Abstract
Psoriasis is a chronic, systemic, inflammatory disease that is often associated with serious comorbid conditions such as cardiovascular disease, obesity, and diabetes. Many patients with moderate-to-severe psoriasis receive either no treatment or receive topical therapy only and report dissatisfaction with treatment, poorly managed symptoms, and continued impact of the disease on quality of life. Patients currently receiving topical monotherapy can benefit from systemic therapies, which are more effective in reducing clinical symptoms, achieving treatment efficacy targets, and improving quality of life. An array of systemic treatment options with varying mechanisms of action are available, including conventional and newer oral systemic agents and biologics. Each option presents a unique set of benefits, safety risks, dosing schedules, and monitoring requirements. The aim of the current review is to better optimize treatment outcomes in patients with psoriasis by presenting a rationale for when to consider systemic therapy in this patient population. The authors discuss the barriers to use of systemic agents and highlight the central importance of each patient’s perspective when assessing disease severity. Additionally, practical strategies for selecting and safely initiating systemic therapy to optimize the treatment of patients with psoriasis are identified.
Keywords: Psoriasis, systemic treatment, phosphodiesterase 4 inhibitor, apremilast, biologic therapy, etanercept, adalimumab, secukinumab, ustekinumab, infliximab, ixekizumab, brodalumab, guselkumab, tildrakizumab, certolizumab
Psoriasis is a chronic, systemic, inflammatory disease with symptoms affecting primarily the skin. At least one-third of patients with psoriasis also experience joint involvement (e.g., psoriatic arthritis [PsA]),1 and many have comorbid diseases with underlying inflammatory or immunologic components.2–4 Psoriasis has been identified as an independent risk factor for metabolic syndrome, cardiovascular disease (CVD), and major cardiovascular events, including myocardial infarction5–10 and renal disease.11–13 The proportion of patients with such comorbid conditions generally increases with the severity of their psoriasis.5–7,11 In line with these comorbidities, all-cause and CVD-related mortality risks are higher among patients with psoriasis versus matched reference groups of people without psoriasis.8,13
Treatment options available to manage psoriatic disease include topical therapy, phototherapy, oral treatment, and biologic therapy.14 Choice of treatment is largely based on disease severity, the presence of comorbid conditions, such as PsA, and treatment history (e.g., responsiveness, tolerability).14–17 For assessing disease severity, a consensus study from the National Psoriasis Foundation (NPF) found that extent of body surface area (BSA) involvement is currently the most preferred severity assessment instrument used in clinical practice. Additionally, NPF concluded that use of BSA should be complemented with assessments of patient-reported measures of quality of life (QOL) and symptoms, such as pruritus and pain, which are known to impact patient perception of disease severity.18,19 Using BSA, the NPF defines severity categories as mild (<3% BSA); moderate (3–10% BSA); and severe (>10% BSA).20,21 Separately, the American Academy of Dermatology (AAD) defines mild severity as less than five percent BSA; moderate, 5- to 9-percent BSA; and severe, 10-percent or greater BSA. The AAD also classifies psoriasis as moderate to severe in patients with a BSA of less than five percent if the psoriasis involves sensitive areas such as the hands, feet, face, or genitals because psoriasis lesions in highly visible or difficult-to-treat areas can be painful, interfere with sleep, impair daily function, and adversely affect QOL to a greater degree than lesions on other bodily locations.14–16
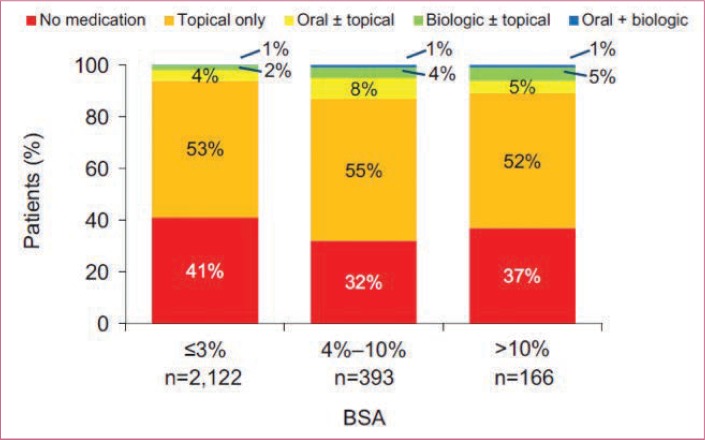
Many people with psoriasis across all levels of severity remain untreated or undertreated, particularly those with moderate disease.19,22 Most patients with moderate or severe disease receive topical monotherapy or no therapy (Figure 1).19,20,23 For example, in one recent United States (US) national analysis, 32 percent of patients diagnosed with moderate-to-severe psoriasis did not receive any treatment over the course of the five-year study period; among those who had received treatment within the previous year, 42 percent were prescribed a topical therapy alone.20,23 Despite the availability of numerous systemic therapies for the treatment of psoriasis, patients who might be candidates for systemic therapies remain undertreated with topical therapy alone or receive no treatment. In the current review, we provide the rationale for systemic therapy for psoriasis and discuss potential barriers to initiating systemic therapies. The importance of both healthcare provider- and patient-rated assessments of disease severity is also addressed. Additionally, the article reviews strategies for identifying appropriate patients for systemic therapy and setting treatment goals. Finally, an overview of the current systemic treatment options for patients with psoriasis is provided.
FIGURE 1.
Frequency of current treatment type according to psoriasis-involved body surface area (BSA)
Adapted with permission from Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–881
RATIONALE FOR SYSTEMIC THERAPY IN PSORIASIS
The understanding that many patients with psoriasis may be appropriate candidates to consider for systemic therapy has grown tremendously. First, our understanding of psoriatic pathophysiology has advanced significantly in recent years, revealing that psoriasis is a chronic, systemic immune-mediated disease; skin plaques are the dermatologic manifestation of the disease process that in about one-third of cases may also involve the joints (PsA).2,24 Psoriasis is one of the most common autoimmune conditions in the US, affecting an estimated 7.4 million adults.25,26 One of the earliest steps in the pathology of psoriasis occurs when activated myeloid dendritic cells produce interleukin (IL)-12, IL-23, and tumor necrosis factor (TNF)-α, recruiting and expanding populations of T-helper (Th)-1 and Th-17 cells that drive production of psoriatic cytokines, including IL-17. These cytokine signals, in turn, lead to the proliferation and differentiation of keratinocytes and production of other effector substances,27,28 giving rise to the cutaneous symptoms of psoriasis, including epidermal thickening and remodeling, angiogenesis, erythema, pruritus, and nail disease.2,28 Moreover, proinflammatory substances can be released into systemic circulation, potentially affecting insulin signaling, angiogenesis, adipogenesis, and lipid metabolism.2 Thus, the chronic Th-1 and Th-17 inflammation observed with psoriasis can affect other organ systems and comorbid conditions, such as obesity, diabetes, and atherosclerosis.2,29 In one analysis of more than 400,000 patients with psoriasis, the most commonly observed comorbidites were hyperlipidemia, hypertension, depression, Type 2 diabetes mellitus, and obesity.30
