouth, genitals, vocal cords) as well as joints, hair, and nails at T60, for the purpose of assessing previously documented effects of MCN ingredients on these areas.4,10,14–16,21
FIGURE 2.
Questionnaire for subject’s reported outcomes on non-skin tissues at Day 60
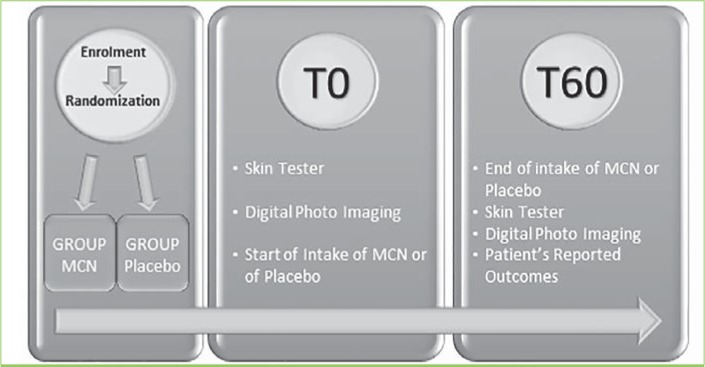
Figure 3 depicts the flowchart and timetable of the RCT. Subjects were fully informed about the study aims and protocols and provided signed informed consent regarding their participation in the study, including photoconsent. The study was performed adhering to ethics protocol as per Helsinki Declaration.
FIGURE 3.
Diagram showing the randomized controlled study design; MCN: multicomponent nutraceutical; TO: Day 0; T60: Day 60
The patients were invited to report any side effects throughout the study period and were educated regarding the necessity of maintaining a stable nutrition regimen and lifestyle, without intake of any other medications or supplements or use of topical substances (e.g., creams, oils) with known activity on the investigated parameters. All data were collected and stored for the relative subsequent statistical analysis, which was performed using the Microsoft Office Excel software program (Microsoft Corp., Redmond, Washington). P-values were calculated for the comparison of all variables using a t-paired test and the Wilcoxon test, and a p-value of 0.05 was considered to be the cutoff for statistical significance.
RESULTS
Groups A and B comprised homogenous subjects with respect to age, body mass index, and phototype. The statistical analysis showed no significant difference between the two groups (Table 1). At the final T60 investigation, one patient in the MCN group was lost to follow-up due to inability to adhere to follow-up timing. The final statistical analysis included 24 subjects in Group A (MCN) and 25 subjects in Group B (placebo).
TABLE 1.
Comparison between the two randomized groups
| CHARACTERISTIC | MCN | PLACEBO | P_VALUE |
|---|---|---|---|
| Smoking | 7 | 3 | ns |
| Contraceptive pill | 2 | 2 | ns |
| Age (mean) (years) | 49.29 | 47.85 | p=0.16 |
| Body mass index (kg/m2) | 21.73 | 21.55 | p=0.45 |
| Skin phototype | |||
| Type 2 | 4 | 6 | ns |
| Type 3 | 15 | 13 | ns |
| Type 4 | 5 | 6 | ns |
| MCN: multicomponent nutraceutical; ns: not signficant | |||
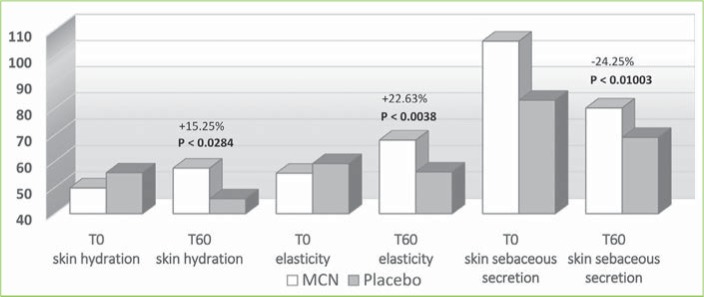
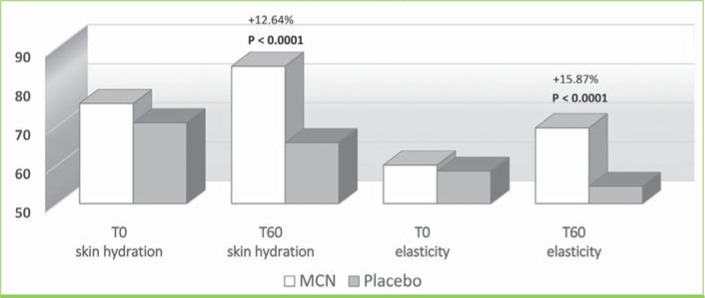
