Abstract
Objectives: Investigators sought to evaluate the antioxidant capacity of a comprehensive topical antioxidant (WEL-DS), its ability to protect skin against the oxidizing effects of UVA/UVB radiation, and to assess the effectiveness and tolerability of WEL-DS for visible improvements in facial photodamage. Study Designs: In-vitro testing utilized a hydrogen peroxide assay to detect activity in human skin explants following application with WEL-DS, a leading antioxidant serum (L-AOX), and a saline control. Clinical studies included a minimal erythema dose (MED) trial in female subjects, aged 35 to 60 years. Skin was initially irradiated to determine each subject’s MED. WEL-DS was applied for four days to one site on the lower back of subjects; the other site remained untreated. Both sites were irradiated with 1X, 2X and 3X each subject’s MED, digital images were obtained, and punch biopsies were collected from the 3X MED irradiated areas for histological analysis. A second clinical study evaluated efficacy and tolerability of twice daily application of WEL-DS in female subjects, aged 25 to 65 years with mild-to-moderate photodamage. Changes in fine lines/ wrinkles, dyschromia, erythema, skin tone, pores, and tolerability were assessed at baseline and Weeks 4, 8, and 12. A subset of subjects were evaluated through Week 16. Results: Skin treated with WEL-DS neutralized up to 53 percent more oxidative stress relative to L-AOX. WEL-DS-treated skin demonstrated significantly less UV-induced erythema at 1X, 2X, and 3X MED and demonstrated cellular protective effects versus untreated irradiated skin (N=5). WEL-DS demonstrated average improvements from baseline of 37 percent, fine lines/ wrinkles; 17 percent, skin tone; 13 percent, dyschromia; 18 percent, erythema; and four percent, pores (N=21; Week 12). Continued improvements were demonstrated in all parameters in an extension study (n=14; week 16). WEL-DS was well-tolerated. Conclusion: These studies demonstrate WEL-DS’s innate ability to quench free radicals, protect skin from the oxidizing effects of UV radiation, and reduce the visible effects of facial photodamage.
Keywords: Antioxidants, oxidative stress, UV-induced erythema, MED, Photoprotection, facial aging
Skin aging is a complex process influenced by intrinsic and extrinsic factors that lead to cumulative and structural changes affecting the appearance of facial skin.1 Three percent of the factors associated with the skin aging process are genetic or physiological in nature, whereas 97 percent are extrinsic in nature and trigger the generation of reactive oxygen species (ROS) or free radicals.1,2 Free radicals are unstable molecules that take electrons from molecules, rendering them nonfunctional or dysfunctional, which results in cumulative damage to skin cells.3 In addition to the natural formation of free radicals through normal metabolic processes, exogenous atmospheric factors, such as ultraviolet (UV) light, and environmental factors, such as irritants, pollution, and smoke, can trigger the production of free radicals.3–5 Eighty percent of free radical damage is thought to be caused by UV-A and -B light exposure to the skin, and the damaging effects of UV light and infrared radiation (IR) to the skin are well documented.3,4,6
Skin has the ability to protect itself against the harmful effects of UV radiation and other environmental factors through an elaborate antioxidant defense system.3,4,7–11 However, as human skin ages, the generation of free radicals increases while the natural endogenous defenses of the skin decrease in efficacy.4,11 The inability of skin to counteract or repair the cumulative effects of free radical damage leads to oxidative stress.12 Coupled with everyday environmental exposure and the bombardment of free radicals, internal defenses can become overwhelmed and lose the ability to function efficiently, which can lead to accelerated skin aging.4 The resulting cumulative and structural changes to the skin manifest in the development of fine lines/wrinkles, dyschromia, sallowness, dehydration, and dryness.11,13,14
Supplementing skin with topical antioxidants can replenish depleted antioxidant levels,15 which can enhance the skin’s natural antioxidant defenses. In contrast to sunscreens, which work on the top layer of skin, topical antioxidants penetrate the skin to stabilize or deactivate free radicals before they damage cells.16–18 Topical antioxidants counteract free radical damage caused by UV light (290–400nm), visible light (400–700nm), and IR radiation (>800nm) as well as other environmental insults (e.g., smog, ozone, particulate matter) that sunscreens are unable to neutralize.5,19 Topical antioxidants are instrumental in skin protection and repair and provide multiple skin health benefits.19
Topical antioxidants are derived from numerous sources and possess unique properties that are thought to benefit the skin and provide varying levels of support in combating free radicals.15,20 Hydrophilic antioxidants, such as vitamin C, protect the water-containing portions of cells, interior cell structures, and interstitial fluid.15,20 Enzymatic antioxidants, such as superoxide dismutase and ubiquinone, support the body’s internal defense system and protect mitochondria. Hydrophobic antioxidants, such as vitamin E, protect the lipid-rich cell components, such as the cell membrane.15,20 Comprehensive skin protection can be achieved by using combinations of antioxidants to facilitate their synergistic interaction and provide broad-based protection from different types of ROS at all cellular levels of the skin.6,18,21
Alto Defense Serum™ (WEL-DS; skinbetter science, Phoenix, Arizona) comprises a balanced ratio of 19 water-soluble, enzymatic, and/or lipid-soluble antioxidants selected with the aim of providing the skin synergistic, comprehensive cellular protection against a broad range of free radicals. As part of a research program evaluating the efficacy and tolerability of WEL-DS, we first compared WEL-DS’s innate antioxidant capacity with that of a well-known antioxidant serum (C E Ferulic®, SkinCeuticals, Dallas, Texas) comprising 15% L-ascorbic acid, 1% vitamin E [alpha tocopherol], and 0.5% ferulic acid [L-AOX]), and a saline control in human skin explants.22 Testing was performed on replicates of mid-dermal grafts from excised human abdominal skin from a single female donor. Two identical, independent experiments were conducted, each involving three test groups: WEL-DS (n=9 skin grafts), L-AOX (n=9 skin grafts), and a saline control (n=4 skin grafts). Grafts were mounted onto a transcutaneous flux apparatus, and test samples and saline were applied to the grafts and left on donor surfaces for up to 20 hours. A standardized hydrogen peroxide/ peroxidase assay (20μL of 01 mM) was used to assess oxidative stress and peroxide activity in washed, homogenized skin tissue over time. Absorbance was measured at baseline (pre-spike), immediately after spike (Time 0), and at one minute (Time 1), two minutes (Time 2), three minutes (Time 3), four minutes (Time 4), five minutes (Time 5), 10 minutes (Time 6), and 15 minutes (Time 7) after spike. Reductions in oxidative stress in WEL-DS, L-AOX, and saline were compared. Repetition of the assay was performed (Test 2) to validate results and ensure reproducibility.