Growing evidence indicates that systemic treatments for psoriasis that reduce inflammation might help reduce the risks of cardiovascular events and improve the pathology associated with CVD.31–33 Wu et al31 reported that patients with psoriasis who received TNF-α inhibitors (e.g., adalimumab, etanercept, or infliximab) for 12 months exhibited a significantly lower rate of major cardiovascular events (1.45%), including myocardial infarction, compared with patients who received methotrexate (4.09%; P<0.001). Over a median follow-up period of 24 months, every six months of cumulative exposure to TNF-α inhibitors was associated with an 11-percent reduction in the risk of developing major cardiovascular events (P=0.02). At Exposure Years 1, 2, and 3, hazard reductions were 21.3 percent, 38.0 percent, and 51.2 percent, respectively.31 Pina et al32 found that after six months of treatment with the TNF-α inhibitor adalimumab, patients with severe psoriasis exhibited improved endothelial function and reduced arterial stiffness. Other research suggests that nonbiologic systemic treatments might also reduce the incidence of cardiovascular events.33,34 Likewise, in a study evaluating Danish patients with severe psoriasis, the five-year incidence (per 1,000 patient-years) of cardiovascular events was significantly decreased among those receiving methotrexate (6.3) versus patients receiving topical therapy, phototherapy, or climate therapy (14.6; P=0.002). Separately, incidence (per 1,000 patient-years) of all-cause mortality was significantly lower with both methotrexate (7.3; P<0.001) and biologics (4.1; P=0.04) versus topical, climate, or phototherapies (24.0).34 An earlier study, from the same group of patients with severe psoriasis, reported generally similar findings; the lowest risk of cardiovascular events or CVD-related death occurred in patients treated with methotrexate or biologics.35 Although much of the research assessing the effect systemic therapies have on psoriasis and comorbidites, to date, focuses on TNF-α inhibitors and CVD, research is underway (NCT02187172, NCT03082729, NCT02690701) to explore the potential benefits on comorbid disease processes with other systemic treatments in patients with psoriasis. Although the clinical benefits of systemic therapies for managing comorbidities in patients with psorisis have not yet been adequately studied, the available evidence indicates that decreasing the inflammatory burden of psoriasis with systemic therapy has the potential to decrease the risk of associated comorbidities.36
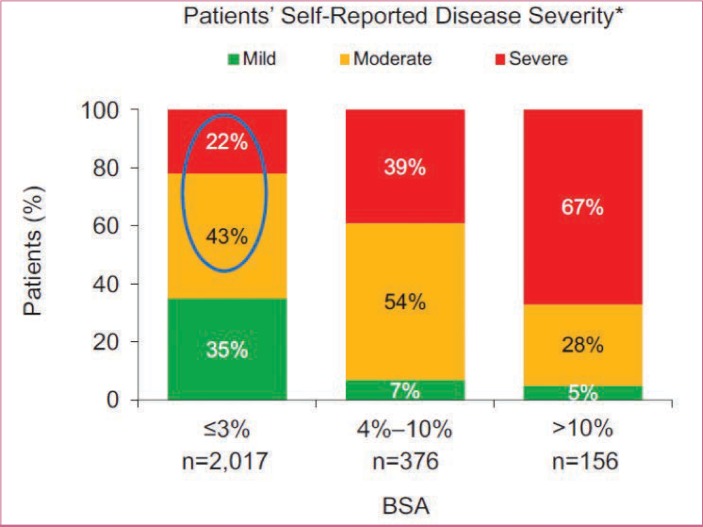
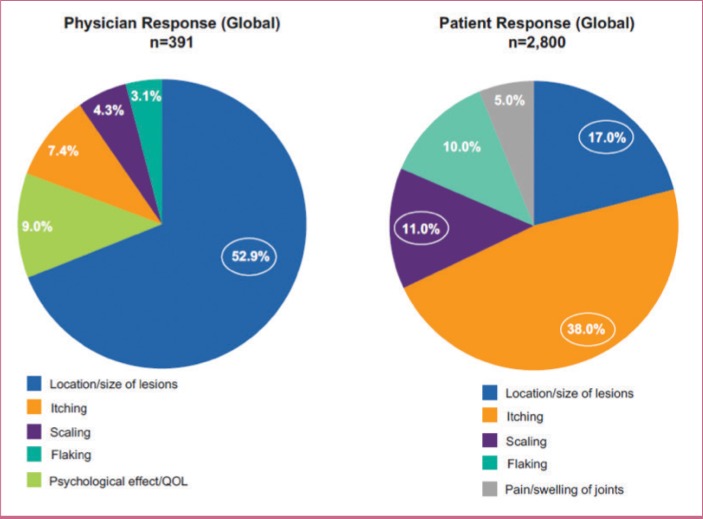
Patients with psoriasis often rate their disease as more severe than assessments that rely solely on BSA involvement.19 For example, many patients who are rated, based on physician-based assessments of affected BSA, as having mild psoriasis self-describe their disease as moderate or severe (Figure 2) and thus may be potential candidates for systemic therapy.19 In one survey of patients with psoriasis, 71 percent of respondents reported that their psoriasis had a moderately to extremely high impact on their daily lives.37 In a multinational survey, more than half of surveyed patients with BSA of three percent or less considered their psoriatic disease to be either moderate or severe.19 Some patients in this subgroup reported having manifestations of psoriasis on their hands, feet, scalp, nails, and/or anogenital area, which has been shown to severely impact daily function and QOL although the skin area involved is limited. Physician-assessed disease severity, based largely on what can be observed on the skin, might not adequately reflect the full scope of the disease.19,38,39 Furthermore, patient and physician perceptions of which factors contribute to disease severity in psoriasis differ greatly.19,22 In surveys of patients with psoriasis and physicians (dermatologists and rheumatologists), the patients most commonly reported itching as the most important factor contributing to disease severity, whereas physicians most commonly reported size or location of lesions (Figure 3).19,22 According to current treatment guidelines, severe impairments in QOL and daily function (particularly with hand or foot involvement) in patients with psoriasis warrant consideration of systemic therapy, even if BSA involvement is limited.14–16
FIGURE 2.
Patient-perceived severity of psoriasis according to psoriasis-involved body surface area (BSA) ; *at its worst
Adapted with permission from Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–881
FIGURE 3.
Top 5 most important factors contributing to psoriasis severity as reported by physicians and patients—circles indicate factors mentioned by more than 10% of respondents; additional predefined factors included bleeding, lack of sleep, and other (physicians: 23.3%; patients: 19.0%); QOL: quality of life
Adapted with permission from van de Kerkhof PCM, Reich K, Kavanaugh A, et al. Physician perspectives in the managment of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29(10):2002–2010
Many patients with psoriasis who are receiving topical therapy alone report inadequate symptom improvement.19,20,37 For example, in a large multinational survey of patients with psoriasis, approximately two-thirds reported ongoing clinical symptoms, such as flaking, itching, scales, and redness, and most of the surveyed patients with moderate-to-severe psoriasis were receiving topical monotherapy.19 In a US survey of patients with psoriasis, 169 of 193 patients currently taking prescription medication were receiving topical monotherapy, and 71 percent of patients reported impaired QOL.37 This evidence suggests that many patients are not achieving adequate symptom relief or meeting treatment goals with ongoing topical therapy.
Rates of overall dissatisfaction with psoriasis treatment regimens are substantial across all levels of disease severity. In one patient survey, the percentage of patients dissatisfied with treatment was nearly 40 percent among those with mild disease severity, more than 50 percent among those with moderate disease severity, and more than 40 percent among those with severe psoriasis.20 The lowest levels of treatment satisfaction have been linked to the use of topical medications versus systemics and phototherapy.40,41 In one large survey using the Treatment Satisfaction Questionnaire for Medication (possible scores ranged from 0=extremely dissatisfied to 100=extremely satisfied), patients with psoriasis receiving topical monotherapy reported lower treatment satisfaction overall (66.7) compared with patients receiving most conventional oral or biologic systemic medications (range: 75.0–83.3).40 Patients treated with topical therapy also had lower treatment effectiveness scores (50.0) versus those treated with conventional oral or biologic systemic medications (range: 66.7–83.3).39 Of patients with psoriasis receiving prescription topical medication, 45 percent were nonadherent to their medication regimen; the most frequently cited reasons for being nonadherent were perceived lack of efficacy and lack of convenience (e.g., takes too much time to apply; is too messy, oily, or sticky; have to apply it too many times per day).37
Low rates of treatment satisfaction, coupled with high rates of undertreatment, particularly in areas of the body considered bothersome and/or difficult-to-treat (e.g., scalp, nails), indicate that many patients with psoriasis who are potential candidates for systemic therapy might be overlooked in regard to receiving this type of treatment. In summary, patients with psoriasis who report low treatment satisfaction, have affected BSA in difficult-to-treat areas, and/or are not improving on topical therapy alone might be appropriate candidates for systemic therapy to alleviate the chronic symptoms of this systemic inflammatory disorder, and warrant closer consideration.
With so many treatment options available, it might be difficult to understand why psoriasis undertreatment is so prevalent. Is treatment beyond topicals not offered? Do patients not seek systemic treatment? Do patients not fill their prescriptions? Is a lack of insurance coverage and/or cost a barrier? In this next section, we review healthcare provider, patient, and payor factors that might contribute to the undertreatment of patients with psoriasis.