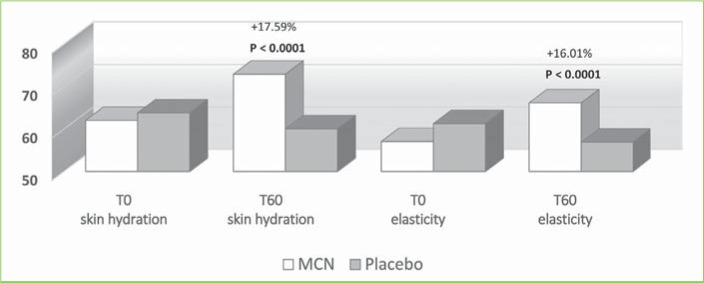
The instrumental examination performed using the Multi Skin Test Center MC 750 system revealed an overall improvement in the investigated parameters at T60 in the MCN group. Conversely, subjects taking the placebo showed no significant improvement in facial skin hydration, elasticity, or sebum. More specifically, at T60, skin hydration and elasticity of the glabella area were improved by 15.2 and 22.6 percent in the MCN group (p=0.03 and p=0.004, respectively); in the placebo group, the values for these parameters were not significantly different versus at baseline. Additionally, sebaceous secretion was reduced at the level of the glabella for the MCN group by 24.2 percent, with a statistically significant difference (p=0.010), while no improvement was observed in the placebo group. In the periocular zone, skin hydration and elasticity increased by 12.6 and 15.9 percent, respectively, in the MCN group, with a statistically significant difference (p<0.001 for both parameters). The placebo group did not show a statistically significant improvement in the periocular zone. In the oral commissural area, skin hydration and elasticity were increased by 17.6 and 16 percent, respectively (p<0.001 for both parameters) in the MCN group, but no statistically significant variation was measured in the placebo group. Figures 4, 5, and 6 show the results and the relative statistical values for all parameters.
FIGURE 4.
Skin tester results in the glabella; MCN: multicomponent nutraceutical; TO: Day 0; T60: Day 60
FIGURE 5.
Skin tester results in the periocular area; MCN: multicomponent nutraceutical; TO: Day 0; T60: Day 60
FIGURE 6.
Skin tester results in the commissural area; MCN: multicomponent nutraceutical; TO: Day 0;T60: Day 60
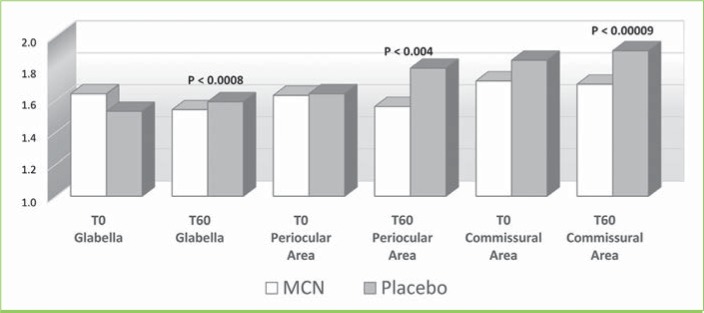
Analysis of the VAS score of DPI showed a slight improvement of wrinkles in the three regions in the MCN group (Figure 7), but the variation was statistically significant only in the periocular region (p=0.043). Figure 10 shows one of the most improved at T60 cases of the periocular zone. No statistically significant variation of wrinkles on digital images was observed in the placebo group.
FIGURE 7.
Wrinkle depth visual analog scale (VAS) assessment; MCN: multicomponent nutraceutical; TO: Day 0; T60: Day 60
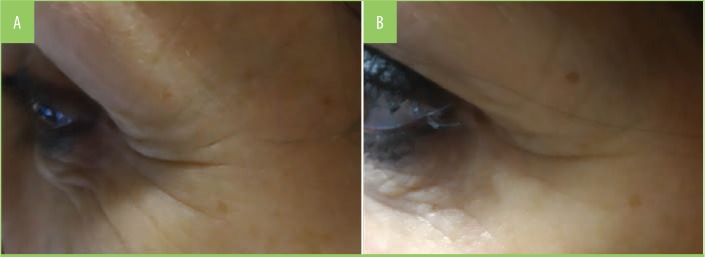
FIGURE 8.