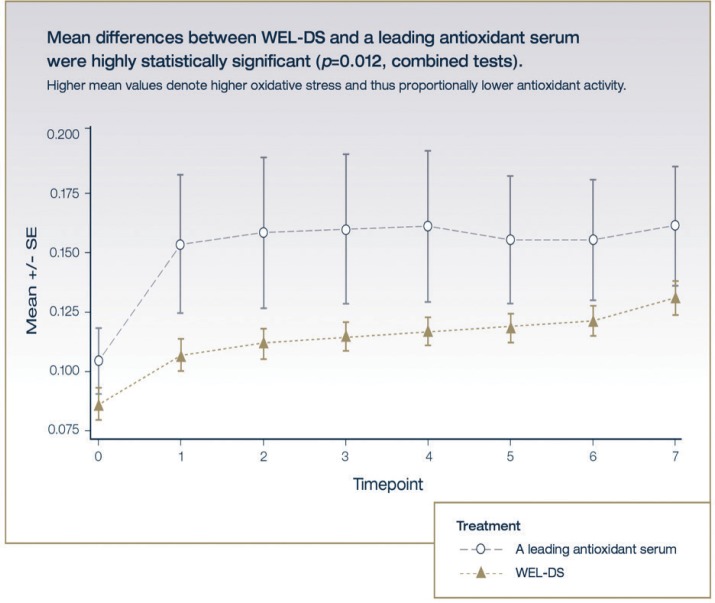
Using a fixed-timepoint analysis, both WEL-DS and L-AOX demonstrated significant antioxidant capacity in quenching peroxide versus the saline control (WEL-DS: p=0.0003 and p=0.003 for Tests 1 and 2, respectively; L-AOX: p=0.001 and p=0.03 for Tests 1 and 2, respectively) (Table 1). Skin treated with WEL-DS neutralized 53 percent and 41 percent more oxidative stress relative to L-AOX in Tests 1 and 2, respectively. The difference between WEL-DS and L-AOX was statistically significant in each test (p=0.0106 and p=0.0051 for Tests 1 and 2, respectively) and for both tests combined (p=0.0001) (Figure 1). Differences in treatment effect within each test and when the tests were combined were significant, with WEL-DS demonstrating greater ability to neutralize peroxide in comparison with L-AOX.
TABLE 1.
Average performance of WEL-DS, L-AOX, and saline: Tests 1 and 2*
| TEST | WEL-DS | L-AOX | SALINE | |
|---|---|---|---|---|
| Test 1 | Average** | 0.088 | 0.135 | 0.314 |
| t-test vs. saline | p=0.0003 | p=0.0012 | N/A | |
| Oxidative stress vs. WEL-DS | 100% | 153% | 363% | |
| Test 2 | Average** | 0.130 | 0.180 | 0.33 |
| t-test vs. saline | p=0.0029 | p=0.0316 | N/A | |
| Oxidative stress vs. WEL-DS | 100% | 141% | 258% | |
| *Higher values denote higher levels of oxidative stress and proportionally lower antioxidant capacity. | ||||
| **Average is based on 8 time points for WEL-DS and L-AOX and 4 time points for saline control. | ||||
| WEL-DS: Alto Defense Serum™; L-AOX: 15% l-ascorbic acid, 1% vitamin E, 0.5% ferulic acid; N/A: Not applicable | ||||
FIGURE 1.
Mean variable stress over time
Once the antioxidant capacity of WEL-DS was established, we sought to assess, in two clinical studies, 1) the ability of WEL-DS to protect skin against the oxidizing effects of UVA-UVB radiation (Minimal Erythema Dose [MED] study) and 2) its effectiveness in improving the appearance of photodamaged facial skin (Facial photodamage study).
DESIGN AND METHODS
Study ethics. These studies were conducted in accordance with all applicable guidelines for the protection of human subjects for research as outlined in 21 CFR 50, the accepted standards for Good clinical Practice (GCP), and approved by IntegReview IRB, Austin Texas (MED study) and Chesapeake IRB, Columbia, Maryland (facial photodamage study).
MED study. This five-day, single-center, randomized, controlled trial enrolled healthy women, 35 to 60 years of age, with Fitzpatrick Skin Types II to III. Eligible subjects were enrolled in the study if they were generally in good health, nonsmokers, and willing to adhere to the requirements of the study and provide informed consent. Subjects were excluded from the study if they had known allergies to skincare products; were pregnant, nursing, or planned on becoming pregnant during the study; had a history of skin cancer or a health or dermatologic condition on their back; had used oral retinoids or steroids during the prior six months; were using anti-inflammatory medication or medication with photo-sensitizing potential; had a dermatologic condition that, in the opinion of the investigator, that might influence test results; or individuals with known abnormal responses to sunlight or artificial light, known sensitivity to sunscreens, or who had been instructed by a healthcare professional to avoid sunlight as a result of a medical condition.