POTENTIAL BARRIERS TO INITIATING SYSTEMIC THERAPIES IN PATIENTS WITH PSORIASIS
There are several potential provider- and patient-related barriers to initiating systemic therapy in patients with psoriasis. Paramount among these are safety concerns, by both patient and clinician, surrounding the use of systemic therapies. In the dermatology setting, patients report concerns about safety with conventional oral systemic treatments22 and with biologics.19,20 In one NPF patient survey, 18 percent of respondents reported they chose to use topical therapy due to fewer adverse events (Ab) compared with other therapies, and approximately 25 percent of patients reported discontinuing a biologic because of an AE, medical complication, or drug interaction.20 Likewise, in a multinational survey, 43 percent and 25 percent of psoriasis patients who had used and then discontinued a conventional oral systemic or biologic treatment, respectively, had discontinued the treatment because of a tolerability or safety issue.19 Patients with comorbid conditions, such as CVD or hypertension, are comparatively more concerned about safety and side effects with biologics than are patients without such comorbidities.42 Similar concerns were reported by dermatologists in a multinational survey, in which 76 percent of participating dermatologists reported not prescribing conventional oral systemic treatments and 91 percent reported discontinuing systemic treatment due to concerns or issues with long-term safety, tolerability, drug interactions, side effects, and/or because of patient refusal/concerns.22 Such concerns are not without merit, as conventional oral systemic therapies, including methotrexate, cyclosporine, and acitretin, are associated with organ toxicities, tolerability issues, and requirements for routine laboratory monitoring.43–45 Likewise, despite biologic use in psoriatic disease beginning almost two decades ago, some dermatologists still express concerns surrounding the long-term safety of biologics.46,47
Another potential barrier to use of systemic therapy is that providers might view psoriasis as a cutaneous or localized disease that is appropriately treated with topical therapy. They also might be more familiar with topical agents than with systemic treatments for psoriasis and might view topical agents as adequate for patients with limited BSA involvement. Moreover, providers might consider topical treatments more convenient to prescribe (e.g., no need for laboratory monitoring, fewer barriers to insurance coverage) and involving fewer safety and tolerability concerns compared with systemic treatments.
Dermatologists and patients also note several burdens associated with conventional oral systemic and biologic treatments that might limit their use. These burdens include issues with high costs and barriers to or lack of coverage by payors as well as additional requirements for safely prescribing conventional oral systemics and biologics, such as prior authorization, extra paperwork, laboratory testing, and patient education.22 In addition, many patients avoid biologic therapies due to anxiety/fear of injections.19
THE IMPORTANCE OF USING PHYSICIAN-AND PATIENT-RATED ASSESSMENTS OF DISEASE SEVERITY
Current tools for assessing psoriasis disease severity (e.g., Physician Global Assessment [PGA], Psoriasis Area and Severity Index [PASI]) are generally impractical for clinical use, and most tools fail to capture patient perspectives.48 In typical clinical dermatology settings in the US, providers most often assess psoriasis severity and set treatment goals based on BSA. Recent guidance on assessing percentage of affected BSA in burn patients might provide more clinically relevant parameters for estimating BSA in patients with psoriasis. The patient’s hand (palm area including digits, with thumb tucked to the side) equates to roughly 0.8 percent of BSA while the palm, excluding digits, represents 0.5 percent BSA.49 For more extensive disease, Wallace Rules of Nines, when applied to psoriatic lesions, might be a useful aid for clinicians to more quickly determine the percentage of affected BSA.49 Regardless, clinical assessment tools, such as BSA, PGA, and PASI, do not incorporate the location of affected skin or patient perspectives on physical symptoms (e.g., itch) and QOL issues.
To more accurately assess disease severity, it is imperative that providers ask their patients with psoriasis about any subjective symptoms (e.g., itch, pain), sleep quality, and impact on QOL, including social, interpersonal, and occupational functioning. Clinicians should also ask patients about their out-of-pocket expenses and time spent treating their psoriasis, as well as any adverse effects or inconveniences they experience with any topical therapy, as any one of these factors can affect patient adherence and product efficacy. Switching to a different agent or a different formulation with a different vehicle might improve tolerability and clinical outcome. Treatment decisions should be individualized to the patient and should reflect his or her personal priorities for symptom relief, safety, convenience, and costs.
IDENTIFYING APPROPRIATE PATIENTS FOR SYSTEMIC THERAPY AND SETTING TREATMENT GOALS
Systemic therapy should be considered for patients with extensive psoriasis (i.e., >3% BSA involvement), those whose daily functioning is adversely affected by psoriasis, those with PsA, and/or those whose QOL is impaired due to the disease.16 Systemic treatments might also be appropriate for patients with “mild” psoriasis (≤3% BSA involvement) if they fail to respond adequately to topical formulations or phototherapy, if phototherapy is impractical, or if their QOL is negatively affected to the extent that the benefits of systemic therapy outweigh the potential risks.16 The goals of therapy should be defined according to the treatment priorities of the individual patient (i.e., reduction in psoriasis symptoms, improved QOL, safety and tolerability issues, costs, treatment burden). With newer systemic therapies, the clinician might consider asking the patient with psoriasis to reassess his or her treatment goals and to consider the possibility of achieving near-complete skin clearance (BSA ≤1%).18 According to a recent NPF consensus statement, with the availability of highly effective treatments, three-percent or less BSA or 75-percent or more improvement in BSA from baseline might be an acceptable level of response after three months of treatment, with a treat-to-target goal of one percent or less BSA; adjustments to target goals might be necessary based on patient life circumstances.18 The clinician should seek a therapy that best aligns with each patient’s goals and personal priorities and switch treatments thereafter if the initial therapy is inadequate.
INITIATING A SYSTEMIC PSORIASIS THERAPY
Algorithms for selecting the most appropriate therapy among the newer systemic options for patients with psoriasis are lacking; however, a checklist of general factors to consider before initiating therapy might provide guidance when selecting an appropriate treatment for patients with psoriasis (Figure 4).16,50 The pretreatment screening procedures for patients with psoriasis are similar to those recommended for patients with rheumatoid arthritis, because they might receive similar pharmacotherapies.16,50 In broad terms, before the initiation of treatment with a conventional systemic agent, a thorough physical examination should be performed and a patient and family history should be obtained, particularly with regard to comorbid and/or uncontrolled renal, cardiac, vascular, and hepatic diseases or symptoms. Patients should be assessed for exposure to risks of hepatitis, human immunodeficiency virus (HIV), or other recurrent or chronic infection; malignancy; excessive alcohol intake; hypertension, Type 2 diabetes mellitus; and dyslipidemia. Signs and symptoms potentially related to PsA should be assessed. Testing for tuberculosis should be performed. Metabolic syndrome should be evaluated.16 Metabolic syndrome is defined as three or more of the following: elevated waist circumference (≥40 inches in men or ≥35 inches in women), reduced HDL-cholesterol (<40mg/dL in men or <50mg/dL in women or on current treatment for reduced HDL-cholesterol), hypertension (or current treatment with an antihypertensive), and/or elevated fasting glucose (≥100mg/dL or current treatment for elevated glucose).51 Current medications should be carefully reviewed. Serum chemistry evaluations should include electrolyte, renal, and hepatic panels, as well as complete blood counts. With both methotrexate and acitretin, female patients of childbearing potential must be educated about reproductive restrictions; with acitretin, they also must agree to stringent adherence to birth control measures before treatment. Because acitretin must not be used in women who are pregnant or who intend to become pregnant during therapy or for at least three years following the discontinuation of acitretin, clinicians should refrain from using acitretin in female patients of childbearing potential unless they have severe psoriasis that is unresponsive to other treatment options or there are contraindications preventing the use of other treatment options.45 In patients treated with methotrexate, pregnancy should be avoided if men or women are receiving methotrexate (men for a minimum of three months after treatment discontinuation, and women for at least one ovulatory cycle after treatment discontinuation).43 Table 1 lists online resources for the identification, treatment, and monitoring of patients with psoriatic disease, based on the major treatment guidelines.14,18,62–64 Specific screening recommendations for individual agents are detailed in Tables 2, 3, 4, and 5.17,43–45,52–61 Based on the consensus among psoriasis experts, for patients beginning any treatment for psoriasis (e.g., topical therapy, phototherapy, systemic treatment), a follow-up evaluation should be performed three months after treatment initiation to assess tolerability and clinical response, with regular follow-up evaluations occurring every six months thereafter.18 If at any point in the treatment course the treatment response fails to meet at least “acceptable response” criteria, defined recently by the NPF as an achievement of BSA of less than three percent or BSA improvement of 75 percent or more from baseline, then alternative treatment options should be discussed with the patient.18
FIGURE 4.