Comparison of periocular wrinkles—A) baseline and (B) Month 2 (T60) following treatment with multicomponent nutraceutical (MSN) intake
The qualitative/quantitative VAS assessment of the PRO regarding wrinkle appearance and the patient satisfaction at T60 indicated an overall improvement in the MCN group, but not in the placebo group. Table 2 shows the detailed patient-reported subjective outcomes. During VAS assessment, the subjects taking MCN reported a 1.98-point improvement in wrinkle appearance (“slight improvement”), while the placebo group reported an 0.96-point improvement (“no improvement”). The MCN group also reported overall satisfaction (2.83–3.25; from “satisfied” to “very satisfied”) with facial skin changes. Conversely, the placebo group patients reported a significantly lower degree of satisfaction with the results (1.73–2.34; from “very unsatisfied” to “unsatisfied”).
TABLE 2.
Subject-reported outcomes
| PARAMETER | MCN | PLACEBO |
|---|---|---|
| Wrinkle improvement | 1.98 | 0.96 |
| Beneficial change in facial skin | 2.83 | 1.73 |
| Younger-looking face | 2.83 | 1.88 |
| Continuation of tablet intake | 3.25 | 2.19 |
| Recommendation of the product to a friend | 3.23 | 2.34 |
| Family judgement of the outcomes | 2.76 | 1.73 |
| Improvement of hair/joints/mucosae/nails | 2.92 | 0.92 |
| MCN: multicomponent nutraceutical | ||
Concerning effects of MCN on the nonskin tissues, subjects reported a slight improvement in joint pain and in mucosae appearance and hydration, most notably in hair appearance and texture (average score of 2.92). Subjects in the placebo group did not report similar results in hair improvement (average score was 0.92). Table 2 summarizes all data from the subject self-assessment of the outcomes.
One case of epigastralgia and one case of atypical vaginal itching (the latter was not believed to be linked to intake of the active substances) were recorded in two subjects taking MCN, without any other side effects reported by patients in either group.
DISCUSSION
Skin aging is related to several factors, including a progressive reduction of hydration, elasticity, sebum regulation, and other negative pathophysiology mechanisms. Multicomponent nutraceuticals have been shown to improve facial skin health and appearance;4,5,7,22 the nutraceuticals hyaluronic acid,3,8,9 carnosine,11,12,23–26 and MSM27,28 have been identified as beneficial for skin and mucosae hydration, elasticity, sebum secretion, wrinkle formation, tonicity, and pigmentation.
In the present study, the lack of any significant positive outcome in the placebo group corroborates the results of the present study on the investigated MCN. The impact of the placebo effect on aesthetic and well-being measures is well known;25 hence, the comparison of active and placebo interventions a study such as ours is instrumental to obtaining scientifically sustainable outcomes. In the patients who received the MCN supplement, the skin-tester results showed a statistically significant decrease in sebum secretion in the glabella area, a possible result of the hyaluronic acid action7,29 and improved control of oxidative stress and glycation. However, the significant difference in baseline sebum levels between the two groups makes this comparison of limited significance with regard to the efficacy of the MCN on sebum secretion.
Separately, the overall increased hydration and elasticity in the facial skin of the subjects who received the MCN is likely due to the synergistic action of the three components. Conversely, the subjects given placebo tablets experienced no statistically significant improvement in facial skin hydration and elasticity at T60. Wrinkle depth assessment by means of DPI resulted in a statistically significant improvement only in the periocular area in the MCN group, without any significant change in the placebo group.
The overall subject satisfaction (average of 3 points on a 4-point scale) in the MCN group is of interest, as PROs represent important items in the assessment of any treatment related to skin aesthetics and well-being. The psychological and subjective component has a strong impact on the assessment of any type of skin-targeting therapy.25 Our PROs matched both the instrumental and the medical-assessed results for both the MCN and placebo patients.
The positive subjective assessment of the MCN effect on the joints, mucosae, nails, and hair might be a result of the proprieties of its three components on these nonskin tissues.10,12,16,21,30 These findings suggest that a separate trial to confirm any possible beneficial effects of the MCN on nonskin targets would be of value.