Treatment with WEL-DS was randomly applied for four consecutive days to one of two sites (Site 1 or Site 2) on the lower back of each subject; the other site remained untreated (control). In an effort to establish each subject’s MED, a single-port solar simulator irradiated an untreated area of skin. Treated and untreated sites were then irradiated with 1×, 2×, and 3× each subject’s MED. On Day 5, individual sites were digitally photographed using a Canfield Twinflash System (Canfield Scientific, Fairfield, New Jersey) and analyzed based on erythema reduction values (a*) and the amount of visible erythema and/or edema for both treated and untreated irradiated sites. Additionally, 3mm punch biopsies were collected from three different sites on the lower back of each subject: the treated irradiated (3× MED) site, the untreated irradiated (3× MED) site, and the untreated unirradiated site. Analysis of biomarkers indicative of skin damage were histologically evaluated using thymine dimers, matrix metalloproteinase 9 (MMP-9), cluster of differentiation (CD)1a Langerhans cells, sunburn cells, and p53 as measures.
Facial photodamage study. This 12-week, single-center, clinical study evaluated the efficacy and tolerability of twice-daily application of WEL-DS in female subjects, 25 to 65 years of age, with mild-to-moderate facial photoaging and no known medical conditions that, in the investigator’s opinion, might interfere with study participation. In addition to providing written informed consent (including photoconsent), subjects had to agree to practice sun avoidance and daily use of sunscreen. Subjects were excluded if they were pregnant, breast feeding, or planning to become pregnant during the study; had any previous hypersensitivity reaction to any of the ingredients in the study product(s); or were currently using or had continuously used for more than two weeks during the previous six months any cosmetic products containing alpha hydroxy acids, retinoids, peptides, growth factors, and/or potent antioxidants.
Enrolled subjects were instructed to apply the study product to clean facial skin twice daily (AM and PM) for 12 weeks. Subjects followed the application of the study product with a supplied moisturizer (AM and PM) and sunscreen (AM), followed by application of their routine makeup products, if applicable. To ensure adherence to protocol and application instructions, participants were instructed to bring the study product with them to each clinic visit to be weighed.
Expert-graded assessments of facial photodamage, including changes in fine lines/ wrinkles, dyschromia, erythema, skin tone, and pore size, were based on a six-point scale, where 0=none and 5=severe, using digital photography (Canfield Olé system; Canfield Scientific, Fairfield, New Jersey). Expert or physician evaluations also included global improvement (5-point grading scale [0=none to 4=severe]), and subjects completed a self-assessment questionnaire comprising 21 questions in which they agreed or disagreed to statements regarding perceived changes in the appearance of redness, pigmentation, lines and wrinkles, and skin brightness and texture, as well as their impressions regarding the feel and texture of the study product. Adverse events (AEs) were monitored and recorded throughout the study period. A subset of subjects (n=14) were evaluated in an extension study and continued using the study product through Week 16.
RESULTS
MED study. Six female subjects were enrolled and five subjects completed the MED study. One subject had insufficient erythema for MED determination. The mean age of the subjects was 42.6 years, and all were Fitzpatrick Skin Types II or III. No adverse events (AEs) were observed or reported during the study.
Digital photographs of test areas were evaluated for changes in erythema, as described previously. Treatment with WEL-DS demonstrated significantly less UV-induced erythema at 1×, 2×, and 3× MED exposures compared with untreated irradiated skin (p=0.025, p<0.001, and p=0.004, respectively) (Table 2 and Figure 2).
TABLE 2.
Erythema reduction in irradiated skin treated with WEL-DS versus irradiated untreated skin
| MED LEVEL | COMPARISON | ESTIMATED DIFFERENCE (SE) | P-VALUE* |
|---|---|---|---|
| 1X MED | WEL-DS vs. Untreated | -3.55 (1.02) | 0.025 |
| 2X MED | WEL-DS vs. Untreated | -6.71 (0.77) | <0.001 |
| 3X MED | WEL-DS vs. Untreated | -5.19 (0.87) | 0.004 |
| *Calculated from paired t-test. Testing hypothesis based on mean measurement being equal between treatments | |||
| WEL-DS: Alto Defense Serum™; MED: Minimal erythema dose | |||
FIGURE 2.
Top: erythema in irradiated skin treated with WEL-DS (2× MED); bottom: erythema in irradiated untreated skin (2× MED)
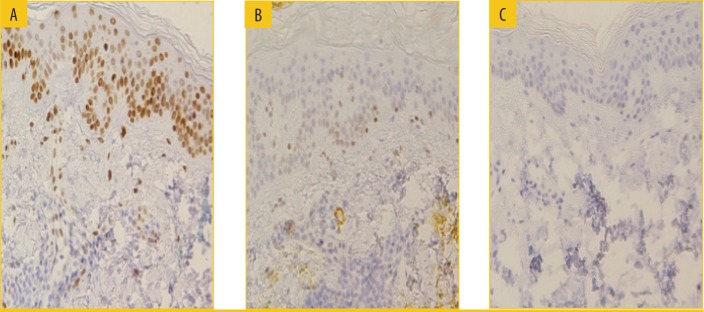
Biopsies obtained from subjects at 3× MED-irradiated treated sites revealed reductions in thymine dimers (p<0.02), MMP-9 (p<0.005), p53, and sunburn cells, as well as a lack of reduction in Langerhans cells (p<0.008), compared with untreated sites, suggesting the protective ability of WEL-DS on a cellular level against sun damage to the skin (Figure 3).23 In addition, Langerhans cells from WEL-DS-treated irradiated sites exhibited markedly less morphological changes compared to untreated irradiated sites, in which cells were atypically less dendritic and appeared ovoid in shape.
FIGURE 3.
Photoprotective effect of WEL-DS on thymine dimers (3× MED)—A) irradiated untreated, B) irradiated WEL-DS, and 3) unirradiated
Facial photodamage study. Twenty-two female subjects with an average age of 56 years and predominantly Fitzpatrick Skin Type II were enrolled in this study. Twenty-one subjects completed the study through Week 12. Due to possible sun exposure, one subject was not evaluated for erythema, skin tone, or dyschromia. A subset of subjects (n=14) continued in the four-week extension study, for a total of 16 weeks of treatment.