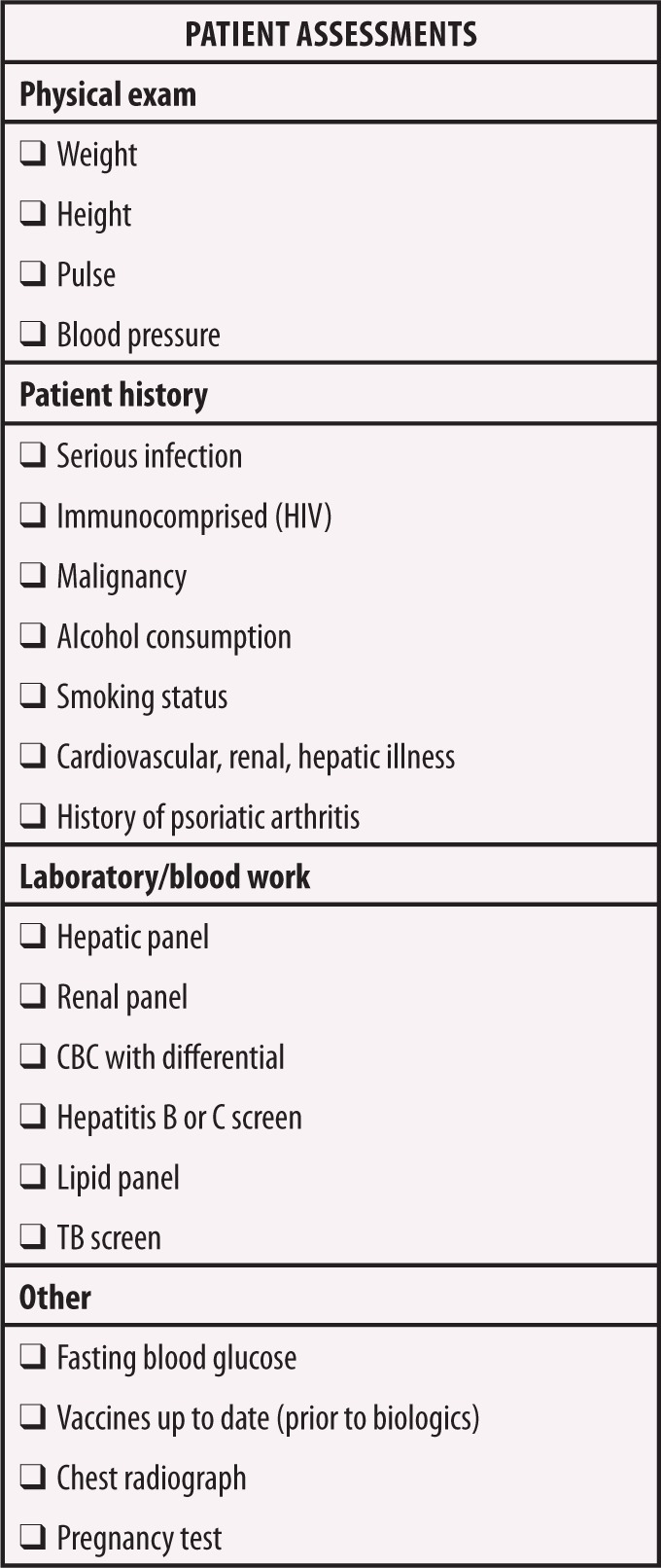
Assessments to be made before the initiation of systemic therapy for psoriasis16,50
TABLE 1.
Major guidelines for psoriatic disease screening, treatment, and monitoring
| ORGANIZATION | REFERENCE | ONLINE RESOURCES |
|---|---|---|
| American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF)14,15 | Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol.2019;80(4):1029–1072. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113. |
AAD-NPF Joint Guidelines: |
| NPF18 | Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290–298. |
|
| European Academy of Dermatology and Venereology (EADV)62 | Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris: update 2015, short version. EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29(12):2277–2294. | European S3 (EADV) Guidelines: https://onlinelibrary.wiley.com/doi/pdf/10.1111/jdv.13354 |
| European League Against Rheumatism (EULAR)63 | Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. | Full EULAR Guidelines: http://ard.bmj.com/content/annrheumdis/75/3/499.full.pdf |
| Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)64 | Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis: treatment recommendations for psoriatic arthritis 2015. Arthritis Rheumatol. 2016;68(5):1060–1071. | Access to GRAPPA Mobile App and Psoriatic Arthritis Pocket Guide: https://psa.guidelinecentral.com |
TABLE 2.
Overview of systemic agents for the treatment of chronic plaque psoriasis, oral systemic agents17,43-45,52-61
| DRUG FACTORS | METHOTREXATE | CYCLOSPORINE | ACITRETIN | APREMILAST |
|---|---|---|---|---|
| Contraindications |
|
|
|
|
| Baseline testing |
|
|
|
|
| Ongoing monitoring |
|
|
|
|
| Patient education |
|
|
|
|
| Dosing schedule |
|
|
|
|
| Drug interactions | PPIs, salicylates, NSAIDs, phenylbutazone, Phenytoin, sulfonamides, probenecid, cisplatin, mercaptopurine, oral antibiotics, other potential hepatotoxins, theophylline | Antibiotics, NSAIDs, melphalan, amphotericin B, antifungals, calcium channel blockers, Cimetidine, ranitidine, tacrolimus, fibric acid derivatives, methotrexate, methylprednisone, allopurinol, amiodarone, bromocriptine, colchicine, anticonvulsants, bosentan, octreotide, orlistat, sulfinpyrazone, terbinafine, ticlopidine, St. John’s Wort, SSRIs, boceprevir, telaprevir | Methotrexate, phenytoin, tetracyclines, oral retinoids, vitamin A supplements, microdose progestin minipill | Strong CYP450 inducers: rifampin, phenobarbital, carbamazepine, phenytoin |
| NSAID: Nonsteroidal anti-inflammatory drug; PPI: Proton pump inhibitor; CYP450: Cytochrome P450; CBC: Complete blood count; BP: Blood pressure; BUN: Blood urea nitrogen; TB: Tuberculosis; BID: Twice daily; LFT: Liver function test | ||||
TABLE 3.
Overview of systemic agents for the treatment of chronic plaque psoriasis, anti-TNF-α agents17,43-45,52-61
| DRUG FACTORS | ETANERCEPT56 | INFLIXIMAB | ADALIMUMAB59 | CERTOLIZUMAB61 |
|---|---|---|---|---|
| Contraindications |
|
|
||
| Baseline testing |
|
|
|
|
| Ongoing monitoring |
|
|
|
|
| Patient education |
|
|
|
|
| Dosing schedule |
|
|
|
|
| Drug interactions |
|
|
|
|
| *As of June 30, 2015, the United States Food and Drug Administration no longer includes pregnancy letter categories (i.e., A, B, C, D, and X) in prescription drug labeling; prescription drugs and biologic products approved or with label updates after June 30, 2015, no longer have pregnancy letter categories. | ||||
| TB: Tuberculosis; CBC: Complete blood count; LFT: Liver fuction tests; PPD: Purified protein derivative | ||||
TABLE 4.
Overview of systemic agents for the treatment of chronic plaque psoriasis, anti-IL-23 agents17,43-45,52-61
| DRUG FACTORS | USTEKINUMAB | GUSELKUMAB | TILDRAKIZUMAB |
|---|---|---|---|
| Contraindications |
|
|
|
| Baseline testing |
|
|
|
| Ongoing monitoring |
|
|
|
| Patient education |
|
|
|
| Dosing schedule |
|
|
|
| Drug interactions |
|
|
|
| *As of June 30, 2015, the United States Food and Drug Administration no longer includes pregnancy letter categories (i.e., A, B, C, D, and X) in prescription drug labeling; prescription drugs and biologic products approved or with label updates after June 30, 2015, no longer have pregnancy letter categories. | |||
| PPD:Purified protein derivative; TB: Tuberculosis | |||
TABLE 5.