Supplementation with carnosine, hyaluronic acid, and MSM is generally considered safe according to existing literature data,3,8,9,26,27 and our own experience confirms the absence of any significant adverse effects in the investigated cohort of subjects. The two cases of transient and possibly MCN-related side effects (one of them of probable casual concomitant onset) were not severe enough to cause the volunteers to discontinue use of the nutraceutical.
Limitations. Generally, the overall positive influence of any dietary supplement on skin metabolism and appearance should be investigated for a longer time interval than the present study due to the intrinsic pathophysiology mechanisms and metabolism of the dermal and subdermal tissues. The short duration of the investigative and treatment period of our study might have limited our results, especially in terms of wrinkle changes.
CONCLUSION
The administration of a multicomponent food supplement of hyaluronic acid, carnosine, and MSM has demonstrated efficacy for facial skin hydration, elasticity, and sebaceous secretion in a short-term follow-up period, with a statistically significant improvement between T0 and T60; conversely, the placebo showed no improvement in the same parameters at T60. Skin wrinkles showed a statistically significant improvement only in the periocular area of the MCN patients, while not at all in the placebo group. The degree of subject satisfaction regarding skin appearance, well-being, and quality of life was significantly higher in MCN subjects. No significant adverse effects were reported in either group. Some generic improvements of hair, joints, and mucosae were subjectively reported by participants the MCN group. While a study with longer term follow-up is needed to confirm our initial findings, our results suggest that the MCN product is safe and effective, at least short term, for improving facial skin aesthetics and well-being. We believe the MCN product could potentially be a beneficial option for those seeking rejuvenation.
REFERENCES
- 1.Puizina-lvic N. Skin aging. Acta Dermotoven. 2008;17(2):47–54. [PubMed] [Google Scholar]
- 2.Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermato-Endocrinology. 2012;4(3):259–270. doi: 10.4161/derm.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawada C, Yoshida T, Yoshida H, et al. Ingested hyaluronan moisturizes dry skin. Nutr J. 2014;13:70. doi: 10.1186/1475-2891-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yagoda M, Gans E. A nutritional supplement formulated with peptides, lipids, collagen and hyaluronic acid optimizes key aspects of physical appearance in nails, hair and skin. J Nutr Food Sci. 2014;S5:002. [Google Scholar]
- 5.Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27(1):47–55. doi: 10.1159/000351376. [DOI] [PubMed] [Google Scholar]
- 6.Choi SY, Ko EJ, Lee YH, et al. Effects of collagen tripeptide supplement on skin properties: a prospective, randomized, controlled study. J Cosmet Laser Ther. 2014;16(3):132–137. doi: 10.3109/14764172.2013.854119. [DOI] [PubMed] [Google Scholar]
- 7.Di Cerbo A, Laurino C, Palmieri B, lannitti T. A dietary supplement improves facial photoaging and skin sebum, hydration and tonicity modulating serum fibronectin, neutrophil elastase 2, hyaluronic acid and carbonylated proteins. J Photochem Photobiol B. 2015;144:94–103. doi: 10.1016/j.jphotobiol.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Papakonstantinou E, Roth M, Karakiulakis G Hyaluronic acid. A key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawada C, Yoshida T, Yoshida H, et al. Ingestion of hyaluronans (molecular weights 800 k and 300 k) improves dry skin conditions: a randomized, double blind, controlled study. J Clin Biochem Nutr. 2015;56(1):66–73. doi: 10.3164/jcbn.14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tashiro T, Seino S, Sato T, et al. Oral administration of polymer hyaluronic acid Alleviates Symptoms of Knee Osteoarthritis: A Double-Blind, Placebo-Controlled Study over a 12-Month Period. ScientificWorldJournal. 2012;2012:167928.. doi: 10.1100/2012/167928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy VP, Garrett MR, Perry G, Smith MA. Carnosine: a versatile antioxidant and antiglycating agent. Sci Aging Knowledge Environ. 2005;2005(18):pe12. doi: 10.1126/sageke.2005.18.pe12. [DOI] [PubMed] [Google Scholar]
- 12.Budzeń S, Rymaszewska J. The biological role of carnosine and its possible applications in medicine. Adv Clin Exp Med. 2013;22(5):739–744. [PubMed] [Google Scholar]