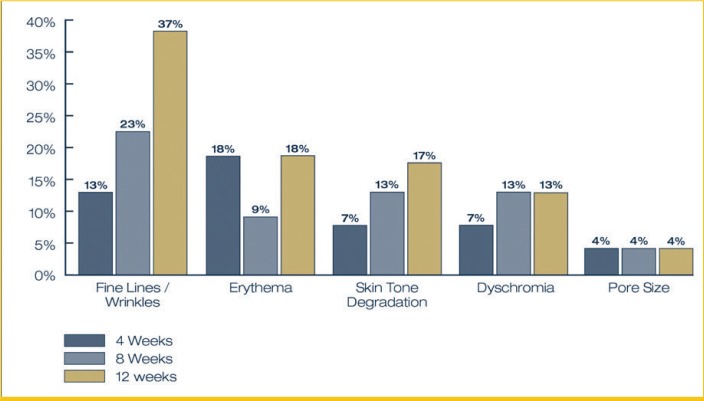
Expert-graded assessments of facial photodamage (6-point scale [0=none to 5=severe]) reported average visible improvements from baseline to Week 12 in fine lines/wrinkles (37%), erythema (18%), skin tone (17%), and dyschromia (13%) (Figures 4 and 5). Improvement in the appearance of pore size remained relatively stable, with an average four-percent reduction in the appearance of pores from baseline to Week 12.
FIGURE 4.
Average percent improvement in appearance of facial photodamage from baseline to Week 12 based on expert assessment
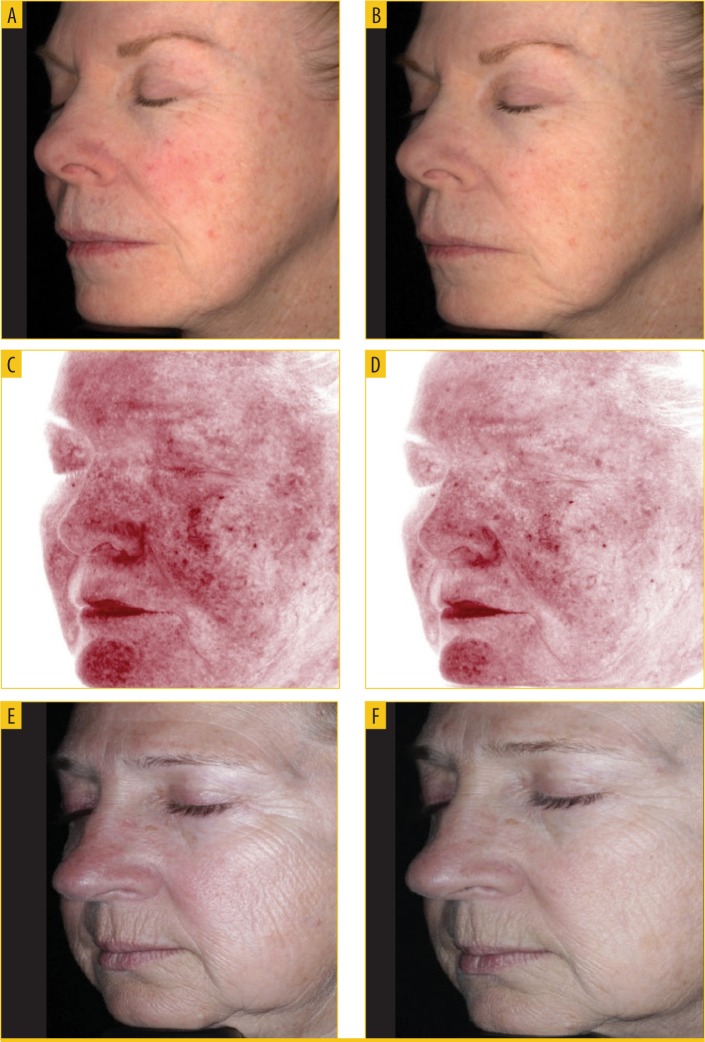
FIGURE 5.
Improvement in the appearance of erythema and fine lines and wrinkles—A) baseline to B) Week 4, C) baseline to D) Week 12, and E) baseline to F) 16 weeks
Expert-graded assessments of facial photodamage reported progressive improvement in fine lines/wrinkles over the 12-week study period, with average improvements of 13 percent at Week 4, 23 percent at Week 8, and 37 percent at Week 12. Eighty-six percent (86%) of subjects demonstrated at least a 1-grade improvement in the appearance of fine lines/wrinkles relative to baseline severity. Forty percent (40%) of subjects demonstrated at least a 1-grade improvement in the appearance of erythema from baseline severity. Skin tone showed continued, progressive improvement from baseline with seven percent, 13 percent, and 17 percent average improvement findings from baseline to Week 12. Fifty percent (50%) of subjects demonstrated at least a 1-grade improvement in the appearance of skin tone relative to baseline severity. An average visible improvement in dyschromia of 13 percent was observed at Week 8 and was maintained through Week 12, with 45 percent of subjects demonstrating at least a 1-grade improvement in the appearance of dyschromia from baseline. Ninety-five percent (95%) of subjects demonstrated at least a 1-grade increase in global improvement at Week 12.
Based on expert-graded assessments of facial photodamage, as described previously, subjects evaluated through Week 16 (n=14) demonstrated progressive average visible improvements of 39 percent in fine lines/ wrinkles, 32 percent in erythema, 27 percent in skin tone and dyschromia, and 21 percent in pore size. All subjects demonstrated a minimum 1-grade increase in global improvement at the end of the 16-week study period.
All subjects reported that the study product had a light texture and feel and was absorbed quickly into the skin. WEL-DS was well-tolerated, with subjects reporting only mild, transient AEs that were deemed possibly related to study products (n=9; dryness and one blemish). No subject discontinued the study due to an AE.