Overview of systemic agents for the treatment of chronic plaque psoriasis, anti-IL-17 agents16,13-45,52-61
| DRUG FACTORS | SECUKINUMAB | IXEKIZUMAB | BRODALUMAB |
|---|---|---|---|
| Contraindications |
|
|
|
| Baseline testing |
|
|
|
| Ongoing monitoring |
|
|
|
| Patient education |
|
|
|
| Dosing schedule |
|
|
|
| Drug interactions |
|
|
|
| *As of June 30, 2015, the United States Food and Drug Administration no longer includes pregnancy letter categories (i.e., A, B, C, D, and X) in prescription drug labeling; prescription drugs and biologic products approved or with label updates after June 30, 2015, no longer have pregnancy letter categories. | |||
| IBD: Inflammatory bowel disease; PPD: Purified protein derivative; TB: Tuberculosis; CYP450: Cytochrome P450 | |||
For patients with inadequate response to an oral systemic medication, escalation to a biologic medication might be considered. As with conventional systemic agents, in broad terms, before beginning treatment with a biologic agent, patients should undergo a physical examination, including patient and family history. Screening steps for patients with psoriasis closely mirror the American College of Rheumatology screening recommendations for patients with rheumatoid arthritis.50 In general, patients should be screened for tuberculosis and assessed for risk of serious or chronic infection. In most cases, treatment with live vaccines should be avoided during course of treatment with a biologic; therefore, immunizations should be up to date prior to initiation of treatment.65 Patients can safely continue to receive nonlive influenza, pneumococcal, and hepatitis B vaccines.50 With brodalumab, patients should be screened for a history or risk of depression. With anti-TNF-α agents, pretreatment clinical laboratory screening should include tests for tuberculosis, hepatitis B and C, a hepatic panel, and a complete blood count. Specific screening recommendations for individual biologic agents are detailed in Tables 3–5. Recommended timing of initial and ongoing follow-up visits (initial visit at 3 months, then 6 months thereafter) is the same as for conventional oral systemic medications.18
CONSIDERATIONS FOR SPECIAL PATIENT POPULATIONS
Pregnancy. Before initiating any treatment, female patients of childbearing age should be advised of any contraindications before becoming pregnant or if there are risks or safety concerns associated with treatment during pregnancy. Female patients who later become pregnant or experience an unplanned pregnancy should be counseled again on the potential risks associated with their current treatment versus untreated severe psoriasis (e.g., increased risk of preterm and low birth weight infants as well as spontaneous abortion)66,67 and the potential risks posed by use of systemic treatments. Maintaining disease control (i.e., clear or almost-clear skin) before, during, and after pregnancy might optimize maternal and fetal outcomes.68 Although nearly one-half of pregnancies are unplanned,69 for those patients who are trying to conceive, reproductive counseling might be helpful. Counseling can help with patient treatment decisions regarding use of alternate medications, nonpharmacologic options, and options for switching to another pharmacologic treatment if the medication they are currently taking is contraindicated during pregnancy or breast-feeding or if the potential treatment risks outweigh the treatment benefits. For drugs that are contraindicated during pregnancy, consider timing drug discontinuation for the first trimester, and ensure patients have up-to-date vaccinations.66 Information on use during pregnancy from the prescribing information of the individual systemic agents is listed in Tables 2–5. The 2014 Food and Drug Administration (FDA) updated Pregnancy and Lactation Labeling Rule (PLLR) now requires labels to summarize available information on maternal and fetal risks of treatment during pregnancy, the presence of the drug in human milk, and its effects on milk production and breastfed children. The PLLR replaces the pregnancy category lettering system (A, B, C, D, X) and requires labels to state whether there is a pregnancy registry. The PLLR will likely encourage enrollment in pregnancy registries and facilitate provider and patient discussion about treatment during pregnancy.70,71 For medications that were FDA-approved before the removal of the pregnancy category lettering system, the letter categories are provided in Tables 2–5. Although the FDA no longer uses the pregnancy category lettering system, clincians might still find it useful to discuss the definitions of the categories with their patients of childbearing potential who are using or considering use of a treatment that has a pregnancy letter category.
In general, with regard to fetal safety, effective treatments that have been available for years are preferable to newer treatments that have fewer data. There are no published human data on pregnancy outcomes with apremilast; use of this agent should be avoided during pregnancy or lactation unless the potential benefits outweigh the potential risks. The conventional systemic agents acitretin and methotrexate are clearly contraindicated. Cyclosporine should be avoided during pregnancy unless the potential benefits outweigh the potential risks.66 Biologics appear to pose relatively lower risk of fetal harm. To gather human data regarding pregnancy risks while receiving systemic treatment for psoriasis, a number of ongoing pregnancy registries monitor pregnancy outcomes in women with psoriasis who are exposed to newer agents during pregnancy.
Cancer. With the development of more effective cancer treatments, it is increasingly common to treat patients with psoriasis who are cancer survivors. The safety of immunosuppressive drugs in such patients has not been widely studied. However, studies of patients with rheumatoid arthritis and guidelines for the treatment of rheumatoid arthritis provide some insight. One recent investigation of patients with rheumatoid arthritis showed that the risk of a second malignancy was not increased in those who had survived a primary cancer and received subsequent treatment with a biologic DMARD.72 Guidelines for rheumatoid arthritis might not necessarily apply to psoriasis; however, current American College of Rheumatology recommendations for patients with rheumatoid arthritis state that while patients with histories of solid tumors might receive the same standard-of-care treatment as those without histories of solid tumors based on disease severity, treatments other than TNF-α inhibitors should be considered in patients with a history of a lymphoproliferative disorder due to potential increased risk of lymphoma with some TNF-α inhibitors.50
Immunocompromised patients. For immunocompromised patients (e.g., those with HIV) topical therapy, phototherapy in conjunction with antiretrovirals, and acitretin are recommended options.73 Immunocompromised patients generally should only receive immunosuppressive agents, such as cyclosporine, or biologic agents, in circumstances of severe or recalcitrant psoriasis, and their treatment should be coordinated with their infectious disease physician.73,74 Given the increased understanding of the pathophysiology of HIV and a growing number of FDA-approved treatments for psoriasis, updated guidelines are needed to assist clinicians in making informed treatment decisions with their patients who have HIV or other immunodeficiencies and psoriasis.
Surgical patients. According to recommendations published by the NPF, patients with psoriasis who plan to undergo routine or low-risk surgery can safely continue using certain systemic therapies, including methotrexate, cyclosporine, and TNF-α inhibitors (e.g., etanercept, infliximab, adalimumab).75 For moderate- to high-risk operations, treatment decisions should be based on each individual patient’s risk factors and comorbidities.75 Updated recommendations are needed to reflect the availability of more recently approved systemic agents, such as apremilast, and newer biologics (e.g., inhibitors of IL-17, IL-23, IL-12/23).
APPROVED SYSTEMIC AGENTS
A brief overview of current options for systemic therapy with respect to baseline screening and monitoring is presented in Tables 2–5. In general, the available systemic agents have all demonstrated efficacy but vary widely with regard to drug class and proposed mechanism of action (MOA) in psoriasis.
Methotrexate. Mechanism(s) of action. Methotrexate43,52,76 is an antimetabolite that acts as an inhibitor of dihydrofolic acid reductase; its MOA in psoriasis is thought to be linked to its inhibition of proliferation of lymphoid tissues and cells, such as T-cells and macrophages.52
Contraindications. The prescribing information for methotrexate includes several contraindications, listed in Table 2.43
Dosage and administration. Methotrexate for psoriasis is usually taken by mouth weekly in divided doses and titrated to achieve optimum efficacy (Table 2).
Warnings, precautions, and adverse effects. Warnings and precautions associated with methotrexate include congenital abnormalities or death in exposed fetuses; renal toxicity; myelosuppression; hepatotoxicity; pulmonary fibrosis; hemorrhagic enteritis; malignant lymphoma; severe, potentially fatal skin reactions; and serious infection.43 Folic acid supplements are strongly recommended for patients taking methotrexate to provide prophylaxis against some of the associated toxicities and to improve tolerability.52 Patients should be counseled that continuous daily dosing of methotrexate can be fatal (it should be dosed weekly). They should also be counseled to avoid or strictly limit use of alcohol, to use a reliable form of contraception, to avoid use of nonsteroidal anti-inflammatory drugs (NSAIDs) and salicylates, and to use caution when taking proton pump inhibitors concurrently.43,76 And finally, patients should be counseled to contact a clinician if any sign of infection, skin reaction, diarrhea, coughing, or unusual bleeding occurs (Table 2).43,76
Cyclosporine. Mechanism(s) of action. Cyclosporine is a cyclic polypeptide immunosuppressant; its MOA in psoriasis is thought to be related to its ability to reversibly inhibit the expansion of T-helper cell populations.44
Contraindications. There are some contraindications to cyclosporine use listed in the prescribing information (Table 2).