- 13.Marí M, Morales A, Colell A, et al. Mitochondrial glutathione: a key survival antioxidant. Antioxid Redox Signal. 2009;11(11):2685–700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn PJ, Boldyrev AA, Formazuyk VE. Carnosine: its properties, functions and potential therapeutic applications. Mol Aspects Med. 1992;13(5):379–444. doi: 10.1016/0098-2997(92)90006-l. [DOI] [PubMed] [Google Scholar]
- 15.Van der Merwe M, Bloomer RJ. The influence of methylsulfonylmethane on inflammation-associated cytokine release before and following strenuous exercise. J Sports Med. 2016;2016:7498359.. doi: 10.1155/2016/7498359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brien S, Prescott P, Lewith G. Meta-analysis of the related nutritional supplements dimethyl sulfoxide and methylsulfonylmethane in the treatment of osteoarthritis of the knee. Evid Based Complement Alternat Med. 2011;2011:528403.. doi: 10.1093/ecam/nep045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herane M, Fuenzalida H, Zegpi E, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase—a double-blind, randomized, placebo-controlled study. J Cosmetic Dermatol. 2009;8:181–185. doi: 10.1111/j.1473-2165.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez N, Sanz X, Dengra J, et al. Five-year outcomes, cosmesis, and toxicity with 3-dimensional conformai external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2013;87(5):1051–1057. doi: 10.1016/j.ijrobp.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg L, Crysler C. A single center, pilot, double-blinded, randomized, comparative, prospective clinical study to evaluate improvements in the structure and function of facial skin with tazarotene 0.1% cream alone and in combination with glisodin® Skin Nutrients Advanced Anti-Aging Formula. Clin Cosmet Investig Dermatol. 2014;7:139–144. doi: 10.2147/CCID.S57600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arsiwala S, Tahiliani S, Jerajani H, Chandrashekhar Bs, Aurangabadkar S, et al. Evaluation of topical anti-wrinkle and firming (AWF) for women, antiwrinkle and firming (AFM) for men and deep wrinkles for wrinkles on face and neck. Asian J Pharm Clin Res. 2013;6(3):86–89. [Google Scholar]
- 21.Ekin M, Yaşar L, Savan K, et al. The comparison of hyaluronic acid vaginal tablets with estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Arch Gynecol Obstet. 2011;283(3):539–543. doi: 10.1007/s00404-010-1382-8. [DOI] [PubMed] [Google Scholar]
- 22.De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:4389410. doi: 10.1155/2016/4389410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babizhayev MA, Deyev AI, Savel’yeva EL, et al. Skin beautification with oral non-hydrolized versions of carnosine and carcinine: effective therapeutic management and cosmetic skincare solutions against oxidative glycation and free-radical production as a causal mechanism of diabetic complications and skin aging. J Dermatolog Treat. 2012;23(5):345–384. doi: 10.3109/09546634.2010.521812. [DOI] [PubMed] [Google Scholar]
- 24.Hipkiss AR, Baye E, de Courten B. Carnosine and the processes of ageing. Maturitas. 2016;93:28–33. doi: 10.1016/j.maturitas.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Batres C, Kramer SS, DeAngelis CG, Russell R. Examining the “cosmetics placebo effect.”. PLoS ONE. 2019;14(1):e0210238.. doi: 10.1371/journal.pone.0210238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babizhayev MA. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5(5):116–143. [Google Scholar]
- 27.Butawan M, Benjamin RL, Bloomer RJ. Methylsulfonylmethane: spplications and safety of a novel dietary supplement. Nutrients. 2017;9(3):290. doi: 10.3390/nu9030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amirshahrokhi K, Khalili AR. Methylsulfonylmethane is effective against gastric mucosal injury. Eur J Pharmacol. 2017;811:240–248. doi: 10.1016/j.ejphar.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 29.Jung YR, Hwang C, Ha JM, et al. Hyaluronic acid decreases lipid synthesis in sebaceous glands. J Invest Dermatol. 2017;137(6):1215–1222. doi: 10.1016/j.jid.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Shanmugam S, Baskaran R, Nagayya-Sriraman S, et al. The effect of methylsulfonylmethane on hair growth promotion of magnesium ascorbyl phosphate for the treatment of alopecia. Biomol Ther. 2009;17(3):241–248. [Google Scholar]