DISCUSSION
The need for comprehensive protection against the cumulative effects of sun exposure is well-documented.24 Sunscreens differ mechanistically from topical antioxidants in that they scatter, absorb, or block UV radiation before free radicals are formed in the skin, whereas topical antioxidants work by neutralizing free radicals and inhibiting their capacity to cause cellular damage.19 While broad-spectrum sunscreens offer protection against both UVA and UVB light, to achieve IR photoprotection, sunscreens need to be supplemented with specific antioxidants.6 Topical antioxidants play a complementary role to sunscreens by neutralizing free radicals and inhibiting their capacity to cause cellular damage.16,18,19
Our results from the MED study suggest WEL-DS is effective in protecting skin against the oxidizing effects of UV radiation. Skin treated with WEL-DS exhibited significantly less UV-induced erythema at all MED levels tested (1×, 2×, and 3×) in comparison with untreated irradiated skin. Histology of irradiated skin treated with WEL-DS correlated with clinical observations, demonstrating broad cellular protection, which further supports evidence of the effective percutaneous absorption and bioavailability capability of WEL-DS.
Irradiated skin treated with WEL-DS demonstrated significant reductions in thymine dimer formation and the upregulation of MMP-9 at 3× MED. Thymine dimer mutations occur due to direct UVB absorption and UVA irradiation and have been associated with nonmelanoma skin cancer.28,29 MMP-9, a Type IV collagenase, is upregulated following UV irradiation of the skin, leading to increased breakdown of collagen and elastin. The destruction of the extracellular matrix following UV irradiation is thought to be responsible for photoaging and induction of MMP-9, which degrades basement membranes.30,31 WEL-DS also appeared to protect the skin against UV-stimulated sunburn cells compared to untreated irradiated skin, suggesting its ability to protect skin from UV damage and inhibit cellular apoptosis. WEL-DS also appeared to provide protective effects related to Langerhans cells and p53. Specifically, treatment with WEL-DS appeared to prevent UV-induced reductions in CD1a (Langerhans cells). Langerhans cells are epidermal antigen-presenting cells that initiate an immune response. Sites treated with WEL-DS had lower (improved) mean values of p53, a cellular protein induced by UV irradiation in response to deoxyribonucleic acid (DNA) damage and oxidative stress.32,33 p53 slows the cell cycle for DNA repair and can induce cellular apoptosis if the damage is significant. These findings suggest WEL-DS offers varied modes of cellular protection against the damaging consequences of UV-exposure to the skin.
Correlation of both objective and subjective measures (biomarker and erythema evaluation, respectively) of UV-induced damage demonstrate the potential strength and consistency of WEL-DS in counteracting UVA/UVB-generated ROS in skin.
Prior studies have tested the ability of vitamin C-based mixtures to protect human skin against UV radiation damage.16,34 In one study, nine subjects were treated with a combination of 15% L-ascorbic acid, 1% alpha tocopherol, and 0.5% ferulic acid (CEFer) and exposed to up to 10× MED. Significant protective benefits were first observed at 8× and 10× MED for erythema and at 6× MED for sunburn cells.34 Another study examined the protective effects of a mixture that combined 10% L-ascorbic acid, 0.5% ferulic acid, and 2% phloretin (CFerPhlor).16 In this study, 10 subjects were evaluated over the course of four days at up to 5× MED and biopsies were obtained from the 5× MED site. The CFerPhlor mixture showed a protective effect at 5× MED.16
Although our study included a small sample size (five subjects) and only tested up to 3× MED, significant and noteworthy results were observed, suggesting the potential antioxidative potency of the WEL-DS formulation in counteracting free radical damage as a result of UV exposure.
The skin’s innate system of antioxidants generally provides protection from oxidant stress generated by both intrinsic and extrinsic factors. However, as the skin ages, its natural defenses, including production of protective antioxidants, decline, which can lead to significant oxidative stress; thus, when the skin is exposed to UV light or other environmental stressors, accelerated aging can occur.12,35
Due to the inherent sensitivity of antioxidants, developing an antioxidant formulation that remains stable over time has been challenging.4 Historically, formulations have narrowly focused on a few specific ingredients such as vitamin C. The water-soluble form of vitamin C, L-ascorbic acid, is often used, due to its known cutaneous benefits of promotion of collagen synthesis, photoprotective capabilities against UVA/UVB exposure, and skin brightening capabilities.59 However, L-ascorbic acid is highly unstable and must be formulated at specific concentrations and pH to ensure stability and adequate skin penetration.35,60,61 Over the last two decades, our understanding of other potent antioxidants, their benefits, and the roles they play in defending the skin against oxidative stress has substantially expanded.12 Broad antioxidant protection is essential in counteracting different types of ROS or free radicals at all cellular compartments of the skin. WEL-DS combines a selection of 19 active antioxidants—a balanced ratio of water-soluble, enzymatic, and lipid-soluble substances—to create a stable formulation designed to offer the skin broad-range protection from free radicals triggered by various extrinsic factors. Many of the antioxidants included in WEL-DS are thought to have additional benefits, such as reducing inflammation and erythema, brightening the skin, and facilitating visible improvements in fine lines and wrinkles (Table 3).4,36–58
TABLE 3.