Dosage and administration. For psoriasis, dosing of cyclosporine oral gelatin capsules or solution should begin at 2.5mg/kg, given in two divided doses per day; the daily dose can be increased to a maximum of 4.0mg/kg/day.44 Blood pressure and renal function must be closely monitored during cyclosporine therapy, with downward dose adjustments made in response to signs of hypertension or renal dysfunction.44
Warnings, precautions, and adverse effects. Warnings and precautions associated with cyclosporine include nephrotoxicity, including structural kidney damage, hypertension, hepatotoxicity, and serious infection;44,52 moreover, patients with psoriasis previously treated with other immunosuppressants, methotrexate, psoralen/UVA (PUVA), UVB, coal tar, or radiation therapy are at increased risk of skin malignancies when receiving cyclosporine.44 Of note, the AAD recommends that cyclosporine be used with extreme caution in elderly patients or those who are immunodeficient, pregnant, or have obesity.52 Patients should be informed that numerous drugs can interact with cyclosporine and that they should report the use of and changes in concomitant medications to the healthcare professional providing their treatment (Table 2). Intake of drugs that increase the renal toxicity of cyclosporine should be restricted, and these include aminoglycosides, NSAIDs, and agents that increase potassium levels.52 Patients should be further counseled against drinking grapefruit juice (it increases cyclosporine concentrations) and receiving live vaccinations (Table 2).44 Cyclosporine is not recommended for continuous use in patients with psoriasis for more than one year;44 it should be used only as an interventional treatment.52
Acitretin. Mechanism(s) of action. Acitretin is a retinoid derived from the metabolism of etretinate.45 Although the MOA of acitretin in psoriasis is unknown, retinoid agents are known to modulate epidermal proliferation and differentiation as well as have immunomodulatory and anti-inflammatory effects.52
Contraindications. Contraindications to acitretin use include pregnancy, the presence of severe hepatic or renal impairment, and hyperlipidemia (Table 2).45
Dosage and administration. Acitretin is taken by mouth once daily in capsule form at doses of 25 to 50mg/day. It is recommended that acitretin be taken with the main meal of the day.45 Patients should be counseled about necessary lifestyle changes to prevent or reduce hyperlipidemia associated with acitretin therapy.52
Warnings, precautions, and adverse effects. Warnings and precautions associated with acitretin are severe birth defects in exposed fetuses, hepatotoxicity, hyperlipidemia, liver toxicity and toxic hepatitis, hyperostosis, pancreatitis, and pseudotumor cerebri (benign intracranial hypertension).45 Because acitretin lacks significant immunosuppressive activity, patients with HIV and severe psoriasis might consider it a useful treatment option.52 Women of childbearing potential must not become pregnant during treatment and for at least three years after discontinuing treatment; additionally, patients must not drink alcohol during treatment and for at least two months afterwards. Healthcare providers should counsel patients about several preventive safety measures and lifestyle changes to consider when taking acitretin (e.g., decreased night vision, avoiding donating blood) (Table 2).
Apremilast. Mechanism(s) of action. Apremilast is a small-molecule phosphodiesterase 4 inhibitor specific to cyclic adenosine monophosphate.55 The MOA in psoriasis is not well-defined, but is likely related to its regulation of the expression of a number of proinflammatory and anti-inflammatory cytokines, such as IL-17, IL-23, and TNF-α, which are known to be involved in the pathology of psoriasis.77
Contraindications. Contraindications to apremilast use include hypersensitivity to apremilast (Table 2).55
Dosage and administration. Apremilast is dosed orally in tablet form, with a recommended dose of 30mg twice daily. Dose titration during the first week of treatment can help minimize the gastrointestinal symptoms (diarrhea and nausea) associated with initial therapy.55 Most cases of diarrhea and nausea occurred within two weeks of the first dose, were predominantly mild in severity, and generally resolved within one month.55 No ongoing laboratory monitoring is required.
Warnings, precautions, and adverse effects. Warnings and precautions associated with apremilast include diarrhea, nausea, vomiting, depression, and weight loss.55 Postmarketing reports of severe diarrhea, nausea, and vomiting associated with apremilast treatment, with some cases requiring hospitalization, have predominantly occurred within the first few weeks of treatment.55 Patients who might be more susceptible to such complications include those older than 65 years of age and those taking medications that can lead to volume depletion or hypotension; these patients should be monitored.55 Apremilast dose should be reduced or suspended if a patient develops severe diarrhea, nausea, or vomiting. Patients should be cautioned against concomitant use of strong cytochrome P450 enzyme inducers, such as rifampin, carbamazepine, or Phenytoin, due to potential reduction in apremilast’s efficacy (Table 2). Patients taking apremilast, as well as their family members or caregivers, should also be alert for the emergence of new or worsening signs of depression, suicidal thoughts, or changes in mood as well as unusual weight loss (Table 2).55
Etanercept, infliximab, adalimumab, and certolizumab. Mechanism(s) of action. Etanercept, infliximab, adalimumab, and certolizumab are biologic anti-TNF-α agents that selectively neutralize the activity of TNF-α.53,56,59,61 The likely MOA of all four agents in psoriasis is related to decreasing inflammatory processes related to TNF-α activity.
Contraindications. Contraindications to etanercept, infliximab, and adalimumab include known hypersensitivity to the agents, their components, or any murine proteins; patients with an active infection should not receive these agents (Table 3).53,56,59 Certolizumab has no contraindications listed, though treatment should not be initiated during an active infection.61
Dosage and administration. Etanercept is approved for use in patients with plaque psoriasis four years of age or older. Etanercept, adalimumab, and certolizumab are administered via subcutaneous injection weekly or every other week; infliximab is given via intravenous infusion every eight weeks, which requires administration in an outpatient treatment center.56,59
Patients receiving etanercept, adalimumab, or certolizumab must be instructed on how to correctly measure the prescribed dose and self-administer a subcutaneous injection; they should administer their first dose under the supervision of a qualified provider.56,59
Warnings, precautions, and adverse effects. Warnings and precautions associated with the use of biologic anti-TNF-α agents include serious infections, lymphoma or other malignancies; worsening heart failure (in patients with pre-existing moderate-to-severe heart failure); and reactivation of tuberculosis or hepatitis B virus.53,56,59,61 The most common AEs associated with etanercept and adalimumab are injection-site reactions (e.g., pain, swelling, erythema, hemorrhage), which are mostly mild to moderate in severity and tend to decrease in frequency with continued treatment;56,59 patients who experience these events might require reassurance and counseling about management strategies. Patients receiving these agents should be advised to seek medical attention if signs of an infection develop, which should be monitored carefully. Patients should be further advised against receiving any live vaccinations (Table 3) and to be alert for possible allergic infusion/injection hypersensitivity reactions, signs/symptoms of central nervous system (CNS) demyelinating disorders, heart failure, lupus-like syndromes, pancytopenia, or autoimmune hepatitis.53,56,59 Use of TNF-α blockers, including certolizumab and adalimumab, has been associated with rare cases of CNS demyelinating disease, such as multiple sclerosis and optic neuritis, and peripheral demyelinating disease, such as Guillain–Barré syndrome; patients should discontinue treatment if these disorders develop.53,56,59
Ustekinumab, guselkumab, and tildrakizumab. Mechanism(s) of action. Ustekinumab, guselkumab, and tildrakizumab are biologic agents that bind and inhibit the interaction of IL-23 with its receptor on the surfaces of natural killer and T lymphocytes, which are involved in the pathology of psoriasis.60,78,79 Ustekinumab also inhibits the binding activity of IL-12. Risankizumab, a humanized IgG1 monoclonal antibody that selectively inhibits IL-23 by binding to the p19 subunit, is currently undergoing FDA review for the treatment of moderate to severe plaque psoriasis.14
Contraindications. Contraindications of ustekinumab and tildrakizumab include hypersensitivity to the agents or any excipients in the formulations (Table 4).60,78 There are no contraindications to the use of guselkumab.79
Dosage and administration. Ustekinumab is approved for use in patients aged 12 years or older.78 These agents are given via subcutaneous injection, and after an initial loading dose schedule, they are given once every eight weeks (guselkumab) or every 12 weeks (ustekinumab and tildrakizumab).60,78,79
Warnings, precautions, and adverse effects. Warnings and precautions associated with use of these agents include serious infection, such as tuberculosis, malignancy (ustekinumab), and hypersensitivity reactions (ustekinumab and tildrakizumab).60,78 Patients should be tested for tuberculosis before initiating guselkumab or tildrakizumab.60,79 Patients should be advised against receiving any live vaccinations while taking these agents (Table 4). Patients receiving ustekinumab should be monitored for the appearance of nonmelanoma skin cancers (Table 4).
Secukinumab, ixekizumab, and brodalumab. Mechanism(s) of action. Secukinumab, ixekizumab, and brodalumab are biologic agents that inhibit the activity of IL-17A (secukinumab and ixekizumab) or IL-17 receptor A (brodalumab).54,57,58
Contraindications. Contraindications include hypersensitivity to the agent or any excipient in the formulation (secukinumab and ixekizumab) or Crohn’s disease (brodalumab) (Table 5).54,57,58
Dosage and administration. Following an initial loading dose schedule, secukinumab, ixekizumab, and brodalumab are given via subcutaneous injection once every four weeks (secukinumab and ixekizumab) or once every two weeks (brodalumab).54,57,58
Warnings, precautions, and adverse effects. Warnings and precautions associated with their use include serious infections, such as tuberculosis; reactivation or worsening of inflammatory bowel disease (IBD) in patients with a history of IBD or comorbid IBD (use of IL-17 inhibitors should be avoided in patients with a history of or comorbid IBD14);and allergic hypersensitivity reactions, including anaphylaxis.54,57,58 Postmarketing reports of anaphylaxis with ixekizumab have been received.57 Brodalumab has been linked to increased incidence of suicidal ideation and behaviors among patients with a history of depression or suicidal ideation or behaviors.58 Due to these risks, prescription and dispensary of brodalumab is restricted to specially certified healthcare providers and pharmacies. Patients and their caregivers should be counseled to be alert for any emergence, change, or worsening of depression, altered mood, or anxiety.58 With secukinumab, patients with latex sensitivity should not handle the removable syringe cap, which contains latex.54 With secukinumab, ixekizumab, and brodalumab, patients should be advised against receiving any live vaccinations (Table 5).
DISCUSSION
Psoriasis is a systemic immunologic disease that is undertreated in a substantial number of patients. Many patients currently receiving topical therapies might achieve greater benefit from systemic therapies. It is important for dermatologists to consider patient-reported itching, pain, location of lesions, and level of QOL impairment when discussing treatment options with their patients suffering from psoriasis so that potential candidates for systemic therapy can be identified. The needs and preferences of the patient should also be carefully considered. Once the clinician and patient decide to initiate systemic therapy, the clinician should educate the patient on the advantages and disadvangtages of each individual agent. The criteria for introducing systemic therapy following topical and/or phototherapy failure are not clearly defined but should be driven by timely identification of failure of topical therapy and patient subjective factors, preferences, and existing comorbidities.
Overall, patients and providers might have different perceptions regarding which factors contribute most to psoriasis severity. Patients might emphasize subjective perceptions (e.g., itch, QOL, and treatment satisfaction), whereas providers might focus more on the visible features of psoriasis involvement (e.g., BSA, location and severity of erythema, induration, scaling). Discussion between patients and providers should include consideration of comorbidities, possible treatment interactions, and side effects;80 family planning; occupational considerations, such as work-related travel, that might impact the patient’s ability to adhere to a dosing schedule (as in the case of those biologics that require intravenous infusion); patient treatment priorities (e.g., safety, efficacy, and cost); and appropriate laboratory and screening requirements (e.g., vaccines, tuberculosis screening, depression, and hepatic, renal, and CVD). Based on this discussion, providers can identify viable options, taking into consideration contraindications, comorbidities, drug interactions, and insurance coverage.
It is also crucial for dermatology providers to inform patients what to expect in terms of tolerability and efficacy, how to manage or minimize bothersome side effects, typical time to onset of clinical efficacy, the route and schedule of dosing, potential effects on vaccines, potential risk of infection (including tuberculosis), and any required ongoing safety monitoring. If a patient has PsA, systemic agents that are appropriate for both psoriasis and PsA, such as methotrexate, apremilast, and many biologic agents, should be considered as first-line therapy. After initiating systemic therapy, patients should return for timely follow-up evaluation and discussion of efficacy, tolerability, and, in some cases, laboratory monitoring. It is imperative that providers maintain a therapeutic relationship with patients to enhance adherence to therapy and improve patient outcomes. Additional guidance for selecting and safely initiating systemic therapy, based on evidence-based treatment recommendations, was recently published in the joint American Academy of Dermatology-National Psoriasis Foundation (AAD-NPF) guidelines for the management and treatment of psoriasis.14,15
CONCLUSION
Many patients with psoriasis currently receiving only topical therapies might benefit from systemic therapies. Many patients with psoriasis across all levels of severity are either untreated or are undertreated with topical monotherapies; however, patients with moderate psoriasis, in particular, might benefit from a more timely initiation of systemic therapy in order to halt the progression of cutaneous symptoms and potentially decrease systemic manifestations of psoriatic disease.
A practical approach to classifying patients with psoriasis differentiates those who are adequately treated with topicals and/or phototherapy from those who require systemic therapy. This approach needs to move beyond observable symptoms, such as BSA, erythema, and scaling, to more fully consider patient-perceived subjective symptoms, such as itch, pain, locations of lesions, and QOL impairment.
When starting a patient on systemic therapy, attributes of individual systemic therapies should be considered along with patient preferences. Newer treatment options can further address a patient’s desire for safer, more effective, and simpler therapy.