WEL-DS antioxidants
| ANTIOXIDANT | ANTIOXIDANT PROPERTIES |
|---|---|
| Chlorogenic acids | Protects against UV-induced oxidative damage; reduces level of free radicals; enhances superoxide dismutase36–38 |
| Coffee arabica leaf extract | Protects against skin damage as a result of sunburn cell formation and DNA degradation39 |
| Theobroma cacao seed extract (cocoa) | Inhibits lipid peroxidation, glutathione oxidation, chelate redox active metals, and enzymes involved in ROS production; supports the Nrf2 signaling pathway38,40,41 |
| Ergothioneine | Protects DNA and protein from oxidative damage; works synergistically with tetrahexyldecyl ascorbate42 |
| Curcuma longa root extract (turmeric) | Protects against hydroxyl radicals, glycosylation, and lipid peroxidation; scavenges superoxide anion43,44 |
| Euterpe oleracea fruit extract (acai) | Protects against free radical damage to the skin during the inflammatory process43,45,46 |
| Vitis vinifera seed extract (grape) | Reduces lipid peroxidation and inhibits metals from reacting and forming hydroxyl radicals4,45 |
| Buddleja officinalis flower extract | Free radical scavenger; protects against harmful effects of UV, blue light and IR wavelengths47 |
| Camellia sinensis leaf extract (green tea) | Reduces hydrogen peroxide formation, nitric oxide, and copper; scavenges superoxide4,43,48–51 |
| Carnosine | Helps quench hydroxyl radicals and provides IR protection52 |
| Crocus sativus leaf extract (saffron) | Protects against free radical damage53 |
| Olea europaea fruit extract (olive) | Protects against UV exposure and DNA oxidation45 |
| Tetrahexyldecyl ascorbate | Inhibits lipid peroxidation and mitigates damaging effects of UV exposure4,48 |
| Tocopheryl acetate | Reduces formation of free radicals from UV exposure48 |
| Tocopherol | Prevents production of free radicals and protects skin from free radicals due to UV exposure4,48,54 |
| Glycyrrhiza glabra root extract (licorice) | Inhibits the amount of oxidative stress; anti-inflammatory properties43,45,55 |
| Superoxide dismutase (SOD) | Protects cell from superoxide toxicity56 |
| Ubiquinone (CoQ10) | Effective against UVA-mediated oxidative stress4,48,57 |
| Arabidopsis thaliana extract | Supports the transport of antioxidants into skin; promotes DNA repair58 |
| IR: infrared; TEWL: transepidermal water loss; UV: ultraviolet; DNA: deoxyribonucleic acid; ROS: reactive oxygen species; Nrf2: nuclear factor erythroid 2-related factor 2 | |
In this clinical study, based on expert-graded assessments, subjects in the WEL-DS group demonstrated improvements in the appearance of fine lines/wrinkles (37%), erythema (18%), skin tone (17%), dyschromia (13%), and pore size (4%) from baseline to Week 12. Progressive visible improvements from baseline were demonstrated in all categories at Week 12 with the exception of pore size, which remained relatively stable throughout the 12-week study period. Expert-graded evaluations demonstrated that nearly all subjects (95%) achieved at least a 1-grade increase in global improvement at 12 weeks.
Achieving early visible changes to photodamaged skin with the use of topical antioxidants is meaningful and might help foster routine and consistent use by patients. Perception regarding the look, feel, and smell of a topical product is also critical in reinforcing consistent use. Among the subjects in our study, 100 percent reported that WEL-DS had a light texture and feel.
Progressive, substantial improvements in the appearance of fine lines and wrinkles (39%), erythema (32%), skin tone (27%), dyschromia (27%), and pore size (21%) were observed from baseline to Week 16 (n=14).
WEL-DS was well tolerated, with no reports of stinging or burning. Reports of dryness among our patient population could possibly be attributed to the time of year in which the study took place (winter months).
Limitations. This study involved a small number of subjects with predominantly light skin. Additional research in a larger and more diverse population would be beneficial in understanding efficacy and tolerability across different skin types. In addition, future studies examining the effects of use beyond 16 weeks in a larger patient sample would be of value, as longer durations of use will provide additional information regarding safety and efficacy.
CONCLUSIONS
In previous research, WEL-DS appeared to elicit significant antioxidant effects, compared to a saline control, by neutralizing hydrogen peroxide in a human skin model. Additionally, skin treated with WEL-DS neutralized 53 percent and 41 percent more oxidative stress (Tests 1 and 2, respectively) compared to a leading antioxidant serum. In our current two-part study, skin treated with WEL-DS demonstrated significantly less UV-induced erythema, compared to untreated irradiated skin, suggesting it provides substantial cellular protection against sun damage. Additionally, treatment with WEL-DS demonstrated early, progressive improvements in the appearance of facial aging, supporting its use as a treatment for reducing free radical damage to the skin. Together, these studies provide strong evidence supporting the use of WEL-DS as a safe and effective method of skin protection from photodamage as well as a treatment for the improving the appearance of UV-damaged facial skin.
ACKNOWLEDGMENTS
We would like to thank Lynne Kolton Schneider, PhD, for her editorial assistance on this manuscript.
REFERENCES
- 1.Poljšak B, Dahmane RG, Godić A. Intrinsic skin aging: the role of oxidative stress. Acta Dermotovenerologko. 2012;21(2):33–36. [PubMed] [Google Scholar]
- 2.Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxyen species and matrix-degrading enzymes. J invest Dermotol. 2012;132(7):1901–1907. doi: 10.1038/jid.2011.476. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Hu JY, Wang SQ. The role of antioxidants in photoprotection: a critical review. J Am Acad Dermotol. 2012;67(5):1013–1024. doi: 10.1016/j.jaad.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Allemann IB, Baumann L. Antioxidants used in skin care formulations. Skin Therapy Lett. 2008;13(7):5–9. [PubMed] [Google Scholar]
- 5.McDaniel D, Farris P, Valacchi G. Atmospheric skin aging—Contributors and inhibitors. J Cosmet Dermotol. 2018;17(2):124–137. doi: 10.1111/jocd.12518. [DOI] [PubMed] [Google Scholar]
- 6.Grether-Beck S, Marini A, Jaenicke T, et al. Effective photoprotection of human skin against infrared A radiation by topically applied antioxidants: results from a vehicle controlled, double-blind, randomized study. Photochem Photobiol. 2015;91(1):248–250. doi: 10.1111/php.12375. [DOI] [PubMed] [Google Scholar]
- 7.Lotito SB, Frei B. Relevance of apple polyphenols as antioxidants in human plasma: Contrasting in vitro and in vivo effects. Free Radic Biol Med. 2004;36(2):201–211. doi: 10.1016/j.freeradbiomed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Murray JC, Burch JA, lannacchione MA, et al. A topical antioxidant solution containing vitamins C and E with ferulic acid protects human skin from sunlight damage and DNA mutations associated with skin cancer. J Invest Dermatol. 2007;127:S134.. [Google Scholar]
- 9.Ou S, Kwok KC, Ferulic acid. Pharmaceutical functions, preparation and applications in foods. J Sci Food Agric. 2004;84(11):1261–1269. [Google Scholar]
- 10.Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48(1):1–19. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 11.Poljšak B, Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract. 2012;2012:135206. doi: 10.1155/2012/135206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stojilijković D, Pavlović D, Arsić I. Oxidative stress, skin aging and antioxidant therapy. Scientific Journal of the Faculty of Medicine in Niš. 2014;31(4):207–217. [Google Scholar]
- 13.Hozier AM, Athar M, Elmets CA. The other end of the rainbow: infrared and skin. J Invest Dermatol. 2010;130(6):1496–1499. doi: 10.1038/jid.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sander CS, Chang H, Salzmann S, et al. Photoaging is associated with protein oxidation in human skin in vivo. J Investig Dermatol. 2002;118(4):618–625. doi: 10.1046/j.1523-1747.2002.01708.x. [DOI] [PubMed] [Google Scholar]
- 15.Abla MJ, Banga AK. Quantification of skin penetration of antioxidants of varying lipophilicity. Inr J Cosmet Sci. 2013;35(1):19–26. doi: 10.1111/j.1468-2494.2012.00728.x. [DOI] [PubMed] [Google Scholar]
- 16.Oresajo C, Stephens T, Hino PD, et al. Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J Cosmet Dermatol. 2008;7(4):290–297. doi: 10.1111/j.1473-2165.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- 17.Pinnell SR, Yang H, Omar M, et al. Topical L-ascorbic acid: percutaneous absorption studies. Dermatol Surg. 2001;27(2):137–142. doi: 10.1046/j.1524-4725.2001.00264.x. [DOI] [PubMed] [Google Scholar]
- 18.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interventions Aging. 2007;2(2):219–236. [PMC free article] [PubMed] [Google Scholar]
- 19.Mayoral FA, Kenner JR, Draelos ZD. The skin health and beauty pyramid: a clinically based guide to selecting topical skincare products. J Drugs Dermatol. 2014;13(4):414–421. [PubMed] [Google Scholar]
- 20.Alonso C, Rubio L, Touriño S, et al. Antioxidative effects and percutaneous absorption of five polyphenols. Free Radic Biol Med. 2014;75:149–155. doi: 10.1016/j.freeradbiomed.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Pandel R, Poljsak B, Godic A, et al. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermotol. 2013:930164. doi: 10.1155/2013/930164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.22. Waugh JM, McDaniel DH, Wortzman M, et al. Antioxidant capacity of a comprehensive topical antioxidant in comparison to a leading antioxidant serum in a human skin model. Poster no. 6242 American Academy of Dermatology Meeting, February 16–20, 2018. San Diego, CA: [Google Scholar]
- 23.23. McDaniel DH, Mazur C, Wortzman M, et al. Protective effects of a comprehensive topical antioxidant from ultraviolet-induced erythema in human skin. Poster no. 6263 American Academy of Dermatology Meeting, February 16–20 2018. San Diego, CA: [Google Scholar]
- 24.Schroeder P, Krutmann J. What is needed for a sunscreen to provide complete protection. Skin Therapy Lett. 2010;15(4):4–5. [PubMed] [Google Scholar]
- 25.Faurschou A, Wulf HC. The relation between sun protection factor and amount of sunscreen applied in vivo. Br J Dermotol. 2007;156(4):716–719. doi: 10.1111/j.1365-2133.2006.07684.x. [DOI] [PubMed] [Google Scholar]
- 26.Hanson KM, Gratton E, Bandeen CJ. Sunscreen enhancement of UV-induced reactive oxygen species in the skin. Free Radic Biol Med. 2006;41(8):1205–1212. doi: 10.1016/j.freeradbiomed.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Wood M, Raisanen T, Polcari I. Observational study of free public sunscreen dispenser use at a major US outdoor event. J Am Acad Dermatol. 2017;77(1):164–166. doi: 10.1016/j.jaad.2017.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Kappes UP, Luo D, Potter M, et al. Short- and long-wave UV light (UVB and UVA) induce similar mutations in human skin cells. J Invest Dermatol. 2006;126(3):667–675. doi: 10.1038/sj.jid.5700093. [DOI] [PubMed] [Google Scholar]
- 29.Mouret S, Baudouin C, Charveron M, et al. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA. 2006;103:13765–13770. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inomata S, Matsunaga Y, Amano S, et al. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Invest Dermatol. 2003;120(1):128–134. doi: 10.1046/j.1523-1747.2003.12021.x. [DOI] [PubMed] [Google Scholar]
- 31.Onoue S, Kobayashi T, Takemoto Y, et al. Induction of matrix metalloproteinase-9 secretion from human keratinocyts in culture by ultraviolet B irradiation. J Dermatol Sci. 2003;33(2):105–111. doi: 10.1016/j.jdermsci.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Chao C, Saito S, Anderson CW, et al. Phosphorylation of murine p53 at ser-18 regulates the p53 responses to DNA damage. Proc Natl Acad Sci USA. 2000;97(22):11936–11941. doi: 10.1073/pnas.220252297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Méplan C, Richard MJ, Hainaut P. Redox signaling and transition metals in the control of the p53 pathway. Biochem Pharmacol. 2000;59(1):25–33. doi: 10.1016/s0006-2952(99)00297-x. [DOI] [PubMed] [Google Scholar]