REFERENCES
- 1.Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–ii7. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130(7):1785–1796. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermotology. 2012;225(2):121–126. doi: 10.1159/000342180. [DOI] [PubMed] [Google Scholar]
- 4.Heimick CG, Lee-Han H, Hirsch SC, et al. Prevalence of Psoriasis Among Adults in the U.S.: 2003–2006 and 2009–2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47(1):37–45. doi: 10.1016/j.amepre.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermotol. 2012;132:556–562. doi: 10.1038/jid.2011.365. (3 Pt 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 7.Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411–2418. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta NN, Yu Y, Pinnelas R, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124(8):775.e1–e6. doi: 10.1016/j.amjmed.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azfar RS, Seminara NM, Shin DB, et al. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148(9):995–1000. doi: 10.1001/archdermatol.2012.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961.. doi: 10.1136/bmj.f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi CC, Wang J, Chen YF, et al. Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: a nationwide population-based cohort study. J Dermatol Sci. 2015;78(3):232–238. doi: 10.1016/j.jdermsci.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Svedbom A, Dalen J, Mamolo C, et al. Increased cause-specific mortality in patients with mild and severe psoriasis: a population-based Swedish register study. Acta Derm Venereol. 2015;95(7):809–815. doi: 10.2340/00015555-2095. [DOI] [PubMed] [Google Scholar]
- 14.Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologic. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 15.Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113. doi: 10.1016/j.jaad.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 16.16.Van Voorhees AS, Feldman SR, Koo JYM, et al. The Psoriasis and Psoriatic Arthritis Pocket Guide: Treatment Algorithms and Management Options 4th ed. Alexandria, VA: National Psoriasis Foundation; 2016. [Google Scholar]
- 17.Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologic. J Am Acad Dermatol. 2008;58(5):826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290–298. doi: 10.1016/j.jaad.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–881. doi: 10.1016/j.jaad.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149(10):1180–1185. doi: 10.1001/jamadermatol.2013.5264. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong AW, Schupp C, Wu J, Bebo B. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003–2011. PLoS One. 2012;7(12):e52935. doi: 10.1371/journal.pone.0052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Kerkhof PCM, Reich K, Kavanaugh A, et al. Physician perspectives in the managment of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis suney. J Eur Acad Dermatol Venereol. 2015;29(10):2002–2010. doi: 10.1111/jdv.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong AW, Koning JW, Rowse S, et al. Undertreatment of patients with moderate to severe psoriasis in the United States: analysis of mediation usage with health plan data. Dermatology Ther. 2017;7(1):97–109. doi: 10.1007/s13555-016-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–735. doi: 10.1016/j.jaad.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 25.McCain J. The disease burden of the most common autoimmune diseases. Manag Care. 2016;25(7):28–32. [PubMed] [Google Scholar]
- 26.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin. 2015;33(1):13–23. doi: 10.1016/j.det.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Gao H, Loyd CM, et al. Chronic skin-specificinflammation promotes vascular inflammation and thrombosis. J Invest dermatol. 2012;132(8):2067–2075. doi: 10.1038/jid.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77(2):287–292. doi: 10.1016/j.jaad.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 31.Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76(1):81–90. doi: 10.1016/j.jaad.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 32.Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43(11):1267–1272. doi: 10.1111/1346-8138.13398. [DOI] [PubMed] [Google Scholar]
- 33.Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148(11):1244–1250. doi: 10.1001/archdermatol.2012.2502. [DOI] [PubMed] [Google Scholar]
- 34.Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol. 2015;29(6):1128–1134. doi: 10.1111/jdv.12768. [DOI] [PubMed] [Google Scholar]
- 35.Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273(2):197–204. doi: 10.1111/j.1365-2796.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- 36.Carvalho AV, Romiti R, Souza CD, et al. Psoriasis comorbidities: complications and benefits of immunobiological treatment. An Bras Dermatol. 2016;91(6):781–789. doi: 10.1590/abd1806-4841.20165080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman SR. Disease burden and treatment adherence in psoriasis patients. Cutis. 2013;92(5):258–263. [PubMed] [Google Scholar]
- 38.Reich K, Griffiths CE. The relationship between quality of life and skin clearance in moderate-to-severe psoriasis: lessons learnt from clinical trials with infliximab. Arch Dermatol Res. 2008;300(10):537–544. doi: 10.1007/s00403-008-0885-7. [DOI] [PubMed] [Google Scholar]
- 39.Schafer I, Hacker J, Rustenbach SJ, et al. Concordance of the Psoriasis Area and Severity Index (PASI) and patient-reported outcomes in psoriasis treatment. Eur J Dermatol. 2010;20(1):62–67. doi: 10.1684/ejd.2010.0815. [DOI] [PubMed] [Google Scholar]
- 40.Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170(3):672–680. doi: 10.1111/bjd.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finch T, Shim TN, Roberts L, Johnson O. Treatment satisfaction among patients with moderate-to-severe psoriasis. J Clin Aesthet Dermatol. 2015;8(4):26–30. [PMC free article] [PubMed] [Google Scholar]
- 42.Schaarschmidt ML, Kromer C, Herr R, et al. Patient preferences for biologicals in psoriasis: top priority of safety for cardiovascular patients. PLoS One. 2015;10(12):e0144335. doi: 10.1371/journal.pone.0144335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fort Lee, NJ: DAVA Pharmaceuticals, Inc.; 2009. Methotrexate [package insert] [Google Scholar]
- 44.East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013. Neoral [package insert] [Google Scholar]
- 45.Research Triangle Park, NC: Stiefel Laboratories Inc.; 2014. Soriatane [package insert] [Google Scholar]
- 46.Singh JA, Cameron C, Noorbaloochi S, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386(999):258–265. doi: 10.1016/S0140-6736(14)61704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramiro S, Gaujoux-Viala C, Nam JL, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2014;73(3):529–535. doi: 10.1136/annrheumdis-2013-204575. [DOI] [PubMed] [Google Scholar]
- 48.Gottlieb AB, Levin AA, Armstrong AW, et al. The International Dermatology Outcome Measures Group: formation of patient-centered outcome measures in dermatology. J Am Acad Dermatol. 2015;72(2):345–348. doi: 10.1016/j.jaad.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Thorn D. Appraising current methods for preclinical calculation of burn size—A pre-hospital perspective. Bums. 2017;43(1):127–136. doi: 10.1016/j.burns.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Singh JA, Saag KG, Bridges SL, Jr., et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(1):1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 51.Grundy SM, Cleeman Jl, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 52.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659. doi: 10.1016/j.jaad.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 53.Horsham, PA: Janssen Biotech, Inc.; 2013. Remicade (infliximab) [package insert] [Google Scholar]
- 54.East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. Cosentyx [package insert] [Google Scholar]
- 55.Summit, NJ: Celgene Corporation; 2017. Otezla [package insert] June. [Google Scholar]
- 56.Thousand Oaks,CA: Immunex Corporation; 2017. Enbrel [package insert] November. [Google Scholar]
- 57.Indianapolis, IN: Eli Lilly and Company; 2017. Taltz [package insert] January. [Google Scholar]
- 58.Bridgewater, NJ: Valeant Pharmaceuticals North America; 2017. Siliq [package insert] February. [Google Scholar]
- 59.North Chicago, IL: AbbVie Inc.; 2014. Humira (adalimumab) [package insert] [Google Scholar]
- 60.Whitehouse Station, NJ: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.; 2018. Ilumya [package insert] March. [Google Scholar]
- 61.Smyrna, GA: UCB, Inc.; 2018. Cimzia [package insert] May. [Google Scholar]
- 62.Strohal R, Prinz JC, Girolomoni G, Nast A. A patient-centred approach to biological treatment decision making for psoriasis: an expert consensus. J Eur Acad Dermatol Venereol. 2015;29(12):2390–2398. doi: 10.1111/jdv.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 64.Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis: treatment recommendations for psoriatic arthritis 2015. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 65.Research Triangle Park, NC: GlaxoSmithKline; 2017. Shingrix [package insert] October. [Google Scholar]
- 66.Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59(2):86–100. doi: 10.1111/ajd.12641. [DOI] [PubMed] [Google Scholar]
- 67.Pottinger E, Woolf RT, Exton LS, et al. Exposure to biological therapies during conception and pregnancy: a systematic review. Br J Dermatol. 2018;178(1):95–102. doi: 10.1111/bjd.15802. [DOI] [PubMed] [Google Scholar]
- 68.Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Momms Dermatol. 2017;3(1):21–25. doi: 10.1016/j.ijwd.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Final rule. Fed Regist. 2014;79(233):72063–72103. U.S. Department of Health and Human Services. [PubMed] [Google Scholar]
- 71.Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. PT. 2016;41(11):713–715. [PMC free article] [PubMed] [Google Scholar]
- 72.Dreyer L, Cordtz RL, Hansen IMJ, et al. Risk of second malignant neoplasm and mortality in patients with rheumatoid arthritis treated with biological DMARDs: a Danish population-based cohort study. Ann Rheum Dis. 2018;77(4):510–514. doi: 10.1136/annrheumdis-2017-212086. [DOI] [PubMed] [Google Scholar]
- 73.Menon K, Van Voorhees AS, Bebo BF, Jr., et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62(2):291–299. doi: 10.1016/j.jaad.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 74.Armstrong AW, Aldredge L, Yamauchi PS. Managing patients with psoriasis in the busy clinic: practical tips for health are practitioners. J Cutane Med Surg. 2016;20(3):196–206. doi: 10.1177/1203475415623508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi YM, Debbaneh M, Weinberg JM, et al. From the Medical Board of the National Psoriasis Foundation: Perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016;75(4):798–805.e7. doi: 10.1016/j.jaad.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 76.Jones KW, Patel SR. A family physician’s guide to monitoring methotrexate. Am Earn Physician. 2000;62(7):1607–1612,1614. [PubMed] [Google Scholar]
- 77.Schafer PH, Parten A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–2029. doi: 10.1016/j.cellsig.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Horsham, PA: Janssen Biotech, Inc; 2018. Stelara [prescribing information] February. [Google Scholar]
- 79.Horsham, PA: Janssen Biotech, Inc.; 2017. Tremfya [package insert] July. [Google Scholar]
- 80.Schmieder A, Schaarschmidt ML, Umar N, et al. Comorbidities significantly impact patients’ preferences for psoriasis treatments. J Am Acad Dermatol. 2012;67(3):363–372. doi: 10.1016/j.jaad.2011.08.023. [DOI] [PubMed] [Google Scholar]