- 34.Murray JC, Burch JA, Streilein RD, et al. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J Am Acad Dermatol. 2008;59(3):418–425. doi: 10.1016/j.jaad.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Pinnell SR, Oresajo C. Review of photodamage and oxidative stress and protection provided by topical antioxidants. Eur Dermatol. 2010;5:32–35. [Google Scholar]
- 36.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendra R, Ahmad S, Oskoueian E, et al. Antioxidant, anti-inflammatory and cytotoxicity of Phaleria macrocarpa (Boerl.) Scheff fruit. BMD Complementary Alternat Med. 2011;11:110. doi: 10.1186/1472-6882-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scapagnini G, Davinelli S, Di Renzo L, et al. Cocoa bioactive compounds: significance and potential for the maintenance of skin health. Nutrients. 2014;6(8):3202–3213. doi: 10.3390/nu6083202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velazquez Pereda Mdel C, Dieamant Gde C, Eberlin S, et al. Effect of green Coffea arabica L. seed oil on extracellular matrix components and water-channel expression in in vitro and ex vivo human skin models. J Cosmet Dermatol. 2009;8(1):56–62. doi: 10.1111/j.1473-2165.2009.00425.x. [DOI] [PubMed] [Google Scholar]
- 40.Ellam S, Williamson G. Cocoa and human health. Annu Rev Nutr. 2013;33:105–128. doi: 10.1146/annurev-nutr-071811-150642. [DOI] [PubMed] [Google Scholar]
- 41.F’guyer S, Afaq F, Mukhtar H. Photochemoprevention of skin cancer by botanical agents. Photodermatol Photoimmunol Photomed. 2003;19(2):56–72. doi: 10.1034/j.1600-0781.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 42.Bazela K, Solyga-Zurek A, Debowska R, et al. I-Ergothioneine protects skins cells against UV-induced damage. A preliminary study. Cosmetics. 2014;1(1):51–60. [Google Scholar]
- 43.Fowler JF , Jr., Woolery-Lloyd H, Waldorf H, et al. Innovation in natural ingredients and their use in skin care. J Drugs Dermatol. 2010;9(6 Suppl):S72–S81. [PubMed] [Google Scholar]
- 44.Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Aavances. 2015;5(35):27986–28006. [Google Scholar]
- 45.Ribeiro AS, Estanqueiro M, Oliveira MB, et al. Main benefits and applicability of plant extracts in skin care products. Cosmetics. 2015;2(2):48–65. [Google Scholar]
- 46.Schauss AG, Wu X, Prior RL, et al. Phytochemical and nutrient composition of the freeze-dried Amazonian palm berry, Euterpe oleraceae mart. (acai) J Agric Food Chem. 2006;54(22):8598–8603. doi: 10.1021/jf060976g. [DOI] [PubMed] [Google Scholar]
- 47.Acevedo JGA, González AME, De Maria y, Campos DM, et al. Photoprotection of Buddleja cordata extract against UVB-induced skin damage in SKH-1 hairless mice. BMC Complem Altern Med. 2014;14:281.. doi: 10.1186/1472-6882-14-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godic A, Poljšak B, Adamic M, Dahmane R. The role of antioxidants in skin cancer prevention and treatment. Oxid Med Cellul Longev. 2014:850479. doi: 10.1155/2014/860479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katiyar SK, Ahmad N, Mukhtar H. Green tea and skin. Arch Dermatol. 2000;136(8):989–994. doi: 10.1001/archderm.136.8.989. [DOI] [PubMed] [Google Scholar]
- 50.Katiyar SK, Elmets CA. Green tea polyphenolic antioxidants and skin photoprotection. Int J Oncol. 2001;18(6):1307–1313. doi: 10.3892/ijo.18.6.1307. [DOI] [PubMed] [Google Scholar]
- 51.Renu S. Treatment of skin diseases through medicinal plants in different regions of the world. Int J Compr Pharm. 2010;4:1–4. [Google Scholar]
- 52.Babizhayev MA, Nikolayev GM, Nikolayeva JG, Yegorov YE. Biologic activities of molecular chaperones and pharmacologic chaperone imidazole-containing dipeptide-based compounds: natural skin care help and the ultimate challenge: implication for adaptive responses in the skin. Am J Ther. 2012;19(2):e69–e89. doi: 10.1097/MJT.0b013e3181e71fb7. [DOI] [PubMed] [Google Scholar]
- 53.Das I, Das S, Saha T. Saffron suppresses oxidative stress in DMBA-induced skin carcinoma: a histopathological study. Acta Histochem. 2010;112(4):317–327. doi: 10.1016/j.acthis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Mayer P. The effects of vitamin E on the skin. Cosmet Toiletries. 1993;108:99.. [Google Scholar]
- 55.Gillbro JM, Olsson MJ. The melanogenesis and mechanisms of skin-lightening agents—existing and new approaches. Int J Cosmet Sci. 2011;33(3):210–221. doi: 10.1111/j.1468-2494.2010.00616.x. [DOI] [PubMed] [Google Scholar]
- 56.Le Quéré S, Lacan D, Lemaire B, et al. The role of superoxide dismutase (SOD) in skin disorder. Nutrafoods. 2014;13(1):13–27. [Google Scholar]
- 57.Hoppe U, Bergemann J, Diembeck W, et al. Coenzyme Q10, a cutaneous antioxidant and energizer. Biofactors. 1999;9(2):371–378. doi: 10.1002/biof.5520090238. [DOI] [PubMed] [Google Scholar]
- 58.Apone F, Tito A, Carola A, et al. A mixture of peptides and sugars derived from plant cell walls increases plant defense responses to stress and attenuates ageing-associated molecular changes in cultured skin cells. J Biotechnol. 2010;145(4):367–376. doi: 10.1016/j.jbiotec.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Farris PK. Topical vitamin C: a useful agent for treating photoaging and other dermatologie conditions. Dermatol Surg. 2005;31:814–817. doi: 10.1111/j.1524-4725.2005.31725. (7 Pt 2) [DOI] [PubMed] [Google Scholar]
- 60.Pinnell SR, Yang H, Omar M, et al. Topical L-ascorbic acid: percutaneous absorption studies. Dermatol Surg. 2001;27(2):137–142. doi: 10.1046/j.1524-4725.2001.00264.x. [DOI] [PubMed] [Google Scholar]
- 61.Stamford NP. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J Cosmet Dermatol. 2012;11(4):310–317. doi: 10.1111/jocd.12006. [DOI] [PubMed] [Google Scholar]